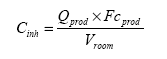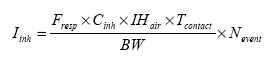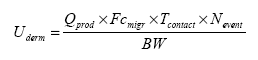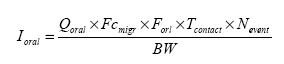|
Survey and release of chemical substances in "slimy" toys 6 Exposure scenarios
6.1 IntroductionTo evaluate the health risk from using slime toys, effects from selected chemical substances are assessed in relation to the relevant exposure period and exposure route for consumers of the toys. Exposure period The exposure period for playing with slimy toys may vary considerably but most commonly is assumed several short-term uses that may vary from a few to several minutes. Assuming a varying exposure period at each use and that the number of use times may also vary, a starting point has been to assume a daily exposure of 60 minutes (1 hour). This assumption is supported, for instance, by an American study on the playing activity of children. In the study was observed average play activity times of 46-70 minutes and 90 percentiles for children 1 to 17 years of age of 120 to 255 minutes (US-EPA 1997). Exposure route During the screening it became clear that many of the substances detected were volatile substances, and a substantial exposure route was via inhalation. Exposure via inhalation takes place from the air borne concentration of the chemical substance in the breathing zone. The substance may then be taken up by the lungs or after ciliary transport across the mucous membranes to the oesophagus taken up by the stomach-intestine canal. Dermal exposure is considered relevant as the slimy toys are specifically intended for ”handling”. The primary exposure is exposure to the skin on hands, but contact to other areas of the body during play can hardly be excluded. Oral exposure is included since contamination of hands or even mouthing the toys cannot be excluded. Due to realistic ”worst case” the calculations of dermal and oral uptake are assuming a body weight of 10 kg for children. Slimy toys may be intended for little older children, but that younger siblings may or will get hold of them cannot be excluded. Uptake Uptake via inhalation, oral or dermal exposure is substance specific and, therefore, dependent of which substances that are found released from the slimy toys. If no information could be found on the specific uptake of the individual substances via inhalation, dermal contact or via mouth or mucous membranes, an uptake of 100% is assumed. In the study 20 specific substances were selected in co-operation with the Danish Environmental Protection Agency. The selection is based on the classification of the substances, measured amounts, etc. (cf. chapter 4). The selected substances have been reviewed individually after presentation of the assessment method, cf. below. Each of the selected substances has been identified by its common name and CAS no. for unambiguously identification. The most common synonyms are stated, and furthermore is mentioned:
Finally, an assessment of the amount of detected released substances has been carried out. This has been performed by calculating / estimating the uptake based on the time of exposure and the body weight of the person (amount/kg body weight/day). If possible, one of the established values for tolerable daily intake (TDI, ADI or RfD) is used for evaluation of the exposure by comparing the values with the obtained analysis results used to estimate the exposure. The basis is the maximum found value, if they appear in several products. The used uncertainty factors are mentioned in the text. In case more TDI, ADI or RfD values exist, the lowest value is preferred. If no TDI, ADI, RfD values are available, a comparison to a concentration where no adverse effects are observed (NOAEL: No Observed Adverse Effect Level) from a relevant long-term study is used. The procedure is mentioned at the individual substances. 6.2 Exposure scenarios6.2.1 IntroductionThe exposure to the consumer from slimy toys will vary considerably according to use duration, which rooms (size, etc.) the toys are used in, ventilation and handling or area of contact and duration of direct contact. To evaluate the exposure in a standardised way, theoretical exposure scenarios have been derived to illustrate the worst possible but realistic exposures. To evaluate the exposure of consumers, the following scenarios have been derived: Exposure via inhalation of volatile substances (exposure via inhalation) Exposure via skin (dermal exposure) Exposure via the mouth (oral exposure) The direct exposure from unwrapping and the first use is assumed to be analogous to the direct exposure measured in the headspace analysis (cf. section on methods). For the evaluation of exposure via inhalation is used a scenario with inhalation of the measured concentration in the breathing zone of 1 m³ and in a room of 20 m³. Dermal exposure is based on measurements of the substance in extractions to artificial sweat. It is assumed that the amount of substance released (migrated) from the toys during an average time of 1 hour extraction (extraction duration 4 hours) corresponds to the potential dermal exposure. Oral exposure is based on measurements of the substance in extractions of artificial saliva. It is assumed that the amount of substance released (migrated) from the toys during an average time of 1 hour extraction (extraction duration 4 hours corresponds to the potential oral exposure. 6.2.2 MethodologyFor the chemical substances detected as evaporated or migrated to sweat or saliva from the slimy toys, an evaluation of which substances appeared to be the most interesting (cf. section 4). Then a selection was made in agreement with the Danish Environmental Protection Agency. Data on the individual substances are retrieved to perform a health hazard evaluation based on known information from previously prepared Danish or foreign monographs, etc. The found data for threshold limit values or toxicity are then compared to the concentrations estimated in the used scenarios. The methodology used is approximately the same as recommended in connection to risk assessment in the European Union (EU) i.e. Technical Guidance Document (TGD 2003). In the TGD the potential risk to the consumer is estimated as the ratio between the predicted no-effect concentration (no-adverse-effect level, NOAEL) and the predicted exposure concentration in the surrounding environment (Predicted Environmental Concentration, PEC), i.e. NOAEL / PEC or the estimated uptake in the exposed humans. NOAEL is based on mammalian data that is often not humans but typically rats, mice and rabbits. Therefore, safety factors are introduced to cover differences extrapolating from other animals to humans. This is expressed either by attaching a fixed safety factor (SF) or by expressing the margin of safety (MOS) which represents the distance between the estimated concentration to the NOAEL. Typically MOS is preferred to be above 100. The safety factor is interpreted as a margin of safety applied to a NOAEL to produce a value below which exposures are presumed to be without health risk. The safety factor is traditionally composed of a factor 10 for extrapolation between species (animal to human, interspecies variation), a factor 10 to protect the most sensitive individuals of the population (intraspecies variation) such as e.g. children. A third factor is applied depending on the data and may vary. For instance 10 is used if LOAEL (lowest observed adverse effect level) is used instead of NOAEL or using subchronic data instead of chronic data. The total safety factor is a result from multiplication of the three factors. The effect level divided with the safety factor is used to evaluate whether there is reason of concern (concern level) or a further refinement of methodology or data is necessary. Thus the assessment may be expressed on basis of concentration divided with the safety factor (such as e.g. ADI, TDI, RfD, RfC) or MOS. In modern society is used many chemical products. It can be difficult for the single consumer to keep track of them all. The handling of the chemical substances is therefore regulated on basis of an extended chemical legislation. In connection with this project no values have been derived for chemical substances already evaluated by national or international experts in the field. The classification authorised in Denmark (Miljøministeriet 2002), which is an implementation of EU classification (28th amendment to EU directive 67/548/EEC), is used in the evaluation. The amendments performed in the 29th amendment and adopted in Directive 2004/73/EC (EC 2004) and not yet implemented in Denmark are included, however, as the implementation may be expected within a short time. For the evaluation of the individual substances is used the threshold limit values mentioned above and explained below. The threshold limit value (TLV) valid for the working environment (AT 2002) is generally not used as it is only valid for the working environment and does not cover the consumer at home. The TLV value is presented for information and comparison, if available. Other limit values included in the health evaluation were:
The effect level for each piece of slimy toy is based on evaluations of individual substances. The established Danish threshold limit values are used when they exist. When no Danish threshold limit values exist, foreign threshold limit values are used including their background, if available. The indoor air quality depends on several factors (ventilation, temperature, etc.) and many sources. In this report, only the contribution from slimy toys is considered but it should be noted that other sources to the same chemical compound may exist in the consumer's resident (e.g. by smoking, cooking, volatiles from paint, lacquers, carpets, etc.). The exposure of the consumer in the home is besides the concentration in the indoor air also dependent on the exposure duration. Because the exposure duration may vary considerably, a maximal exposure of 1 hours is assumed. 6.2.3 Exposure via inhalationThe exposure via inhalation may theoretically extend from the acquisition or purchase of the slimy toys, until it is no longer used (discarded). The substances that the consumer are exposed to during unwrapping and during the initial use period may approximately be assumed to be the substances observed in the “head-space” analyses. The exposure via inhalation is expressed as the concentration of the chemical substance in the air in the breathing zone and expressed as an average concentration over a reference period, e.g. 1 hour or 1 day. For the consumer of slimy toys the exposure period may be extended from the time the slimy toy is unwrapped and used to considerably longer time, where the toy degasses or if more pieces of slimy toys are used and the duration for all emission products to be ventilated out of the room/home. For estimation of the exposure via inhalation, the concentration in the air must be known, the inhalation rate and air volume (the breathing zone or the size of the room). The inhalation rate for an average adult person is set to 20 m³/day corresponding to 0.83 m³/hour (standard in TGD 2003). However, because slimy toys appeal more to children than adults, it is decided to use scenarios for children. For a child several choices are available depending on age and level of activity. For the assessment is chosen a short term scenario with a child at moderate activity and the respiration rate 1.2 m³/time. For the short-term inhalation scenario is used exposure in the breathing zone that in this context is set to 1 m³. Even small children can be assumed to get into contact with the toy or be in the same room where the toy is used. Therefore, based on a reasonable ”worst case” consideration, a long-term exposure scenario is selected using a respiration rate of 8.3 m³/day (child of 3 to 5 years of age, TGD 2003). The concentration in closed rooms is assumed to be higher than in ventilated rooms. For the calculation of the concentration in the room it is assumed that the substance is emitted instantly to the entire room and is homogeneously dispersed. The size of the standard room is set to 8 m² and the height 2.5 meter, i.e. the volume of the room is 20 m³. The concentration in inhaled air can then be calculated according to the equation:
The amount of inhaled substance is then (TGD 2003):
6.2.4 Dermal exposureIn a scenario for dermal exposure is assumed that the product is used by hand, which thereby is primarily exposed. However, slimy toys may also get in contact to other parts of the body and, thus, an actual exposure area may be difficult to establish. Therefore, it is chosen to use the release from the toy per time unit in the migration test as the average amount to which the consumer is exposed. Before percutaneous exposure the chemical compound has to migrate from the toy to the skin. When the compound has reached the skin, the compound may be absorbed percutaneous to the blood stream and then distributed throughout the body. The uptake after contact may be from ”free” chemical compounds released from the toy or from degradation products. The degradation of the compounds may take place in the toy, via bacteria or enzymes on the skin or in the gastrointestinal-tract after absorption. As the chemical compounds are located on the exterior of the toy, or potentially can be released or migrate from the toy, an extraction solution simulating sweat has been used. The substances detected by the extraction are the substances that potentially may be absorbed via the skin by contact to the toy. The exposure can be expressed in the equation (TGD 2003) which is modified to the used exposure scenario:
where:
As basis is assumed that a child plays with the slimy toy one or several times each day. The total dermal exposure duration is assumed to be maximum 1 hour/day. The child's body weight is set to 10 kg. This means that the calculations are performed as released (migrated) substance during an average of 1 hour (based on an analysis extraction period of 4 hours) × exposure duration/day × fraction absorbed/kg body weight: Thus dermal absorption = Weight of toy × µg substance released/hour × 1/4 (hours) × (% absorption/100%) exposure duration × (hour/day) / 10 (kg) = µg/kg body weight per day. The contact time of 1 hour/day through a prolonged period is assumed to be exaggerated. However, if this results in no problems, then no concern is relevant at shorter exposure periods. Absorption After exposure to the skin the chemical compound has to pass the skin, before actual absorption is taking place. Only a few data of percutaneous absorption of the studied compounds have been found. The dermal absorption is therefore estimated. Depending on the exposure and/or the compound's lipophilicity the dermal penetration is assumed to be insignificant for very lipophilic compounds with a log Kow more than 5 (OECD 1993). Also dermal penetration is considered very small for compounds with a log Kow less than -1 (i.e. very hydrophilic) and for compounds with a molecular weight above 700 (Vermeire et al. 1993). According to a Dutch model, the dermal absorption is estimated to 10% for compounds with a molecular weight above 500 g/mol and a log Kow <-1 or >4 (De Heer 1999). The latter values are also included in the TGD (2003). In standard assessments, when no information is available, a typical dermal absorption of 100% is used (TGD 2003). This has been performed with all organic compounds. If information on absorption was available, the information has been used in refining of the estimates. It has been performed by multiplying the dermal exposure (Uderm) with the absorption factor (Fabs): Aderm = Uderm Fabs where:
The dermal absorption of metals is presumed very small. For zinc, the dermal absorption is 2% in liquid zinc compounds and in solids assumed 0.2% in the EU risk assessment (ECB 2004). For chromium low penetration rates of 51Cr have been observed: 0.07% in 3 hours and 0.18% in 50 hours (Fairhurst and Minty 1989). Approximately the same relation is assumed in the scenarios for dermal absorption of metals but modified to 0.2% for all metals. In the latest draft to the risk assessment of nickel, for nickel salts is used 2% as absorbed fraction by dermal contact (nickel sulphate, nickel chloride, nickel nitrate and nickel carbonate). For nickel metal is found a dermal absorption of 0.2% based on an in vivo study on humans (MST 2003). 6.2.5 Oral exposureBy oral exposure the absorption takes place after release (migration) of the compounds from the slimy toy and mixing with the saliva. Uptake is assumed to take place over the epithelium in the mouth cavity or in the gastro-intestinal-tract. As basis for the assessment of the oral intake is used the general equations described in relevant references (TGD 2003, OECD 1993, Bremmer and van Veen 2002). The equations are then adjusted to an equation fit for the actual scenario with measurements of chemical substances migrated to artificial saliva. Surface area of the toys was not used as most were so small that the child with a little dexterity may get in contact with (by licking, sucking or chewing on) the total surface.
where
As basis is assumed that a child plays with the slimy toy one or several times each day. The total oral exposure duration is assumed to be maximum 60 minutes or 1 hour/day. The child's body weight is set to 10 kg. The analysis results represent the amount migrated to the saliva extracts after 4 hours of extraction. The amount is recalculated to release per hour. Thus oral absorption = Weight of toy × µg substance released/hour × 1/4 (hours) × (% absorption/100%) × exposure duration (1 hour/day) / 10 (kg) = µg/kg body weight per day. Oral intake is especially relevant for the product which as lipstick is directly aimed to put on the lips (TO-03, lip gloss). As a worst case all of the product may be taken up orally. It is noted that oral intake also may take place by hand-to-mouth, i.e. hands or fingers, which have touched the product, and then are placed in the mouth. This may result in transference of substance from the fingers to the mouth. Especially for a sticky product like slimy toys such a contribution must be considered likely and not insignificant. As information in the reference literature (Bremmer and van Veen 2002, Green 2002, Kiss 2001) indicate that hand-to-mouth averages 3 to 10 minutes that part is considered included in the selected exposure period of 1 hour. Absorption After exposure to the mouth cavity the chemical substance has to pass the epithelium before actual absorption may take place. Only few data for oral absorption of the selected substances have been found. The oral uptake across the epithelium (oral absorption) is therefore estimated for many for the substances. The same methodology as given for dermal absorption (cf. above) is used. By standard evaluation or where no information is available, a typical oral absorption of 100% (TGD 2003) is used. 6.2.6 Total exposureIf the consumer is exposed to the same substance from the same product via different exposure routes, the total uptake can be added. No assessment of the single slimy toy versus another has been performed. Partly because it was not the purpose to evaluate single slimy toys, and partly because the number was too small. The purpose of the survey was to evaluate which substances and the amount of substances slimy toys released. Finally, the detected concentrations are so low that by the short exposure duration such an exercise would be of limited value by normal consumer patterns. However, it should be noted that the consumer (child) may handle more than one slimy toy simultaneously or at intervals, thus increasing the exposure to one or more chemical substances correspondingly. Other sources of the same chemicals may also be present in the surroundings of the play activity. This may also contribute to the total exposure. 6.3 Evaluation of individual substancesFor the evaluation of individual substances the below mentioned substances have been selected in co-operation with the Danish Environmental Protection Agency. Organic substances Inorganic substances 6.3.1 2-ButanoneIdentification
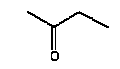
The melting point is -86.6°C. The boiling point is 79.5°C (Budavari 1996). The vapour pressure is 12077 Pa at 25°C (90.6 mmHg). The water solubility is 223 g/l at 25°C (EPI). The partition coefficient log Kow is experimentally determined to 0.29 (Hansch et al. 1995). Use 2-Butanone is used in a number of industrial applications. The primary use of 2-butanone, accounting for approximately 63 percent of all known consumption, is as a solvent in protective coatings. 2-Butanone is also used as a solvent in adhesives; printing inks; paint removers; in the production of magnetic tapes; and in dewaxing lubricating oil. Classification Butanone is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Effects on health Of data on acute effects are found:
*: 1 ppm = 2.95 mg/m³ The substance is irritating to the eyes and mildly to moderately irritating to the skin in rabbits (NCM 1999). Skin irritation studies in rabbit showed a moderately irritation but not enough for a classification. However, the substance was highly irritating in a Draize eye irritating test on rabbits, where the substance had a maximum Draize score at between 1 hour and up to 14 days of exposure. The substance was not sensitising in a guinea pig maximisation test (IUCLID 2000). In humans several case reports and occupational studies indicate neurological effects such as polyneuropathy, after prolonged exposure to 2-butanone vapours. This is supported by the acute exposure studies (NCM 1999). In a repeated dose toxicity test on rats exposed to 0, 1250, 2500 or 5000 ppm 2-butanone via inhalation (vapours) 6 hours/day, 5 days/week for 90 days. After 90 days exposure via inhalation, a depression of mean body weight gain in the 5000 ppm exposure group was observed. The group also had a slight but significant increase in liver weight. LOAEL was 5000 ppm and NOAEL 2500 ppm (equivalent to 7500 mg/m³) (IUCLID). In a mouse developmental study with exposure via inhalation (Schwetz et al. 1991) based on development of skeletal parts after exposure by inhalation for 7 hours/day on days 6-15 of gestation was observed a lowest effect concentration, LEC, of 5202 mg/m³. The study was used for the calculation of RfC (cf. below). In a multigeneration reproductive developmental study, where rats were given 2-butanol in drinking water, was observed based on decreased pup body weight a NOAEL of 594 mg/kg bw/day (0.3% 2-butanol solution) and a LOAEL of 1771 mg/kg bw/day (1% 2-butanol solution) (Cox et al. 1975). Recalculating from the test substance 2-butanol to 2-butanone the 21-day lower 95% confidence interval on the effective dose associated with 5% decrease in body weight (LED05) was 639 mg/kg bw/day. The study was used to calculate the oral RfD, cf. below. Cox et al. (1975) conducted a multigeneration reproductive and developmental toxicity study of 2-butanol. The identification of the critical effect for 2-butanone is based on its metabolic precursor, 2-butanol. Other available pharmacokinetic and toxicological data support the use of 2-butanol as an appropriate surrogate for 2-butanone. The rationale for using 2-butanol is that orally administered 2-butanol almost completely (96%) is converted to 2-butanone and its metabolites within 16 hours (IRIS 2003). No studies examining the subchronic or chronic effects of oral exposure to 2-butanone in humans or experimental animals were identified. Threshold limit values The threshold limit value for the working environment is 50 ppm equivalent to 145 mg/m³ with skin notation, i.e. the substance can be absorbed through the skin (AT 2002). The C-value is 1 mg/m³ (B-værdilisten, Miljøstyrelsen 2002). Inhalation RfC: 5 mg/m³ cf. above. In a developmental study on mice (Schwetz et al. 1991) was observed a lowest effect concentration LEC: 5202 mg/m³, which was recalculated / adjusted to LEC(ADJ) = 5202 mg/m³ × 7 h/24 h = 1517 mg/m³ or adjusted to human equivalent concentration, LEC(HEC): 1517 mg/m³. Using a safety factor of 300 (3 for interspecies, 10 for intraspecies and 10 for data deficiencies) derived an oral RfC 5 mg/m³ (IRIS 2003). Oral RfD: 0.6 mg/kg bw/day In a multigeneration reproductive developmental rat drinking water study was observed a NOAEL of 594 mg/kg/day (0.3% 2-butanol solution) (Cox et al. 1975). Recalculating from the test substance 2-butanol to 2-butanone the lower 95% confidence interval associated with 5% decrease in body weight LED05 was 639 mg/kg bw/day. Using a safety factor of 1000 (10 inter, 10 intraspecies and 10 for data deficiencies) derived at a chronic oral RfD 0.6 mg/kg bw/day (IRIS 2004). Absorption Absorption studies in humans and animals have demonstrated that 2-butanone can be absorbed via the lungs, the skin, and the gastrointestinal system. Pulmonary absorption values range from 41.1% to 55.8% (IPCS 1993). The relative uptake through the lungs by humans was about 53% through a 4 hour exposure at 200 ppm (HSDB, WHO 1993). Oral studies in rats have demonstrated that the peak blood level of 2-butanone (0.95 mg/ml blood) was reached in 4 hours following oral administration of the chemical in water (1690 mg/kg) (US-EPA 1994). 2-Butanone can also be absorbed through intact human skin. A steady state concentration in expired air was reached in 2-3 hours following exposure of the palmar surface of the forearm of volunteers (Krasavage et al. 1982). Absorption is more rapid through moist skin than through dry skin, and the rate of percutaneous absorption has been estimated to range from 5 to 10 micrograms/cm²/min (IPCS 1993). Assessment The assessment of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using expsoure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 53%. Calculation example (The calculation example is only included for the first substance but the same calculation procedure is used for the remaining substances): Evaporation of 2-butanone was measured to 2.3% corresponding to 184 ng from the toy (184 ng in 1 L rilsan bag corresponding to 0.079 mg/m³). The exposure in the breathing zone will be smaller corresponding to a dilution on 1000 compared to the concentration in the rilsan bag. The concentration in the breathing zone is 0.079 µg/m³. Short term exposure will be: 0.079 µg/m³ × 0.53 (absorption) × IH short-term 1.2 m³/h or 8.3 m³/day / 10 kg / 20 m³), i.e.: Short-term exposure: (0.079/1000) × 0.53 × 1.2 / 10 / 1 Long-term exposure: (0.079/1000) × 0.53 × 8.3 /10 / 20 Table 6.1. Uptake via inhalation of 2-butanone
*: Note that the air concentration in the room used for the chronic exposure estimation is 5% (1/20) of the concentration in the tabulated breathing zone 2-Butanone was only found to evaporate from 1 slimy toy but from both the exterior part and the interior liquid. 2-Butanone was not detected in saliva or perspiration (sweat) extracts. Conclusion The concentration of 2-butanone was far below the RfC value of 5 mg/m³ and the C-value of 1 mg/m³. The RfD value of 0.6 mg/kg bw/day was not exceeded either. The margin of safety (MOS) is above (594/2.2×10-6 = ) 2.7×108. It is therefore concluded that the exposure to 2-butanone does not present any health risk to the consumer. 6.3.2 2-ButoxyethanolIdentification
The melting point is –74.8°C. The boiling point is 168.4°C (DOW 1990). The vapour pressure is 117 Pa at 25°C (0.88 mmHg) (DOW 1990). The water solubility is 1 kg/l at 25°C (miscible, DOW 1990). The partition coefficient log Kow is experimentally determined to 0.83 (Hansch et al. 1995). Use 2-Butoxyethanol is used as a solvent in surface coatings and in vinyl and acrylic paint (CICAD 1998). Further is mentioned the use as solvent in printing inks and colorants in the EU risk assessment report, draft 2004 (ECB 2004). Classification 2-Butoxyethanol is classified in the List of dangerous substances (Miljøministeriet 2002):
Effects on health 2-Butoxyethanol is moderately acute toxic, irritating to eyes and skin (but not a skin sensitizer). Eye irritation examinations showed that 30 and 70% concentrations of the substances were irritating to the eyes with increasing irritation with corresponding increasing time of exposure. The skin irritation was mild at 4 hours of exposure of rabbit skin, but the irritation increased with increasing time of exposure (CICAD 1998). The effects have mostly been registered as a haemolytic activity of butoxy-ethanol. The effect was dependent on age with older rats as the most sensitive (CICAD 1998). Acute toxicity:
In a subchronic 90 days inhalation study, rats were exposed to 2-butoxy-ethanol at concentrations of 0, 5, 25, or 77 ppm for 6 hours/day, 5 days/week for 13 weeks. Based on haematotoxic effects, the NOAEL and LOAEL were 25 ppm (121 mg/m³) and 77 ppm (372 mg/m³), respectively (Dodd et al. 1983). In a study on developmental effects, pregnant rats were exposed to 2-butoxyethanol at 0, 25, 50, 100 or 200 ppm (35 per group) for 6 hours/day on days 6-15 of gestation. Based on haematotoxic effects the NOAEL and LOAEL were 50 ppm (242 mg/m³) and 100 ppm (483 mg/m³), respectively (Tyl et al. 1984). In a 13 weeks study with rats groups of 10 of each sex were exposed through the drinking water. Based on the water consumption, the male rats were exposed to 0, 69, 129, 281, 367 or 452 mg/kg/day and female rats to 0, 82, 151, 304, 363 or 470 mg/kg/day. Based on effects of the blood parameter and liver, which were observed at even the lowest concentration, LOAEL was 69 mg/kg/d for males and 82 mg/kg/d for females. When water consumption and body weight from the last week of the exposure is used, LOAEL is converted into 55 mg/kg/d for males and 59 mg/kg/d for females. NOAEL could not be determined in the examination (NTP 1993, IRIS 1999). The result is still used by US-EPA to derive a RfD value of 0.5 mg/kg bw/day (IRIS 2004). 2-Butoxyethanol has been evaluated as potential human carcinogen, Group C (IRIS 2003). Threshold limit values The threshold limit value for the working environment is 20 ppm corresponding to 98 mg/m³ with skin notation, i.e. the substance may penetrate the skin (AT 2002). The C-value is 0.04 mg/m³ (B-værdivejledningen, Miljøstyrelsen 2002). Inhalation RfC value is 13 mg/m³. The value is based on sub-chronic rat inhalation study (Tyl et al. 1984, cf. above). The value is based on NOAEL 242 mg/m³ and calculated with a safety factor 10, 6/24 in order to convert 6 hours' exposure to 24 hours per day, a conversion from rat to human (inhalation rate for rat 0.16 m³/d and for human 22 m³/d, the body weight of rat 0.215 kg and for human 64 kg) (CICAD 1998). The RfC calculated using the mentioned variables is then: RfC = (242/10) (6/24) [(0.16/0.215)/(22/64)] = 13.1 mg/m³. Oral RfD value is 0.5 mg/kg bw/day. The value is based on a 13-week of subchronic study where haematological effects were found as the most sensitive endpoint with a LOAEL of 55 to 59 mg/kg/day for rats (NTP 1993, cf. above). US-EPA converted the value into 5.1 mg/kg bw/day for humans and used a safety factor of 10 for intraspecies sensitivity (US-EPA 1999). Absorption 2-Butoxyethanol is easily absorbed after inhalation, or by oral or dermal exposure (CICAD1998). Consequently, an absorption of 100% has been used. In the ECB (2004), Risk Assessment Report draft is used 61% for absorption via inhalation and 30% dermal absorption. Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using expsoure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.2. Uptake via inhalation of 2-butoxyethanol
*: Note that the air concentration in the room used for the chronic exposure estimation is 5% (1/20) of the concentration in the tabulated breathing zone 2-Butoxyethanol was detected released from 6 slimy toys. The estimated concentrations are more than 1000 times below the RfC value and the estimated uptakes significantly less than the RfD value of 500 µg/kg bw/day. 2- Butoxyethanol was not detected in saliva or sweat extracts. Conclusion From the tables above can be derived that none of the amounts taken up by the use of slimy toys results in a dosage above the RfD value. Neither the inhalation reference value (RfC) of 13.5 mg/kg/day nor the C-value of 40 µg/m³ have been exceeded, as the concentration in the breathing zone was max. 0.24 µg/m³ and the room concentration was max. 0.24/20 = 0.012 µg/m³. The margin of safety MOS is more than (500/0.01=) 50000. Therefore, this substance is evaluated not to provide any health risk. 6.3.3 3-CareneIdentification
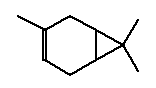
The melting point is <25°C. The boiling point is 170°C. The vapour pressure is estimated to 280 Pa at 25°C (2.09 mmHg). The water solubility is estimated to 4.6 mg/l at 25°C (EPI 2000). The partition coefficient log Kow is measured to 4.38 (EPI). Use 3-Carene belongs to the chemical group terpenes. Terpenes exist in ethereal oils. Terpenes may arise from the use of vegetable oils and resins in products and as solvent in colorants. Classification 3-Carene is not classified. 3-Carene belongs to the group of terpenes. Terpenes or vegetable turpentine (CAS no. 9006-64-2) is classified in the List of dangerous substances (Miljøministeriet 2002):
Effects on health Only few data have been available for 3-carene. Most of the available data were based on acute effects. Acute toxicity:
Exposure of pigs and rats to 3-carene at 5000 mg/m³ for 10-20 minutes induced a marked bronchi-constriction in isolated, perfused and ventilated lungs (Falk-Filipsson 1995). An oxidation product of 3-carene (probably a hydroperoxide) is thought to be the causal factor of the observed irritative and sensitising effects. 3-Carene induces contact allergy in pigs and sensitise guinea pigs. In case studies 3-carene has been found to be the specific sensitiser in the terpenes (Söderkvist 1987, Falk-Filipsson 1995). In humans, a concentration of 450 mg/m³ 3-carene caused discomfort in the eyes experimentally. No effects were found at 225 mg/m³ (Falk-Filipsson 1995). Because the substance is a terpene, the evaluation is based on a general knowledge on terpenes. The terpenes are generally irritants to the mucous membranes. Turpentine from coniferous trees are skin sensitisers. The sensitization, however, is not confirmed for the individual terpenes with the exception of 3-carene (ASS 2000). Monoterpenes, which include among others D-limonene, pinenes and carenes, are described under the common name "turpentine" with CAS no. 8006-64-2. Turpentine consists chemically of 58 to 65% alpha-pinene and beta-pinene and other isomere terpenes. Turpentine from wood extracted from waste wood or sawdust contains 80% alpha-pinene, 15% monocyclic terpenes, 1.5% terpene-alcohols and other terpenes (Bingham et al. 2001). Already in 1939 was demonstrated that Swedish painters more often suffered dermatitis from turpentine compared to French painters. The difference could be traced to the turpentine's content of 3-carene that was considerably higher in Swedish than in French manufactured turpentine. An oxidation product of 3-carene was later identified as the probable cause to these effects (Söderkvist 1978). Effects on health Vapours are irritating by contact to eyes and respiratory tracts. If vapours are inhaled, they may cause headache, vomiting, dizziness and faintness. The liquid irritates the skin, and if ingested may cause irritation to the total digestion system and possibility of kidney lesions. If the liquid substance reaches the lungs, it may cause severe pneumonia (Prager 1996). The lethal dose of turpentine may by ingestion be as low as 110 g. However, survival after ingestion of 120 g has been observed. As little as 15 g has been shown fatal to a child (Bingham et al. 2001). In an experiment with male and female volunteers, the following observations have been reported. Persons with an average age of 35 years were exposed to 0 or 450 mg/m³ of a mixture consisting of 10 parts alpha-pinene, 1 part beta-pinene and 5 parts 3-carene (synthetic turpentine) for 12 hours 4 times over a two-week period. Acute damages to the lungs were observed. Male volunteers exposed for two hours with 450 mg/m³ during easy workout experienced their respiratory passages were influenced by the exposure, and they had breathing difficulties after the termination of the exposure (Bingham et al. 2001). Threshold limit values The threshold limit value for the working environment is 25 ppm equivalent to 140 mg/m³, corresponding to high-boiling aromatic hydrocarbons (terpenes, turpentine) (AT 2002). LCI (Lowest Concentration of Interest) is 0.25 mg/m³ for most terpenes (Larsen et al., 1999). The C-value for turpentine is 1 mg/m³ (C-value guidance, Miljøstyrelsen 2002). Absorption No information has been found on uptake of 3-carene but as turpentine and alpha- and beta-pinene are easily absorbed through lungs, skin and gastro-intestinal canal (Clayton and Clayton 1983) the absorption for 3-carene is set to 100%. Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.3. Uptake via inhalation of 3-carene
Carene was detected in 7 samples of slimy toys analysed for volatile organic compounds (headspace) at the concentrations between 9 and 49 µg/m³. The calculated uptake via inhalation is summarised in the table above. Carene was not detected in the sweat or saliva extractions. Conclusion By comparison of the maximum measured concentration of 49 µg/m³ with the found LCI value of 250 µg/m³ is observed a factor of 5 in difference. Using NOAEL 225 mg/m³ the margin of safety (MOS) is 225/2×10-6 = 1,1×108. This means that the amount released does not imply any health risk. 6.3.4 CyclohexanoneIdentification
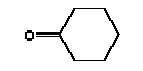
The melting point is -31°C. The boiling point is 155°C (Budavari 1996). The vapour pressure is 577 Pa at 25°C (4.3 mmHg) (Daubert and Danner 1985). The water solubility is 25 g/l at 25°C (Yalkowsky and Dannenfelser 1992). The partition coefficient log Kow is measured to 0.81(Hansch et al. 1995). Use Cyclohexanone is used in the chemical industry for organic synthesis, particularly in the production of adipic acid and caprolactam (ca. 95%), polyvinyl chloride and its copolymers, and methacrylate ester polymers. Classification Cyclohexanone is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Effects on health Acute toxicity:
For humans was observed that the threshold for irritation to the nasal mucous membranes was 0.28 mg/l of air (280 mg/m³ or about 70 ppm). The value was seconded by irritation of eye, nasal, and throat at 0.362 mg/l of air (362 mg/m³ or about 90 ppm). A second exposure 2 weeks after the initial series indicated an increase in the sensory irritation threshold. In this series, the only response recovered was throat irritation at 0.547 mg/l of air (547 mg/m³ or about 136 ppm) (SIDS 1996). Humans exposed for 3-5 minutes found 50 and 75 ppm (200-301 mg/m³ air) irritating to the eyes, nose and throat. A concentration of 25 ppm was unobjectionable (Nelson et al. 1994). Cyclohexanone exhibits low to slight acute toxicity by the oral and inhalation routes and is moderately toxic by the dermal route. Cyclohexanone is an eye and skin irritant; but does not induce skin sensitisation. Upon repeated administration to rats of cyclohexanone in drinking water, the NOAEL was 4700 ppm after 25 weeks, and the LOAEL was 3300 ppm after 2 years. Effects at higher concentrations were primarily body weight decreases. The NOAEL in published repeated dose inhalation studies was 100-900 ppm. Those values were based on either gray mottling of the lungs or ocular irritation and degenerative changes in the liver and kidney at higher concentrations. However, the NOAEL in those studies was not confirmed in later and better inhalation studies, where for reproductive and developmental effects NOAEL values of 650-1000 ppm were observed. In a two-generation reproduction study, decreased fertility was observed in rats exposed via inhalation at 1400 ppm but not at 500 ppm. The effect was found to be reversible following a post-exposure recovery period. (IRIS 2004). In a chronic rat oral study, where rats in groups of 52 animals per dose were exposed to cyclohexanone in drinking water at 3300, 6500, 13000 and 25000 ppm. Based on mortality and decrease in body weight a LOAEL of 6500 ppm corresponding to 910 mg/kg bw/day was found. NOAEL was 3300 ppm corresponding to 462 mg/kg bw/day (Lijinski and Kovatch 1986). Threshold limit values The threshold limit value (TLV) is 10 ppm equivalent to 40 mg/m³ with skin notation (H), i.e. the substance may penetrate the skin (AT 2002) TCA (tolerable concentration in air) is 136 µg/m³ (Baars et al. 2001). The C-value is 0.1 mg/m³ (B-værdivejledningen, Miljøstyrelsen 2002) The oral RfD value is 5 mg/kg bw/day. In a chronic oral rat study was found a NOAEL of 462 mg/kg bw/day (cf. Lijinski and Kovatch 1986 above). Applying a safety factor of 100 (10 for inter- and 10 for intraspecies extrapolation) derived an oral RfD value of 5 mg/kg bw/day. TDI (tolerable daily intake) value is 4.6 mg/kg bw/day (Baars et al. 2001). Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.4 Uptake via inhalation of cyclohexanone
The TDI value of 4.6 mg/kg bw/day was not exceeded. A factor of >8000 to the highest estimated concentration in the breathing zone (1 m³) at acute exposure and a factor of >24000 to the highest estimated concentration in the 20 m³ room at chronic exposure was observed. Cyclohexanone was detected in the sweat extractions from 4 slimy toys. The uptake is calculated below. Table 6.5 Uptake of cyclohexanone by dermal exposure
The TDI value was not exceeded: the difference was approx. a factor of 30 to the TDI value of 4.6 mg/kg bw/day and the RfD value of 5 mg/kg bw/day. Using the NOAEL value of 462 mg/kg bw/day the margin of safety (MOS) is >2900. Cyclohexanone was detected in the saliva extractions from 3 slimy toys. The calculated uptake by oral exposure is summarised in the table below. Table 6.6 Uptake of cyclohexanone by oral exposure
The TDI value was not exceeded. The difference was approx. 80 to the TDI value of 4.6 and the RfD value of 5 mg/kg bw/day. Using the NOAEL value of 462 mg/kg bw/day the margin of safety (MOS) is >7800. Conclusion The maximum total uptake of cyclohexanone was from toy TO-01 at 216 µg/kg bw/day (cf. table below). This uptake was below the TDI value of 4.6 mg/kg bw/day. Table 6.7 Total uptake of cyclohexanone by exposure to slimy toys
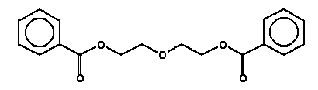
The total uptake by the three exposure routes is below the TDI value of 4.6 mg/kg bw/day. Besides, the total margin of safety is (MOS: 462/0.217 =) > 2000. The release of cyclohexanone is therefore not considered to pose a health problem. 6.3.5 Diethylglycol dibenzoateIdentification
The melting point is 28°C. The boiling point is 225°C (Budavari 1996). The vapour pressure is 573 Pa at 25°C (4.3 mmHg) (Daubert and Danner 1985). (1.7x10-5 Pa, Velsicol 2001). The water solubility is 38.3 mg/l at 30°C (Velsicol 2001). The partition coefficient log Kow is measured to 3.2 (Velsicol, OPPT 2001). Use Diethylglycol dibenzoate is used as plastisiser in polymers and may be recovered in vinyl floors, adhesives and sealants. Diethylglycol dibenzoate is mentioned in the INCI list with a function as emollient. Classification Diethylglycol dibenzoate is not classified. Effects on health The acute toxicity is low:
In a repeated dose toxicity study, where diethylglycol dibenzoate was dietary administered for 13 weeks at the concentration 0, 250, 1000, 1700 or 2500 mg/kg/day, was observed a NOAEL 1000 mg/kg bw/day (OPPT 2001). In a study on developmental toxicity to rat foetuses the test substance was given as oral (gavage) administration at 0, 250, 500 and 1000 mg/kg/day. The exposure period was days 6-19 of gestation inclusively. Maternal toxicity resulted in a NOAEL 1000 mg/kg/day. Prenatal development showed a NOAEL 500 mg/kg/day. Foetal growth and developmental had a NOAEL of 250 mg/kg/day (OPPT 2001). In a study on reproductive toxicity for two-generations, rats were exposed for 38 weeks by dietary administration at 0, 1000, 3300 or 10000 ppm. NOAEL for the developing offspring is considered to be 300 ppm. NOEL for reproductive parameters is considered to be 10000 ppm (OPPT 2001). In a skin sensitisation test on guinea pig, no evidence of skin sensitisation in any of twenty test animals. Evidence of skin sensitisation was produced by hexyl cinnamic aldehyde (HCA) in all ten positive controls thus confirming the sensitivity of the method (OPPT 2001). Threshold limit values No threshold limit values have been available. Absorption No values on absorption were available, therefore, the absorption is set to 100%. Assessment Diethylglycol dibenzoate was not detected as volatile substance in the headspace analyses but as migrated substance in the sweat and saliva extractions. Table 6.8 Uptake of diethylglycol dibenzoate by dermal exposure
Table 6.9 Uptake of diethylglycol dibenzoate by oral exposure
As no threshold limit values are found, the margin of safety to NOAEL from a rat development toxicity study is used (OPPT 2001). The lowest NOAEL observed is 250 mg/kg bw/day and the total uptake from dermal and oral exposure is 43.3+23.9 = 67.2 µg/kg bw/day, i.e. the margin of safety (MOS) is >3700. Thus it is concluded that no health risk from the exposure to diethylglycol dibenzoate existed. Components homologous to diethylglycol dibenzoate Chemical components homologous to diethylglycol dibenzoate were observed even if they could not be specifically identified. However, they could be detected in the sweat and saliva extractions. Measurements of components homologous to diethylglycol dibenzoate resulted in the following uptakes (cf. tables below): Table 6.10 Uptake by dermal exposure
Table 6.11 Uptake by oral exposure
The homologues substances were detected at approximately the same concentrations as the corresponding diethylglycol dibenzoate. Assuming that their toxicity are of a comparable character and level the measured concentrations can be added (cf. table below): Table 6.12 Total Uptake by oral exposure
Based on a margin of safety (MOS) of at least 250/0.130 = 1920 was assessed that they do not pose a health risk to the consumer. 6.3.6 EthylbenzeneIdentification
The melting point is –95°C. The boiling point is 136.2°C (Budavari 1996). The vapour pressure is 1280 Pa at 25°C (9.6 mmHg, Daubert and Danner 1985). The water solubility is 169 mg/l at 25°C (Sanemase et al. 1982; EPI). The partition coefficient log Kow is measured to 3.15 (Hansch et al. 1995). Use Ethylbenzene is used as solvent of resins in printing inks and lacquers. Ethylbenzene is a component of oil products. Classification Ethylbenzene is adopted on the List of dangerous substances and classified(Miljøministeriet 2002):
Effects on health Ethylbenzene has a low acute and chronic toxicity. The acute threshold values are 430 to 860 mg/m³ (100-200 ppm) (IPCS 1996). Ethylbenzene is moderately toxic by oral administration (Lewis 1992). Acute toxicity:
Ethylbenzene is an irritant to skin, eyes and mucous membranes and may affect the central nervous system (Budavari 1996, IPCS 1996). The main part of toxicity studies on ethylbenzene is inhalation studies. Inhalation of 434 mg/m³ may cause irritation. The lowest published toxic concentration for humans is 434 mg/m³ (8 hours exposure) causing irritation of the nose and eyes (Larsen et al. 1999). Based on a 13 weeks inhalation study a NOAEL of 430 mg/m³ (100 ppm) has been derived (IPCS 1996, ATSDR 1999). The NOAEL 430 mg/m³ is based on 6 hours/day, 5 days a week. A back-calculation based on continuous exposure 24 hours/day and 7 days/week results in a concentration of 77 mg/m³. Using an uncertainty factor of 100 (10 for interspecies and 10 for intraspecies extrapolation) resulted in a TCA of 770 µg/m³ (Baars et al. 2001). A recommended threshold limit value (TWA) of 22 mg/m³ (5 ppm) is derived from a 13 weeks inhalation animal study: 2150/(1052) = 22 mg/m³ (IPCS 1996). In a 182 days oral rat study ethylbenzene was administered via gavage in olive oil to rats at the dosis 13.6, 136, 408 and 680 mg/kg/day for 5 days/week. After examination of several toxic effects was set a LOAEL of 408 mg/kg/day based on histopathological changes. Recalculating from 5 days/week to 7 days/week the level was adjusted to (408 ×5/7 =) 291 mg/kg bw/day. Correspondingly was NOAEL of 136 mg/kg bw/day recalculated to 97 mg/kg bw/day (Wolf et al. 1956). Ethylbenzene has been evaluated by IARC who concluded that there was insufficient evidence of ethylbenzene being carcinogenic to humans but sufficient evidence for test animals. Ethylbenzene was therefore classified in group 2B: "possibly carcinogenic to humans" (IARC 2000). Absorption The most important exposure route was inhalation where 44 to 64% is absorbed via the lungs (IPCS 1996). In the assessment is used 100% absorption. Threshold limit values The threshold limit value for the working environment is 50 ppm corresponding to 217 mg/m³ (AT 2002). The C-value is 0.5 mg/m³ (B-værdivejledningen 2002, Miljøstyrelsen 2002). TCA (tolerable concentration in air): 770 µg/m³ (Baars et al. 2001). TDI (tolerable daily intake) value is 100 µg/kg/day (IPCS 1996). The oral RfD value is 97 µg/kg bw/day. The RfD value was derived from a NOAEL in a 182 days oral rat study (Wolf et al. 1956) of 97 mg/kg bw/day. Using a safety factor of 1000 (10 for intraspecies, 10 for interspecies variation and 10 for extrapolating from subchronic to chronic) was derived a NOAEL of 97 µg/kg bw/day. The same study and methodology forms the basis for WHO derivation in the Drinking water programme of a TDI of 97 µg/kg bw/day (IPCS 1996). Assessment Ethylbenzene was detected in the screening test but was quantified together with xylenes in the headspace analyses. In the assessment of inhalation ethylbenzene, therefore, is included in the assessment of xylenes. Ethylbenzene is separated in the qualitative analyses of migration to sweat and saliva. The results are presented below. Table 6.13 Uptake by dermal exposure
Table 6.14 Uptake by oral exposure
By adding the uptakes from sweat and saliva from the toy with the highest migration of ethylbenzene, BR-01, is reached 0.35+0.40 = 0.75 µg/kg bw/day. This value does not exceed the TDI value 100 µg/kg bw/day. The margin of safety (MOS) is calculated to: 97/0.00075 = >10000. Therefore, it is concluded that ethylbenzene does not imply a health risk by handling or placing the toy in the mouth. Relating to inhalation cf. the section on xylene. 6.3.7 2-HexanoneIdentification
The melting point is -55.5°C. The boiling point is 127.6°C (Budavari 1996). The vapour pressure is 1146 Pa at 25°C (11.6 mmHg, Daubert and Danner 1985). The water solubility is 17500 mg/l at 25°C (EPI). The partition coefficient log Kow is measured to 1.38 (Hansch et al. 1995). Use 2-Hexanone is as pure substance a clear, colourless liquid with a sharp odour. The substance dissolves very easily in water, and can evaporate easily into the air as a vapour. The substance is used in paint and paint thinner, to make other chemical substances, and to dissolve oils and waxes. It is used in drying and curing of coatings in which it is contained as a solvent. Classification 2-Hexanone is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Effects on health Acute toxicity:
Most studies concern exposure via inhalation. In a 90-day study, hens were exposed continuously to 2-hexanone. At 200 ppm 1 of 5 hens died after 72 days. At 400 ppm, 2 of 5 hens died by day 27. The cause of death was not stated. No deaths were observed in the groups exposed to 100 ppm and below. The highest NOAEL value (approx. 10 ppm) and a reliable LOAEL value of 50 ppm are recorded in comparable studies (ATSDR 1992). Studies using oral administration of the substances are usually of low quality. However, two studies are presented below and used in the evaluation. The lowest NOAEL was observed in a study on effects following oral administration by gavage in rats. The rats were dosed 5 days/week for 40 weeks. Based on the effects on kidney and liver NOAEL was 400 mg/kg/day (Eben et al. 1979). In a study on hens 2-hexanone was administered orally via gavage 7 days/week for 90 days at doses from 100 mg/kg bw/day and more. Based on neurotoxic symptoms such as ataxia (disturbance of co-ordination or dyssynergy, i.e. movements are badly co-ordinated because they cannot be properly controlled from the brain) and histopathological changes is set a LOAEL of 100 mg/kg bw/day (Abou-Donia et al. 1982). Threshold limit values The threshold limit value for the working environment is 1 ppm corresponding to 4 mg/m³ with skin notation (H), i.e. the substance may penetrate the skin (AT 2002). The C-value is 0.3 mg/m³ (Hexanones in the C-value guidance document (B-værdivejledningen, Miljøstyrelsen 2002)). Absorption 2-Hexanone is easily absorbed after administration via the inhalation route. An analysis of the expired breath from humans who had inhaled 2-hexanone at 10 and 50 ppm for 7.5 hours or 100 ppm for 4 hours indicate that 75 to 95% of the inhaled amount of vapours was absorbed by the lungs and respiratory tract (DiVincenzo et al. 1978). 2-Hexanone also appears to be easily absorbed after oral administration. Humans who ingested a single capsule containing 14C-2-hexanone at 0.1 mg/kg excreted about 40% of the 14C in breath and 26% in urine during the next 8 days (DiVincenzo et al. 1978). This indicates that the excreted and with that absorbed amount averaged at least 40+26=66% of the administered dose. Oral administration of 14C-2-hexanone at doses of 20 or 200 mg/kg by gavage to rats resulted in excretion of about 1.2% of the administered radioactivity in the feces, about 44% in the breath, 38% in urine, and 16% remaining in the carcass (DiVincenzo et al. 1977). The results were similar at either dosage level. These findings suggest that about 98% of the administered dose was absorbed. 2-Hexanone is also absorbed after dermal application. The excretion of 14C in the breath and urine of two human volunteers was measured after a 60-minute occlusive application of 14C-2-hexanone to their shaved forearms (DiVincenzo et al. 1978). Calculated skin absorption rates were 4.8 and 8.0 pg/min/cm²; however, the fraction of 2-hexanone that was absorbed was not calculated. 14C-Hexanone was also applied to the clipped thorax of beagle dogs, and absorption was observed to be slow at first but increased dramatically after 20 minutes. At 60 minutes, 77 mg of 2-hexanone had penetrated the skin (DiVincenzo et al. 1978). The fraction of applied 2-hexanone that was absorbed was not calculated. Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.15 Uptake by exposure via inhalation of 2-hexanone
2-Hexanone was detected in 2 slimy toys. The estimated uptake via inhalation is 3.8 and 3.6 ng/kg body weight. Using the C-value for hexanones of 300 µg/m³ the value is far more (>3000 times) than the estimated inhalation concentration of 0.09 µg/m³ in the breathing zone (1 m³) and the room concentration of 0.09/20 = 0.0045 µg/m³. By comparing the LOAEL from the 90-day rat oral study of 100 mg/kg bw/day the margin of safety (MOS) is 100/3.9×10-6 = >2.6×108. 2-Hexanone was not detected as migrated substance in the sweat or saliva extractions. Conclusion 2-Hexanone is a volatile substance that evaporates fast which is confirmed by the detection of the substance in the headspace analyses only. Because no ADI or similar values were found, the validated C-value and MOS are used for the assessment. As the measured concentrations were above the C-value and MOS is very high, it is considered that no health problems to the consumer are expected by exposure to 2-hexanone. 6.3.8 2-PhenoxyethanolIdentification
The melting point is 14°C. The boiling point is 245°C (Budavari 1996). The vapour pressure is 0.93 Pa at 25°C (0.007 mmHg, Dow 1990) or 4 Pa at 20°C (IUCLID 2000). The water solubility is 26700 mg/l at 20°C (Yalkowsky and Dannenfelser 1992). The partition coefficient log Kow is measured to 1.16 (Hansch et al. 1995). Use 2-Phenoxyethanol is used as solvent in many industrial products. Classification 2-Phenoxyethanol is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Effects on health Acute toxicity:
The substance was not irritating to skin in tests on humans in 48 hours closed patch tests and 24 hours tests 3 times/week for 3 weeks. The substance is found irritating to eyes in rabbits (IUCLID 2000). The substance is not a sensitiser in maximisation tests on guinea pigs and in patch tests on humans (IUCLID 2000). 2-Phenoxyethanol is studied in a repeated dose toxicity test for 13 weeks using oral administration of 2-phenoxyethanol in the feed at the concentrations 0, 50, 100, 200 and 500 mg/kg bw. At the highest concentration was observed a significant decrease in body weight gain and an alteration in blood picture. Thus, NOAEL is set to 200 mg/kg bw (IUCLID 2000). Threshold limit values The threshold limit value for the working environment (TLV) is 20 ppm corresponding to 110 mg/m³ with skin notation (H), i.e. the substance may penetrate the skin (DF 2001). The C-value is 0.1 mg/m³ (Miljøstyrelsen 2002). Absorption Because no value on absorption is available the absorption is set to 100%. Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.16 Uptake by exposure via inhalation of 2-phenoxy-ethanol
2-Phenoxyethanol was detected in 1 slimy toy. The calculated uptake via inhalation was 3.9 ng/kg bw/day. 2-Phenoxyethanol was detected in the sweat extractions of 4 slimy toys. By intake it is assumed that a child uses the toy for 1 hour/day. The weight of the child is 10 kg and the absorption is 100%. Based on this the amount of uptake of the substance is calculated (cf. table below). Table 6.17 Uptake by dermal exposure to 2-phenoxyethanol
Because no TDI value is available the NOAEL value of 200 mg/kg bw/day is used. By comparing the NOAEL to the estimated values the margin of safety (MOS) for dermal uptake was >130. 2-Phenoxyethanol was detected in the saliva extractions of 3 slimy toys. Table 6.18 Uptake by oral exposure to 2-phenoxyethanol
Because no TDI value is available the NOAEL value of 200 mg/kg bw/day is used. By comparing the NOAEL to the estimated values the margin of safety (MOS) for oral uptake was >4300. Conclusion Since no limit values are available the margin of safety to NOAEL from a 90 days rat repeated dose toxicity test is used (IUCLID 2000). The lowest NOAEL found was 200 mg/kg bw/day and the highest total uptake from inhalation, dermal and oral exposure was 1514 µg/kg bw/day, i.e. margin of safety (MOS) is >130. It is assessed that no health risk from the exposure to 2-phenoxyethanol exists. 6.3.9 2-Phenylmethylenoctanal (alpha-Hexylcinnamaldehyd)Identification
* The substance is probably better known as alfa-hexylcinnamaldehyde, therefore, that name is used below. The melting point is 4°C. The boiling point is 304°C. The vapour pressure is 0.027 Pa at 20°C (0.0002 mmHg). The water solubility is estimated to 1.8 mg/l at 25°C (also found estimated to 2.75 mg/l at 25°C based on an estimated log Kow 4.82). The partition coefficient log Kow is measured to 5.3. All data are based on studies or estimates presented in FFHPVC (2000). Use alfa-Hexylcinnamaldehyde is often added as fragrance or flavour in food and cosmetics. Classification alfa-Hexylcinnamaldehyde is not adopted on the List of dangerous substances (Miljøministeriet 2002): Effects on health Acute toxicity:
Of subchronic tests, only a 90 days dermal test was found. The test material was applied percutaneously to the shaved dorsals of 10 male rats at dose levels of 0.125, 0.25, 0.50 and 1.0 g/kg bw/day for 90 consecutive days. Based on multisystemic toxicity in the examined parameters LOAEL was 0.125 g/kg bw/day. Because effects were observed at the lowest applied dose level no NOAEL could be derived (FFHPVC 2000). Thus, LOAEL was 125 mg/kg bw/day. In a similar 90 days test with daily dermal application to the clipped backs of 10 rats, only 1 dose level at 25 mg/kg bw/day was used. No evidence of toxicity induced by treatment was observed. Thus NOAEL was 25 mg/kg bw/day (FFHPVC 2000). In an Ames test, no mutagenic activity was determined with any of the Salmonella typhimurium strains tested (FFHPVC 2000). Contact sensitisation due to exposure with alfa-hexylcinnamaldehyde is rare (De Groot et al. 1994). Still, alfa-hexylcinnamaldehyde is used as positive control for the Local Lymph Node Assay (LLNA). LLNA is used as in vivo confirmation of sensitisation potential of chemical substances (Klink and Meade 2003). In the OECD guideline on skin sensitisation, hexylcinnamaldehyde is mentioned as one of three preferred substances for reliability check of the tests sensitivity, as it is known to have mild-to-moderate skin sensitisation properties (OECD 1993). In vivo geno toxicity was tested in a BASC test on the fruit fly Drosophila melanogaster after oral administration of the test substance. No mutagenic activity was demonstrated (FFHPVC 2000, Wild et al. 1983). The same result was observed in a micronucleus test on mice. Thus NOEL was 756 mg/kg which was the highest dosage used (FFHPVC 2000, Wild et al. 1983). alfa-Hexylcinnamaldehyde is one of the fragrances that according to the Scientific Committee on cosmetic products and non-food products intended for consumers (SCCNFP) is evaluated as allergenic by skin contact, i.e. allergenic (List of undesirable substances Miljøstyrelsen 2004). alfa-Hexylcinnamaldehyde (CAS no. 101-86-0) is a common fragrance allergenic according to the survey by the EU Scientific Committee. The substance is included in the SCCNFP list of the 26 substances there are most often reported as allergenic. The substance belongs to the substances that the EU Parliament in 2002 suggested obligatory to be declared on cosmetic products. From March 11 2005, the substance must be declared in the ingredients list on cosmetic products in the EU, if the concentration exceeds 0.001% (1 mg per 100 gram) in products to remain on the skin (leave-on products) or 0.01% (10 mg per 100 gram) in products that are washed off (rinse-off products) (Directive 2003/15/EC, EC 2003). Threshold limit values No threshold limit values are found. Absorption No values for the absorption via the different exposure routes were available. Thus, an absorption of 100% is assumed. Assessment alfa-Hexylcinnamaldehyde was not detected as volatile substance in the headspace analyses. alfa-Hexylcinnamaldehyde was detected in the sweat extractions in 1 slimy toy. Table 6.19 Uptake by dermal exposure to alfa-hexylcinnamaldehyde
Since no limit values are available, the uptake through skin is assessed by a comparison to NOAEL of 25 mg/kg bw/day from a 90 days dermal rat test. The margin of safety (MOS) was 25/0.00081 = 30800. alfa-Hexylcinnamaldehyde was not detected in the saliva extractions. The effect of oral uptake is thus not assessed. Conclusion alfa-Hexylcinnamaldehyde was found migrated only to sweat with a MOS of 30800. alfa-Hexylcinnamaldehyde, therefore, is assessed not to imply a health risk to the consumer. However, it should be noted that alfa-hexylcinnamaldehyde is used as positive control in studies on skin sensitising. Thus, a risk for skin sensitising can not be excluded. 6.3.10 D-LimoneneIdentification
The melting point is –74.35°C (Lide 1992). The boiling point is 176°C (Budavari 1996). The vapour pressure is 192 Pa at 25°C (1.44 mmHg) (Riddick et al. 1986). The water solubility is 13.8 mg/l at 25°C (Massaldi and King 1973). The octanol/water distribution coefficient log Kow is measured to 4.57 (Li and Perdue 1995). D-Limonene has a high vapour pressure indicating that limonene can be expected to evaporate from dry and wet surfaces Use D-Limonene is used as solvent, in the production of resins and as wetting and dispersing agent. Classification D-Limonene is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Effects on health D-Limonene is irritating to the skin and may be a sensitizer (Budavari 1996, Karlberg and Lindell 1993). D-Limonene is a moderate oral toxicant (Lewis 1992). Examples of effect levels are presented below. D-Limonene is readily oxidised by the air oxygen. Experimental studies show that limonene in itself is not allergenic, but allergenic compounds are formed from limonene by autooxidation (Karlberg et al. 1992, Karlberg and Lindell 1993). Acute toxicity:
In a 13 weeks study on rats, the rats were administered orally at a dosage of 0, 2, 5, 10, 30, and 75 mg/kg bw/day 5 days/week. Based on histological examination changes in the kidneys were observed. On that basis was set a NOEL of 5 mg/kg bw/day. The LOEL for increased liver and kidney weight was 75 mg/kg bw/day. The NOEL for effects in the liver was 10 mg/kg bw/day. The NOAEL for effects in the liver was 30 mg/kg bw/day (Webb et al. 1989, CICAD 1998). In a 13 weeks study on rats, the rats were orally administered 0, 150, 300, 600, 1200 or 2400 mg/kg/day. Based on a dose-related decrease in body weight gain from 600 mg/kg/day NOAEL was 300 mg/kg bw/day (IRIS 2004). In a 2-year study, rats were orally by gavage administered D-limonene 5 days/week at the dosages 0, 300 and 600 mg/kg/day. The mortality was significantly increased at 600 mg/kg/day. Thus, NOAEL was 300 mg/kg bw/day (IRIS 2004). In a 2-year study on mice with oral administration 5 days/week at the dosage 0, 250 and 500 mg/kg/day (male mice); or 0, 500 and 1000 mg/kg/day (female mice). Based on histopathological observations in the liver (multinucleated hepatocytes and cytomegaly) a LOAEL was set at 500 mg/kg bw/day and NOAEL 250 mg/kg bw/day (NTP 1990, IRIS 2004). In the studies, effects on the liver appear to be the effect observed at the lowest dose. Therefore, this effect forms the basis for deriving the TDI value. D-limonene (CAS no. 5989-27-5) is a common fragrance allergenic. The substance is included in the SCCNFP list of the 26 substances there are most often reported as allergenic. From March 11 2005, the substance must be declared in the ingredients list on cosmetic products in the EU, if the concentration exceeds 0.001% (1 mg per 100 gram) in products to remain on the skin (leave-on products) or 0.01% (10 mg per 100 gram) in products that are washed off (rinse-off products) (Directive 2003/15/EC, EC 2003). Threshold limit values Threshold limit values for the working environment (TLV) is 25 ppm corresponding to 140 mg/m³, which is equivalent to high-boiling aromatic carbohydrates such as terpenes and turpentine (AT 2002). In Sweden the threshold limit value NGV (niveaugränsvärde) is 150 mg/m³ (25 ppm) with the remark skin sensitiser (Karlberg and Lindell 1993). The TDI value 0.1 mg/kg bw/day is based on a 13 weeks oral rat study with the lowest level of observed effects on the liver. NOAEL was 10 mg/kg bw/day (Webb et al. 1989). Using a safety factor of 100 (10 for interspecies and 10 for intraspecies variation) derives a TDI at 0.1 mg/kg bw/day (CICAD 1998). D- Limonene is on the List of undesirable substances, because it is suspected to be allergenic (Miljøstyrelsen 2000). Absorption D-Limonene is easily taken up from the lungs after inhalation. Short-time exposure studies show 58 to 70% absorbed after 2 hours of exposure (Karlberg and Lindell 1993, Falk-Filipsson et al. 1993, 1998). Due to the high absorption, the absorption is set at 100% in this study. Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.20 Uptake by exposure via inhalation of D-limonene
D-Limonene was detected in 4 slimy toys with a calculated uptake via inhalation between 1 and 3 ng/kg body weight at short-term exposure and between 0.4 and 1 ng/kg bw/day at prolonged exposure. The TDI value of 0.1 mg/kg bw/day was not exceeded, and the margin of safety (MOS) was 10 x 106. D-Limonene was not detected in the sweat or saliva extractions. Conclusion D-Limonene was measured as volatile substance in 4 slimy toys. NOAEL was 10 mg/kg/day in a 90 days rat study. Thus, the margin of safety is above >1×107, and D-limonene is considered not to present a health problem at prolonged exposure related to the used scenarios. However, it is noted that it is known that D-limonene may oxidise to allergenic compounds. 6.3.11 alpha-PineneIdentification
The melting point is -62.5°C. The boiling point is 156°C (Furia and Bellanca 1975). The vapour pressure is 633 Pa at 25°C (4.75 mmHg, Daubert and Danner 1989). The water solubility is 0.65 mg/l at 250°C (FFHPVC 2002). The partition coefficient log Kow measured to 4.83 (Li and Perdue 1995). Pinene has a high vapour pressure indicating that pinene can evaporate from dry and wet surfaces. Use alpha-Pinene belongs to the chemical group terpenes (cf. further details at 3-carene). Terpenes exist in ethereal oils. Terpenes may arise from the use of vegetable oils and resins in products and as solvent in colorants. Classification alpha-Pinene is not classified under its own name or CAS no. If pinene is considered analogues to vegetable turpentine the classification is (Miljøministeriet 2002):
Effects on health alpha-Pinene is moderately oral toxic but very toxic by inhalation (Lewis 1992) and strongly irritating to eyes, mucous membranes and skin (Budavari 1996, Lewis 1992). Examples on effect levels are presented below. alpha-Pinene is known as contact allergen (Thomsen 1990). Acute toxicity:
Most studies available are based on exposure via inhalation. However, a few studies on the effect on reproduction in rats (1-generation) have been found. All studies are performed by using turpentine containing approx. 20% alpha-pinene. The test substance was administered orally by gavage. In each study, NOAEL was the highest administered dosage, i.e. NOAEL varied between 260 and 600 mg/kg bw/day (FFHPVC 2002). Three examples are presented below: One generation reproduction study on mice. The mice were orally administered by gavage the test material, which was a mixture of 85-90% terpene hydrocarbons and <10% oxygenated terpene hydrocarbons. The major bicyclic terpene hydrocarbon constituents of the formula C10H16 are alpha-pinene (20-25%), beta-pinene (15-18%) and sabinene (38-42%). The animals were exposed on days 6 to 15 of gestation. The doses given were 0 (control), 6, 26, 120, or 560 mg/kg bw/day. NOAEL was 560 mg/kg bw/day (FFHPVC 2002). The same test material was used in hamsters in a 1-generation reproduction study on adult hamsters with oral administration (gavage) on day 6 to 15 of gestation at the dosages 0 (control), 6, 28, 130 or 600 mg/kg bw/day. NOAEL was 600 mg/kg bw/day (FFHPVC 2002). The same test material was used in rats in a 1-generation reproduction study on adult rats with oral administration (gavage) up to day 14 of gestation with the dosages 0 (control), 3, 12, 56 or 260 mg/kg bw/day. NOAEL was 260 mg/kg bw/day (FFHPVC 2002). Threshold limit values TLV is 25 ppm corresponding to 140 mg/m³. The same as high boiling aromatic hydrocarbons (terpenes, turpentine) (AT 2002). The C-value is 0.05 mg/m³ (Miljøministeriet 2002). Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.21 Uptake by exposure via inhalation of alfa-pinene
alfa-Pinene was detected in 9 slimy toys. Using the NOAEL 260 mg/kg bw/day, the margin of safety (MOS) was >3.2×107. alfa-Pinene was not detected in the sweat or saliva extractions. Conclusion alfa-Pinene was measured in 9 out of 17 slimy toys. The MOS was very high and alfa-pinene, therefore, is not considered a potential health problem at prolonged exposure duration. However, it is noted that alfa-pinene is known as contact allergenic. 6.3.12 1,2-PropanediolIdentification
The melting point is -60°C. The boiling point is 187.6°C (Budavari 1996). The vapour pressure is 17.2 Pa at 25°C (0.129 mmHg, Daubert and Danner 1989). The water solubility is high, i.e. miscible at 25°C (EPI). The partition coefficient log Kow is measured to –0.92 (Hansch et al. 1995). Use 1,2 Propanediol is a synthetic liquid substance that absorbs water. The substance is also known as propylene glycol, which is used to make polyester compounds, and as a base for de-icing solutions. The substance is used in chemical, food and pharmaceutical industries. The substance is used to absorb extra water and maintain moisture in certain medicines, cosmetics, or food products. It is used as a solvent for food colours and flavours, and in the paint and plastics industries (Clayton and Clayton 1982, IRIS 2004). Classification The substance is not adopted on the List of dangerous substances and, therefore, not classified (Miljøministeriet 2002). Effects on health 1,2-Propanediol is not especially acute toxic. For instance it has been found that a lethal dosis for humans is given as 15 g/kg body weight (Gosselin et al. 1976). Acute toxicity:
However, studies of humans and animals show that repeated eye, skin, nasal, or oral exposures to 1,2-propanediol for a short time may develop some irritation (ATSDR 1997). Studies indicate that exposure to 1,2-propanediol for a prolonged period may lead to haemolysis of red blood cells (ATDSR 1997). In a 13-week study the effect by exposure of rats via inhalation 6 hours/day, 5 days/week using aerosol concentrations at 0, 51, 321 and 707 ppm is studied. Based on nasal haemorrhaging was set a LOAEL of 51 ppm, which was the lowest concentration used (Suber et al. 1989). In rhesus monkeys and rats, exposed to 1,2-propanediol continuously via inhalation at concentrations in air up to 112 ppm for 13 to 18 months, no adverse effects were observed in the hepatic system (Robertson et al. 1947). In studies on rats exposed via the food for 2 years, no adverse hepatic effects were observed at the highest level of 2500 mg/kg bw/day (Gaunt et al. 1972). Threshold limit values A threshold limit value for the working environment is not set in Denmark: An American value of 50 ppm corresponding to 170 mg/m³ has been found (ACGIH, ATDSR 1997). The C-value is 1 mg/m³ (Miljøstyrelsen 2002). The American MRL (Minimal risk Level) corresponding to RfC is 0.009 ppm corresponding to 0.03 mg/m³. The MRL was based on the LOAEL of 51 ppm for nasal haemorrhaging in rats (Suber et al. 1989). The MRL was obtained by dividing the LOAEL value by 1000 (10 for inter and 10 for intravariability and 10 to extrapolation to NOAEL) and multiplying by factors to adjust the exposure from 6 hours per day (6 or 24) and 5 days per week (5 of 7) to continuous exposure (ATDSR 1997). The ADI value is 25 mg/kg bw/day according to FAO/WHO (1974). RfDsubchronic is 30 mg/kg bw/day. The oral reference dose is based on a NOEL of 6% after oral administration in the diet over 20 weeks to rats. The value is based on adverse effects to the liver and using a safety factor of 100 (10 for intra and 10 for interspecies variation): Oral RfD subchronic 30 mg/kg bw/day (US-EPA 1997). RfDchronic is 20 mg/kg bw/day. The oral reference dose is based on NOEL 50000 ppm administered orally over 2 years in the diet to dogs. The value is based on decreased erythrocyte counts, decreased hematocrit and haemoglobin content in the blood and a safety factor of 100: Oral RfD chronic 20 mg/kg bw/day (US-EPA 1997). Absorption Propylene glycol is readily absorbed by the gastro-intestinal tract (US-EPA 1997). Thus, the absorption is set to 100%. Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.22 Uptake via inhalation by exposure to 1,2-propanediol
1,2-Propanediol was detected as volatile substance from 2 slimy toys. The uptake is calculated to max. 67 ng/kg bw/day. The ADI value of 25 mg/kg bw is not exceeded. Using NOAEL 2500 mg/kg bw/day the margin of safety (MOS) is more than 3.6 x 107. 1,2-Propanediol was not detected in the sweat or saliva extractions. Conclusion 1,2-Propanediol is assessed not to imply a health problem to the consumer. 6.3.13 2-Propenoic acid 2-methyl-methylester (methyl methacrylate)2-Propenoic acid 2-methyl-methylester is better known under the name methyl methacrylate which is used below. Identification
The melting point is -48°C. The boiling point is 100°C. The vapour pressure is 3600-4700 Pa at 20°C. The water solubility is 16000 mg/l at 20°C. The partition coefficient log Kow is measured to 1.38 at 20°C. (All values from ECB 2002). Use The substance is mainly used as an intermediate in production of polymers, copolymers, adhesives, reactive resins, as well as in other polymers used for consumer products (ECB 2002, RAR vol 22). Classification Methyl metacrylate is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Effects on health Acute toxicity:
The acute toxicity is low regardless of exposure route as based on the available values. However, the substance is an irritant and classified as such. For irritating effects a NOAEC of 100 ppm corresponding to 410 mg/m³ (ECB 2002) is found. The substance has a moderate to strong sensitising potential in experimental animals. Cases of contact dermatitis have been reported for workers exposed to the substance (SIDS 2003). The main effect caused by inhalation is a degeneration of the olfactory region of the nose. For this effect in a two-year inhalation study in rats a NOAEC of 25 ppm corresponding to 104 mg/m³ was identified (SIDS 2003). Based on the results from the same study by Lomax et al. (1997) US-EPA derives a RfC of 0.7 mg/m³ (IRIS 2004). Oral administration to rats resulted in a NOAEL of 200 mg/kg bw/day (SIDS 2003). In a 2-year oral rat study, the rats were administered via the drinking water at the doses 6, 60 and 2000 mg/l. The highest exposure level was recalculated to animal body weight: 2000 mg/l × 0.0313 l/rat/day divided with the rat body weight. A NOAEL of 146 mg/kg bw/day for females, while NOAEL was 121 mg/kg bw/day for males, which was the highest concentration tested on males (Borzelleca et al. 1964). Absorption Methyl methacrylate is rapidly absorbed after inhalatory or oral administration. Besides, the substance can easily be absorbed through skin (SIDS 2003, ECB 2002). Threshold limit values The threshold limit value for the working environment is 25 ppm corresponding to 102 mg/m³ with skin notation H, i.e. the substance may penetrate the skin (AT 2002). The C-value is 0.03 mg/m³ (B-værdivejledningen, Miljøstyrelsen 2002). The RfC value is 0.7 mg/m³ (cf. above). TDI (tolerable daily intake) is 1.2 mg/kg/day. The lowest NOAEL value from the 2-year rat study by Borzelleca et al. (1964) is used to derive a TDI using a safety factor of 100 (10 for intra and 10 for interspecies variation), i.e. TDI is 121/100 = 1.2 mg/kg bw/day (CICAD 1998). The RfD value is 1.4 mg/kg bw/day. The RfD value is derived from the same study used for TDI. However, an average body weight of the rats of 0.462 kg is used. Thus, the combined NOAEL is 136 mg/kg bw/day and the RfD 1.4 mg/kg bw/day (IRIS 2004). Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.23 Uptake by exposure via inhalation of methyl methacrylate
Methyl methacrylate was detected as evaporated substance from 1 slimy toy. The uptake was calculated to 7 ng/kg bw/day. Thus the TDI value of 1.2 mg/kg bw is not exceeded. Using NOAEL 121 mg/kg bw/day the margin of safety (MOS) is 1.6x107. Methyl methacrylate was not detected in the sweat or saliva extractions. Conclusion Methyl methacrylate was assessed not to imply a health risk to the consumer. 6.3.14 N-PropylbenzamideIdentification
The melting point is 100°C. The boiling point is 328°C (Budavari 1996). The vapour pressure is estimated to 0.01 Pa at 25°C (7.8×10-5 mmHg). The water solubility is estimated to 2247 mg/l at 25°C (EPI). The partition coefficient log Kow is measured to 1.72 (Hansch et al. 1995). Classification N-Propylbenzamide is not adopted on the List of dangerous substances (Miljøministeriet 2002). Effects on health No information to describe the effects to humans of the substance was available. Threshold limit values No threshold limit values are found. Assessment N-Propylbenzamide and N-acetylbenzamide were not detected in the headspace as volatile substances. N-Propylbenzamide and N-acetylbenzamide were not determined quantitatively in sweat and saliva extracts. Therefore, the somewhat more uncertain qualitative measurements from 2 products are used in the assessment. Table 6.24 Uptake by exposure to N-propylbenzamide + N-acetylbenzamide based on the results from the screening of sweat and saliva extracts
Conclusion No data were available for an evaluation of effects and thereby a conclusion. The analyses results show that the substance is detected as migrated at concentrations between 1 and 36 µg/g and the estimated uptake to maximum 13.2+0.88 = 14.1 µg/kg bw/day. The missing toxicological data mean that it is not possible to assess potential risk to the consumers of the toy. However, the substances are not expected to pose an immediate potential health risk at such low concentrations. 6.3.15 N-AcetylbenzamideIdentification
The estimated melting point is 172°C. The boiling point is estimated to 400°C (Budavari 1996). The vapour pressure is estimated to 1.5 x 10-5 Pa at 25°C (Daubert and Danner 1985). The water solubility is estimated to 17400 mg/l at 25°C (EPI). The partition coefficient log Kow is estimated to 0.68. Classification N-Acetylbenzamide is not adopted on the List of dangerous substances (Miljøministeriet 2002): Effects on health No information to describe the effects to humans of the substance was available. Threshold limit values None found. Assessment Both benzamides are considered above under N-propylbenzamide. N-Acetylbenzamide was not detected in the headspace as volatile substance. N-Acetylbenzamide was not determined quantitatively in sweat and saliva extracts. Therefore, the somewhat more uncertain qualitative measurements from 2 products are used in the assessment. Conclusion Cf. N-propylbenzamide 6.3.16 StyreneIdentification
The melting point is –30.6°C. The boiling point is 145.2°C. The water solubility is 300 mg/l at 25°C. The vapour pressure is 867 Pa at 25°C. The octanol/water partition coefficient is measured to log Kow 3.02 (ECB 2002). Use Styrene is used to a large extent in the plastic (polystyrene) and rubber industry but also in many other products. Classification Styrene is classified in the List of dangerous substances (Miljøministeriet 2002):
Effects on health Styrene is not acute toxic based on acute toxicity data. Of these are mentioned:
The problematic health effect is that styrene is considered neurotoxic. Affecting the neurological development seems to be the most sensitive endpoint observed. In young rats exposed to 260 mg/m³ effects were observed on behaviour and biochemical parameters in the brain (Kishi et al. 1992 in WHO 2000). In occupationally exposed humans, minor effects were observed such as effects on verbal abilities and disturbances to the vision at air concentrations of 107-213 mg/m³. Using the lowest value for precautionary reasons and recalculated from working hours to continuous exposure with a factor 4.2 and apply a further safety factor of 10 for inter-individual variation and 10 for extrapolating from LOAEL to NOAEL a value of 107/(4.210) = 0.26 mg/m³ (weekly average) is derived (WHO 2000). Mutti et al. (1984) examined in a cross-sectional study the neuropsychological function in 50 workers whose mean duration of styrene exposure was 8.6 (SD of 4.5) years. Styrene exposure was assessed by the authors to correspond to air concentrations ranging from 10-300 ppm as a mean daily exposure. This was based on the concentration-response relationship between urinary metabolite concentration (mandelic acid and phenylglyoxylic acid levels normalised to creatinine in "morning-after" urine). There were observed a significant effect level in the subgroup whose urine contained 150-299 mmole urinary metabolites/mole creatinine. Workers with metabolite concentrations of up to 150 mmoles/mole appeared to have no significant effects. This level is therefore designated as the NOAEL in this study. The authors state that this level of urinary metabolites corresponds to a mean daily 8-hour exposure to air styrene of 25 ppm (106 mg/m³). 95% confidence interval is calculated for an 8-hour exposure at 100 ppm, and the lower limit of the confidence calculation was 88% of the mean styrene exposure. This factor is applied to the correction of NOAEL: 25 ppm 0.88 = 22 ppm (94 mg/m³). In a subchronic oral study on dogs, where the effect of styrene on red blood cells and the liver was studied after oral administration for 560 days, LOAEL was observed at 400 mg/kg/day and NOAEL set to 200 mg/kg/day (Quast et al. 1979). In a 2-year three-generation rat study, rats were exposed to 125 mg/l (corresponding to 7.7 mg/kg/day for males and 12 mg/kg/day for females) and 250 mg/l (corresponding to 14 mg/kg/day for males and 21 mg/kg/day for females) in drinking water. The body weight was affected at 21 mg/kg/day, while male and female reproduction was not affected. Therefore, NOAEL was 14 mg/kg/day for males and 12 mg/kg/day for females (Van Appeldoorn et al. 1986). There are only weak indication that styrene should be carcinogenic. However, IARC has evaluated the substance as possible carcinogenic to humans and placed the substance in group 2B (inadequate evidence in humans and limited evidence in experimental animals for the carcinogenicity of styrene: IARC 1994, WHO 2000). Apparently, the carcinogenicity potential of styrene is related to the metabolite styrene oxide, which is quickly transformed into styrene glycols (WHO 2000). Threshold limit values The threshold limit value for the working environment is 25 ppm equivalent to 105 mg/m³ with notation LHK. L means that the threshold limit value is a ceiling value, which at no time must be exceeded. H means that the substance can penetrate the skin. K means that the substance is adopted on the list of substances that may be carcinogenic (AT 2002). WHO has given a 24 hours air quality guideline value of 800 µg/m³ (IPCS 1983) and for continued exposure 260 µg/m³ air (WHO 2000). The C-value is 0.2 mg/m³ (Miljøstyrelsen 2002). The inhalation RfC value is set on basis of effects to the central nervous system (Mutti et al. 1984) with a NOAEL 94 mg/m³ (cf. above). The value is calculated to continuous exposure and assuming that 10 m³ air was the respiration rate during the working hours: 94 mg/m³ 10/20 m³/day 5/7 days = 34 mg/m³ (NOAEL HEC, human equivalent concentration). The safety factor of 30 was based on the application of 10 for intraspecies variation and 3 for data deficiencies, thus, RfC = 34/30 = 1 mg/m³ (IRIS). The oral RfD value is based on a subchronic oral study on dogs for 560 days, where NOAEL was observed to 200 mg/kg/day (Quast et al. 1979). Applying a safety factor of 1000 (10 for inter-, 10 for intraspecies variation and 10 for extrapolation subchronic to chronic effects the resulting RfD is: 200/1000 = 0.2 mg/kg bw/day (IRIS). TDI is 120 µg/kg bw/day. A Dutch value (Van Appeldoorn et al. 1986, cf. above) is based on a 2-year rat study with a safety factor of 100 (Baars et al. 2001). Absorption Styrene is absorbed easily from the lungs. In different studies uptake has been measured to vary between 45 and 93% (IPCS 1983). Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.25 Uptake via inhalation by exposure to styrene
Considering inhalation the concentrations are below the RfC value of 1 mg/m³ and below the WHO air quality guideline value of 0.26 mg/m³. Considering the amount taken up the RfD is 0.2 mg/kg bw/day. The calculated values are a factor of 1x106 lower. The calculated values are below the found TDI value of 0.12 mg/kg bw/day. Using NOAEL 12 mg/kg bw/day the margin of safety (MOS) is more than 2.7x106. Conclusion Based on the calculated scenarios no health risks to the consumers are expected due to the presence of styrene in slimy toys. 6.3.17 TolueneIdentification
The melting point is -95°C. The boiling point is 111°C. The vapour pressure is 3800 Pa at 25°C. The water solubility is 515 mg/l. The partition coefficient log Kow is experimentally determined to 2.65 (ECB 2003). Classification Toluene is classified in the List of dangerous substances (Miljøministeriet 2002):
Effects on health Toluene is irritating to the skin and harmful to the health. Toluene is suspected to be toxic to reproduction, i.e. possible risk of harm to the unborn child. Acute toxicity Of acute data several have been found. Of those are mentioned:
Data on acute toxicity via inhalation was between 22 and 24 g/m³, which do not present any immediate reason to concern. However, it was also observed that toluene even at low concentrations (from 285 mg/m³) may induce headaches, dizziness, irritation and sleeplessness (ECB 2003). An inhalation value for humans with a LOEL 25 mg/m³ has been found (Lewis 1999). In humans experimentally exposed to toluene a concentration at and above 75 ppm (285 mg/m³) resulted in headache, dizziness, a feeling of intoxication, irritation and sleeplessness. A NOAEC of 40 ppm (150 mg/m³) is set for these effects (ECB 2003). A study concerns the neurological effects in occupationally exposed persons. No NOAEL could be established. LOAEL was observed to be 332 mg/m³ (88 ppm). LOAEL adjusted to continuous exposure was: 332 10/20 5/7 = 119 mg/m³ (Foo et al. 1990). In a 2-year rat study with chronic inhalation a degeneration of the nasal epithelium was observed (NTP, 1990). NOAEL could not be established. LOAEL was 2261 mg/m³ (600 ppm). Recalculating LOAEL to 24 hour/day and 7 days a week results in: 2261 mg/m³ 6.5/24 hours 5/7 days = 437 mg/m³. Adjusting the effect in the extra-thoracic region assuming that the respiration rate for rats was 0.27 m³/day and that the epithelium in rats was 11.6 cm² and 177 cm² in humans, a resulting value would be: 437 (0.24/20 m³/day) (177/11.6 cm²) = 79 mg/m³. In humans, toluene is a known respiratory irritant with central nervous system (CNS) effects. Available studies could not provide a NOAEL concentration for either of these effects that should have been used in the evaluation of a potential basis for the RfC calculation. Consequently, the study of Foo et al. (1990) was used for the CNS effects, and that of the National Toxicology Program (NTP, 1990) for the irritant effects. Because the CNS effect was judged to be a more severe and relevant endpoint, the LOAEL for this effect was used for deriving the RfC. Further, this effect is supported by a number of other occupational studies that show effects around 100 ppm. In a 13 week study on mice a LOAEL of 312 mg/kg/day was observed (WHO 2000). The result is based on a subchronic study (NTP 1989), where 10 rats/sex/group were orally gavage administered toluene dissolved in corn oil at dosage levels of 0, 312, 625, 1250, 2500, or 5000 mg/kg for 5 days/week for 13 weeks. All animals receiving 5000 mg/kg died within the first week. One female and 8 males in the 2500 mg/kg group died, but 2 of these were due to gavage errors. No deaths occurred at lower doses. Based on liver and kidney weight changes in male rats at 625 mg/kg the NOAEL was 312 mg/kg/day. The toxicological significance of these organ weight changes is strengthened by the occurrence of histopathologic changes in both the liver and kidney at higher doses. Because the exposure was for 5 days/week, LOAEL is recalculated to 7 days: (625×5/7=) 446 mg/kg/day and NOAEL correspondingly 325×5/7 = 223 mg/kg/day (IRIS 2004). A 90 days oral rat study with NOAEL of 625 mg/kg/day and a 2-year rat inhalation study with a NOAEC 300 ppm (1125 mg/m³) (ECB 2003). Toluene is classified as reproduction toxic category 3 (Dir. 2004/74/EC, EC 2004), meaning that indications have been observed of possible risk of harm to the unborn child. Indication on repeated contact may cause allergic contact dermatitis has also been observed. Absorption Data have been found on the dermal exposure, and the uptake fraction is low. Dermal uptake at exposure to toluene vapours is measured to approx. 1% of the amount of toluene taken up via inhalation at exposure to the same concentrations (Riihimäki and Pfäffli 1978, Piotrowski 1967). Uptake via inhalation is studied in humans. The uptake after 3 hours of exposure when at rest was approx. 50% of the inhaled amount of toluene. During work the uptake may be significantly higher. It was concluded that toluene is fast taken up by inhalation and that the amount depended on the respiration rate. Threshold limit values The threshold limit value for the working is 94 mg/m³ equivalent to 25 ppm (AT 2002). The inhalation RfC value 0.4 mg/m³ is based on the Foo et al. 1990 study mentioned above where a LOAEL was found and recalculated to 119 mg/m³. Applying a safety factor of 300 (10 for intraspecies variation, 10 for extrapolating from LOAEL to NOAEL and 3 due to data deficiencies) results in RfC = 119/300 = 0.4 mg/m³. The C-value is 0.4 mg/m³ (B-værdivejledningen 2002). Oral RfD value is 0.2 mg/kg bw/day based on a 13 weeks oral rat study where styrene was administered by gavage. In the study changes in liver and kidney weight were observed. LOAEL was 625 mg/kg bw/day and NOAEL 312 mg/kg bw/day. Because the exposure was for 5 days/week NOAEL was adjusted to 312 x 5/7 = 223 mg/kg bw/day. A safety factor of 1000 was applied (for inter and intraspecies variation, for subchronic to chronic extrapolation and for limited number of data on reproduction and development). The TDI value 223 µg/kg bw/day is based on LOAEL 312 mg/kg/day in a 13 week study on mice (WHO 2000). Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Table 6.26 Uptake via inhalation by exposure to toluene
The RfD and TDI value of 223 µg/kg bw/day is not exceeded. Toluene was also observed in the analyses of migrated substances. The results are presented below. Table 6.27 Uptake by dermal exposure to toluene
Table 6.28 Uptake by oral exposure to toluene
The total contribution from the three exposure routes is calculated below by addition of each contribution. Table 6.29 The total contribution by exposure to toluene
Conclusion The toluene release from the tested slimy toys did not reach concentrations that could cause health problems to the consumer. The highest uptake was from K-01 where the uptake was 1.7 µg/kg bw/day. The TDI value is 223 µg/kg bw/day. Using the NOAEL 223 mg/kg bw/day the margin of safety is more than (223/0.0017 =) 130000. However, it should be noted the amount of toluene in air may also be caused by other sources than the studied toys. The actual indoor air concentration of toluene may therefore be higher than the estimated concentration. 6.3.18 XyleneXylene is used as solvent and in the production of colorants. Xylene consists of a mixture of the three isomers: o-, m-, and p-xylene with m-xylene as the dominant part (ratio approx. 20:40:20, respectively). Identification
The boiling point of xylene (mixture) is 138.5°C. The vapour pressure is 1065 Pa at 25°C (7.99 mmHg, Daubert and Danner 1985). The water solubility is 106 mg/l at 25°C (Yalkowski and Dannenfelser 1992). The partition coefficient log Kow is experimentally determined to 3.12 (Hansch et al. 1995). Classification Xylene (and isomers) is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
Effects on health Of acute and chronic data are found:
ATSDR (1995) points out that both animals and human data indicate that the mixtures of xylenes: m-, o- and p-xylene, all results in similar effects but that the single isomers not necessarily are equal potent relating to a specific effect. Therefore, the evaluation is based on xylene mixture. Prolonged exposure to organic solvents may cause brain damage. Generally, concentrations around 100 ppm are observed to be the NOEL for brain damages. 10 mg/m³ is observed to be NOEL for teratogenic effects in animal studies (Hass and Jacobsen 1993). Korsak et al. (1992) exposed groups of 12 rats to toluene, m-xylene, or a 1:1 mixture for 6 hours per day, 5 days per week at a concentration of 0 or100 ppm for 6 months or 1000 ppm for 3 months. In a second study, Korsak et al. (1994) exposed groups of 12 rats by inhalation to 0, 50, or 100 ppm m-xylene, n-butyl alcohol or a 1:1 mixture for 6 hours per day, 5 days per week for 3 months and evaluated similar endpoints as in the earlier study (Korsak et al., 1992). Sensitivity to pain was assessed by placing the animal on a hot plate (54°C) and measuring the time until the animal starts licking its paws. Rats exposed to 50 or 100 ppm m-xylene alone had statistically significantly increased sensitivity to pain at the end of the 3-month exposure period. LOAEL is set to 100 ppm and NOAEL 50 ppm. Condie et al. (1988) has performed an oral rat study. A LOAEL of 150 mg/kg/day could be established, but the effects were minor, and there was no reason to believe that the NOAEL would be very different. The study is supported by NTP (1986), which in a chronic oral rat study observed a NOAEL of 179 mg/kg/day (cf. RfD below). Xylenes are not classified for their carcinogicity. IARC has placed xylenes in Group 3, i.e. ”not classifiable as to its carcinogenicity to humans” (IARC 1999). Threshold limit values The threshold limit value for the working environment is 25 ppm equivalent to 109 mg/m³ with notation H, i.e. may penetrate the skin (AT 2002). The C-value is 0.1 mg/m³ (B-værdivejledningen Miljøstyrelsen 2002). The TCA (tolerable concentration in air): 870 µg/m³ (Baars et al. 2001). TCA is a guidance threshold limit value based on LOAEL 870 mg/m³ (200 ppm) observed in an inhalation study, where the critical endpoint was reproduction toxicity (Hass and Jacobsen 1993) and the application of a safety factor of 1000 (IPCS 1997). The LCI value 100 µg/m³ is based on an animal study with a NOEL for teratogenic effect of 10 mg/m³ (LCI = NOEL/10101) (Larsen et al.1999). The RfC: 0,. mg/m³ is based on Korsak et al. (1992). In the study, impaired motor co-ordination was observed with a NOAEL 50 ppm equivalent to 217 mg/m³, which was recalculated to 217 mg/m³ 6/24 hours × 5/7 days = 39 mg/m³. Applying a safety factor of 300 (10 for inter- and 10 for intraspecies variation and 3 for LOEL extrapolation to NOEL): 39/300 = 0.1 mg/m³. The TDI (tolerable daily intake): 150 µg/kg bw/day (based on Condie et al. 1988). The oral RfD value 0.2 mg/kg /day is based on a 2-year rat study where rats were administered xylene mixture daily for 5 days per week. A LOAEL was observed at 500 mg/kg/day and a NOAEL at 250 mg/kg/day (NTP 1986). Adjusted to chronic exposure the NOAEL value corresponded to 250 5/7 days = 179 mg/kg/day. Applying a safety factor of 1000 the RfD is derived at: RfD = 179/1000 = 0.2 mg/kg bw/day. Absorption Uptake via inhalation is found to be approx. 60% (ATSDR 1995, IPCS 1997). Because no further information was found, the evaluation is based on 100% absorption. Assessment The evaluation of inhalation is based on short term exposure with 1 m³ air in the breathing zone and exposure for 1 hour, and a long-term exposure scenario using exposure in a 20 m³ room for 1 hour per day. Both scenarios using exposure to a child of 10 kg body weight (bw). The absorption by inhalation is set to 100%. Because xylene (= dimethylbenzene) and ethylbenzene were hardly separable in the screening analysis (headspace), they are considered together for the exposure via inhalation. However, they are separated in the quantitative analyses on migration to sweat and saliva. Table 6.30 Uptake by exposure via inhalation of xylenes and ethylbenzene
The uptake via inhalation is below the TDI value of 150 µg/kg bw/day. Xylene was also detected in the analyses of migrated substance. The results are presented below. Table 6.31 Uptake by dermal exposure to xylene
The dermal uptake is below the TDI value of 150 µg/kg bw/day. Table 6.32 Uptake by oral exposure to xylene
Oral uptake via the mouth cavity is below the TDI value of 150 µg/kg bw/day. Even if the contribution from the two exposure routes dermal and oral are added, the largest calculated contribution of approx. 1.4 µg/kg/day is still below the TDI value. The total contribution is made complicated, as the contribution from inhalation includes both xylene and ethylbenzene. Therefore, the total contribution from the three exposure routes is calculated by addition of each contribution from both xylene and ethylbenzene, cf. below. Table 6.33 The total uptake by exposure to xylene and ethylbenzene
Using the lowest TDI value of 100 µg/kg bw/day (TDI for ethylbenzene) for the assessment, it is observed that the highest total uptake of 2.6 µg/kg bw/day is from product BR-01. Thus, the amount is still below the TDI value. Using the NOAEL 179 mg/kg bw/day the margin of safety (MOS) is more than: 179/0.00256 = 69000. Conclusion The conclusion is that xylene (and ethylbenzene) is not released to an extent to pose a health risk to the consumer. 6.3.19 BoronIdentification
Boron has a melting point of 2300°C. Boric acid Identification
Boric acid has a melting point of 171°C. The water solubility is 47.2 g/l at 20°C (IUCLID 2000) and 63.5 g/l at 30°C (WHO 1998). The vapour pressure is very low (9.9 µPa, ECB 2004). The partition coefficient is -0.757 at 25°C (IUCLID 2000). Classification Neither boron nor boric acid is classified currently (Miljøministeriet 2002). However, a new EU classification is suggested for boric acid, borax and other boron compounds (Miljøstyrelsen 2004):
According to the draft of 30th ATP (Adaptation to Technical Progress of Directive 67/548/EEC, draft, Jan. 2005) the later proposal is even more rigorous:
Use Boric acid and sodium salts of boron (primarily borax, or disodium tetraborate decahydrate) are used for the manufacture of glass, fibreglass insulation, polymers, laundry products, etc. (Woods 1994). Elemental boron has only limited applications. Effects on health Boron is a non-metallic element. Because boric acid is a weak acid with a pKa value of 9.2 the substance exists primarily as the undissociated acid (H3BO3) in aqueous solutions as do the borate salts. Therefore, the toxicity associated with these compounds is expected to be similar based on boron equivalents (US-EPA 2004). Most data on effects of boron are found in studies using boric acid and then recalculating to boron. Acute toxicity:
Boric acid is slightly irritating to the skin and to the eyes but not to a degree that requires classification (IUCLID 2000). The data regarding developmental and reproductive toxicity show that lower foetal body weights in rats is the critical effect. In a study on rat foetal development the female rats were administered boric acid from mating to 20 days later in the diet. The doses are calculated to 0, 78, 163, or 330 mg boric acid/kg/day (0, 13.6, 28.5 or 57.7 mg B/kg/day). Among several examined parameters the foetal weight was the most sensitive. LOAEL for decreased foetal weight was 13.6 mg B/kg bw/day (Heindel et al. 1992). A similar study performed in the same way but using the doses 19, 36, 55, 76, and 143 mg boric acid/kg/day (3.3, 6.3, 9.6, 13.3, and 25 mg B/kg/day). In the study was observed that for the foetal weight gain LOAEL was 76 mg boric acid/kg bw/day (13.3 mg B/kg bw/day) and NOAEL 55 mg boric acid/kg bw/day corresponding to 9.6 mg B/kg bw/day (Price et al. 1996). It is noted that boric acid is undergoing risk assessment in the EU with Austria as the Member State responsible. Threshold limit values The threshold limit value for the working environment is 10 mg B/m³ (ACGIH, boric acid as nuisance dust). The C-value is administratively set to 0.003 mg B/m³ (B-værdilisten Miljøstyrelsen 2002). The RfD value is 0.2 mg/kg bw/day (IRIS 2004, cf.- below). Based on the studies by Heindel et al. (1992) and Price et al. (1996) US-EPA has calculated a ”Benchmark Dose Level” with effect in the foetal weight decrease of 59 mg boric acid/kg bw/day (10.3 mg B/kg bw/day) and using a safety factor of 66 derived a RfD value of 0.2 mg B/kg bw/day (IRIS 2004). The TDI is 0.4 mg B/kg bw/day (WHO 1998). The recommended TI (Tolerable Intake) is 0.4 mg/kg bw/day (9.6/25), where it is also recommended that consumer products are allocated 5% of this value = 0.02 mg/kg bw/day (IPCS 1998). Absorption Boron is absorbed during inhalation exposure (US-EPA 2004). Boron apparently is not absorbed across intact skin. However, there is evidence that boron can be absorbed through damaged skin, especially from an aqueous vehicle (Nielsen 1970). Boron is well absorbed from the gastro-intestinal tract in humans. Schou et al. (1984) observed that after oral administration of boric acid in both water and water-emulsifying ointment an average of 92-94% of administered boron was excreted in the urine within 96 hours (US-EPA 2004). Assessment Boron was determined as total substance (cf. section 5). The results are presented below. Table 6.34 Content of boron in positive screening analysis (remaining samples below detection limit of 1 µg/g)
Table 6.35 Content of boric acid in positive quantitative analysis (remaining samples below the detection limit of 0.5-1 µg/g)
The estimates of uptake from the interior (liquid) part of the slimy toy DK-01 is based on the assumption that the liquid may come into contact with the skin or as worst case directly into the mouth. The absorption is set to 23% via the skin (contact fraction 0.001) and 100% absorption via the mouth with a contact fraction estimated to 1%. Thus, the dermal uptake is: 0.5 × 8400 × 0.23 × 0.001/10 = 0.097 µg B/kg bw/day and oral uptake: 0.5 × 8400 × 1.0 × 0.01/10 = 4.2 µg B/kg bw/day. The estimates of the uptake from the analysed exterior part of the slimy toy are based on 1 o/oo migration and 23% uptake via the skin and 100% absorption orally. The RfD value of boron is 0.2 mg B/kg bw/day. Because the value is based on boric acid in the study, the value is considered valid. This means that if more than 1% of the liquid in the toy is swallowed, there may be a risk of a harmful effect. Using the NOAEL 9.6 mg B/kg bw/day the margin of safety (MOS) is more than: 9.6/0.0043 = 2230. The highest concentration found was 8400 µg/g equivalent to 8400 mg/kg or 0.8% of the product. Boric acid is regulated by the Statutory Order on Cosmetics according to which it is not to be used in products to children under 3 years of age. In mouthwash products maximum is 0.1% calculated boric acid, mass/mass and in other products max. 3%, both calculated as boric acid (Statutory Order no. 74 of 14/01/2005). This means that if the consumer by accident (release the liquid inside the toy) may ”wash his mouth” with the content before spitting it out, the consumer has been exposed to a concentration of boron higher than allowed in mouthwash products. Conclusion The content of boron may potential pose a health problem to the consumer. Especially by contact to or by swallowing the liquid inside the toy, where the highest concentration of boron was measured. 6.3.20 NickelIdentification
The melting point of nickel is 1455°C. Classification Nickel is classified in the List of dangerous substances (Miljøministeriet 2002):
Most nickel compounds are classified for sensitisation potentials as R43, May cause sensitization by skin contact. Effect on health Skin contact with nickel is essential, as most nickel compounds may cause allergic reactions by sensitised persons. An assessment of skin contact would therefore be relevant. However, it has not been possible to find relevant data for such an assessment. EU has included nickel in the risk evaluation programme for existing substances but this has not yet been completed (ECB 2002). Oral RfD is based on the results from a 2-year rat study (Ambrose et al. 1976), where the rats were exposed to 0, 100, 1000 or 2500 ppm nickel in the food (estimated to 0, 5, 50 and 125 mg/kg bw). A significant reduction of body weight was observed and in the females a significant higher heart to body weight ratio and a lower liver to body weight ratio than in controls both in 1000 and 2500 ppm groups. No significant effects were observed in the 100 ppm group. LOAEL was therefore set to 1000 ppm (50 mg Ni/kg bw) while NOAEL was 100 ppm (5 mg Ni/kg bw). The study was supported by a subchronic study performed later (1986), which also finds a NOAEL of 5 mg Ni/kg bw/day (IRIS 2004). Threshold limit values The threshold limit value for the working environment is 0.05 mg/m³ (AT 2002). The TCA (tolerable concentration in air) is 0.05 µg/m³ (Baars et al. 2001). The C-value is 0.0001 mg/m³ (B-værdivejledningen 2002). The tolerable daily intake by oral ingestion (TDI) has been calculated to 5 µg/kg bw/day (WHO 1996). However, a Dutch assessment on nickel suggests 0.05 mg/kg bw/day i.e. 50 µg/kg bw/day based on a NOAEL of 5 mg/kg/day from a chronic rat study, where rats were exposed to nickel sulphate in the food and using a safety factor of 100 (Baars et al. 2001). The RfD value 0.02 mg/kg bw/day is based on a 2-year rat study by Ambrose et al. (1976). A safety factor of 300 was used (IRIS 2004). Absorption The bioavailability by oral intake is estimated to 5% (Baars et al. 2001). By dermal uptake the absorption is shown to be 0.2% for humans (MST 2003). Assessment Nickel was measured as total nickel in the screening. The quantitative measurements resulted only in a small increase. The results are presented below. Table 6.36 Content of nickel in the screening analyses with a positive result (remaining samples had nickel below the detection limit of 1 µg/g
The uptake is estimated based on the fact that all nickel is bioavailable. Calculation example (assuming that all nickel migrates): Dermal uptake: 2.9612.20.002/10 = 0.0072 µg/kg bw/day Oral uptake: 2.9612.20.05/11 = 0.1798 µg/kg bw/day Table 6.37 Content of nickel in quantitative analyses with positive result (remaining samples had nickel below the detection limit of 1 µg/g
This means that the total uptake (dermal and oral) is at maximum 0.2 µg/kg bw/day. The calculation demonstrates that even by the unrealistic assumption of total release the amounts will be below the TDI value. The highest concentration was 2.96 µg Ni/g equivalent to 310-4 %. The WHO derived TDI value was 5 µg/kg body weight as the lowest of the two available TDI values. From the calculation in the table above is observed that none of the amount intakes or uptakes result in a dosis above 5 µg/kg bw/d. Using the NOAEL 5 mg/kg bw/day the margin of safety (MOS) is more than: 5/0.00018 = 28000. Thus it is assessed that nickel does not imply any health risk. Conclusion Nickel does not pose a health risk by uptake at the amounts determined by analyses of the selected toys to most consumers. Nickel is a known allergen. No information was found as to the levels necessary to exclude the possibility as it is individual and depends of the sensitivity of the exposed person. Therefore, a reservation is made relating to particularly sensitive persons. 6.4 Conclusion of health assessmentFor the health assessments, parameters relating to small children are used in the scenarios, as the consumers in this context especially is related to children. For the selected chemical substances, the table below summarises the released concentrations in the breathing zone (1 m³), migration to artificial sweat and saliva, the calculated uptakes, the used NOAEL, margin of safety (MOS) and conclusions. It should be noted that the room air concentration used to estimate uptake and compared to NOAEL is 5% (1/20) of the tabulated concentration in the breathing zone. Table 6.38 Summary of conclusions for the evaluated substances
N = Number of discoveries over the detection limit
Table 6.37 Contact allergens from the SCCNFP list detected in the analyses of slimy toys
D-Limonene was detected in 3 products and hexylcinnamaldehyde in 1 product. Of other substances that are potentially allergenic, 3-carene, alfa-pinene and nickel were detected. All 3 terpenes: D-limonene, 3-carene (7 products) and alfa-pinene (8 products), were only detected in the headspace analyses. Whether the effect from contact allergenes is the same by exposure via inhalation as by dermal contact is somewhat uncertain but the substances may also be found in aerosols, which potentially also may reach skin areas. Boron was detected in 3 products at maximum 0.8% of the product. It is evaluated that by exposure to the liquid inside one of the products a health risk could not be excluded. Nickel was detected in 2 products at maximum of 0.0003% of the toy. It is evaluated that the nickel content poses no immediate concern, unless the consumer is specifically sensitive (nickel allergy). For the remaining substances were found that none was detected neither as volatile nor as migrated to sweat or saliva at such concentrations that uptake would cause any concern to let children play with the products. However, it should be noted that the consumer (child) may handle more than one slimy toy simultaneously or at intervals, thus increasing the exposure to one or more chemical substances correspondingly. Other sources of the same chemicals may also be present in the surroundings of the play activity. This may also contribute to the total exposure.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||