|
More environmentally friendly alternatives to PFOS-compounds and PFOA 3 Chemistry
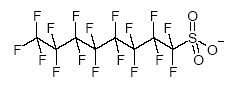
Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and other related compounds are part of a chemical family called fluorinated surfactants. Surfactant is an abbreviation of the term surface-active agent. A surfactant is a substance, which even at low concentrations effectively lowers the surface tension of its medium by selective adsorption on the interface. Fluorinated surfactants are surfactants where the hydrophobic part of the surfactant molecule contains fluorine. At least one hydrogen atom in the hydrophobic part of the molecule has been replaced with fluorine. Perfluorinated surfactants, which are the group that PFOS and PFOA belong to, are fully fluorinated surfactants, where all hydrogens in the hydrophobic part of the molecule have been replaced by fluorine (Kissa 2001). Perfluorinated surfactants have the unique ability to dramatically lower aqueous surface tension, improve wetting and levelling, and remain chemically stable under harsh use conditions. Furthermore, fluorinated surfactants are much more surface active than their hydrogen counterparts and exhibit surface activity in organic systems. Additionally, they are stable to heat (fire resistant), acids, and bases as well as reducing and oxidising agents. Because of these unique properties they are often irreplaceable in many applications (Kissa 2001). Some surfactants may not be perfluorinated but contain both perfluorocarbon and hydrocarbon functionality. These may provide low surface tension, wetting and levelling properties, but would not be expected to be as chemically and thermally stable as their perfluorinated analogue (e.g. F(CF2)7COOH versus F(CF2)6CH2 CH2SCH2 CH2COOH). Alternatively, perfluorinated alkyl chains can be appended to hydrocarbon polymer backbones, such as acrylates creating "comb-like" structures where the backbone is a hydrocarbon and some of the tines contain perfluorinated chains. These "fluorinated polymers" are used as surface modification coatings. The backbone adheres to the surface while the chains align perpendicular. These polymers provide oil and water repellency and stain resistance to substrates like textiles or paper. In addition, fluoropolymers may also be perfluorinated materials. Common fluoropolymers are polytetrafluoroethylene (PTFE) and polyvinylidene fluoride (PVDF). They are often manufactured using a perfluorocarboxylic acid ammonium salt as a processing aid. The most common processing aids are based on octanoic and nonaoic acids. Fluoropolymers are fundamentally different from the "fluorinated polymers" described above. Perfluorooctanyl sulfonate compounds (called "PFOS-related compounds") are members of a large family of anthropogenic chemicals that all are derivatives of and can degrade to perfluorooctanyl sulfonate (PFOS). They have had broad use in consumer and industrial products (US EPA Administrative Record). The anion perfluorooctanyl sulfonate illustrated in Figure 3.1 has no specific CAS number (OECD 2002). Figure 3.1: Structural formula of PFOS - perfluorooctane sulfonate (C8F17SO3)
Well-known brands for consumer products based on polyfluorinated chemistry are Teflon, Scotchgard, Gore-Tex, Silverstone and Stainmaster (US EPA 2003). PFOS-related substances and PFOA are members of the larger family of perfluoroalkylated substances (PFAS). PFAS compounds cover a wide array of chemical substances that may contain perfluoroalkyl chains, including perfluorinated compounds with shorter carbon chains, e.g. C6, C4, and C2, which obviously cannot degrade to PFOS (the eight carbon chain) or PFOA in the environment. OECD has drawn up a list of 175 different perfluoro-substances divided in 22 different categories. All 175 substances contain perfluoro structures, i.e. also perfluoroalkylsulfonates and not just PFOS-related compounds, but not all substances contain sulfur. Non-sulfur containing substances are included in the list of PFOS-substances as they represent a risk of formation of perfluorooctanyl sulfonate at decomposition in a sulfurous environment. This definition by the OECD is somewhat confusing compared to other uses of the term "PFOS-related substances". The 22 different categories of PFOS-related compounds as reported by the OECD are listed in Table 3.1 (Statens forureningstilsyn 2004; Havelund 2002). Table 3.1: The classification of PFOS-compounds in 22 categories according to OECD (Havelund 2002).
Great Britain has worked specifically with the family of perfluorooctanyl sulfonyl fluoride (the POSF family), "PFOS-related compounds", creating a list of 96 different PFOS-related substances for use in their risk reduction strategy. OSPAR [2] has a list of 30 PFOS-related compounds. Furthermore, 88 PFOS-related compounds are a part of the American SNUR rules (the US EPA). The different lists have an overlap in certain cases, but compounds do only exist on one list and not on another. E.g. of the 88 compounds on the American lists, 48 compounds cannot be found on the list made by OECD (Kemikalieinspektionen 2004; RPA 2004). The difference in the classification of PFOS-related substances lies in the fact that some organisations consider compounds only to be PFOS-related if a C8F17 group is directly linked to a sulfonyl group (-SO2), as they assume that only the presence of the full C8F17SO2 group in the original molecule may allow the potential degradation of that molecule to PFOS in the environment. Others (e.g. OECD) do, on the other hand, consider compounds to be PFOS-related if they represent a risk of formation of perfluorooctanyl sulfonate at decomposition in a sulfurous environment (RPA 2004; Statens Forureningstilsyn 2004; Havelund 2002). Category number 16 in Table 3.1, the fluoroalcohols, represents the telomer alcohols, which are also discussed in this report. According to the OECD classification, the telomer alcohols are hence considered as a part of the PFOS-compounds. However, in this report the short-chained telomer-based compounds are not considered as part of the "PFOS-related substances" as they per definition cannot degrade to PFOS. The technical properties of PFOS (perfluorooctane sulfonate) are described in Table 3.2. Table 3.2: Technical properties of PFOS (perfluorooctane sulfonate). Most properties represent the potassium salt of PFOS (OECD 2002).
Some of the most important PFOS-related compounds are listed in table Table 3.3 together with their commonly used acronyms. Table 3.3: Polyfluorinated compounds
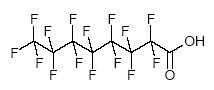
A related substance is perfluorooctanoic acid (PFOA), which is the acid of perfluorooctane. The structural formula of PFOA (CAS 335-67-1) is illustrated in Figure 3.2 (US EPA 2003). Figure 3.2: Structural formula of PFOA - perfluorooctanoic acid (C8HF15O2)
PFOA is used as a processing aid in the manufacture fluoropolymers. PFOA is also an item of commerce and well documented as a significant contaminant in PFOS-related chemicals. PFOA is used as a polymerisation aid in order to improve the physical properties of the polymer and increase the rate of polymerisation (Hekster et al. 2002; ENVIRON 2004). The abbreviation PFOA is used as a group name for perfluorooctanoic acid and its salts. According to the preliminary risk assessment of PFOA carried out by the US EPA the PFOA-compounds listed in Table 3.4 are relevant. However, the ammonium, sodium, potassium and silver salts of PFOA are of primary interest (US EPA 2003a). Table 3.4: Relevant PFOA compounds (PFOA and its salts) (US EPA 2003a).
The technical properties of PFOA are described in Table 3.4. Table 3.5: Technical properties of PFOA (perfluorooctanoic acid) (US EPA 2002; ENVIRON, 2004).
3.1 Manufacturing of PFOA, PFOS, PFOS-related compounds and telomersThe total world production of PFOS, PFOS-related compounds, telomers and PFOA derivatives during the years and the emissions to the environment are unknown. In May 2000 the 3M Company announced that it would voluntarily cease manufacturing materials based on perfluorooctanesulfonyl fluoride (POSF) after a metabolite of this compound (PFOS) was found to be widespread in human population and wildlife (Olsen et al. 2003). Based on a production of 3,000 ton in 2000 by 3M alone (Giesy & Kannan 2002), the total annual world production is likely to have been more than 5,000 ton. Many of the applications of the POSF-related compounds lead to emissions to the environment during or shortly after use. Three different manufacturing processes exist for production of fluorinated compounds (Kemikalieinspektionen 2004; Kissa 2001). However, the first two mentioned processes are the ones, which are normally used (Moody et al. 2000; Hekster et al. 2002): 1. Direct fluorination using the electro-chemical fluorination (ECF). 2. Telomerisation. 3. Oligomerisation (the chemical process of creating oligomers (molecules of just a few monomers) from larger or smaller molecules). Both PFOA and PFOS-related compounds and their salts are produced by use of the above-mentioned methods. However, whereas PFOS and PFOS-related compounds are used commercially for numerous applications, PFOA is primarily used as its salts (primarily the ammonium and sodium salts) as non-reactive processing aids in the production of fluoropolymers and fluoroelastomers and in other surfactant uses. PFOS may be formed by degradation of PFOS-related chemicals. PFOA has been used as a surfactant in a number of commercial applications beyond fluoropolymers and may be formed through the transformation or metabolism of other polyfluorinated chemicals (such as telomer alcohols). However, as already stated, there is an ongoing discussion at the moment, whether the occurrence of PFOA in the environment is due to breakdown of the telomer alcohols, due to impurities in the telomer products or due to direct releases from PFOA use as an industrial chemical. 3.1.1.1 Direct fluorination using electro-chemical fluorinationThe ECF process – also called the Simons Electro Chemical Fluorination - was used by the 3M Company to manufacture PFOS and PFOA. The starting point of this process is 1-octanesulfonyl fluoride, which is dispersed in liquid hydrogen fluoride. An electric current is passed through the solution causing the hydrogen atoms on the octanesulfonyl fluoride to be replaced by fluorine (Kissa 2001; 3M 1999; Hekster et al. 2002). C8H17SO2F + 17 HF →C8F17SO2F + 17 H2 + by-products The electrochemical fluorination process yields about 35-40% straight chain (normal) perfluorooctane sulfonyl fluoride (POSF), and a mixture of by-products and waste of unknown and variable composition, e.g. branched-chain, straight chain or cyclic perfluoroalkylsulfonyl fluorides with various chain lengths (3M 1999). Despite low to moderate yields of perfluorinated compounds and the many side products, the ECF process is economically attractive because of the relatively low costs of electricity and the hydrogen fluoride reagent (Moody et al. 2000). POSF is a commercially viable product, but is primarily an important intermediate in the synthesis of many other fluorochemical products. 3M used POSF to produce PFOA, PFOS and other PFOS-related compounds (3M 1999). 3.1.1.2 TelomerisationDuPont, Daikin, Clariant and Ashai Glass and others use the telomerisation process. It yields a linear, even perfluorocarbon chain length product (Kissa 2001; Kemikalieinspektionen 2004). Commercial products manufactured through the telomerisation process are generally mixtures of polyfluorinated straight-chain compounds with ranges of even carbon numbers, but distillation can be used to obtain pure compounds (US EPA 2002). C2F5I + 3 C2F4→ C8F17I→ C8F17-surfactants A telomer is defined as a low molecular weight polymer chain in which the terminal group of the chain-like molecule is not the same as the side group (About 2004). Fluorotelomers are starting materials for the manufacturing of perfluorinated compounds and used as agents to incorporate a fluorinated carbon skeleton into polymeric materials. Polyfluorinated telomers are used in production of fire-fighting foams, cleaning agents, and oil-, stain-, and grease-repellent surface treatment agents for carpets, textiles, leather and paper (US EPA 2003b). PFOA is an unintended reaction by-product, present at trace levels in some telomer-based products (DuPont 2004). Furthermore, information indicates that certain polyfluorinated telomers, especially telomer alcohols, are transformed to PFOA in the environment or can be metabolised to PFOA, if absorbed by living organisms (US EPA 2003b; Renner 2004). If so, PFOA can be a widespread problem, as telomers are used in a wide range of applications. The ongoing discussion today is whether the occurrence of PFOA in the environment is due to impurities of PFOA in the telomers, due to telomer degradation to PFOA in the environment or due to direct releases of PFOA because of use as an industrial chemical. The US EPA has initiated a study (June 2004) to throw light on this aspect (Hogue 2004). 3.1.1.3 OligomerisationOligomerisation is the chemical process of creating molecules of just a few monomers (oligomers) from larger or smaller molecules. However, this process is not as widespread as the ECF process or the telomerisation process. 3.2 Producers of PFOS-compounds and PFOAThe largest global producer of PFOS-compounds has for long been the 3M Company. However, they have recently ceased their production of PFOS (but have continued the production of other perfluoro-compounds with shorter chain length). On the European market this has resulted in buyers shifting to other producers that have carried on the production of the PFOS-compounds (Renner 2001). OECD has identified the following producers of PFOS-compounds: Table 3.6: List of producers of PFOS-compounds according to OECD (OECD 2002).
The 3M Company was formerly the largest global manufacturer of PFOA. In 2002, the 3M Company stopped external sale of PFOA and now only manufactures for their own internal use. DuPont produces the ammonium salt of PFOA in the US for use as a processing aid in fluoropolymer manufacture (US EPA 2002; Personal communication, DuPont 2004a). OECD has identified the following producers of PFOA: Table 3.7: List of producers of PFOA according to OECD (US EPA 2002).
Footnotes[2] OSPAR is the current instrument guiding international cooperation on the protection of the marine environment of the North-East Atlantic.
|