|
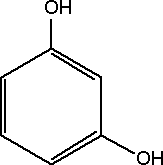
Substance Flow Analysis of Resorcinol 1 Introduction1.1 Purpose of the analysis 1.1 Purpose of the analysisResorcinol has been prioritized as being a chemical compound of high concern because studies have provided evidence that resorcinol may cause endocrine disruption (BKH, 2000). In order to provide an overview of the flows of resorcinol in Denmark and its potential for exposure of the environment and humans, the Danish Environmental Protection Agency has initiated the present substance flow analysis of resorcinol in Denmark. The purpose of the analysis is to investigate the use, consumption and disposal of resorcinol in order to provide an overview of its dissemination and exposure potential. Resorcinol is known to have a potential for human exposure through pharmaceuticals, different types of cosmetics used on the skin, and by inhalation of wood and cigarette smoke. However, the extent of the exposure in Denmark has not previously been investigated. 1.2 Methodology and limitationsThe investigation is made as an overview analysis of the flows of resorcinol in Denmark. The main fields of application and the sources of environmental exposure related to resorcinol are identified. The basic principles of the investigation are similar to those defined in the paradigm for substance flow analysis developed for the Danish EPA (Lassen & Hansen, 2000). However, due to limited statistical information, the main flows of resorcinol have also been estimated by direct contact to Danish industries. The two types of information are combined to give a low and a high estimate for flows of resorcinol in Denmark. The EU Technical Guidance Document for estimation of emissions in relation to risk assessment has been used to establish figures for emissions to air, water and soil from all resorcinol-related activities in Denmark. This is not an element in the paradigm, but was judged to be applicable despite – and because – of the limited amount of information. The report thus provides both a realistic estimate of the range for resorcinol consumption in Denmark, and a fairly conservative estimate of exposure of the environmental compartments air, water, and soil as well as amounts and fate of resorcinol-containing waste. By establishing both types of information, the report is better suited for decision-support, e.g. in relation to the need for a risk assessment or potential regulatory measures. The main limitation of the study is the uncertainty regarding the completeness of the statistical information. Some information may be missing and other figures may be over- or underestimated due to changes in consumption patterns. On the exposure side, the main limitation of the study is the lack of knowledge regarding the state of resorcinol when emitted with waste water, i.e. has the compound reacted with other substances or is it still in its active form? 1.3 What is Resorcinol?Resorcinol is the meta (1,3) isomer of dihydroxybenzene. The molecular structure is illustrated below.
Figure 1-1 The molecular structure of Resorcinol. The hydrogen atom surrounded by the two meta-hydroxyl groups can be substituted much more easily than the other ring hydrogens. Compared to catechol and hydroquinone, resorcinol has the highest reactivity toward formaldehyde (Ullmann's, 2002). Resorcinol is produced with benzene as the main feedstock. In The United States resorcinol is produced using the “classical” route via 1,3-benzenedisulfonic acid. In Japan, resorcinol is produced via 1,3-diisopropylbenzene (Ullmann's, 2002). 1.3.1 SynonymsVarious synonyms and abbreviations of resorcinol are used in the literature. In this report the trivial name resorcinol is used. Some of the synonyms used include 1,3–Benzenediol, 1,3-Dihydroxybenzene, 1,3–Benzoldiol, m-Hydroquinone and Resorcin (HSDB, 2003). 1.3.2 Chemical and physical propertiesResorcinol [Cas. No. 108-46-3], C6H6O2 is a dihydric phenol and exhibits the typical reactivity of a phenol. Its most important reaction is with formaldehyde to form phenolic resins (Ullmann's, 2002). Resorcinol is a white to off-white needle-like crystals, flakes or powder. When exposed to light and air resorcinol crystals acquire a pink tint (NTP, 2003). It has a weak odor and a bittersweet taste. Resorcinol does not occur naturally, but has been found in waste effluents from coal gasification and as a pollutant in filtered ground and surface water at a waste treatment plant (Spectrum Laboratories, 2003). It has been produced industrially for more than 100 years (Ullmann's, 2002). The typical chemical and physical properties of resorcinol are presented below (NTP, 2003).
1.4 International market and trends in consumption1.4.1 Global market and consumptionGlobal production of resorcinol reached 46,000 metric tons in year 2000, representing a total value of $233 million. The United States is the largest producer and consumer of resorcinol. In 2000, the U.S. plant operated near full capacity, while Japanese production exceeded its nameplate capacity (Hajduk, 2001). In addition, there are three small plants in China, two in India and an additional plant in Japan (Spectrum laboratories, 2003). Western Europe's last remaining resorcinol plant was closed in late 1991 because of environmental concerns. Since then, periodic announcements state that a new resorcinol plant will be built in Europe. However, no such plant is currently under construction and all plans appear to be postponed indefinitely. Japanese resorcinol production is primarily for export (80% of production was exported in 2000). Capacity in Japan was last increased in late 1993. Trade among regions is significant: more than one-half of the world production is exported (about 29 thousand metric tons in 2000). From 2000 to 2005, world consumption of resorcinol is expected to grow at a 0.6% average annual rate, with Japan exhibiting no growth, Europe contracting and the developing regions growing at 3-4% (Hajduk, 2001). The primary consumer of resorcinol at the global level (more than 50 %) is the rubber industry. In the production of tires and other reinforced rubber products (conveyor belts, driving belts),
resorcinol-phenol-formaldehyde condensates are used to enhance adhesion between cord and rubber (dip formulations, dry bonding agents). Furthermore, some rubber mixtures contain resorcinol to
improve properties after curing.
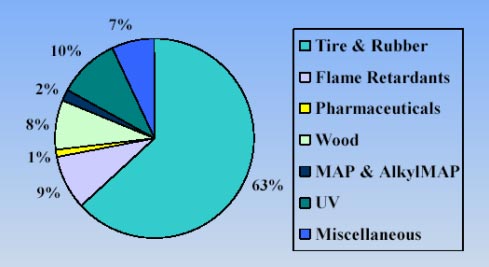
1.4.2 EU market, consumption and trendsThe European Community imported approximately 13,000 tons of resorcinol in year 2001. From 1979 to 1990 the import of resorcinol to Western Europe grew from 2,300 to 3,300 tons. From 1991 and 1995 the import was much higher and fluctuated between 7,000 to 10,700 tons. From 1996 to 1999 the import has grown from 9,000 to 9,600 tons, which is equivalent to about 1.6% annually (RTF, 2002). The distribution of the imported resorcinol for use in different industries can be seen in Figure 1-2 below. As can be seen, the consumption pattern differs somewhat from the overall international consumption, the main uses being in tire and rubber production, but with a lower use in wood production, only 8% compared to the 25% observed internationally.
Figure 1-2. Illustration of the use of resorcinol according to industry in the European Community. Reprinted from (RTF, 2002). (MAP = m-aminophenyl production).
|
||||||||||||||||||