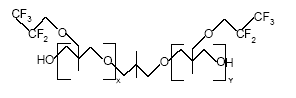|
Environmental Project no. 1013 More environmentally friendly alternatives to PFOS-compounds and PFOAContents
4 Use of PFOS-related compounds, PFOA, and their alternatives
5 Technical assessment of alternatives
6 Environment and health assessment of PFOS and PFOA and other PFAS substances
7 Environment and health assessment of alternatives to PFOS/PFOA related compounds
Appendix A – Fluorine-containing substances – Terminology and definitions
Appendix B – Method used in search for alternatives
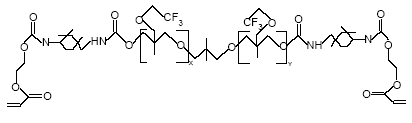
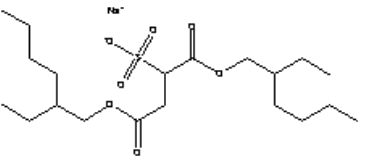
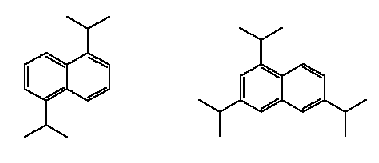
Appendix C – Use of PFOS-related compounds in Denmark Appendix D – Use of PFOA ammonium salt in Denmark Appendix E – Use of telomer alcohols in Denmark Appendix F – Identified alternatives
PrefaceThis project was initiated by the Danish Environmental Protection Agency as a part of the Program for Cleaner Products, Area 1.7 Collection of knowledge and mapping of alternatives to problematic substances. The report was drafted in the period January 2004 - November 2004, and it contains references to scientific papers being published until October 2004. Frank Jensen from the Danish EPA supervised the project. The growing environmental concern of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) derivatives is due to the fact that these potential harmful compounds now are global environmental pollutants distributed in air, water, soils and biota, including in polar bears living in remote arctic areas. In addition, PFOS and PFOA have been observed in human blood samples of the general population in many countries. The reason for this widespread occurrence seems to be that these perfluorinated substances are environmentally persistent and bioaccumulative. PFOS and a range of related perfluorinated compounds are used in numerous industrial products and consumer products because of their special chemical properties, for instance the ability to repel both water and oils. PFOA is used as a processing aid in the manufacture of fluoropolymers. PFOA is a well-documented contaminant in PFOS-related chemicals and fluorotelomers and may be found as an impurity in products containing PFOS-related chemicals. This project report contains the newest information about the properties, use and occurrences of PFOS, PFOA and related polyfluorinated compounds. In addition, a search for and a preliminary assessment of possible alternatives to these types of chemicals were done. The report focuses mainly on the Danish situation but may also be a contribution to the PFOS/PFOA discussion at European level. March 2005 Summary and conclusions
Background and purposePerfluorooctane sulfonate (PFOS) and a range of related perfluorinated compounds are used in numerous industrial products and consumer products because of their special chemical properties, for instance the ability to repel both water and oil. Perfluorooctanoic acid (PFOA) is used as a processing aid in the manufacture of fluoropolymers. PFOA is a well-documented contaminant in PFOS-related chemicals and telomers and is hence found as an impurity in various products. It is a growing concern that these potential harmful compounds now are found as widespread global environmental pollutants distributed in air, water, soils and biota, which indicates that perfluoroalkyl substances are environmentally persistent and bioaccumulate in wildlife and in humans. The implications of these occurrences and exposures are still not fully explored and understood but preliminary results have in some cases revealed unwanted adverse effects. Many more studies are underway, and in the future the knowledge will be improved considerably. If the substances are considered harmful, substitution with less harmful substances should take place, if possible. However, are there fit-for-use and less-hazardous alternatives available? That is what this report is about. The usages of PFOS and related substances in Denmark and elsewhere are described, and possible alternative substances to PFOA and PFOS-related substances are identified. The environmental properties and health risks are assessed both for PFOS and PFOA related substances and for potential substitutes. However, for most of the alternatives the knowledge level was rather scarcely. The project has mainly been based on scientific and technical literature, national reports and easily accessible information from the Internet or from personal interviews. Where relevant, the information has been supplemented with searches in the Danish Product Register of specific compounds and with contact to relevant producers and suppliers of products containing PFOS, PFOA, fluorotelomers or other related compounds. Furthermore, the Internet has been searched for possible alternatives, and the relevant companies have been contacted for further information. Project resultsSummary of the use of PFOS-related substances and alternativesDifferent recent surveys from different countries have mapped the use of PFOS-related substances. These surveys and information from the industry and producers of the substances have provided the picture of the use of PFOS-related substances and their alternatives as shown in Table 0.1. Table 0.1: Summary of the use areas of PFOS-related substances.
In most cases the alternatives to PFOS-related substances are other fluorinated chemicals with shorter chain length, such as C6-fluorotelomers or perfluorobutane sulfonate (PFBS). These chemicals fall under the larger chemical family called perfluoroalkyl substances (PFAS). The reason for this continuous use of fluorinated compounds is that polyfluorinated surfactants have superior properties compared to other and less expensive surfactants. Today the largest use areas of PFOS-based compounds seem to be:
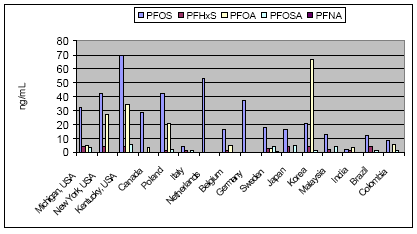
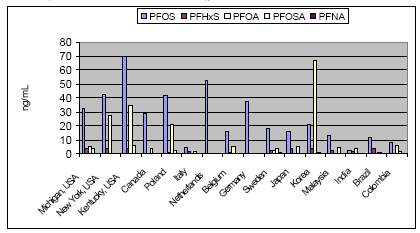
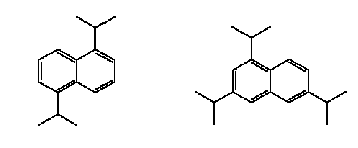
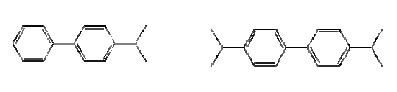
Not many alternatives to polyfluorinated compounds have been identified during this or other projects. The identified alternatives were primarily silicone-based products or hydrocarbon based surfactants for the paint and varnish area. In this area silicone-based products and hydrocarbon surfactants (such as aliphatic alcohols, sulfosuccinates, and propylated aromatics) are also used as alternatives, but in general it seems that these alternatives cannot be used, where extreme demands regarding low surface tension are needed. In these cases fluorinated surfactants seem to be the only substances that can reach the very low surface tension levels. Summary of the use of PFOA-related substances todayPFOA and its salts are used as a processing aid in the manufacture of fluoropolymers such as polytetrafluoroethylene (PTFE), a process not occurring in Denmark. DuPont has for the last 30 years investigated possible alternatives to PFOA as processing aid in the production of fluoropolymers. Several fluorohydrocarbons have been tested, but the results showed that the presence of hydrogen in the surfactant resulted in problems with the polymerisation. Supercritical carbon dioxide as solvent has also been tested in a pilot-scale facility. However, DuPont does not expect that this process will ever evolve into a technology that would have the capability to totally replace the current water-based polymerisation process. The conclusion so far from testing over the last 30 years is that there are no viable alternatives to PFOA. In Denmark only the ammonium salt of PFOA is found in very small quantities in a few products. Other PFOA-related substances were not found via a search in the Danish Product Register. The PFOA ammonium salt was registered for fluxing agents (used in plumbing) and in a primer and topcoat used for fluoroplastic coating. The use in fluxing agents is very limited and will cease, when leaded plumbing are banned from the year 2006, as lead-free plumbing do not require the use of these fluxing agents. Most important use area from an environmental perspectiveEmissions to the environment (air, soil and water) of PFOS and other polyfluorinated substances may happen directly from the production and processing plants. However, most important is releases during use (indoor or outdoor) and disposal of products containing these substances. Other PFAS-based chemicals such as PFBS and perfluorohexane sulfonate (PFHxS) with shorter chain length, precursor of PFOS such as perfluorooctane sulfonamide (PFOSA) and perfluorinated carboxylic acids (PFCA), including PFOA and perfluorononanoic acid (PFNA), are also found in the environment. Furthermore, the fluorotelomers, which in some cases are used as alternatives to PFOS-based compounds are also found in the environment and they seem to be long-range transported and degraded to PFOA and other PFCAs in the environment. Environmental sources of fluorinated telomers are currently unknown but these substances may be released at manufacturing of polyfluorinated compounds and at the decomposition of polymeric materials and consumer products that incorporate telomers. The most important of the above use areas for PFOS-related compounds is from an environmental perspective the use as surfactant in waxes, and floor polish. In this area only some substitution has been carried out, whereas a more substantial substitution has been taken place within the cleaning agent area. Common for the cleaning agents, waxes and floor polishes are that the PFOS-related compounds are ingredients of the cleaning agents. The widespread use of these products therefore also results in a widespread emission to the environment of these substances. Impregnation products, fire-fighting foams, the photographic industry and hydraulic oils within the airplane industry are still use areas that contribute to the total PFOS/PFOA concentration in the environment as these uses predominantly seem to use fluorotelomers or PFAS-compounds with shorter chain length (like PFBS) as alternatives to the former PFOS-compounds. However, the photographic industry does not seem to be the biggest problem, as this industry represents a smaller use area, and as the PFAS compounds are not a part of the final products. No information has been found on the size of the hydraulic oil use area. However, this area is not expected to be that large. Fire-fighting foams represent the area with the largest risk of a huge accidential leak directly to the environment. These foams are primarily used for oil-gasoline related fires, including at airports, air bases, offshore oil platforms, oil refineries and oil storage tanks at harbours. Use of fire-fighting foams to fight large fires and accidental spills may cause considerable local, persistent contamination of ground and surface waters. A shift to the non-fluorinated training foams, which some organisations are beginning to make, is therefore a way forward to avoid unnecessary emissions to the environment of the fluorinated compounds. The use of impregnation agents to protect domestic products such as clothes and carpets may be one of the most important exposure ways for the human population. Measurements have confirmed that PFOA and PFOS can be found in vacuum cleaner dust in private households. The most important human exposure may be through inhalation of air and the dust in private homes and offices. Health and environmental exposure and effects of polyfluorinated substancesPerfluoroalkylated substances are present in the environment primarily in the form of the most stable PFOS and PFOA, which are the final degradation products of various perfluorooctyl compounds. Recently, when the many PFOS uses have been phased out, the environmental concentrations of other related substances such as PFBS, PFHxS and PFNA have been increasing. PFOS, PFOA and other perfluorinated compounds are now considered as global environmental contaminants. They have been found in indoor air, outdoor air, soil, ground water, surface waters and even at 1000 m depth in the Pacific Ocean. Perfluorinated compounds are widely distributed in wildlife. PFOS has been detected in blood and liver samples from various species of aquatic animals (seal, otter, sea lion, dolphin, polar bear, mink), birds, fish and amphibians. Some samples also contained other related substances such as PFOSA, PFHxS, PFNA and PFOA. Some perfluorinated substances have even been found in blood and liver samples from the general human population (see Figure 0.1). Whereas PFOS and PFOA and perfluorinated acids with longer alkyl chain bioaccumulate in wildlife and human tissue, perfluorinated acids with fully fluorinated chain lengths of C5 and below do not seem to accumulate significantly in wildlife and human tissues. . Figure 0.1: Human blood levels of PFOS-related substances in various countries
One of the puzzling aspects about the PFAS contamination in the environment has been that substances such as PFOS and PFOA have been identified in remote Arctic areas, for example in polar bears, and even in relatively high concentration compared to the concentration levels found for other contaminants. The long-range transportation of specifically PFOA has been puzzling, as the low volatility and the ability of the substances to bind to water does not make it probable that PFOA will spread easily in the environment. However, the latest research indicates that the long-range air transportation of PFOA can be explained by the fact that fluorotelomer alcohols (FTOH) and some other non-polar PFAS are more volatile and less water-soluble and therefore have a greater tendency to escape the water phase. Short-chained FTOHs have an atmospheric lifetime of 20 days, which makes them able to travel about 7000 km. They may consequently be long-range transported and hereby reach remote arctic areas, where they can degrade to the most stable compounds PFOA and PFOS. In addition to the air transportation seawater, wildlife and humans may also move these chemicals to the arctic. Studies show that PFOS and other polyfluorinated chemicals are readily absorbed in the body. Both PFOA and PFOS are considered to be metabolically inert, and other perfluorocarboxylic acids and perfluorosulfonic acids do have similar properties, which means that their functional derivatives may be transformed to the parent compound. For example, the fluorotelomer alcohol 8:2 FTOH is transformed to PFOA in rats. Once absorbed in the body, PFOA and PFOS may bind to proteins and accumulate in various body tissues, including blood and liver; for PFOS also in testis and brain. The half-life of PFOA is about 2-4 years in humans and 1 month in monkeys. The half-life of PFOS is longer than for PFOA – about 200 days in monkeys. The half-life value in humans was not found. The acute lethal toxicities of PFOS and PFOA are moderate corresponding to a classification as harmful, if swallowed. PFOS is more toxic than PFOA, and the toxicity of related substances increases with the length of the alkyl chain. The liver is the primary target organ for polyfluorinated compounds, and these chemicals cause peroxisome proliferation in the rodent liver as well as induction of various enzymes involved in lipid metabolism. PFOS seems to be more active than PFOA concerning this effect but again PFDA with a longer alkyl chain is even more active. Toxic effects have been reported, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain. PFOA also affects the serum levels of various hormones, i.e. reducing testosterone and increasing estradiol in rats. Thus, it can be considered as an endocrine disruptor. Although the fluorinated chemicals do not seem to be mutagenic, PFOA has induced testis cancer, and PFOS and EtFOSE have induced liver cancer in experimental animals. USEPA classifies PFOA as a carcinogen in animals. PFOS and PFOA cause developmental effects, including reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. However, the structural abnormalities were only found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams. Thus the relevance may be questioned. Other tested PFAS (PFBS and PFHxS) had no significant effect on reproduction even at high doses. In general, the information in open literature about the toxicology of the polyfluorinated compounds is rather sparse, and it will take some time and efforts, before sufficient information for evaluation of the full impact of the present levels in humans is available. The experience from the work environment has not indicated any important adverse health effects among exposed workers, besides a retrospective cohort mortality study of a perfluorooctanesulfonyl fluoride (PFOSF) production workforce, which reported an excess of bladder cancer at high-exposure jobs. With respect to aquatic toxicity PFOS is considered to be moderately acute toxic and slightly chronically toxic to aquatic organisms. PFOA is practically non-toxic. EtFOSA is slightly acute toxic to daphnids. There seems to be large species difference in the biological response, because PFOS was three orders of magnitude more toxic to the aquatic midge Chironomus tentans than to most other aquatic organisms. The scarce database indicates a need for further studies. Environmental exposure and effects of non-fluorinated alternativesIn general very little information about the specific substances was available. The alternative hydrocarbon surfactants seem to be ready biodegradable. The fatty alcohol polyglycolether sulfate is readily biodegradable and does not seem to be toxic to aquatic organisms. The sulfosuccinates are likewise easily biodegradable, do not seem to bioconcentrate, but are harmful to aquatic organisms. The biphenyls and the naphthalene derivatives are potentially bioaccumulative. The biphenyl moiety seems to be easily biodegradable, whereas the naphthalene moiety only slowly biodegrade. The sparse information suggests that the biphenyls are acutely toxic to aquatic organisms, whereas the naphthalene's have no acute toxic effects in the investigated fish species. Of the investigated alternatives, the silicone polymers seem to have the more adverse environmental effects. The specific compound investigated is classified as environmentally harmful (R51/53 "Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment"), as the substance is toxic to aquatic organisms and is bioaccumulative. Health effects of non-fluorinated alternativesIn general very sparse information about the health effects of the alternative substances is found. Therefore, most of the information is based on the material safety data sheets (MSDS). The fatty alcohol polyglycolether sulfate are acutely toxic by ingestion but are not considered to be irritating. The sulfosuccinates are irritants to eyes, skin and the respiratory system. Dermatitis has been observed as a long-term effect as well as CNS depression. The substance is mildly harmful to toxic, if swallowed. The naphthalene- and biphenyl derivatives are irritating substances, and the biphenyl compounds may produce skin sensitisation or dermatitis. Furthermore, one of the biphenyl compounds is known to cause CNS damage as well as liver and kidney damage. The parent compound naphthalene is classified as possible carcinogenic in humans (IARC Group 2B). However, no carcinogenicity has been identified for the specific naphthalene derivatives used as alternatives to polyfluorinated compounds. The silicone polymers are irritating substances and are harmful by inhalation. Main conclusionsThe information obtained from the different surveys have shown that PFOS-related substances have been replaced in most application areas, or at least in the largest application areas. Still some use areas exist, where until now it has not been possible to identify possible alternatives to the PFOS-related substances, and in these areas it is predicted by industry that it may take as long as 5-10 years to find suitable alternatives. Even though non-fluorinated alternatives, such as different hydrocarbon surfactants and silicone products, have been identified and are in use within specific areas, the general picture is that in most cases or at least in the larger application areas, other fluorinated compounds are used instead. Generally, the reason for this is that the non-fluorinated alternatives do not work as well, especially in situations, where extremely low surface tension is needed. The fluorinated compounds, used as alternatives, are typically fluorinated compounds with shorter chain length such as fluorotelomer alcohols (mainly C6 chain length), PFBS (perfluorobutane sulfonate), or perfluorinated polyethers based on a CF3 or a C2F5 structure. Even though the alternative telomer alcohols mainly are based on a chain length of C6, the products are a mixture of telomer alcohols of different chain length, including C8 and C10 compounds. The new finding that telomer alcohols may break down to PFOA in the environment means that the use of telomer alcohols still may be a source of PFOA (and other PFCA) in the environment. Among the polyfluorinated alkyl compounds the bioaccumulation potential and hazard increase by increasing length of the alkylated alkyl group. Polyfluorinated compounds with an alkyl chain length of C5 or below do not seem to be significant bioaccumulative and toxic. They are, however, still substances that will persist in the environment for decades, and the implications for human health and the environment are unclear, as the toxicity and ecotoxicity of these shorter chained fluorinated compounds are yet to be examined. Sammenfatning og konklusioner
Baggrund og formålPerfluoroctanesulfonat (PFOS) og en række beslægtede perfluorerede forbindelser bruges i talrige industriprodukter og forbrugerprodukter på grund af deres specielle kemiske egenskaber, for eksempel evnen til at afvise vand og olie. Perfluoroctansyre (PFOA) anvendes som et proceshjælpemiddel ved fremstilling af fluorpolymere. PFOA er en veldokumenteret kontaminant i PFAS-relaterede kemikalier og findes derfor som en urenhed i produkter, der indeholder PFOS-relaterede kemikalier. Der er en stigende bekymring for disse potentielle skadelige forbindelser, der nu findes som globalt udbredte forureninger i luft, vand, jord samt i flora og fauna, hvilket indikerer, at disse perfluorerede stoffer er miljømæssigt persistente og akkumulerer i dyr og mennesker. Opdagelsen af disse stoffers forekomst i miljøet er ny og ikke helt udforsket og forstået, men de foreløbige resultater tyder på, at stofferne i nogle tilfælde kan have uønskede skadelige effekter. Hvis disse stoffer er miljø- og sundhedsskadelige, bør erstatning med mindre skadelige stoffer finde sted, hvis muligt. Men findes der brugbare og mindre skadelige alternativer? Det er, hvad denne rapport blandt andet handler om. Anvendelsen af PFOS, PFOA og beslægtede stoffer i Danmark og i andre lande og mulige alternative stoffer er beskrevet. De miljømæssige egenskaber og sundhedsrisici er vurderede både for PFOS, PFOA og beslægtede stoffer samt for mulige erstatningsstoffer. For de fleste alternativer er erfaringsgrundlaget dog meget sparsomt. Projektet er hovedsageligt baseret på oplysninger fra videnskabelig og teknisk litteratur, nationale rapporter og lettilgængelig information på Internettet samt personlige interviews og med kontakt til relevante producenter og leverandører. Hvor det har været relevant, er oplysningerne blevet suppleret med søgninger i Produktregistret. ProjektresultaterSammenfatning af anvendelsen af PFOS og beslægtede stoffer samt alternativerForskellige nyere undersøgelser fra forskellige lande har kortlagt anvendelsen af PFOS-relaterede stoffer. Disse undersøgelser og oplysninger fra industrien og producenter af stofferne har bidraget til billedet af anvendelsen af PFOS-relaterede stoffer og deres alternativer, som vises i Table 0.1. Tabel 0.1: Sammenfatning af anvendelsesområder for PFOS-relaterede stoffer.
I de fleste tilfælde er alternativerne til PFOS og beslægtede stoffer andre fluorholdige kemikalier med kortere kædelængder, som C6-fluortelomere eller PFBS (perfluorbutansulfonat). Disse kemikalier hører under den større kemikaliefamilie kaldet perfluoralkyl stoffer (PFAS). Årsagen til den fortsatte anvendelse af fluorerede forbindelser er, at disse overfladeaktive stoffer har optimale egenskaber sammenlignet med de andre og meget billigere overfladeaktive stoffer uden fluor. I dag ser det ud til, at de største anvendelsesområder for PFOS-baserede forbindelser er:
Ikke mange alternativer til fluorholdige forbindelser er blevet identificeret i dette eller i andre projekter. De identificerede alternativer er primært silikone-baserede produkter eller carbonhydrider til området for maling og lak. I dette område anvendes f.eks. alifatiske alkoholer, sulfosuccinater og propylsubstituerede aromatiske forbindelser som alternativer, men generelt ser det ud til, at disse alternativer ikke kan anvendes, hvor der er ekstreme krav vedrørende lav overfladespænding. I disse tilfælde ser det ud til, at fluorholdige overfladeaktive stoffer er de eneste stoffer, som kan nå de meget lave niveauer af overfladespænding. Sammenfatning af anvendelsen af PFOA-relaterede stoffer i dagPFOA og dets salte anvendes som et proceshjælpemiddel ved fremstillingen af fluorholdige polymere som f.eks. polytetrafluorethylen (PTFE), en proces der dog ikke forekommer i Danmark. DuPont har i de sidste 30 år undersøgt mulige alternativer til PFOA som proceshjælpemiddel i produktionen af fluorpolymere. Adskillige fluorhydrocarboner er blevet testet, men resultaterne viste, at tilstedeværelsen af hydrogen i det overfladeaktive stof resulterede i problemer med polymeriseringen. Superkritisk kuldioxid som overfladeaktivt stof er også blevet undersøgt i en pilottest. DuPont forventer dog ikke, at denne proces nogensinde vil blive udviklet til en teknologi, der vil have kapacitet til at erstatte den nuværende vandbaserede polymeriseringsproces fuldt ud. Indtil nu er konklusionen fra afprøvningerne gennem de sidste 30 år, at der ikke er nogle anvendelige alternativer til PFOA. I Danmark findes ammoniumsaltet af PFOA kun i meget små mængder og i meget få produkter. Andre PFOA-relaterede stoffer blev ikke fundet via en søgning i det danske Produktregister. Ammoniumsaltet af PFOA var registreret til flusmidler til lodning og i en grunder og dækfarve til anvendelse ved fluorplastisk overfladebehandling. Anvendelsen i flusmidler er meget begrænset og vil stoppe, når blylodning forbydes fra år 2006, da blyfri lodning ikke kræver brug af disse flusmidler. De vigtigste anvendelsesområder for PFOS og beslægtede stoffer set fra et miljømæssigt synspunktEmissioner til miljøet (luft, jord og vand) af PFOS og andre perfluorerede stoffer kan ske direkte fra produktions- og procesanlæg. Vigtigst er dog afgivelser ved brug (indendørs eller udendørs) samt bortskaffelse af produkter, der indeholder disse stoffer. Andre fluorholdige kemikalier som f.eks. PFHxS (perfluorhexansulfonat) og PFBS (perfluorbutansulfonat) med kortere kædelængde samt perfluorinerede carboxylsyrer (PFCA) inklusive PFOA findes også i miljøet. Desuden bruges fluortelomere i nogle tilfælde som alternativer til PFOS-baserede forbindelser. Fluortelomere ser ud til at blive transporteret over lange distancer og nedbrudt til PFOA og andre perfluorcarboxylsyrer i miljøet. Miljømæssige kilder af fluorerede telomerer er p.t. ukendte, men disse stoffer kan formentlig blive frigjort ved fremstilling af perfluorinerede forbindelser og ved nedbrydning af polymere materialer og forbrugerprodukter, som inkorporerer telomere. Det vigtigste af ovennævnte anvendelsesområder for PFOS-relaterede forbindelser set fra et miljømæssigt synspunkt er anvendelsen som overfladeaktivt stof i voks og gulvpolermiddel. På dette område er der kun foretaget begrænset substitution, hvorimod en noget større erstatning har fundet sted på området for rengøringsmidler. Fælles for rengøringsmidlerne, voks og gulvpolermidlerne er, at de PFOS-relaterede forbindelser er ingredienser i rengøringsmidler. Den udbredte anvendelse af disse produkter resulterer derfor også i en udbredt udledning af disse stoffer til miljøet. Imprægneringsmidler, brandslukningsskum, den fotografiske industri og hydraulikolier brugt i flyindustrien er fortsat brugsområder, der bidrager til miljøbelastningen med PFOS og PFOA. Indenfor disse områder er det fortrinsvis fluortelomere eller PFAS-forbindelser med kortere kædelængde end C8, der anvendes. Den fotografiske industri synes ikke at være et stort problem, da den repræsenterer et mindre forbrug, og de fluorholdige stoffer er ikke en del af slutproduktet. Det har ikke været muligt at finde oplysninger vedrørende brugen af mængden af hydraulikolie, men den er formodentlig begrænset. Brandslukningsskum repræsenterer området med den største risiko for store udslip direkte til miljøet. Disse midler bruges fortrinsvis til at slukke olie- og benzinbrande i lufthavne, offshore anlæg, olieraffinaderier, olieledninger og tankanlæg. Brugen sådanne steder kan forårsage en meget betydelig lokal forurening, der spredes over et større område. Mange steder bruges midler uden fluor til træningsformål. Anvendelsen til imprægnering af forbrugervarer som beklædning, møbelstoffer og tæpper synes at være en af de vigtigste udsættelser af mennesker, og betydelige mængder af PFOS er konstateret i opsamlet støv fra støvsuger i private hjem og i kontorer. Miljø- og sundhedsaspekter af polyfluorerede stofferPerfluorerede stoffer forekommer i miljøet primært som de stabile PFOS og PFOA forbindelser, som er slutprodukt for nedbrydning af diverse perfluorforbindelser. Disse stoffer ses nu som globalt udbredte forureninger, som forekommer selv i afsides arktiske områder. I den senere tid, hvor mange anvendelser af PFOS er stoppet, har koncentrationen i miljøet af de andre stoffer som fx PFBS, PFHxS og PFNA været stigende. Stofferne forekommer i indeluft, udeluft, jord, grundvand, overfladevand og selv i 1 km dybde i Stillehavet. Desuden er stofferne fundet i blod- og leverprøver fra diverse vandlevende dyr (sæl, odder, søløve, delfin, isbjørn og mink) samt i fugle, fisk og mennesker. Nogle analyserede prøver indeholdt udover PFOS og PFOA også PFOSA, PFHxS, PFNA og PFOA. Det tyder derimod på, at perfluorerede stoffer med en kædelængde på C5 og derunder ikke akkumuleres i større omfang i mennesker og dyr. Et af de overraskende fund mht. PFAS kontaminering er, at PFOS og PFOA forekommer i afsides egne af kloden og i relativt høje koncentrationer i forhold til andre forureninger. Stofferne er ikke særligt flygtige. Ny forskning tyder på, at det er mere flygtige fluortelomere og perfluoroctansulfonamider, som langtransporteres med luften til disse afsides egne og dér nedbrydes til PFOS, PFOA og beslægtede stoffer. Kortkædede telomere har faktisk en atmosfærisk levetid på 20 dage, hvorved stofferne kan transporteres 7000 km med vinden. Der kan dog også være tale om et lokalt forbrug til fx brandslukning eller transport med havstrømme og fisk. PFOS og beslægtede stoffer optages let i kroppen, hvor de kan bindes til proteiner og især akkumulerer i blod og lever, men for nogle forbindelsers vedkommende også i testikler og hjernevæv. Halveringstiden i kroppen synes at være flere år. Den akutte giftighed af PFOS og PFOA er moderat, og førstnævnte er mest sundhedsfarlig. Giftigheden af de beslægtede stoffer vokser med kædelængden. Leveren er det primære målorgan for perfluorforbindelser, og de fremkalder peroxisom proliferation i rottelever såvel som induktion af forskellige enzymer involveret i fedtstofskiftet. PFOS synes at være mere aktiv end PFOA mht. denne effekt, men igen er PFDA med en længere alkylkæde endnu mere aktiv. PFOA og PFOS har også en indvirkning på blodniveauet af diverse hormoner, fx ved at nedsætte testosteronkoncentrationen og øge koncentrationen af estradiol i rotter. Stofferne må derfor anses for at være hormonforstyrrende (endocrin disruptor). PFOS, PFOA og beslægtede stoffer er ikke mutagene, men PFOA fremkalder testikelkræft, og PFOS og EtFOSE fremkalder leverkræft i forsøgsdyr. De amerikanske miljømyndigheder klassificerer PFOA som kræftfremkaldende i forsøgsdyr. PFOS og PFOA kan i forsøgsdyr fremkalde udviklingsforstyrrelser med nedsættelse af fødselsvægt. Meget høje doser af PFOS kan fremkalde strukturelle misdannelser, men relevansen kan diskuteres. Andre undersøgte PFAS-stoffer (PFBS og PFHxS) havde derimod ingen effekt ved de høje doser. I al almindelighed er der begrænset viden om toksikologien af disse perfluorholdige stoffer, og det vil tage nogle år, før der er tilstrækkelig viden til at vurdere den fulde konsekvens af udsættelsen for disse stoffer. Erfaringen fra arbejdsmiljøet tyder ikke på væsentlige effekter på mennesker, bortset fra en enkelt undersøgelse der viser en overhyppighed af blærekræft i arbejdere med betydelig udsættelse for perfluoroctansulfonylfluorid (PFOSF). Med hensyn til toksicitet over for organismer, der lever i vand, så anses PFOS for moderat akut giftigt, EtFOSA er endnu mindre akut giftigt, mens PFOA er praktisk talt ugiftigt. Der synes at være betydelige artsforskelle, da PFOS var tre størrelsesordener mere giftigt for en bestemt vandmide i forhold til de fleste andre akvatiske organismer. Der er behov for mere viden på dette område. Miljøaspekter for de ikke-fluorholdige alternativerDer savnes generelt viden om miljøaspekterne ved de anvendte og foreslåede alternativer. De fleste alternativer synes at være bionedbrydelige. Det gælder carbonhydriderne, alkoholpolyethersulfater samt sulfosuccinater, men sidstnævnte er toksiske for vandorganismer. Biphenyl- og naphthalen-derivaterne er potentielt bioakkumulerende, og mens biphenyl synes at være letnedbrydelig og akut giftig for fisk, så er naphthalen-derivaterne meget langsomt nedbrydelige og mindre giftige for vandorganismer. Af de undersøgte alternativer virker silikone polymererne til at have de værste miljøeffekter. Den specifikke forbindelse, der er vurderet, er klassificeret som miljøfarlig med R51/53 "Giftig for organismer, der lever i vand; kan forårsage uønskede langtidsvirkninger i vandmiljøet". Sundhedseffekter af de ikke-fluorholdige alternativerGenerelt var det ikke muligt at finde mange relevante oplysninger om sundhedseffekter af de alternative stoffer undtagen fra leverandørbrugsanvisninger (MSDS). § Alkohol polyglycolethersulfater er akut giftige ved indtagelse, men er ikke lokalirriterende. § Sulfosuccinater irriterer øjne, hud og luftveje. Hudeksem og effekt på centralnervesystemet er set ved langtidseksponering. Ved indtagelse er de sundhedsfarligt til giftigt. § Naphthalen- og biphenyl-derivater er lokalirriterende stoffer, og biphenylstofferne kan fremkalde allergisk eksem; mulige effekter på centralnervesystemet og nyrerne. § Silikone polymer er lokalirriterende stoffer, som er sundhedsskadelige ved indånding. HovedkonklusionerDen foreliggende information viser, at PFOS-beslægtede stoffer allerede er blevet erstattet i de fleste anvendelser. Nogle anvendelser fortsætter, og industrien vurderer, at det kan tage 5-10 år, før tilfredsstillende alternativer findes i alle tilfælde. Selvom alternativer uden fluor findes, er billigere og er i brug på visse områder, så er det generelle billede, at de fleste vigtige anvendelser fortsat er med polyfluorforbindelser, men det er de mere kortkædede (kortere end C8) fx fluortelomeralkoholer med C6, PFBS (perfluorbutansulfonat) eller perfluorerede polyetherere baseret på en CF3 eller C2F5 struktur. Grunden er, at alternativer uden fluor ikke har så ekstrem lav en overfladespænding, som ofte behøves. Undersøgelser tyder på, at bioakkumuleringsevne og farlighed af de perfluorerede forbindelser øges med voksende længde af alkylkæden. Med en kædelængde af C5 eller derunder synes stofferne ikke at være så farlige, men deres persistens i miljøet betyder, at de vil forblive i miljøet i årtier eller måske for evigt. Konsekvenserne af dette kan ikke vurderes, da disse stoffer er meget dårligt undersøgt for miljø- og sundhedseffekter. Hvis uventede skadevirkninger eventuelt dukker op, er det for sent, da stofferne kan ikke tilbagekaldes. 1 Terminology and definitionsIn order to assist the reader of this report, the classes of chemicals discussed and their inter-relationship are defined. More specific terms are provided in Appendix A. Perfluoroalkylated substance (PFAS) A general term that describes a substance bearing a perfluorocarbon unit, F(CF2)n-R where n is an integer and R is not a halogen, or hydrogen. Examples include F(CF2)6CH2CH2OH, F(CF2)6SO2N(CH3)CH2CH2OH, and p-F(CF2)6-C6H4OH. Perfluorosulfonates Perfluorooctane sulfonate (PFOS) is a chemical, which is part of the larger perfluorooctyl sulfonyl fluoride (POSF) family. It is believed that many POSF chemicals can degrade to PFOS. The POSF family of chemistry had a wide array of uses. For the purpose of this report "PFOS-related chemicals" are synonymous with the POSF family of chemicals. Recently, perfluorobutane sulfonate (PFBS) and a family of PFBS –based products have been introduced as alternatives. In this report, the PFBS substances are not considered as a part of the family of "PFOS-related chemicals" (as used in this report), because they have a shorter chain length than PFOS. Perfluorocarboxylates Perfluorocarboxylic acids (PFCAs) are a group of chemicals, which have been manufactured as industrial products used principally as processing aids in the manufacture of fluoropolymers such as polytetrafluoroethylene, PTFE. Perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA) are the most commonly used processing aids. Telomers Fluorotelomers or "telomers" are a family of speciality chemicals, which have similar function to the PFAS family of chemicals but are chemically different. As a result of their similar uses, they are of interest too. Further, some telomer-based substances can degrade to PFOA. Telomer-based substances are probably the most prevalent alternatives to PFAS-related chemicals. 2 Introduction2.1 The purpose of the projectA survey of perfluorooctanyl sulfonate (PFOS) and PFOS-related compounds in consumer products carried out by the Danish EPA in 2001 and 2002 has revealed that PFOS-related compounds are used in numerous products, and that several PFOS-related compounds are used in products in Denmark (Havelund 2002). Therefore, The Danish EPA initiated a survey that should map the use of PFOS-related compounds in impregnating agents, wax and floor polish products (Vejrup et al. 2002). Furthermore, the Danish EPA has initiated a survey that will map the use of compounds in shoe care products, and hereby also the use of PFOS-related compounds. The common purpose of these projects is to map the dissemination of these problematic PFOS-related compounds. The purpose of this present project is to collect know-how about the existing technical alternatives to PFOS/PFOS-related compounds and perfluorooctanoic acid (PFOA)/PFOA-related compounds. Finally, the purpose of this project is to carry out an environmental and health assessment of PFOS-related substances and of the possible alternatives to PFOS- and PFOA-related compounds. 2.2 HistoryThis is a short summary of the most important aspects in the history of fluorinated chemicals. If not otherwise described, the information is from the website of the Fluoride Action Network (Fluoriede Action Network 2004). The history of fluorinated chemistry starts in 1938, when the fluoropolymer PTFE (polytetrafluoroethylene) was discovered by the company DuPont and later introduced to the market in 1949. In 1951 ammonium perfluorooctanoate, the ammonium salt of PFOA (also called C8) starts to be used to make PTFE and related polymers. In 1954 France's Conseil Supérieur de l'Hygiène Publique officially cleared PTFE for use on frying pans by the company TEFAL. In 1958 the French ministry of agriculture approved the use of PTFE in food processing (Funderburg 2000). In 1962 the American Food and Drug Administration (FDA) grants a final approval to PTFE cookware. In 1967 the FDA approves Zonyl®, DuPont's leading brand of fluorinated telomers, for use in food packaging. Already in 1954 some employees at DuPont started to express concerns about the toxicity of C8. In 1978, 3M reported that C8 was detected in the blood of its workers, and DuPont began to express concern internally about the possible toxic effects of C8. 3M and DuPont obtained further knowledge about C8 in the early 1980's:
In May 2000 3M announced, under pressure from the U.S. EPA, that they will begin phasing out PFOS and US production of a related chemical (PFOA) due to principles of responsible environmental management. In September 2002 the U.S. EPA began the review of data that are produced to understand the hazards and risks associated with the use of PFOA. Animal studies showed that exposure to C8 could result in a variety of effects including developmental/reproductive toxicity, liver toxicity and cancer. In June 2003, the 3M Company replaced PFOS in their Scotchgard brand with a C4 chemical (PFBS – perfluorobutanesulfonate). In February 2004 the U.S. Federal Agency (NIEHS) announced that they would conduct a four-year study of blood levels of residents in the affected Ohio communities. Furthermore, the U.S. Federal Housing and Urban Development (HUD) Agency announced in March 2004 that they would conduct a two-year study of young children's exposures in their Florida homes to selected chemicals (pesticides) including polyfluorinated chemicals. In April 2004 DuPont launched a $1 million study to compare the health of employees, who work directly with C8, and those who do not. According to DuPont the objective of the study includes multiple health endpoints. The study will also evaluate liver function, the prime target of PFOA as observed in animal studies (Personal communication, DuPont 2004a). In June 2004, the US EPA announced that they would conduct a study of how PFOA gets into human blood (EWG, 2004a). The study will also include degradation studies of telomers. The goal of the degradation studies is to determine whether PFOA comes from the breakdown of telomers' carbon chains or from impurities in telomer products (Hogue, 2004). In July 2004 the US EPA Enforcement Division files a complaint against DuPont, as the US EPA alleges that DuPont has failed to report information about the hazards of C8. In August 2004 DuPont responded to EPA's complaint providing facts and information that refute the allegations made by the agency (DuPont website 2004). 2.3 The PFOS/PFOA problemPolyfluorinated substances are generally persistent in the environment. Carbon-fluorine bonds are extremely strong, and as a result they do not break easily. The problem with different perfluorochemiclas, including a large number of PFOS-related compounds and PFOA-compounds, is that the chemicals may degrade in the environment to PFOS and PFOA respectively, but no further degradation of PFOS or PFOA will occur, as they are chemically and biologically inert and very stable. Furthermore, PFOS, PFOA and related substances are found to bioaccumulate in wildlife and in humans. Recent observations show that PFOS, PFOA and related substances have been found in mammals, birds and fish in large part of the world, including polar bears in the Arctic (Giesy & Kannan 2001; Giesy & Kannan 2002). Furthermore, both PFOS and PFOA have been observed in blood samples of the general population in USA, India and Italy (Kannan et al. 2003). The growing concern of PFAS and derivatives is due to the fact that these compounds are globally distributed in the environment, are environmentally persistent, are bioaccumulative, are magnifying in food chains and are potentially harmful for animals and Man (OECD 2002; Giesy & Kannan 2001). OSPAR [1] decided to add PFOS to the OSPAR list of chemicals for priority action (OSPAR HSC 2003), whereas PFOA was not added to any OSPAR list at that stage on the basis of the data provided by industry (APME 2002). This decision will be revisited in the near future, as the full dataset is now available from the further testing programme on PFOA (APME 2004). The pathways of PFOS and PFOA to remote locations as the Arctic are virtually unknown. In comparison with the typical persistent organic pollutants (POPs) some PFOS and PFOA related substances are much more water-soluble and a little more volatile. Recent studies suggest that fluorotelomer alcohols, which are volatile and more readily can be transported to remote locations, can be degraded to PFOA in the environment, and hence explain the discovery of PFOA in remote locations (US EPA 2003b; Renner 2004; Ellis et al. 2004). Other potential sources of PFOS and PFOA are the direct use of fire-fighting foams in the Arctic, and a recent hypothesis is that via ocean currents direct long-range transport of PFOS and PFOA may occur to the Arctic (Taniyasu, 2004). A discussion is ongoing at the moment, whether the occurrence of PFOA in the environment isdue to:
Future research will address this in more details. 2.4 Method usedThis project is primarily based on a literature study and on easily accessible information on the Internet. This information has though, where relevant, been supplemented with more specific information as searches in the Danish Product Register on specific compounds and contact to relevant producers and suppliers of products containing PFOS-related compounds or PFOA-compounds. The more specific initiatives taken in order to search for alternatives to PFOS-related compounds are described in details in Appendix B. In brief, an Internet search has been performed and the relevant producers have been contacted by email. Furthermore, relevant Danish importers of products containing PFOS- or PFOA-related compounds have been contacted in order to learn more about the use and possible alternatives (see Appendix D and E). With regard to the environment and health assessment of the PFOS-related compounds and PFOA, a detailed literature study has been performed. By personal contact to Professor John P. Giesy (Zoology Department at Michigan State University) and Professor Scott A. Mabury (Department of Chemistry at the University of Toronto) – some of the leading scientists in this area - it was possible to get access to the newest possible information about the subject. Furthermore, representatives for DuPont has been helpful and provided useful information for this project. 3 Chemistry
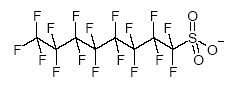
Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and other related compounds are part of a chemical family called fluorinated surfactants. Surfactant is an abbreviation of the term surface-active agent. A surfactant is a substance, which even at low concentrations effectively lowers the surface tension of its medium by selective adsorption on the interface. Fluorinated surfactants are surfactants where the hydrophobic part of the surfactant molecule contains fluorine. At least one hydrogen atom in the hydrophobic part of the molecule has been replaced with fluorine. Perfluorinated surfactants, which are the group that PFOS and PFOA belong to, are fully fluorinated surfactants, where all hydrogens in the hydrophobic part of the molecule have been replaced by fluorine (Kissa 2001). Perfluorinated surfactants have the unique ability to dramatically lower aqueous surface tension, improve wetting and levelling, and remain chemically stable under harsh use conditions. Furthermore, fluorinated surfactants are much more surface active than their hydrogen counterparts and exhibit surface activity in organic systems. Additionally, they are stable to heat (fire resistant), acids, and bases as well as reducing and oxidising agents. Because of these unique properties they are often irreplaceable in many applications (Kissa 2001). Some surfactants may not be perfluorinated but contain both perfluorocarbon and hydrocarbon functionality. These may provide low surface tension, wetting and levelling properties, but would not be expected to be as chemically and thermally stable as their perfluorinated analogue (e.g. F(CF2)7COOH versus F(CF2)6CH2 CH2SCH2 CH2COOH). Alternatively, perfluorinated alkyl chains can be appended to hydrocarbon polymer backbones, such as acrylates creating "comb-like" structures where the backbone is a hydrocarbon and some of the tines contain perfluorinated chains. These "fluorinated polymers" are used as surface modification coatings. The backbone adheres to the surface while the chains align perpendicular. These polymers provide oil and water repellency and stain resistance to substrates like textiles or paper. In addition, fluoropolymers may also be perfluorinated materials. Common fluoropolymers are polytetrafluoroethylene (PTFE) and polyvinylidene fluoride (PVDF). They are often manufactured using a perfluorocarboxylic acid ammonium salt as a processing aid. The most common processing aids are based on octanoic and nonaoic acids. Fluoropolymers are fundamentally different from the "fluorinated polymers" described above. Perfluorooctanyl sulfonate compounds (called "PFOS-related compounds") are members of a large family of anthropogenic chemicals that all are derivatives of and can degrade to perfluorooctanyl sulfonate (PFOS). They have had broad use in consumer and industrial products (US EPA Administrative Record). The anion perfluorooctanyl sulfonate illustrated in Figure 3.1 has no specific CAS number (OECD 2002). Figure 3.1: Structural formula of PFOS - perfluorooctane sulfonate (C8F17SO3)
Well-known brands for consumer products based on polyfluorinated chemistry are Teflon, Scotchgard, Gore-Tex, Silverstone and Stainmaster (US EPA 2003). PFOS-related substances and PFOA are members of the larger family of perfluoroalkylated substances (PFAS). PFAS compounds cover a wide array of chemical substances that may contain perfluoroalkyl chains, including perfluorinated compounds with shorter carbon chains, e.g. C6, C4, and C2, which obviously cannot degrade to PFOS (the eight carbon chain) or PFOA in the environment. OECD has drawn up a list of 175 different perfluoro-substances divided in 22 different categories. All 175 substances contain perfluoro structures, i.e. also perfluoroalkylsulfonates and not just PFOS-related compounds, but not all substances contain sulfur. Non-sulfur containing substances are included in the list of PFOS-substances as they represent a risk of formation of perfluorooctanyl sulfonate at decomposition in a sulfurous environment. This definition by the OECD is somewhat confusing compared to other uses of the term "PFOS-related substances". The 22 different categories of PFOS-related compounds as reported by the OECD are listed in Table 3.1 (Statens forureningstilsyn 2004; Havelund 2002). Table 3.1: The classification of PFOS-compounds in 22 categories according to OECD (Havelund 2002).
Great Britain has worked specifically with the family of perfluorooctanyl sulfonyl fluoride (the POSF family), "PFOS-related compounds", creating a list of 96 different PFOS-related substances for use in their risk reduction strategy. OSPAR [2] has a list of 30 PFOS-related compounds. Furthermore, 88 PFOS-related compounds are a part of the American SNUR rules (the US EPA). The different lists have an overlap in certain cases, but compounds do only exist on one list and not on another. E.g. of the 88 compounds on the American lists, 48 compounds cannot be found on the list made by OECD (Kemikalieinspektionen 2004; RPA 2004). The difference in the classification of PFOS-related substances lies in the fact that some organisations consider compounds only to be PFOS-related if a C8F17 group is directly linked to a sulfonyl group (-SO2), as they assume that only the presence of the full C8F17SO2 group in the original molecule may allow the potential degradation of that molecule to PFOS in the environment. Others (e.g. OECD) do, on the other hand, consider compounds to be PFOS-related if they represent a risk of formation of perfluorooctanyl sulfonate at decomposition in a sulfurous environment (RPA 2004; Statens Forureningstilsyn 2004; Havelund 2002). Category number 16 in Table 3.1, the fluoroalcohols, represents the telomer alcohols, which are also discussed in this report. According to the OECD classification, the telomer alcohols are hence considered as a part of the PFOS-compounds. However, in this report the short-chained telomer-based compounds are not considered as part of the "PFOS-related substances" as they per definition cannot degrade to PFOS. The technical properties of PFOS (perfluorooctane sulfonate) are described in Table 3.2. Table 3.2: Technical properties of PFOS (perfluorooctane sulfonate). Most properties represent the potassium salt of PFOS (OECD 2002).
Some of the most important PFOS-related compounds are listed in table Table 3.3 together with their commonly used acronyms. Table 3.3: Polyfluorinated compounds
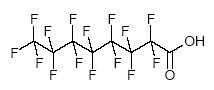
A related substance is perfluorooctanoic acid (PFOA), which is the acid of perfluorooctane. The structural formula of PFOA (CAS 335-67-1) is illustrated in Figure 3.2 (US EPA 2003). Figure 3.2: Structural formula of PFOA - perfluorooctanoic acid (C8HF15O2)
PFOA is used as a processing aid in the manufacture fluoropolymers. PFOA is also an item of commerce and well documented as a significant contaminant in PFOS-related chemicals. PFOA is used as a polymerisation aid in order to improve the physical properties of the polymer and increase the rate of polymerisation (Hekster et al. 2002; ENVIRON 2004). The abbreviation PFOA is used as a group name for perfluorooctanoic acid and its salts. According to the preliminary risk assessment of PFOA carried out by the US EPA the PFOA-compounds listed in Table 3.4 are relevant. However, the ammonium, sodium, potassium and silver salts of PFOA are of primary interest (US EPA 2003a). Table 3.4: Relevant PFOA compounds (PFOA and its salts) (US EPA 2003a).
The technical properties of PFOA are described in Table 3.4. Table 3.5: Technical properties of PFOA (perfluorooctanoic acid) (US EPA 2002; ENVIRON, 2004).
3.1 Manufacturing of PFOA, PFOS, PFOS-related compounds and telomersThe total world production of PFOS, PFOS-related compounds, telomers and PFOA derivatives during the years and the emissions to the environment are unknown. In May 2000 the 3M Company announced that it would voluntarily cease manufacturing materials based on perfluorooctanesulfonyl fluoride (POSF) after a metabolite of this compound (PFOS) was found to be widespread in human population and wildlife (Olsen et al. 2003). Based on a production of 3,000 ton in 2000 by 3M alone (Giesy & Kannan 2002), the total annual world production is likely to have been more than 5,000 ton. Many of the applications of the POSF-related compounds lead to emissions to the environment during or shortly after use. Three different manufacturing processes exist for production of fluorinated compounds (Kemikalieinspektionen 2004; Kissa 2001). However, the first two mentioned processes are the ones, which are normally used (Moody et al. 2000; Hekster et al. 2002): 1. Direct fluorination using the electro-chemical fluorination (ECF). 2. Telomerisation. 3. Oligomerisation (the chemical process of creating oligomers (molecules of just a few monomers) from larger or smaller molecules). Both PFOA and PFOS-related compounds and their salts are produced by use of the above-mentioned methods. However, whereas PFOS and PFOS-related compounds are used commercially for numerous applications, PFOA is primarily used as its salts (primarily the ammonium and sodium salts) as non-reactive processing aids in the production of fluoropolymers and fluoroelastomers and in other surfactant uses. PFOS may be formed by degradation of PFOS-related chemicals. PFOA has been used as a surfactant in a number of commercial applications beyond fluoropolymers and may be formed through the transformation or metabolism of other polyfluorinated chemicals (such as telomer alcohols). However, as already stated, there is an ongoing discussion at the moment, whether the occurrence of PFOA in the environment is due to breakdown of the telomer alcohols, due to impurities in the telomer products or due to direct releases from PFOA use as an industrial chemical. 3.1.1.1 Direct fluorination using electro-chemical fluorinationThe ECF process – also called the Simons Electro Chemical Fluorination - was used by the 3M Company to manufacture PFOS and PFOA. The starting point of this process is 1-octanesulfonyl fluoride, which is dispersed in liquid hydrogen fluoride. An electric current is passed through the solution causing the hydrogen atoms on the octanesulfonyl fluoride to be replaced by fluorine (Kissa 2001; 3M 1999; Hekster et al. 2002). C8H17SO2F + 17 HF →C8F17SO2F + 17 H2 + by-products The electrochemical fluorination process yields about 35-40% straight chain (normal) perfluorooctane sulfonyl fluoride (POSF), and a mixture of by-products and waste of unknown and variable composition, e.g. branched-chain, straight chain or cyclic perfluoroalkylsulfonyl fluorides with various chain lengths (3M 1999). Despite low to moderate yields of perfluorinated compounds and the many side products, the ECF process is economically attractive because of the relatively low costs of electricity and the hydrogen fluoride reagent (Moody et al. 2000). POSF is a commercially viable product, but is primarily an important intermediate in the synthesis of many other fluorochemical products. 3M used POSF to produce PFOA, PFOS and other PFOS-related compounds (3M 1999). 3.1.1.2 TelomerisationDuPont, Daikin, Clariant and Ashai Glass and others use the telomerisation process. It yields a linear, even perfluorocarbon chain length product (Kissa 2001; Kemikalieinspektionen 2004). Commercial products manufactured through the telomerisation process are generally mixtures of polyfluorinated straight-chain compounds with ranges of even carbon numbers, but distillation can be used to obtain pure compounds (US EPA 2002). C2F5I + 3 C2F4→ C8F17I→ C8F17-surfactants A telomer is defined as a low molecular weight polymer chain in which the terminal group of the chain-like molecule is not the same as the side group (About 2004). Fluorotelomers are starting materials for the manufacturing of perfluorinated compounds and used as agents to incorporate a fluorinated carbon skeleton into polymeric materials. Polyfluorinated telomers are used in production of fire-fighting foams, cleaning agents, and oil-, stain-, and grease-repellent surface treatment agents for carpets, textiles, leather and paper (US EPA 2003b). PFOA is an unintended reaction by-product, present at trace levels in some telomer-based products (DuPont 2004). Furthermore, information indicates that certain polyfluorinated telomers, especially telomer alcohols, are transformed to PFOA in the environment or can be metabolised to PFOA, if absorbed by living organisms (US EPA 2003b; Renner 2004). If so, PFOA can be a widespread problem, as telomers are used in a wide range of applications. The ongoing discussion today is whether the occurrence of PFOA in the environment is due to impurities of PFOA in the telomers, due to telomer degradation to PFOA in the environment or due to direct releases of PFOA because of use as an industrial chemical. The US EPA has initiated a study (June 2004) to throw light on this aspect (Hogue 2004). 3.1.1.3 OligomerisationOligomerisation is the chemical process of creating molecules of just a few monomers (oligomers) from larger or smaller molecules. However, this process is not as widespread as the ECF process or the telomerisation process. 3.2 Producers of PFOS-compounds and PFOAThe largest global producer of PFOS-compounds has for long been the 3M Company. However, they have recently ceased their production of PFOS (but have continued the production of other perfluoro-compounds with shorter chain length). On the European market this has resulted in buyers shifting to other producers that have carried on the production of the PFOS-compounds (Renner 2001). OECD has identified the following producers of PFOS-compounds: Table 3.6: List of producers of PFOS-compounds according to OECD (OECD 2002).
The 3M Company was formerly the largest global manufacturer of PFOA. In 2002, the 3M Company stopped external sale of PFOA and now only manufactures for their own internal use. DuPont produces the ammonium salt of PFOA in the US for use as a processing aid in fluoropolymer manufacture (US EPA 2002; Personal communication, DuPont 2004a). OECD has identified the following producers of PFOA: Table 3.7: List of producers of PFOA according to OECD (US EPA 2002).
4 Use of PFOS-related compounds, PFOA, and their alternatives
It could be assumed that all the focus on the environmental problems of PFOS and PFOA would result in a shift away from using these products. However, this is not necessarily the case – at least not for all uses and at least not yet. A comprehensive survey of the world production of PFOS-related substances does not exist, for which reason it is difficult to say exactly if the use of these substances is beginning to decrease. Even though the 3M Company started to phase out the production of PFOS chemicals and PFOA in the year 2000 and today has replaced the C8 chemical perfluorooctane sulfonate (PFOS) with a C4-chemical (PFBS – perfluorobutane sulfonate), the production of these substances has not necessarily fallen drastically. After the 3M Company has ceased their production of PFOA, DuPont has initiated a production of PFOA for internal use (though in smaller scale than the former manufacture by 3M). It is likely that other companies also have continued the production of PFOS and PFOA. Nevertheless, fact is that the 3M Company has stopped the production of PFOS-related compounds and now focuses on the production of C4- compounds. Dyneon/3M, DuPont, Miteni, Ashai Glass and Daikin produce PFOA principally for use in fluoropolymer processing (Personal communication DuPont 2004a). A quick search in the database of the United States Patent and Trademark Office (www.uspto.gov) shows that patents are still granted today with the use of PFOS and PFOA. A search (in May 2004) on "perfluorooctane sulfonate" resulted in 190 patents, where the most recent patent, which concerns photo-acid generators, is dated 25 May 2004. Actually, 21 of the 190 patents mentioning "perfluorooctanesulfonate" have been issued in 2004. Likewise, a search in the same database on "perfluorooctanoic acid" resulted in 358 patents, where the most recent patent, which concerns applications for perfluorovinyl ethers, is dated 18 May 2004. This specific patent describes that perfluorooctanoic acid is a more effective processing aid for the production of the mentioned fluoropolymers. In all, 11 of the 358 patents mentioning "perfluorooctanoic acid" have been issued in 2004. A hit on the words "perfluorooctane sulfonate" and "perfluorooctanoic acid" in the patent database does not necessarily mean that the issued patent utilise PFOS or PFOA for production or similar. However, it is striking that both PFOS and PFOA are mentioned in so many new patents. The following pages describe the properties and general uses of PFOS-compounds and PFOA, as well as known alternatives to PFOS-related compounds and PFOA. The primary purpose of this project was to collect know-how about the technical alternatives to PFOS-related compounds and to PFOA. The method used in identifying alternative compounds is described briefly in chapter 2. In short, a combination of contacting producers, search in literature, and search on the Internet for information on alternatives has been used (see Appendix B for details). In general, telomer-based products are the most common alternatives to PFOS-related compounds. Other compounds that have been identified as alternatives to PFOS-related compounds are presented in details in Appendix F. The alternative compounds identified in this project are presented in more details in the following paragraphs for the specific use categories. Other alternatives found in literature and other surveys on PFOS-related compounds are mentioned and discussed as well. 4.1 Use of PFOA and PFOA-related compoundsAs described earlier, PFOA is primarily used as its salts (primarily the ammonium and sodium salts) as non-reactive processing aids in the production of fluoropolymers and fluoroelastomers and in other surfactant use. Normally, PFOA is not, as PFOS, a part of the products but can be formed through the transformation or metabolism of other perfluorochemicals (like e.g. telomer alcohols) or can be released from the products under use (ENVIRON 2004). A search in the Danish Product Register for use of PFOA and the PFOA-salts (mentioned in Table 3.4) was performed in this project (see detailed description in appendix D). The search showed that only the PFOA ammonium salt was found in products reported to the Danish Product Register. The PFOA ammonium salt is found in fluoroplastic coatings – a primer and a topcoat. It is present in the maximum concentration of 0.2% and the total amount of the PFOA ammonium salt in products found in the Danish Product Register is maximum 35 kg per year. No alternatives to PFOA have been identified. Several producers of PFOA were asked about alternative options to PFOA, and those who replied had no alternative options to PFOA. DuPont has for the last 30 years investigated possible alternatives to PFOA as processing aid in the production of fluoropolymers. Several fluorohydrocarbons have been tested, but the results showed that the presence of hydrogen in the surfactant resulted in problems with the polymerisation. Supercritical carbon dioxide as reaction medium has also been tested in a pilot-scale facility. However, DuPont does not have the expectation that this process will ever evolve into a technology that would have the capability to totally replace the current water-based polymerisation process (Personal communication DuPont 2004a). The conclusion so far is that DuPont by alternative methods is able to produce only one type fluoropolymer and in limited quantitites, but it does not appear to be feasible to make all products by this route. The conclusion from testing over the last 30 years is that there are no viable alternatives to PFOA (Personal communication DuPont 2004a). Therefore, PFOA is not discussed in details, as the primary application is as a non-reactive processing aid to produce other fluorinated and perfluorinated compounds. Instead the focus in this chapter will be on PFOS and PFOS-related compounds. 4.2 Use of PFOS and PFOS-related compoundsThe majority of PFOS-related chemicals are high molecular weight polymers in which perfluorooctane sulfonate represents a fraction of the total molecular weight (OECD 2002). Perfluorinated compounds with long carbon chains, like e.g. PFOS, have the ability to repel both water and oil. Because of these special surface-active properties, the PFOS-related compounds are used as surface-active compounds in a range of applications (Kissa 2001; Kemikalieinspektionen 2004). In addition, the PFOS has a unique thermal stability and is stable to acids, bases, oxidants and reductants as well (Moody et al. 2004; Kissa 2001). Only very few countries have mapped the use of PFOS and PFOS-related chemicals. According to an inventory of the use of PFOS-related chemicals in the United Kingdom, the use of PFAS-related [3] chemicals for carpet and textile treatment was clearly the largest area, representing about 49% of the total use of PFAS-related compounds in the UK in 2001 (see Table 4.1). This also matches the fact that 48% of the production of PFOS-related chemicals produced by 3M in the year 2000 was used for surface treatment purposes (Kemikalieinspektionen 2004; RPA 2004; Hekster et al. 2002). Otherwise, paper and paperboard treatment and fire-fighting chemicals represent relatively large single uses of PFAS-related chemicals - 15% and 16% respectively in the UK in 2001. The remaining use (20% in the UK in 2001) of PFAS-related chemicals is represented by speciality surfactants and chemical intermediates. Table 4.1: Application areas for PFAS-related chemicals in the United Kingdom (2001), compared with the former global 3M production of PFOS-related chemicals (2000) (Hekster et al. 2002; OECD 2002; Kemikalieinspektionen 2004; RPA 2004).
As shown above, the PFOS-related chemicals are used in a variety of products within three main categories (OECD 2002):
The category performance chemicals can be divided further into more specific use areas, which are described in more details below:
4.2.1 Impregnation of textiles, leather and carpetsFluorinated chemicals are extensively used by the textile industry and by private consumers to form a coating on textiles, leather and carpets, which will repel both water and oil. The products used are polymers based on fluorinated acrylates and methacrylates. Textiles used for e.g. tablecloth, upholstery, rainproof clothing and bed linen are treated with these chemicals. 2-3% perfluorochemicals (of the fibre weight) are necessary to obtain water repellence. Bayer, DuPont, 3M and Daikin are some of the important suppliers of the chemicals (Hekster et al. 2002). In the production of textiles, PFAS substances are also used as wetting agents to e.g. enhance dyeing and as a binder in non-woven fabrics. PFAS substances are also used as e.g. antifoaming agents in textile treatment baths, as emulsifying agents for fibre finishes, and as penetration aids for bleaches (RPA 2004). For carpets PFOS-related substances have in addition to impregnating agents also been used as carpet spot cleaners (RPA 2004). Well-known soil repellents for carpets are Scotchgard® (3M), Zonyl® (DuPont), Baygard® (Bayer) and Foraperle® (Atofina/DuPont). An average of 15% fluorinated polymers is used for carpet protection (Hekster et al. 2002). The PFOS-related compounds typically used for textile and carpet surface treatment applications are the acrylate, adipate and urethane polymers of N-ethyl perfluorooctane sulfonamidoethanol (FOSE) (RPA 2004). Polymeric fluorochemicals are used as water repellents for treatment of leather. Water repellent consumer sprays are also available for leather products. Only about 0.025-0.05% perfluorochemicals (of the leather weight) is necessary to obtain water repellence (Kissa 2001). RPA – Risk & Policy Analysts Limited, which has prepared the examination behind the risk reduction strategy of United Kingdom for perfluorooctane sulfonate, concludes in their analysis that impregnating agents for use for textiles, leather and carpets today must be regarded as a historical use area. The use of PFOS-related chemicals in impregnating agents is very limited today or is ceased (RPA 2004). This is in line with the Swedish risk reduction strategy survey on PFOS-related compounds. In 2002, 28% of the total PFOS-related compounds were used as impregnating agents for textiles and leather. However, according to the Swedish suppliers and users, which were asked during the survey, PFOS or PFOS-related compounds are no longer used in impregnating agents (Kemikalieinspektionen 2004) However, this is somewhat in contrast with the Danish survey on PFOS-related chemicals. According to the Danish survey on use of PFOS-related products (see appendix C) impregnating products for textiles and leather represent somewhere between 16.5% and 30% of the total use of PFOS-related compounds registered in the Danish Product Register [4]. According to the survey impregnating products for leather is the largest group representing between 15% and 28% of the total use of PFOS-related compounds registered in the Danish Product Register (Havelund 2002). However, these numbers do not include imported products and products with a content of PFOS-related substances that are not labelled as dangerous substances. In most cases, a substitution of PFOS-related chemicals is not carried out in this area in Denmark, even though producers on the market state that impregnation of textile and leather can be carried out without the use of PFOS-related chemicals. Suppliers of chemicals for the textile industry (including production of carpets) state that they have commercially available alternatives to the PFOS-containing products. However, the survey was not able to identify the used alternatives (Havelund 2002). As the Danish survey is two years prior to the UK and the Swedish surveys, this may on the other hand explain the difference, and the possible substitutions can be graining ground on the Danish market as well. The Danish EPA has initiated a study on different shoe care products with the purpose to identify the substances used in this type of products. The study has been carried out at the same time as this present project, and the results are hence not yet published. The study has examined different shoe care products like e.g. shoe polish and impregnating agents. Impregnating agents represent about 20% of the market of shoe care products. In all 42 different impregnating agents on the Danish market were identified. It is estimated that the identified impregnating products represent the majority of the impregnating products for shoes on the Danish market. Technical data sheets and material safety data sheets were obtained from the suppliers. On the basis of this information, four of the 42 different impregnating products were analysed for a content of eight different PFOS-compounds, due to a suspicion of a possible content of PFOS-compounds. The chemical analysis showed that only one of these four products contained PFOS-compounds in a total concentration of 0.00015% (1.5 mg/kg). This low concentration suggests that the PFOS-compounds are impurities, probably because of a use other fluorinated compounds with shorter chain length or fluorinated telomers. The test results can therefore support the fact that PFOS-related compounds have been substituted in impregnating products for shoes found on the Danish market. However, it was not examined how many of the investigated impregnation products that contained other fluorinated compounds, such as fluorinated telomers (Engelund et al. 2004). 4.2.1.1 Possible alternatives for impregnation of textiles, leather and carpetsAccording to the Swedish risk reduction strategy on PFOS-related compounds, the alternatives used today in impregnating agents for textiles and leather are based on other highly fluorinated compounds like e.g. polytetrafluoroethylene (PTFE [5]), which is produced by use of PFOA (Kemikalieinspektionen 2004) For water repellence a mixture of silicones and stearamidomethyl-pyridine-chloride can be used alone as an alternative to PFOS-related compounds or together with a combination of carbamide (urea) and melamin resin (Kemikalieinspektionen 2004). In June 2003, the 3M Company replaced the PFOS-compound in their Scotchgard products by PFBS (perfluorobutane sulfonate). 3M's Scotchgard products are cleaners and stain protectors for carpets, leather, furniture, automotives, hard surfaces and other apparels. After the phase-out of PFOS in the Scotchgard product, the 3M Company first presented a product in an aerosol-can based on non-perfluoro chemistry. However, the product worked on water but not grease. Therefore, 3M now uses the perfluoro-compound with a shorter chain length – C4 (Fluoride Action Network 2004). The technical properties of PFBS are presented in appendix F. The Norwegian mass flow analysis of PFAS substances states that regarding impregnating agents for textiles, leather and carpets probably no PFOS-related chemicals are used today. This is based on the fact that 3M has stopped the production of PFOS-related compounds (produces PFBS instead), and that the products by Bayer and DuPont for impregnating carpets neither contain PFOS-related chemicals (but are based on telomers instead with shorter chain length). However, this means that the compounds used instead probably are fluoroalkyl polymers based on telomer-monomers (Statens Forureningstilsyn 2004). Furthermore, the Norwegian mass flow analysis of PFAS substances states that impregnating agents based on silicone can be used to impregnate textiles (leisurewear and sports wear) and leather (footware). Normally, tablecloths are not impregnated with PFAS substances. Instead polytetrafluoroethylene, also known under trademarks as Teflon® [6], Fluon®, Polyflon®, Vaflon®, is used. Furthermore, a new technology makes it possible to produce carpets with dirt- and water-repellant properties. These properties can be built-in in the synthetic fibres (polypropylene), which are used in the production of carpets. Thereby, use of impregnating agents is unnecessary. However, this technology does not seem to be used yet (Statens Forureningstilsyn 2004). The possible alternatives identified for impregnation of textiles, leather and carpets are:
4.2.2 Impregnation of paper and cardboardFluorinated chemicals are used in the paper industry to produce water and greaseproof paper. PFOS-related compounds are used both in food contact applications (plates, food containers, bags, and wraps) and in non-food contact applications (folding cartons, containers, carbonless forms, and masking papers) (OECD 2002; RPA 2004). The main suppliers of fluorochemicals in the paper industry are 3M (Scotchban®), Bayer (Baysize-S/Baysynthol®), Ciba (Lodyne®), Clariant (Cartafluor®) and DuPont (Zonyl®). Their brand names are listed in brackets. It is estimated that 1.0-1.5% fluorochemical (based on the dry weight of the fibres) is needed for paper protection (Hekster et al. 2002). Paper protection can be achieved by using two different classes of chemistries. One is to use the mono-, di- and triphosphate esters of N-ethyl perfluorooctane sulfonamidoethanol (FOSE) in rough proportions of 10%, 85% and 5% respectively. The second is to use N-methylperfluorooctane sulfonamidoethanol acrylate polymer (RPA 2004). As for impregnation agents for textiles, leather and carpets, RPA concludes that use of PFOS-related chemicals in impregnating agents is very limited today or is ceased in the UK, but the trend is also believed to have occurred throughout Europe (RPA 2004). The same conclusion can be seen in the Norwegian mass flow analysis of PFAS substances. No PFAS products are registered in the Norwegian Product Register today, but previously there have been. One producer of greaseproof paper exists in Norway, and according to this producer no PFAS-related substances are used (i.e. no fluorotelomers or perfluorinated substances with shorter chains are used either). The alternatives used instead are not listed (Statens Forureningstilsyn 2004). This is, however, somewhat in contrast to the Danish survey on PFOS-related chemicals. According to this survey (see appendix C) impregnating products for paper and cardboard represent somewhere between 15% and 28% of the total use of PFOS-related compounds registered in the Danish Product Register. These numbers do not include imported products or products with a content of PFOS-related substances that are not labelled as dangerous substances (Havelund 2002). According to the Danish survey, a substitution of PFOS-related chemicals is not carried out in most cases, even though players on the market state that impregnation of paper can be carried out without the use of PFOS-related chemicals. However, the survey was not able to identify the used alternatives (Havelund 2002). As the Danish survey is two years prior to the UK and the Norwegian surveys, this may on the other hand explain the difference, and the possible substitutions can be gaining ground on the Danish market as well. 4.2.2.1 Possible alternatives for paper and cardboardNone of the surveys mentioned above has identified the used or available alternatives. The Norwegian survey claims that no PFAS-substances are used in Norway within this area. According to DuPont telomer-based substances are used as alternatives to PFOS-compounds within this area, as DuPont themselves produces telomer-based substances for use in this application area (Personal communication DuPont, 2004a). The possible alternatives identified for impregnation of paper and cardboard are telomer-based substances. 4.2.3 Cleaning agents, waxes and floor polishes – industrial and consumer productsPFOS-related substances are used/have been used in a variety of industrial and household cleaning products as surfactants. PFOS-related substances are used in cleaning agents, automobile waxes, alkaline cleaners, denture cleaners and shampoos to improve wetting, used in floor polish to improve wetting and levelling, and used in dishwashing liquids and carwashes as a rine-aid (PFA 2004). According to the UK risk reduction strategy for PFOS, the use of PFOS-related compounds as cleaning agents – both industrial and consumer product – must be regarded as a more or less historical use in the UK. This statement is made, even though it is not clear what alternatives are used today (RPA 2004). This statement is in line with the Norwegian survey on PFAS-substances that states that PFAS-substances formerly have been used, but are no longer used in cleaning agents. However, in some sprayproducts for cleaning of glass, in waxes and in floor polishes and similar products PFAS-substances are still used. There is no indication if the used substances are PFOS-related substances or other PFAS-substances (with shorter chain length). One producer claims to use a fluorocompound, which is not a PFAS product (Statens Forureningstilsyn 2004). This statement is somewhat in contrast to the Danish investigation of PFOS-related compounds in Danish products. The Danish survey showed that cleaning agents and polishing compounds are one of the larger use areas. Cleaning agents are alone responsible for about 8% of the total use of PFOS-related compounds, whereas polishes and waxes (including shoe and furniture polish) are responsible for between 0.3% and 9% of the total use of PFOS-related compounds registered in the Danish Product Register. However, according to the survey a substantial amount of substitution has taken place within cleaning products for industrial use, whereas PFOS-related compounds still are used in ordinary cleaning products for the consumer market (Havelund 2002). According to the Swedish survey of PFOS-related compounds, cleaning agents and floor polish products were one of the larger uses, where PFOS-related compounds are used. According to the survey of the Swedish Product Register 6% of the total amount of PFOS-related compounds was used within cleaning agents and floor polish products in 2002 (Kemikalieinspektionen 2004). The Swedish survey showed that only one specific PFOS-compound was used in cleaning agents, floor polish and auto polish products. This compound is the potassium salt of glycine, N-ethyl-N-[(heptadecafluorooctyl)sulfonyl] (CAS-no. 2991-51-7). The content in the final product is in general between 0.005 – 0.01% (Kemikalieinspektionen 2004). The Norwegian survey lists a content of fluorinated compound of below 0.01% (Statens Forureningstilsyn 2004). The Danish survey lists a concentration interval, which is about 10 times higher (0.06 – 0.1%), and several different PFOS-related compounds were found in different cleaning agents, waxes and polishes (Havelund 2002). Today, fluorinated surfactans (such as PFOS) are allowed in the Swan eco-labelled filmforming floor care products. However, the concentration limit is set at 0.01%, and it is indicated that in the future it will be investigated whether it is possible to tighten this requirement (Nordic Ecolabelling 2004). According to the UK risk reduction strategy on PFOS, fluorinated surfactants are accepted in the ecolabelling scheme, as they are difficult to replace (RPA 2004). 4.2.3.1 Possible alternatives for cleaning agents, waxes and floor polishesAccording to the website of the 3M Company, 3M produces some alternatives to PFOS-related chemicals, called Novec compounds (se appendix F for further details), which are used in products for commercial and industrial cleaning. These alternative compounds are all fluorinated compounds based on a C4 structure. Examples are methyl nonafluorobutyl ether (CAS no. 163702-07-6) and methyl nonafluoroisobutyl ether (CAS no. 163702-08-7). One of the contacted companies in search for alternatives, OMNOVA Solutions Inc., manufactures a line of fluorosurfactants (fluorinated polyether) called PolyFox are used in about 40 polish products in the USA, Europe and Asia. According to this company their PolyFox product line can, besides being used as an alternative to PFOS-based products, also be used as an alternative to the long chained telomer based products. The entire PolyFox family of fluorosurfactants are polymers with a molecular weight greater than 1,000. The PolyFox polymers are based on ether links – both the polymer backbone linkages and the link between the backbone and the perfluoroalkyl pendant side chains. The PolyFox fluorosurfactants are synthesized from perfluoroalkyl starting materials with a fully fluorinated carbon chain length of C4 or less. The current first generation products are all made with C2F5 or CF3 perfluoroalkyl side chain structures (Personal communication OMNOVA 2004). The basic structure and the technical properties of the PolyFox products are presented in Appendix F. Fluorinated surfactants are especially used in water-based floor polish products, as these compounds lower the surface tension and contribute to the formation of a hard film with a good adhesion to the floor, mainly on floors of PVC and linoleum. The advantage of the fluorinated surfactants is that they do not alter any of the other properties of the product (Kemikalieinspektionen 2004). However, in the last decade the trend has been to use softer waxes, which are a combination of cleaning agents and polish. In these products, the fluorinated surfactants are substituted with non-ionic or anionic surfactants, which have good wetting properties. The advantage of the soft waxes is that they can be applied on top of the old layer of wax, whereas the old and hard waxes (based on PFOS-related compounds) had to be removed before a new layer was added. According to the Swedish sector of cleaning agents, waxes and floor polish, the sector aims to developed non-fluorinated products, but the time where all products will be free from fluorinated compounds could not be determined (Kemikalieinspektionen 2004). According to the earlier Danish survey on PFOS-related compounds it is assessed that the use of PFOS-related compounds in wax and polish is very difficult to replace. The substitutes may have to be added in a much higher concentration than the fluorinated compounds. Acrylates have been suggested as a general alternative to PFOS-related compounds, also within products as waxes. Furthermore, as the fluorinated compounds have the same function in waxes as in paint, it may be possible to use the same substitutes as within the paint and varnish industry (Havelund 2002). The possible alternatives identified for cleaning agents, waxes and floor polishes are:
4.2.4 Paint and varnishPFOS-related chemicals have several uses in paint and varnishes. For example, they can be used as wetting, levelling, and dispersing agents, and may also be used to improve gloss and antistatic properties. They can be used as additive in dyestuff and ink, e.g. as foam generators. Furthermore, they can be used as pigment grinding aids or as agents to combat pigment flotation problems (Kemikalieinspektionen 2004; RPA 2004). According to BASF, fluorosurfactants are, in coatings application, mainly used for substrate wetting, levelling and reduction of surface tension (e.g. in spray applications) (Personal communication BASF 2004). The information received from different suppliers within the paint and varnish industry suggests that fluorinated surfactants in general are much more expensive alternatives compared to other surfactants. Therefore, fluorosurfactants are only used for special purposes in paint and varnishes, where it is necessary to gain such a low surface tension, which no other (non-fluorinated) alternatives can achieve, e.g. in product where an extremely smooth surface is necessary. According to the former Danish survey on PFOS-related compounds (Havelund 2002) – see appendix C for further details – paint and varnish represents somewhere between 11% and 12.5% of the total use of PFOS-related compounds registered in the Danish Product Register. Furthermore, the group of printing ink, toner and additives for printing ink additionally represents between 6.7% and 12.6% of the total use of PFOS-related compounds registred in the Danish Product Register. However, the survey also state that a substitution of PFOS-compounds in the paint and varnish industry by and large have been carried out in Denmark. PFOS-related compounds can, however, be present in imported products. The substitution has been carried out over a long period and often entirely new recipes have been developed. Therefore it is not possible precisely to determine, which substances that have substituted the used PFOS-compounds in paint and varnish (Havelund 2002). This statement is in line with the Swedish investigation on use of PFOS-related substances. Even though the use of PFOS-related compounds was large within the Paint and Varnish Industry in Sweden in 2002, information today from different suppliers in Sweden suggests that PFOS-related compounds no longer are ingredients in paint and varnish products (Kemikalieinspektionen 2004) According to the UK risk reduction strategy for PFOS, the use of PFOS-related compounds as additive in dyestuff must be regarded as a more or less historical use in the UK. This statement is made, even though it is not clear what the alternatives are today (RPA 2004). In contrast, PFAS-substances are used in several paint products on the Norwegian market. The substances are used both in waterbased and solventbased paints and varnishes. The amount used is low – below 0.01% (w/w) (Statens Forureningstilsyn 2004). Perhaps the difference lies in the difference between the investigated substances in the different surveys (PFOS-related substances versus PFAS-substances), hereby implying that a use of perfluorinated substances with shorter chain length may still be used in other countries as well? DuPont confirms that telomer-based surfactants are used within this area (Personal communication DuPont 2004a). 4.2.4.1 Possible alternatives for paint and varnishAs mentioned under impregnating agents for textiles, leather and carpets the 3M Company has replaced their PFOS-compounds with C4 compounds, where PFBS (perfluorobutane sulfonate) is the basis. This replacement has also been made within coating products – especially within the area of electronic coating (3M webpage 2004). The UK risk reduction strategy on PFOS (RPA 2004) mentions that a company in the UK has satisfactorily replaced a fluorosurfactant with a polyether-modified polydimethyl siloxane. As mentioned under cleaning agents, waxes and floor polishes OMNOVA Solutions Inc. manufactures a line of fluorosurfactants called PolyFox™. The PolyFox polymers can also be used as alternatives to PFOS-related compounds in coating formulations. The PolyFox polymers are based on ether links – both the polymer backbone linkages and the link between the backbone and the perfluoroalkyl pendant side chains. The PolyFox flurosurfactants are synthesized with C2F5 or CF3 as the starting material (Personal communication OMNOVA 2004). For details see the description of the PolyFox compounds in appendix F. Rütgers Kureha Solvents produces different propylated aromatics (naphthalenes and biphenyls), which can be used as water repelling agents for different applications. For example rust protection systems, marine paints, resins, printing inks, coatings, electrical applications, electronically and mechanical applications (Personal communication RKS 2004). The different propylated aromatics and their technical properties are presented in details in appendix F. Other possible replacements to fluorosurfactants are silicone surfactants or surfactants based on aliphatic alcohols. BASF produces a range of aliphatic alcohols, both anionic and non-ionic surfactants. However, especially the effect of the non-ionic surfactants is mixed, because these products also are used as defoamers or emulsifiers. So the non-ionic surfactants are difficult to use as replacements for fluorosurfactants. BASF puts emphasis on the following anionic surfactants: a fatty alcohol polyglycolether sulfate and a sulfosuccinate, which under special circumstances may be alternatives to fluorosurfactants (Personal communication BASF 2004). The technical properties of these products are described in details in appendix F. Worlée-Chemie produces silicone polymers, which in the paint and ink industry in several cases can be used as alternative wetting agents to fluorosurfactants. Two products are emphasized, one being a product based on a non-ionic modified silicone polyether, and the second product is a mixture of a silicone polyether and a diocylsulfosuccinate in ethanol and water (Personal communication Worlée-Chemie 2004). The technical properties of these products are described in details in Appendix F. The companies Münzing Chemie, Cognis and BASF have all mentioned sulfosuccinates as possible alternatives to fluorosurfactants within the paint and varnish area. Sulfosuccinates are esters of succinic acid (HOOC-CH2-CH2-COOH) in reaction with hydrogen sulfite. Münzing Chemie has been able to replace fluorobased wetting agents, e.g. in wood primers, with a product based on a sulfosuccinate derivative in ethanol (19%) and water (12.5%) (Personal communication with Münzing Chemie 2004). The technical properties of this product are described in Appendix F. Cognis has been able to replace fluorobased surfactants based on sulfosuccinates in printing inks, where they are also approved for food contact. Their product is based on the sodium salt of di-2-ethylhexyl sulfosuccinate in ethanol (5%) and water (20%) (Personal communication with Cognis 2004). The technical properties of this product are described in Appendix F. BASF produces a sulfosuccinate product, which under special circumstances can be used as an alternative to fluorosurfactants within the coating industry. The product is based on di-octyl-sulfosuccinate (di(2-ethylhexyl)-sulfosuccinate) (Personal communication BASF 2004). The technical properties of this product are described in Appendix F. The possible alternatives identified for paints and varnishes are:
4.2.5 Pesticides and insecticidesAccording to the UK risk reduction strategy for PFOS, PFOS-related compounds have formerly been used in the manufacture of baits for ants and in insecticides against beetles and ants. However, today both associations in the UK and in the EU indicate that its members are not involved in the use of PFOS-related substances in the manufacture of pesticides. Use of PFOS-related compounds in pesticides is thus regarded as a more or less historical use. This statement is made, even though it is not clear what is used (as alternatives) today (RPA 2004). This is in line with the Danish survey on PFOS-related compounds, where no PFOS-related compounds were found in pesticides products (Havelund 2002). However, the investigation may not cover imported products. 4.2.5.1 Possible alternatives for pesticidesAs the use of PFOS-related compounds already has ceased within this area, it has been irrelevant to search for alternatives. No indications have been found in literature regarding alternatives to PFOS-related substances today. 4.2.6 Fire-fighting foamsThere are several different types of fire-fighting foams, both fluorine containing foam types and fluorine-free types. Fire-fighting foams containing fluorines are (RPA 2004):
According to the UK risk reduction strategy on PFOS any of the above types of fire-fighting foams may contain PFOS-related substances or surfactants (RPA 2004). Aqueous film forming foams (AFFF) were developed in the 1960's for the purpose of extinguishing flammable liquid fuel fires. Alcohol-resistant aqueous film forming foams (AR-AFFF) were developed in the 1980's to deal with water miscible liquids such as alcohol and petrol containg up to 20% alcohol. They provide a fire-extinguishing film consisting of foam, when mixed with water and air. Monomeric perfluorinated salts are used to contribute to the performance of AFFF as the primary fire-extinguishing chemical and as vapour sealants that prevent re-ignition of fuel and solvent (Moody et al. 2000; RPA 2004). The concentration of perfluorinated compounds in fire-fighting foams is about 0.5-1.5% (Hekster et al. 2002). Use of fire-fighting foams represents less than 2% of the total fluorochemical use, worldwide according to Buckeye 2001. However, the UK survey of PFOS-related substances states that the use of PFOS-related compounds in fire-fighting foams was 16.3% of the total use of PFOS-related compounds in the UK in 2001 (RPA 2004). In Norway more than 50% of the total use of PFAS-related substances are used for fire-fighting purposes (Statens Forureningstilsyn 2004). In contrast, fire extinguishants represent between 0.3% and 1.1% of the total use of PFOS-related compounds in Denmark according to the data registered in the Danish Product Register (Havelund 2002). However, today in the UK all fire-fighting foams are manufactured through use of PFOS-free fluorochemicals. As fire-fighting foams have a long shelf life (10<-20 years) PFOS-containing fire-fighting foams are still used in the UK. According to the UK survey only the AFFF and the AR-AFFF foams do contain PFOS-related compounds today, and these foams were manufactured before the 3M Company withdrew their PFOS-chemicals from the market (RPA 2004). The use of foam-forming fire extinguishers can be divided in two groups: Mobile hand-held fire extinguishers and stationary fire-fighting systems. For the mobile fire extinguishers more environmentally friendly foam has been introduced, at least in Holland where the eco-labelling scheme Millieukeur has been established for these types of products. However, products with the Millieukeur label do not need to be PFOS-free, but contain less PFOS compared to other fire-fighting foams (Hekster et al. 2002). According to a Swedish survey on PFOS-related compounds, new hand-held fire extinguishers do not contain surfactants based on PFOS-related compounds (Kemikalieinspektionen 2004). However, the search for alternatives shows that fluorosurfactants are still used. Now they are just based on a C4 or a C6 chain, which means they are not in the category of PFOS-related compounds (Personal communication DuPont 2004b). The stationary fire-fighting systems can be based on five different agents (Hekster et al. 2002):
However, when it comes to extinguish a liquid fuel fire, fire-fighting foams with fluorosurfactants are the most effective (Kemikalieinspektionen 2004; Moody et al. 2000). Normally, a mixture of fluorinated surfactant and a hydrocarbon-based surfactant are used in AFFF, as this combination is more cost-effective and performs better than either surfactant separately (Moody et al. 2000). According to Moody et al. (2000) the use of fluorinated compounds in fire-fighting foams have been reduced, as fire-fighting foams with the sole purpose of using them during training exercises have been developed. These training foams have become popular, as they are cheaper, due to the absence of the expensive fluorinated surfactants. According to the survey on use of PFOS and PFOS-related substances in Denmark, carried out in 2001 (see a short summary in Appendix C), PFOS and PFOS-related substances were registered in fire-fighting foams in Denmark. In all about 0.3 and 1.1% of the total use of PFOS-related substances registered by the Danish Product Register comes from fire extinguishants. According to the survey, based on information from the Danish Product Register in 2000 [7], perfluoroalkyl sulfonamide aminopropyl derivates and perfluoroalkyl sulfonates are used in fire-fighting foams (Havelund 2002). 4.2.6.1 Possible alternatives for fire-fighting foamsThe alternatives to the PFOS-based fluorosurfactants used in existing fire-fighting foams are (RPA 2004):
- Synthetic detergent foams (often used for forestry and high expansion applications) - Protein foams (mainly used for training, but also some marine use) - Other fluorine free-foams The fluorine containing fire-fighting foams is used in many cases as they are relatively fluid and provide fast fire extinction. However, Moody et al. (2000) suggests returning to previously used technology such as the protein-based foams, to avoid the use of fluorinated surfactants in fire-fighting foams. The silicone and hydrocarbon based surfactants are often used in combination with fluorosurfactants to achieve higher performance levels in actual fire situations. Used alone the silicone and hydrocarbon based surfactants provide the lowest technical suitability of the potential alternatives and hence are not real alternatives to fluorosurfactants (RPA 2004). It is important to notice that foams without fluorinated surfactants cannot reach the level of performance obtained with foams containing fluorinated surfactant compounds. Fire-fighting foams made from fluorinated surfactants have technically shown to be the only technology, which can quickly and effectively extinguish fires from highly combustible and flammable materials (Fire-fighting Foam Coalition 2001). Therefore, the only real alternative to the PFOS-based fire-fighting foams is the PFAS-based fluorosurfactants. The PFOS-free fire-fighting foams used today are unlikely free from fluorinated compounds, but are based on PFAS-compounds with C6 or C4 chain length. As described earlier, the 3M Company started to phase out the production of PFOS chemicals in the year 2000 and manufactures today perfluorinatede chemicals with shorter carbon chains (compared to PFOS – a C8-chemical). According to the website of 3M a C6-fluorinated compound is used in their Fire Protection Fluid. The compound is dodecafluoro-2-methylpentan-3-one (CF3-CF2-C(=O)-CF(CF3)2). (See more specific description in Appendix F) (3M webpage, 2004). DuPont manufactures telomer-based compounds of shorter carbon chains – e.g. C6-compounds. According to DuPont, their fluorosurfactants used for fire-fighting foams are based predominantly of a C6-telomer. Depending on the product the content of fluorinated C6-telomer is between 65 and 95% (Personal communication DuPont 2004b). This means that the rest (5-35%) of the fluorinated telomers in the products is either fluorinated telomers with higher or lower carbon chain (possibly a part may still be C8?). No such information about the purity of the C6 compound of 3M was available (See more specific description in Appendix F). The survey on PFOS-compounds from the UK states that the fluorine-free foams are a relatively new technology today. These fluorine-free foams are used in training exercises, for shallow spill fires, but are also suitable for flammable liquid fires in line with the PFOS based or telomer based fire-fighting foams. Some information suggests that the fluorine-free foams may not currently achieve the same standards of PFOS based fire-fighting foams on a few chemical properties, but an European foam producer indicates that it produces fluorine-free foams that perform as well during testing as PFOS based foams. The producer indicates that these foams are widely used in Australia, Singapore, New Zealand and other parts of the world (RPA 2004). A Swedish investigation on the subject shows that fire-fighting foams with the content of PFOS-related compounds were removed from the (Swedish) market in 2003. The alternatives were highly fluorinated telomers, but no CAS-numbers could be identified. The fire-fighting foams are today predominantly based on C6F13, but it is possible that the fire-fighting foam contains C8F17 – a PFOS-related compound (Kemikalieinspektionen 2004). Similarly, the Norwegian survey on PFAS-compounds states that PFOS-related compounds are not likely to be used anymore in fire-fighting foams, as fire-fighting foams now most likely are based on telomer-compounds with shorter chain length. In Norway the AFFF fire-fighting foams are mainly used in offshore installations, at oil refineries, at tankers, at airports and similar places where kerosene products and other flammable liquids are used and stored. Over 50% of the total use of PFAS-compounds [8] in Norway is due to the use of fire-fighting foams, and use of fire-fighting foams in offshore installations represents more than half of the use of fire-fighting foams (Statens Forureningstilsyn 2004). According to Falck Denmark (the department Falck Teknik), Denmark uses about 10 tons of fire-fighting foam for land use per year, i.e. not counting use on ships and oil platforms. Formerly, the annual amount was as high as 30 tons for land use, but with a stop of fire-fighting foam for use in fire training exercises, the use has fallen drastically (Personal communication Falck Teknik 2004). Falck Denmark alone uses less than 5 tons fire-fighting foam annually. Falck Denmark imports and sells fire-fighting foams to the fire department of Copenhagen (Københavns Brandvæsen). According to Falck, no fluorinated compounds are present in the fire-fighting foams that Falck imports from Sthamer in Germany. Their fire-fighting foam is based on either protein foam or syntethic foam (Personal communication Falck Teknik 2004). Two other companies cover the last 40-50% of the fire-fighting foam market in Denmark. One of these companies was contacted (wanted to be anonymous). Formerly, their fire-fighting foams did contain polyfluorinated compounds supplied by the 3M Company. However, when 3M stopped its production, they in stead imported fire-fighting foams from Solberg Skandinavien, a company located in Norway. The Danish importer was not aware that the fire-fighting foams contained fluorinated compounds or not. According to the website of Solberg Skandinavien [9], they sell fire-fighting foams both with and without fluorinated compounds According to Solberg Skandinavien their fire-fighting foams AFFF and AR-AFFF are not based on PFOS-related compounds. The fluorinated surfactants, which according to their MSDS's all are based on a polyfluoroalkyl betaine, are telomer based (Personal communication Solberg Skandinavien 2004). It is not clear, which telomers their fire fighting foams are based on, but they are most likely a blend of telomers with different chain lengths as is the case for the DuPont produced telomers. Copenhagen Airports (which covers both the airports in Kastrup and in Roskilde) solely use AFFF foams for fire-fighting purposes. The foams are purchased by Solberg Skandinavien and are PFOS-free, but fluorotelomer based. However, for training purposes special training fire-fighting foams – foams without fluorine - are used. An estimated amount of 1000 litres of training foam is used annually. The vehicles used by Copenhagen Airports for fire extinguishing and training purposes contain both AFFF foam with fluorine, and the special training foam without fluorine. Twice a year Copenhagen Airports are obligated to check the vehicles. In these cases the AFFF foam with fluorine is used, but the foam is gathered by use of coal filters, which are then send to special chemical treatment (at KommuneKemi, Denmark). For each check of the vehicles it is estimated that about 50-70 litres of fluorine containing AFFF foam are used (Personal communication Copenhagen Airports 2004). Part of the offshore industry in Denmark, which operates in the North Sea, has also been contacted. One (of three) company has informed that they use AFFF fire-fighting foams at the offshore installations. The AFFF foams used are all containing fluorosurfactants, and their supplier informs that the fluorosurfactants are based on fluorinated telomers (i.e. mainly C6 fluorinated compounds) (Personal communication 2004). The Danish Navy was contacted in order to learn about their use of fire-fighting foams (Personal communication the Navy 2004). Four different fire-fighting foams are currently used in the Danish Navy, in the Danish Air force and in the Danish Army. Three of the four fire-fighting foam products are AFFF products, which means they are based on fluorinated surfactants. The Navy and the Air force are using two and one of the three different AFFF products, respectively. All three organisations also use a fire-fighting foam product called Sthamex F-15 (from Sthamer in Germany). According to Sthamer in Germany this product does not contain any fluorinated surfactants (Personal communication Sthamer 2004). Sthamex F-15 is used by the Navy in real fire situations on board ships, and is also used by the Army in real fire situations. Common for the Navy and the Army is that all relevant personnel is educated at a training school HVIMS, which is using a non-fluorinated fire-fighting foam called Biofoam T (obtained from Hauberg Technique) for education and training purposes (Personal communication, the Navy 2004). According to Hauberg Technique, supplier of the Biofoam T fire-fighting foam, none of their fire-fighting foams contain fluorinated compounds. (Personal communication, Hauberg Technique 2004). The possible alternatives identified for fire-fighting foams are:
4.2.7 Photographic industryIn the photographic industry PFOS-related compounds are used in the manufacturing process of film, photo paper and plates. The PFOS-related compounds function as dirt rejecters and friction control agents. Furthermore, they reduce surface tension and static electricity. Imaging materials that are more sensitive to light (i.e. high speed films) are more in need of the properties provided by PFOS based materials (RPA 2004). The concentration of PFOS-related substances in coatings in films, paper and plates is in the range of 0.1-0.8 μg/cm2. Due to a reduction of the use of films (caused by use of digital cameras), the use of PFOS within this area is not expected to grow (RPA 2004). Electronic products as cameras, printers, scanners etc. do not contain PFOS-related compounds (RPA 2004). Furthermore, PFOS-related compounds have been used in developers for photo films according to a Swedish survey. However, the survey suggests that this use is no longer relevant. No information about the alternative products was found (Kemikalieinspektionen 2004). In Denmark between 0.2% and 1.1% of the total use of PFOS-related compounds registered in the Danish Product Register is due to the use of photo developers (Havelund 2002). 4.2.7.1 Possible alternatives for the photographic industryAccording to the UK reduction strategy for PFOS-related compounds, the work of substituting PFOS-related compounds within the photographic industry has been ongoing since the year 2000 (where the 3M Company ceased their production of PFOS compounds). This work has resulted in a reduction of 83% of the use of PFOS-related compounds within this industry. This reduction is primarily due to a change to digital techniques, where a dry process technique is used (RPA 2004). The alternatives that have replaced the PFOS-related compounds are telomer products and chemicals with short perfluorinated chains such as C3 and C4, but also non-fluorinated chemicals such as hydrocarbon surfactants and silicones (RPA 2004) For the remaining use of the PFOS-related compounds within the photographic industry no alternatives have been identified so far. These uses are surfactants, electrostatic charge control agents, friction control agents, dirt repellent agents and adhesion control agents for mixtures used in coatings applied to films, papers, and printing plates (RPA 2004). The possible alternatives identified for the photographic industry are:
4.2.8 Manufacturing of semiconductorsPFOS/PFAS based chemicals are used in the fabrication of imaging devices such as digital cameras, cell phones, printers, scanners etc. Manufacture of print plates is a lithographic process. In this process PFOS-related compounds are used because of their surface-active properties, and as they are able to resist the strongly acidic conditions. The PFOS compounds are used in photo-acid generators (PAGs), in antireflective coatings (ARC), and as surfactants in developers, etch mixtures and commercial photoresists (Kemikalieinspektionen 2004; RPA 2004) The PFOS-related compounds are a part of the process chemicals and are not a part of the final products (RPA 2004) 4.2.8.1 Possible alternatives for manufacturing of semiconductorsAccording to the European Semiconductor Industry Association new techniques are being developed where PFOS-related substances are not being used. However, these techniques are not yet ready for commercial use and will not be for the next 2 to 5 years. The most critical manufacturing processes are photoresist (PAG) and anti-reflex treatment (Kemikalieinspektionen 2004; RPA 2004). 4.2.9 Hydraulic oils within the airplane industryHydraulic oils are used in airplanes to cause a break pressure. However, the early used hydraulic oils could cause fires for which reason perfluorinated surfactants are added. Use of perfluorinated compounds in the hydraulic oils also has the function that they prevent corrosion by lowering the surface tension. Hydraulic oils with a content of perfluorinated compounds are used in both civil and military airplanes all over the world (Kemikalieinspektionen 2004). A specific compound is used in the hydraulic oil – the potassium salt of perfluoroethyl cyclohexyl sulfonate (CAS-no. 67584-42-3). Per definition, this compound is not a PFOS-related compound, but is a part of the large PFAS group (perfluoroalkyl substances) (RPA 2004). The content of the substance in the used hydraulic oils is about 0.1% (Kemikalieinspektionen 2004). The total global market for fluorinated compounds in aircraft hydraulic fluids is about 2.2 tonnes per year (RPA 2004). 4.2.9.1 Possible alternatives for hydraulic oils within the airplane industryA search for alternatives within this area has been going on for 30 years and about 2500 different compounds have been tested. Unfortunately, no compounds have been useful, meaning that no alternatives have been identified. The not fully fluorinated telomers have not met the required demands and cannot be used as alternatives even though the chemistry is similar to that of PFOS based compounds (Kemikalieinspektionen 2004). Today the only possible alternative to the above mentioned fluorinated substance is potassium perfluorooctane sulfonate, and there is no known alternative chemistry, which will provide adequate protection to the aircrafts (RPA 2004). A change in the formulation of the hydraulic oils seems to be the only alternative solution. This will, however, demand a comprehensive testing together with an approval from the airplane manufacturers, which may take as long as 10 years, as the safety measures within this industry are very high (Kemikalieinspektionen 2004). According to the UK risk reduction strategy on PFOS, manufacturers of hydraulic fluids used in the airplane industry are looking for manufactures that would be willing to produce the needed PFOS substances, when the existing stocks are exhausted. The 3M Company formerly produced the potassium salt of perfluoroethylcyclohexyl sulfonate used in hydraulic fuels, but they also withdrew the production of this substance from the market (RPA 2004). 4.2.10 Metal surface treatmentFor the metal surface treatments called chromium plating and chromating PFOS-related compounds are used to lower the surface tension of the chromium bath. The PFOS-related compounds act as a barrier over the chromium bath and will prevent an emission of chromium (VI) aerosols. The aerosols are a health risk, as chromium (VI) is carcinogenic (RPA 2004). PFOS-related substances used for mist suppression in metal plating baths are the potassium, lithium, diethanolamine, and ammonium salts of perfluorooctane sulfonic acid, as well as quaternary ammonium salts and amines. According to the UK survey the quaternary ammonium salt of PFOSA (CAS No. 56773-42-3) seems to be used the most. Typically, 10% solutions of PFOS-related substances are used for metal plating. An estimation of the PFOS-related substances for use within the metal plating industry in the EU is between 8.6-10 tonnes per year. Important applications for chromium plating include aircraft, medical industries, vehicles and general engineering (RPA 2004). In Sweden about 3% of the PFOS-related compounds used in 2002 was used for metal surface treatment (Kemikalieinspektionen 2004). According to the Danish report on use of PFOS-related chemicals, between 0.6 and 2.3% of the total use of PFOS-related chemicals was used for metal surface treatment. Another 3.5% of the total use of PFOS-related compounds registered in the Danish Product Register was used within the area of electroplating and galvanization (Havelund 2002). Besides the use for chromium plating, which is the main use, fluorinated surfactants are also used as (RPA 2004):
In the area of surface coating, PFOS-related compounds and PFOA are found in products, which are surface coated with fluoroplastics (PTFE, PVDF and other fluoropolymers). At the moment no PFOA-free PTFE-dispersion is available on the market, but all the big suppliers of PTFE have started to work on possible alternatives. However, at the moment there seems to be no satisfying alternative to PFOA in the production-process of PTFE (Personal communication – surface treatment company 2004; Personal communication DuPont 2004a). 4.2.10.1 Possible alternatives for metal surface treatmentAt the moment, EU funded research is trying to identify alternatives, as use of chromium(VI) is a serious health risk (carcinogenic) in the chromium-plating and chromating processes. If alternatives to chromium(VI) can be found the use of PFOS-related chemicals is most likely eliminated within the area of metal surface treatment (Kemikalieinspektionen 2004; RPA 2004). An alternative process already exists for decorative chromium plating. In this process chromium(III) is used instead and no PFOS-chemicals are necessary. However, the problem is that the process with chromium(III) does not function as well for hard plating. Instead larger closed tanks or increased ventilation are suggested as (expensive) alternative solutions for the applications where a use of chromium(III) is not possible yet (Kemikalieinspektionen 2004; RPA 2004) 4.2.11 Plumbing – fluxing agentsThe search in the Danish Product Register for use of PFOA salts and telomer alcohols, performed in this project (see detailed description in Appendix D and E), shows that the PFOA ammonium salt and the telomer alcohol perfluorooctanol are the only two substances, which are found in products reported to the Danish Product Register of the PFOA salts and of the telomer alcohols. These two compounds are both found in fluxing agents, which are used for plumbing with leaded soldering tin. According to the Danish survey on use of PFOS-related compounds fluxing agents for plumbing represent about 0.3% of the total use of PFOS-related compounds registered in the Danish Product Register (Havelund 2002). 4.2.11.1 Possible alternatives for plumbing – fluxing agentOne of the companies selling fluxing agents with a content of fluorinated compounds was contacted (see Appendix E for a more detailed description). According to this company the fluxing product will automatically go out of use in a couple of years as the "European Directive 2002/95/EC of January 27 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment" states that after July 1st 2006 lead is no longer permitted in electrical and electronic equipment (Directive 2002/95/EC 2003). Today plumbing is carried out with the use of soldering tin that consists of 63% lead and 37% tin. When using this leaded soldering tin it is necessary to use a soldering flux to prepare the surface for the plumbing. Fluxing agents are used in automatically closed systems and are sprayed on the object, which is heated (to remove the fluxing agent), and then the plumbing is carried out. The alternatives to leaded soldering tin are use of soldering tin of either pure tin or soldering tin with a small percentage of silver and copper, 0.7% and 3.5% respectively. With a use of lead-free soldering tin it is, however, possible to use water-based fluxing agents instead of the solvent based fluxing agents (with perfluoro compounds). This means that the use of perfluoro compounds in fluxing agents automatically will cease with the implementation of lead-free plumbing. 4.2.12 Other usesThe risk reduction strategy of PFOS-related compounds made by the UK has identified a few other uses of PFOS-related compounds. These are:
These applications have been identified outside the EU and as more historical uses for which reason they are not described in further details (RPA 2004). Furthermore, telomer alcohols are used in chemical analysis. Fluorous Technologies, Inc. describes that fluorous techniques are used for the synthesis and separation of organic molecules in reaction mixtures (Fluorous Technologies 2004). 5 Technical assessment of alternatives
This assessment of alternatives is based on information from technical data sheets describing the usage of the compounds. Furthermore, the comments and discussion are mostly based on experience with surface coatings. The alternatives identified in this project are described shortly in Table 5.1, and in more details in Appendix F. Table 5.1: Alternatives to PFOS-compounds identified in this project.
5.1 General information on surfactants for coatingsA coating needs to fulfil a number of technical demands. The demands depend both on the method of application, the substrate and the demands of the coated surface. To achieve certain technical properties a number of additives are used especially within water-borne coatings. Water-borne systems including both emulsions (binder) and dispersions (e.g. pigment) normally contain several surface-active agents like emulsifiers, wetting agents, defoamers etc. It should also be noted that water-borne systems generally are more demanding with regard to wetting and adhesion than solvent-based systems basically due to the fact that water has a very high surface tension. Normally the binder system will, at least to some extent, determine the surface tension of the coating as water-borne binder systems include emulsifiers that are surface active. Non-adsorbing substrates like metal and plastics are a challenge for water-borne systems, as the surface tension needs to fit to the surface tension of the substrate to get proper wetting as well as adhesion. For this reason the surface tension of the coating needs to be in the same order or lower than that of the substrate. This can be achieved by using surfactants as they lower the surface tension of the coating. But it should be noted that different substrates demand different surface tensions of the coating system, and that substrates, which do not adsorb the coating, are more demanding compared to substrates that adsorb the coating. To get a proper wetting and adhesion of a new coating layer at already coated surfaces, the surface tension of the new coating needs to be in the same order of the already coated layer or even lower. Therefore, the surfactants present in a coating may influence the possibility to recoat the already coated surface, especially if the coating has a very low surface tension, as it often is the case when fluorinated surfactants are used. The presence of surfactants in water-borne coating may cause problems with regard to keeping the raw materials compatible. If the surfactants are not compatible with each other, the system may become unstable due to competitive adsorption. Other surfactant-induced problems may occur during levelling and drying of the coating. Examples are craters and orange peel. Foaming is a well-known problem for water-borne coating systems. In some cases extremely stable foam can be produced especially during mixing and applications like spraying. Too stable foam in a coating inevitably leads to surface defects in the coating, as for instance craters. Foaming is counteracted in coatings by adding defoamers, which are another category of surface-active agents. Generally a low content of surfactant is to be preferred, if possible, to avoid surface defects. It is also necessary that the needed surface tension can be achieved quickly especially in industrial processes where speed is a parameter. Some industrial applications may even demand instant decrease in the surface tension. This is why the static surface tension is not an accurate prediction on flow and levelling. Dynamic processes are thus better described by the dynamic surface tension. Often industrial processes have very high demands for the application of coatings and the surface quality. Examples are coating of airplanes, cars and corrosion protection. The same accounts for printing on plastics at high speeds or inkjet applications, where low viscosity systems are used. In inkjet applications the surface tension for application is even dependent on the type of used printing head. It is also claimed that surface tension gradients during levelling of the coating can form surface defects, due to a dynamic behaviour. Solvent-based products do also include surface-active compounds, but the sensitivity of these coatings towards application methods and substrate is much less and therefore the number of surfactants used in a solvent-based system is normally lower. 5.2 Conventional fluorinated surfactantsIn fluorinated surfactants, the hydrophobic part of the surfactant molecule contains fluorine. In perfluorinated surfactants all hydrogens in the hydrophobic segment have been replaced by fluorine. In partially fluorinated surfactants the hydrophobic part of the surfactant molecule contains both fluorine and hydrogen atoms. The hydrophobic part of the fluorinated surfactant does not only repel water but repels oil and fat as well. Fluorinated surfactants are much more surface-active than their hydrocarbon counterparts. Fluorinated surfactants exhibit surface activity in organic systems and are stable to heat, acids, bases, as well as oxidising agents. The achievable minimum surface tension is much lower for fluorinated surfactants than for non-fluorinated surfactants (Kissa 2001) The conventional fluorinated surfactants are by producers of new alternatives (OMNOVA) claimed to have some basic problems involving foaming, too low surface tension, incompatibility with other components and lack of UV curing capability. On the other hand it should be noted that foaming also is dependent on the formulation itself, and therefore it is not necessarily due to the fluorinated surfactant. In general, non-ionic fluorinated surfactants, like hydrocarbon-type non-ionic surfactants, produce less foam and less persistent foam than ionic fluorinated surfactants. It should be noted that even though foaming should be minimised in water-borne coatings to avoid surface defects, foaming might be important in other application, e.g. fire-fighting foams. The level of the surface tension needed in a coating is decided both by the application procedure and the type of substrate that should be wetted. This means that a broad product range of surfactants need to be available to cover the application needs ranging from very low and to moderate surface tensions. The fluorinated surfactants are mainly used for coatings where there is a demand for extremely low surface tension. 5.3 The "new generation" of fluorinated surfactantsThe "new generation" of fluorinated surfactants is basically built up of a polymer and small fluorine molecules, which probably are very quick with regard to orientation towards a surface interface. The Novec products are polymeric anionic fluorinated surfactants, which are based on perfluorobutane compounds (perfluorobutane sulfonates). These compounds are claimed to have a low dynamic surface tension or rather a rapid surface migration, which is important in high speed coating processes or in low viscosity systems. Generally, this surfactant has a lower surface tension than hydrocarbon silicone surfactants. It can also be used in a less amount than the hydrocarbon surfactants. The compounds are said to influence the second layer coating adhesion less than silicon or conventional fluorinated surfactants. Besides the paint and coatings industry the Novec products should also be useful as surfactants in electronic coating, in industrial commercial cleaning, and cleaners for solder flux residue. They have also been identified as alternatives in connection with impregnation of textiles, leather and carpets. The Polyfox products are polyethers with a short perfluoroalkyl side chain (C2 or C3). It seems that these surfactants have a moderate surface tension, which is not quite as low as the conventional fluorinated surfactants. The new surfactants are claimed to have a broad processing window, where less interference with other compounds is experienced. Coating quality is improved as reduced foaming is achieved. The last item is an important factor in producing and processing water-borne coatings. Polyfox products have been tested in a high solid 2-component acrylate urethane for automotive topcoats with success. This surfactant has also potential for use in polyester/melamine coatings where it can achieve higher solids and reduce VOC use. It can also be used in pigment dispersions, inks lacquers etc. It should be noted that there also are Polyfox surfactants for a number of applications with non-aqueous systems including Powder coatings as well as UV curing systems. As the end group chemistry can be variated, tailor made substances with different affinity can be achieved for different applications. Three examples from the technical data sheets are given below:
Polyfox products are available with the following end group functionality: hydroxyl, carboxylic acid, sulfate, and acrylate. DuPont™ Forafac® products with C6 fluorinated compounds based on perfluorohexylethyl sulfonamide betaines are used for fire extinguishing formulations. There has not been identified any non-fluorinated alternatives. 5.4 Non-fluorinated surfactants5.4.1 Silicone polyethersA low viscous non-ionic special modified silicone polyether from Worlée is suggested for improvement of surface wetting of aqueous systems also on difficult substrates like polyethylene and polypropylene or contaminated substrates. It has a low surface tension and is claimed to be highly efficient improving wetting, spreading and levelling of water-borne coatings and eliminating surface defects without foam stabilising. It is further claimed that the compound normally has no negative effect on recoating. Another alternative within this group is a surfactant that combines silicone polyether and dioctylsulfosuccinate. This surfactant can be used to improve wetting properties of aqueous coatings for different substrates where the penetration into absorbing surfaces also is improved. 5.4.2 SulfosuccinateA sodium salt of di(2-ethylhexyl) sulfosuccinate manufactured by Cognis can be used as a wetting agent in aqueous coating systems and is particularly suitable for difficult-to-wet substrates like plastics, metal, cellulose film, silicone treated papers and glass. This surfactant may also be used as an emulsifier for emulsion polymerization. Another area where it can be used as an alternative to fluorinated surfactants is in optimising the colour acceptance of aqueous pigment concentrates in different coatings. The product has a medium foam formation. A sulfosuccinate from Münzing Chemie, which can be used as wetting agent for aqueous systems is claimed to have good wetting properties, no increase in foam and good recoatability. The surface tension is claimed to be moderate. Application areas are decorative paint, wood and furniture coatings, automotive and repair coating, industrial coatings, printing inks and overprint varnishes. In formulations of detergents and cleaners, anionic surfactants like sodium dioctylsulfosuccinate dissolved in a glycol and water are used as wetting agents. Sulfonates can also be used. Basically, some of the same chemistry as in coatings can be used. 5.4.3 Aliphatic alcoholsEsters of fatty alcohol ethoxylates are also used in the area of industrial cleaners. BASF produces a range of aliphatic alcohols, both anionic and non-ionic surfactants. These substances are effective wetting agents. The anionic surfactants can be used as detergent and cleaning agent for the chemical industry. Non-ionic surfactants can be used as defoamers or emulsifiers. 5.4.4 Propylated aromaticsDifferent isopropylnaphthalenes and isopropylbiphenyls are very hydrophobic substances that are compatible with almost all raw materials as follows: Epoxy resins, polyurethane resins, resin esters, hydrocarbon resins, polystyrene, elastomers, dispersions, emulsions, styrene-acrylate-copolymers, vinyl acetate and ethylene vinyl acetate polymers, mineral oils, bitumen, etc. The compound can be used as water repelling agents for different applications. For example rust protection systems, marine paints, resins, printing inks, coatings, electrical applications, electronically and mechanical applications. They may also act as plasticizers and film forming aids in emulsion paints and adhesives. 5.5 Discussion of alternatives5.5.1 Paint and coatingsIn the future it will still be very attractive (or even unavoidable) to use fluorinated compounds as ingredients in specific applications. It can be assumed that especially the new fluorinated surfactants with small low surface tension groups that can orientate very quickly upon application, together with a polymeric chain that can immobilise the additive after drying, will be a very effective combination. The biggest advantage is probably due to the fact that they can be tailor-made for specific applications, where the affinity towards specific molecules and the dynamic surface tension is crucial. It should be noted that for different applications different surface tension is needed. Furthermore, it is claimed that these alternative fluorinated compounds can reduce foaming, which is important especially in water-borne coating applications. Silicone products (also combined with other molecules) may give problems when recoating is necessary, but this may be a question about a low or a moderate surface tension. There are alternatives to fluorinated compounds based on sulfosuccinate, which are very versatile, but they are not claimed to have the same ability to reduce foam in the same manner as the new alternative fluorinated compounds. It seems that a number of versatile solutions are available for the coatings industry. It should be noted that in general it is very difficult to substitute substances where the demands on the dry coating are very high. It is also important to remember that it may include major formulation work when a surfactant is substituted, as the use of surfactants is system dependent also with regard to compatibility towards other raw materials. It is probably possible to use the "new generation" of fluorinated surfactants instead of the conventional ones. It will probably depend on the demand for a low surface tension, if it is possible for the sulfosuccinates to substitute the old generation of fluorinated surfactants. As a comparison, even though volatile organic compounds are unwished, they are still used for specific industrial applications where the technical demands are high. 5.5.2 Floor polish, waxes and cleaning agentsThere are alternatives within the range of the "new generation" of fluorinated surfactants that can be used within the area of floor polishing. Of course it is possible to formulate products where the fluorinated surfactants may be avoided due to the use of softer waxes or acrylics in floor polishes, but it must be noted that specific harsh demands (resistance to wear) may make fluorinated surfactants unavoidable in some cases. Even though conventional fluorinated compounds may be used extensively in traditional cleaning agents it is probably possible to use the experiences from the coatings area to determine which substitution possibilities being available. 5.5.3 Impregnation of textiles, leather, and carpets.There are alternatives within the range of the "new generation" of fluorinated surfactants that can be used within the area of impregnation. Furthermore, silicones combined with stearamidomethyl-pyridine-chloride may do the job. It is very possible that substitution in impregnation of paper and board may be very similar to impregnation of textiles. So the first choice would be to use telomer-based alternatives. 5.5.4 Fire-fighting foamNo non-fluorinated compounds have been identified for use in fire-fighting foam. The "new generation" of fluorinated compounds (C6 or lower compounds) are though already in use in fire-fighting foam today. Of course, it is of course possible to go back and use previous technology such as protein-based foams. But the disadvantages with this previous technology (the cause of technology change) should then be taken into account. 5.5.5 General commentIt is not possible to conclude that we can substitute all PFOS compounds in near future. But at the same time it is clear that material development can make it possible to more or less avoid the conventional PFOS products entirely. Basically, the development in the future will probably go towards more and more tailor-made products, where different molecules with different properties are combined. This can be achieved with many compounds but the effect both technically and environmentally will depend on the molecular size and shape. 5.6 Economical assessment of alternativesAccording to an article on perfluorinated surfactants (Moody et al. 2000), the cost of fluorinated surfactants is higher than that of hydrocarbon surfactants. Within a specific application, fluorinated surfactants are typically cost effective, as their relatively high price is offset by the low concentrations needed to achieve the desired effect. Nevertheless, the authors of the article assess, that due to the high prices of fluorinated surfactants, fluorosurfactant applications are limited to problems that the conventional, lower-priced surfactants cannot address. Kissa (2001) makes the same statement, and DuPont also confirms this statement – fluorinated compounds are only used, if no other alternatives are found fit for use (Personal communication DuPont, 2004a). According to the UK risk reduction strategy on PFOS (RPA 2004) PFAS substances have a limited use in the UK paper industry because they are considered to be very costly. The same survey states that for fire-fighting foams the fluorine-free alternatives are approximately 5-10% more expensive than the fluorosurfactant based foams. According to a manufacturer of the fluorine-free alternatives the price would fall if the market size increased. A more deliberately shift towards fluorine-free fire-fighting foam alternatives will probably eliminate the difference in cost. In general, very little information about the price of the alternatives was found, even though the producers of alternative products were asked specifically about this information. However, the sparse information received suggests that the alternatives are about the same price as the PFOS-related compounds or even cheaper. One company mentioned in particular that the price of the alternatives intentionally is kept at the same level as the PFOS-related compounds. The information received from different suppliers specifically within the paint and varnish industry suggests that fluorinated surfactants in general are much more expensive alternatives compared to other surfactants. Therefore, fluorosurfactants are only used for special purposes in paint and varnishes, where it is necessary to gain such a low surface tension, which no other (non-fluorinated) alternatives can achieve, e.g. in product where an extremely smooth surface is necessary. Furthermore, it must be assumed that the "new generation" of fluorinated compounds are at the same price level as the PFOS-related compounds. 6 Environment and health assessment of PFOS and PFOA and other PFAS substances
6.1 Environmental fate6.1.1 Physical-chemical propertiesMost perfluoroalkyl substances (PFAS) are very stable compounds, which have low vapour pressures, surface energies, and special surface-active properties. There are similarities to persistent organic pollutants (POPs) in being stable and hydrophobic but differ with PFAS having both oleophobic properties in one end of the molecule and sometimes polar/hydrophilic properties in the other functional end, where POPs are lipophilic and non-polar. Hence, PFAS will not accumulate in fatty tissues and will often occur dissociated as anions and interacts with polar sites in membranes and in sediments. For PFOA a reported octanol-water partition coefficient (Log Pow) is 5 (3M 2000), but the special solubility profiles of PFOS and PFOA make environmental fate predictions based on octanol-water partition coefficients irrelevant for these chemicals. For the individual PFOS and PFOA-related substances, large differences exist between the water solubility and vapour pressure etc. Some data examples are shown in Table 5.1. It should be noted that commercial products are not pure substances, and can contain several % of branched isomers and traces of other PFAS. Perfluorinated carboxylic acids (PFCA) are stronger acids than their non-fluorinated counterparts and have the corresponding lower pKa. For PFOA the pKa is 2.80 (Kissa 2001). PFOA is dissociated in water and does not evaporate from the water phase. Water is believed to be the target compartment for PFOA. Fluorotelomers (linear, long-chain, polyfluorinated alcohols) have higher calculated vapour pressures than the parent alcohol; for example 10:2 FTOH is 1000 times more volatile than dodecanol, possibly because of the unique molecular geometry (Stock et al. 2004). Examples are given in Table 6.1: Table 6.1: Water solubility and vapour pressure for some PFAS (Heckster et al. 2003; Stock et al. 2004; Shoeib et al. 2004)).
6.1.2 Abiotic degradationThe perfluorocarbon chain, with the very strong C-F bindings, is extremely resistant to heat, UV-radiation and to chemical attacks by acids and bases, and to reducing and oxidizing agents. The functional end groups (sulfonamides, alcohol etc.) will on the other hand be more readily transformed in the environment and in organisms, and the more complex compounds will finally be degraded to the most persistent sulfonates (e.g. PFOS) and carboxylates (e.g. PFOA). Fluorinated organic polymers are very stable to hydrolysis resulting in half-lives from 1-5 years to 500 years (3M). However, heating of fluoropolymers such as polytetrafluorethylene (PTFE) to more than 350oC, degradation in smaller molecules occurs, including a small (0.01%) formation of PFOA (Ellis et al. 2001). Smoke chamber experiments with fluorotelomer alcohols (4:2, 6:2 and 8:2 FTOH) exposed to chlorine atoms as a surrogate for OH-radicals indicate that these chemicals in the atmosphere can oxidise/degrade to series of perfluorinated carboxylic acids (Ellis et al. 2004). The yields from 8:2 FTOH was mainly PFNA [10] (1.6%) and PFOA (1.5%) and to a lesser extend shorter chain acids. Atmospheric lifetime of short chain FTOHs, as determined with its reaction with OH-radicals, was approximately 20 days making the molecule able to travel about 7000 km (Ellis et al. 2004). 6.1.3 BiodegradationThe 175 polyfluorinated substances on a list developed by Canadian authorities were studied with a computer program simulating microbial degradation. The prediction was that 109 substances might be degraded to PFOS and 61 to PFOA (Dimitrov et al. 2004). In a laboratory test using a microbial enrichment culture 8:2 FTOH aerobic degradation occurred. Telomer acids and PFOA were identified as metabolites. 85% of the telomer alcohol was degraded after a week. The half-life was about one day (Schröder 2003). There was no aerobic degradation of either PFOS or PFOA derivatives. However, microbiological degradation of PFOS and PFOA in contaminated sludge occurred under anaerobic conditions. PFOS biodegraded easier than PFOA. The former compound disappeared within 2 days and the later in 25 days. The degradation products were not identified (Schröder 2003). Dinglasan et al. (2004) examined the aerobic biodegradation of the 8:2 Telomer alcohol (8:2 FTOH) using a mixed microbial system. The initial measured half-life of the 8:2 FTOH was 0.2 days mg-1 of initial biomass protein. Volatile metabolites and nonvolatile metabolites were identified and quantified. Telomer acids (CF3(CF2)7CH2COOH; CF3(CF2)6CFCHCOOH) and PFOA were identified as metabolites during the degradation, the unsaturated telomer acid being the predominant metabolite measured. 6.1.4 SorptionTable 6.2 provides the results summary table from the adsorption/desorption study of ammonium perfluorooctanoate (APFO) to soil according to OECD test guideline 106: Adsorption-Desorption Using a Batch Equilibrium Method (APME, 2004). These results indicates that the Koc values obtained for APFO are in the low end of the range of Koc values used for the recommended reference substances from the OECD test guideline 121: HPLC screening method based on soil adsorption data. This range goes from a low log Koc value of 1.25 (Koc ~ 18) for Acetanilide, to a high log Koc value of 5.63 (Koc ~ 426580) for DDT. Table 6.2: Summary of the results from the adsorption/desorption study of ammonium perfluorooctanoate (APFO) to soil according to OECD test guideline 106.
Soils tested at 1:1 soil:solution ratio; Wilmington sludge tested at 1:5 solids:solution ratio 24 hour equilibration time 6.2 Environmental exposure and occurrence6.2.1 Environmental releaseEmissions of PFOA, PFOS and related chemicals to the environment (air and water) may happen directly from production and processing plants, e.g. release during product use, e.g. fire-fighting foam, and as product impurities or degradation products, or release of PFOA during the production of fluoropolymers such as PTFE . It should be realised that the use of fire fighting foams on e.g. offshore oil platforms in the North Sea, may be a potential direct water pollution risk. Sources of FTOH are currently unknown, although it is likely that they may be released from the decomposition of polymeric and non-polymeric materials that incorporate FTOHs or from release of residual amounts of the FTOHs themselves that failed to be covalently linked to the polymer during production (Ellis et al. 2003) It is expected that PFAS is present in the environment primarily in the form of the final stable degradation products PFOS and PFOA. 6.2.2 Long-range transportationThe binding to water and the low volatility make it less likely that PFOS and PFOA will be spread long-range with the air by the "grass-hopping" and cold condensation mechanisms as persistent organic pollutants (POPs) in general can. The occurrence of PFOS and PFOA in remote areas such as the arctic and in arctic animals is therefore puzzling. However, the prevailing hypothesis is that long-range transport to the arctic occurs via volatile precursors of both PFOS and PFOA, with subsequent degradation to these stable products. Volatile PFOS-precursors are a.o. MeFOSE, EtFOSE (OECD, 2002). It has been hypothesized that fluorotelomer alcohols (FTOH) may be long-range transported and hereby reach remote arctic areas, where they can degrade to the more stable PFOA (Ellis et al. 2004). Alternatively or in addition, direct use of fluorosurfactants in fire-fighting foams used in remote regions may constitute a source of PFOS and PFOA. Recently a hypothesis has also been brought forward that ocean current transport may in part explain the presence of PFOS and PFOA in the arctic (Yamaguchi et al. 2004; Caliebe et al. 2004). 6.2.3 Levels in airSix different perfluorinated substances have been detected in the air at a highly urbanized site of Toronto, Canada. The mean concentrations ranged from 14 pg/m3 EtFOSA to 205 pg/m3 EtFOSE, and totally 260 pg/m3. At a rural site (Long Point) in Canada the levels were 2-3 fold less (74 pg/m3) and only one substance was found (Martin et al. 2002). Polyfluorinated sulfonamides and fluorinated telomer alcohols have been measured by Stock et al. (2004) in six North American cities – Reno (NV), Griffin (GA), Cleves (OH), Winnipeg (MB), Long Point (ON) and Toronto (ON). Mean concentrations of total perfluorinated sulfonamides (EtFOSA, MeFOSE and EtFOSE), ranged from 22 pg/m3 (Winnipeg) to 403 pg/m3 in Griffin. Mean concentrations of total FTOHs (6:2, 8:2 and 10:2) ranged from 11 pg/m3 (Winnipeg) to 165 pg/m3 (Toronto). Surface treatment products, previously manufactured by 3M Company for soil, stain and water protection of home furnishings – including carpets, were primarily MeFOSE-based polymers. It was in line with the fact that the highest mean and single levels of MeFOSE in the air (359 pg/m3 and 1549 pg/m3) were measured in Griffin, a location of carpet production. Three perfluoroalkyl sulfonamides (MeFOSE, EtFOSE and MeFOSEA) used in surface treatment formulation for textile and paper products to impact oil and water resistance were measured in indoor and outdoor air. Levels of MeFOSE and EtFOSE in outdoor air were 16-32 pg/m3 and 8,5-10 pg/m3, respectively. MeFOSEA was not detected. Indoor air from 4 houses and an old laboratory ranged 667-8315 pg/m3 and 289-1917 pg/m3, respectively. Thus, indoor air levels were about 100 times higher than outdoor values. Indoor levels of MeFOSEA in three of the houses were 5-283 pg/m3 (Shoeib et al. 2004). The high indoors air levels of perfluoroalkyl sulfonamides compared to outdoors levels were confirmed in the recent study by Shoeib et al. (2004) using passive air samplers showing 25 times higher levels indoors of MeFOSE (geometric mean 1968 pg/m3) and 13 times higher levels of EtFOSE (geometric mean 1033 pg/m3). Results for EtFOSA (geometric mean 54 pg/m3) and MeFOSEA (geometric mean 38 pg/m3) were a magnitude lower. The content of PFOS in airborne dust along Japanese roads was up to 427 ng/g. The air concentrations ranged 0.1-2.1 pg/m3 in a rural town and 2.3 -22 pg/m3 in an urban city (Sasaki et al 2003). Higher concentrations were observed in the summer than in the winter. FTOH and polyfluorinated sulfonamides detected in tropospheric concentrations typically ranging from 7 to 106 pg/m3 and from 14 to 393 pg/m3; higher concentrations were observed in urban locations relative to rural locations (Martin et al. 2002). Tropospheric measurements in the arctic in support of the long-range transport hypothesis have not yet been published. Table 6.3: Levels of PFAS in the air (pg/m3). 6.2.4 Levels in ground- and drinking waterFluorotelomer sulfonates, primarily polyfluoroalkylthioamido sulfonates, are used as surfactant to lower the surface tension of fire-fighting foams used to fight hydrocarbon-fuelled fires (aqueous film forming foams (AFFF)). These surfactants and their degradation products may occur in ground water as a result of this use. The concentrations will vary with time and distance from the use place (Moody & Field 1999; Moody et al. 2003; Schultz et al. 2004). A study at a former Air Force base in Michigan showed that ground water from wells around a fire-training area contained perfluorinated degradation products (PFOS, PFHxS, PFOA and PFHxA) five or more years after its last known use ranging in concentrations from 3-120 μg/L (Moody et al. 2003). In a follow-up study, including two another former military facilities in Nevada and Florida, where such fire foams were used, these four degradation products were found in ground water from all three facilities. In addition, even fluorotelomer sulfonates were measured at the Michigan (<0.6-182 μg/L) and Florida facilities (1,100-14,600 μg/L). In the Nevada and Florida facilities some ground water samples also contained PFBS, PFPS and PFHpA (Schultz et al. 2004). In tap water delivered from 8 waterworks in Japan the PFOS levels were 0.1-4 ng/L. In tap water delivered by another waterwork, which obtained the raw water from a polluted river, levels of PFOS were about 50 ng/L (Harada et al. 2003). For guidance purposes, 3M scientists, taking into account the US Environmental Protection Agency (USEPA) risk assessment approach, have developed a lifetime drinking water health advisory for PFOS. This advisory of 1000 ng/L (1 ppb) represents the concentration of PFOS in drinking water that is not expected to cause any adverse (non-cancer) effect over a lifetime of exposure, with a substantial margin of safety. It was assumed that 2L of water is consumed daily, and that drinking water represents 20% of the daily exposure to PFOS (Hansen et al. 2002). A team of scientific experts and governmental regulatory agencies – including US EPA – developed screening levels for PFOA protective of human health. Their recommended safe level for PFOA content in water was 150 ug/L (150 ppb) (CATT, 2002). The US Ohio EPA independently reviewed and confirmed the CATT findings. 6.2.5 Levels in surface waterHansen and co-workers (2002) have measured PFOS and PFOA in the Tennessee River in Alabama. Upstream Baker's Creek, which receives influents from several manufacturing facilities including a fluorochemicals manufacturing plant, PFOS levels in the river water averaged 32±11 ng/L and ranged from 17 to 54 ng/L. Upstream PFOA levels were below the detection limit of 25 ng/L. Downstream concentrations averaged 114±19 ng PFOS/L and 394±128 ng PFOA/L and maximum values reached 144 ng PFOS/L and 598 ng PFOA/L. In June 2000 at the international airport at Toronto, Canada, an accidental spill happened, and 22,000 litre of fire-fighting foam containing perfluorinated surfactants including about 300 kg PFOS entered into Etobicoke Creek. During a 153 days period 54 samples of creek water were obtained and analysed for perfluorohexane sulfonate (PFHxS), perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA), and total perfluorinated surfactant concentrations. Upstream levels of PFHxS and PFOS were below the detection limit (<0.01 μg/L), and PFOA levels were also very low (0.008-0.033 μg/L). Downstream levels were <0.02 –134 μg/L for PFHxS, <0.016-2210 μg/L for PFOS, and <0.02-10.6 μg/L for PFOA. Total perfluorinated surfactant concentrations upstream ranged between 0.011 and 0.028 μg/L, and downstream the concentrations ranged from 2 to 17,000 μg/L. The highest levels were measured on day one and day two after the accident and until 15 km downstream (Moody et al. 2002). On September 26, 2003, magnitude 8.3 earthquakes strucked Hokkaido Island in Japan and initiated a. o. major fires at an oil storage facility at a refinery (Yamashita et al. 2004). At least 40,000 litre of fire-fighting foam containing perfluorinated compounds were used to control the fires. The runoff waters contained 3,669 ng PFOS/L, 149 ng PFHxS/L, 200 ng PFOSA/L, 300 ng PFNA/L, 162 ng PFOA/L and smaller levels of PFBS, PFUnA, PFDeA, PFHpA and PFHxA. Since October 2003 the environmental levels of these chemicals were monitored in waters and snow around. Mean concentrations of PFOS decreased from 42 ng/L to 4 ng/L from October to December. High levels in soil and snow in December indicate some persistent airborne sources. In Japan PFOS concentrations in 126 samples of surface waters, mainly from rivers, ranged 0.2-157 ng/L with a geometric mean of 2.37. In effluents from a wastewater plant concentrations were between 300 and 440 ng/L (Harada et al. 2003). Another Japanese study found PFOS in nine out of 25 surface water samples and reported levels of PFOS in the Tokyo Bay surface water ranging from 8 to 59 ng/L with a mean of 26 ng/L (Taniyasu et al. 2003). In other bay water samples levels were lower and below the detection limits. In Osaka Bay and Ariake Bay mean levels of PFOS were respectively 8.7 (range: <4-21) and 4.8 ng/L (range <9-11). In Lake Biwa PFOS levels ranged <4-7.4 ng/L with a mean of 3.8 ng/L. Levels of PFHxS and PFBS were below detection limits. In seawater from South China Sea and Sulu Sea PFOS levels ranged 0.01-0.11 ng/L. Concentrations of PFOA were 4-7 times higher than those of PFOS. Overall concentration of PFOS in open ocean waters was 60 to 600 times lower than coastal waters of Japan (Yamashita et al. 2003). Coastal waters of Hong Kong, the Pearl River Delta, including South China Sea, and Korea have been analysed for PFOS and PFOA (So et al. 2004). The ranges of PFOS concentrations were 0.09-3.1, 0.02-12, and 0.04-730 ng/L, respectively, and the ranges of PFOA concentrations were 0.73-5.5, 0.24-16, and 0.24-320 ng/L, respectively. Thus highest concentrations analysed in Asia were in coastal waters around Korea. In a newer study with a lower limit of quantification highest levels were found in seawater samples from Tokyo Bay with up to 192 ng PFOA/L, 71 ng PFNA/L, 58 ng PFOS/L and 5.6 ng PFHxS/L (Taniyasu et al. 2004). Coastal area of Hong Kong, China and Korea had about 10 times lower maximum levels, though PFNA levels were 100 times lower. Even lower levels of PFOA (less than 0.4 ng PFOA/L), PFOS and PFHxS but not PFNA were detected in open Pacific Ocean waters. Similar low levels of PFOA, PFOS and PFHxS were also determined in the deep (>1000 m) ocean waters indicating widespread contamination. The "background" surface water levels of perfluorinated compounds in the Pacific Ocean were in general 5-10 timeslower than in the Atlantic Ocean. The first data for perfluorooctane surfactants in Great Lakes water have recently been published (Boulanger et al. 2004). Sixteen samples from Lake Erie and Lake Ontario were analysed for eight perfluorinated chemicals. Concentrations of PFOS in the two lakes ranged from 21-70 and 27-50 ng/L, respectively. Analysis also showed the presence of PFOS precursors, NEtFOSAA [11] (range of 4.2-11 ng/L) and FOSA [12] (range of 0.6-1.3 ng/L). Perfluorooctane sulfinate, another precursor, was identified at six of eight locations in concentrations up to 17 ng/L. Other precursors such as EtFOSE, PFOSAA and EtFOSA were not detected. In Michigan surface water samples background concentrations were between 2 and 5 ng/L for PFOS and <8-16 ng/L for PFOA with maxima of 29 and 36 ng/L, respectively (Sinclair et al. 2004). Seven perfluorinated compounds have in 2003 been measured in water sampled in the depth of 5 m at 15 locations in the North Sea (Caliebe et al. 2004). The highest levels, about 20 ng/L, were found for PFOS and PFOA in samples from the mouth of River Elbe while the other detectable compounds (PFHxA, PFHxS, PFHpA, PFNA, PFDeA and PFOSA) range from 1-3 ng/L. Relatively high levels of PFOA and PFOS were also found in samples from the Dutch and German Waddensea (5-10 ng/L). One sampling station at the northern end of the island of Sild will be representative for the Danish Waddensea. In open waters levels were below 1 ng/L, and only PFOA could be quantified. In River Elbe waters the concentration of PFOS was higher than of PFOA. In seawaters the relation was opposite. In a recent Nordic screening project a few samples of water from Norway, Denmark, Sweden, Finland, Iceland and Faroe Islands were investigated for perfluorinated compounds (Berger et al. 2004; Kallenborn et al. 2004). The levels of PFOA were highest with median levels of 5.2 ng/L in seawater, 7.8 ng/L in lake water, 13.1 ng/L in rainwater, 20.5 ng/L in sewage effluent and 297 ng/L in landfill effluent. 6.2.6 Levels in sediment and sludgeThe binding of some PFAS to sediments and sludge is strong and stable, which means a high potential for accumulation herein. In a 3M study of six urban locations in the USA, where some PFAS was detected in various media the highest levels occurred in sewage sludge from cities with production or industrial use of the PFAS (3M 2001). In a recent Nordic screening project a few samples of sewage sludge from Norway, Denmark, Sweden, Finland, Iceland and Faroe Islands were investigated for perfluorinated compounds (Berger et al. 2004; Kallenborn et al. 2004). PFOS was the dominating substance in Denmark, Norway and Sweden and PFOA was highest in Iceland and Faroe Islands. Highest maximum levels of PFOS were found in Sweden (2644 pg/g ww), Denmark (1041 pg/g ww) and Norway (1023 pg/g ww). Somewhat lower levels in Finland (925 pg/g ww), and in Island and Faroe Islands the levels were much lower at 220-241 pg/g ww. In Finland PFHxA was the second most abundant, and PFNA was found in Norwegian, Danish and Finnish sludge. Sediment samples had in general lower levels than sludge. The highest level of PFAS was 1150 pg/g ww in a Finnish sample. Contamination was also high in Norway but not in Sweden and other countries. 6.2.7 Levels in biota and wildlifeJohn P. Giesy and co-workers at Michigan State University did report for the first time the global contamination and widely distribution of PFOS in wildlife (Giesy & Kannan 2001). Tissues of various species of aquatic animals (seals, otter, sea lion, dolphin, polar bear, mink), birds, fish and amphibians collected in the 1990's for other purposes were scanned and analysed for PFOS, PFOSA, PFHxS and PFOA. However, in most samples only PFOS could be quantified in concentrations > 1 ng/g (ppb) and reported. There was no difference between levels in male and female animals as for the lipophilic POPs. PFOS is also found in bird and fish eggs (up to 250 ng/g) suggesting possible maternal transfer during yolk formation (Giesy & Kannan 2002). Other studies were initiated and in Europe PFOS levels in the biota were especially high at 36 ng/g to 1700 ng/g in the rivers and coast near Antwerp in Belgium, where a fluorochemical factory is located (Van de Vijver at al. 2002; Hoff et al. 2003). Where the PFOS level in seals is higher in Europe than the US, it is opposite with the levels in dolphins and seabirds. Kannan and Giesy (2002) have studied the frequency of detection (detection limit: 1-35 ng/g ww) and the maximum concentrations of PFOS, PFOA, FOSA and PFHxS in animal tissues (see Table 6.4): Table 6.4: max. conc. (ng/g ww or ppb) and frequency of detection (%) in animal tissues
6.2.7.1 Levels in zooplankton and shrimpsComposites of zooplankton from Frobisher Bay in the Eastern Canadian arctic contained 1.1-2.1 ng PFOS/g ww and 1.7-3.4 ng PFOA/g ww. Levels in shrimps from the same area were about 5 times lower (Tomy et al. 2003). 6.2.7.2 Bioaccumulation in fishAnalysis of existing wildlife data shows that organisms consuming fish contain greater concentrations than their food sources, and a PFOS liver accumulation ratio was measured at 22 for mink feeding on fish meals (Giesy & Kannan 2001). In two separate laboratory experiments juvenile rainbow trout (Oncorhynchus mykiss) were exposed either via water or diet to a mixture of a homologous series of perfluoroalkyl carboxylates and sulfonates during 32 days followed by a 41 days depuration period (Martin et al. 2003ab). The carcass bioconcentration factors increased with increasing chain length and ranged from 4.0 to 23,000 (Martin et al. 2003a). The substances were efficient absorbed from the feed but only carboxylates with more than 6 perfluoroalkyl carbons and sulfonates with more than 4 perfluoroalkyl carbons were detectable in fish tissues at all given sampling times. The perfluorinated acids accumulated to the greatest extend in blood > kidney > liver > gall bladder. Under the circumstances of the study no biomagnification was observed because the half-lives did not exceed 46 days, thus uptake via water (bioconcentration) is probably more important (Martin et al 2003b). Data from the two papers of Martin and co-workers are shown in Table 6.5 The depuration carcass half-lives for rainbow trout ranged from 3 days for PFOA to 13 days for PFOS and to 32 days for PFTA. The food chain bioaccumulation factor (BAF) ranged from 0.038 (PFOA) to 1.0 (PFTA). Thus both half-lives and bioaccumulation factors did increase with the length of the perfluoroalkyl chain. Table 6.5: Accumulation of PFAS in fish carcass (Martin et al. 2003ab).
Clearance of PFOA from fathead minnows took more than 15 days (3M 1995). In a recent study Martin et al. (2004) analyzed for PFOS, the homologous series of PFCAs ranging from 8 to 15 carbons in chain length, and the PFOS-precursor heptadecafluorooctane sulfonamide (FOSA) in various organisms from a food web of Lake Ontario. PFOS was the dominant acid in all samples, but long chain PFCAs, ranging in length from 8 to 15 carbons, were also detected in most samples between <0.5 and 90 ng/g. By accounting for the known diet composition of lake trout, it was shown that bioaccumulation was indeed occurring at the top of the food web for all perfluoroalkyl compounds except PFOA. It is clear that perfluoroalkyl sulfonates have greater BCF, BAF, BMFs, half-lives and uptake rates than corresponding acids. The extent of bioconcentration of PFAS appeared to be highly structure dependent, and PFOA with less than seven and PFOS with less than six fluoroalkylcarbons did not accumulate in rainbow trout. 6.2.7.3 Accidental release of PFOSIn June 2000 at the international airport at Toronto, Canada, an accidental spill happened, and 22,000 litre of fire retardant foam containing perfluorinated surfactants including about 300 kg PFOS entered into Etobicoke Creek. Three weeks after the accident 6 fishes (common shiner, Notropus cornutus) were catched in the river, and the livers analysed for perfluorinated compounds (Moody et al. 2002). Total perfluoroalkane sulfonate concentrations in the fish livers ranged 2-73 μg/g and it was mainly PFOS. Two samples contained small levels of PFBS (<0.009 μg/g) and four samples of PFHxS (<0.062 μg/g). Total levels of seven determined perfluorocarboxylates in fish livers ranged 0.07-0.40 μg/g thus 100 times lower. The most abundant of these was PFDA with 0.032-0.14 μg/g, followed by PFUnA with 0.013-0.083 μg/g, PFHpA with 0.005- 0.048 PFOA with 0.006-0.04 μg/g, PFHxA with <0.002-0.040 μg/g, PFTA 0.009-0.034 μg/g and PFPeA with <0.002-0.013 μg/g. A half year later 3 additional fishes were catched and analysed. Total perfluoroalkane sulfonate concentrations in the fish livers then ranged 9.2-40 μg/g, thus no considerable decrease. However, total levels of perfluorocarboxylates ranged 0.82-1.02 μg/g, thus a considerable increase. Based on the data in this study the bioaccumulation factor (BAF) for PFOS in fish livers was calculated to range between 6,300-125,000 (Moody et al. 2002; 2003). This range is much larger than the result of the previously mentioned experimental study of rainbow trout. 6.2.7.4 Levels in fishIn their original paper Giesy & Kannan (2001) reported significant levels (up to 380 ng/g ww) of PFOS in fish (eggs, liver and plasma) from Michigan waters (see Table 6.6). PFOS levels in tuna fish from the Northern Pacific were lower than the limit of quantification, while tuna liver from the Mediterranean contained 21-87 ng PFOS/g ww. In livers from tuna fish taken in the North Pacific the levels were below the detection limit of 7 ng/g (Giesy & Kannan 2002). The levels were clearly higher in populated and industrialised area than in remote locations. In a later paper (Kannan et al. 2002c) PFOS levels in blood from tuna and swordfish from the Mediterranean Sea were reported. Levels ranged 27-52 (mean 40) ng/mL and 4-14 (mean: 7.2) ng/mL, respectively. Levels of PFOSA ranged 13-19 ng/mL (mean: 15) and 1.1-28 ng/mL (mean: 15), respectively. However, levels of PFOA and PFHxS were below the quantification limit. The same was the case for all the perfluorochemicals in Atlantic salmon. In Frobisher Bay in the Eastern Canadian arctic the highest levels of PFOA (2.9-57 ng/g ww) were in deepwater redfish (Tomy et al. 2003). This fish also contained an extremely high level of EtFOSA. Various fish species from Japan did contain from 3 to7,900 ng/g ww PFOS in the livers and 1 to 834 ng/mL in the blood. Some of them also contained PFHxS in levels up to 18 ng/g liver and 121 ng/mL blood (Taniyasu et al. 2003). In all samples PFBS levels were below the detection limit. A recent study identified both PFOS and PFOA in biota from the Canadian arctic (Martin et al. 2004). Fish were collected at various locations. PFOS was the major contaminant and levels of FOSA were alsorelatively high but all samples contained also perfluorinated carboxylic acids (PFCA) ranging in length from 9 to 15 carbons. In general, odd-length PFCAs exceeded the concentration of even-length PFCAs and concentration decreased with increasing chain length. Levels were generally lower than levels for similar animals in the USA. Sinclair et al. (2004) found PFOS (ranges: <7 – 381 ng/g ww; means: 43-263 ng/g ww) in livers, muscle and eggs of Chinook salmon, lake whitefish, brown trout and other Michigan fish. No other perfluorinated compound was present. PFOS ranged from 27 to 52 ng/mL (mean: 40) and 4 to 21 ng/mL (mean: 10), respectively, in blood from bluefin tuna and swordfish catched in the Italian part of the Mediterranean (Corsolini & Kannan 2004). In livers PFOS ranged 21-87 ng/g ww (mean: 47) and <1-13 ng/ ww (mean: 7), respectively. The levels of FOSA in bluefin tuna were 2-4 fold less than for PFOS. It was opposite in swordfish blood, where FOSA concentrations were 1.5-fold greater. In a recent Nordic screening project a few samples of fish were investigated for perfluorinated compounds (Berger et al. 2004; Kallenborn et al. 2004). The distribution was characterized by a high variability. PFOS followed by PFOSA dominated over PFOA in freshwater fish. The highest levels of 551 ng PFOS/g ww and 141 ng PFOSA/g ww were measured in a Finnish pike sample. Marine fish samples from Faroe Island had the lowest contamination, and PFOSA was the most abundant substance. Otherwise PFOS dominated in samples from other areas. In all the Icelandic samples PFDS and PFHxA were present. In the Danish samples PFHxA, PFHpA and PFOA were present. Levels of PFOS in Danish flounders and herrings were around 20 ng/g ww. Table 6.6: Perfluorinated substances in fish. 6.2.7.5 ShellfishPFOS was measured in oysters (Crassostrea virginica) collected in 1996-1998 from 77 locations in the Gulf of Mexico and Chesapeake Bay. In 51 of these locations PFOS was detected in concentrations ranging from <42 to 1225 ng/g dry weight with a median of 387 ng/g dw (Kannan et al. 2002b). Only one of the twelve samples from Chesapeake Bay had measurable levels and that was as high as 1106 ng/g. In Puerto Rica PFOS levels averaged 437 ng/g dry weights. 6.2.7.6 AmphibiansA few frogs and turtles have been studied by Giesy & Kannan and the levels were relatively high (see Table 6.7). Table 6.7: PFOA and PFOS in Amphibians.
6.2.7.7 BirdsIn their original paper Giesy & Kannan (2001) reported significant levels of PFOS in birds (egg yolk, plasma and liver) from various places (see Table 6.8). The highest levels were found in blood plasma from bald eagles collected in Midwestern USA (1-2570 ng PFOS/g ww). The levels were clearly higher in populated and industrialised areas than in remote locations. Levels in birds from Antarctica are around or below the quantification limit. PFOS was determined in 161 samples of liver, kidney, blood, or egg yolk from 21 species of fish-eating water birds from the USA and the remote Midway Atoll in the northern Pacific Ocean (Kannan et al. 2001b). Highest PFOS concentrations in blood plasma were from bald eagles collected in the Midwestern USA ranging from 13 to 2220 ng/mL (mean: 330 ng/mL). Concentrations were higher in blood plasma than in whole blood. The highest liver-PFOS concentration (1780 ng/g ww) was found in a cormorant from San Diego. PFOS in blood serum from albatrosses collected at Midway ranged 3 to 34 ng/mL. In livers and kidneys from the albatrosses levels were below the quantification limit. Livers from 83 birds collected from Japan and Korea were analysed. PFOS was found in 95% of the samples in levels up to 650 ng/g ww and concentrations were within the ranges reported from the USA and Europe. Black-tailed gulls from Hokkaido, Japan, contained 2-12 ng PFOS/mL. PFOA and PFHxS were found in 5-10% of the samples in concentrations up to 21 and 34 ng/g ww, respectively. Perfluorooctane sulfonamide (FOSA) was detected only in all common cormorants from the Sagami River in Japan in levels up to 215 ng/g ww. Highest levels of PFOS were also at that location. There was no correlation between levels of PFOS and FOSA; the latter was only distributed locally. Levels of PFOS were lower in Korea compared to Japan (Kannan et al. 2002b). In the Sardinian Sea in Italy cormorant livers contained 32-150 ng PFOS/g (mean: 61) and 29-450 ng PFOA/g (mean: 95); thus surprisingly PFOA was more abundant than PFOS. Levels of PFOSA and PFHxS were lower than the quantification limit (Kannan et al. 2002c). In 44 white-tailed sea eagles collected near the Baltic coast of Poland and East Germany in the years 1979 to 2000, PFOS levels in livers averaged 36 ng/g ww. There was an upward trend in levels during the years. Levels of PFOA, FOSA or PFHxS were below the quantitation limit. PFOS levels in various birds living in Tokyo Bay were 68-1200 ng/g liver and 0.3-167 ng/mL blood (Taniyasu et al. 2003). In a few samples low levels of PFHxS were also found. A recent study identified both PFOS and PFOA in biota from the Canadian arctic. Common loons, northern fulmars and black guillemots were collected at various locations. PFOS was the major contaminant but common loon also contained significant levels of FOSA. In contrast to mammals and fish, perfluorinated carboxylic acids were not detected in birds. Levels were generally lower than levels for similar birds in the USA (Martin et al. 2004). In a recent Nordic screening project two pooled samples of fulmar eggs from Faroe Islands were investigated for perfluorinated compounds (Berger et al. 2004; Kallenborn et al. 2004). Levels were 33 and 40 ng/g ww. PFOS stood for 95% of the PFAS levels followed by PFNA and PFOSA. Table 6.8: PFOA and PFOS in birds.
6.2.7.8 Aquatic mammalsIn their original paper Giesy & Kannan (2001) reported significant levels of PFOS in aquatic mammals from various places (see Table 6.9). The highest levels were found in mink (970-3680 ng PFOS/g ww) and river otters (34-990 ng/g ww) from the western USA. High levels were also found in Alaskan polar bear (180-680 ng/g ww) and seals (16-230 ng/g ww) from the Baltic Sea and in dolphins (65-430 ng/g ww) from the Mediterranean Sea. The levels were clearly higher in populated and industrialised aresa than in remote locations. Levels in animals from Antarctica are around or below the quantification limit. Kannan et al. (2001a), investigated 247 tissue samples (liver and blood) from 15 species of marine animals collected from Florida, California and Alaskan coastal waters, the northern Baltic Sea, the Arctic (Spitzbergen) and Sable Island in Canada. In seals from the Canadian and Norwegian arctic the blood levels were 3-50 ng PFOS/mL. The highest PFOS concentration in livers was 1520 ng/g ww in a stranded bottlenose dolphin from Sarasota Bay in Florida, and the highest concentration in blood was 475 ng/mL in a ringed seal from the Bothnian Bay. Ringed seals were more contaminated than grey seals. (The same is occurring for PCB and dioxin in the Baltic, and it is explained by the fact that ringed seals eat more bottom living organisms). No age-dependent increase was observed. A few brain and kidney samples contain non-quantifiable levels of PFOS. In dolphins from the Mediterranean Sea blood levels of FOSA (mean 223 ng/g ww) were surprisingly greater than levels of PFOS (mean: 143 ng/g ww), which again were much higher than levels of PFOA (mean: 3.1 ng/g ww) and PFHxS (mean: 4.5 ng/g ww). Pilot whales had also high levels of both PFOS and FOSA (Kannan et al. 2002c). For seals from the northern Baltic Sea levels of PFOS in the livers were very high and up to 1100 ng/g ww. Levels in ringed seals were about the double of levels in grey seals. Another study of Mediterranean dolphins has recently been published by Corsolini & Kannan (2004). The samples were from 1991-1998 and from around Italy. The blood from captive bottlenose dolphins contained 42-210 ng PFOS /mL and livers from similar dolphins but wild-living animals contained PFOS on the same scale. Levels in striped dolphin livers were about the half, while muscle and liver tissues from a common dolphin had 77 and 940 ng PFOS/g ww, respectively. Most of the highly contaminated dolphins contained also considerable levels of FOSA. Van de Vijver et al. (2003) studied perfluorinated chemicals in livers and kidneys from mammals stranded along the southern North Sea coast. PFOS was predominant in terms of concentration, and the highest concentrations were found in mammals and displayed the highest trophic position. Levels were mostly higher in liver compared to kidney. Harbour seal levels were higher compared to white beaked dolphin > harbour porpoise > grey seal > sperm whale > white-sided dolphin > striped dolphin > fin whale/hooded seal. In 10% of the samples levels were below the detection limit of 10 ng PFOS/g wet weight. Juvenile porpoises contained more PFOS than adult animals, and there were no significant sex differences. Thus PFOS is not depleted during lactation as lipophilic POP chemicals. Perfluoroundecanoic acid (PFUA) was determined in the liver from a single harbour porpoise (110 ng/g ww), a sperm whale (50 ng/g ww), a white-sided dolphin (60 ng/g ww) and in three white-beaked dolphins (50-150 ng/g ww). Perfluorononanoic acid (PFNA) was found in a sperm whale (240 ng/g ww) and a white-beaked dolphin (480 ng/g ww) only. Perfluorodecanoic acid (PFDeA) was found in three white-beaked dolphins (90-120 ng/g ww). PFOA and perfluorododecanoic acid (PFDoA) were not detected. In Frobisher Bay in the Eastern Canadian arctic the highest levels of PFOS (8.7-14.3 ng/g ww) were found in beluga whales. Narwhales had also rather high PFOS levels, while FFOA levels in whales were one-tenth of PFOS levels (Tomy et al. 2003). Contamination with perfluorinated chemicals has been measured in livers from wild mink and river otters in the USA (Kannan et al 2002d). PFOS was detected in all mink samples, while PFOA, FOSA and PFHxS were detected only in a few samples. Mink from Midwestern USA (Illinois) contained greater PFOS concentrations in their livers (up to 5140 ng/g ww) than mink from South Carolina (up to 3110 ng/g ww), Louisiana (up to 320 ng/g ww) and Massachusetts (up to 1100 ng/g ww)). A laboratory study with mink, where carps from a polluted river were substituted for marine fishes at levels of 10, 20 and 40% in the fed, showed a dose-related sharp increase in PFOS concentrations of the livers. FOSA, which was not detectable in the carps, was measurable in the mink livers. PFOS was also measured in all 20 liver samples (up to 994 ng/g ww) from river otters living in the states of Washington and Oregon, USA. FOSA was measured in 90% of the samples (up to 72 ng/g ww). PFOA (up to 19 ng/g ww) and PFHxS (up to 76 ng/g ww) were only detected in a few samples. A recent study identified both PFOS and PFOA in biota from the Canadian arctic. Polar bears, ringed seals, arctic fox, and mink were collected at various locations. PFOS was the major contaminant but all samples contained also perfluorinated carboxylic acids (PFCA) ranging in length from 9 to 15 carbons. In all samples except for minks PFOS dominated. In minks perfluorononanoate (PFNA) was highest. Mammals feeding at higher trophic levels had greater contamination. Highest mean levels were 3100 ng PFOS/g, 180 ng PFNA/g and 8.6 ng PFOA/g in polar bears. In general, odd-length PFCAs exceeded the concentration of even-length PFCAs and concentration decreased with increasing chain length. Levels were generally lower than levels for similar animals in the USA (Martin et al. 2004). In a recent Nordic screening project a few samples of marine mammals from Denmark, Sweden, Iceland and Faroe Islands were investigated for perfluorinated compounds (Berger et al. 2004; Kallenborn et al. 2004). Grey seals from Sweden and harbour seals from Denmark were most contaminated with PFOS as dominating substances (up to 1 μg/g ww). Minke whales from Iceland were lower contaminated than pilot whales from Faroe Island. In pilot whales PFOSA levels were sometimes exceeding PFOS levels. Table 6.9: PFOA and PFOS in marine mammals.
6.2.8 Levels in terrestrial animalsWild wood mice (Apodemus sylvatica) captured in a nature reserve close by a fluorochemicals factory in Belgium had extremely high PFOS levels in the livers ranging from 470-17,855 ng/g ww with a median of 506 ng/g ww and mean of 2618 ng/g ww (Hoff et al. 2004). Wood mice living and captured 3 km away had hundred times lower average levels (mean: 280 ng/g ww). The result suggested sex independence of PFOS levels, increased levels in older mice, and maternal PFOS transfer to the young. PFOS levels in blood serum from two pet rabbits from Tokyo ranged 6-9 ng/mL (Taniyasu et al. 2003). The accumulation of 13 different perfluorinated chemicals (PBFS, PFHxS, PFOS, PFPeA, PFHxA, PFHpA, PFOA, PFNA, PFDeA, PFUnA, PFDoA, PFOSA, THPFOS) was studied in cattle from Japan by analysis of blood plasma (Guruge et al. 2004). There was no clear age-dependency, and the levels were on that scale lower than in fish. 6.3 Human exposureThe pathways leading to human exposure to PFOS and PFOA are not well known. Protections of clothes and carpets are among the most important uses of these chemicals and precursors. Thus exposure to dust indoor may be important. Vacuum cleaner dust from Japanese homes also contained between 11 and 2,500 ng PFOS/g dust (mean: 200 ng/g; median: 24.5 ng/g) and between 69 and 3,700 ng PFOA/g dust (mean: 380 ng/g; median: 165 ng/g), respectively. PFOA levels were in general higher than PFOS levels. The highest concentrations of both were in the same sample, and there was an association between PFOS and PFOA in all samples; an indication of a similar origin (Moriwaki et al. 2003). The daily intake of PFOS from air exposure in Japan was calculated to 10-100 pg/day, which would result in 1.2-12 ng/L excess of plasma PFOS levels (Sasaki et al. 2003). Food intake may be another exposure route. N-Ethylperfluorooctane sulfonamide (N-EtFOSA) is present (or at least has been present [13]) in some oil- and water repellent coatings for paper and paperboard used in food packaging. A Canadian study of fast food composites collected before 2000 revealed that more than 55% of the composites contained this chemical (detection limit: 10 pg/g). The highest level measured (23,500 pg/g) was in a pizza. PFOS was also detected in three samples. Samples after 2000 were free of these contaminants (Titlemeier et al. 2003). A Japanese study has shown that consumption of drinking water obtained from a polluted river may lead to a significantly increased daily intake of 0.2-1 mg PFOS/day and may contribute 8-16 μg PFOS/L to blood serum levels and result in a 25-50% rise in normal levels (Harada et al. 2003). 6.4 Levels in humansPerfluorochemicals in human blood have for decades been detected in occupational exposed individuals. In a worker handling the ammonium salt of PFOA the blood contained up to 71 g organic fluorine/ml blood (Ubel et al. 1980). Serum PFOS concentrations among production workers in PFOS-related processes averaged 0.5-2 ppm (mg/L) and ranged <0.1-12 ppm (Olsen et al. 2003). Normally, exposure to the stable fluoropolymers will not give rise to increased free fluoride concentration in the blood. Human samples from 1998-2000 showed average PFOS levels of 17-53 ng/mL for PFOS and 3-17 ng/mL for PFOA (OECD 2002). Mean concentration in 645 sera samples from six different states in the USA was 34.9 ng/mL (range: <4.3-1660 ng/mL) (Olsen et al. 2003b). Thus occupational levels may be 100-1000 times higher than levels in the general population. In 65 commercial available human serum samples the average level of PFOS was 28.4 ng/mL with a range of 6.7-81.5 ng/ml. PFOA and PFHxS were quantified in half of the samples and PFOSA only in one sample and at much lower levels (Hansen et al. 2001). Blood serum and livers in 31 American blood donors were analysed for fluorochemicals by Olsen et al. (2003a). Liver PFOS concentrations ranged between <4.5 to 57 ng/mL, and serum PFOS ranged between <6.1 to 58.3. PFOS was determined in all but one serum sample. However, in about half of the liver samples levels were below the limit of quantification. The means of the positive samples were 28.0 ng/g ww for liver and 18.3 ng/mL for serum. Among the 23 human donors with paired mean liver and serum PFOS concentrations were 20.8 ng/g (ppb) and 18.2 ng/ml (ppb), respectively. The mean serum to liver ratio was 1.3. Only a few sample had levels of FOSA and PFHxS above the quantification limits, and only 1 liver was found with measurable amount of PFOA. In blood serum the mean levels for PFOA, FOSA and PFHxS were 3.1, 4.5, and 2.4 ng/mL, respectively. A larger study characterised PFOS and six other related fluorochemicals in serum samples taken in 2000-2001 from a 645 adult American blood donors from cities in six states (Olsen et al. 2003b). The levels ranged from the lower limit of determination of 4.1 ppb (ng/mL) to 1,656 ppb with a geometric mean of 34.9 ppb. The mean was a little higher among males than females but no substantial difference was observed by age. The levels of the other fluorochemicals were on that scale lower. The mean of PFOA was 4.3-5.3 ppb (Olsen et al. 2003b). In a recent study of an elderly population (ages 65-96) PFOS in the blood ranged <3.4-175 ng/mL with a geometric mean of 31 ng/mL (Olsen et al. 2004). This is inside the normal range (30-40 ng/mL) for non-occupational exposed individuals. The most elderly had slightly lower concentrations but no differences between sexes were noted (Olsen et al. 2004). In the same study levels of PFOA, PFHxS, PFOSAA and M570 ranged respectively <1.4-16.7 ng/mL (mean: 4.2), <1.4-40.3 ng/mL (mean: 2.2), <1.6-21.1 (mean: 1.5) and <1.0-6.6 (mean: 1.2). A study of historic and recent serum samples from Japan revealed that the levels were influenced by several factors (Harada et al. 2004). Sex and residential area are the most influential factors, while age and smoking status are not so important. In general males had 50-100% higher concentrations than females. The PFOS and PFOA mean levels in sera in 2003 ranged from 3.5 to 28.1 μg/L and from 2.8-12.4 μg/L, respectively. Highest levels of PFOS and PFOA were measured in Kinki, an area with known contamination of surface water. Historic data seems to indicate that levels of PFOS reach a maximum in the mid 1990's while PFOA concentrations are still increasing. Perfluorinated compounds were investigated in maternal and in cord blood in 15 pregnant Japanese women (Inoue et al. 2004). PFOS concentrations in maternal samples ranged from 4.9 to 17.6 μg/L, whereas those in foetal samples ranged from 1.6 to 5.3 μg/L, or one-third of maternal levels. In contrast PFOSA was not detected in all samples while PFOA was determined in 3 out of 15 samples ranging 0.5-2.3 μg/L. The presence of perfluorinated compounds in 23 archived pooled samples of blood plasma from females of the northern population in Canada was studied by Tittlemier et al (2004). Average levels were 36.9 ng PFOS/mL and 2.2 ng PFOA/mL. All samples also contained PFNA (0.11-1.98 ng/mL), while 70% of the samples contained detectable levels of PFHpA and only one sample contained PFHxA. Levels in cord blood plasma were about the half of maternal blood. There was no difference between levels in Inuit and Caucasian people as there are for PCB and dioxin, thus the source of exposure may not be food. In a recent study from Sweden 66 whole blood samples from the general population were analysed for 12 perfluorinated compounds with chain length 4 to 14 (Kärmann et al. 2004). PFOS, PFOA, PFOSA, PFHxS and PFNA were found in 92-100% of the samples, while traces of PFDeA and PFDoA were found in 65% of the samples. Besides this, PFDeS, PFHxA, PFDoA and PFTeA were detected in 3-8% of the samples. Levels of PFOS dominated with a range of 1.7-37 ng/mL and a mean of 18.2 ng/mL. The mean sum of all perfluorinated compounds was 24.6 ng/ml. Rather high levels of perfluorinated chemicals were found in blood and semen from Sri Lanka (Guruge et al. 2004). The levels in the capital Colombo were a little higher than in a rural area. Levels in semen were ten times lower than in serum. Maximum serum concentrations were 18 ng PFOS/mL and 23 ng PFOA/mL. PFOS and other perfluorinated chemicals were determined in blood serum from the general population of the USA, India and Italy by Kannan et al. (2003). PFOS levels (mean: 32 ng/mL) in Americans are 7-8 times higher than in Italians who again have twice the levels of Indians. In the majority of samples from the USA PFHxS, PFOA and PFOSA were also detected (means: 3.9, 5.1, and 3.4 ng/mL, respectively). In contrary, one third of the samples from Italy and India had detectable levels of PFHxS while PFOA and PFOSA only were detectable in a few or zero samples. The concentration of PFNA and PFDA was between 0.5 and 1 ng/mL. No variation in sex or age was seen. In a recent study Kannan et al. (2004) reported results of perfluorinated compounds in blood from additional countries. In all countries besides Korea PFOS was far the dominating chemical with means ranging from 66 ng/mL in Kentucky, USA, to 2.3 ng/mL in India. In Korea mean concentration of PFOA (88 ng/mL) in females was higher than that of PFOS (15 ng/mL). Curiously, PFOA was only determined in 19-25% of the samples. This may be caused by individual exposure to a local contamination. These data from Korea were also published separately by Yang et al. (2004), who analysed 50 whole blood samples from Daegu, the fourth largest city in Korea. Figure 6.1: Perfluorinated chemicals in human blood from various countries (Kannan et al. 2004; OECD 2002; Kärmann et al. 2004)
Table 6.10: Perfluorochemicals in human samples.
6.5 Animal toxicityThe effects of PFOA on biological systems have been extensively studied in rodents (Kennedy et al. 2004). PFOA causes peroxisome proliferation as well as induction of various enzymes involved in lipid metabolism. Toxic effects have been reported, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain. PFOA also affects the serum levels of various hormones, i.e. reducing testosterone, and increasing estradiol in rats. Subchronic exposure of animals to PFOS may lead to significant weight loss accompanied by hepatotoxicity and reduction of serum cholesterol and thyroid hormones. When evaluating animal studies it has to be taken into account that control animals can be exposed to the general PFOS contamination through fish-based feedstuff. 6.5.1 Toxicokinetics and metabolism6.5.1.1 AbsorptionPFOS is readily absorbed in the body (Lau et al 2004), as is PFOA (Kennedy et al 2004). 6.5.1.2 TransformationPFOA and PFOS are both considered being metabolically inert (Clark et al. 1973). Other PFCAs and PFASs do have similar properties, and their functional derivatives may be transformed to PFCAs and PFASs. For example, the fluorotelomer alcohol 8:2 FTOH is transformed to PFOA in rats (Hagen et al. 1981). 6.5.1.3 AccumulationPFOA and PFOS may bind to proteins and accumulate in various body tissues, including blood and liver of exposed organisms. PFOS also accumulates in the testis and brain (Vanden Heuvel et al 1992; Austin et al. 2003). In the blood these chemicals are bound to serum proteins, mainly albumin but also sex-hormone binding globulins and corticosteroid-binding globulins, which regulate and maintain the steady state of free hormones in the blood. The potential of perfluorinated carboxylic acids (PFBA, PFOA, PFDA) and sulfonic acids (PFBS, PFHxS, PFOS) of varying chain length to displace hormones from specific serum binding protein (receptor) in blood serum from carps, male fowl and bald eagle was studied by Jones et al. (2003b). In carp serum a very small displacement of testosterone was seen for PFOS, PFBA, PFOA, and PFDA but not for PFBS and PFHxS. In bird serum corticosterone was slightly displaced with increasing carbon chain length and the sulfonic acids were more potent than the carboxylic acids. Under environmentally relevant conditions no effect will appear. 6.5.1.4 EliminationOnce absorbed in the body PFOA is eliminated as the free carboxylic acid mainly with urine and to a less extent in faeces. Thus the renal elimination is critical for detoxification (Vanden Heuvel et al. 1991). The biological half-life of PFOA in male rats (5.6 days) is 70 times longer than that in female rats (2 hrs). The difference is mainly due to the difference in renal clearance, which was significantly reduced by probenezid, suggesting that PFOA is excreted by organic anion transporters. Castration of male rats caused a 14-fold increase in renal clearance, comparable with female rats, which again was reduced by treatment with testosterone. Treatment with estradiol also increased the renal clearance. In female rats ovariectomy increased the renal clearance (Kudo et al. 2002). Perfluorocarboxylic acids (PFCA) with a shorter carbon chain length than PFOA are more rapidly eliminated in urine and PFHpA (0.10/0.05) > PFOA (5.6/0.08) >PFNA (30/2.4) >PFDA (40/59 days) for male/female rats (Kudo et al. 2001; Ohmori et al. 2003). The sex differences were most marked for PFOA. The half-life of PFOS in the monkey was about 200 days (Seacat et al. 2002), and the half-life of PFOA in monkeys is about 1 month (Burris et al. 2002). 6.5.2 Animal toxicityThe oral rat LD50 for PFOS is 251 mg/kg bw (US EPA 2000). For PFOA and PFDA the rat intraperitoneal LD50's are 189 and 41 mg/kg, respectively. Thus PFDA is much more toxic and do have delayed effects (Olson and Anderson 1983). The rat oral LD50 for PFOA is between 430 and 1800 mg/kg (Kennedy et al. 2004). Female rats were injected intraperitoneal with 0, 1 and 10 mg PFOS/kg bw for two weeks. The PFOS exposure decreased food intake and body weight in a dose-dependent manner (Austin et al. 2003). 6.5.2.1 Liver toxicitySeveral toxicological studies have demonstrated that the liver is the primary target organ for PFOS and PFOA. Liver cell hypertrophy and reduction in serum cholesterol are early responses to PFOS in experimental animals. Kudo & Kawashima (1997) found that fish oil-feeding prevents PFOA induced fatty liver in mice. In an in vitro test various perfluorinated chemicals were tested for interference with the liver-fatty acid binding protein (L-FABP). The most potent chemical was PFOS followed by EtFOSA, EtFOSE, and PFOA (Luebker et al. 2002). This interference may contribute to the toxicity of these chemicals. In a sub-chronic dietary toxicity study of potassium perfluorooctane sulfonate rats were exposed to up to 20 ppm for 14 weeks (Seacat et al. 2003). Decreased serum glucose and serum cholesterol increased liver weight and alanin transferase (ALT) activity, hepatocytic hypertrophy and cytoplasmic vacuolisation were observed. Serum and liver PFOS concentrations were proportional to dose, and serum concentrations were higher in female than in males. The serum to liver PFOS ratios ranged from approx. 3:1 to 12:1. The no-observed-adverse effect level (NOAEL) was 5 ppm corresponding to 44 ppm (mg/L) in males and 64 ppm in females and mean liver PFOS concentration of 358 ppm (mg/kg) and 370 ppm. PFOS concentrations associated with the no adverse effect (0.15 mg/kg/d) in a 6-month monkey study were 59-70 mg/kg in the liver and 67-83 mg/L for serum (Seacat et al. 2002a). Rats seem to tolerate somewhat higher liver concentrations of PFOS than monkeys. PFOA is considerably less reactive than PFOS (PFOA LOEL of 3 to 10 mg/kg/d), and the target is different (Butenhoff, 2002). The only site of change in the monkey was liver enlargement at the doses cited. 6.5.2.2 Peroxisome proliferationPeroxisome proliferation is a well-known toxicological mechanism in rodents but not in humans. It may cause lipid accumulation in the liver, uncoupling of the mitochondrial oxidative phosphorylation, and reduction of thyroid hormone in circulation. Many hypolipidemic drugs, solvents and environmental chemicals can cause peroxisome proliferation, for example Clofibrate, DEHP, chloroform, perchloroethylene, trichloroethylene, HFC-123, and MTBE. Perfluorocarboxylates (PFCA), particularly PFOA and PFDA, are highly potent peroxisome proliferators in rodent livers and affect mitochondrial, microsomal, and cytosolic enzymes and proteins involved in lipid metabolism (Ikeda et al. 1985; Vanden Heuvel 1996; Upham et. al. 1998). PFOA is a peroxisome proliferator in rodents, whereas PFOS is much less reactive in rodents, but EtFOSE has no effect (Berthiaume and Wallace 2002). Peroxisome proliferation is a less relevant endpoint to humans. The PFOS derivatives FOSA and EtFOSA may also uncouple the oxidative phosphorylation (Schnellmann & Manning, 1990) Administration of PFOA (0.0025-0,04% ww) and PFDA (0.00125-0.01%) in the feed for a week to male rats induced various enzyme systems and significantly increased accumulation of fats in the liver, increased cell size and proliferation of peroxisomes in a dose-dependent manner. At highest exposure concentrations PFDA was more liver toxic than PFOA (Kawashima et al. 1995). Increase in hepatic fatty acid -oxidation activity is a biochemical measure of peroxisome proliferation. Kudo and co-workers (2001) studied PFCAs with different chain length (C7, C8, C9 and C10) in male liver. The result was that the liver concentration and not the chain length was decisive, but the longer the chain the more of the compound was accumulated in the liver. In vitamin A deficient mice, PFOA had a stronger effect and caused a 3-6 times increase in the β-oxidation of fatty acids (Sohlenius et al. 1995). 6.5.2.3 Cellular effectsSome of the observed effects of perfluorinated compounds may be due to alterations in cell membrane fluidity, which is a measure of the relative mobility of the phospholipid bilayer of the cell membrane. The selectively permeable cell membrane forms the first barrier that separates the cell from exogenous exposures. Effects on the permeability status of the cell membrane could play an important role in mediating the adverse effects of a number of environmental contaminants. In some in vitro assay systems PFOS - but not PFBS and PFHxS – significantly increased in a dose-dependent manner membrane fluidity of fish leukocytes, and decreased mitochondrial membrane potential determined by flow cytometry. PFOS alone did not induce cytochrom P450 1A1 activity but at co-exposure of cells to TCDD ("dioxin") and PFOS the cytochrom P450 1A1 activity induced by TCDD was increased (Hu et al. 2003). Gap junction intercellular communication (GJIC) is the major pathway of intracellular signal transduction, and it is thus important for normal cell growth and function. Defects in this communication may lead to teratogenesis, neuropathy, infertility, diabetes, autoimmune disorders, cancer, and other diseases (Trosko et al. 1998). Upham and co-workers (1998) showed that perfluorinated carboxylic acids with carbon chain length of 7-10 can rapidly and reversibly inhibit gap junction intercellular communication in a dose-dependent manner in vitro and with PFDA inhibiting more than PFOA. In in vitro and in vivo test systems PFOS, PFOSA, and PFHxS – but not PFBS - inhibit gap junction intercellular communication in a dose-dependent fashion, and this inhibition occurred rapidly and was reversible (Hu et al. 2002). Cellular effects such as cell membrane fragility and gap junction communication are but two of the hypothetical explanations for the effects of these molecules. These are not the hallmark effects, such as interference with liver function or lipid metabolism. 6.5.3 MutagenicityAmmonium perfluorooctanoate was found to be non-mutagenic in an Ames test using five strains of Salmonella typhimurium and in a single strain of Saccharomyces cerevisiae (Griffith and Long 1980). Several other mutagenicity studies of PFOA published by contract laboratories supports the inactivity of PFOA (Kennedy et al. 2004). No result of mutagenicity testing of PFOS was found in the open literature. 6.5.4 CancerLeydig cells in the testis are the main sites for testosterone biosynthesis. In animal studies with CD rats, a strain that has a low spontaneous incidence of these tumours, PFOA produced a dose-dependent increase in Leydig cell adenomas, and PFOA modified Leydig cell steroidogenesis in vitro (Biegel et al. 1995; Liu et al. 1996). The tumours may be a result of endocrine changes, because a reduced aromatase activity and a sustained increase in serum estradiol were observed (Biegel et al. 2001). However, US Environmental Protection Agency classifies PFOA as a carcinogen in animals (US EPA 2000). A finished two-years feeding study with PFOS has only been briefly reported (Seacat et al. 2002b). A modest liver tumour response was observed in the high dose group (20 ppm PFOS potassium salt). EtFOSE, for which 20% of an oral dose is converted to PFOS, has already been studied and a dietary dose of 100 ppm caused increase of hepatocellular adenomas in females and thyroid follicular cell adenomas in males (Thomford et al. 2002). 6.5.5 Impacts on reproduction and developmentTeratological studies have been conducted in rat, rabbit and mouse with potassium and lithium salts of PFOS (Lau et al. 2003; 2004). The findings are in agreement between laboratories and across species examined, and are generally unremarkable, when maternal effects are taken into consideration. Observed developmental effects include reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. These structural abnormalities were found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams. The no-observed-effect-level (NOEL) for PFOS in rabbits was 0.1 mg/kg/d (Case et al. 2001). High doses of EtFOSE also caused reduced maternal body weight and foetal weight in rodents and had effects quite similar to its metabolite PFOS (Lau et al. 2004). Both PFBS and PFHxS have been assessed for developmental and reproductive effects. Maternal exposure to PFBS potassium salt did not produce any adverse effect on embryo/foetal development, and no significant alterations were noted in a two-generation study in rats at doses as high as 1 g/kg. PFHxS is only examined in a screening system at lower doses without any effect observed (Lau et al. 2004). Very high doses (30 mg/kg/d) of PFOA resulted in a reduction of maternal weight and a significant increase of mortality in the pups but at lower concentrations no significant effects were noted (Lau et al 2004). PFDA and PFOA had no teratogenic effect in mice (Lau et al. 2004). In a 2-generation reproductive toxicity study with PFOA, no structural changes were observed, and marginal changes in survival were seen at 30 mg/kg and not at 10 mg/kg (Butenhoff, 2004). 6.5.6 Endocrine disruptionFemale rats were injected intraperitoneally with 0, 1 and 10 mg PFOS/kg bw for two weeks. The estrous cyclicity was affected, serum corticosterone level was increased, and serum leptin concentration and norepinephrine concentration in the paraventricular nucleus of the hypothalamus were decreased. It was concluded that PFOS could affect the neuroendocrine system in rats and be an endocrine disruptor (Austin et al. 2003). Endocrine changes were studied in adult male rats fed at 25 mg ammonium perfluorooctanoate per kg by gavage (Biegel et al. 1995). Serum testosterone levels and testicular interstitial fluid testosterone levels were decreased, and serum estradiol levels were increased. The reason was an induction of a hepatic aromatise by PFOA, which converts testosterone to estradiol. In a two-week gavage study ammonium perfluorooctanoate decreased serum and testicular interstitial fluid testosterone levels, decreased relative accessory sex organ weights and increased serum estradiol levels (Cook et al. 1992). Several other studies have shown that PFOA can cause hormonal changes in males expressed as increased oestrogen level (Biegel 1997). In contrast, several other studies show no such effects. Functional reproduction was not altered in rats exposed through 2-generations at APFO levels upto and including 30 mg/kg/day (Butenhoff, 2004). Further, in a 6-months oral study in monkeys, daily doses upto 20 mg/kg/day did not produce any changes in hormone levels, including oestrogen (Butenhoff, 2002) 6.6 Human toxicityChemical plant workers exposed to fluorochemicals in the air (up to 3.9 mg/m3) for years had about 100 times higher levels of organic fluorine in their blood (1-71 mg/L serum) than normal people but no ill effects were attributable to exposure to these fluorochemicals. The half-life of PFOA in humans was estimated to be 18-24 months (Ubel et al. 1980). Findings in animal studies suggest that PFOA affects hormonal states and metabolism of lipids in humans. However, no significant correlation has been observed between serum levels of PFOA (mean 5-7 mg/L) and any biological parameter in ammonium perfluorooctanoate plant workers (Olsen et al. 1998; 1999; 2000). A retrospective cohort mortality study of a perfluorooctanesulfonyl fluoride (PFOSF) production workforce reported an excess of bladder cancer based on 3 deaths (SMR 12.77) at high-exposure jobs (Alexander et al. 2003). Workers at a PFOA production plant in the USA whose serum levels averaged 5 ppm had no excess of cancer mortality related to PFOA (Olsen et al. 2004). Olsen et al. (1998) concluded there was reasonable assurance of no substantial hormonal changes associated with PFOA at serum levels measured among these male production employees. 6.7 Ecotoxicity6.7.1 Terrestrial organismsRecent avian toxicology studies have for bobwhites revealed a reproductive LOAEL of 10 ppm in the diet or 0.77 mg PFOS/kg bw/day for male and female birds. This corresponded to 8.7 μg PFOS/mL and 4.9 μg PFOS/g in female serum and liver, respectively, and to 141 μg PFOS/mL and 86 μg PFOS/g in male serum and liver (Giesy & Jones 2004). In mallards the LOAEL was the same (10 ppm) for males but 50 ppm for females. 6.7.2 Aquatic organismsFrom Table 6.11 it is seen that PFOS is moderately acute toxic and slightly chronically toxic to aquatic organisms. PFOA is practically non-toxic. N-EtFOSA is slightly acute toxic to daphnids. Hoff and co-workers (2003) studied the toxicological effects of PFOS on selected biochemical endpoints in the common carp (Cyprinus carpio). PFOS was administered by a single intraperitoneal injection. The results suggested that PFOS induces a rise in serum transaminase levels indicative for disruption of hepatocyte membrane integrity that may be related to cell necrosis. PFOS was three orders of magnitude more toxic to the aquatic midge Chironomus tentans than to most other aquatic organisms. The NOEC was 0.49 g/L (MacDonald et al. 2004). The actual concentrations in the environment are still two orders of magnitude lower. PFOA had no impact on these organisms. The authors hypothesized that the high sensitivity to PFOS may be explained by PFOS-haemoglobin interactions. Table 6.11: Aquatic toxicity of PFAS (adapted from Heckster et al. 2003 and amended)
6.8 Summary of the environmental and health effects of PFOS and PFOA and other polyfluoroalkyl substances6.8.1 Environmental chemistryPolyfluoroalkyl substances (PFAS) are used because of their surfactant properties. They have both hydrophobic and oleophobic properties and are chemically and thermally inert, especially the fluorocarbon chain is extremely resistant to heat and chemical attack, e.g. by acids and bases, and reducing and oxidizing agents. The C-F binding is very strong. Thus the perfluoro part of the molecules is a stable identity, which in practice is non-degradable by all means. On the other hand the functional end group will be more readily transformed in the environment and in organisms, and therefore the compounds will be degraded to the persistent sulfonates (e.g. PFOS) and carboxylates (PFOA) in the end. The sulfonates and carboxylates are polar species, which will not accumulate in fatty compartments but in blood and liver, and these substances will often interact with polar sites in sediments. 6.8.2 Environmental releasePFOS and PFOA are intermediates and by-products from various industrial processes involving PFAS. Emissions to the environment (air and water) may happen directly from production and processing plants or released during product use, e.g. fire-fighting foam, and as product impurities or degradation products. Environmental sources of fluorinated telomers are currently unknown but these substances may be released, when manufacturing perfluorinated compounds and at the decomposition of polymeric materials that incorporate telomers. 6.8.3 Environmental exposureIt is expected that PFAS id present in the environment primarily in the form of the final stable degradation products PFOS and PFOA. In the recent years PFOS, PFOA and other perfluorinated compounds have been discovered as global environmental contaminants. They have been found in indoor air, outdoor air, soil, ground water, surface waters and even at 1000 m depth in the Pacific Ocean, in wildlife and human tissues. 6.8.3.1 Long-range transportationThe occurrence of PFOS and PFOA in the wildlife in remote areas such as the Arctic is puzzling. The binding to water and the low volatility make it less likely that PFOS and PFOA will be spread long-range with the air by the "grass-hopping" and cold condensation mechanisms as persistent organic pollutants (POPs). However, the prevailing hypothesis is that long-range transport to the arctic occurs via volatile precursors of both PFOS and PFOA, with subsequent degradation to these stable products. Volatile PFOS-precursors are a.o. MeFOSE and Et FOSE. It has been hypothesized that fluorotelomer alcohols (FTOH) may be long-range transported and hereby reach remote arctic areas, where they can degrade to the more stable PFOA. Atmospheric lifetime of short chain FTOHs, as determined with its reaction with OH-radicals, is approximately 20 days making the molecule able to travel about 7000 km. Therefore, they may be long-range transported and hereby reach remote areas, where they can degrade to PFOA. On the other hand products containing perfluorinated substances may also be used for example in fire-fighting foam at bases in remote areas. Recently a hypothesis has also been brought forward that ocean current transport may in part explain the presence of PFOS and PFOA in the arctic. 6.8.4 Air levelsData on levels of perfluorinated substances in ambient air exist only from the USA, Canada and Japan. The substances identified in pg/m3 have mainly been MeFOSE and EtFOSE with lower levels of PFOS, MeFOSEA and FTOH. In urban areas levels were often 10 times higher than in rural areas, and in cities with carpet production the levels were even higher. Higher concentrations were seen more in the summer than in the winter. Indoor levels are especially high and may be 10-100 times higher than outdoors, and since the modern individual spends much time indoor, this exposure will really count. 6.8.4.1 Levels in ground and drinking waterIn Japan tap water contained between 0.1-4 ng PFOS/L. Use of fire-fighting foam at military facilities in the USA has resulted in several episodes with serious pollution of groundwater with perfluorinated compounds in concentrations up to 15 mg/L. The found substances were PFOS, PFHxS, PFBS, PFPS, PFOA, PFHxA, and PFHpA. 6.8.5 Levels in surface watersIn water from the Great Lakes both PFOS and some precursors (EtFOSAA and FOSA) were detected in unpolluted waters. Concentrations of PFOS were 20-70 ng/L. River water downstream an US fluorochemicals plant contained up to 144 ng PFOS/L and 598 ng PFOA/L. Upstream PFOS levels were below 50 ng/L and PFOA could not be detected. Similar high levels were determined in effluents from a Japanese wastewater plant. In waters samples taken from remote Pacific Ocean areas low levels (<0.4 ng/L) of PFOA, PFOS and PFHxS were detected. Similar levels were found in samples from 1000 m depth. The background levels were 5-10 times higher in water samples from the Atlantic Ocean. Coastal seawater from China, Korea and Japan contained 20 to 1000 times higher levels and PFNA could also be detected. In most cases the concentration of PFOA was higher than PFOS. Use of fire-fighting foams has also caused serious pollution of surface waters. An accidental spill at Toronto Airport polluted a creek. Upstream levels of perfluorinated substances were <0.03 μg/L while downstream concentrations ranged 2 to 17,000 μg/L. The substances measured were: PFOS, PFHxS, and PFOA. After a fire at an oil storage facility at a refinery in Japan the runoff waters contained 3,669 ng PFOS/L, 149 ng PFHxS/L, 200 ng PFOSA/L, 300 ng PFNA/L, 162 ng PFOA/L and smaller levels of PFBS, PFUnA, PFDeA, PFHpA and PFHxA. The levels in the waters decreased by time (dilution?) but it is likely that the use of fire-fighting foams is an important source of soil and water pollution. The highest levels of PFOA and PFOS found in samples from the North Sea were about 20 ng/L in samples from the mouth of River Elbe while the other detectable compounds (PFHxA, PFHxS, PFHpA, PFNA, PFDeA and PFOSA) ranged from 1-3 ng/L. Relatively high levels of PFOA and PFOS (5-10 ng/L) were also found in samples from the Dutch and German Waddensea, representative for the Danish Waddensea. In open waters levels were below 1 ng/L, and only PFOA could be quantified. In River Elbe waters the concentration of PFOS was higher than of PFOA. In seawaters the relation was opposite. In a few samples of water from Norway, Denmark, Sweden, Finland, Iceland and Faroe Islands investigated for perfluorinated compounds, levels of PFOA were highest with median levels of 5.2 ng/L in seawater, 7.8 ng/L in lake water, 13.1 ng/L in rainwater, 20.5 ng/L in sewage effluent and 297 ng/L in landfill effluent. Background levels of perfluorinated compounds in surface water from diffuse polluted areas seem to be about 20 ng/L in comparison to 0.2 ng/L in unpolluted areas. 6.8.6 Levels in sediment and sewage sludgeThe binding of PFAS to sediment sewage sludge is in general strong and stable, which means a high potential for accumulation herein. In a few samples of sewage sludge from the Nordic countries PFOS was the dominating substance in samples from Denmark, Norway and Sweden, and PFOA was highest in Iceland and Faroe Islands. Highest maximum levels of PFOS were found in Sweden (2644 pg/g ww), Denmark (1041 pg/g ww) and Norway (1023 pg/g ww). Somewhat lower levels in Finland (925 pg/g ww), and in Island and Faroe Islands the levels were much lower at 220-241 pg/g ww. In Finland PFHxA was the second most abundant, and PFNA was found in Norwegian, Danish and Finnish sludge. In general sediment samples had lower levels than sludge. The highest level of PFAS was 1150 pg/g ww in a Finnish sample. Contamination was also high in Norway but not in Sweden and other countries. 6.8.7 Levels in biotaMany perfluorinated compounds are global contaminants, which bioaccumulate and are widely distributed in wildlife. PFOS has been detected in concentrations > 1 ng/g (ppb) in most samples analysed from various species of aquatic animals (seals, otter, sea lion, dolphin, polar bear, mink), birds, fish and amphibians. Some samples also contained PFOSA, PFHxS and PFOA. There was no difference between levels in male and female animals as for the lipophilic POPs. PFOS is also found in bird and fish eggs suggesting possible maternal transfer during yolk formation. Where the PFOS level in seals is higher in Europe than the US, it is opposite with the levels in dolphins and seabirds. The extent of bioconcentration of PFAS appears to be highly structure dependent. Whereas PFOS and PFOA and perfluorinated compunds with longer alkyl chain are considered to be bioaccumulative in wildlife and sometimes biomagnifying, perfluorinated acids with fully fluorinated chain lengths of C5 and below seem not to bioaccumulate. 6.8.8 Levels in fishSome perfluorinated compounds accumulate in fish liver with a bioaccumulation factor of more than 6,000. At the previous mentioned Toronto Airport spill fishes in the creek were highly contaminated. Total perfluoroalkane sulfonate concentrations in the fish livers ranged 2-73 μg/g and it was mainly PFOS. A few samples also contained small levels of PFBS, PFHxS, PFOA, PFPeA, PFHpA, PFDA, PFUnA and PFTA. Normal levels in fish are much lower at 20-100 ng/g ww. In a recent Nordic project levels of PFOS followed by PFOSA dominated over PFOA in freshwater fishes. The highest levels of 551 ng PFOS/g ww and 141 ng PFOSA/g ww were measured in a Finnish pike sample. Marine fish samples from Faroe Island had the lowest contamination, and PFOSA was the most abundant. Otherwise PFOS dominated. In all the Icelandic samples PFDS and PFHxA were present. In the Danish samples PFHxA, PFHpA and PFOA were also present. Levels of PFOS in Danish flounders and herrings were around 20 ng/g ww. PFOS was the major contaminant in fish from the Canadian Arctic, and levels of FOSA were also rather abundant but all samples contained also perfluorinated carboxylic acids (PFCA) ranging in length from 9 to 15 carbons. In general, odd-length PFCAs exceeded the concentration of even-length PFCAs, and the concentration decreased with increasing chain length. Levels were generally lower than levels for similar animals sampled in the USA. 6.8.9 Levels in birdsSignificant levels of PFOS in birds (egg yolk, plasma and liver) have been reported from various places. The levels were clearly higher in populated and industrialised areas than in remote locations. Levels in birds from Antarctica are around or below the quantification limit. The highest levels were found in blood from bald eagles collected in Midwestern USA (2570 ng PFOS/g ww). PFOS was the major contaminant in birds from the Canadian Arctic but common loon also contained significant levels of FOSA. In contradiction to mammals and fish perfluorinated carboxylic acids were not detected in birds. Levels were generally lower than levels for similar birds in the USA. In fulmar eggs from Faroe Islands PFAS levels were about 40 ng/g ww and 95% of that was PFOS followed by PFNA and PFOSA. 6.8.10 Levels in mammalsSignificant levels of PFOS have been reported in aquatic mammals from various places. The highest levels were found in mink (3680 ng PFOS/g ww) and river otters (990 ng/g ww) from the western USA. High levels were also found in Alaskan polar bear (680 ng/g ww) and seals (230 ng/g ww) from the Baltic Sea and in dolphins (430 ng/g ww) from the Mediterranean Sea. The levels were clearly higher in populated and industrialised areas than in remote locations. Both PFOS and PFOA have been identified in polar bears, ringed seals, arctic fox, and mink from the Canadian Arctic. PFOS was the major contaminant but all samples contained also perfluorinated carboxylic acids (PFCA) ranging in length from 9 to 15 carbons. In all samples except for minks PFOS dominated. In minks Perfluorononanoic acid (PFNA) was highest. Mammals feeding at higher trophic levels had greater contamination. Highest mean levels were 3100 ng PFOS/g, 180 ng PFNA/g and 8.6 ng PFOA/g in polar bears. In general, odd-length PFCAs exceeded the concentration of even-length PFCAs and concentration decreased with increasing chain length. In the Nordic area grey seals from Sweden and harbour seals from Denmark were mostly contaminated with PFOS as the dominating substances (up to 1 g/g ww). Minke whales from Iceland were lower contaminated than pilot whales from Faroe Island. In pilot whales PFOSA levels were sometimes exceeding PFOS levels. 6.8.11 Human exposureThe pathways leading to human exposure to PFOS and PFOA are not well known. Protections of clothes and carpets are among the most important uses of these chemicals and precursors. Thus exposure to indoor air and dust may be important. High concentrations of perfluorinated compounds have been measured in indoor air (see earlier). Dust collected from vacuum cleaners in Japanese homes also contained very high levels of PFOS and PFOA. PFOA levels were in general higher than PFOS levels. The daily intake of PFOS from air exposure in Japan was calculated to 10-100 pg/day, which would result in a 1.2-12 ng/L excess of plasma PFOS levels. Food intake may be another exposure route. Some perfluorinated compounds e.g. EtFOSA may be present in some oil- and water repellent coatings for paper and paperboard used in food packaging. A Canadian study of fast food composites collected before 2000 revealed that more than 55% of the composites contained this chemical (detection limit: 10 pg/g). The highest level measured (23,500 pg/g) was in a pizza. PFOS was also detected in three samples. Samples after 2000 were free of these contaminants. A Japanese study has shown that consumption of drinking water obtained from a polluted river may lead to a significantly increased daily intake of up to 1 mg PFOS/day and may contribute 8-16 μg PFOS/L to blood serum levels and result in a 25-50% rise in normal levels. 6.8.12 Human levelsFor decades perfluorochemicals have been detected in human blood from occupational exposed individuals. Serum PFOS concentrations among production workers in PFOS-related processes averaged 0.5-2 mg/L (ppm) and ranged <0.1-12 mg/L (ppm). These occupational levels are 100-1000 times higher than present serum and liver levels of 15-50 μg PFOS/L (ppb) in the general population. Levels of PFOA, PFHxS, PFOSAA and PFOSA are a magnitude lower (<5 μg/L). A study of historic and recent serum samples from Japan revealed that sex and residential area are the most important factors influencing the levels while age and smoking status had no influence. It seems that levels of PFOS reached a maximum in the mid 1990's, while PFOA concentrations are still increasing. PFOS concentrations in foetal samples were only one-third to a half of maternal levels. Levels of PFOS and PFOA in blood samples from the Canadian Arctic were similar to other parts of Canada and seemed not to be related to food habits as it is for PCB and dioxins. All samples also contained PFNA and a few samples also PFHpA and PFHxA. In Sweden blood samples from the general population were analysed for 12 perfluorinated compounds with chain length from 4 to 14. PFOS, PFOA, PFOSA, PFHxS and PFNA were found in almost all samples, while traces of PFDeA and PFDoA were found in most samples. Besides this, PFDS, PFHxA, PFDoA and PFTeA were detected in a few samples. The mean sum of all perfluorinated compounds was 25μg/L, and levels of PFOS dominated with a mean of 18 μg/L. 6.8.13 Animal toxicityPFOA and PFOS may bind to proteins and accumulate in various body tissues of exposed organisms, including in blood and liver, and for PFOS also in testes and brain. Although the perfluoroalkane sulfonic acids and carboxylic acids are closely related structurally, these chemicals elicit different biological responses in vitro and in vivo. The acute lethal toxicity is moderate corresponding to a classification as harmful. PFOS is more toxic than PFOA, and the toxicity increases with the length of the alkyl chain. The liver is the primary target organ for perfluorinated compounds. PFCAs cause peroxisome proliferation in the rodent liver as well as induction of various enzymes involved in lipid metabolism. PFDA with a longer alkyl chain seems even more active than PFOA. Toxic effects have been reported, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain. PFOA also affects the serum levels of various hormones, i.e. reducing testosterone, and increasing estradiol in rats. Thus it acts as an endocrine disruptor. In in vitro and in vivo test systems PFOS, PFOSA, and PFHxS – but not PFBS - inhibit gap junction intercellular communication in a dose-dependent fashion, and this inhibition occurred rapidly and was reversible. Perfluorinated carboxylic acids with carbon chain length of 7-10 can also rapidly and reversibly inhibit gap junction intercellular communication in a dose-dependent manner in vitro, and with PFDA inhibiting more than PFOA. Gap junction intercellular communication (GJIC) is the major pathway of intracellular signal transduction, and it is thus important for normal cell growth and function. Defects in this communication may lead to teratogenesis, neuropathy, infertility, diabetes, autoimmune disorders, cancer, and other diseases. Although the fluorinated chemicals do not seem to be mutagenic, PFOA induces testis cancer and PFOS and EtFOSE induce liver cancer in experimental animals. USEPA classifies PFOA as a carcinogen in animals. PFOS causes developmental effects including reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. However, the structural abnormalities were found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams. Thus the relevance may be questioned. PFOA causes reduction of foetal weight. Other PFAS (PFBS and PFHxS) have neither a significant effect on reproduction or development even at high doses. 6.8.14 Human toxicityIn general the knowledge about the toxicology of the perfluorinated compounds is rather sparse, and it will take some time and efforts, before we will have sufficient information for evaluation of the full impact of the present levels in humans. The experience from the work environment has not indicated any important adverse health effects among exposed workers, besides a retrospective cohort mortality study of a perfluorooctanesulfonyl fluoride (PFOSF) production workforce, which reported an excess of bladder cancer at high-exposure jobs. 6.8.15 EcotoxicityOnly a few studies and mainly concerning acute toxicity to aquatic organism exist in open literature. PFOS is moderately acute toxic and slightly chronically toxic to aquatic organisms. PFOA is practically non-toxic. N-EtFOSA is slightly acute toxic to daphnids. There seems to be large species difference in the biological response, because PFOS was three orders of magnitude more toxic to the aquatic midge Chironomus tentans than to most other aquatic organisms. The scarce database indicates a need for further studies. 7 Environment and health assessment of alternatives to PFOS/PFOA related compoundsA search for different ecotox and human tox information has been performed for the identified alternatives to PFOS/PFOA related compounds. The search has been performed in different common databases, such as Toxnet, NTP, IARC, Ecotox, Chemfate, and N-class. The search results are presented in Table 7.1 below. As can be seen from the table very little information about the environmental and health effects of the substances is available. A few abstracts were identified for most of the substances, but none of the abstracts gives relevant information. For three of the substances (two groups of alternatives) a record in HSDB (Hazardous Substances Data Bank) was identified. For a single substance two records were found in the Ecotox database, but no records of aquatic toxicity (LC50 values) were available. For about half of the substances a few results about the chemical fate of the substances were available in the Chemfate database. The sparse information found for the different alternatives is presented below. Table 7.1: Search results in different common ecotox and human tox databases.
7.1.1 Fluorinated polyetherOMNOVA Solutions Inc. produces series of fluorinated polyethers (PolyFox™ products), which can be used as a surfactant and as flow, level, and wetting additive for coating formulations. The PolyFox formulation is currently being used as a surfactant in floor polish products in the USA, Europe and Asia. The entire PolyFox family of fluorosurfactants are polymers with a molecular weight greater than 1,000. The PolyFox polymers are based on ether links – both the polymer backbone linkages and the link between the backbone and the perfluoroalkyl pendant. The PolyFox fluorosurfactants are synthesized from perfluoroalkyl starting materials with a fully fluorinated carbon chain length of C4 or less. The current first generation products are all made with C2F5 or CF3 perfluoroalkyl side chain structures (Personal communication OMNOVA 2004). The basic structure of two of the different PolyFox™ compounds is illustrated in the figures below. Other and further details can be found in appendix F (Personal communication, PoraTek 2004). Figure 7.1: The basic structure of PolyFox™ 3320 compound (Personal communication, PoraTek 2004). x+y equals about 20.
Figure 7.2: The basic structure of PolyFox™ 656 with C2F5 as the basic perfluoralkyl group. (Personal communication, PoraTek 2004). x+y equals about 6.
7.1.1.1 Health effectsNo information other than the data given in the MSDS's has been found for these fluorinated polyethers (CAS numbers are proprietary). According to the MSDS's the PolyFox products must be labelled R43: "May cause sensitisation by skin contact". Furthermore, the products may be irritating to the respiratory system (by inhalation) and the gastrointestinal tract (if swallowed). A LD50 (oral, rat) value of > 2.0 g/kg indicates that the products have low acute toxicity by ingestion. 7.1.1.2 Environmental effectsNo information other than the data given in the MSDS's has been found for these fluorinated polyethers. For some of the PolyFox products 48hLC50 values > 100 mg/L for daphnias were reported, which indicates that the products have low acute toxicity to aquatic organisms (MSDS's from PolyFox products 2004). However, OMNOVA states that their fluorinated polyethers have a high molecular weight that makes them less available for transport across bio-membranes and therefore less biologically available (in contrast to the relatively small molecules of PFOS and telomer-based fluorosurfactants). Furthermore, the polymer backbone linkage of the PolyFox molecules is an ether link, which is more environmentally stable than e.g. the ester linkages of PFOS and telomer-based fluorosurfactants. This makes the PolyFox molecule more resistant to degradation to lower molecular carboxylic acids. Additionally, the PolyFox products are made with shorter fluorochain lengths (C2F5 or CF3 structures), which means they cannot produce the longer perfluorinated acids such as PFOA but only the less hazardous perfluoroacetic and perfluoropropionic acids (Personal communication OMNOVA 2004). The paper by Martin et al. (2003) confirms that perfluorinated acids with fully fluorinated chain lengths of C5 and below do not accumulate (see Chapter 5). 7.1.2 Fatty alcohol polyglycolether sulfateBASF produces a fatty alcohol polyglycolether sulfate (Emulphor FAS 30), which can be used as levelling and wetting agent in paints and coatings (See further details in Appendix F). 7.1.2.1 Health effectsNo information other than the data given in the MSDS has been found for this fatty alcohol polyglycolether sulfate (no CAS number available). According to the MSDS this fatty alcohol polyglycolether sulfate is a non-irritating and non-hazardous substance. A LD50 (oral, rat) value > 2.0 g/kg is given, which indicates that the substance is acute toxic by ingestion. No other information is available (MSDS Emulphor® FAS 30 2003). 7.1.2.2 Environmental effectsNo information other than the data given in the MSDS has been found for this fatty alcohol polyglycolether sulfate. According to the MSDS the fatty alcohol polyglycolether sulfate is readily biodegradable (>70% elimination according to OECD 301E), and it does not seem to be acute toxic to aquatic organisms as the reported 96hLC50 value for fish (Leuciscus idus) is > 100 mg/L (MSDS Emulphor® FAS 30 2003). 7.1.3 Silicone polymersWorlée-Chemie produces silicon polymers, which can be used as wetting agents as replacement for fluorinated surfactants in several cases in the paint and ink industry. Their products contain 3-(polyoxyethylene) propylheptamethyl trisiloxane (CAS No 67674-67-3) (See further details in Appendix F). 7.1.3.1 Health effectsNo information other than the data given in the MSDS has been found for the silicone polymers. According to the MSDS the silicone polymer must be classified as irritating and harmful with the R-phrases, R20 ("Harmful by inhalation") and R41 ("Risk of serious damage to the eyes"). Prolonged and frequent skin contact can cause skin irritation (MSDS Worlée Add® 340 2004). 7.1.3.2 Environmental effectsNo information other than the data given in the MSDS has been found for the silicone polymers. According to the MSDS the silicone polymer must be classified as environmentallay dangerous with the R-phrases R51/53 ("Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment"). The R-phrase R53 indicates that the substance is bioaccumulative (MSDS Worlée Add® 340 2004). 7.1.4 SulfosuccinateBASF and Cognis both produce a surfactant based on 50-75% of the sodium salt of di(2-ethylhexyl) sulfosuccinate (see Appendix F: "Identified alternatives" for further details), which can be used as a wetting agent for paints and coatings. In one product the sulfosuccinate is mixed with water and ethanol, and in the other the sulfosuccinate is mixed with water and 2,2-dimethylpropane-1,3-diol (Personal communication BASF 2004; Personal communication Cognis 2004). The product with the lowest content of sulfosuccinate (containing water and 2,2-dimethylpropane-1,3-diol) is classified as a local irritant (Xi) with R-phrases R38 ("Irritating to skin") and R41 ("Risk of serious damage to eyes") (MSDS Lutensit® A-BO 2003). Münzing Chemie also produces a surfactant based on a sulfosuccinate derivative in ethanol and water, which also can be used as a wetting agent for paints and coatings. The type of sulfosuccinate (or CAS No.) is not specified. Figure 7.3: The chemical structure of the sodium salt of di(2-ethylhexyl) sulfosuccinate (CAS No. 577-11-7).
7.1.4.1 Health effectsAccording to the MSDS's of the sulfosuccinate products, the products can cause skin, eye and respiratory irritation, especially on prolonged or repeated contact. Dermatitis has been observed as a long-term effect. Other possible long-term effects are central nervous system (CNS) depression, as well as injuryto heart, liver and blood-forming organs. The acute toxicity of the di-2-ethylhexyl sulfosuccinate (LD50 (oral, rat) = 1.9 g/kg) indicates that the substance is mildly harmful, if swallowed. A LD50 (oral, rat) value of > 2.0 g/kg is also reported, but this value may be for the entire product and not for the single substance di(2-ethylhexyl) sulfosuccinate (MSDS Hydropalat 875 2002; MSDS Lutensit® A-BO 2003). Information found in the HSDB database suggests that di(2-ethylhexyl) sulfosuccinate is mildly toxic (by ingestion) to humans with a probable oral lethal dose (human) of 0.5-5 g/kg. The substance is in rare cases reported to produce skin rash and allergies (HSDB database 2004). 7.1.4.2 Environmental effectsAccording to the MSDS's, and the Chemfate and HSDB databases the substance di-2-ethylhexyl sulfosuccinate is easily biodegradable and not likely to bioconcentrate. Only one LC50 value of the substances has been found in a MSDS (MSDS Lutensit A-BO 2003). This 96hLC50 value of 10-100 mg/l for Leuciscus idus (small fresh-water cyprinoid fish) shows that the sulfosuccinate is harmful to aquatic organisms. Table 7.2: Environmental data for di(2-ethylhexyl) sulfosuccinate (Chemfate database 2004; HSDB database 2004; MSDS Lutensit A-BO 2003).
7.1.5 Propylated aromaticsAs presented in Appendix F: "Identified alternatives", Rütgers Kureha Solvents produces different propylated aromatics (naphthalenes and biphenyls), which can be used as water repelling agents for different applications, such as corrosion protection systems, marine paints, resins, printing inks, coatings, electrical, electronical and mechanical applications (Personal communication RKS 2004). The presented propylated aromatic products are all colourless liquids with a boiling point at about 300 °C. Their flash point lies all about 140 °C. The substances have a very low solubility in water. Common for the substances is that none of them is classified as hazardous substances. Figure 7.4: The chemical structure of Ruetasolv DI (CAS No. 38640-62-9) – to the left, and Ruetasolv TTPN (CAS No. 35860-37-8) – to the right.
Figure 7.5: The chemical structure of Ruetasolv BP 4103 (CAS No. 25640-78-2) – to the left, and Ruetasolv BP 4201 (CAS No. 69009-90-1) to the left. Both products are mixtures of isomers.
7.1.5.1 Health effectsAccording to the MSDS of one of the products (MSDS Ruetasolv DI 2003), the acute toxicity towards rats (LD50) of the substance bis(1-methylethyl)-naphthalene (CAS No. 38640-62-9) is higher than 3900 mg/kg, which means no classification of the substance as harmful. Neither irritating nor sensitising effects of the substance have been identified. Furthermore, the substances have been found without mutagenic effects in the Ames test and in vivo in mice, no carcinogenic effects in rats, teratogenic effects in mice or foetotoxicity in mice. Somewhat conflicting information was found for the substance in the HSDB database, where naphthalenes in general are found to be possibly irritating to eyes, skin and mucous membrane. Repeated exposure may cause symptoms like headache and vomiting. Prenatal exposure to naphthalene has been harmful to the unborn child. According to IARC (International Agency for Research on Cancer) naphthalene is possibly carcinogenic to humans (Group 2B). Ruetasolv DI (bis(1-methylethyl)naphthalene) is belonging to the chemical group of napthalenes, but no carcinogenicity has been identified for this specific naphthalene compound (HSDB database 2004). The substances diisopropyl-1,1'-biphenyl (CAS No. 69009-90-1) and (1-methylethyl)-1,1'-biphenyl (CAS No. 25640-78-2) can according to the MSDS cause irritation of the skin at long-term exposure. Furthermore, irritation of the mucous membrane of the eyes is possible. No sensitising effect is known for the substance (MSDS Ruetasolv BP 4201 2003; MSDS Ruetasolv BP 4103 2004). The HSDB database confirms that (1-methylethyl)-1,1'-biphenyl is an irritant of eyes, nose, throat, mucous membrane and the respiratory tract. At repeated skin contact the substance may produce skin sensitisation or dermatitis. Central nervous system (CNS) damage, liver and kidney damage have been reported as chronic effects. Oral LD50 values for rats of 4.7 and 8.5 g/kg indicate that the chemical has low acute toxicity and does not need a classification as harmful (HSDB database 2004). The substance tris(1-methylethyl)naphthalene (CAS No. 35860-37-8) has according to the MSDS no irritating or sensitising effects (MSDS Ruetasolv TPPN 2004). 7.1.5.2 Environmental effectsRuetasolv DI (bis(1-methylethyl)naphthalene; CAS No. 38640-62-9) is according to the MSDS easily biodegradable and has a low bioaccumulation potential. The acute aquatic toxicity for fishes is measured above the saturation point. After 96 hours the concentration of 0.5 mg/L (above saturation) showed no lethal effect. Therefore, the substance has no acute toxic effect in the investigated fish species. Conflicting information was found in the HSDB and Chemfate databases. According to this information (see Table 7.3), the substance bis(1-methylethyl)naphthalene only slowly biodegrades and has a tendency to bioaccumulate. In the Ecotox database a LD50 value of 2 ml/kg (14 days) for yellow tails (Seriola quinqueradiata) is reported for bis(1-methylethyl)naphthalene. As can be seen from Table 7.3 that environmental data about the substances ability to bioaccumulate was found for three of the propylated aromatics in the Chemfate database. All three substances have an octanol/water partition coefficient (log KOW) higher than three, and since the bioconcentration factor (BCF) for the substances is greater than 100, the substances are considered to be potential bioaccumulative. Furthermore, the biphenyl moiety seems to be easily biodegradable, whereas the naphthalene moiety only slowly biodegrades. The LC50 value for the biphenyl compound shows that the compound is acutely toxic to aquatic organisms. Table 7.3: Environmental data for some of the propylated aromatics. (Chemfate database 2004; HSDB database 2004).
No other ecotoxicological information was found for the other products either in databases or given in the MSDS. 8 Discussion and conclusions
Different reports have recently mapped the use of PFOS-related compounds in Europe and in the particular countries. These are:
Our survey of the use of PFOS- and PFOA-related substances and its alternatives is based on information in the reports mentioned above and on additional information mainly gathered during the project period by searching databases, contacting individuals and companies, mainly Danish. Our survey is described in Chapter 4 and summarised in Table 8.1: Table 8.1: Summary of the use areas of PFOS and related substances and their alternatives.
The overall picture is that the formerly most used PFOS-related substances in general have been phased out and substituted with other fluorinated surfactants, such as C6-fluorotelomer-based products or short-chained perfluorinated compounds like PFBS (perfluorobutane sulfonate). The reason for this continuous use of fluorinated compounds is that polyfluorinated surfactants have superior properties compared to other and less expensive surfactants. 8.1 Discussion of the different use areas for PFOS-related compoundsToday, the former major use areas for PFOS-related compounds like impregnation of textiles, leather and carpets as well as impregnation of paper and cardboard seem to be more or less historical as other alternatives are in use today. The Danish survey from 2002 does, however, register use of PFOS-related substances for the impregnation area. This may be explained by the fact that the Danish survey is older than the Swedish and UK surveys. Therefore, the use of PFOS-related compounds in impregnating agents in Denmark may already be historical or the use can be assumed to disappear in the near future. The substances used instead of the PFOS-related compounds for impregnation products seem to be other highly fluorinated compounds like PFBS or telomer-based polymers. Silicone based products have also been mentioned as alternatives to PFOS-related compounds. For impregnation of paper and cardboard the Norwegian survey claims that other highly fluorinated compounds are not used instead, as all PFAS-based products seem to have gone out of use. However, telomer-based substances are used as alternatives, as stated by DuPont. Cleaning agents have been one of the larger use areas for PFOS-related compounds, but this is an area, where the use of PFOS-related substances seems to be more or less historical. The Norwegian survey states that in some specific cleaning products for cleaning of glass PFAS-substances are still used. The Danish survey has registered a use of PFOS-related substances for cleaning agents, but also states that substitution has taken place within cleaning products for industrial use. As the Danish survey is older than the other surveys, the use of PFOS-related compounds in cleaning agents in Denmark may also already be historical or the use can be assumed to disappear in the near future. None of the surveys have identified, which alternative compounds that are used instead of PFOS or PFAS-based compounds, but the alternatives may be non-PFAS-based products as the Norwegian survey has examined the use of all PFAS-based products. Waxes and floor polishes have been and still are one of the larger use areas for PFOS-related compounds. Some substitution has been carried out, where fluorinated compounds of lower chain length have been used, e.g. C4-perfluorinated compounds, fluorotelomer-based substances or fluorinated polyethers have been used. In some waxes and floor polishes PFAS compounds are still used. In fact, PFOS-related surfactants are still today allowed in small concentrations in the Swan eco-labelled film-forming floor care products. The argument is that these surfactants are difficult to replace. The paint and varnish area has formerly been one of the major use areas for PFOS-related compounds. Even though the Danish survey has identified a large use of PFOS-related compounds in paint, ink and varnish products, the survey also states that the substitution of PFOS-compounds by and large has been carried out in Denmark. This is in line with the other surveys that also claim that the paint and varnish industry no longer uses PFOS-related compounds. The substances used instead are other highly fluorinated compounds with shorter chain length (like PFBS), telomer-based substances or non-fluorinated compounds such as propylated aromatics, aliphatic alcohols, silicones or sulfosuccinates. According to the information received it may be difficult in short term to avoid fluorinated surfactants for very special purposes, where an extreme low surface tension is needed. In other cases other non-fluorinated surfactants may do the job, but reformulation of the products may be necessary. Fire-fighting foams have been one of the quantitatively smaller use areas of PFOS-related compounds but a use area with a high risk for environmental exposure. Today, the fire-fighting foams on the market are PFOS-free, and have been replaced by fluorotelomers, predominantly C6-telomers. However, information received indicates that fluoroalkyl compounds of higher chain length also occur as impurities in the fire-fighting foams. As fire-fighting foams have a long shelf life, there may still be foams in stock, which are based on PFOS-compounds. However, an important factor within this area is that many fire-fighting foam users are using fluorine-free synthetic foams or protein foams for training exercises. The photographic industry is one of the smaller use areas of PFOS-related compounds. A shift to digital techniques has reduced the use of PFOS-related compounds drastically (with about 80%), but for the remaining uses no alternatives have been identified so far. The alternatives that have replaced the PFOS-related compounds are fluorotelomers based products (C3 or C4) and non-fluorinated compounds like hydrocarbon surfactants and silicone products. Manufacturing of semiconductors is also one of the smaller use areas of PFOS-related compounds. PFOS-related compounds are still being used, but new techniques are being developed within this field that do not require PFOS-related compounds. However, it may take up to 5 years before these PFOS-free techniques are ready for commercial use. Hydraulic oils for the airplane industry are also one of the smaller areas, where PFAS-related compounds are still being used, as fluorotelomers have been tested as alternatives, but cannot be used. PFAS-free alternatives are not yet available and it may take up to ten years before a PFAS-free solution has been found. Metal surface treatment – especially chromium plating and chromating – covers some of the larger use areas of PFOS-related compounds. For some purposes – decorative chromium plating – solutions that do not require PFOS-compounds have been identified, but for chromating and hard chromium plating the industry is still working on alternatives. Plumbing is one of the minor use areas of PFOS-related compounds. PFOS-related compounds are being used in fluxing agents in the process of plumbing with leaded soldering tin. However, as lead is being phased-out in electrical and electronic equipment from 2006, this will automatically end the use of PFOS-related compounds. Today the largest use areas of PFOS-based compounds therefore seem to be:
For manufacturing of semiconductors no PFOS-free techniques are available yet and PFOS-free solutions do not seem to be found in the near future. The PFOS-related compounds are not found in the semiconductor products, but are only used as processing chemicals, which means that the spreading of PFOS-based chemicals within this area is more controllable. The same can be concluded for the use of PFOS-related chemicals within metal surface treatment and the photographic industry. The PFOS-related compounds will not be a part of the end product, for which reason the emission of PFOS-related chemicals to the environment to some extent can be controlled. In contrast, the PFOS-related chemicals are ingredients of the cleaning agents, the waxes and the floor polishes. In these specific use areas the emission of PFOS-related chemicals to the environment is much more diffuse and much more difficult to control. As most of the cleaning agent area today are PFOS-free, the most important area with respect to emission of PFOS-related compounds today seems to be the use area of waxes and floor polishes. 8.2 Discussion of the use of PFOA and saltsPFOA and its salts are used as a processing aid in the manufacture of fluoropolymers such as polytetrafluoroethylene, a process not occurring in Denmark. DuPont has for the last 30 years investigated possible alternatives to PFOA as processing aid in the production of fluoropolymers. Several fluorohydrocarbons have been tested, but the results showed that the presence of hydrogen in the surfactant resulted in problems with the polymerisation. Supercritical carbon dioxide as reaction medium has also been tested in a pilot-scale facility. However, DuPont does not expect that this process will ever evolve into a technology that would have the capability to totally replace the current water-based polymerisation process. The conclusion so far from testing over the last 30 years is that there are no viable alternatives to PFOA. In Denmark only the ammonium salt of PFOA is found in very small quantities in a few products. Other PFOA-related substances were not found via a search in the Danish Product Register. The PFOA ammonium salt was registered for fluxing agents (used in plumbing) and in a primer and topcoat used for fluoroplastic coating. The use in fluxing agents is very limited and will cease, when leaded plumbing are banned from the year 2006, as lead-free plumbing does not require the use of these fluxing agents. 8.3 Release to the EnvironmentEmissions to the environment (air, soil and water) of PFOS-related and other perfluorinated substances may happen directly from the production and processing plants. However, most important is releases during use (indoor or outdoor) and disposal of products containing these substances. Environmental sources of fluorinated telomers are currently unknown but these substances may be released at manufacturing of perfluorinated compounds and at the decomposition of polymeric materials and consumer products that incorporate telomers. PFOS-related chemicals are not the only environmental problem. Other PFAS-based chemicals such as PFHxS and PFBS with shorter chain length and perfluorinated carboxylic acids (PFCA) including PFOA are also found in the environment. Furthermore, the fluorotelomers, which in some cases are used as alternatives to PFOS-based compounds are also found in the environment and they seem to be long-range transported and degraded to PFOA and other PFCAs in the environment Impregnation products, fire-fighting foams, the photographic industry and hydraulic oils within the airplane industry are still use areas that contribute to the total PFOS/PFOA concentration in the environment as these uses predominantly seem to use fluorotelomers or PFAS compounds with shorter chain length (like PFBS) as alternatives to the former PFOS-compounds. However, the photographic industry does not seem to be the biggest problem, as this industry represents a smaller use area, and as the PFAS compounds are not a part of the final products. No information has been found on the size of the hydraulic oil use area. However, this area is not expected to be that large. The PFAS emissions from the photographic industry are more controllable compared to the diffuse emissions that the fire-fighting foams, the impregnating products and the hydraulic oils represent. The fire-fighting foams represent a huge leak to the environment during use. With regard to the hydraulic oils, most of the oils can be assumed to be collected at regular check-ups and repairs, but some of the oil may also be leaked to the environment by accident. The use of impregnation agents to protect domestic products such as clothes and carpets may also be one of the most important exposure ways for the human population. Measurements have confirmed that PFOA and PFOS can be found in vacuum cleaner dust in private households. The most important human exposure may be through inhalation of air and the dust in private homes and offices. On the other hand, fire-fighting foams represent the area where there is the largest risk of a huge leak directly to the environment. Studies have shown that the fluorinated surfactants used in fire-fighting foams and their degradation products may occur in ground water and surface waters five or more years after its last known use. It is important to notice that fire-fighting foams made from fluorinated surfactants is the only technology, which can quickly and effectively extinguish fires from highly combustible and flammable materials like oils and gasoline. Therefore, the only real alternative to the PFOS-based fire-fighting foams is the other fluorosurfactants. However, a shift to the non-fluorinated training foams is a way forward to avoid unnecessary emissions to the environment of the fluorinated compounds. 8.4 Health and environmental aspects of PFOS, PFOA and other polyfluorinated substancesPerfluoroalkylated substances are present in the environment primarily in the form of the most stable PFOS and PFOA, which are the final degradation products of various perfluorooctyl compounds. Recently, when the many PFOS uses have been phased out, the environmental concentrations of other related substances such as PFBS, PFHxS and PFNA have been increasing. PFOS, PFOA and other polyfluorinated compounds are now considered as global environmental contaminants. They have been found in indoor air, outdoor air, soil, ground water, surface waters and even at 1000 m depth in the Pacific Ocean. Perfluorinated compounds are widely distributed in wildlife. PFOS has been detected in blood and liver samples from various species of aquatic animals (seal, otter, sea lion, dolphin, polar bear, mink), birds, fish and amphibians. Some samples also contained other related substances such as PFOSA, PFHxS, PFNA and PFOA. Some perfluorinated substances have even been found in blood and liver samples from the general human population. Whereas PFOS and PFOA and perfluorinated acids with longer alkyl chain are considered to be bioaccumulative in wildlife and human tissues, perfluorinated acids with fully fluorinated chain lengths of C5 and below do not seem to accumulate significantly. The occurrence of PFOS and PFOA in the wildlife in remote areas such as the Arctic is puzzling. The binding to water and the low volatility make it less likely that PFOS and PFOA will be spread long-range with the air by the "grass-hopping" and cold condensation mechanisms as persistent organic pollutants (POPs). However, the prevailing hypothesis is that long-range transport to the arctic occurs via volatile precursors of both PFOS and PFOA, with subsequent degradation to these stable products. Volatile PFOS-precursors are a.o. MeFOSE and EtFOSE. It has been hypothesized that fluorotelomer alcohols (FTOH) may be long-range transported and hereby reach remote arctic areas, where they can degrade to the more stable PFOA. Atmospheric lifetime of short chain FTOHs, as determined with its reaction with OH-radicals, is approximately 20 days making the molecule able to travel about 7000 km. Therefore, they may be long-range transported and hereby reach remote areas, where they can degrade to PFOA. On the other hand products containing perfluorinated substances may also be used for example in fire-fighting foam at bases in remote areas. Recently a hypothesis has also been brought forward that ocean current transport may in part explain the presence of PFOS and PFOA in the arctic. Studies show that PFOS and other perfluorinated chemicals are readily absorbed in the body. Both PFOA and PFOS are considered to be metabolically inert, and other perfluorocarboxylic acids and perfluorosulfonic acids do have similar properties, which means that their functional derivatives may be transformed to the parent compound. For example, the fluorotelomer alcohol 8:2 FTOH is transformed to PFOA in rats. Once absorbed in the body, PFOA and PFOS may bind to proteins and may accumulate in various body tissues, including blood and liver; for PFOS also in testis and brain. The half-life of PFOA is about 2-4 years in humans and 1 month in monkeys. The half-life of PFOS is longer than for PFOA – about 200 days in monkeys. The half-life in humans was not found. The acute lethal toxicities of PFOS and PFOA are moderate corresponding to a classification as harmful, if swallowed. PFOS is more toxic than PFOA, and the toxicity of related substances increases with the length of the alkyl chain. The liver is the primary target organ for perfluorinated compounds, and these chemicals cause peroxisome proliferation in the rodent liver as well as induction of various enzymes involved in lipid metabolism. PFOS seems to be more active than PFOA concerning this effect but again PFDA with a longer alkyl chain is even more active. Toxic effects have been reported, such as induction of fatty liver and uncoupling of the mitochondrial respiratory chain. PFOA also affects the serum levels of various hormones, i.e. reducing testosterone and increasing estradiol in rats. Thus, it is considered an endocrine disruptor. Although the fluorinated chemicals do not seem to be mutagenic, PFOA has induced testis cancer, and PFOS and EtFOSE have induced liver cancer in experimental animals. USEPA classifies PFOA as a carcinogen in animals. PFOS and PFOA cause developmental effects, including reduction of foetal weight, cleft palate, oedema, delayed ossification of bones, and cardiac abnormalities. However, the structural abnormalities were only found in the highest PFOS dose groups, where significant reductions of weight gain and food consumption were also observed in the pregnant dams. Thus the relevance may be questioned. Other tested PFAS (PFBS and PFHxS) had no significant effect on reproduction even at high doses. In general, the information in open literature about the toxicology of the perfluorinated compounds is rather sparse, and it will take some time and efforts, before sufficient information for evaluation of the full impact of the present levels in humans is available. The experience from the work environment has not indicated any important adverse health effects among exposed workers, besides a retrospective cohort mortality study of a perfluorooctanesulfonyl fluoride (PFOSF) production workforce, which reported an excess of bladder cancer at high-exposure jobs. With respect to aquatic toxicity PFOS is considered to be moderately acute toxic and slightly chronically toxic to aquatic organisms. PFOA is practically non-toxic. EtFOSA is slightly acute toxic to daphnids. There seems to be large species difference in the biological response, because PFOS was three orders of magnitude more toxic to the aquatic midge Chironomus tentans than to most other aquatic organisms. The scarce database indicates a need for further studies. 8.5 Health and environmental aspects of the identified non-polyfluorinated alternativesNot many alternatives to perfluorinated compounds have been identified during this project. The same was the case in the different surveys from Holland, the UK, Norway and Sweden, which also have investigated this subject. The mentioned alternatives were primarily silicone-based products or hydrocarbon based surfactants. The area, where most non-fluorinated alternatives were identified within this project, was the paint and varnish area. In this area silicone-based products and hydrocarbon surfactants (such as aliphatic alcohols, sulfosuccinates, and propylated aromatics) are also used as alternatives, but in general it seems that these alternatives cannot be used, where extreme demands regarding low surface tension are needed. In these cases fluorinated surfactants seem to be the only substances that can reach the very low surface tension levels. These specific alternatives have been investigated closer for their environmental and health effects, but in general very little information about the specific products was available. Most information was found in the material safety data sheets for the specific substances, supplemented with very few data from different search databases as Toxnet, HSDB, ECOTOX and Chemfate. As no alternative for PFOA is found, it is impossible to review the health and environmental aspects of these substances. However, PFOA use is mainly limited to be a polymerisation aid for fluoropolymers. 8.5.1 Environmental effectsIn general the alternative hydrocarbon surfactants seem to be readily biodegradable. The fatty alcohol polyglycolether sulfate is readily biodegradable and does not seem to be toxic to aquatic organisms. The sulfosuccinates are likewise easily biodegradable, do not seem to bioconcentrate, but are harmful to aquatic organisms. The biphenyls and the naphthalene derivatives are potentially bioaccumulative. The biphenyl moiety seems to be easily biodegradable, whereas the naphthalene moiety only slowly biodegrades. The sparse information suggests that the biphenyls are acutely toxic to aquatic organisms, whereas the naphthalene has no acute toxic effects in the investigated fish species. Of the investigated alternatives, the silicone polymers seem to have the most adverse environmental effects. The specific compound investigated is classified as environmentally dangerous (R51/53 "Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment"), as the substance is toxic to aquatic organisms and is bioaccumulative. 8.5.2 Health effectsIn general very sparse information about the health effects of the alternative substances is found. Therefore, most of the information is based on the material safety data sheets. The fatty alcohol polyglycolether sulfate is acutely toxic by ingestion but is not considered to be irritating. The sulfosuccinates are irritants to eyes, skin and the respiratory system. Dermatitis has been observed as a long-term effect as well as CNS depression. The substance is mildly harmful to toxic, if swallowed. The naphthalene- and biphenyl compounds are irritating substances, and the biphenyl compounds may produce skin sensitisation or dermatitis. Furthermore, one of the biphenyl compounds is known to cause CNS damage as well as liver and kidney damage. The parent compound naphthalene is classified as possible carcinogenic in humans (IARC Group 2B). However, no carcinogenicity has been identified for the specific naphthalene derivatives used as alternatives to perfluorinated compounds. The silicone polymers are irritating substances and are harmful by inhalation. 8.6 ConclusionsThe results of the different surveys of PFOS and other perfluoroalkylated compounds indicate that the former, and for some areas present, use of these substances has generated a global environmental contamination problem. A recent Nordic screening investigation has shown that the pollution in Denmark with these chemicals seems to be comparable with other industrialised countries but more studies are needed to really describe the situation and trends. In most of these areas the industry is trying to find suitable alternatives. For some major use areas PFOS-related substances have already been substituted and diminished the problems. Today, PFOS-related substances are primarily used in areas, where it has not been possible yet, clearly to identify alternatives to the PFOS-related substances. The environmental assessments of the non-fluorinated alternatives show that in most cases these alternatives are not of a similar environmental concern as they are easily biodegradable. However, the silicone polymers may be more problematic as they are persistent, bioaccumulative and at the same time are toxic to aquatic organisms. The survey of the use of PFAS-related substances shows that it is possible to reduce the use by simple means. The use of non-fluorinated fire-fighting foams for training purposes is a good example of, how it is easily possible to eliminate unnecessary use of fluorinated surfactants. However, some fire-fighting facilities are still using fluorinated fire-fighting foams for both training exercises and "real fire" situations. Furthermore, it is likely that many of the facilities using fire-fighting foams may have stocks of earlier generation of fire-fighting foams still being used. Although the use of PFOS-related substances has been reduced, the use of other perfluorinated substances is still considerable, as the PFOS-related chemicals in many cases have been replaced with other perfluorinated compounds with shorter chain length like PFBS or telomer-based substances with an alkyl chain length of C6 or below. Furthermore, the used telomers are not limited to C6-compounds, as the telomerisation process yields a mixture of fluorotelomers with different chain length. Therefore products based on fluorotelomer compounds also contain some compounds with longer chains, even though the telomer mixture predominantly is based on C6. The large use of C6-telomers in products like fire-fighting foams and impregnating agents may still be an environmental problem. A shift to telomer compounds instead of perfluoroalkylated substances (PFAS) does not eliminate the problems with PFAS, but only reduces the problem, as PFAS may still be formed in the environment due to the part of the telomers containing the longer than C6-chain lengths. Even though the short-chained fluorinated compounds (of an alkyl chain length of C5 or less) have been identified as less bioaccumulative and toxic, they are still substances that will persist in the environment for decades. The toxicity and ecotoxicity of the shorter chained fluorinated compounds are yet to be examined, and many studies are underway. Nevertheless, the implications for human health and the environment are unclear, and if adverse effects are discovered in the future, it will not be possible to "call back" the already emitted substances. 9 References3M. The science of organic fluorochemistry. February 5, 1999. 3M webpage. Found at www.3m.com in 2004. About, Definition of Telomer, CRC Press LCC. Found at www.composite.about.com/library/glossary in May 2004. Alexander BJ, Olsen GW, Burris JM, Mandel JH, Mandel JS. Mortality of employees of a perfluorooctanesulfonyl fluoride manufacturing facility. Occup Environ Med 2003; 60: 722-729. APME. OSPAR factsheet APFO, Brussels, 2002. APME. Further data submission to OSPAR Secretariat, 2004. Austin ME, Kasturi BS, Barber M, Kannam K, MohanKumar PS, MohanKumar SMJ. Neuroendocrine effects of perfluorooctane sulfonate in rats. Environ. Health Perspec 2003; 111: 1485-1489. Berger U, Järnberg U, Kallenborn R. Perfluorinated alkylated substances (PFAS) in the European Nordic environment. Organohalogen Compounds 2004; 66: 4046-4052. Berthiaume J, Wallace KB. Perfluorooctanoate, perfluorooctanesulfonate, and N-ethyl perfluorooctanesulfonamido ethanol; peroxisome proliferation and mithocondria biogenesis. Toxicol Lett 2002; 129: 23-32. Biegel LB, Hazard characterization for human health C8 exposure CAS registry no. 3825-26-1. 1997. DuPont Biegel LB, Liu RCM, Hurtt ME, Cook JC. Effects of ammonium perfluorooctanoate on Leydig cell function: in vitro, in vivo and ex vivo studies. Toxicol Appl Pharmacol 1995; 134: 18-25. Biegel LB, Hurtt ME, Frame SR, O'Connor JC, Cook JC. Mechanisms of extrahepatic tumor induction by peroxisome proliferators in male CD rats. Toxicol Sci 2001; 60: 44-55. Boudreau TM, Wilson CJ, Cheong WJ, Sibley PK, Mabury SA, Muir DCG, Solomon KR. Response of the zooplankton community and environmental fate of perfluorooctane sulfonic acid in aquatic microcosms. Environ Toxicol Chem 2003: 22(11): 2739-2745. Boulanger B, Vargo J, Schnoor JL, Hornbuckle KC. Detection of perfluorooctane surfactants in Great Lakes water. Environ Sci Technol 2004: 38: 4064-4070. Burris JM, Lundberg JK, Olsen GW, Simpson C, Mandel J. Interim Report No. 2. Determination of serum half-lives of several fluorochemicals. 3M, 2002 (cit. from Olsen GW et al. 2003b) Buckeye Fire Equipment. Technical bulletin Bueckeye Fire Fighting Foam Products and the Environment – Miscellaneous. Found at www.buckeye.com. Butenhoff J, York R, Seacat A, Luebker D. Perfluorooctanesulfonate-induced perinatal mortality in rat pups is associated with a steep dose-response. Toxicologist 2002; 66(1-S): 25 (abstract). Butenhoff JL, Gaylor DW, Moore JA, Olsen GW, Rodricks J, Mandel JH, Zobel LR. Characterization of risk for general population exposure to perfluorooctanoate. Regul Toxicol Pharmacol 2004; 39: 363-380. Caliebe C, Gerwinski W, Hühnerfuss H, Theobald N. Occurrence of perfluorinated organic acids in the water of the North Sea. Organohalogen Compounds 2004; 66: 4074-4078. Case MT, York RG, Christian MS. Rat and rabbit oral developmental toxicology studies with two perfluorinated compounds. Int J Toxicol 2001; 20: 101-109. CATT. Final ammonium perfluorooctanoate (C8) assessment of toxicity team (CATT) report. West Virginia Department of Environmental Protection. August, 2002. Chemfate database. Found at http://www.syrres.com/esc/chemfate.htm. Search performed in June 2004. Clark LC, Becattini F, Kaplan S, Obrock V, Cohen D, Becker C. Perfluorocarbons having a short dwell time in the liver. Science 1973; 181: 680-682 Cook JC Murray SM, Frame RS, Hurtt ME. Induction of Leydig cell adenomas by ammonium perfluorooctanoate: a possible endocrine-related mechanism. Toxicol Appl Pharmacol 1992; 1134: 209-217. Corsolini S, Kannan K. Perfluorooctanesulfonate and related fluorochemicals in several organisms including humans from Italy. Organohalogen Compounds 2004; 66: 4079-4085. Dimitrov S, Kamenska V, Walker JD, Windle W, Purdy R, Lewis M, Mekenyan O. Predicting the biodegradation products of perfluorinated chemicals using Catabol. SAK QSAR Environ Res 2004; 15: 69-82. Dinglasan MJA, Ye Y, Edwards EA, Mabury SA. Fluorotelomer alcohol biodegradation yields poly- and perfluorinated acids. Environ Sci Technol 2004; 38: 2867-2864. Directive 2002/95/EC of January 27 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment. Official Journal of the European Union, 13.2.2003. DuPont Telomer Information. Found at www.telomerinform.dupont.com in May 2004. DuPont Website. Found at www.dupont.com. Ellis DA, Martin JW, De Silva AO, Mabury SA, Hurley MD, Andersen MPS, Wallington TJ. Degradation of fluorotelomer alcohols: a likely atmospheric source of perfluorinated carboxylic acids. Environ Sci Technol 2004; 38: 3316-3321. Ellis DA, Mabury SA, Martin JW, Muir DCG. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 2001; 412: 321-324. Engelund B. et al. Kortlægning og sundhedsmæssig vurdering af kemiske stoffer i skoplejemidler. Title in English: Mapping and toxicological evaluation of chemical substances in shoe care products. 2004. To be published by the Danish EPA, probably in the spring 2005. ENVIRON International Coporation. Product-specific exposure assessment and risk characterization for perfluorooctanoate in select consumer articles. Prepared by ENVIRON for DuPont Wilmington, Delaware, May 2004. Environmental Working Group, PFCs – A Family of Chemicals that Contaminate the Planet, March 2003. Found at www.ewg.org/reports/pfcworld/. Environmental Working Group (2004a). EPA moves to break industry logjam on Teflon chemical studies – Agency to conduct research to identify sources of Teflon chemicals in humans and the environment. June 24, 2004. Found at http://www.ewg.org/issues/PFCs/20040624/index.php. Environmental Working Group (2004b). EPA alleges DuPont failed to report information about the hazards of C8. Payne D. Pittsburgh News, July 9, 2004. Found at http://www.ewg.org/news/story.php?id=2829. Fire-fighting Foam Coalition. AFFF Fire fighting foams. US EPA public docket. Presentation to the US EPA, 18 September 2001. Fluoride Action Network. Timeline for PFOS and PFOA perfluorinated chemicals, The Pesticide Project. Found at www.fluorideaction.org/pesticides/effect.PFOS.class.timeline.htm in May 2004. Fluorous Technologies, Inc. FTI Primer: A short introduction to fluorous techniques for the synthesis and separation of organic molecules. Yeske P. Found at www.fluorous.com in July 2004. Funderberg AC. Making Teflon stick. Invention & Technology: Summer 2000 Volume 16, Number 1. Giesy J, Jones P. Toxicological perspectives on perfluorinated compounds in avian species. Organohalogen Compounds 2004; 66: 4086-4089. Giesy JP, Kannan K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ Sci Technol 2001; 35: 1339-1342. Giesy JP, Kannan K. Perfluorochemical surfactants in the environment. Environ Sci Technol 2002; 36: 147A-152A. Griffith FD, Long JE. Animal toxicity studies with ammonium perfluorooctanoate. Am Ind Hyg Assoc J 1980; 41: 576-583. Guruge K, Taniyasu S, Yamashita N, Miyazaki S, Yamanaka N, Wijeratna S, Seneviratne H. Perfluorinated compounds in human serum and seminal plasma from an urban and rural population in Sri Lanka. Organohalogen Compounds 2004; 66: 4004-4008. Gurunge KS, Taniyasu S, Miyazaki S, Yamanaka N, Yamashita N. Age dependent accumulation of perfluorinated chemicals in beef cattle. Organohalogen Compounds 2004; 66: 4029-4034. Hansen KJ, Clemen LA, Ellefson ME, Johnson HO. Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ Sci Technol 2001; 35: 766-770. Hansen KJ, Johnson HO, Eldridge JS, Butenhoff JL, Dick LA. Quantitative characterization of trace levels of PFOS and PFOA in the Tennessee River. Environ Sci Technol 2002; 36: 16681-1685. Harada K, Saito N, Sasaki K, Inoue K, Koizumi A. Perfluorooctane sulfonate contamination of drinking water in the Tama River, Japan: Estimated effects of resident serum levels. Bull Environ Contam Toxicol 2003; 71: 31-36. Harada K, Saito N, Inoue K, Yoshinaga T, Watanabe T, Sasaki S, Kamiyama S, Koizumi A. The influence of time, sex and geographical factors on levels of perfluorooctane sulfonate in human serum over the last 25 years. J Occup Health 2004; 46: 141-147. Havelund S., Kortlægning af perfluorooktanylsulfonat og lignende stoffer i forbrugerprodukter – fase 1, COWI Rådgivende Ingeniører A/S, Miljøprojekt Nr. 605, 2001, Miljøstyrelsen (in Danish). Title in English: Survey of perfluorooctanylsulfonate and similar substances in consumer products – phase 1. Havelund S., Kortlægning af perfluorooktanylsulfonat og lignende stoffer i forbrugerprodukter – fase 2, COWI Rådgivende Ingeniører A/S, Miljøprojekt Nr. 691, 2002, Miljøstyrelsen (in Danish). Title in English: Survey of perfluorooctanylsulfonate and similar substances in consumer products – phase 2. Heckster FM, Laane RWPM, Voogt P de. Environmental and toxicity effects of perfluoroalkylated substances. Rev Environ Contam Toxicol 2003; 179: 99-121. Hekster FM, Voogt P de, Pinjenburg AMCM, Laane, RWPM. Perfluoroalkylated substances – Aquatic environmental assessment. Report RIKZ/2002.043, 1 July 2002. National Institute for Coastal and marine Management/RIKZ, 2002. Hoff PT, Van Dongen W, Esmans EL, Blust R, De Coen WM. Evaluation of the toxicological effects of perfluorooctane sulfonic acid in the common carp (Cyprinus carpio). Aquatic Toxicol 2003; 62: 349-359. Hoff PT, Scheirs J, Van de Vijver K, Van Dongen W, Esmans EL, Blust R, De Coen WM. Biochemical effects evaluation of perflurooctane sulfonic acid-contaminated wood mice (Apodemus sylvaticus). Environ Health Perspec 2004; 112: 681-686. Hogue C. Fluorotelomers to the test. Chemical & Engineering News, p. 6, July 5, 2004. HSDB database (Harzardous Substances Data Bank). Found at http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB. Search performed June, 2004. Hu W, Jones PD, Upham BL, Trosko JE, Lau C, Giesy JP. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci 2002; 68: 429-436. Hu W, Jones PD, , De Coen W, King L, Fraker P, Newsted J, Giesy JP. Alteratikons in cell membrane properties caused by perfluorinated compounds. Ikeda T, Aiba K, Fukuda K, Tanaka M. The induction of peroxisome proliferation in rat liver by perfluorinated fatty acids, metabollically inert derivatives of fatty acids. J Biochem 1985; 98: 475-482 Inoue K, Okada F, Ito R, Kato S, Sasaki S, Nakajima S, Uno A, Saijo Y, Sata F, Yoshimura Y, Kishi R, Nakazawa H. Perfluorooctane sulfonate (PFOS) and related perfluorinated compounds in human maternal and cord blood samples: assessment of PFOS exposure in a susceptible population during pregnancy. Environ Health Perspec 2004; 112: 1204-1207. Jones PD, Newsted J, Giesy JP. Toxicological perspectives on perfluorinated compounds. Organohalogen Compounds 2003a; 62: 311-315. Jones PD, Hu W, De Coen W, Newsted J, Giesy JP. Binding of perfluorinated fatty acids to serum proteins. Environ Toxicol Chem 2003b; 22: 2639-2649. Juhler S., Giftige Teflonpander (Title in English: Toxic Teflon pans), Article in the Danish Magazine Mad og Sundhed (English: Food and Health), June 13, 2003. Found at http://www.madogsundhed.dk/12_presseresume/9235mos1.htm. Kallenborn R, Berger U, Järnberg U, Dam M, Glesne O, Hedlund B, Hirvi J-P, Lundgren A, Mogensen BB, Sigurdsson AS. Perfluorinated alkylated substances (PFAS) in the Nordic environment. Nordic Council of Ministers, September 2004. Kannan K, Koistinen J, Beckmen K, Evans T, Gorzelany JF, Hansen KJ, Jones PD, Helle E, Nyman M, Giesy JP. Accumulation of perfluorooctane sulfonate in marine mammals. Environ Sci Technol 2001a; 35: 1593-1598. Kannan K, Franson JC, Bowerman WW, Hansen KJ, Jones PD, Giesy JP. Perfluorooctane sulfonate in fish-eating water birds including bald eagles and albatrosses. Environ Sci Technol 2001b; 35: 3065-3070. Kannan K, Hansen KJ, Wade TL, Giesy JP. Perfluorooctane sulfonate in oyster, Crassostrea virginica, from the Gulf of Mexico and the Chesapeake Bay, USA. Arch Environ Contam Toxicol 2002; 42: 313-318. Kannan K, Choi J-W, Iseki N, Senthikumar K, Kim DH, Masunaga S, Giesy JP. Concentrations of perfluorinated acids in livers of birds from Japan and Korea. Chemosphere 2002b; 49: 225-231. Kannan K, Corsolini S, Falandysz J, Fillmann, Kumar KS, Loganathan BG, Mohd MA, Olivero J, Van Wouwe N, Yang JH, Aldous KM. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 2004; 38: 4489-4495. Kannan K, Corsolini S, Falandysz J, Oehme G, Focardi S, Giesy JP. Perfluorooctanesulfonate and related fluorinated hydrocarbons in marine mammals, fishes, and birds from coasts of the Baltic and the Mediterranean Sea. Environ Sci Technol 2002c; 36: 3210<-3216. Kannan K, Newsted J, Halbrook RS, Giesy JP. Perfluorooctanesulfonate and related fluorinated hydrocarbons in mink and river otters from the United States. Environ Sci Technol 2002d; 36: 2566-2571. Kannan K, Giesy JP. Global distribution and bioaccumulation of perfluorinated hydrocarbons. Organohalogen Compounds 2002; 59: 267-270. Kannan K, Kumar KS, Corsolini S, Aldous KM. Perfluoroalkylated compounds in human blood. Organohalogen Compounds 2003; 64: 29-32. Kawashima Y, Kobayashi H, Miura H, Kozuka H. Characterisation of hepatic responses of rat to administration of perfluorooctanoic and perfluorodecanoic acids at low levels. Toxicology 1995; 99: 169-178. Karsten E., Lackrohstoff-Tabellen. Bearbeidet von Olaf Lückert, 10. Aufl., Hannover, Vincentz Verlag, 2000 (In German). Kärrman A, van Bavel B, Järnberg U, Hardell L, Lindström G. Levels of perfluoroalkylated compounds in whole blood from Sweden. Organohalogen Compounds 2004; 66: 4058-4062. Kemikalieinspektionen, Sverige, PFOS-relaterede ämnen – Strategi för utfasning. Juni 2004. Kennedy GL, Butenhoff JL, Olsen GW, O'Connor JC, Seacat AM, Perkins RG, Biegel LB, Murphy SR, Farrar DG. The toxicology of perfluorooctanoate. Crit Rev Toxicol 2004; 34: 351-384. Kissa E. Fluorinated surfactants and repellents, 2.ed. Marcel Dekker, New York, 2001. Kubwabo C, Vais N, Benoit FM. A pilot study on the determination of perfluorooctanesulfonate and other perfluorinated compound in blood of Canadians. J Environ Monit 2004: 6: 540-545. Kudo N, Bandai N, Suzuki E, Katakura M, Kawashima Y. Induction by perfluorinated fatty acids with different carbon length of peroxisomal -oxidation in the liver of rats. Chem-Biol Interact 2000; 124: 119-132. Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem-Biol Interact 2002; 139: 301-316. Kudo N, Kawashima Y. Fish oil-feeding prevents perfluorooctanoic acid-induced fatty liver in mice. Toxicol Appl Pharmacol 1997; 145: 285-293. Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem-Biol Interact 2001; 134: 203-216. Kuklenyik Z, Reich JA, Tully, JS, Needham LL, Calafat AM. Automated solid-phase extraction and measurement of perfluorinated organic acids and amides in human serum and milk. Environ Sci Technol 2004; 38: 3698-3704. Lau C, Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Stanton ME, Butenhoff JL, Stevenson LA.Exposure to perfluorooctane sulfonate during pregnancy in rat and mouse II: Postnatal evaluation. Toxicol Sci 2003; 74: 382-392. Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol 2004; 198: 231-241. Liu RCM, Hurtt ME, Cook JC, Biegel LB. Effect of the peroxisome proliferators, ammonium perfluorooctonate (C(), on hepatic aromatise activity in adult male Crl:CD BR (CD) rats. Fundam Appl Toxicol 1996; 30: 220-228. Luebker DJ, Hansen KJ, bass NM, Butenhoff JL, Seacat AM. Interactions of fluorochemicals with rat liver fatty-acid binding protein. Toxicology 2002; 176: 175-185. MacDonald MM, Warne AL, Stock NI, Mabury SA, Solomon KR, Sibley PK. Toxicity of perfluorooctane sulfonic acid perfluorooctanoic acid to Chironomus tentans. Environ Toxicol Chem 2004; 23: 2116-2123. Martin JW, Muir DCG, Moody CA, Ellis DA, Kwan WC, Solomon KR, Mabury SA. Collection of airborne fluorinated organics and analysis by gas chromatography/chemical ionization mass spectrometry. Anal Chem 2002; 74: 584-590. Martin JW, Mabury SA, Solomon KR, Muir DCG. Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mukiss). Environ Toxicol Chem 2003a; 22: 196-204. Martin JW, Mabury SA, Solomon KR, Muir DCG. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mukiss). Environ Toxicol Chem 2003b; 22(1): 189-195. Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DCG, Mabury SA. Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ Sci Technol 2004; 38: 373-380. Martin JW, Kanan K, Berger U, De Voogt P, Field J, Franklin J, Giesy JP, Harner T, Muir DCG, Scott B, Kaiser M, Järnberg U, Jones KC, Mabury SA, Schroeder H, Simcik M, Sottani, C, van Bavel B, Kärrman A, Lindström G, van Leeuwen S. Analytical challenges hamper perfluoroalkyl research. Environ Sci Technol 2004; 38: 248A-255A. Moody CA, Field JA. Determination of perfluorocarboxylates in groundwater impacted by fire-fighting activity. Environ Sci Technol 1999; 33: 2800-2806. Moody CA, Field JA. Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams. Environ Sci Technol 2000; 34: 3864-3870. Moody CA, Hebert GN, Strauss SH, Field JA. Occurrence and persistence of perfluorooctanesulfonate and other perfluorinated surfactants in ground water at a fire-training area at Wurtsmith air force base, Michigan, USA. J Environ Monit 2003; 5: 341-345. Moody CA, Martin JW, Kwan WC, Muir DCG, Mabury S. Monitoring perfluorinated surfactants in biota and surface water samples following an accidental release of fire-fighting foam into Etobicoke Creek. Environ Sci Technol 2002; 36: 545-551. Moriwaki H, Takata Y, Arakawa R. Concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in vacuum cleaner dust collected in Japanese homes. J Environ Monit 2003; 5: 753-757. MSDS Hydropalat 875, Cognis Corporation, 26.11.2002. MSDS Lutensit A-BO, BASF Aktiengesellschaft, 15.05.2003. MSDS Emulphor FAS 30, BASF Aktiengesellschaft, 11.04.2003. MSDS's for several PolyFox products (656, 3320, 6320, 636, 6520), OMNOVA Solutions Inc, all dated 26 or 27.4.2004. MSDS Ruetasolv DI, Rütgers Kureha Solvents GmbH, 14.01.2003. MSDS Ruetasolv BP 4201, Rütgers Kureha Solvents GmbH, 11.04.2003. MSDS Ruetasolv BP 4103, Rütgers Kureha Solvents GmbH, 06.01.2004. MSDS Ruetasolv TTPN, Rütgers Kureha Solvents GmbH, 19.02.2004. MSDS WorléeAdd 340, Worlée-Chemie GmbH, 26.3.2004. Nordic Ecolabelling. Ecolabelling of filmforming floor care products, 14 December 2000 – 11 December 2006, Version 2.3. 8 March, 2004. Oakes KD, Sibley PK, Solomon KR, Mabury SA, Van der Kraak GJ. Impact of perfluorooctanoic acid on fathead minnow (Pimephales promelas) fatty acyl-COA oxidase activity, circulating steroids, and reproduction in outdoor microsoms. Environ Toxicol Chem 2004; 23: 1912-1919. OECD, Hazard Assessment of perfluorooctane sulfonate (PFOS) and its salts, Co-operation on existing chemicals, Organisation for Economic Co-operation and Development (OECD), ENV/JM/RD(2002)17/FINAL, November 21 2002. Ohmori K, Kudo N, Katayama K, Kawashima Y. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology 2003; 184: 135-140. Olsen GW, Burris JH, Burlew MM. Plasma cholecystokinin and hepatic lipoproteins in ammonium perfluorooctanoate production workers. Drug Chem Toxicol 2000; 23: 603-620. Olsen GW, Gilliland FD, Burlew MM, Burris JH, Mandel JS, Mandel JH. An epidemiologic investigation of reproductive hormones in men with occupational exposure to perfluorooctanoic acid. J Occup Environ Med 1998; 40: 614-622. Olsen GW, Burris JH, Mandel JH, Zobel LR. Serum perfluorooctane sulfonate and hepatic and lipid clinical chemistry tests in fluorochemical production employees J Occup Environ Med 1999; 41: 799-806. Olsen GW, Hansen KJ, Stevenson LA, Burris JM, Mandel JH. Human donor liver and serum concentrations of perfluorooctane sulfonate and other perfluorochemicals. Environ Sci Technol 2003a; 37: 888-891. Olsen GW, Church TR, Miller JP, Burris JM, Hansen KJ, Lundberg JK, Armitage JB, Herron RM, Medhdizadehkashi Z, Nobiletti JB, O'Neill EM, Mandel JH, Zobel LR. Perfluorooctane sulfonate and other fluorochemicals in the serum of American Red Cross adult blood donors. Environ Health Perspec 2003b; 111: 1892-1901. Olsen GW, Church TR, Larson EB, van Belle G, Lundberg JK, Hansen KJ, Burris JM, Mandel JH, Zobel LR. Serum concentrations of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington. Chemosphere 2004; 54: 1599-1611. Olson CT, Andersen ME. The acute toxicity of perfluorooctanoic and Perfluorodecanoic acids in male rats and effect on tissue fatty acids. Toxicol Appl Pharmacol 1983; 70: 362-372. OSPAR. Summary record of the meeting of the hazardous substances committee (HSC), The Hague, 7-11 April 2003. Personal communication with Poul Erik Andersen, Product Register, Danish Labour Inspectorate (Produktregisteret, Arbejdstilsynet), 11.02.2004. Personal communication with Frank Jensen, Danish EPA (Mijløstyrelsen), March and June 2004. Personal communication with Watze de Wolf, Manager, Environmental Sciences Europe, DuPont Coordination Center, Belgium, June-October 2004 (2004a) Personal communication by email with Martin Stephan, Global Business Manager, Surfactants for fire-fighting foam, DuPont, May and June 2004 (2004b). Personal communication by email with Bill Beers, Akron Technology Center, OMNOVA, May 2004. Personal communication by email with Poul Rasmussen, PoraTek I/S, May 2004. PoraTek is a representative for OMNOVA in Denmark. Personal communication by email with Sandra Gurt, Münzing Chemie GmbH, May and June 2004. Personal communication by email with Heinz-Guenther Schulte, Cognis Deutschland GmbH & Co, May 2004. Personal communication by mail with Christa van Aalten, Rütgers Kureha Solvents (RKS) GmbH, May 2004. Personal communication by email with Christian Wulff, BASF, May 2004. Personal communication by email with Stefan Mansel, Worlée-Chemie, May 2004. Personal communication with a company that sells fire-fighting foams in Denmark, June 2004. Personal communication with Jørgen Damsgaard, Falck Teknik, June 2004. Personal communication with Jens Søgaard, the Navy (Søværnets Materiel Kommando), October 2004. Personal communication with Frank Hauberg, Hauberg Technique, October 2004. Personal communication with Dr. M. Prall, Dr. Richard Sthamer GmbH & Co. KG, October 2004. Personal communication with a surface treatment company in Denmark, July 2004. Personal communication with Finn Ryan, Teleinstruments A/S, July 2004. Personal communication with Jens Peter Larsen, Copenhagen Airports A/S, September 2004. Personal communication with Reidar Ladehaug, Solberg Skandinavien, September 2004. www.articfoam.com Press release from EPA's office of R&D, 12 October 2004. EPA Partners With American Chemistry Council Study Young Children's Exposures to Household Chemicals. Found at www.epa.gov/cheers/ (http://www.epa.gov/cheers/images/news_release_101204.pdf). Reagan WK, Lindstrom KR, Thompson KL, Flaherty JM. Analytical techniques and method validation for the measurement of selected semivolatile and nonvolatuile organofluorochemicals in air. J Occup Environ Hyg 2004; 1: 559-569. Renner R. Evidence of toxic effects and environmental impacts has sent researchers scrambling to obtain data. Environ Sci Technol 2001; 35: 154A-160A. Renner R. Perfluorinated sources: Factories outside, consumer products inside?. Science News, January 23, 2004. Risk & Policy Analysts Limited (RPA) in association with BRE Environment. Perfluorooctane Sulphonate – Risk reduction strategy and analysis of advantages and drawbacks. Final Report prepared for Department for Environment, Food and Rural Affairs and the Environment Agency for England and Wales, August 2004. Sasaki K, Harada K, Saito N, Tsutsui T, Nakanishi S, Koizumi A. Impact of airborne perfluorooctane sulfonate on the human body burden and the ecological system. Bull Environ Contam Toxicol 2003; 71: 408-413. Sanderson H, Boudreau TM, Mabury SA, Solomon KR. Effects of perfluorooctane sulfonate and perfluorooctanoic acid on the zooplanktonic community. Ecotoxicol Environ Safety 2004; 58: 68-76. Schröder HF. Determination of fluorinated surfactants and their metabolites in sewage sludge samples by liquid chromatography with mass spectrometry and tandem mass spectrometry after pressurised liquid extraction and separation on fluorine-modified reverse-phase sorbents. J Chromatography 2003; 1020: 131-151. Schnellmann RG, Manning RO. Perfluorooctane sulfonamide: a structurall novel uncoupler of oxidative phosphorylation. Biochim Biophys Acta 1990; 1016: 344-348. Schröder HF. Determination of fluorinated surfactants and their metabolites in sewage sludge samples by liquid chromatography with mass spectrometry and tandem mass spectrometry after pressurised liquid extraction and separation on fluorine-modified reverse-phase sorbents. J Chromatography 2003; 1020: 131-151. Schultz MM, Barofsky DF, Field JA. Quantitative determination of fluorotelomer sulfonates in groundwater by LC MS/MS. Environ Sci Technol 2004; 38: 1828-1835. Seacat AM, Thomford PJ, Hansen KJ, Olsen GW, Case MT, Butterhof JL. Subchronic toxicity studies on perfluorooctane sulfonate potassium salt in Cynomolgus monkeys. Toxicol Sci 2002; 68: 249-264. Seacat AM, Thomford PJ, Butenhoff JL. Terminal observations in Sprague-Dawley rats after lifetime dietary exposure to potassium perfluorooctanesulfonate Toxicologist 2002b; 66: 185 (abstract). Seacat AM, Thomford PJ, Hansen KJ, Clemen LA, Eldrigde SR, Elcombe CR, Butterhof JL. Sub-chronic dietary toxicity of potassium perfluorooctane sulfonate in rats. Toxicology 2003; 183: 117-131. Shoeib M, Harner T, Ikonomou M, Kannan K. Indoor and outdoor air concentrations and phase partitioning of perfluoroalkylsulfonamides and polybrominated diphenyl ethers. Environ Sci Technol 2004; 38: 1313-1320. Shoeib M, Harner T, Wilford B, Jones K, Zhu J. A survey of perfluoroalkyl sulfonamides in indoor and outdoor air using passive air samplers. Organohalogen Compounds 2004; 66: 3999-4003. Sinclair E, Taniyasu S, Yamashita N, Kannan K. Perfluorooctanoic acid and perfluorooctane sulfonate in Michigan and New York waters. Organohalogen Compounds 2004; 66: 4069-4073. So MK, Taniyasu S, Yamashita N, Giesy JP, Zheng J, Fang Z, Im SH, Lam PKS. Perfluorinated compounds in coastal waters of Hong Kong, south China, and Korea. Environ Sci Technol 2004; 38: 4056-4063. Sohlenius A-K, Andersson K, Olsson J, DePierre JW. Peroxisome proliferation and associated effects caused by perfluorooctanoic acid in vitamin A-deficient mice. Chem-Biol Interact 1995; 98: 45-50. Statens Forureningstilsyn (Norwegian Pollution Control Authority). Bruken af PerFluorAlkylStoffer (PFAS) i produkter i Norge – Materialstrømsanalyse. English title: Use of perfluoralkyl substances (PFAS) in products in Norway – Mass Flow analysis. Statens Forureningstilsyn, Oslo, September 2004. Stock NI, Lau FK, Ellis DA, Martin JW, Muir DCG, Mabury SA. Polyfluorinated telomer alcohols and sulfonamides in the North American troposphere. Environ Sci Technol 2004a; 38(4): 991-996. Stock NI, Ellis DA, Deleebeck L, Muir DCG, Mabury SA. Vapor pressures of the fluorinated telomer alcohols – limitation of estimation methods. Environ Sci Technol 2004b; 38(6): 1693-1699. Szostek B, Prickett KB, Buck RC. DuPont Haskell Laboratory. Determination of Telomer B Alchols by Liquid chromatography-Tandem Mass Spectometry in Environmental Matrices. Abstract for American Society for Mass Spectrometry 52nd Conference, 2004. Taniyasu S, Kannan K, Horii Y, Hanari N, Yamashita N. A survey of perfluorooctane sulfonate and related perfluorinated organic compounds in water, fish, birds, and humans from Japan. Environ Sci Technol 2003; 37(12): 2634-2639. Taniyasu S, Yamashita N, Kannan K, Horii Y, Sinclair E, Petrick G, Gamo T. Perfluorinated carboxylates and sulfonates in open ocean waters of the Pacific and Atlantic Oceans. Organohalogen Compounds 2004; 66: 4035-4040. Taves DR. Evidence that there are two forms of fluoride in human serum. Nature 1968; 217: 1050-1051. Thibodeaux JR, Hanson RG, Rogers JM, Grey BE, Barbee BD, Richards JH, Butenhoff JL, Stevenson LA, Lau C. Exposure to Perfluorooctane Sulfonate during Pregnancy in Rat and Mouse. I: Maternal and Prenatal Evaluations. Toxicol Sci 2003; 74: 369-81. Thomford PJ, Seacat AM, Butenhoff JL. Terminal observations in Sprague-Dawley rats after lifetime dietary exposure to N-ethyl perfluorooctane sulfonamide ethanol. Toxicologist 2002; 66: 185 (abstract). Titlemeier SA, Edwards L, Pepper K. Concentrations and temporal trends of two perfluorooctylsulfonamides in fast food composites collected during the Canadian total diet study. Organohalogen Compounds 2003; 62: 315-319. Tittlemeier S, Ryan JJ, Van Oostdam J. Presence of anionic perfluorinated organic compounds in serum collected from northern Canadian populations. Organohalogen Compounds 2004; 66: 4009-4014. Tomy G, BudaKOWski W, Halldorson T, Helm P, Stern G, Tittlemier S. Fluorinated organic compounds in an eastern arctic marine food web. Organohalogen Compounds 2003; 62: 323-327. Trosko JE, Chang C-C, Upham B, Wilson M. Epigenetic toxicology as toxicant-induced changes in intracellular communication. Toxicol Lett 1998; 102-103: 71-78. Ubel FA, Sorenson SD, Roach DE. Health status of plant workers exposed to fluorochemicals – a preliminary report. Am Ind Hyg Assoc J 1980; 41: 584-589. Upham BL, Deocampo ND, Wurl B, Trosko JE. Inhibition of gap junctional intercellular communication by perfluorinated fatty acids is dependent on the chain length of the fluorinated tail. Int J Cancer 1998; 78: 491-95. US EPA Administrative Record AR226-0550. Fluorochemical use, distribution and release overview. Prepared by 3M Company, May 26, 1999. US EPA (2002), Revised draft – Hazard assessment of Perfluorooctanoic Acid and Its Salts, Office of Pollution Prevention and Toxics, Risk Assessment Division, November 4, 2002. US EPA (2003a), Preliminary Risk Assessment of the Developmental Toxicity Associated With Exposure to Perfluorooctanoic Acid and its Salts, Office of Pollution Prevention and Toxics, Risk Assessment Division, April 10, 2003. US EPA (2003b), PFOA Q's & A's. April 14, 2003. US EPA, OPPT Fact Sheet. Vanden Heuvel, Kuslikis B, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J Biochem Toxicol 1991; 6: 83-92. Vanden Heuvel JP, Kuslikis BI, Peterson RE. Covalent binding of perfluorinated fatty acids to proteins in the plasma liver and testes of rats. Chem-Biol Interact 1992; 82: 317-328. Vanden Heuvel JP. Perfluorodecanoic acid as a useful pharmacologic tool for the study of peroxisome proliferation. Gen Pharmacol 1996; 27: 1123-1129. Van de Vijver KI, Hoff PT, Das K, Van Dongen W, Esmans EL, Jauniaux T, Bouquegneau J-M, Blust R, de Coen W. Perfluorinated chemicals infiltrate ocean waters: link between exposure levels and stable isotope ratios in marine mammals. Environ Sci Technol 2003; 37: 5545-5550. Vejrup K.V., Lindblom B., Analysis of perfluorooctanesulfonate compounds in impregnating agents, wax and floor polish products, National Environmental Research Institute, Survey of Chemical Substances in Consumer Products, Survey No. 17, 2002, Danish EPA. Yamashita N, Kannan K, Taniyasu S, Horii Y, Hanari N, Okazawa T, Petrick G. Environmental contamination by perfluorinated carboxylates and sulfonates following the use of fire-fighting foam in Tomakomai, Japan. Organohalogen Compounds 2004; 66: 4063-4068. Yamashita N, Taniyashu S, Horii Y, Kannan K, Gamo T. Perfluorooctane sulfonate and related compounds in the South China Sea, Sulu Sea and Japanese environmental samples. Organohalogen Compounds 2003; 62: 339-343. Yang JH, Kannan K, Kim S-Y, Shin I-H. Levels of perfluorooctansulfonate and related fluorochemicals in human blood from the general population of Korea. Organohalogen Compounds 2004; 66: 4041-4045. Appendix A Fluorine-containing substances – Terminology and definitions
FluorochemicalA general, non-specific, term used for a broad description of all chemicals containing the element fluorine. Specifically, the term is used most commonly to describe small (1-8 carbon length) fluorinated molecules, which are most often used for refrigeration, as fire suppression agents and as specialty solvents. Fluorinated chemicalA general, non-specific term used synonymously with "fluorochemical". FluorotelomerA specific term used to describe an oligomer created by reaction of tetrafluoroethylene (TFE) with perfluoroethyl iodide CF3CF2I to produce F(CF2CF2)n-I [n = 3-6, avg. 4], a linear, even carbon number chain length oligomer; the term "telomer" is often used synonymously with fluorotelomer. Fluorotelomer alcohol (FTOH)A general term which describes a class of alcohols of the general structure F(CF2CF2)nCH2CH2OH, where n is an integer. FluoropolymerA general term used to describe a polymer which has fluorine attached to the majority of carbon atoms which comprise the polymer chain backbone. Common fluoropolymers are: polytetrafluoroethylene (PTFE), polyvinylidene fluoride (PVDF), fluorinated ethylene-propylene (FEP), etc. These are typically high molecular weight polymers used in high performance applications where chemical resistance and thermal stability are essential. Fluorinated (organic) polymerA general term used to describe a polymer which has a hydrocarbon backbone (polyamide, polyester, polyurethane, etc.) to which a fluorinated carbon chain is appended, also known as a fluorinated alkyl chain; an example would be a polymer such as -[CH2CH(C(=O)OCH2CH2(CF2)8F)]n-. Perfluoro- / perfluorinatedDescribes specifically a substance where all hydrogen atoms attached to carbon atoms are replaced with fluorine atoms – CFn.. Perfluoroalkylated substance (PFAS)A general term, which describes a substance that bears a perfluorocarbon unit F(CF2)n-R where n is an integer and R is not a halogen, or hydrogen. Examples include F(CF2)6CH2CH2OH, F(CF2)6SO2N(CH3)CH2CH2OH, and p-F(CF2)6-C6H4OH. FluorosurfactantA non-specific, general term used to describe a surface active, low molecular weight (<1000), substance where carbons bear fluorine in place of hydrogen. Examples would include CF3(CF2)7SO3-K+, H(CF2)7COO-NH4+, F(CF2CF2)3CH2CH2SO3-NH4+, CH3CH2CF2CF2CH2COO-NH4+, etc. Perfluorocarboxylic acid (PFCA)A general term which describes a class of fully fluorinated carboxylic acids and their salts of the general structure F(CF2)n-COOX (X = H, NH4+, Group I alkali metal, Group II alkaline earth). These are generally manufactured by the electrochemical fluorination process or from fluorotelomer-based raw materials. The most common industrially used fluoropolymer polymerization aids are: ammonium perfluorooctanoate (CAS# 3825-26-1) and perfluoro fatty acids, C7-13, ammonium salts (CAS# 72968-3-88). Polyfluorinated substancesA term for substances with more fluoro-substituents. Appendix B Methods used in search for alternatives
Several steps were taken in order to identify alternatives for PFOS-related compounds and PFOA. The method and sources used are listed and described in more details below.
Search on the InternetFirst of all, a search on the Internet was carried out in order to identify alternatives for PFOS-compounds and PFOA. Search words like "PFOS", "PFOA" and "alternative(s)", "replacing", "replacement", etc. were used at ordinary search sites and more scientific search sites. This preliminary search identified a few alternatives, and several links to companies stating that they use "PFOS-free" products. This angle was therefore persecuted in the process of contacting producers. Participation in a conference in PragueFORCE Technology participated in a conference held by SETAC [14] Europe in Prague, 18-22 April 2004. One session (five presentations) was entirely on polyfluorinated substances, and 17 posters were presented on the topic. Presentations were made from both universities and industry. The latest research indicates that the presence of PFOA in the environment is caused by the decomposition of telomer alcohols. We made some personal contacts, which were used in the process of contacting producers for information about possible alternatives. Participation in a conference in Helsingør (Elsinore)FORCE Technology participated in a conference held by the Department of Chemistry at the University of Copenhagen, in Helsingør (Elsinore), Denmark, 11-13 June 2004. The conference was the Fifth Informal Conference on Reaction Kinetics and Atmospheric Chemistry. The first two lectures held on June 11 were entirely on the subject of PFOS, PFOA and fluorinated telomers. Scott A. Mabury from the University of Toronto in Canada held a lecture on "The fabulously fascinating environmental chemistry of fluorinated compounds – why atmospheric fate is so important?". Tim J. Wallington from Ford Motor Company in Michigan, USA held a lecture on "Atmospheric oxidation of fluorotelomer alcohols: A likely source of perfluorinated acids in the global environment?". Scott Mabury is one of the world's leading experts on perfluorinated compounds. We received his latest work and have used this in the environmental assessment of PFOS and PFOA. Participation in a conference in BerlinFORCE Technology participated in the Dioxin04 conference held in Berlin, Germany, 6-10 September 2004. One session was entirely on perfluorinated substances, where among other things the Nordic report on levels of PFOS-related substance in the Nordic countries was presented. Professor John P. Giesy from Michigan State University, USA, participated in the conference as well. He and his group of scientists discovered the PFOS-related substances in the nature, and Giesy was the one who convinced the 3M Company to stop the use of the substances. The latest information about the PFOS-related substances was achieved by personal contact to Giesy. Furthermore, the company DuPont was present at the conference. Contact to producers for information about alternativesThe preliminary search on the Internet identified some alternatives. The producers of these alternatives were contacted in order to learn more about the mentioned alternatives (price, physical/chemical properties, etc.) and perhaps learn more about other possible alternatives. Furthermore, the companies at the moment producing PFOS-related compounds and PFOA were contacted in order to learn more about their knowledge about the possible alternative options. The lists produced by OECD for both PFOS and PFOA producing companies were used (U.S. EPA, 2003a), (OECD, 2002). Table 9.1: List of producers of PFOS-compounds according to OECD (OECD, 2002).
Table 9.2: List of producers of PFOA according to OECD (US EPA, 2002).
Finally, the companies stating that they use "PFOS-free" products were contacted in order to learn more about the identity of the substances or formulations that they use instead of PFOS. All the identified relevant producers received either an email with questions about alternatives to PFOS-related compounds and PFOA or the questions were send by use of a form on the company website, if any. In order to reach as many producers of alternatives as possible, an alternative route was also used. The project group was in possession of a raw materials catalogue of the paint, ink and varnish industry (Karsten, 2000). In this catalogue the raw materials are divided after their function. Some of the fluorotensides from DuPont (the Zonyl products) were listed under different functions (levelling agent, water repelling agent, wetting agent and other coating additives) in this raw materials catalogue. An email was therefore sent to all the listed producers (if an email could be found) of products within the same function category. All these producers were asked the question if their product can be used as an alternative to PFOS compounds as they were listed with the same function as fluorotensides. In all, about 60 emails were sent or forms filled in to different producers that hopefully would be able to supply the project with more information about possible alternatives to PFOS and PFOA. The number of emails sent was distributed as presented in Table 9.3. The generated list of producers was longer (in all about 25 companies more), but some companies had been bought by other companies already on the list, or for some companies it was simply im possible to find either a website or an email address, and therefore no email was sent. Some of the about 60 emails sent were returned because of an unknown user address. In these cases an effort was made to find a new email address or a website of the company in order to fill in a questionnaire form. This means that about 60 companies should have received an email with questions about possible alternatives to PFOS-compounds and PFOA. Table 9.3: Number of emails sent to and received from producers.
However, not many replied, as shown in Table 9.3. In all, we received 12 replies, where eight of the replies contained information about possible alternatives to PFOS-compounds. The results are discussed in the chapter about alternatives to PFOS-compounds in the report. Use of personal international contactsThe project group had some personal international contacts, which were used in order to collect all the latest possible information about PFOS-compounds. These personal contacts were:
These personal contacts delivered the latest scientific research on the PFOS, PFOA and telomer alcohol subjects. Use of confidential information from the Danish Product RegisterThe search in the Danish Product Register for use of PFOA-compounds and telomer alcohols gave primarily confidential information, as only very few companies use these compounds. However, these companies were contacted in order to learn more about the use of the PFOA-compounds and telomer alcohols and their possible alternatives. The outcome of the search for these compounds in the Danish Product Register and the contact to the companies is described in more details in appendix D and E. Appendix C Use of PFOS-related compounds in DenmarkIn 2001/2002 a project funded by the Danish EPA was carried out in order to gain an overview of which types of products on the Danish market that may contain PFOS and PFOS-related compounds (Havelund, 2002). The project was a survey of PFOS and similar substances in consumer products. The survey showed that 75 of the 175 identified PFOS-compounds on OECD's list were registered in the Danish Product Register. These 75 PFOS-compounds were from 17 of the total 22 different categories of PFOS-compounds (as described in the main report). The search in the Danish Product Register showed that PFOS-compounds were most widespread in the following types of products:
As to products with small consumption, the following types of products are relevant:
A total list of application areas for PFOS-related compounds according to the search in the Danish Product register can be found in Table 9.5 below. The search in the Danish Product Register showed that the total registered sale in Denmark of PFOS-compounds contained in products is 8-16 tons per annum. Products for impregnating leather and paper as well as paint/varnishes and reprographic agents represent the largest amounts. However, a search in the Danish Product Register is not necessarily complete with respect to products containing PFOS-compounds as the Danish Product Register only registers products that must be labelled as dangerous products. Most PFOS-compounds are not considered as dangerous substances. Products containing PFOS-compounds will, however, be registered if the products contain other substances, which contribute to the classification of the product as a dangerous substance. In other words, the Danish Product Register does not register all products containing PFOS-compounds on the Danish market, and the registered amounts do not give an adequate picture of the total sales in Denmark. Finally, imported final products containing PFOS-compounds are not registered in the Product Register. Therefore, a market survey was initiated in order to quantify the non-registered amount of PFOS-compounds in products. This survey concluded that the total Danish consumption of PFOS-compounds in products probably was between 5 to 50 tons per year. The market survey carried out showed that in some areas substitutions of PFOS-compounds have been made, whereas the use of PFOS-compounds in other areas was unchanged (see Table 9.4 below). Table 9.4: Use of PFOS-compounds for different products according to the Danish survey on PFOS-compounds in consumer products (Havelund, 2002).
A more recent survey on PFOS-compounds in impregnating agents as well as wax and polish for floors showed that PFOS-compounds were found in 3 of 21 products. The PFOS-compounds were found in one impregnating agent for leather and textiles, in one impregnating agent for textiles and in one floor caring product with wax and polish. However, the producers stated, when contacted, that the products containing the PFOS-compounds either are or will be removed from the market. (Vejrup et al, 2002). Furthermore, the survey showed that some of the problems in mapping the use of PFOS-compounds are:
Table 9.5: List of products with PFOS-related compounds registered in the Danish Product Register (Havelund, 2002). The application areas are listed in descending order. The application area with the highest calculated content (in kilos) in the sold products listed first.
Please notice that for some product groups the calculated content of PFOS-compounds is negative in Table 9.5. The numbers are taken directly from the reference (Havelund, 2002), where there is no explanation for the negative numbers. Appendix D Use of PFOA ammonium salt in DenmarkOne of the tasks of this project was to make a survey of the use of PFOA in Denmark, similar to the earlier survey of PFOS (Havelund 2002). As described in chapter 3 (Table 3.4) seven PFOA-compounds and salts of PFOA are relevant. (US EPA, 2003a). A preliminary search was carried out in the SPIN [15] database – a database of product registers in the Nordic countries. This search showed that only the PFOA compound itself and the ammonium salt of the compounds listed in Table 3.2 in chapter 3 were registered in the product register of the Nordic countries. The search in the SPIN database also showed that only the ammonium salt of PFOA is registered in the Danish Product register, i.e. is used in chemical products produced in Denmark (which must be labelled as dangerous). A contact to the Danish Product Register revealed the following about the use of the ammonium salt of PFOA with CAS number 3825-26-1 (Andersen, 2004; Jensen, 2004):
Further information about the use of PFOA ammonium salt from the Danish Product Register is confidential, which means that three or fewer companies have registered products with a content of the PFOA ammonium salt. The information from the Danish Product Register shows that the production of products containing PFOA is very small (35 kilo per year) – also compared to the amount of PFOS-compounds in consumer products in Denmark (5-50 tons per year). Still, this is what could be expected, as the problem with PFOA primarily seems to be the release of PFOA during use. Normally, PFOA is not present in the product after production and will not be registered in the Product Register. Furthermore, the information from the Danish Product Register only refers to products that are produced in Denmark and do not reveal the possible content of PFOA in imported products. PFOA may in a larger degree than PFOS be a contaminant in imported finished fluoropolymeric products. This information can be obtained by collecting import data from Statistics Denmark for the products expected to either contain PFOA or to release PFOA in use. However, as PFOA can be released from numerous products, it will be difficult to calculate a correct amount of imported products from where PFOA can be released when used. First of all, the nomenclature used by EU (The Combined Nomenclature - KN-codes) are not detailed enough to be able to distinguish between e.g. different kitchen utensils, such as pots and pans. Secondly, it is not possible to distinguish between for example Teflon coated products and non-Teflon coated products. A calculation of the imported amount of products expected to be able to release PFOA is therefore problematic and will necessarily be based on educated guesses. Hence, the calculation will be very uncertain. Therefore, no statistical information about products expected to release PFOA when used is obtained in this project. Instead the companies – only two, which according to the Danish Product Register sell products with a content of PFOA ammonium salt, were contacted. 9.1 Information from a surface treatment company in DenmarkOne of the Danish companies with a registered use of PFOA was contacted. The company is one of the leading applicators in Europe within the field of utilisation of PTFE fluoroplastics. The company carries out coating of all sorts of products with fluoroplastics. Several different layers of fluoroplastics are added in a closed automatic process, where the primer and topcoat layers are added by spraying. According to this company, the ammonium salt of PFOA is found in two of their products – a primer and a topcoat. The company does not produce the fluoroplastic, but imports the products. The PFOA ammonium salt is in the products due to impurities, as PTFE is manufactured by use of PFOA ammonium salt. The specific content of PFOA ammonium salt in the products is unknown to the company, but the content is very small – less than 0.1% of the total product weight. The purchase of the PFOA ammonium salt containing products was in 2002 115 kg and 147 kg respectively. The purchase in 2003 was 130 kg and 0 kg respectively. This means that the company utilises less than 250 grams of PFOA per year. The company contacted their supplier, which states that at the moment no PFOA-free PTFE-dispersion is available on the market, but all the big suppliers of PTFE have started to work on possible alternatives. However, at the moment there seems to be no satisfying alternative to PFOA in the production-process of PTFE. (Personal communication – surface treatment company, 2004). 9.2 Information from Teleinstrument A/STeleinstrument A/S was the other company with a registered use of PFOA. According to the Danish Product Register this company uses PFOA ammonium salt in fluxing agents for the purpose of plumbing. The company contacted their British suppliers and got the reply that the PFOA ammonium salt no longer is used in their products. In other words, it is a mistake that this use has not been deleted from the Danish Product Register. (Personal communication Teleinstrument, 2004). Appendix E Use of telomer alcohols in DenmarkAs the newest research indicate that telomer alcohols may be degraded to PFOA in the environment, a search for the use of telomer alcohols in the Danish Product Register was also performed. A preliminary search was carried out in the SPIN [16] database – a database of product registers in the Nordic countries. The preliminary search involved the most typical telomer alcohols as listed in Table 0.1. Table 0.1: List of telomer alcohols.
This search showed that only the perfluorooctanol compound (6:2 FTOH) of the compounds listed in Table 0.1 was registered in the product register of the Nordic countries with a production/sale in Denmark, i.e. is used in chemical products produced in Denmark (which must be labelled as dangerous). A contact to the Danish Product Register revealed the following about the use of perfluoroctanol with CAS number 647-42-7 (Personal communication Jensen, 2004):
The company, which had registered a use of the compound perfluorooctanol, was contacted in order to learn more about the use and possible alternatives. It turned out that the compound was used in four products, but only one of the products was used today. As listed above, this one product represents a total annual sale of 1 kilo of perfluorooctanol. The company sells about 3-4,000 litres of the fluxing agent, which means that perfluorooctanol is added to the product in a concentration of about 0.03% (assuming a density of 1 kilo/litre). According to the company the fluxing product will automatically go out of use in a couple of years as the "European Directive 2002/95/EC of January 27 2003 on the restriction of the use of certain hazardous substances in electrical and electronic equipment" states that after July 1st 2006 lead is no longer permitted in electrical and electronic equipment. (Directive 2002/95/EC, 2003). This means that plumbing with lead is not longer permitted by this date. Today plumbing is carried out with the use of soldering tin that consists of 63% lead and 37% tin. When using this leaded soldering tin it is necessary to use a soldering flux to prepare the surface for the plumbing. Fluxing agents are used in automatically closed systems and are sprayed on the subject, which is then heated (to remove the fluxing agent) and then the plumbing is carried out. The alternatives to leaded soldering tin are use of soldering tin of either pure tin or soldering tin with a small percentage of silver and copper, 0.7% and 3.5% respectively. With a use of lead-free soldering tin it is, however, possible to use water-based fluxing agents instead of the solvent based fluxing agents (with perfluorocompounds). This means that the use of perfluorocompounds in fluxing agents automatically will cease with the implementation of lead-free plumbing. Appendix F Identified alternatives
Telomer-based products are in general alternatives to PFOS-related compounds. However, a search for other alternatives was carried out during the project. First of all, the Internet was used. Secondly, the producers of relevance were contacted by email in order to learn more about the alternatives (see a more detailed description in appendix B). Furthermore, a different approach was used within the paint and varnish area, as EnPro ApS (a laboratory within the area of paint, ink and varnish) participated in this project. A raw materials catalogue was used to identify possible producers of alternatives. An email was sent to the companies that produced products with the same function as the listed fluorosurfactants in the catalogue. Therefore, more alternatives are presented within this area. The following compounds have been identified as alternatives to PFOS-related compounds during the project. No alternatives to PFOA have been identified. Several producers of PFOA were asked about alternative options to PFOA, and those who replied had no alternative options to PFOA. However, as described in the report, use of PFOA does not seem to be the biggest problem. This will be the production of telomer alcohols that in the environment can degrade to PFOA. One company mentioned that their products (fluorinated) can be used as alternatives for telomer based products as well. Table 0.1: Alternatives to PFOS-compounds
Each of the alternatives described above is presented more detailed in the following paragraphs. PFBS – PerfluorobutanesulfonateIn June 2003, the 3M Company replaced the PFOS-compound in their Scotchgard products with PFBS (perfluorobutanesulfonate). 3M's Schotchgard products are cleaners and stain protectors for carpets, leather, furniture, automotives, hard surfaces and other apparels. After the phase-out of PFOS in the Scotchgard products, the 3M Company first presented an aerosol-can based on non-perfluoro chemistry. However, the product worked on water but not on grease. Therefore, 3M now uses the perfluoro-compound with a shorter chain length – C4 instead of the former C8 PFOS compound. (Flouride Action Network, 2004). In the following table properties of PFBS are presented. The technical and environmental properties are found in different product information at 3M's website (www.3m.com). Table 0.2: Properties of the alternative PFBS
C6-fluorinated compoundAccording to 3M's website, a C6-fluorinated compound is used in their Fire Protection Fluid. The compound is dodecafluoro-2-methylpentan-3-one (CF3-CF2-C(=O)-CF(CF3)2). This compound is also a part of the Novec product series. Table 0.3: Properties of the alternative C6 – fluorinated compound
Fluorotelomer-based productsDuPont manufactures a range of fluorotelomers especially designed for fire-fighting foam formulations – DuPont FORAFAC®. These fluorotelomer products are predominantly based on C6 molecules (more than 80%). According to DuPont, their FORAFAC® 1157 and 1183 products are based on more than 95% C6 telomer, whereas FORAFAC® 1157N is based on more than 65% C6 telomer. (Personal communication DuPont, 2004). Table 0.4: Properties of DuPont's Forafac
Fluorinated polyetherOne of the contacted companies in search for alternatives gave the following information about their alternative, which is a fluorinated polyether. This has specifically been used in about 40 floor polish products in the USA, Europe and Asia. According to OMNOVA the PolyFox product line is, besides an alternative to PFOS-based products, also an alternative to the long chained telomer based products (which have showed to have the potential to degrade to PFOA in the environment). (Personal communication OMNOVA, 2004). OMNOVA Solutions Inc. manufactures a line of fluorosurfactants called PolyFox™. The entire PolyFox family of fluorosurfactants is polymers with a molecular weight greater than 1,000. The PolyFox polymers are based on ether links – both the polymer backbone linkages and the link between the backbone and the perfluoroalkyl pendant side chains. The PolyFox flurosurfactants are synthesized from perfluoroalkyl starting materials with a fully fluorinated carbon chain length of C4 or less. The current first generation products are all made with C2F5 or CF3 perfluoroalkyl side chain structures. (Personal communication OMNOVA, 2004). The fluorinated polyethers are either hydroxy-terminated or acrylate-terminated according to the MSDS's found for PolyFox products. The basic structure of some of the different PolyFox™ compounds is illustrated in the figures below. (Personal communication, PoraTek, 2004). Figure 0.1: The basic structure of PolyFox 3320 compound (Personal communication, PoraTek, 2004). x+y equals about 20. 
Figure 0.2: The basic structure of PolyFox 136 A with CF3 as the basic perfluoralkyl group (Personal communication, PoraTek, 2004). x+y equals about 6.
Figure 0.3: The basic structure of PolyFox 156 A with C2F5 as the basic perfluoralkyl group (Personal communication, PoraTek, 2004). x+y equals about 6.
Figure 0.4: The basic structure of PolyFox 656 with C2F5 as the basic perfluoralkyl group. The technical properties of this compound are described in Table 0.5 (Personal communication, PoraTek, 2004). x+y equals about 6.
The technical properties listed in the table below are for PolyFox 656, which is shown in Figure 7.2 just above. However, all PolyFox compounds have similar technical properties. Table 0.5: Properties of the alternative fluorinated polyether
Propylated aromaticsRütgers Kureha Solvents produces different propylated aromatics (naphthalenes and biphenyls), which can be used as water repelling agents for different applications. For example rust protection systems, marine paints, resins, printing inks, coatings, electrical applications, electronically and mechanical applications. (Personal communication RKS, 2004). The different propylated aromatics are presented in table Table 0.6 below and some of the compounds are illustrated in the figures below. Figure 0.5: The chemical structure of Ruetasolv DI (CAS-No. 38640-62-9) – to the left, and Ruetasolv TTPN (CAS-No. 35860-37-8) – to the right.
Figure 0.6: The chemical structure of Ruetasolv BP 4103 (CAS-No. 25640-78-2) – to the left, and Ruetasolv BP 4201 (CAS-No. 69009-90-1) to the left. Both products are mixtures of isomers.
Table 0.6: Properties of the alternative propylated aromatics
Aliphatic alcoholsBASF produces a large range of surfactants for different purposes. In coatings application fluorosurfactants are, according to BASF, mainly used for substrate wetting, levelling and reduction of surface tension (e.g. in spray applications). The key to the often-superior performance of the fluorosurfactants is the extremely low surface tension, which cannot be matched with other surfactants. However, due to high prices of the fluorosurfactants and due to environmental reasons, other surfactants can be used as alternatives if it is not a technical must to achieve such low surface tension levels. Possible replacements to fluorosurfactants are silicone surfactants, which BASF does not produce, or surfactants based on aliphatic alcohols. BASF produces a range of aliphatic alcohols, both anionic and non-ionic surfactants. However, especially the effect of the non-ionic surfactants is mixed, because these products can also be used as defoamers or emulsifiers. So the non-ionic surfactants are difficult to use as replacements for fluorosurfactants. Therefore, only the anionic surfactants of BASF (Emulphor® FAS 30 and Lutensit® A-BO) are described in details. The Emulphor product from BASF is a fatty alcohol polyglycolether sulfate, whereas the Lutensit A-BO product is a sulfosuccinate. The Lutensit A-BO product is therefore described together with the other sulfosuccinate products. (Personal communication BASF, 2004). Table 0.7: Properties of the alternative fatty alcohol ethoxylate
SulfosuccinateInformation from Münzing ChemieMünzing Chemie produces wetting agents for water-based applications, which are used to lower the surface tension of water to wet substrates or pigment surfaces. With some of their products they are able to replace fluorobased wetting agents, e.g. in wood primers. The best product for this kind of applications is their product EDAPLAN LA 451, which is based on a sulfosuccinate derivative in ethanol (19%) and water (12.5%). Sulfosuccinates are esters of succinic acid (HOOC-CH2-CH2-COOH) in reaction with hydrogen sulfite. The product EDAPLAN 451 contains a mixture of chemicals. (Personal communication with Münzing Chemie, 2004) Table 0.8: Properties of the alternative Sulfosuccinate (by Münzing Chemie)
Information from CognisCognis produces surfactants, wetting agents and dispersing agents for the paint and coating industry. The group of products technically competing with fluorosurfactants are sulfosuccinates. However, the sulfosuccinates do not reach such low surface tensions as it is possible with fluorinated surfactants, but nevertheless sulfosuccinates produced by Cognis are widely used in printing inks, where they also are approved for food contact. The best product for competing with fluorinated surfactants is their product Hydropalat® 875, which is based on the sodium salt of di(2-ethylhexyl) sulfosuccinate in ethanol (5%) and water (20%). (Personal communication with Cognis, 2004) Table 0.9: Properties of the alternative Sulfosuccinate (by Cognis)
Information from BASFAs described in the previous section BASF also produces a sulfosuccinate products, which can be used as an alternative to fluorosurfactants within the coating industry. The Lutensit® A-BO product of BASF contains a di-octyl-sulfosuccinate (di(2-ethylhexyl) sulfosuccinate), which has the following structure.
Table 0.10: Properties of the alternative sulfosuccinate
Silicone polymersWorlée-Chemie produces silicon polymers, which in the paint and ink industry in several cases can be used as alternative wetting agents to fluorosurfactants. Two products are emphasized: WorléeAdd® 340 and WorléeAdd® 345. The first product is a non-ionic modified silicone polyether, where the latter is a mixture of a silicone polyether and a dioctylsulfosuccinate in ethanol and water. (Personal communication Worlée-Chemie, 2004). Table 0.11: Properties of the alternative silicone polymers
Footnotes[1] OSPAR is the current instrument guiding international cooperation on the protection of the marine environment of the North-East Atlantic. [2] OSPAR is the current instrument guiding international cooperation on the protection of the marine environment of the North-East Atlantic. [3] Please notice that the UK survey maps the use of PFAS chemicals and not just only PFOS-related compounds. This may explain why the fire-fighting foams are represented with such a relatively high percentage, as this is an area where the use of PFAS substances with shorter chaing length (not PFOS) is gaining ground today. [4] It is not indicated in the report (Havelund, 2002) when the information from the Danish Product Register is from, but it is probably from the year 2000. [5] Teflon®, Fluon®, Polyflon®, Vaflon® are all examples of brand names of PTFE. [6] It should be noted that Teflon® is a consumer brand which describes performance in consumer products. While PTFE (polyetetrafluorethylene) containing cookware is often branded Teflon®, many Teflon® branded items do not contain any fluorinated materials. [7] The report does not indicate when the information from the Danish Product Register is from, but it is probably from the year 2000. [8] Please notice that this survey calculates the total use of PFAS-compounds where other surveys focus on the use of PFOS-related compounds that do not include lower carbon chain products as C4 and C6 fluorinated compounds. [10] Perfluorononanoic acid [11] 2-(N-Ethylperfluorooctane sulfonamido) acetic acid [12] Perfluorooctane sulfonamide [13] Surveys indicate that the use of PFAS compounds for impregnation of paper and cardboard is more or less historical. [14] Society of Environmental Toxicology and Chemistry [15] Substances in Preparations in Nordic Countries - www.spin2000.net/spin.html. [16] Substances in Preparations in Nordic Countries - www.spin2000.net/spin.html.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||