|
| Front page | | Contents | | Previous |
More environmentally friendly alternatives to PFOS-compounds and PFOA
Appendix F Identified alternatives
Telomer-based products are in general alternatives to PFOS-related compounds. However, a search for other alternatives was carried out during the project. First of all, the Internet was used. Secondly, the
producers of relevance were contacted by email in order to learn more about the alternatives (see a more detailed description in appendix B). Furthermore, a different approach was used within the paint and
varnish area, as EnPro ApS (a laboratory within the area of paint, ink and varnish) participated in this project. A raw materials catalogue was used to identify possible producers of alternatives. An email was
sent to the companies that produced products with the same function as the listed fluorosurfactants in the catalogue. Therefore, more alternatives are presented within this area.
The following compounds have been identified as alternatives to PFOS-related compounds during the project. No alternatives to PFOA have been identified. Several producers of PFOA were asked about
alternative options to PFOA, and those who replied had no alternative options to PFOA. However, as described in the report, use of PFOA does not seem to be the biggest problem. This will be the
production of telomer alcohols that in the environment can degrade to PFOA.
One company mentioned that their products (fluorinated) can be used as alternatives for telomer based products as well.
Table 0.1: Alternatives to PFOS-compounds
| Alternative compound |
Product trade name |
Company |
Used in / used for |
Perfluorobutanesulfonate (PFBS) - C4
or based on different C4-perfluorocompounds
|
Novec® |
3M |
Paint and coatings industry. As electronic coating. Industrial
and commercial cleaning. Cleaner for solder flux residue. Degreasing
applications. |
Dodecafluoro-2-methylpentan-3-one
(CF3-CF2-C(O)-CF(CF3)2)
|
Novec® |
3M |
Fire-fighting fluid |
| C6 fluorocompounds (predominantly 80%) |
FORAFAC® |
DuPont |
Fire-fighting foam |
| CF3 or C2F5 pendant fluoroalkyl
polyethers |
PolyFox® |
OMNOVA Solutions Inc. |
Surfactant and flow, level, and wetting additive for coating formulations.
Also used in floor polish. |
| Propylated aromatics (naphthalenes or biphenyls) |
Ruetasolv® |
Rütgers Kureha Solvents Gmbh |
Water repelling agents for rust protection systems, marine paints,
coatings, etc. |
| Aliphatic alcohols (sulphosuccinate and fatty alcohol ethoxylates) |
Emulphor®, Lutensit® |
BASF |
Levelling and wetting agents |
| Sulfosuccinate |
(EDAPLAN® LA 451) |
Münzing Chemie |
Paint and coating industry: Wetting agents for water based applications
– e.g. wood primers |
| Sulfosuccinate |
(HYDROPALAT® 875) |
Cognis |
Paint and coating industry: Wetting and dispersing agents |
| Silicone polymers |
WorléeAdd® |
Worlée-Chemie |
Wetting agents in the paint and ink industry |
Each of the alternatives described above is presented more detailed in the following paragraphs.
PFBS – Perfluorobutanesulfonate
In June 2003, the 3M Company replaced the PFOS-compound in their Scotchgard products with PFBS (perfluorobutanesulfonate). 3M's Schotchgard products are cleaners and stain protectors for
carpets, leather, furniture, automotives, hard surfaces and other apparels. After the phase-out of PFOS in the Scotchgard products, the 3M Company first presented an aerosol-can based on non-perfluoro
chemistry. However, the product worked on water but not on grease. Therefore, 3M now uses the perfluoro-compound with a shorter chain length – C4 instead of the former C8 PFOS compound.
(Flouride Action Network, 2004).
In the following table properties of PFBS are presented. The technical and environmental properties are found in different product information at 3M's website (www.3m.com).
Table 0.2: Properties of the alternative PFBS
| Technical properties |
PFBS – perfluorobutane sulfonate |
| Trade name |
3M's Novec™ |
| CAS number |
Trade secret (the specific compound used in Scotchgard)® |
| Molecular formula |
C4F9SO3 |
| Structural formula |
CF3-CF2-CF2- CF2-S(=O)(=O)O-
|
| Appearance |
Yellow viscous liquid
(a product Novec™ with 50% fluoropolymer)
|
| Melting point |
Not applicable |
| Boiling point |
200 °C (a product Novec™ with 90% fluoropolymer) |
| Flash point |
64 °C (a product Novec™ with 50% fluoropolymer)
82 °C (a product Novec™ with 90% fluoropolymer)
|
| Vapour pressure |
0.29 mm Hg at 20 °C
(a product Novec™ with 90% fluoropolymer)
|
| Solubility in water |
Dispersable in all proportions |
| Specific gravity |
1.06 kg/l (a product Novec™ with 50% fluoropolymer)
1.14 kg/l (a product Novec™ with 90% fluoropolymer)
|
| Viscosity |
30 cps (a product Novec™ with 50% fluoropolymer)
4000-6000 cps (a product Novec™ with 90% fluoropolymer)
|
| Surface tension |
25 dynes/cm at 25 °C in toluene, 0.5% surfactant by weight
(a product Novec™ with 50% fluoropolymer)
|
| Recommended use level |
Between 0.1% and 0.5% active surfactant |
| Environmental properties |
|
| Classification |
? |
| Bioconcentration factor |
<1 |
| Acute ecotoxicity |
LC50 96-hr. (fathead minnow) > 1000 mg/l |
| Toxicity |
Acute oral LD50 (rats) > 2000 mg/kg |
| Log KOW |
- |
| Air/water partition coefficient in pure water |
- |
| Degradability |
- |
C6-fluorinated compound
According to 3M's website, a C6-fluorinated compound is used in their Fire Protection Fluid. The compound is dodecafluoro-2-methylpentan-3-one (CF3-CF2-C(=O)-CF(CF3)2). This compound is also
a part of the Novec product series.
Table 0.3: Properties of the alternative C6 – fluorinated compound
| Technical properties |
C6-fluorinated compound |
| Trade name |
3M's Novec (Novec™ 1230) – consists of 99.9% of the fluorocompound |
| CAS number |
756-13-8 |
| Molecular formula |
C6F12O |
| Structural formula |
CF3-CF2-C(O)-CF-(CF3)2 |
| Appearance |
Clear colorless liquid |
| Melting point |
-108 °C |
| Boiling point |
49 °C |
| Flash point |
- |
| Vapour pressure |
244 mmHg at 20 °C (0.404 bar) |
| Solubility in water |
Nil |
| Specific gravity |
1.6 (water = 1) |
| Viscosity |
0.6 centipoise at 25 °C |
| Surface tension |
- |
| Recommended use level |
4-6% |
| Environmental properties |
|
| Classification |
- |
| Bioconcentration factor |
- |
| Acute ecotoxicity |
- |
| Toxicity |
- |
| Log KOW |
- |
| Air/water partition coefficient in pure water |
- |
| Degradability |
- |
Fluorotelomer-based products
DuPont manufactures a range of fluorotelomers especially designed for fire-fighting foam formulations – DuPont FORAFAC®. These fluorotelomer products are predominantly based on C6 molecules (more
than 80%). According to DuPont, their FORAFAC® 1157 and 1183 products are based on more than 95% C6 telomer, whereas FORAFAC® 1157N is based on more than 65% C6 telomer. (Personal
communication DuPont, 2004).
Table 0.4: Properties of DuPont's Forafac
| Technical properties |
|
| Trade name |
Forafac® 1203 |
| CAS number |
161278-39-3 (EC-No. 500-631-6) for fluoropolymer used |
| Molecular formula |
|
| Structural formula |
|
| Appearance |
Brown liquid
(a product FORAFAC® 1203 with 8-14% fluoropolymer)
|
| Melting point |
-22 °C (with 8-14% fluoropolymer) |
| Boiling point |
95 °C at 1013 hPa (with 8-14% fluoropolymer) |
| Flash point |
> 100 °C (with 8-14% fluoropolymer) |
| Vapour pressure |
- |
| Solubility in water |
Completely soluble |
| pH |
8.5 |
| Relative density |
1.07 at 20°C |
| Viscosity |
22 Mpas at 1000 s-1 (with 8-14% fluoropolymer) |
| Surface tension |
16.5 mN/m at 25 C in toluene, 3% surfactant (with 8-14% fluoropolymer) |
| Recommended use level |
0.5 g FORAFAC per liter
3% solution
|
| Environmental properties |
|
| Classification |
R52/53 Harmful to aquatic organisms, may cause long-term adverse
effects in the aquatic environment |
| Bioconcentration factor |
- |
| Acute ecotoxicity |
- |
| Toxicity |
Acute oral LD50 (rats) > 5000 mg/kg |
| Log KOW |
- |
| Air/water partition coefficient in pure water |
- |
| Degradability |
- |
Fluorinated polyether
One of the contacted companies in search for alternatives gave the following information about their alternative, which is a fluorinated polyether. This has specifically been used in about 40 floor polish
products in the USA, Europe and Asia. According to OMNOVA the PolyFox product line is, besides an alternative to PFOS-based products, also an alternative to the long chained telomer based products
(which have showed to have the potential to degrade to PFOA in the environment). (Personal communication OMNOVA, 2004).
OMNOVA Solutions Inc. manufactures a line of fluorosurfactants called PolyFox™. The entire PolyFox family of fluorosurfactants is polymers with a molecular weight greater than 1,000. The PolyFox
polymers are based on ether links – both the polymer backbone linkages and the link between the backbone and the perfluoroalkyl pendant side chains. The PolyFox flurosurfactants are synthesized from
perfluoroalkyl starting materials with a fully fluorinated carbon chain length of C4 or less. The current first generation products are all made with C2F5 or CF3 perfluoroalkyl side chain structures. (Personal
communication OMNOVA, 2004).
The fluorinated polyethers are either hydroxy-terminated or acrylate-terminated according to the MSDS's found for PolyFox products.
The basic structure of some of the different PolyFox™ compounds is illustrated in the figures below. (Personal communication, PoraTek, 2004).
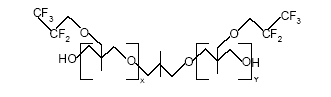
Figure 0.1: The basic structure of PolyFox 3320 compound (Personal communication, PoraTek, 2004). x+y equals about 20.
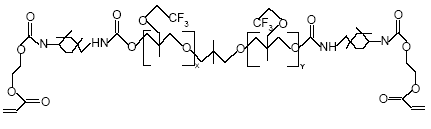
Figure 0.2: The basic structure of PolyFox 136 A with CF3 as the basic perfluoralkyl group (Personal communication, PoraTek, 2004). x+y equals about 6.
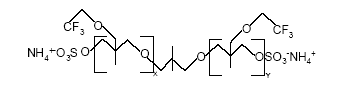
Figure 0.3: The basic structure of PolyFox 156 A with C2F5 as the basic perfluoralkyl group (Personal communication, PoraTek, 2004). x+y equals about 6.
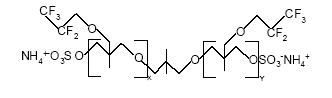
Figure 0.4: The basic structure of PolyFox 656 with C2F5 as the basic perfluoralkyl group. The technical properties of this compound are described in Table 0.5 (Personal communication, PoraTek, 2004).
x+y equals about 6.
The technical properties listed in the table below are for PolyFox 656, which is shown in Figure 7.2 just above. However, all PolyFox compounds have similar technical properties.
Table 0.5: Properties of the alternative fluorinated polyether
| Technical properties |
Fluorinated polyether |
| Trade name |
OMNOVA's PolyFox (PolyFox™ 656 Fluorosurfactant) |
| CAS number |
Proprietary |
| Molecular formula |
- |
| Structural formula |
- |
| Appearance |
Liquid colourless viscous oil |
| Melting point |
< -16 °C |
| Boiling point |
> 150 °C |
| Flash point |
139 °C |
| Vapour pressure |
< 1 mm at 20 °C |
| Solubility in water |
< 1% |
| Specific gravity |
1.28 |
| Viscosity |
- |
| Surface tension |
- |
| Recommended use level |
150 ppm active material (for PolyFox PF-136A) |
| Environmental properties |
|
| Classification |
R43 May cause sensitisation by skin contact |
| Bioconcentration factor |
- |
| Acute ecotoxicity |
- |
| Toxicity |
Acute oral LD50 (rats) > 2000 mg/kg |
| Log KOW |
- |
| Air/water partition coefficient in pure water |
- |
| Degradability |
- |
Propylated aromatics
Rütgers Kureha Solvents produces different propylated aromatics (naphthalenes and biphenyls), which can be used as water repelling agents for different applications. For example rust protection systems,
marine paints, resins, printing inks, coatings, electrical applications, electronically and mechanical applications. (Personal communication RKS, 2004). The different propylated aromatics are presented in
table Table 0.6 below and some of the compounds are illustrated in the figures below.
Figure 0.5: The chemical structure of Ruetasolv DI (CAS-No. 38640-62-9) – to the left, and Ruetasolv TTPN (CAS-No. 35860-37-8) – to the right.
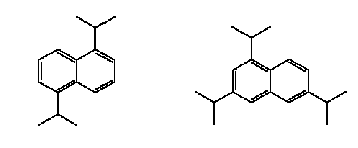
Figure 0.6: The chemical structure of Ruetasolv BP 4103 (CAS-No. 25640-78-2) – to the left, and Ruetasolv BP 4201 (CAS-No. 69009-90-1) to the left. Both products are mixtures of isomers.
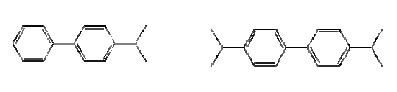
Table 0.6: Properties of the alternative propylated aromatics
| Technical properties |
Propylated aromatics |
| Trade name |
Ruetasolv DI |
Ruetasolv BP 4201 |
Ruetasolv BF 4103 |
Ruetasolv TPPN |
1-/2-Isopropyl-naphthalene |
| CAS number |
38640-62-9 |
69009-90-1 |
25640-78-2 |
35860-37-8 |
38640-62-9 |
| Molecular formula |
C16H20 |
C18H22 |
C15H16 |
C19H26 |
C13H14 |
| Structural formula |
(CH3)2-CH-C5H3-C5H3-CH-(CH3)2 |
Mixture of isomers |
Mixture of isomers |
|
|
| Appearance |
Colourless liquid |
Colourless liquid |
Colourless liquid |
Colourless liquid |
Colourless liquid |
| Melting point |
- |
< -10 °C |
18 °C |
- |
< -9 C |
| Boiling point |
290-300 °C |
300-335 °C |
293-315 °C |
325-350 °C |
255-265 °C |
| Flash point |
> 140 °C |
> 140 °C |
> 140 °C |
> 160 °C |
118 °C |
| Vapour pressure at 20 °C |
0.0003 kPa |
0.0002 kPa |
- |
0.0002 kPa |
0.0069 hPa |
| Solubility in water at 20 °C |
< 0.02 mg/l |
Not miscible |
Not miscible |
Not miscible |
Not miscible |
| Specific gravity at 15 °C |
0.960 kg/l |
0.965 kg/l |
0.988 kg/l |
0.935 kg/l |
0.982 kg/l |
| Viscosity at 20 °C |
12 mPas |
26 mPas |
- |
75 mm2/s |
4 mm2/s |
| Viscosity at 40 °C |
6 mPas |
11 mPas |
4.2 mPas |
18-24 mm2/s |
2-3 mm2/s |
| Surface tension at 20 °C |
33.4 mN/m |
- |
- |
- |
- |
| Recommended use level |
- |
- |
- |
- |
- |
| Environmental properties |
|
|
|
|
|
| Classification |
None |
None |
None |
None |
None |
| Bioconcentration factor |
Low bio-accumula-tion |
- |
- |
- |
- |
| Acute ecotoxicity - LC50 96-hr. (fish) |
LC0 value 0.5 mg/l above sat. |
- |
- |
- |
- |
| Toxicity - Acute oral LD50 (rats) |
> 3900 mg/kg |
- |
- |
- |
- |
| Log KOW |
> 4 |
- |
- |
- |
- |
| Air/water partition coefficient in pure water |
- |
- |
- |
- |
- |
| Degradability |
Easily degradable |
- |
- |
- |
- |
Aliphatic alcohols
BASF produces a large range of surfactants for different purposes. In coatings application fluorosurfactants are, according to BASF, mainly used for substrate wetting, levelling and reduction of surface
tension (e.g. in spray applications). The key to the often-superior performance of the fluorosurfactants is the extremely low surface tension, which cannot be matched with other surfactants. However, due to
high prices of the fluorosurfactants and due to environmental reasons, other surfactants can be used as alternatives if it is not a technical must to achieve such low surface tension levels.
Possible replacements to fluorosurfactants are silicone surfactants, which BASF does not produce, or surfactants based on aliphatic alcohols. BASF produces a range of aliphatic alcohols, both anionic and
non-ionic surfactants. However, especially the effect of the non-ionic surfactants is mixed, because these products can also be used as defoamers or emulsifiers. So the non-ionic surfactants are difficult to
use as replacements for fluorosurfactants. Therefore, only the anionic surfactants of BASF (Emulphor® FAS 30 and Lutensit® A-BO) are described in details. The Emulphor product from BASF is a fatty
alcohol polyglycolether sulfate, whereas the Lutensit A-BO product is a sulfosuccinate. The Lutensit A-BO product is therefore described together with the other sulfosuccinate products. (Personal
communication BASF, 2004).
Table 0.7: Properties of the alternative fatty alcohol ethoxylate
| Technical properties |
Fatty alcohol polyglycol ether sulfate, sodium salt |
| Trade name |
Emulphor® FAS 30 |
| CAS number |
- |
| Molecular formula |
- |
| Structural formula |
- |
| Appearance |
Yellowish liquid |
| Melting point |
- |
| Boiling point |
> 100 °C |
| Flash point |
> 100 °C |
| Vapour pressure |
- |
| Solubility in water |
Soluble (5% in distilled water at 23 °C) |
| Specific gravity |
1.057 kg/l |
| Viscosity |
100 mPas at 23 °C |
| Surface tension |
46 mN/m at 23 °C |
| Recommended use level |
- |
| Environmental properties |
|
| Classification |
None |
| Bioconcentration factor |
- |
| Acute ecotoxicity |
LC50 96-hr. (leuciscus idus): > 100 mg/l |
| Toxicity |
Acute oral LD50 (rats) > 2000 mg/kg |
| Log KOW |
- |
| Air/water partition coefficient in pure water |
- |
| Degradability |
Readily biodegradable (>70% elimination OECD 301E) |
Sulfosuccinate
Information from Münzing Chemie
Münzing Chemie produces wetting agents for water-based applications, which are used to lower the surface tension of water to wet substrates or pigment surfaces. With some of their products they are able
to replace fluorobased wetting agents, e.g. in wood primers. The best product for this kind of applications is their product EDAPLAN LA 451, which is based on a sulfosuccinate derivative in ethanol (19%)
and water (12.5%). Sulfosuccinates are esters of succinic acid (HOOC-CH2-CH2-COOH) in reaction with hydrogen sulfite. The product EDAPLAN 451 contains a mixture of chemicals. (Personal
communication with Münzing Chemie, 2004)
Table 0.8: Properties of the alternative Sulfosuccinate (by Münzing Chemie)
| Technical properties |
Sulfosuccinate |
| Trade name |
EDAPLAN® (LA 451) |
| CAS number |
- |
| Molecular formula |
- |
| Structural formula |
- |
| Appearance |
Yellow fluid |
| Melting point |
Not determined |
| Boiling point |
78 °C |
| Flash point |
27 °C |
| Vapour pressure |
Steam pressure of 57 hPa at 20 °C |
| Solubility in water |
Fully miscible with water |
| Specific gravity |
- |
| Viscosity |
200 s at 20 °C |
| Surface tension |
27.0 mN/m (1% in water) |
| Recommended use level |
0.1 to 1% |
| Environmental properties |
|
| Classification |
? |
| Bioconcentration factor |
- |
| Acute ecotoxicity |
- |
| Toxicity |
- |
| Log KOW |
- |
| Air/water partition coefficient in pure water |
- |
| Degradability |
- |
Information from Cognis
Cognis produces surfactants, wetting agents and dispersing agents for the paint and coating industry. The group of products technically competing with fluorosurfactants are sulfosuccinates. However, the
sulfosuccinates do not reach such low surface tensions as it is possible with fluorinated surfactants, but nevertheless sulfosuccinates produced by Cognis are widely used in printing inks, where they also are
approved for food contact. The best product for competing with fluorinated surfactants is their product Hydropalat® 875, which is based on the sodium salt of di(2-ethylhexyl) sulfosuccinate in ethanol (5%)
and water (20%). (Personal communication with Cognis, 2004)
Table 0.9: Properties of the alternative Sulfosuccinate (by Cognis)
| Technical properties |
Sulfosuccinate |
| Trade name |
Hydropalat® 875
Chemical compound: di(2-ethylhexyl) sulfosuccinate, sodium salt
|
| CAS number |
577-11-7 |
| Molecular formula |
C20H37NaO7S |
| Structural formula |
(C4H9-CH-(C2H5)-CH2-O-C(O)-CH2)2
–CHSO3- Na+ |
| Appearance |
Clear liquid |
| Melting point |
153-157 °C |
| Boiling point |
Not determined |
| Flash point |
45 °C (113 F) |
| Vapour pressure |
Not determined |
| Solubility in water |
Appreciable (>10%) |
| Specific gravity |
1.07 |
| Viscosity |
- |
| Surface tension |
- |
| Recommended use level |
0.1-1% calculated of the finished coating
1-3% calculated on monomer content for polymerisation
|
| Environmental properties |
|
| Classification |
? |
| Bioconcentration factor |
- |
| Acute ecotoxicity |
- |
| Toxicity |
Acute oral LD50 (rats) = 1900 mg/kg |
| Log KOW |
- |
| Air/water partition coefficient in pure water |
- |
| Degradability |
- |
Information from BASF
As described in the previous section BASF also produces a sulfosuccinate products, which can be used as an alternative to fluorosurfactants within the coating industry.
The Lutensit® A-BO product of BASF contains a di-octyl-sulfosuccinate (di(2-ethylhexyl) sulfosuccinate), which has the following structure.
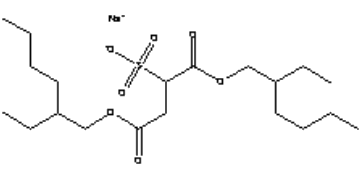
Table 0.10: Properties of the alternative sulfosuccinate
| Technical properties |
Sodium di(2ethylhexyl) sulfosuccinate |
| Trade name |
Lutensit® A-BO
- 50-60% di(2ethylhexyl) sulfosuccinate
- 5-10% 2,2-dimethylpropane-1,3-diol
|
| CAS number |
577-11-7 |
| Molecular formula |
C20H37NaO7S |
| Structural formula |
|
| Appearance |
Yellowish liquid |
| Melting point |
- |
| Boiling point |
- |
| Flash point |
> 100 °C |
| Vapour pressure |
20 mbar at 20 °C, 97 mbar at 50 °C |
| Solubility in water |
Forms cloudy solution |
| Specific gravity |
1.11 kg/l |
| Viscosity |
250 mPas at 23 °C |
| Surface tension |
29 mN/m (0.1% in destilled water) |
| Recommended use level |
- |
| Environmental properties |
|
| Classification |
Xi, R38 Irritating to skin, R41 Risk of serious damage to eyes |
| Bioconcentration factor |
- |
| Acute ecotoxicity |
LC50 96-hr. (leuciscus idus): 10-100 mg/l |
| Toxicity |
Acute oral LD50 (rats) > 2000 mg/kg |
| Log KOW |
1.0 |
| Air/water partition coefficient in pure water |
- |
| Degradability |
Readily eliminated from water (>90% elimination OECD 303A) |
Silicone polymers
Worlée-Chemie produces silicon polymers, which in the paint and ink industry in several cases can be used as alternative wetting agents to fluorosurfactants. Two products are emphasized: WorléeAdd® 340
and WorléeAdd® 345. The first product is a non-ionic modified silicone polyether, where the latter is a mixture of a silicone polyether and a dioctylsulfosuccinate in ethanol and water. (Personal
communication Worlée-Chemie, 2004).
Table 0.11: Properties of the alternative silicone polymers
| Technical properties |
Silicone polymers |
| Trade name |
WorléeAdd® 340
100% active substance
|
WorléeAdd® 345
71% active substance
|
| CAS number |
67674-67-3 |
67674-67-3 (10-15%) and dioctylsulfosuccinate (50-55%) |
| Molecular formula |
|
|
| Structural formula |
|
|
| Appearance |
Clear to slightly hazy, yellow coloured fluid |
Slightly turbid yellowish liquid |
| Melting point |
- |
- |
| Boiling point |
> 35 °C |
- |
| Flash point |
>100 °C |
>23 °C |
| Vapour pressure |
- |
- |
| Solubility in water |
- |
- |
| Specific gravity |
1.03 kg/l |
1.0 kg/l |
| Viscosity at 20 °C |
55-85 mPas |
80-160 mPas (117 mPas) |
| Surface tension |
28 mN/m (0.5%) |
32 mN/m (0.5%) |
| Recommended use level |
0.1 – 1.0% |
0.3-1.5% on total formulation |
| Environmental properties |
|
|
| Classification |
Xn R20 Harmful by inhalation
N R51/53 Toxic to aquatic organisms, may cause long term adverse effects in the
aquatic environment
Xi R41 Risk of serious damage to eyes
|
Xi R10 Flammable
R52/53 Harmful to aquatic organisms, may cause long term adverse effects in the
aquatic environment
R41 Risk of serious damage to eyes
|
| Bioconcentration factor |
- |
- |
| Acute ecotoxicity |
- |
- |
| Toxicity |
- |
- |
| Log KOW |
Not determined |
Not determined |
| Air/water partition coefficient in pure water |
- |
- |
| Degradability |
- |
- |
| Front page | | Contents | | Previous | | Top |
Version 1.0 June 2005, © Danish Environmental Protection Agency
|






