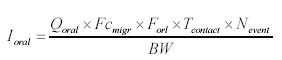|
Migration and health assessment of chemical substances in surface treated wooden toys 4 Health assessment
A screening of potential health hasardous effects from substances released (migrated) from surface treated wooden toys has been performed. A literature screening on the substances identified at the qualitative migration examinations is performed. It has the purpose to make sure that the substances focused on at the qualitative analyses are the most relevant substances. After the quantitative analyses are carried out the appeared results are evaluated. Data on individual substances such as NOAEL, LOAEL or other relevant data are used to the extent they are available. Alternatively, QSAR data are used for substances where no data are found. 4.1 IntroductionTo the assessment of the health risk from the use of wooden toys, a selection of effects on health by the detected substances are evaluated in relation to the relevant exposure duration and exposure route to the consumer of the toy. 4.1.1 Exposure durationThe average potential exposure duration, i.e. the period where the child is awake and not eating is 10 hours/day for children under 2 years of age and 10.7 hours/day for children between 2 and 3 years of age in an American study (Kiss 2001). In a Dutch study, the period was 8 to 9 hours for children between 3 and 36 months of age (Groot et al. 1998, table 5). The oral intake strongly depends on the exposure duration, i.e. how often and for how long the toy is placed in the mouth. That question has been studied especially in connection to the assessment of the exposure to phthalates in plastic toys. Studies on the behaviour of children mouthing subjects including fingers have for instance been performed in several studies. Groot et al. (1998) studied 42 children between 3 and 36 months of age and Juberg et al. (2001) studied 385 children between 0 and 36 months of age. Their studies show that the youngest children mouth subjects more often than older children do (table 5). Table 5 Average period of toys in the mouth (minutes) and exposure duration (hours) per day (Groot et al. 1998)
*: Other than comforter, teething ring etc. In an American observation study of the mouthing behaviour of children 0 to 6 years of age, the behaviour of 491 children was studied by parents and 169 children at the age if 0 to 36 months by trained observers. In conclusion it was observed that for all objects mouthed except pacifiers the average mouthing period was 70 minutes/day for children between 3 months and 1 year, 48 minutes for children between 1 and 2 years, and 37 minutes for children between 2 and 3 years of age (Kiss 2001) In a statistical analysis of the same numbers, Greene (2002) has examined the behaviour in 169 children between 3 and 36 months of age. He found a skewness in exposure as few children mouthed objects for a long period and many children mouthed objects for a short time or not at all. This observation was supported by e.g. a previous American study on the play activity of children. The study finds average times of 46 to 70 minutes and 90 percentiles for play activity of children (1 to 17 years of age) between 120 and 255 minutes per day (US-EPA 1997, US-EPA 2002). In a British study on the mouthing behaviour of children, 236 children at the age of 1 month to5 years were studied (DTI 2002). The study found a peak at the age of 9 months when only observing the period of 0 to 3 years of age (table 6). Table 6 Estimated maximum daily oral exposure (hours : min) (DTI 2002)
In the DTI study 50% of the toys were made of plastic and 6% of wood. However, that distribution depends on the availability of toys and can therefore not be included in the evaluation. The exposure duration for playing with wooden toys may vary considerably but most common is assumed to be several short-term uses that may vary from a few to many minutes. This study especially focuses on the oral exposure, i.e. exposure after mouthing the toy and sucked on it, biting it etc. by children of 0 to 36 months of age. In the CSTEE evaluation on exposure to plasticisers was recommended an exposure period of 3 hours in the assessment of migrated phthalates to children (CSTEE 1998). Based on the above observations/studies which indicate that children at the age of 3 to 36 months mouth toys at an average of 70 minutes and at maximum (90 percentile) of 3 hours and the recommendation of CSTEE, the assessment in this project has selected an exposure duration of 3 hours/day as a reasonable worst case scenario. 4.1.2 Body weightSince the exposure is given in mg/kg body weight it is evaluated which body weight that would be an appropriate average for children. Studies by Bremmer and van Veen (2002) and US-EPA (2002) have been obtained. The results are presented in table 7, below). Table 7 Body weight of children
Whether the differences between Dutch and American children is actual or a result of the selection of children and variation in numbers are unknown. The study by Bremmer and van Veen covers children of "well educated" parents and a small number whereas the American study covers a wider section of children and a far higher number of children. In the CSTEE evaluation of the exposure to plasticisers was recommended a body weight of 8 kg in the assessment of migrated phthalates on children (CSTEE 1998). As the study especially relates to very small children that weight may be a little too low for the target group of this project. The study further is indicated to be based on the Bremmer and van Veen / Groot et al. studies. In regard of realistic worst case, this project therefore in the calculations uses an assumed body weight of 10 kg for children based on the far more extensive American study (US-EPA 2002) and a peak of mouthing behaviour at 9 months of age (DTI 2002). That wooden toys may be purchased or intended for a little older child does not exclude the possibility that younger children may or will get hold of the toys. 4.1.3 Exposure routeDuring the screening it was realised that some the detected substances were volatile substances and an exposure via inhalation possible. However, this exposure route is considered to be less essential in this context. Exposure of the skin (dermal exposure) is considered relevant as wooden toys are specifically intended to "handling". The primary exposure is to the skin of the hands. Intake vi the mouth (oral intake) is assumed potentially to present the largest concern for children under 3 years of age, partly because this age group is known to mouth objects but they may also suck on fingers after having touched the toy and thereby transfer potential contamination from the hands to the mouth. 4.1.4 UptakeUptake via inhalation, oral or dermal exposure will be substance specific and therefore depend on which substances that are detected released from the toy. If no information was available on the specific uptake of the individual substances via inhalation, by dermal contact or via moth / mucous membranes an uptake of 100% is assumed. In the study, 14 specific substances have been selected in co-operation with the Danish Environmental Protection Agency. The selection is based on the classification of the substances, number of detections and the level of the measured concentration in the saliva extracts. The selected substances have been reviewed individually after a presentation of the assessment method (cf. below). Each of the selected substances has been identified by its common name and CAS no. for unambiguously identification. The most common synonyms are stated and furthermore is mentioned:
Finally, an assessment of the amount of detected released substances has been carried out. This has been performed by calculating / estimating the uptake based on the duration of exposure and the body weight of the person (amount/kg body weight/day). If possible, one of the established values for tolerable daily intake (TDI, ADI or RfD) is used for evaluation of the exposure by comparing the values with the obtained analysis results used to estimate the exposure. The used uncertainty factors (safety factors) are mentioned in the text. In case more TDI, ADI or RfD values exist, the lowest value is preferred. If no TDI, ADI, RfD values are available, a comparison to a concentration where no adverse effects are observed (NOAEL: No Observed Adverse Effect Level) from a relevant long-term study is used. The procedure is mentioned at the individual substances. 4.2 Exposure scenarios4.2.1 IntroductionThe exposure to the consumer from wooden toys will vary considerably according to use duration, handling or area of contact and duration of direct contact. To evaluate the exposure in a standardised manner, theoretical exposure scenarios have been derived to illustrate the worst possible but realistic exposures. To evaluate the exposure of consumers, the following scenario has been derived for the exposure via the mouth (oral exposure). Oral exposure is based on measurements of the substance in extractions of artificial saliva. It is assumed that the amount of substance released (migrated) from the toys during an average time of 2 hour extraction x 1.5 = 3 hours corresponds to the potential maximum oral exposure per day. (Note that the extraction is performed for 2 hours according to the standard EN71-3 and recalculated to 3 hours exposure period as the target period). 4.2.2 MethodologyFor the chemical substances detected as migrated to saliva from the wooden toys, an evaluation of which substances appeared to be the most interesting (cf. section 3). Data on the individual substances are retrieved to perform a health hazard evaluation based on known information from previously prepared Danish or foreign monographs, etc. The obtained data for threshold limit values or toxicity are then compared to the concentrations estimated in the used scenario. The methodology used is approximately the same as recommended in connection to risk assessments in the European Union (EU) described in the Technical Guidance Document, TGD (EC 2003). In the TGD the potential risk to humans is estimated as the ratio between the predicted no-effect concentration (no-adverse-effect level, NOAEL) and the predicted exposure concentration in the surrounding environment (Predicted Environmental Concentration, PEC), i.e. NOAEL / PEC or the estimated uptake in the exposed humans. NOAEL is based on mammalian data that is often not humans but typically rats, mice and rabbits. Therefore, a safety factor is introduced to cover differences extrapolating from other animals to humans. This is expressed either by attaching a fixed safety factor (SF) or by expressing the margin of safety (MOS) which represents the distance between NOAEL and the estimated uptake. Typically MOS is preferred to be above 100. The safety factor is interpreted as a margin of safety applied to a NOAEL to produce a value below which exposures are presumed to be without health risk. The safety factor is traditionally composed of a factor 10 for extrapolation between species (animal to human, interspecies variation), a factor 10 to protect the most sensitive individuals of the population (intraspecies variation) such as e.g. children. A third factor is applied depending on the data and may vary. For instance 10 is used if LOAEL (lowest observed adverse effect level) is used instead of NOAEL or using subchronic data instead of chronic data. The total safety factor is a result from multiplication of the three factors. The effect level divided with the safety factor is used to evaluate whether there is reason of concern (concern level) or a further examination of methodology or data is necessary. Thus the assessment may be expressed on basis of concentration divided with the safety factor (such as e.g. ADI, TDI, RfD, RfC) or MOS. In modern society is used many chemical products. It can be difficult for the single consumer to keep track of them all. The handling of the chemical substances is therefore regulated on basis of an extended chemical legislation. In connection with this project no values have been derived for chemical substances already evaluated by national or international experts in the field. The classification authorised in Denmark (Miljøministeriet 2002), which is an implementation of EU classification (28th amendment to EU directive 67/548/EEC), is used in the evaluation. The amendments performed in the 29th amendment and adopted in Directive 2004/73/EC (EC 2004) and not yet implemented in Denmark are included, however, as the implementation may be expected within the near future. For the evaluation of the individual substances is used the threshold limit values mentioned above and explained below. The threshold limit value (TLV) valid for the working environment in Denmark (AT 2002) is generally not used as it is only valid for the working environment and does not cover the consumer at home. The TLV value is presented for information and comparison, if available. Other limit values included in the health evaluation were:
The effect level for each piece of wooden toy is based on evaluations of individual substances. The established Danish threshold limit values are used when they exist. When no Danish threshold limit values exist, foreign threshold limit values are used including their background if available. 4.2.3 Exposure via the mouthBy oral exposure the absorption takes place after release (migration) of the compounds from the wooden toy and mixing with the saliva. Uptake is assumed to takes place over the epithelium in the mouth cavity or in the gastro-intestinal-tract. As basis for the assessment of the oral intake is used the general equations described in relevant references (TGD 2003, OECD 1993, Bremmer and van Veen 2002). The equations are then adjusted to an equation fit for the actual scenario with measurements of chemical substances migrated to artificial saliva. The weight of the surface area of the toy (10 cm2 x sample depth x density) which the child may have in the mouth for a prolonged time is used in the exposure assessment.
where
As basis is assumed that a child plays with the wooden toy one or several times each day. The total oral exposure duration is assumed to be maximum 180 minutes or 3 hour/day. The child's body weight is set to 10 kg. The analysis results represent the amount migrated to the saliva extracts after 2 hours of extraction according to the standard EN 71-3. The amount is recalculated to release per hour and multiplied by 3 (3 hours average). The migration is calculated as release per cm2. According to the standard EN71-10 the sample should be 3 mm deep. Using the density of the wood used to make the wooden toy (based on information on the density of dry wood) the released amount per cm2 is calculated. Table 8 Density of dry wood, 12% moisture (Danish Technological Institute, Wood Technology, Træteknik 2004)
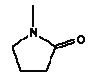
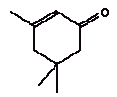
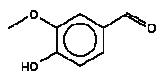
The released amount/cm2 is the measured concentration x sample depth x density of sample: Qoral (μg/cm2) = Fcmigr (μg/g) sample depth (0.3 cm) density (0.7 g/cm3) The calculations are performed as released substance per 10 cm2/hour (based on the analysis extraction over 2 hours) exposure duration/day absorption/kg body weight: Thus oral absorption = Weight of exposed toy μg substance released/hour 1/2 (hours) exposure duration (3 hour/day) (% absorption/100%) / 10 (kg) = μg/kg body weight per day. It is noted that oral intake also may take place by hand-to-mouth, i.e. hands or fingers, which have touched the product, and then are placed in the mouth. This may result in a transfer of substance from the fingers to the mouth. As information in the reference literature (Bremmer and van Veen 2002, Green 2002, Kiss 2001) indicate that hand-to-mouth averages 3 to 10 minutes that part is considered included in the selected exposure period of 3 hour. Absorption After exposure to the mouth cavity the chemical substance has to pass the epithelium before actual absorption may take place. Data for oral absorption have not been available for all of the selected substances. The oral uptake across the epithelium (oral absorption) is therefore estimated or set to 100% for many for the substances (TGD, EC 2003). 4.3 Evaluation of individual substancesFor the evaluation of individual substances the below mentioned chemical substances have been selected in co-operation with the Danish Environmental Protection Agency. Of organic substances: 2-Butoxyethanol 2-(2-Butoxyethoxy)ethanol Cyclohexanone 2,6-Dimethoxybenzoquinone 3,6-Dimethyl-1,4-dioxane-2,5-dione (3,6-Dimethyl-2,5-dioxo-1,4-dioxane) 2-Ethoxyethanol 2-(2-Ethoxyethoxy)ethanol Formamide Furfural 4-Hydroxy-3,5-dimethoxy-benzaldehyde N.Methyl-2-pyrrolidone 3,5,5-Trimethyl-2-cyclohexen-1-one (= Isophorone) Vanillin Of inorganic substances: Barium 4.3.1 2-ButoxyethanolIdentification
The melting point is –74.8°C. The boiling point is 168.4°C (DOW 1990). The vapour pressure is 117 Pa at 25°C (0.88 mmHg, DOW 1990). The water solubility is 1 kg/l at 25°C (miscible, DOW 1990). The partition coefficient is log Kow is experimentally determined to 0.83 (Hansch et al. 1995). Use 2-Butoxyethanol is used as a solvent in surface coatings and in vinyl and acrylic paint (CICAD 1998). Further is mentioned the use as solvent in printing inks and colorants in the EU risk assessment report, draft 2004 (ECB 2004). Classification 2-Butoxyethanol is classified in the List of dangerous substances (Miljøministeriet 2002):
Effects on health 2-Butoxyethanol is moderately acute toxic, irritating to eyes and skin (but not a skin sensitizer). Eye irritation examinations showed that 30 and 70% concentrations of the substances were irritating to the eyes with increasing irritation with corresponding increasing exposure duration. The skin irritation was mild at 4 hours of exposure of rabbit skin, but the irritation increased with increasing exposure duration (CICAD 1998). The effects have mostly been registered as a haemolytic activity of 2-butoxy-ethanol. The effect was dependent on age with older rats as the most sensitive (CICAD 1998). Acute toxicity:
In a subchronic 90 days inhalation study, rats were exposed to 2-butoxyethanol at concentrations of 0, 5, 25, or 77 ppm for 6 hours/day, 5 days/week for 13 weeks. Based on haematotoxic effects, the NOAEL and LOAEL were 25 ppm (121 mg/m3) and 77 ppm (372 mg/m3), respectively (Dodd et al. 1983). In a study on developmental effects, pregnant rats were exposed to 2-butoxyethanol at 0, 25, 50, 100 or 200 ppm (35 rats per group) for 6 hours/day on days 6 to 15 of gestation. Based on haematotoxic effects the NOAEL and LOAEL were 50 ppm (242 mg/m3) and 100 ppm (483 mg/m3), respectively (Tyl et al. 1984). In a 13 weeks study with rats, groups of 10 of each sex were exposed through the drinking water. Based on the water consumption, the male rats were exposed to 0, 69, 129, 281, 367 or 452 mg/kg/day and female rats to 0, 82, 151, 304, 363 or 470 mg/kg/day. Based on effects of the blood parameter and liver, which were observed at even the lowest concentration, LOAEL was 69 mg/kg/d for males and 82 mg/kg/d for females. When water consumption and body weight from the last week of the exposure is used, LOAEL is converted into 55 mg/kg/d for males and 59 mg/kg/d for females. NOAEL could not be determined in the examination (NTP 1993, IRIS 2004). The LOAEL value from the rat study of 55 mg/kg bw/day was recalculated assuming 1.5 l /day drinking water and a body weight of 60 kg to a human equivalent dose, HEC, of 5.1 mg/kg bw/day (IRIS 2004). In the EU risk assessment report, the same value was recalculated assuming 2 l/day of drinking water and a body weight of 70 kg to a LOAEL (HEC) 7.6 mg/kg bw/day (ECB 2004). Because several studies demonstrates that rats are much more sensitive than human to the haemolytical effects of 2-butoxyethanol LOAEL values is accepted as NOAEL (ECB 2004). 2-Butoxyethanol has been evaluated as potential human carcinogen, Group C (IRIS 2004). Threshold limit values The threshold limit value for the working environment is 20 ppm corresponding to 98 mg/m3 with skin notation, i.e. the substance may penetrate the skin (AT 2002). The C-value is 0.04 mg/m3 (B-værdivejledningen, Miljøstyrelsen 2002). The inhalation RfC value is 13 mg/m3. The value is based on a subchronic rat inhalation study (Tyl et al. 1984, cf. above). The value is based on NOAEL 242 mg/m3 and calculated with a safety factor 10, 6/24 in order to convert 6 hours' exposure to 24 hours per day, a conversion from rat to human (inhalation rate for rat 0.16 m3/d and for human 22 m3/d, the body weight of rat 0.215 kg and for human 64 kg) (CICAD 1998). The RfC calculated using the mentioned variables is then: RfC = (242/10) (6/24) [(0.16/0.215)/(22/64)] = 13.1 mg/m3. Oral RfD value is 0.5 mg/kg bw/day. The value is based on a 13-week of subchronic study where haematological effects were found as the most sensitive endpoint with a LOAEL of 55 to 59 mg/kg/day for rats (NTP 1993, cf. above). US-EPA used the PBPK model of Corley (1994) to "back-calculate" human equivalent dose (HEC) of 5.1 mg/kg bw/day for humans. Using a safety factor of 10 for intraspecies variation an orall RFD value of 0.5 kg/kg bw/day was derived (IRIS 2004). Absorption 2-Butoxyethanol is easily absorbed after inhalation, or by oral or dermal exposure (CICAD1998). Consequently, an absorption of 100% has been used. In the ECB (2004), Risk Assessment Report draft is used 61% for absorption via inhalation and 30% dermal absorption. No information on oral absorption is presented. Assessment The assessment of oral exposure is based on exposure for 3 hours to a child of 10 kg body weight (bw). The absorption is set to 100%. Calculation example (lab.no. 31342-4): Migration: 322 0.3 (cm) 0.7 (g/cm3) = 67.6 μg/cm2 Total migration: 67.6 10 / 2 = 338 μg/10 cm2/hour Oral uptake: 338 3 (h) 1 (100%) / 10 = 101 μg/kg bw/day Table 9 Uptake of 2-butoxyethanol by oral exposure
2-Butoxyethanol was detected in 12 wooden toys. The calculated uptake is equal to or more than 5 times below the RfD value of 500 μg/kg lgv/day. Using the NOAEL value 7.6 mg/kg bw/day and the highest estimated uptake the margin of safety (MOS) is equal to or more than: 7.6/0.1 = 76. Conclusion From the above table is observed that none of the amounts taken up by mouthing the wooden toy result in exceeding the RfD value. Thus the substance is assessed not to cause a health risk to the consumer The lowest margin of safety is lower than the 100 usually preferred as the lowest MOS. Thus, the migration of the substance in at least 1 product may cause health concern. 4.3.2 2-(2-Butoxyethoxy)-ethanolIdentification:
The melting point is -68°C. The boiling point is 230°C at 1013 hPa. The water solubility is high (miscible with water). The vapour pressure is 2.7 Pa at 20°C. The octanol/water partition coefficient is measured to log Kow 0.56. All data are from the EU risk assessment of the substance (RAR vol. 2, ECB 2000). Classification 2-(2-Butoxyethoxy)ethanol is classified in the List of dangerous substances (Miljøministeriet 2002):
Use The substance belongs to the group of glycol ethers that is mainly used as solvents. 2-(2-Butoxyethoxy)ethanol is used as solvent in paints, dyes and colorants in both aqueous and non-aqueous systems thereof a large part in surface coatings. The amount in paint is noted to be maximum 5% but typically at 1 to 4%. Besides the substance is used in a series of other industrial processes and products (ECB 2000). Effects on health 2-(2-Butoxyethoxy)ethanol has a low acute toxicity by oral and dermal exposure routes. Some data on acute toxicity have been found (IUCLID 2000). Of these are mentioned:
By acute exposure no mortalities were observed even at exposure to saturated vapours (IUCLID 2000). The substance is evaluated to be eye irritating and may be skin irritating after repeated exposure to high concentrations of 2000 mg/kg bw/day (ECB 2000). Of studies with prolonged exposure was found a 5-week subacute study on rats exposed to 0, 13, 39 and 117 mg/m3 for 6 hours/day, 5 days/week for 5 weeks. Based on effects on the liver NOAEL was 39 mg/m3. Because the same effects were not observed in a 90-day study where rats were exposed to 0, 13, 40 and 94 mg/m3, 6 hours/day, 5 days/week the NOAEL was established at 94 mg/m3 (ECB 2000). In a 13 weeks subchronic dermal study rats were exposed for 13 weeks at 0, 200, 600 and 2000 mg/kg bw/day. No systemic effects were observed at the highest concentration even if concentration dependent skin irritations (erythema) were observed. NOAEL was established at 2000 mg/kg bw/day (ECB 2000). The oral toxicity is based on a one-generation reproduction study by Nolen et al. (1985). The rats were administered the substance via gavage directly into the stomach at 0, 250, 500 and 1000 mg/kg bw/day. Since no effects on fertility were observed NOAEL for maternal toxicity was established at the highest test dosis of 1000 mg/kg bw/day. Based on reduced body weight gain in rat pups a reproduction toxicity NOAEL of 500 mg/kg bw/day was established (ECB 2000). Threshold limit values The threshold limit value for the working environment is 100 mg/m3 (AT 2002). The C-value is 0.02 mg/m3 (Miljøstyrelsen 2002). Absorption The dermal absorption has been studied. In rats 2-(2-butoxyethoxy)-ethanol relatively easy is absorbed through the skin with between 30 and 50% of the applied radioactive labelled substance (Boatman 1993). No values for oral absorption were available but presuming it is easier than via the skin, a 100% absorption by oral exposure is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 10. Uptake of 2-(2-Butoxyethoxy)ethanol by oral exposure
2-(2-Butoxyethoxy)ethanol was detected in the extracts from 5 products Because no threshold limit values for effects based on amount taken up after oral exposure NOAEL is used for the calculation of the margin of safety (MOS). Using a NOAEL of 500 mg/kg bw/day MOS is:(500/0.0167 =) at least 30000. Conclusion With a margin of safety of at least 30000, 2-(2-butoxyethoxy)ethanol is assessed not to cause any health concern to the consumers (children mouthing the wooden toy). 4.3.3 CyclohexanoneIdentification
The melting point is -31°C. The boiling point is 155°C (Budavari 1996). The vapour pressure is 577 Pa at 25°C (4.3 mmHg, Daubert and Danner 1985). The water solubility is 25 g/l at 25°C (Yalkowsky and Dannenfelser 1992). The partition coefficient log Kow is experimentally determined to 0.81(Hansch et al. 1995). Use Cyclohexanone is used in the chemical industry for organic synthesis, particularly in the production of adipic acid and caprolactam (ca. 95%), polyvinyl chloride and its copolymers, and methacrylate ester polymers. Classification Cyclohexanone is adopted on the List of dangerous substances (Miljøministeriet 2002) and classified:
Effects on health Acute toxicity:
For humans was observed that the threshold for irritation to the nasal mucous membranes was 0.28 mg/l of air (280 mg/m3 or about 70 ppm). The value was seconded by irritation of eye, nasal, and throat at 0.362 mg/l of air (362 mg/m3 or about 90 ppm). A second exposure 2 weeks after the initial series indicated an increase in the sensory irritation threshold. In this series, the only response recovered was throat irritation at 0.547 mg/l of air (547 mg/m3 or about 136 ppm) (OECD 1996). Humans exposed for only 3 to 5 minutes found 50 and 75 ppm (200-301 mg/m3 air) irritating to the eyes, nose and throat while a concentration of 25 ppm was unobjectionable (Nelson et al. 1994). Cyclohexanone exhibits low to slight acute toxicity by the oral and inhalation exposure routes and is moderately toxic by the dermal route. Cyclohexanone is an eye and skin irritant but does not induce skin sensitisation. Upon repeated administration to rats of cyclohexanone in drinking water, the NOAEL was 4700 ppm after 25 weeks and the LOAEL was 3300 ppm after 2 years. Effects at higher concentrations were primarily body weight decreases. The NOAEL in repeated dose inhalation studies was 100-190 ppm. Those values were based on either gray mottling of the lungs or ocular irritation and degenerative changes in the liver and kidney at higher concentrations. However, the NOAEL in those studies was not confirmed in later and better inhalation studies where for reproductive and developmental effects NOAEL values of 650-1000 ppm were observed. In a two-generation reproduction study, decreased fertility was observed in rats exposed via inhalation at 1400 ppm but not at 500 ppm. The effect was found to be reversible following a post-exposure recovery period (IRIS 2004). In a chronic rat oral study, rats in groups of 52 animals per dose were exposed to cyclohexanone in drinking water at 3300, 6500, 13000 and 25000 ppm. Based on mortality and decrease in body weight a LOAEL of 6500 ppm corresponding to 910 mg/kg bw/day was found. NOAEL was 3300 ppm in the drinking water corresponding to 462 mg/kg bw/day (Lijinski and Kovatch 1986). Threshold limit values The threshold limit value (TLV) is 10 ppm equivalent to 40 mg/m3 with skin notation (H), i.e. the substance may penetrate the skin (AT 2002) The C-value is 0.1 mg/m3 (B-værdivejledningen, Miljøstyrelsen 2002). The oral RfD value is 5 mg/kg bw/day. In a chronic oral rat study was found a NOAEL of 462 mg/kg bw/day (cf. Lijinski and Kovatch 1986 above). Applying a safety factor of 100 (10 for inter- and 10 for intraspecies extrapolation) derived an oral RfD value of 5 mg/kg bw/day. TDI (tolerable daily intake) value is 4.6 mg/kg bw/day (Baars et al. 2001). Assessment Cyclohexanone was detected in the saliva extracts from 2 wooden toys. The calculated uptake by oral exposure is summarised in the table below. The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 11. Uptake of cyclohexanone by oral exposure
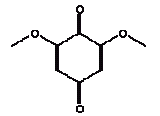
No exceeding of the TDI value was found (the margin was a factor of approx. 500 to the TDI value of 4.6 and the RfD value of 5 mg/kg bw/day). Using a NOAEL value of 462 mg/kg bw/day the margin of safety (MOS) is >54000. Conclusion With a margin of safety of more than 54000, cyclohexanone is assessed not to cause health concern to the consumers (children mouthing the wooden toy). 4.3.4 2,6 DimethoxybenzoquinoneIdentification:
The melting point is 256°C. The boiling point is estimated to 286°C. The water solubility is estimated to 70600 mg/l at 25°C (EPI). The vapour pressure is estimated to 3 mPa at 25°C (2.2 x 10-5 mmHg). The octanol/water partition coefficient is measured to log Kow –0.06 (Hansch et al. 1995). Classification 2,6-Dimethoxybenzoquinone is not classified in the List of dangerous substances (Miljøministeriet 2002). However, the substance is on the Danish EPA's Advisory list for self-classification with the classification:
Use No information on the use of the substance has been available. The few available information on the substance indicates that the substance occurs naturally in several plants. Quinones and derivated substances are used in a series of products among others in the manufacture of dyes (IPCS 1994). Effects on health Only a few informations were found on 2,6-dimethoxybenzoquinone. Based on information on quinones or hydroquinones. They seem to have similar effects. Quinones such as 2,6-dimethoxybenzoquinone are the main reason to contact dermatitis in certain plants and are regarded as common in leaf trichomes and pollen (Lovell 1993, Rasmussen 1986, MacAuley 1997). Quinone (CAS no. 106-51-4) may cause dermatitis (Budavari 1996). Therefore, information has been searched on quinone and hydroquinones, which are analogous structures. It is discovered that hydroquinones can be metabolised to 1,4-benzoquinone. For hydroquinones the acute oral LD50 values vary between 300 and 1300 mg/kg bw. Of prolonged studies a 13 weeks rat study was found with oral administration of hydroquinone. Kidney damages, reduced body weight and adverse effects to the central nervous system were observed. NOAEL was 20 mg/kg bw/day (IPCS 1994). Hydroquinones are sensitising to both humans and animals (IPCS 1994). Quinone appears to be more toxic than hydroquinone. Acute data indicate an acute oral toxicity of 25 to 50 mg/kg. No data on prolonged studies were available (HSDB 2004). Threshold limit values No threshold limit values, ADI values or similar for 2,6-dimethoxy-benzoquinone are available. For p-benzoquinone (quinone, CAS no. 106-51-4) the threshold limit value for the working environment is 0.1 ppm corresponding to 0.4 mg/m3 (AT 2002). For hydroquinones (CAS no. 123-31-9, 1,4-benzenediol) the threshold limit value for the working environment is 2 mg/m3 with notation LK. L means that the threshold limit value is a ceiling value that must not be exceeded at any time. K means that the substance is adopted on the list of substances considered to be carcinogenic (AT 2002). Absorption No values on the absorption of 2,6-dimethoxybenzoquinone are found. Hydroquinones are readily absorbed through the skin where it is metabolised to 1,4-benzoquinone and distributed to all tissues (IPCS 1994). Therefore 100% absorption is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 12. Uptake of 2,6-dimethoxybenzoquinone by oral exposure
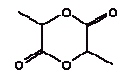
2,6-Dimethoxybenzoquinone was detected in the extracts from 7 products. The estimated uptake by oral exposure is calculated to vary between 1.5 and 10 μg/kg bw/day. Since no threshold limit values for effects from amounts taken up by the body or NOAEL values were available then based on the available information an actual assessment can not be performed The self-classification is based on modelled molecular structure analysis and together with the suspicion that the substance is released from the wood and not from the surface treatment the detection is not considered an immediate cause for concern. However, it should be noted that quinones are recognised allergenic substances that may cause contact dermatitis. The determined concentrations may not result in problems unless the child is particularly sensitive or already sensitive to the substance. 4.3.5 3,6-Dimethyl-1,4-dioxane-2,5-dione3,6-Dimethyl-1,4-dioxane-2,5-dione and 3,6-dimethyl-2,5-dioxo-1,4-dioxane are discussed together. The names indicate that it may be the same substance although they have individual CAS and EINECS numbers. The differences may be only stereoisomeric. Identification:
Identification:
Because no specific data could be found the physico-chemical data are estimated based on the molecular structure. As the molecular structure is identical for the two substances the estimated values are the same. The melting point is estimated to 26°C. The boiling point is estimated to 308°C. The water solubility is estimated to 3200 mg/l at 25°C. The vapour pressure is estimated to 0,17 Pa at 25°C (0,00126 mmHg). The octanol/water partition coefficient is estimated to log Kow 1.6 (EPI). Classification Neither 3,6-dimethyl-1,4-dioxane-2,5-dione nor 3,6-dimethyl-2,5-dioxo-1,4-dioxane is classified in the List of dangerous substances (Miljøministeriet 2002). However, both substances are included in the Danish Environmental Protection Agency's Advisory list for self-classification of dangerous substances with the classification:
Use No information on use is available. Since the chemical compound is detected in almost all extracts a possible explanation could be that the substance/substances are occurring naturally in wood. Effects on health No data on the toxicity of the substance are available. Threshold limit values No threshold limit values, ADI or corresponding values are found. Absorption No values are found and therefore 100% absorption is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 13. Uptake of 3,6-Dimethyl-1,4-dioxane-2,5-dione by oral exposure
The substance was detected in the extracts from 14 out of 15 products. The estimated uptake by oral exposure is calculated to vary between 3 and 31 μg/kg bw/day. No threshold limit values for effects from amounts taken up by the body or NOAEL values were available. Thus based on the available information an actual assessment can not be performed 4.3.6 2-EthoxyethanolIdentification:
The melting point is -70°C. The boiling point is 135°C. The water solubility is high (miscible with water, DOW 1990). The vapour pressure is 706 Pa at 25°C (5.3 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow -0.32 (Hansch et al. 1995). Classification 2-Ethoxyethanol is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Use The substance is used for instance in paint, dye and lacquer industry as solvent for nitrocellulose and lacquers and to increase the stability of emulsions. Effects on health Some data on acute toxicity have been found. Of these are mentioned:
Of studies with prolonged exposure a 13-week inhalation study is found where rats were exposed via inhalation to 0, 25, 103 and 403 ppm corresponding to 0, 92, 380 and 1485 mg/m3 for 6 hours/day, 5 days/week. At the highest concentration was observed a significant decrease in the weight of pituitary in males and decreased leukocyte count in females. NOAEL is therefore set to 103 ppm (380 mg/m3) (Barbee et al. 1984). In a 13 weeks study dogs were exposed by oral administration of the doses 0, 46, 93 and 186 mg/kg bw/day. Based on observation of testicular oedema, a reduction in haemoglobin concentration and haematocrit value a NOAEL was set to 93 mg/kg bw/day (IUCLID 2000). In studies on reproduction toxicity with oral administration severe effects on testicles, spermatid count and semen quality at doses of 300 mg/kg bw/day and above (IUCLID 2000). In a 14 weeks fertility study where mice were exposed for 18 days before mating to the test substance in drinking water at 750-2600 mg/kg bw/day. Mice of both sexes were infertile at 2600 mg/kg lgv/day, the fertility in both sexes was reduced at 1500 mg/kg bw/day and at 750 mg/kg bw/day no effects on fertility was observed (Lamb et al. 1984). In a study with exposure to pregnant female rats via inhalation in the gestation period, increased mortality in the foetuses, deformities and other effects were observed. NOAEL for teratogenic effects was set to 10 ppm corresponding to 36 mg/m3 (IUCLID 2000). Threshold limit values The threshold limit value for the working environment is 5 ppm corresponding to 18.5 mg/m3 with notation H. H indicates that the substance may penetrate the skin (AT 2002). The C-value is 0.2 mg/m3. The value is under revision, a new proposal is 0.01 mg/m3 (Miljøstyrelsen 2002). The inhalation reference concentration, RfC, is set by US-EPA to 0.2 mg/m3. The value is derived from a subchronic rat study with NOAEL 380 mg/m3 (Barbee et al. 1984). The NOAEL was recalculated from 6 hours/day, 5 days/week to 24 hours/day for 7 days/week, i.e. NOAEL adjusted to (380 6/24 hours 5/7 days =) 68 mg/m3. Using a safety factor of 300 (3 for interspecies and 10 for intraspecies variation and 10 for the use of a subchronic study) RfC is derived at 68/300 = 0.2 mg/m3 (IRIS 2004). Absorption The substance can be absorbed via inhalation, the skin and via gastro-intestinal tract. After oral intake of the substance 76 to 80% was excreted in the urine within 96 hours (IPCS 1990). Therefore 100% absorption is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 14. Uptake of 2-ethoxyethanol by oral exposure
2-Ethoxyethanol was detected in extracts from 2 products. Because no limit values for effects from amounts taken up in the body are found NOAEL is used to calculate the margin of safety (MOS). Using a NOAEL of 94 mg/kg bw/day MOS is at least: 94/0.0054 = 17400. Conclusion Based on a high MOS value 2-ethoxyethanol is considered not to pose a health risk to the consumer. 4.3.7 2-(2-Ethoxyethoxy)-ethanolIdentification:
The melting point is -76°C. The boiling point is 196°C. The water solubility is high (miscible with water, Riddick et al. 1986). The vapour pressure is 16.8 Pa at 25°C (0.126 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow -0.54 (Funasaki et al. 1984). Classification 2-(2-Ethoxyethoxy)-ethanol is not classified in the List of dangerous substances (Miljøministeriet 2002). Use 2-(2-Ethoxyethoxy)-ethanol is used as solvent in paint, dyes and lacquers (Buadavari 1996). Effects on health Some data on acute toxicity have been available. Of these are mentioned:
2-(2-Ethoxyethoxy)-ethanol is a moderate irritant to eyes (Grant 1986). In a dermal study rabbits were applied 0.1, 0.3, 1 and 3 ml/kg/day on the skin 5 times/week for 90 days. Based on observations of kidney damages NOAEL was set to 0.3 ml/kg/day or approx. 300 mg/kg bw/day (IUCLID 2000). In a 30 days oral rat study 2-(2-ethoxyethoxy)-ethanol was administered in the drinking water at doses from 410 to 3200 mg/kg bw/day. Reduced weight gain was observed at 1830 mg/kg bw/day and unspecified micropathological changes at all doses. NOAEL is set to 590 mg/kg bw/day (IUCLID 2000). In a 90 days study, pigs were orally administered the test substance at the doses 0, 167, 500 and 1500 mg/kg. Damages to the kidney and changes to the blood picture at 1500 mg/kg and damages to the liver at 500 mg/kg were observed. NOAEL is set to 167 mg/kg bw/day (Gaunt et al. 1968). Threshold limit values No threshold limit values for the working environment was available. The C-value is 1 mg/m3 (Miljøstyrelsen 2002). Absorption No values on absorption of the substance are found and therefore 100% absorption is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 15. Uptake of 2-(2-ethoxyethoxy)-ethanol by oral exposure
2-(2-Ethoxyethoxy)-ethanol was detected in extracts from 4 products. Because no limit values for effects from amounts taken up in the body are found NOAEL is used to calculate the margin of safety (MOS). Using a NOAEL of 167 mg/kg bw/day MOS is more than: 167/0.0189 = 8800. Conclusion Based on a high MOS it is assessed that 2-(2-ethoxyethoxy)-ethanol is not considered to pose a health risk to the consumer of the wooden toy. 4.3.8 FormamideIdentification:
The melting point is 2.25°C. The boiling point is 210°C at 1013 hPa (Budavari 1996, Hsdb). The water solubility is high at 25°C (miscible with water, IUCLID 2000). The vapour pressure is 8.1 Pa at 25°C (0.061 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow -0.82 (IUCLID 2000). Classification Formamide is adopted on the List of dangerous substances (Miljøministeriet 2002) and classified:
Use The substance is used in the production of several chemical compounds as solvent and in adhesives (HSDB). Effects on health Some data on acute toxicity have been available. Of these are mentioned:
The substance is moderately irritating by contact to the skin, eyes and mucous membranes (Lewis 1996). Formamide is experimentally demonstrated to be teratogenic following oral or percutaneous use (ILO 1983). However, later studies indicate that the observations were caused by very high concentrations, i.e. undiluted test substance or 50% dilutions (ACGIH 1991). The effect of formamide on reproduction is studied in a reproduction toxicity test in mice where formamide was administered orally in drinking water at the doses of 0, 100, 350 and 750 mg/l corresponding to approx. 24 to 195 mg/kg bw/day. Effects on reproduction such as reduced fertility and litter size were observed at 750 mg/l in the parental generation and in the first litter generation. Therefore NOAEL is set to 350 ppm corresponding to 87 mg/kg bw/day (NTP 1992). In a reproduction study on rats, the rats were administered formamide orally via gavage at the doses of 0, 50, 100 or 200 mg/kg bw/day on gestational days 6 to 19 after the mating. Reduced weight of gravid uterine weight at 200 mg/kg bw/day and reduced foetal body weight at 100 mg/kg bw/day and above was observed, i.e. NOAEL was 50 mg/kg bw/day (NTP 1998). The effect of formamide on foetal development is studied on rabbits where formamide was administered orally via gavage at the doses 0, 35, 70 or 140 mg/kg bw/day in the days 6 to 29 of gestation. At the highest dosis significantly reduced weight of uterus, mean litter size and reduced foetal body weight were observed. NOAEL is therefore set to 70 mg/kg bw/day (NTP 2001). Threshold limit values The threshold limit value for the working environment is 10 ppm corresponding to 18 mg/m3 with notation H. H indicates that the substance may penetrate the skin (AT 2002). The C-value is 0.01 mg/m3 (Miljøstyrelsen 2002). Absorption Formamide is absorbed directly through the skin in guinea pigs (Patty 1963). Following oral administration at 2-4 g/rabbit 39% of the dose was recovered unchanged (Snyder 1990). No further information are found and therefore a 100% absorption is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 16. Uptake of formamide by oral exposure
Because no limit values for effects from amounts taken up in the body are found NOAEL is used to calculate the margin of safety (MOS). Using a NOAEL of 50 mg/kg bw/day MOS is more than: 50/0.022 = 2300. Conclusion Based on the calculated MOS value that is calculated from the extract containing the highest concentration of the substance, formamide is not considered to pose an immediate health risk to the consumer. 4.3.9 FurfuralIdentification:
The melting point is –36.5°C. The boiling point is 161.7°C. The water solubility is 77000 mg/l at 25°C (Yalkowsky and Dannenfelser 1992). The vapour pressure is 295 Pa at 25°C (2.21 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow 0.41 (Hansch et al. 1995). Classification Furfural is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
It is noted that the classification is dependent of the concentration:
Furthermore, the classification is proposed included R38: Irritating to skin by the 30th ATP (ECB 2005). Use Furfural is used as solvent and in the manufacture of resins and adhesives (CICAD 2000) and dyes (ECB 2004). Effects on health 2-Furfural is acute toxic by oral intake and by exposure via inhalation. A few data have been found on acute toxicity. Of those are mentioned:
Irritation of the respiratory system and eyes are observed at single as well as by repeated exposure. By exposure to the skin the effects seem moderate or irreversible (CICAD 2000). In 13-week studies inhalation studies, NOAEL values of 80 mg/m3 in hamsters and 208 mg/m3 in rabbits for neoplastic effects were found (CICAD 2000). No reports on irritation to respiratory tract and eyes in humans at exposure to 40 mg/m3 (10 ppm) during 8 hours or at 80 mg/m3 during 4 hours were found (CICAD 2000). In a 13 weeks inhalation study, Syrian golden hamsters were exposed to furfural vapours for 6 hours/day, 5 days/week in 13 weeks at the doses 0, 20, 115 and 552 ppm equivalent to 0, 80, 460 and 2208 mg/m3. Based on observations of harmful effects to the olfactory epithelium NOAEL was 20 ppm equivalent to 77 mg/m3 (JECFA 1999, CICAD 2000). A 13 weeks diet toxicity study on rats is performed. The measured exposure doses were 0, 26, 53, 82 and 160 mg/kg bw/day. At 82 mg/kg changes in the liver in 5 out of 10 male rats were observed while there were no changes at 53 mg/kg bw/day (measured concentration). NOAEL was therefore set to 53 mg/kg bw/day (JECFA 2001, ECB 2004). A 90 days rat study from the American National Toxicology Program (NTP) was found. On basis of reduced body weight gain and histopathology, a LOAEL was set to 11 mg/kg bw/day. As this was the lowest concentration used, no NOAEL could be established (NTP 1981 study referenced in IRIS 2004). In a 2-year study on oral administration malignant tumours were observed in rats at 60 mg/kg bw/day and in mice at 50 mg/kg bw/day (CICAD 2000). In CICAD (2000) is mentioned that furfural was genotoxic in vitro in mammalian cells but the genotoxic potential in vivo could not be finally concluded on. The possibility that the genotoxicity may contribute to the carcinogenic process prevents the establishment of a reliable NOAEL (CICAD 2000). At a later evaluation by the Scientific Committee on Cosmetic and Non-Food Products intended for consumers (SCCNFP) was concluded that furfural was not genotoxic in vivo (SCNNP 2004). IARC has evaluated furfural and concluded that there is limited evidence for the carcinogenic effect in experimental animals and inadequate evidence for carcinogenic effect in humans. This means that furfural by IARC (1995) is not classifiable as to its carcinogenicity to humans (Group 3) while furfural by EU is classified Carc.Cat. 3;R40, i.e. Limited evidence of carcinogenic effects. Furfural is found irritating to skin and mucous epithelium and reports on both eczema and allergic skin sensitizing and photo sensitising are found (SCCNFP 2004). Furfural is under the EU program for risk assessment on existing substances but report not finalised yet (the latest draft on human health part from October 2004, ECB 2004). Threshold limit values The threshold limit value for the working environment is 2 ppm equivalent to 7.9 mg/m3 with notation HK. H means that the substance may penetrate skin. K means that the substance is on the list of substances that is considered carcinogenic (AT 2002). The C-value is 0.002 mg/m3 (Miljøstyrelsen 2002). The ADI 0.5 mg/kg bw/day is set on the basis of NOAEL 53 mg/kg in the 13 weeks rat study and applying a safety factor of 100 (JECFA 2001). The RfD-value is set on the basis of the 90-days rat study by NTP. Based on LOAEL 11 mg/kg/day and applying a safety factor of 3000 (10 for inter- and 10 for intraspecies variation, 10 for extrapolating from subchronic to chronic data and a factor of 3 for using a LOAEL) an oral reference dosis (RfD) of 11/3000 = 0.003 mg/kg bw/day was derived (IRIS 2003). Absorption Following oral administration in rats, 14C-2-furfural was readily absorbed and up to 85% recovered in the urine within 24 hours and approx. 7% in the exhaled air. In humans, absorption of the vapour via skin and the lungs and dermal absorption of the substance in liquids has been demonstrated (CICAD 2000). In the evaluation an absorption of 100% is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 17. Uptake of furfural by oral exposure
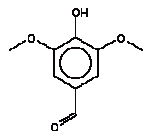
Furfural was detected in the extracts from 4 products. The RfD value of 3 μg/kg/day is not exceeded. Both JECFA (2001) and the EU risk assessment report (ECB 2004) reach the same conclusion that the NOAEL value of 53 mg/kg bw/day is the preferred value for risk characterisation. Using NOAEL 53 mg/kg bw/day the margin of safety (MOS) is more than: 53/0.00145 = 36000. Conclusion Furfural does not exceed the RfD value even though the calculated highest oral uptake of 1.5 μg/kg bw/day is at the same level as the RfD of 3 μg/kg bw/day. However, the MOS is more than 36000 and the substance is concluded not to pose a health problem to the consumer. Considering that the substance is found a possible carcinogenic the question is whether the presence of the substance is undesirable in this context. 4.3.10 4-Hydroxy-3,5-dimethoxy-benzaldehydeIdentification:
The melting point is 113°C. The boiling point is estimated to 305°C. The water solubility is estimated to 9500 mg/l at 25°C. The vapour pressure is estimated to 8.6 mPa at 25°C (6.510-5 mmHg). The octanol/water partition coefficient is estimated to log Kow 0.88 (EPI). Classification 4-Hydroxy-3,5-dimethoxy-benzaldehyde is not classified in the List of dangerous substances (Miljøministeriet 2002) but included in the Danish EPA's Advisory list for self-classification of dangerous substances (Miljøstyrelsen 2001) with the classification:
Use No informations on the use are available. Effects on health No data on acute toxicity or other relevant data on 4-hydroxy-3,5-dimethoxy-benzaldehyde have been found. Threshold limit values No threshold limit values for the working environment are available. Absorption No values on absorption have been found and therefore a 100% absorption is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 18. Uptake of 4-hydroxy-3,5-dimethoxy-benzaldehyde by oral exposure
Since no effect values, threshold limit values or similar are found no specific evaluation of the substance could be performed. For a group of benzaldehyde analogous substances a group-ADI has been set at 5 mg/kg bw/day (WHO 1996). However, the evaluation is based on benzaldehyde, CAS no. 100-52-7, where a reproduction toxicity study has been available with the NOAEL of 5 mg/kg bw/day (IUCLID 2000). Besides for benzaldehyde a RfD value of 0.1 mg/kg bw/day is available (IRIS 2004). Because these values are below the WHO group-ADI they are considered a little safer to use. Because no limit values for effects from amounts taken up in the body are found NOAEL is used to calculate the margin of safety (MOS). Using a NOAEL of 5 mg/kg bw/day MOS to the highest estimated uptake is: 5/0.0063 = 790. Conclusion Based on MOS values at and above 790 and that none of the amounts taken up are close to the oral chronic RfD for benzaldehyde, which is evaluated to be more toxic than 4-hydroxy-3,5-dimethoxy-benzaldehyde, 4-hydroxy-3,5-dimethoxy-benzaldehyde is assessed not to pose a health risk to the consumer. 4.3.11 N-Methyl-2-pyrrolidoneIdentification:
The melting point is -24°C. The boiling point is 202°C. The water solubility is high at 25°C (miscible with water). The vapour pressure is 25.3 Pa at 25°C (Riddick et al. 1986). The octanol/water partition coefficient is measured to log Kow –0.46 (IUCLID 2000). Classification N-Methyl-2-pyrrolidone is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Use The substance has several uses within the chemical industry among others in paints, dyes and lacquers. The substance is used as solvent and in the production of pigment and printing inks (IUCLID 2004, CICAD 2001). Effects on health N-Methyl-2-pyrrolidone has a low acute toxicity. Some data on acute toxicity have been found. Of those are mentioned:
Uptake of acute toxic doses by oral intake, dermal exposure or exposure via inhalation causes functional disorders and affects the central nervous system. Irritation of the respiratory system by exposure via inhalation and of the gastro-intestinal tract after oral administration has been observed (CICAD 2001). N-Methyl-2-pyrrolidone has a low potential for skin irritation and a moderate potential for eye irritation in rabbits (IUCLID 2000). In a 13 week study with exposure via inhalation rats were exposed to 0, 500, 1000 and 3000 mg/m3 for 6 hours/day, 5days/week. Nasal irritation and crust formations on nasal edges were observed at 1000 mg/m3. At 3000 mg/m3, decreased body weight and decreased relative weight of testis were observed. The NOAEL was then set to 500 mg/m3 (CICAD 2001). Of studies on prolonged duration of oral exposure, a 28-day study where rats were orally administered (via gavage) the doses 0, 257, 514, 1028 and 2060 mg/kg bw/day was found. A dose-dependent increase in relative liver and kidney weights was observed at 1028 mg/kg bw/day. Thus, the NOAEL was 514 mg/kg bw (CICAD 2001). In a 90-day study, rats were administered the substance in the diet at the doses 0, 3000, 7500, and 18 000 mg/kg diet/day corresponding to 0, 169, 433 and 1057 mg/kg bw/day in males and 0, 217, 565 and 1344 mg/kg bw/day in females. A dose-related decrease in body weight and effects on the central nervous system was observed at 433 and 565 mg/kg bw/day in males and females, respectively. The NOAEL was 169 mg/kg bw/day in males and 217 mg/kg bw/day in females (CICAD 2001). Threshold limit values The threshold limit value for the working environment is 5 ppm corresponding to 20 mg/m3 (AT 2002). The C-value is 0.5 mg/m3 (Miljøstyrelsen 2002). The TDI value is 0.6 mg/kg bw/day. The value is derived from the 90-day oral study with a NOAEL of 169 mg/kg bw/day divided with a safety factor of 300 (10 for interspecies and 10 for intraspecies variation and 3 for adjusting from a 90 day subchronic to chronic) (CICAD 2001). Absorption N-Methyl-2-pyrrolidone is absorbed easily after inhalation, oral or dermal exposure and is distributed to the whole body. Approximately 80% of the administered dosis are excreted via the urine within 24 hours (Åkesson and Paulsson 1997). Therefore, an absorption of 100% is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 19. Uptake of N-methyl-2-pyrrolidone by oral exposure
N-Methyl-2-pyrrolidone was detected in extracts from 5 products. The TDI value of 600 μg/kg bw/day is not exceeded. The highest uptake is a factor of 30 below the TDI value. Using the 90-day diet toxicity NOAEL of 169 mg/kg bw/day the margin of safety (MOS) is at least: 169/0.019 = 8900. Conclusion N-Methyl-2-pyrrolidone is assessed not to pose a health risk to the consumer of the toy. 4.3.12 3,5,5-Trimethyl-2-cyclohexen-1-one (= isophorone)Identification:
The melting point is -8,1°C. The boiling point is 215°C. The water solubility is 12000 mg/l at 20°C (IUCLID 2000). The vapour pressure is 58 Pa at 25°C (0.438 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow 1.67 (IUCLID 2000). Classification 3,5,5-Trimethyl-2-cyclohexen-1-one is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Use 3,5,5-Trimethyl-2-cyclohexen-1-one is used as solvent in synthetic resins, polymers, polyacrylates and cellulose derivatives and has some use in certain paints and printing inks (IPCS 1995). Effects on health 3,5,5-Trimethyl-2-cyclohexen-1-one has a low to moderate acute toxicity. Some data on acute toxicity have been available. Of these are mentioned:
3,5,5-Trimethyl-2-cyclohexen-1-one is evaluated to be irritating to eyes, nose and throat but not to skin (IPCS 1995, OECD 2003). Several studies on prolonged exposure exist. In a 91 days oral repeated dose toxicity study, rats were administered 3,5,5-trimethyl-2-cyclohexen-1-one via gavage at the doses 0, 62.5, 125, 250, 500 and 1000 mg/kg bw/day for 5 days/week. Effects were only observed as one mortality at the highest dose. NOAEL is therefore set at 500 mg/kg bw/day (IUCLID 2000). In a 91 days oral repeated dose toxicity study, mice were administered 3,5,5-trimethyl-2-cyclohexen-1-one via gavage at the doses 0, 62.5, 125, 250, 500 and 1000 mg/kg bw/day for 5 days/week. Effects were only observed as mortality at the highest dose and dosis related decrease in body weight in males at 250 mg/kg bw/day and above. NOAEL is set to 125 mg/kg bw/day (IUCLID 2000). In a 90 days oral study on beagle dogs, which daily for 7 days/week were administered the substance in gelatine capsules at the doses 0, 35, 75 and 150 mg/kg bw/day, no essential substance related effects at any of the used concentrations were observed. NOAEL is set to 150 mg/kg bw/day (IUCLID 2000). In a 2-year oral carcinogenicity study on mice and rats were administered the substance via gavage 5 days/week at the doses 250 and 500 mg/kg bw/day. Some evidence of carcinogenic effects (tumours) was observed by pathological examinations of the kidneys in males but not in females at the lowest dose (NTP 1984). Threshold limit values The threshold limit value for the working environment is 5 ppm corresponding to 25 mg/m3 with notation LK. L means that the threshold limit value is a ceiling value that must not be exceeded at any time. K means that the substance is included on the list of substances that is considered carcinogenic (AT 2002). The C-value is 0.03 mg/m3 (Miljøstyrelsen 2002). The oral RfD value is 0.2 mg/kg bw/day. The value is based on the 90-day study on dogs with NOAEL 150 mg/kg bw/day and the 2-year carcinogenicity studies with a recalculation of the 250 mg/kg bw administered 5 days/week to 7 days/week (250 x 5/7 =) 179 mg/kg bw/day. Using a safety factor of 1000 (10 for inter-, 10 for intraspecies variation and 10 for subchronic to chronic (dog) or 10 for LOAEL to NOEL in the rat study) is derived an oral RfD at 0.2 mg/kg bw/day (IRIS 2004). Absorption By oral administration of radioactive labelled 3,5,5-trimethyl-2-cyclohexen-1-one, 93% was recovered in the urine and exhaled air within 24 hours. The uptake of the substance through the skin is fast indicating a readily dermal absorption (IPCS 1995). Based on this information a 100% absorption is assumed. Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 20. Uptake of 3,5,5-trimethyl-2-cyclohexen-1-one by oral exposure
3,5,5-Trimethyl-2-cyclohexen-1-one does not exceed the RfD value of 200 μg/kg bw/day. The highest calculated oral uptake of 6.6 μg/kg bw/day is a factor 30 below the RfD value. Using the 91-day mice diet toxicity NOAEL of 125 mg/kg bw/day the margin of safety (MOS) is at least: 125/0.0066 = 19000. Conclusion 3,5,5-Trimethyl-2-cyclohexen-1-one is assessed based on the RfD value and the MOS not to pose a health risk to the consumer of the toys. 4.3.13 VanillinIdentification
The melting point is 81.5°C. The boiling point is 284°C (Kirk-Othmer 1991). The vapour pressure is 0.016 Pa at 25°C (0.00012 mmHg, Yaws 1994) or 0.33 Pa (OECD 1996). The water solubility is 11000 mg/l at 25°C (Yalkowsky and Dannenfelser 1992). The partition coefficient log Kow is measured to 1.23 (OECD 1996). Classification Vanillin is not classified (Miljøministeriet 2002). Use Vanillin is present as a natural compound in plants and is identified in plant oils, balsams, resins and wood. The best known natural source is the plant Vanilla planifolia in the Orchid family (Kirk-Othmer 1991, Ullmann 1993). Vanillin is also manufactured synthetically from guaiacol but mainly from lignin, which is the main component in waste from sulphite pulp in the paper industry (Hocking 1997). Vanillin is used in food ("vanilla"), pharmaceuticals and in the cosmetics and perfume industry. Effects on health Vanillin is a phenol aldehyde with reactive aldehyde and hydroxyl moieties in the molecule. Acute toxicity:
Acute toxicity was not observed after inhalation of vapours from saturated solutions. However, irritation was observed after application to the skin and mucous membranes (Kirk-Othmer 1991). A 30 days inhalation test with exposure for 4 hours/day, 5 days a week to saturated vapours at 20°C showed no mortality but reduction in body weight and effects to liver and to the haemoglobin level (ECB 2000). A 4 months inhalation study showed that 15 mg/m3 (LOAEL) affected nerves and cardiovascular systems, liver and blood systems. Therefore, NOAEL is set to 0.5 mg/m3 (ECB 2000). Several diet toxicity tests are performed and the summarised in IUCLID (ECB 2000) and SIDS (OECD 1996). The most essential are presented below: In a 14 weeks study with oral administration via gavage of 300 mg vanillin/kg to rats, twice weekly, no adverse effects were observed. Rats fed diets containing vanillin at levels of 20 mg/kg/day for 18 weeks had no adverse effects while 64 mg/kg/day for 10 weeks caused growth depression and damage to the myocardium, liver, kidney, lung, spleen and stomach. Rats fed diets containing vanillin for 13 weeks exhibited growth depression and enlargement of the liver, kidney and spleen at dosage levels 5% (2500 mg/kg/day*), mild changes at 1% (500 mg/kg/day*), and no changes at 0.3% (150 mg/kg/day*). Four to six week old rats maintained for 91 days on diets containing vanillin exhibited no adverse effects at rates of 3000 ppm (150 mg/kg/day*), mild adverse effects at 10,000 ppm (500 mg/kg/day*) and growth depression and enlargement of the liver, kidney and spleen at 50,000 ppm (2500 mg/kg/day*). Rats fed dietary levels of vanillin of 10,000 ppm (500 mg/kg/day*) for 16 weeks, 1000 ppm (50 mg/kg/day*) for 27-28 weeks, 20,000 or 50,000 ppm (1000 or 2500 mg/kg/day*) for 1 year, or 5000, 10,000, or 20,000 ppm (250, 500, or 1000 mg/kg/day*) for 2 years exhibited no adverse effects on growth or haematology and produced no macroscopic or microscopic changes in tissues (Hagan et al. 1967). Rats fed for 5 weeks on a diet of vanillin at 0.5 g/kg of the diet showed symptoms of intoxication, including decreases in adrenal vitamin C and in liver protein (Kirwin and Galvin 1993). *Based on a food factor of 0.05 for a 0.35 kg rat (US-EPA 1985). The highest NOEL from the 1-year repeated dose toxicity rat study with oral administration was 50000 ppm corresponding to 2500 mg/kg/day. NOEL in the 2 years rat study was 1000 mg/kg bw/day. The latter value from Hagan et al. (1967) is used by WHO to derive an ADI-value. Teratogenicity, genotoxicity and carcinogenicity tests are performed that were all negative, i.e. there was no indications of such effects (OECD 1996). Threshold limit values A threshold limit value for the working environment is not available (AT 2002). An ADI (Acceptable Daily Intake) of 10 mg/kg has been agreed between FAO/WHO and EU (OECD 1996) based on NOEL 1000 mg/kg bw/day from a 2-year rat study (Hagan et al. 1967). JECFA has placed vanillin in structure class I together with other terpenoid substances, i.e. vanillin is applied a group-ADI of 0.05 mg/kg/day. Absorption No data are available on adsorption. Therefore 100% adsorption is used Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100%. Table 21. Uptake of vanillin by oral exposure
From the table above is seen that none of the amounts taken up by oral exposure results in a dosis above the ADI value of 50 μg/kg bw/day. The WHO recommended ADI of 10 mg/kg bw/day is not exceeded either. Using the NOAEL 1000 mg/kg bw/day from the 2-year rat study the margin of safety is more than: 1000/0.0047 = 200000. Conclusion Vanillin does not pose any health risk to the consumer by oral exposure to the migrated amounts determined by analyses of the selected toy products. 4.3.14 BariumIdentification:
The melting point is 710°C. The boiling point is 1600°C (Budavari 1996). The vapour pressure is assumed to be very low. The water solubility also assumed to be very low. Classification Barium and barium compounds are not classified. Use Some barium compounds among others barium acetate is used in colorants (Budavari 1996). Barium compounds are used in several industries but also in cosmetics, pharmaceuticals and in paints and dyes (CICAD 2001). Effects on health The toxic effects are based on the assumption that the barium-ion is the active substance since the toxicity studies usually are performed with water soluble barium salts and the results then recalculated to barium equivalents. Acute oral LD50 values for rats administered barium chloride, - carbonate, and –sulphide are between 118 and 800 mg/kg bw (IPCS 1990). In a 13 weeks oral rat study, the rats were administered barium chloride in drinking water at daily doses of 0, 125, 500, 1000, 2000 and 4000 mg/l corresponding to 0, 10, 30, 65, 110, and 180 mg/kg/bw. Based on kidney effects NOAEL was set to 65 mg/kg bw/day in a NTP study from 1994 (CICAD 2001). In a chronic oral rat study, rats were dosed via the drinking water for 2 years with 0, 500, 1250 and 2500 mg barium chloride/l corresponding to 0, 15, 45 and 75 mg/kg bw for females and 0, 15, 30 and 60 mg/kg for males. Based on a relative increase of the kidney weight NOAEL was set to 45 mg/kg bw/day (CICAD 2001). The critical effect in humans appears to be increased blood pressure (hypertension) and harmful effects to the kidneys (CICAD 2001). In an oral study on humans, barium chloride was administered in drinking water at gradually increased doses for 4-week periods while the blood picture was examined continuously. NOAEL was set to 10 mg Ba/l/day when the test volunteers were given1.5 l/day. Assuming a mean body weight of 70 kg this corresponds to a NOAEL of 0.21 mg/kg bw/day (Wones et al. 1990). In an epidemiological study of population groups in USA, a NOAEL of 7.3 mg/Ba/l/day was calculated. Assuming an intake of 2 l/day and 70 kg body weight 7.3 mg Ba/l corresponds to 0.21 mg/kg bw/day (Brenniman and Levy 1984). Threshold limit values The threshold limit value for working environment is 0.5 mg/m3 (AT 2002). The C-value is preliminary set to 0.005 mg/m3 (Miljøstyrelsen 2002). RIVM has derived an acceptable daily breathing air concentration (HAC) to 0.001 mg/m3 (Baars et al. 2001). US-EPA has suggested an acceptable daily oral intake dosis (RfD) to 0.070 mg/kg bw/day based on several studies but as principal studies the studies by Wones et al. (1990), Brenniman and Levy (1984), and NTP (1994). Applying a safety factor of 3 they derived an RfD value of (0.21/3 = 0.07 mg/kg bw/day (IRIS 2002). WHO has suggested a TDI of 0.02 mg/kg bw/day based on Wones et al. (1990) applied a safety factor of 10 for insufficient data and a potential difference between children and adults, i.e. (0.21/10 = 0.02 mg/kg bw/day (WHO 1996, CICAD 2001). RIVM has calculated a tolerable daily intake TDI 0.020 mg/kg bw/day based on the same considerations (Baars et al. 2001). According to the Statutory Order on toys the bioavailability of barium as a consequence of the use of the toy must not exceed 25 μg/day (Bkg. 1116, 2003). Absorption The absorption following oral intake is strongly influenced by e.g. food in the gastro-intestinal tract that may bind barium, the duration of the study period and animal species. Absorption values varying from 0.7 to 85% have been found (CICAD 2001). In a study on humans it was observed that the absorption was approx. 10% with 90 to 98% excreted via faeces and 2 to 10% via the urine (Cember et al. 1961, Tipton et al. 1969). In a Dutch evaluation is estimated that the bioavailability by inhalation is 75%. The bioavailability by oral intake is estimated to 10% (Baars et al. 2001). Assessment The assessment of oral exposure is based on the exposure for 3 hours of a child of 10 kg body weight (bw). The absorption is set to 100% and 10%. Table 22. Uptake of barium by oral exposure
The determined concentrations for oral uptake vary between 9 and 28 μg/kg lgv/day when 100% absorption is assumed. Using the Dutch bioavailability value, which states the absorption to be 10%, the calculated uptakes are the values mentioned in right column of the table above. This reduces all calculated values to below the TDI value of 20 μg/kg bw/day. Using the human oral NOAEL of 10 mg/kg bw/day the margin of safety (MOS) is more than: 10/0.0284 = 350 with 100% absorption and 3500 with 10% absorption. According to the Statutory Order on toys the bioavailability of barium must not exceed 25 μg/day. The value 25 μg/day is based on an assumed oral intake of 8 mg of the toy, i.e. the maximum bioavailable concentration of barium in the toy must be max. 3125 mg Ba/kg toy (CSTEE 2004). In the standard EN 71-3 is given the limit value 1000 mg Ba/kg toy (EN 71-3). Assuming a bioavailability of 10% the measured migration of 29-90 μg/g (= mg/kg) thus far below 1000 mg Ba/kg toy. On the other hand it must be noted that the major source to metal exposure to children is via the food. Other sources to exposure are not included in the assessment. If for instance the limit of the contribution from toys is set to 10% of the TDI value of 20 μg/kg bw/day then at least 3 products exceed the value. Conclusion Based on MOS and assuming the 10% absorption is the most realistic it is assessed that barium does not pose a potential health risk to the consumer of wooden toy.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||