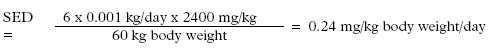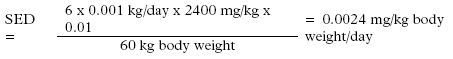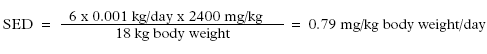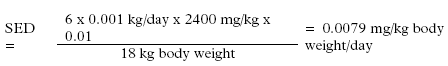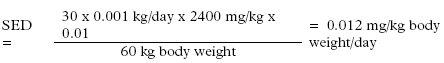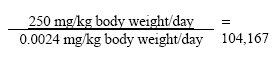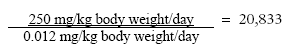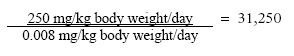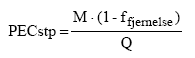|
Survey of chemical substances in consumer products no. 69, 2006 Survey of liquid hand soaps, including health and environmental assessmentsContents
9 Effects in the aquatic environment
PrefaceThe products included in the project "Survey of liquid hand soaps, including health and environmental assessments" was collected in the spring 2005. As stated in the survey new legislation was enforced in March 2006 regarding declaration of 26 allergenic fragrance substances. The 26 particularly allergenic fragrance substances must be stated separately on the product label if they appear in concentrations above 0.01% in hand soaps sold after 11th March 2005. Since several of the included products were produced earlier than 11th March 2005 some of the products did not declare content of the 26 fragrances even though they were identified in the analysis. The survey was sent to the producers of the included products for commenting ahead of the publication of the survey. Due to this hearing the Danish EPA has received some comments regarding the analysis, the labelling of the products and the safety evaluation of the products from the producers. Some of these comments are now included as text in the survey or included as footnotes. The conclusions of the received comments regarding the specific products are described below. Product no. 3: The composition of the product has been changed. The product no longer contains the environmental problematic substance Cocamide DEA and also the composition of the used fragrance mix is changed so that it no longer contains musks or phthalates. Furthermore, the content of all the 26 allergenic fragrance substances are below the declaration level. Product no. 6: The producer's own analyses show a lower content of the 26 fragrances the Danish EPA's analysis. This might be due to differences in analytical methods or uncertainties. The product do however no longer contains any of the 26 allergenic fragrance substances since the composition of the product has been changed. Product no. 7: Since the product was produced before 1st march 2005 there was no declaration of the 26 allergen fragrance chemicals. Products produced after 1st marts 2005 are declared correctly according to the legislation. The Danish EPA's analyses show a content of Linalool just above 0,01%. The supplier of the fragrance mix has confirmed that the content is below 0,01% and due to this there is no obligations to declare it. Since the uncertainties on the EPA's analysis are 10-15% this can explain the difference. The producer furthermore states that the product will be taken of the market due to decreasing demands. Product no. 8: The Danish EPA has received documentation regarding the content of MG (Methyldibromo glutaronitril). The content is below the detection limit of the analysis so the declaration of MG on the product was correct. The producer has subsequently chosen to phase out the use of MG and other allergenic preservatives from their soaps, so today none of these are used in the producer's liquid soaps. Product 8 has not been produced after 11th March 2005 and is therefore no longer on the Danish market. The EPA did also receive a safety evaluation of the product. Product no. 9: The retailer no longer sells this product. Product no. 13: The composition of the product has been changed, Sodium Benzoate is currently used as preservative in the product. Product no. 18 and 21: Are no longer on the market Summary and conclusionsIn the spring of 2005, DTC and DHI have carried out a survey of liquid hand soaps for sale partly via retail distribution (consumer products) and partly for occupational use. In May 2005, 25 liquid hand soaps were purchased in retail outlets. In addition, information has been obtained on 25 liquid hand soaps for professional use. Product ingredients have been identified based on the products' list of ingredients and product safety data sheets, and a survey was carried out to determine if the products contain fragrance chemicals and preservatives reported as contact allergens. Fifteen of the products were selected for chemical analysis of 26 particularly sensitizing fragrance chemicals. Furthermore, 3 of the 15 products were selected for analysis of the preservative Methyldibromoglutaronitrile. 11 of the 15 products were consumer products, 4 products were for occupational use. The result of the survey of liquid hand soaps showed that the content of fragrance chemicals reported as contact allergens was listed on the product label of a few products (both consumer products and products for occupational use), and that different (potentially) preservatives reported as contact allergens are used in liquid hand soaps. The result of the chemical analyses showed discrepancies between the lists of ingredients and the actual content, as 6 of the 15 analysed products contained fragrance chemicals reported as contact allergens in concentrations > 0.01% that was not listed on the product label. Methyldibromoglutaronitrile was not detected in the 3 product which were analysed for the substance. According to the EU Cosmetics Directive the concentration of 26 fragrance chemicals reported as contact allergens must be listed on the products' INCI list of ingredients if the fragrance chemicals appear in concentrations > 0.01% in rinse-off products. This is to ensure that particularly sensitive consumers can avoid products with specific fragrance chemicals and thereby to reduce the number of cases of allergy. To assess the safety of the products concerning health effects, 6 of the fragrance chemicals contained in high concentrations in the products were selected for further evaluation. When calculating the exposure to the fragrance chemicals reported as contact allergens the amount of liquid hand soap used when washing hands was determined at 1 g soap per wash. The exposure calculations showed a very low daily exposure and a fully acceptable safety margin (MoS) for fragrance chemicals for both adults and children alike. However, because of the sensitizing risk of perfumery materials and as there is no lower concentration limit for the sensitizing effects of the substances, it may be concluded that particularly sensitive persons, including children and people with allergy, have a risk of developing allergy when using products for which the obligation of labelling of fragrance chemicals apply. To assess the environmental properties of the products, 8 substances were selected for further exposure assessment that were representative or of particularly interest. The 8 substances comprised 4 surfactants and 4 preservatives. The exposure calculations showed that the use of liquid hand soaps may cause harmful effects in the aquatic environment by discharge of wastewater to areas characterised by a limited exchange of water, such as the inner part of an inlet. There is no predicted risk of effects in open waters with a regular water flow. 1 IntroductionFor the past 10-15 years consumer habits have changed concerning the use of hand soaps. Formerly, mainly solid products were used that were made of fatty acids and with few ingredients. Today, the use of liquid products or foam products is more widespread. These have a more complex composition and contain for instance preservatives to a much greater extent. In many cases, perfumery materials and pigments have also been added. Many different products are for sale on the Danish market, both liquid and solid products, and the market includes various manufacturers and importers for both consumers (retail distribution) and for occupational use (I&I products [1]). Hand soaps are covered by the Danish "kosmetikbekendtgørelsen" [2]. All ingredients in cosmetics must be stated on the product label and listed in descending order according to their weight percentage in the product. Wherever possible the ingredients should be stated by the common nomenclature for cosmetics (INCI name). Fragrance chemicals must be listed as "parfume" or "parfum". As of 11 March 2005, 26 specified fragrance chemicals reported as contact allergens must be declared separately on products marketed after this date. Products already for sale on 11 March 2005 are excepted from the obligation of labelling. There are no legal requirements for environmental risk assessment of substances and products. However, environmental criteria for the environmental properties of cosmetic products are established under the Nordic eco-label as common criteria for all cosmetics covered by the EU cosmetics directive. Consumers are exposed daily to potentially problematic substances in connection with washing their hands. Especially, fragrance chemicals and preservatives have been brought into focus as these substances are frequently the cause of contact dermatitis. Furthermore, many preservatives and fragrance chemicals have not been adequately investigated concerning their impact on the environment after discharge. Consequently, there is a demand for generally comprehensible knowledge of the potential risk of using the products, both from a health perspective and from an environmental perspective. In this project a survey has been carried out of liquid hand soaps for sale in retail distribution and for occupational use (trade and industry as well as the health sector). When mapping the product ingredients focus has been on biocides, preservatives, fragrance chemicals, and surfactants. Moreover, a comparison has been made of ingredients typical of liquid hand soaps for sale in retail distribution and of liquid hand soaps for occupational use. The survey results have been used to assess the exposure of both users and the environment to chemical substances in liquid hand soaps, and consequently to assess the risk involved in using liquid hand soaps. 2 PurposeThe purpose of the project " Survey and environmental and health assessment of liquid hand soaps" was:
3 Allergens in cosmeticsMany of the fragrance chemicals and preservatives that are used in consumer products such as cosmetics, detergents, cleaning materials, air fresheners, etc. may cause allergic reactions. In this connection, cosmetics are of particular interest as the products are applied on the skin or in the hair resulting in an immediate exposure to the chemical substances in the products. There are two product types: "leave-on" products (creams, make-up, lipcare, etc.), which stays on the skin, and "rinse-off" products (hand soaps, shampoos, balms, etc.), which are washed off.. A typical allergic reaction in connection with the use of cosmetics is contact dermatitis. The Danish association of persons sensitive to fragrances and chemicals (Foreningen for Duft og Kemikaliefølsomme) estimates that 4% of the Danish population are allergic to chemicals, also known as MCS (Multiple Chemical Sensitivity) (www.dkmcs.dk). An interview study carried out by the Danish knowledge centre on allergy (Videncenter for Allergi) in March 2005 showed that more than 40% of the respondents experienced symptoms at least once a year when using fragranced products (www.videncenterforallergi.dk). The EU Scientific Committee on Consumer Products (SCCP) has compiled a list of 26 fragrance chemicals reported as contact allergens, which have been included in annex 3 of the Danish "kosmetikbekendtgørelsen" (1). According to "kosmetikbekendtgørelsen", the 26 fragrance chemicals must be stated separately on the product label of all cosmetics, which are marketed after 11 March 2005, if the fragrance chemicals appear in concentrations above 0.001 % in leave-on products or above 0.01% in rinse-off products. Various preservatives are known to cause contact dermatitis. Some preservatives may have an indirect allergen effect, e.g. formaldehyde donors. Formaldehyde can cause allergic reactions in a small number of the population, who are particularly sensitive to formaldehyde. In cosmetics a concentration limit has been established for both formaldehyde and for formaldehyde donors. Table 3.1 gives an overview of the 26 fragrance chemicals reported as contact allergens and potentially sensitizing preservatives that are often found in cosmetics. Table 3.1: (Potentially) sensitizing substances in cosmetics
4 Survey4.1 Definition of products in the surveyLiquid hand soaps make up a large number of products in retail distribution. Apart from products sold as hand soaps, several products on the retail market are sold as both hand, hair and body soaps (all-over soaps, body soaps). The market for occupational use also includes a wide range of hand cleaning agents and hand disinfectants. Typically, hand cleaning agents are used for removal of filth such as oil, grease, printing ink, oil paint, metal and cement dust, etc. Hand disinfectants are used when there are particular demands to hygiene, e.g. in the hospital and care sector, in day nurseries, and in the food industry. As one of the purposes of the survey is to compare products for sale in retail distribution and for occupational use respectively, the survey has been limited to liquid hand soaps (including creams, gels and foams). In addition, a few all-over soaps have been included as they are regarded as hand soaps on the basis of their packing and size. Hand cleaning agents and hand disinfectants have not been included as these are primarily for occupational use and not products for the general consumer. Consequently, there is no real basis for a comparison of consumer products and products for occupational use. 4.2 The surveyIn May 2005 a survey was carried out of hand soaps for sale on the Danish retail market and for occupational use (I&I products). The survey was carried out by collecting information from the list of ingredients on the products purchased in retail distribution and per mail order and through contact to selected manufacturers and suppliers of products for occupational use. 4.2.1 Products on the retail marketHand soaps on the retail market have been identified at the following distributors:
In March 2005 the Danish Information Centre for Environment & Health (Informationscentret for Miljø og Sundhed, IMS) made a screening of the Danish retail market for liquid hand soaps. The content of chemical substances were examined in 45 products on the basis of the products' list of ingredients. The survey had special focus on potentially sensitizing substances but also on substances hazardous to human health or causing environmental impacts. The result of the market screening is available in Danish on IMS's webpage (www.miljoeogsundhed.dk) (2). Products are listed with remarks, if any, on the content of potentially sensitizing substances or other substances with unwanted effects on human health or the environment. The survey in May 2005 showed that IMS's market screening still covered a large part of the liquid hand soaps for sale on the retail market. As a result, the IMS survey has been used as a supplement to this survey. From 10 – 26 May, 25 different products were purchased during visits to the above shops, with the exception of pharmacies, and via the Internet. Nine products are identical to products/trade names in IMS' report that were given a remark due to their content of unwanted substances. The products were purchased to demonstrate if the unwanted substances, in particular the very sensitizing preservative Methyldibromoglutaronitrile (MG), can still be found in the products. The remaining products in the IMS survey have not been purchased again. The survey has concentrated on products containing perfume and only a limited number of hand soaps without perfumes were bought. Eco-labelled products have not been purchased as the 26 fragrance chemicals reported as contact allergens must not total more than 0.01% in products such as hand soaps. The range of hand soaps on the retail market is large and changeable, but this survey supplemented with the above market screening from IMS is believed to cover the whole market in the spring of 2005. Several manufacturers market products with the same name but with different fragrances. In these cases mainly one fragrance of the product has been purchased. The prices of the 25 purchased products range from DKK 12.50 – 115.00 apiece or DKK 25.00 – 460.00 per litre. Table 4.1 lists the products purchased in retail distribution, stating the form of the product and if potentially allergenic fragrance chemicals or preservatives have been identified on the basis of the product's list of ingredients. Table 4.1 Purchased products
# Product 3 is now marketed in a new formulationing * Product 13 is now marketed in a new formulation without Methylchloroisothiazolinone og Methylisothiazolinone **Product is no longer on the market 4.2.2 Products for occupational useProducts for occupational use have been identified through contacts with the I&I trade and with The Association of Danish Cosmetics, Toiletries, Soap and Detergent Industries (Brancheforeningen for Sæbe, Parfume og Teknisk/kemiske artikler, SPT). Selected manufacturers estimated to have a large market share in hand soaps for occupational use have been contacted. In addition, inquiries have been made to suppliers to investigate who produces the hand soaps that they sell. Manufacturers/suppliers have been contacted by phone in order to obtain information on the products for sale on the Danish market, where the products are sold, the form of the product (foam, gel, cream, etc.), and if possible information on market shares. The contact was followed up by an e-mail with more elaborate details of the survey and the information requested on the products. On the basis of the communication with manufacturers and suppliers 25 products for occupational use have been included in the survey. These products are grouped in two categories:
Information on product composition has been obtained from safety data sheets or other product information. The selected products are estimated to cover >50% of the market for hand soaps for occupational use. Table 4.2 lists the products for occupational use, stating the form of the product and if potentially allergen fragrance chemicals or preservatives have been identified on the basis of the product safety data sheets. Two products (nos. 27 and 30) are used primarily in the hotel and catering trade whereas the remaining 23 products are used in all I&I trades. Table 4.2 Selected products for occupational use
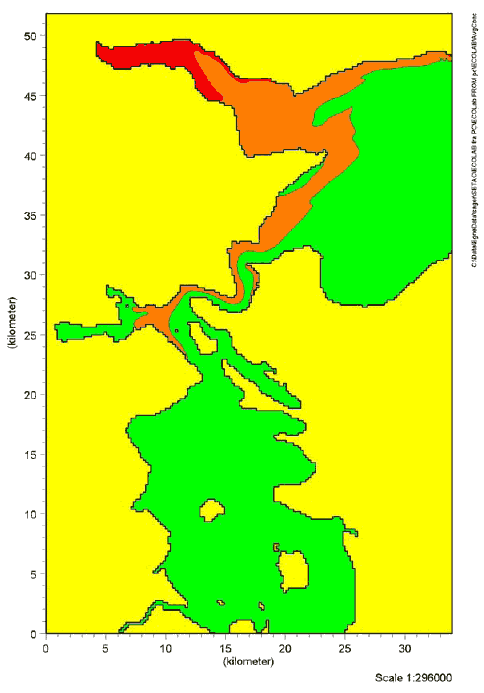
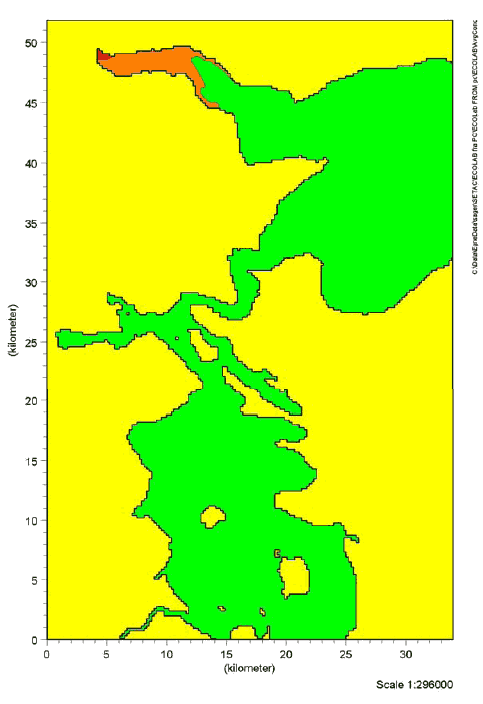
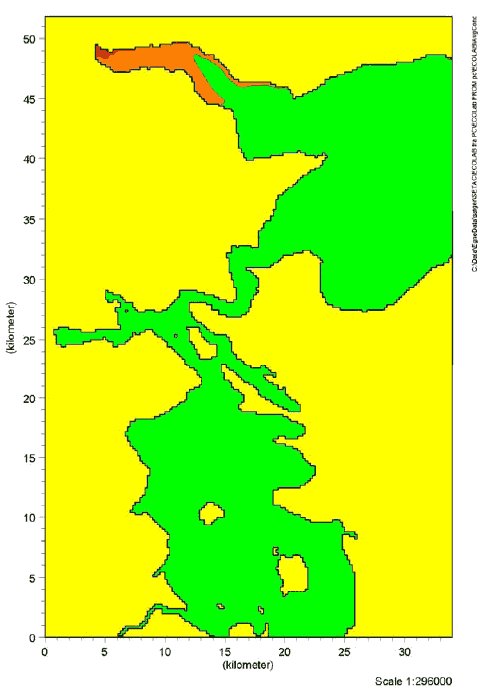
4.3 Ingredients and product labelling4.3.1 Labelling requirementsHand soaps are covered by the Danish "kosmetikbekendtgørelsen" (Danish statutory order no. 74 of 14/01/2005) (1). This means that the products must be labelled as follows:
The 26 particularly allergen fragrance chemicals must be stated separately on the product label if they appear in concentrations above 0.01% in hand soaps delivered after 11 March 2005. Tables 4.1 and 4.2 above list the fragrance chemicals in the products in the spring of 2005. 4.3.2 Registration of products in the surveyAll products purchased in retail distribution and the selected products for occupational use have been registered in a database. The following general product information has been recorded:
To identify preservatives, fragrance chemicals and surfactants in the products the products' list of ingredients or safety data sheets have been examined. Preservatives, fragrance chemicals and surfactants have been identified by their INCI name, chemical name and CAS number. Remaining product ingredients have been stated with INCI name as "other ingredients". 4.3.3 Fragrance chemicals, preservatives and surfactants in the productsTable 4.3 lists fragrance chemicals identified in products in retail distribution and for occupational use, respectively, according to the products' list of ingredients or safety data sheets. Table 4.3 Fragrance chemicals identified in the products
* Included on EU's list of 26 allergen fragrance chemicals Fragrance chemicals, included on EU's list of 26 allergen fragrance chemicals, are stated on the product label of two of the purchased retails products and four of the products for occupational use. Content of perfume was listed on the majority of the products purchased in retail distribution (21 of 25 products) and particular attention was paid to perfumed products in connection with purchase. Based on the product supply, it is estimated that in general far more perfumed than perfume-free products are marketed in retail distribution. Among the products for occupational use approx. half of the products were perfumed. Table 4.4 lists preservatives identified in products for retail distribution and for occupational use, respectively, according to the products' list of ingredients or safety data sheets. Table 4.4 Identified preservatives in the products.
* Potentially sensitizing substances ** Found in anti-bacterial soaps *** Included on EU's list of 26 allergen fragrance chemicals It appears from table 4.4 that several of the products contain preservatives which can be described as potentially sensitizing substances, including 2-Bromo-2-Nitropropane-1,3-Diol, DMDM Hydantoin, Imidazolidinyl Urea, Methyldibromoglutaronitrile, Methylchloroisothiazolinone and Methylisothiazolinone. These substances are found in more products for sale in retail distribution than in the selected products for occupational use. A number of the preservatives used in products for retail distribution are identical with preservatives used in I&I products. Generally, various different preservatives are used in products for retail distribution. 22 different preservatives have been identified in the 25 products for retail distribution whereas 15 different preservatives have been identified in the 25 products for occupational use. In addition, different preservatives are used in combination in the retail products whereas I&I products in general only contain one preservative (according to the product safety data sheets). Four of the 25 examined I&I products can be characterized as anti-microbial soaps. These products contain biocides such as Benzalkonium Chloride, Chlorhexidin Digluconate, Triclosan, and Undecylenic acid that are not found in liquid soaps for common use. Table 4.5 lists surfactants identified in products for retail distribution and for occupational use, respectively, according to the products' list of ingredients or safety data sheets. Table 4.5 Identified surfactants in the products.
As appears from table 4.5 it is largely the same type of surfactants that are used in retail products and in I&I products. The surfactants Cocamidopropyl Betaine and Sodium Laureth Sulfate occur often in the products. 4.3.4 Comparison of retail products and products for occupational useWhen comparing the content of the most important ingredients (surfactants, preservatives and fragrance chemicals) in consumer products and in products for occupational use, there is no noticeable difference of ingredients in the two groups. Both product types contain fragrance chemicals and preservatives reported as contact allergens, and based on available data it is not possible to assess if allergen substances appear more frequently in products for private consumers than in products for occupational use. It is estimated that the supply of perfume-free products is larger for products for occupational use than for products for retail distribution. In general, it appears that slightly more preservatives are used in consumer products compared with products for occupational use. The most common preservatives in consumer products are Benzoic acid, Dehydroacetic acid, Phenoxyethanol, and Sodium benzoate. In products for occupational use the most common preservative is Sodium benzoate. Anti-bacterial soaps for occupational use also contain biocides which are not found in liquid soaps for private consumption. Concerning surfactants, particularly Sodium laureth sulfate and Cocamidopropyl betain appear frequently in both product groups. However, a number of the ingredients identified in the two product groups are only found in a small number of the products, and consequently it is difficult to compare the products. 4.3.5 Products in IMS' market screeningThe 45 liquid hand soaps in IMS' market screening were screened mainly for content of (potentially) sensitizing substances. The screening showed that several of the products contained the following substances (preservatives):
In connection with this survey nine of the 45 products from IMS's screening (2) were purchased. Methyldibromoglutaronitril had been phased out from one of the products, but the remaining products still listed the problematic ingredients identified by IMS. 5 Chemical analyses
5.1 Selection of products for chemical analysisThe main purpose of the survey of hand soaps is to investigate if the products contain fragrance chemicals and preservatives reported as contact allergens. The criteria for selection of products for chemical analysis were laid down in consultation with the Danish Environmental Protection Agency and comprise:
From the initial screening of chemical ingredients in liquid hand soaps, 15 products were selected for chemical analysis. The programme included analysis of fragrance chemicals and the preservative Methyldibromoglutaronitril. When selecting the products the aim was to find products partly with a large distribution on the market and partly with a stated content of both well-known and unknown fragrance chemicals. As a result, the most neutrally smelling products were not selected for analysis. The 15 products were analysed for the 26 fragrance chemicals listed on EU's list of fragrance chemicals reported as contact allergens (3). In addition, three products with scent of roses were analysed for Methyl eugenol. Methyl eugenol is a natural component of rose oil. Methyl eugenol has been found to be genotoxic and carcinogenic (4). According to the Danish "kosmetikbekendtgørelse", annex 2, the substance is prohibited in concentrations > 0.001% in rinse-off products and consequently relevant for further scrutiny. Moreover, three products were analysed for the preservative Methyldibromoglutaronitril (MG) as a result of the substance's contact allergen properties. 5.2 Analytical methods5.2.1 Methyl eugenol and MethyldibromoglutaronitrilA part sample of the product is extracted with dichloromethane for one hour on shaking table and left to stand over night. A part sample of the extract is taken and analysed directly at combined gaschromatography and mass spectrometry (GC/MS). The content is calculated quantitatively. The analyses are performed as true double determinations. Methyl eugenol: Methyldibromoglutaronitril: Uncertainty is 20% RSD. The increased uncertainty is due to use of a technical product as reference standard. The limit of detection is 100 mg/kg. 5.2.2 Fragrance chemicalsA part sample of the product is taken and extracted with water and tert-butylmethylether by means of shaking, heating, and standing during a period of approximately 16 hours. A part sample of the extract is taken and analysed directly at combined gaschromatography and mass spectrometry (GC/MS). The analyses are performed as true double determinations. The limit of detection is 10 mg/kg and uncertainty is 10-15% RSD. It is not possible to determine a limit of detection for Oak moss extract and Tree moss extract, as these are natural extracts with many components and not merely one single substance. An exact limit of detection cannot be calculated as the content of these natural extracts vary. Instead the limit is given as "Not determined". 5.3 Results5.3.1 Methyl eugenolThree products were analysed for methyl eugenol and the result of the analyses is given in table 1. Methyl eugenol was not detected in the products. The analyses have been performed in double thus 2 results (A and B) are given in the table. The unit is mg/kg and the limit of detection is 10 mg/kg. Table 5.1 Results of the analysis for Methyl eugenol. The results are given in mg/kg.
<.: Means less than the stated limit of detection # Product 3 is now marketed in a new formulationing 5.3.2 MethyldibromoglutaronitrilThree products were included in the analysis for Methyldibromoglutaronitril. The samples were analysed in double determinations (A and B). Content above the limit of detection could not be determined in the products. Unit is mg/kg and the limit of detection is 100 mg/kg. Table 5.2 Result of the analysis for Methyldibromoglutaronitril. The results are given in mg/kg.
<.: Means less than the stated limit of detection **Product is no longer on the market 5.3.3 Fragrance chemicalsA total of 26 substances were analysed in the 15 liquid soaps. The result of the analyses is given in table 3. Result A and B indicate double determinations. The 26 fragrance chemicals were detected in 14 of the 15 products. The total content varies from 8 to 2600 mg/kg corresponding to a range from 0.0008 to 0.26 weight%. Table 5.3 Result from the analysis for fragrance chemicals. Unit is mg/kg. Two results indicate double determinations.
LOD: Means limit of detection Table 5.3 continued. Result from the analysis for fragrance chemicals. Unit is mg/kg. Two results indicate double determinations.
LOD: Means limit of detection Table 5.3 continued. Result from the analysis for fragrance chemicals. Unit is mg/kg. Two results indicate double determinations.
LOD: Means limit of detection Table 5.3 continued. Result from the analysis for fragrance chemicals. Unit is mg/kg. Two results indicate double pdeterminations.
LOD: Means limit of detection 5.4 Summary of analytical results5.4.1 Methyl eugenolMethyl eugenol is not on the list of the 26 fragrance chemicals reported as contact allergens. However, the substance has been included in the analysis in the light of its carcinogenic effects and its natural occurrence in rose oil. The three products that were analysed for Methyl eugenol had a scent of roses but did not contain detectable concentrations of Methyl eugenol. 5.4.2 MethyldibromoglutaronitrilMethyldibromoglutaronitril was stated on the list of ingredients of three products, however, the substance was not detected in the products at the detection limit of 100 mg/kg (0.01%). Consequently, the concentration in the products is estimated at being less than 0.01%. In the literature concentrations of 0.0075 – 0.06% are mentioned for the substance (5). Maximum tolerated concentration in cosmetics is 0.1% in rinse-off products (1). 5.4.3 Fragrance chemicalsIn one of the products (no. 16) none of the fragrance chemicals could be detected and in another product (no. 1) only one of the fragrance chemicals was detected. Between 3 and 12 of the tested fragrance chemicals were found in the remaining 13 products. The following 7 fragrance chemicals were not found in any of the analysed products: Anisyl alcohol, Amylcinnamyl alcohol, Benzylcinnamate, Cinnamal, Methyl heptin carbonate, Oakmoss, and Treemoss. Occurrence of the remaining 19 fragrance chemicals in the products is shown in table 5.4. Table 5.4 Occurrence of fragrance chemicals in the 15 analysed products.
As appears from table 5.4 some of the most commonly used fragrance chemicals in the analysed hand soaps are Linalool, Geraniol, Citronellol, Hexylcinnamaldehyde, Lilial, Benzyl alcohol, Benzyl salicylate and Eugenol. These are the substances found in the largest concentrations in the products. The highest content of a single fragrance chemical is 2400 mg/kg for D-limonen. The total content of the 26 fragrance chemicals in the tested products is between 1 mg/kg and up to 2600 mg/kg. Table 5.5 is a summary of the analytical results showing occurrence in number of products, minimum and maximum measured values, and the maximum value as a percentage by weight in the products. Table 5.5 Summary of analytical results.
5.5 Agreement between analytical results and list of ingredients/safety data sheetsAs mentioned above, the 26 allergen fragrance chemicals must be stated on the product label of hand soaps (rinse-off products) if the concentration of a single substance is above 0.01%, equal to 100 mg/kg, for products marketed after 11 March 2005. The products, which were selected for chemical analysis, did not state content of allergen fragrance chemicals on the label with the exception of product nos. 45 and 50. This may be because the products had been in store for a long time prior to being sold. However, the chemical analysis showed that one or more of the 26 fragrance chemicals were identified in 9 of the 15 analysed products in concentrations > 0.01% (>100 mg/kg). Table 5.7 lists fragrance chemicals identified in the products in concentrations > 0.01%. Table 5.6 Fragrance chemicals, which are identified in the products by chemical analysis (content > 0.01%), must be stated on the product label.
* Stated on the product label As can be seen from the above product nos. 5, 6, 7, 8, 15 and 21 contain allergen fragrance chemicals in concentrations above > 0.01%, which are not stated on the list of ingredients. The formulation of product 6 has been changed after the analysis were carried out so that the product no longer contains any of the 26 allergen fragrances. Produkt 7 is now labelled according to the regulation with content of Hexylcinnamaldehyde, D-limonen and the product does not contain Linalool in concentration above 0,01% according to the information the Danish EPA has received. Product 21 and 8 is no longer on the market. Only a safety data sheet is available for product no. 34, and consequently the list of ingredients on the product label could not be verified. In order to label the products correctly it is a prerequisite to have information from the raw material suppliers on the content and concentration of the fragrance chemicals reported as contact allergens in the perfumery raw materials. Although Methyldibromoglutaronitril was stated on the product label of three products (no. 8, 15, and 21), the content of the substance could not be detected in concentrations > 100 mg/kg in the products. This may be due to the fact that the substance is used in lower concentrations in the products. 6 Health assessment
6.1 Selection of substances for health assessmentWhen selecting the substances for health assessment, particular emphasis has been placed on the risk of developing allergy when considering the product content of allergen substances. Selection of fragrance chemicals for health assessment has been carried out based on the occurrence of the substances in the products and the result of the chemical analysis. The most frequently used fragrance chemicals in the products were in descending order: Linalool, Geraniol, Citronellol, Hexylcinnamaldehyde, and Lilial. Table 6.1 Content and occurrence of substances in the hand soaps
Linalool and Geraniol were assessed in connection with a previous survey for the Danish Environmental Protection Agency (6). When comparing the information on content and occurrence of substances in hand soaps, cf. table 5.6, with the previous assessments the following substances have been selected for health assessment:
Methyldibromoglutaronitril will not be assessed as the substance was not detected in the products. Toxicological profiles of the selected fragrance chemicals have been drawn up below. Information on each substance has been found in toxicological reference books and databases as well as in scientific articles. Based on the published data a NOAEL/NOEL or LOAEL has been identified for the user exposure in chapter 7. 6.2 HexylcinnamaldehydeOccurrence and useHexylcinnamaldehyde is used as a fragrance ingredient in perfumes, often in flowery perfumes. Occur naturally in for instance boiled rice. Hexylcinnamaldehyde is the main component of jasmine fragrances. The consumption of the substance is estimated at 87 μg/person/day or 1 μg/kg body weight/day in Europe and at 11 μg/person/day or 0.2 μg/kg body weight/day in the USA (7). IdentificationHexylcinnamaldehyde is an aldehyde.
Physical-chemical properties (11)
Acute toxicityLD50 [3] values have been found to be 3100 and 4650 mg/kg body weight in rats orally exposed to Hexylcinnamaldehyde (11,12). By oral exposure of mice LD50 is approx. 2300 mg/kg body weight (12). Observed toxic effects were dose dependent and included somnolence (generally reduced activity) and effects on lungs (respiratory depression) (12). LD50 is stated as > 3000 mg/kg body weight in rabbit skin exposed to Hexylcinnamaldehyde (11). The body weight was found to be affected in rat experiments in which the animals breathed in a concentration of 2.12 mg Hexylcinnamaldehyde/L air (nominal concentration was 5.00 mg/l). At a microscopical examination 14 days after exposure enlarged bronchial lymph nodes were found, sometimes accompanied by pulmonary congestion or with many grey-green pinpoint foci in the lungs. LC50 [4] was determined at > 5 mg/L (11). Local irritationExposure to undiluted Hexylcinnamaldehyde on shaved rabbit skin for 24 hours in doses of 0.1 g and 0.5 g caused moderate to severe irritation (11,12). On guinea pig skin 0.1 g exposure to undiluted Hexylcinnamaldehyde for 24 hours caused severe irritation (12). Skin irritation or sensitization were not observed in humans, to whom 12-12.5% Hexylcinnamaldehyde in Ethanol or Petrolatum was applied repeatedly (11). AllergyHexylcinnamaldehyde is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens but less frequently reported as contact allergens. In 3 studies with 20, 119 and 179 patients respectively with cosmetics eczema, 1,1 and 7 cases of contact allergy were reported, which corresponds to 5, 0.8 and 3.9% of the patients (13). Hexylcinnamaldehyde is one of the positive control substances in OECD's guideline for animal sensitization tests (13,14). Repeated exposureNo reports on the effect of repeated oral exposure.Several reports on dermal experiments with mice. Exposure of 750 mg/kg body weight/day for 3 days caused skin sensitization. Exposure of 1800 mg/kg body weight/day for 3 days caused skin sensitization and skin inflammation. TDLo [5] in these two studies is 750 and 1800 mg/kg body weight respectively (12). Reduced growth in females, effects on the gastrointestinal tract and blood effects at the lowest dose were observed in groups of 15 rats of each sex, which were exposed dermally to 125, 250, 500 and 1000 mg Hexylcinnamaldehyde/kg body weight/day for 90 days. Irritation of the gastrointestinal tract, increased liver and kidney weight, and microscopical changes in these organs were observed at higher doses. At 1000 mg/kg body weight/day the mortality exceeded 50% (8/15). In the light of this a LOAEL [6] of 125 mg/kg body weight/day was determined (11). Hexylcinnamaldehyde is tested negative (did not cause mutations) in Ames test in doses up to 3.6 mg/plate with or without metabolic activation with S-9 (11). The substance did not cause chromosome changes in vivo in a Drosophila melanogaster test at doses of 2163 mg/L (10 mmol) (11) or in the mouse bone marrow chromosome aberration test at 657 mg/kg body weight (7). Critical effects The critical effect of Hexylcinnamaldehyde is contactallergy. Because of the sensitizing effects of Hexylcinnamaldehyde, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.2 NOAEL used for calculation of MoS for Hexylcinnamaldehyde
6.3 LilialOccurrence and useLilial is the trade name of a synthetically made substance that is used widely as a fragrance ingredient in perfumes, often in flowery perfumes (cyclamen, lily of the valley). IdentificationLilial is an aldehyde.
Physical-chemical properties
Acute toxicityLD50 values have been found to be 1390 mg/kg body weight in rats orally exposed to Lilial (11,12), which means the substance is hazardous to human health. LD50 values at intraperitoneal (i.p.) [8] exposure of mice is approx. 700 mg/kg body weight. Observed toxic effects included somnolence, generally reduced activity, and respiratory depression (11,12). At skin exposure of rats the LD50 value is stated at > 2000 mg/kg body weight (11) and >5000 mg/kg body weight respectively. In rats Lilial may be absorbed through the skin (19% of a dose during 120 hours) and is secreted mainly through the kidneys. The substance could not be detected in the blood 30 minutes after application of approx. 0.2 g Lilial on the skin (9 cm2). The highest concentration in the blood was measured after 60 minutes at 484 ng/mL, and after 6 hours the blood concentration went down quickly (11). Local irritationUndiluted Lilial on shaved rabbit skin for 24 hours caused moderate irritation (11). Skin irritation or sensitizing of human skin was not observed after repeated application of first 5% and later 4% Lilial in vaseline (11). Undiluted Lilial in rabbit eyes did not cause irritation (11). AllergyLilial is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens but less frequently reported as contact allergens. Two and five cases respectively of contact dermatitis have been reported in two studies of 167 og 179 patients with cosmetics eczema, which corresponds to 1.2% and 2.8% of the patients (13). In a number of guinea pig tests for the sensitizing potential of Lilial, the substance was found to be sensitizing in 5/8 studies (11). In human studies Lilial caused contact allergy in one out of 8 studies (11). Lilial did not cause photo sensitization in guinea pig studies (11). Repeated exposure Oral exposure (by stomach tube) of groups of 8 male rats of 25, 50, 100, 200 and 400 mg Lilial/kg body weight/day in sunflower oil for 5 days caused effects on the testicles, epididymes, and the sperm ducts. A NOEL [9] of 25 mg/kg body weight/day was found for this effect (11). No effects observed after oral dosing of male mice, male guinea pigs, and male rhesus monkeys with 100 mg Lilial/kg body weight/day for 5 days, nor on testicles (11). Oral exposure of pregnant female rabbits with 7020 mg/kg body weight/day on gestation day 7-19 caused changes in the skeletal muscles of the foetuses (12). No effects observed in dogs after doses of up to 200 mg/kg body weight/day (in gelatine capsules) for up to 90 days (11). In two studies male rats and male mice were exposed dermally to Lilial. A dose of 2000 mg/kg body weight/day was applied to the rat skin for 5 days causing effects on testicles, epididymes, and the sperm ducts. In the mice study, a dose of 750 mg/kg body weight/day was applied to the mice skin causing skin sensitization (12). A NOAEL of 25 mg/kg body weight/day has been determined based on a 90-day oral exposure study with rats, in which reduced choline and acetylcholinesterase in the plasma was observed but not in the brain or in the red blood cells/erythrocytes. Moreover, male rats had disorders in the spermatozoon formation (11). Lilial was tested negative (did not cause mutations) in Ames' test (11). No available data for inhalation of Lilial. Critical effects The critical effect of Lilial is contactallergy. Because of the sensitizing effects of Lilial, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.3 NOAEL used for calculation of MoS for Lilial
6.4 AmylcinnamalOccurrence and useAmylcinnamal is used as a fragrance ingredient in perfumes. Amylcinnamal occur naturally in for instance soy beans. Amylcinnamal is a synthetically made substance and has a jasminelike odour (16). Consumption of the substance is estimated at 25 μg/person/day or 0.42 μg/kg body weight/day in Europe and at 23 μg/person/day or 0.38 μg/kg body weight/day in the USA (7). Amylcinnamal is one of the constituents of Fragrance Mix (FM), a perfume blend used in dermal clinics for diagnosing contact allergy to fragrances. Identification Amylcinnamal is an aldehyde (17).
Physical-chemical properties (18)
WHO has determined a NOAEL for Amylcinnamal of 290 mg/kg body weight/day for male rats and 320 mg/kg body weight/day for female rats (7). Acute toxicityLD50 values have been found at 3730 mg/kg body weight/day in rats orally exposed to Amylcinnamal (12). Observed toxic effects included effects on sense organs, somnolence and generally depressed activity (12). No available data for the toxicity of Amylcinnamal after dermal exposure. Local irritation100 mg undiluted Amylcinnamal on shaved rabbit skin caused severe irritation. A 5% solution on guinea pig skin for 2 weeks caused mild irritation, while undiluted Amylcinnamal on guinea pigs for 24 hours caused moderate irritation (12). Irritation and sensitization of human skin not observed after repeated application of 20% Amylcinnamal (14). AllergyAmylcinnamal is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens and for which many reports on user allergy are available. Five cases of contact allergy are reported from two studies of 1072 and 167 patients with cosmetics eczema, which corresponds to 0.47% and 3% of the patients. The patients were exposed to 1% and 5% Amylcinnamal in Vaseline. Of 179 patients with putative cosmetics allergy, 7 (3.9 %) reacted positively when tested with 10% Amylcinnamal in vaseline (13). Amylcinnamal is a known allergen. It is part of the Fragrance Mix for diagnostic testing. The substance is responsible for 2-3% of the reactions of this blend (1.9% in Italy, 2.3% in Denmark and 2.5% in France) (13). Amylcinnamal is identified as the cause of allergic reactions in persons with fragrance cosmetic allergy. In 78 European consumers with fragrance eczema, 2.6% reacted positively when tested with 2% Amylcinnamal (13). Repeated exposureOral exposure of rats with 500 mg/kg body weight/day for 64 days caused enzyme changes in the liver and increased the liver weight (7). Groups of 15 rats were exposed through the feed to 0, 80, 400 and 4000 mg Amylcinnamal/kg feed for 14 days. In the highest dose group a statistically significant increased liver weight in male and female rats was observed, including an increased kidney weight in male rats in proportion to the body weight. Microscopical changes in livers and kidneys were not observed in the animals. Based on this a NOAEL of 290 (320) mg/kg body weight/day for male rats (female rats) was determined (7). Effects concerning growth, consumption, blood or clinical chemistry were not observed after oral exposure with Amylcinnamal through the feed of 15 rats of each sex for 90 days corresponding to 6.1 mg and 6.6 mg/kg body weight/day for male and female rats. Moreover, no toxic effects of the microscopical examination were observed after termination of the study. NOAEL was determined at 6.1 mg/kg body weight/day for male rats and 6.6 mg/kg body weight/day for female rats (7). Amylcinnamal was tested negative (did not cause mutations) in Ames' test in concentrations up 1000 μg/plate with or without metabolic activation with S-9 mixture. The substance did not cause chromosome changes in vivo in a Drosophila melanogaster test at concentrations of up to 2023 mg/L (10 mmol/L) or in the mouse bone marrow chromosome aberration test when exposed to 1213 mg/kg body weight/day (7). No available data for inhalation of Amylcinnamal. Critical effects The critical effect of Amylcinnamal is contactallergy. Because of its sensitizing effects, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.4 NOAEL used for calculation of MoS for Amylcinnamal
6.5 CoumarinOccurrence and useCoumarin has a pleasant odour and is used as a fragrance ingredient in perfumes and as a flavouring agent in food. The substance occurs naturally in tonka beans (seeds of Dipteris odorata) and as an ingredient in essential oils, e.g. cassia leaf oil (Chinese cinnamon, Cassia fistula) (up to 83.300 mg/kg), cinnamon leaf oil (40.600 mg/kg), cinnamon bark oil (7000 mg/kg), and in lavender and peppermint oil (7000 mg/kg) (19). Coumarin is a lactone produced by enzymatic hydrolysis of the glucoside melilotoside. Coumarin glucosides occur in plants such as woodruff (Asperula odorata), rue (Ruta graveolens), and in lovage (Levisticum officinale). Melilotoside occurs in plants such as golden melilot (Melilotus altissima) and meadow melilot (Melilotus arvensis) (20). IdentificationCoumarin is a e.
Physical-chemical properties (22)
EFSA, the European Food Safety Authority, states a NOAEL for Coumarin of 10 mg/kg bodyweight/day based on the liver toxicity of the most sensitive species, dog. A safety factor of 10 allowing for variation between the species and another safety factor of 10 for variation between individuals gives a tolerable daily intake (TDI) of 0-0.1 mg coumarin/kg body weight/day (19). Acute toxicityLD50 oral, mice, rat, guinea pig: 196-680 mg/kg body weight/day (22). Coumarin has been used in the treatment of lymphoedema. From this it is known that most humans tolerate single doses of at least 400 mg. However, a few, 17 out of 2173 patients in a clinical-toxicological study, suffered injuries on the liver after repeated dosing resulting in increased liver enzyme figures (23). No available data for acute toxicity after dermal exposure. Local irritationNo available data. AllergyCoumarin belongs to the group of fragrance chemicals which in 1999 were reported most frequently as the cause of contact allergy (13). Coumarin is not considered a photoallergen. It is only coumarin derivatives which are found to be photoallergens (24). Repeated exposureSeventeen out of 2173 patients, of which the majority received oral doses of 100 mg Coumarin daily for a month followed by 50 mg daily for two years, had increased liver enzyme figures. None of the patients had permanent injuries on the liver. In five of the 17 patients, who continued to take Coumarin, the liver enzyme figures fell to a normal level. In five studies, supported by the Lymphedema Association in Australia, 1106 patients received 400 mg Coumarin daily for an average of 14.6 months resulting in two cases of liver damage (23). Both the European Food Safety Authority (EFSA) as well as IARC have previously concluded that Coumarin is carcinogenic in rats and possibly in mice. However, in 2004 EFSA's scientific panel for food additives etc. concluded that it must be a non-genotoxic mechanism as a covalent linkage of Coumarin to DNA could not be proved in rat livers and rat kidneys (19). Critical effects The critical effect of consumption and absorption is liver toxicity. The critical effect of skin contact is contact allergy. Table 6.5 NOAEL used for calculation of MoS for Coumarin
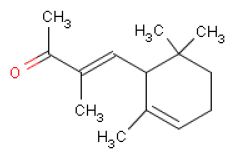
6.6 α-IsomethyliononOccurrence and useα-Isomethylionon is used as a fragrance ingredient in perfumes, often in flowery perfumes. In addition, it is used as a flavour additive in foods. The consumption of the substance is estimated at 0.09 μg/kg body weight/day in Europe and at 0.02 μg/kg body weight/day in the USA (25). No available data on natural occurrence. Identificationα-Isomethylionon is a cyclohexanol ring with a ketone side chain.
Physical-chemical properties
WHO states a NOEL for α-Isomethylionon of < 4 mg/kg body weight/day (25). Acute toxicityLD50 values have been found to be > 5000 mg/kg body weight/day at oral exposure of rats with a mixing of Methyl-α-ionon and α-Isomethylionon. Based on data for a similar substance, β-ionon, WHO estimates that most likely α-Isomethylionon is transformed into harmless substances in the body (25). No available data for absorption of α-Isomethylionon through the skin or for inhalation of α-Isomethylionon. Local irritationNo available data concerning the irritating effect of -Isomethylionon on skin and eyes. Allergy-Isomethylionon is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens but less frequently reported as contact allergens. Three patient studies of allergy to cosmetics are described in which 2 of 179 patients (1.1 %), one of 75 patients (1.3 %) and one of 119 patients (0.8 %) showed allergic reactions to -Isomethylionon (13). Repeated exposure-Isomethylionon was administered daily to groups of 15 rats of each sex in their feed for 90 days in doses of 4 mg/kg body weight/day. No effects reported on kidneys, livers or the blood that differed from the control group or were outside normal. A NOEL was stated to be more than 4 mg/kg body weight/day (25). No available data on mutagenic effects for α-Isomethylionon. An analogous substance, Methyl--ionon, was not mutagenic in Ames' test and in tests with Drosophila melanogaster (25). Critical effects The critical effect of -Isomethylionon is allergy. Because of the sensitizing effects of α-Isomethylionon, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.6 Summary of data used for calculation of MoS for α-Isomethylionon
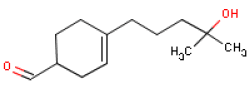
6.7 Lyral®Occurrence and useLyral® with the chemical name 4-(4-Hydroxy-4-methyl pentyl)-3-cyclohexene-1-carboxaldehyde is used as a fragrance ingredient in perfumes. Lyral® is also described as a blend of 3- and 4-(4-Hydroxy-4-methyl pentyl)-3-cyclohexene-1-carboxaldehyde. Lyral® is not a natural substance but is a synthetic fragrance chemical. IdentificationLyral® is an aldehyde.
Physical-chemical properties (12)
Acute toxicityAt oral exposures of rats to Lyral®, LD50 have been found to be 3250 mg/kg body weight. Observed toxic effects were lacrimation, strong somnolence and tremor (12). At exposure of Lyral® to rabbit skin, LD50 is 11300 mgL/kg body weight. Observed toxic effects were somnolence and changes in the structure or function of the salivary glands (12). No available data concerning inhalation of Lyral®. Local irritationThe substance is indicated to be mildly irritating and patch tests on humans have seldom shown irritation. An irritation test of 0.5 ml Lyral® on shaved rabbit skin for 4 hours showed a mild reaction (12). A study of Lyral®, 5% in petrolatum, on humans showed irritation of the skin in 4 out of 3245 patients (0.1 %) from dermal clinics (26). Application of 100 mg in rabbit eyes for an unspecified period of time caused mild irritation of the eyes (12). AllergyThe EU scientific committee SCCNFP has listed Lyral® on the list of fragrance chemicals which are known allergens and are most frequently reported as contact allergens. Lyral® has been identified as the cause of contact allergy in 2-3% of patients with eczema who were examined by lap tests. Today the substance is included in standard lap tests in many dermal clinics (27). There are several reports on Lyral® studies on patients from dermal clinics. One study of 106 patients showed a positive reaction in 3 (2.8 %) patients with Lyral®, 5% in petrolatum, and in 1 (0.9 %) patient with Lyral® 1% Clinical relevance has not been clearly demonstrated but was probably relevant in 2 patients. The last of the patients with a positive reaction may have had a skin irritation reaction (3). A study of 1855 patients with eczema who were tested with a screening series of 11 fragrance chemicals showed positive reactions in 50 (2.7%) patients with Lyral®, 5% in petrolatum, of which probably 2/3 were relevant (3). A study from 2003 has shown that Lyral® in dilutions from 6 ppm to 6% caused allergic reactions in almost all persons who were sensitized with Lyral®, and that a reduction in the use of the fragrance chemical was necessary to avoid contact allergy (27). Today, IFRA has limited the use of Lyral® to 1.5% in both leave-on products and rinse-off products, including household products such as detergents and cleaning materials (15). Repeated exposureNo available data on repeated exposure at oral administration of Lyral®. No available data on mutagenic effects, carcinogenic effects or reproductive effects of Lyral®. Critical effects The critical effect of Lyral® is contactallergy. Because of the sensitizing effects of Lyral®, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.7 NOAEL used for calculation of MoS for Lyral®
7 User exposure7.1 Exposure assessmentExposure assessment of the hand soaps is based on exposure to the 26 fragrance chemicals, which the EU has assessed as contact allergens, and exposure to Methyleugenol and Lyral®. The guidelines for amount per application and frequency of use as stated in the EU Technical Guidance Document (TGD) (28) and in SCCNFP's guidelines (29), toxicological profiles of the fragrance chemicals as described above in chapter 6, and the analytical results in chapter 5 have been used in the following to estimate the exposure in a worst-case scenario for two standard persons: an adult of 60 kg and a child of 18 kg (3-5 years). The daily exposure has been calculated based on the highest measured content of fragrance chemicals in the tested hand soaps. 7.1.1 Used amounts of liquid hand soapsThe typical amount of hand soap used when washing hands is determined in the EU Technical Guidance Document (TGD) to 0.8 g for solid soap bars and with a user frequency of 3-6 times daily (28). No value has been determined for the amount of liquid hand soap used when washing hands (28,29). To calculate the exposure to liquid hand soaps the dosage was based on the measurement of the actual consumption when washing hands. In an exposure test at two workplaces the amounts used of selected hand soaps were measured. In one workplace an assessment was carried out of the dosage per hand wash based on the hand soaps that were accessible at the workplace. By using an automatic dispenser the amount used per hand wash was calculated at 0.6 g for a foam product in which the density is assumed to be lower than for gel/cream products. In a laboratory the dosage of a liquid hand soap with a dispenser device was measured at 1.8 g per wash. Kitchen staff was assessed to be among the working groups who wash their hands most frequently and when interviewing them it was informed that they wash their hands with soap 20-30 times daily on average. Two exposure assessments were carried out in a workplace during a period of five days with five selected liquid hand soaps purchased for this survey. The foam product was not included as only one foam product had been selected and purchased. The liquid hand soaps were placed at the lavatories. All products contained fragrance and had different viscosities. All the soaps had a pumping device which, however, varied from product to product. In one of the products it was particularly easy to pump the soap. Adults (both female and male) from 28-61 years participated in the test. None of the test persons required washing hands after particularly dirty labour. Each of the test persons recorded the amount used each time they washed their hands. The amount used was found as an average of the recorded number of dosages for each product. The amounts used of the five hand soaps appear from table 7.1. Table 7.1 Amounts used for five of the hand soaps
*: Single determination only The study was carried out on a selected user group and is only indicative of the consumption. The average consumption was 0.92 g/hand wash. The study indicated that the consumption of liquid hand soaps per hand wash was higher than the TGD value of 0.8 g for solid soap bars. Furthermore, it is possible that the fragrance of the product may influence the amount used when washing hands. Also the actual pumping device may influence the amount used. The average amount of soap used per hand wash has been determined at 1.0 g in the below exposure scenario by comparing the average dosage of 0.92 g with the amounts used of 0.6 g and 1.8 g in occupational use. 7.1.2 Exposure scenariosBased on the used amount of soap per hand wash of 1 g found in the above study, realistic "worst-case" scenarios have been drawn up for exposure to fragrance chemicals of adults and children at skin contact. The exposure scenarios are based on common use of the products with the TGD frequency of 6 times per day from. The exposed area is the surface of the hands. Data for skin absorption of fragrance chemicals have not been found in literature and as a worst-case scenario it is assumed that 100% of the substances is absorbed through the skin. However, this will clearly give too high results as the products are rinsed off with water. Therefore, the EU has introduced a term "Retention factor" which takes into account the rinsing-off and dilution of the hand soap in connection with common use. For products such as hand soaps the EU has set a retention factor of 0.01 (29). Exposure results, in the EU termed SED or Systemic Exposure Dosage (29), is stated in mg substance per kg body weight at a time and/or day based on the following data:
Daily amount of exposure, D-limonen, adult:
Daily amount of exposure to D-limonen in hand soap for an adult, dilution taken into account (Retention factor: 0.01):
Daily amount of exposure to D-limonen in hand soap, child 3-5 years:
Daily amount of exposure to D-limonen in hand soap for a child, dilution taken into account (Retention factor: 0.01):
For kitchen staff the exposure is not 6 times daily but informed to be up to 30 times daily.
Dilution taken into account (Retention factor: 0.01):
The daily exposure to the 19 fragrance chemicals that were found in the analysis is estimated per kg body weight per day for the two standard persons. The results appear in table 7.2. Table 7.2 Content and daily exposure to the 19 fragrance chemicals that were found in the analysed hand soaps.
* : The maximum measured weight-% is above 0.01% which is the EU limit for labelling of the 26 allergen fragrance chemicals in rinse-off products ** bw: body weight The chemical analyses show that the concentrations of the total content of the 19 allergen fragrance chemicals in the examined hand soaps are from 0.0013% - 0.24 weight-%. The content of fragrance chemicals reported as contact allergens in the hand soaps is low compared with the 0.5 – 1% which is stated in the literature as the typical fragrance content in shampoos and liquid soaps (31). It is possible that other fragrance chemicals than those reported as contact allergens are also found in the products. 7.2 Safety assessment of selected substancesWhen calculating the Margin of Safety (MoS) of a substance the estimated daily exposure (= SED) of each fragrance chemical is used in the following:
7.2.1 D-limonenOf the 26 fragrance chemicals reported as contact allergens D-limonen occurs in the largest amount in the hand soaps. The substance has been evaluated in a previous survey for the Danish Environmental Protection Agency (32). With a NOEL value for D-limonen of 250 mg/kg body weight/day for liver injuries and based on the estimated daily exposure of 0.0024 for adults and 0.008 for children gives the following safety margins: MoS, adult:
MoS, adult, occupational use:
MoS, child:
7.2.2 Other fragrance allergensFor the fragrance chemicals reported as contact allergens Hexylcinnamaldehyde, Lilial, Amyl cinnamal, Coumarin, a-Isomethylionon and Lyral®, which are assessed in chapter 6 of this survey, the NOAEL is given in table 7.3. When calculating the MoS, the SEDadult and SEDchild from table 7.2 are used. The MoS results also appear from table 7.3. Table 7.3 NOAEL used for calculation of MoS for selected fragrance chemicals
*: occupational use MoS should at least be 100 to take into account a safety factor of 10 for extrapolation of data from animals to humans and a safety factor of 10 to take into account particularly sensitive consumers. The calculations show that the safety margins are far from being exceeded, both for consumers and in connection with frequent occupational use. However, it must be emphasized that the MoS has not been calculated for allergy but for other critical effects, cf. the health assessments in chapter 6. The fragrance chemicals have all been reported by SCCNFP as contact allergens at skin contact. As there is no "zero effect level" for allergy it is essential to point out that skin contact with these fragrance chemicals should be avoided (3). 7.2.3 Conclusion of safety assessmentThe exposure assessments showed very low daily exposure and a high safety margin for the hand soaps for both adults and children and in connection with frequent occupational use. As it concerns assessment of substances reported as contact allergens it must be concluded that for particularly sensitive consumers including persons with allergy the use of products with a high content of fragrance chemicals may cause adverse health effects. This is supported by the fact that the EU has determined an obligation of labelling of these fragrance chemicals above a certain concentration in the finished products. In table 7.2 can be seen the fragrance chemicals which exceed this limit (indicated by an *) and which must thus be stated on the product label. 8 Environmental assessment8.1 Selection of substancesWhen selecting substances for assessment of the environmental effects of the use of liquid hand soaps, a preliminary screening of the environmental hazard of the substances was carried out. This screening has been compared with the frequency with which the substances are contained in the products. In addition, an assessment of the approximate concentrations of the substances in the products has been carried out based on dispensaries from other consumer products from previous surveys. When combining the hazard of the chemical substances with the frequency with which they are found in the products, it gives an impression of which substances are expected to cause possible impacts on the aquatic environment. From an environmental aspect surfactants and preservatives are the most interesting substances. Surfactants are the active substances found in the highest concentrations in the products. The vast majority of surfactants used in cosmetics are readily biodegradable but are often highly acutely toxic to aquatic organisms. Furthermore, some surfactants are potentially bioaccumulative. Many preservatives are toxic to aquatic organisms in very low concentrations and are also very difficult to degrade due to their toxic effect on the degrading bacteria. 8.1.1 SurfactantsAll surfactants identified in the hand soaps are assessed to be readily biodegradable but the toxicity of the surfactants vary within the different groups of surfactants. As described earlier, particularly two surfactants are found in the majority of the products. The anionic surfactant Sodium laureth sulfate is found in 39 out of 50 products, and the amphoteric surfactant Cocamidopropyl betaine is found in 31 out of 50 products. These two substances cannot be characterized as environmentally hazardous but they have been included in the environmental assessment as they occur with the highest frequency and in the highest concentrations in the products (apart from water). In addition, Cocamide DEA and Cocamide MEA have been selected as they are found in several of the products (14 and 8 products, respectively). The majority of the remaining surfactants are only found in a few products and are not estimated to contribute significantly to the overall chemical impact. 8.1.2 PreservativesMost of the identified preservatives are found to have a low frequency in the products. The most common preservatives are Sodium benzoate (15 of 50 products), Phenoxyethanol (12 of 50 products) and Dehydroacetic acid (7 of 50 products). None of these three preservatives can be described as critical to the environment. Each of the remaining preservatives are only found in few of the products. The preservatives with the most critical environmental properties are 2-Bromo-2-Nitropropane-1,3-Diol, Methylchloroisothiazolinone and Methylisothiazolinone (Kathon) (found in common liquid hand soaps) as well as Chlorhexidine Digluconate, Benzalkonium Chloride and Triclosan (found in anti-bacterial hand soaps). Only limited data are available on DMDM Hydantoin and Imidazolidonyl Urea (found in common liquid hand soaps). Focus have been concentrated on some of the preservatives in common hand soaps as these make up the largest product volume. 8.1.3 Environmental assessment of selected substancesThe following 8 substances have been selected for further environmental assessment (table 8.1): Table 8.1 Substances selected for environmental assessment
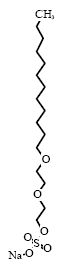
* Estimated from dispensaries from liquid hand soaps collected in previous projects ** Of the 50 products covered by the survey. The environmental properties of the selected substances are described according to their biodegradability, acute toxicity to aquatic organisms, and the potential for bioaccumulation according to the regulations for classification and labelling of chemical substances (33). Below the physical/chemical properties of the substances have been estimated by means of the program EPIWIN v. 3.12 (USEPA, 2004). The water/octanol partition coefficient (log Pow) has been used as a measure for the potential for bioaccumulation of the substances. According to the regulations on classification and labelling of chemical substances and products (32), a substance is considered potentially bioaccumulative when the log Pow > 3. 8.2 Environmental profiles of the selected substances8.2.1 Sodium laureth sulfateOccurrence and useSodium laureth sulfate is an anionic surfactant (group: alkyl ether sulfate) which is found in 39 of the 50 products in the survey. Typically, Sodium laureth sulphate is used in cosmetics such as liquid hand soaps, liquid body soaps and shampoos (34). IdentificationSodium laureth sulfate is composed of an alkyl chain, typically with 12-14 carbon atoms which are combined with a number of ethoxylate (EO) groups via an ester linkage.
* Generic structure: Sodium laureth sulfate C12, 2EO Physical-chemical properties *
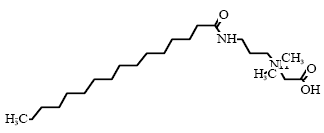
Environmental assessment Sodium laureth sulfate is fully degradable in a 28-day standard test for ready biodegradability. Sodium laureth sulfate is also degradable under anaerobic conditions. The acute effect of Sodium laureth sulfate to aquatic organisms can be described as toxic to moderately toxic with EC/LC50 values between 1.2-32 mg/l. The lowest EC50 value of 1.2 mg/l is found for daphnia in a 96-hour test. NOEC values < 1 mg/l have been found in chronic tests with daphnia (21 days) and fish (365 days) (34). With an estimated log Pow value of 1.14 the substance is not assessed to bioaccumulate. As the substance is expected to be fully degraded in waste water treatment plants, Sodium laureth sulfate is assessed not to cause long-term adverse effects in the aquatic environment. 8.2.2 Cocamidopropyl betainOccurrence and useCocamidopropyl betain is an amphoteric surfactant (group: betains) which is found in 31 of the 50 products in the survey. Cocamidopropylbetain is typically used in products for personal care such as liquid hand soaps, liquid body soaps, cleansing creams, and shampoos but it is also used in detergents and cleaning materials (34). IdentificationCocamidopropyl betain is composed of an alkyl chain which is combined with a quaternary nitrogen atom through an amide linkage.
* Generic structure, C16 alkyl chain Physical-chemical properties *
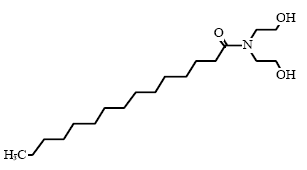
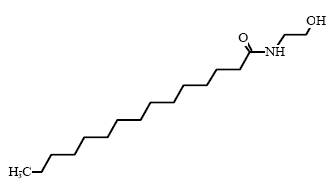
* Estimated data for Cocamidopropylbetain, C16 (ref: EPISUITE v. 3.12, USEPA, 2004) Environmental assessment Cocamidopropyl betain is fully degradable in a 28-day standard test for ready biodegradability. Cocamidopropyl betain is also degradable under anaerobic conditions. The acute effect of Cocamidopropyl betain to aquatic organisms can be described as toxic to moderately toxic with EC/LC50 values between 1.8-22 mg/l. The lowest EC50 value of 1.8 mg/l is found for algae in a 72-hour test. There is no available data on the chronic toxicity of Cocamidopropyl betain (34). With an estimated log Pow value of 2.65 the substance is not assessed to bioaccumulate. As the substance is expected to be fully degraded in waste water treatment plants, Cocamidopropyl betain is assessed not to cause long-term adverse effects in the aquatic environment. 8.2.3 Cocamide DEA/Cocamide MEAOccurrence and useCocamide DEA and Cocamide MEA are nonionic surfactants (group: fatty acid amides) and they are found in 14 and 8 respectively of the 50 products in this survey. Typically, fatty acid amides are used in products for personal care such as liquid hand soaps, liquid body soaps, shaving creams, and shampoos (34). IdentificationCocamide DEA/Cocamide MEA is composed of an alkyl chain which is combined with two or one amide group through an C-N linkage.
* Generic structure, C15 alkyl chain Physical-chemical properties *
* Estimated data for Cocamide DEA, C15 (ref: EPISUITE v. 3.12, USEPA, 2004)
* Generic structure, C15 alkyl chain Physical-chemical properties *
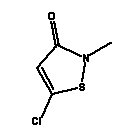
* Estimated data for Cocamide MEA, C15 (ref: EPISUITE v. 3.12, USEPA, 2004) Environmental assessment Both Cocamide DEA and Cocamide MEA are fully degradable in a 28-day standard test for biodegradability and are thus described as readily biodegradable (34). Cocamide MEA is found to be anaerobically biodegradable (34) whereas Cocamide DEA in a test for anaerobic biodegradability had an inhibiting effect on the degrading bacteria (35). Cocamide DEA and Cocamide MEA can be described as toxic to moderately toxic to aquatic organisms with EC/LC50 values between 2-6 mg/l for Cocamide DEA and 24->100 mg/l for Cocamide MEA. The lowest EC50 value for Cocamide DEA of 2.3 mg/l is found for algae in a 96-hour test. Generally, Cocamide MEA is less toxic with the lowest EC50 value of 26 mg/l for algae (34). There is no available data on the chronic toxicity of neither Cocamide DEA nor Cocamide MEA. With estimated log Pow values >4 for both Cocamide DEA and Cocamide MEA, the substances are assessed to be potentially bioaccumulative. Cocamide DEA will thus be classified as hazardous to the environment with N; R51/53 (Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment) because of the acute toxicity of the substance and its potential for bioaccumulation. However, Cocamide MEA will not be classified as hazardous to the environment as the substance is only moderately toxic (>10 mg/l). 8.2.4 Methylchloroisothiazolinone/Methylisothiazolinone (Kathon)Occurrence and useMethylchloroisothiazolinone and Methylisothiazolinone form part of the commercial product Kathon, which is often used as a preservative in cosmetics and cleaning materials. Kathon is found in 4 of the 50 products in this survey. IdentificationMethylchloroisothiazolinone and Methylisothiazolinone are heterocyclic aromatic compounds.
Physical-chemical properties *
* Estimated data (ref: EPISUITE v. 3.12, USEPA, 2004)
Physical-chemical properties *
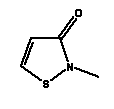
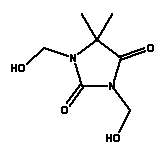
* Estimated data (ref: EPISUITE v. 3.12, USEPA, 2004) Environmental assessment Methylisothiazolinene and Methylchloroisothiazolinone are not readily biodegradable and have not been proven to be degradable under anaerobic conditions. Both Methylisothiazolinene and Methylchloroisothiazolinone have a high acute toxicity and consequently they show inhibiting effects on the degrading bacteria in tests for ready biodegradability. In a modified test for ready biodegradability in which very low concentrations of 14C labelled Methylisothiazolinene and Methylchloroisothiazolinone were used, a relatively high degree of degradability was observed corresponding to approx. 40-60% of the added radioactivity (34). Both substances have high acute toxicity to aquatic organisms with EC/LC50 values <1 mg/l. The lowest EC50 value of 0.003 mg/l is found for algae for the commercial product Kathon. There is no available data on the chronic toxicity of Methylisothiazolinene and Methylchloroisothiazolinone. None of the substances are assessed to be bioaccumulative as the estimated partition coefficients (log Pow) values are <0. Kathon is classified as hazardous to the environment with N; R50/53 (Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment) on the list of unwanted substances. 8.2.5 DMDM HydantoinOccurrence and useDMDM Hydantoin is found in 4 of the 50 products in this survey. DMDM Hydantoin is typically used as a preservative in cosmetics. IdentificationDMDM Hydantoin is a heterocyclic aromatic compound.
Physical-chemical properties *
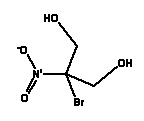
* Estimated data (ref: EPISUITE v. 3.12, USEPA, 2004) Environmental assessment There is no available data neither for the ready biodegradability nor the anaerobic biodegradability of DMDM Hydantoin. QSAR calculations of the biodegradability of DMDM Hydantoin under aerobic conditions indicate that the substance can be expected to degrade quickly in the environment (EPISUITE v. 3.12, USEPA, 2004). There are few experimental data on the aquatic toxicity of DMDM Hydantoin. EC50 values of 37 mg/l for daphnia have been found in a 96-hour test, and LC50 values for fish between 173-515 mg/l have been found in a 96-hour test (36). The substance is assessed to be hazardous to aquatic organisms as the lowest EC50 value is below 100 mg/L. It has not been possible to find data for the chronic toxicity of DMDM Hydantoin. Based on the estimated partition coefficient (log Pow) value of -2.37, DMDM Hydantoin is not assessed to be bioaccumulative. Provided that DMDM Hydantoin is degraded quickly in the environment, the substance is assessed not to be critical to the aquatic environment. However, additional data for the biodegradability and for the aquatic toxicity to algae are required to finally assess whether DMDM Hydantoin can be expected to cause adverse effects in the aquatic environment. 8.2.6 2-Bromo-2-Nitropropane-1,3-Diol (Bronopol)Occurrence and use2-Bromo-2-Nitropropane-1,3-Diol is found in 3 of the 50 products in this survey. 2-Bromo-2-Nitropropane-1,3-Diol is used as a preservative in cosmetics and in cleaning materials (34). Identification2-Bromo-2-Nitropropane-1,3-Diol is a nitro-substituted compound.
Physical-chemical properties *
* Estimated data (ref: EPISUITE v. 3.12, USEPA, 2004) Environmental assessment There is no available data for the ready biodegradability of 2-Bromo-2-Nitropropane-1,3-Diol. A standard test for ready biodegradability shows an inhibition of the degrading bacteria as a result of the high toxicity of 2-Bromo-2-Nitropropane-1,3-Diol (34). 2-Bromo-2-Nitropropane-1,3-Diol is classified with N; R50 (Very toxic to aquatic organisms) on the list of unwanted substances and consequently it is assumed to be easily biodegradable as it is not classified with R53 (May cause long-term adverse effects in the aquatic environment). 2-Bromo-2-Nitropropane-1,3-Diol has high acute toxicity to aquatic organisms. The lowest EC50 value of 0.37 is found in a 72-hour test with algae. EC50 values between 1-10 mg/l have been found for crustaceans, while fish are less sensitive to 2-Bromo-2-Nitropropane-1,3-Diol with typical LC50 values between 20-60 mg/L (34). No available data for the chronic toxicity of 2-Bromo-2-Nitropropane-1,3-Diol have been found. With an estimated partition coefficient (log Pow) value of -0.64, 2-Bromo-2-Nitropropane-1,3-Diol is not assessed to be bioaccumulative. 9 Effects in the aquatic environment
9.1 Selected substances for simulation of effects in the aquatic environmentThe environmental assessment of the 8 selected substances (chapter 8) showed that 4 of the substances have characteristics which have to be assessed further in a riskassesment to asses if they can cause critical effects in the aquatic environment. These 4 substances, Cocamide DEA, Methylchloroisothiazolinone/ Methylisothiazolinone (Kathon), and 2-Bromo-2-Nitropropane-1,3-Diol, are consequently included in an assessment of the environmental effects of the use of liquid hand soaps. 9.2 The fate of chemical substances in liquid hand soapsChemical substances used in liquid hand soaps will primarily be discharged to the environment through treated waste water from municipal waste water treatment plants. The hand soaps are washed out to the sewerage system and are via sewers lead to the waste water treatment plant. In the waste water treatment plant the chemical substances will be subject to various processes such as degradation under aerobic and anaerobic (anoxic) conditions, sorption to sludge particles, evaporation, hydrolysis, etc. As a result, the share of substances discharged with the treated waste water depend on the fate of the chemical substances in the waste water treatment plants. Furthermore, different biological and abiotic elimination processes in the aquatic environment will affect the concentration of the chemical substances. In addition, the concentration will depend on hydraulic parameters such as mixing/dilution and conditions of the water currents. 9.3 Total estimated use of chemical substances in the productsStatistics are not available of the annual consumption of liquid hand soaps in Denmark. The use of the selected substances is thus estimated indirectly on the basis of the average dose of soap used when washing hands and the average daily frequency of handwashing for normal consumers. The estimated maximum consumption is based on the following assumptions:
Table 9.1 states the estimated amounts of the selected chemical substances used in liquid hand soaps. Table 9.1 Estimated consumption of the selected substances in liquid hand soaps.
9.4 Estimate of Predicted Environmental Concentration (PEC) and Predicted No Effect Concentration (PNEC)In order to estimate the environmental risk resulting from discharge of the selected substances in the liquid hand soaps, the Predicted Environmental Concentration (PEC) is compared with the Predicted No Effect Concentration (PNEC) of the substances in the aquatic environment. The concentration of substances from the waste water treatment plant discharge (PECstp) is estimated based on amounts of consumption of the substance (M), the degree of elimination in the waste water treatment plant (fremoval) and the annually discharged waste water in Denmark (Q):
Q = 611 mill m3/year (37) fremoval is based on references in the EU Technical Guidance Document (TGD) (28). fremoval is a function of the octanol-water partition coefficient (log Pow), Henry's constant (H) and the biodegradability of each substance. The estimated PECstp values are shown in table 9.2 Table 9.2 Estimated PEC values
The highest concentrations expected not cause adverse effects in the aquatic environment, PNEC, are estimated based on data on the toxicity of the substances to aquatic organisms with application of an assessment factor as described in the EU Technical Guidance Document (28). The estimated PNEC values for the selected substances are shown in table 9.3. Table 9.3 Estimated PNEC values
9.5 Estimate of risk quotientsThe estimated risk quotients (RQ) for the selected substances are shown in table 9.4. RQ is estimated as PEC/PNEC. Table 9.4 Estimated risk quotients
* Risk quotients for Kathon is estimated as the sum of the risk quotients for the two components Methylchloroisothiazolinone and Methylisothiazolinone A risk quotient > 1 indicates a probability of effects in the aquatic environment. A standard dilution factor of 10 after discharge of the treated waste water to the aquatic environment is anticipated. A risk quotient less than 10 thus indicates that there is no risk of adverse effects in the aquatic environment. From table 9.4 it appears that the risk quotient in the discharge from the waste water treatment plant of Cocamide DEA, 2-Bromo-2-Nitropropane-1,3-Diol and Kathon is between 47 and 422. Discharge of substances in the calculated concentrations can thus be expected to cause a risk of adverse effects in the aquatic environment. To assess the effect in the aquatic environment, simulations of the dilution of the substances and their transformation in the environment have been carried out in a defined exposure scenario. 9.6 Exposure scenario: LillebæltTo estimate the concentration (PEC) of the selected chemical substances, a fate model describing the degradation (biological degradation, hydrolysis, photolysis), evaporation, and sedimentation, is used. All processes are described by a first order reactions as regards substance concentration. The process descriptions have been put into a template in the modeling tool ECOLAB developed by DHI. To describe the transport of the substances the fate model is linked to a hydraulic model, which models the water flow in defined waterways. In the following example the two-dimensional model MIKE 21 has been used (concentration in depth is assumed to be distributed evenly). Lillebælt (the Danish strait of Little Belt) has been chosen as a representative exposure scenario, which describes near-shore waters in Denmark. The area covered by the model is approx. 35 km x 50 km. To ensure a kind of equilibrium a simulation period of 2 months has been used. Weather conditions observed during the first week of April 2004 were repeated in the simulation approx. 10 times. The substances are discharged to Lillebælt from 5 waste water treatment plants. Their characteristics and locations appear from table 9.5 and figure 9.1. Table 9.5 Characteristics of waste water treatment plants with discharge into Lillebælt
* M: mechanical; B: biological; N: nitrification; D: denitification; C: Chemical depositing.
Figure 9.1: Location of outlet from waste water treatment plants, Lillebælt The PEC (Predicted Environmental Concentration) of the selected chemical substances are estimated by linking the fate of the chemical substances in the waste water treatment plants and the aquatic environment with the water flow conditions in Lillebælt. The PEC values are compared with the PNEC (Predicted No Effect Concentration) values of the substances, which is the highest concentration at which no adverse effects in the aquatic environment are expected, and a risk quotient RQ (=PEC/PNEC) for the substances is estimated after discharge to the aquatic environment. During the simulation period, there are great variations in the concentrations of the chemical substances in the aquatic environment as a result of the natural variations of the water flows. In order to assess possible chronic effects, the average concentration of the substances during the simulation period has been estimated and compared with PNEC. To assess possible acute effects, the maximum concentration of the substances during the simulation period has been estimated and compared with 10PNEC, as it is generally assumed that PNEC for acute effects is a factor 10 higher than PNEC for chronic effects. The results of the simulations of the substances Cocamide DEA, 2-Bromo-2-Nitropropane-1,3-Diol and Kathon are illustrated graphically with risk quotients in the intervals RQ ≤ 0,1; RQ 0,1 - 1 and RQ ≥ 1 for the waters in Lillebælt. The area of Lillebælt where there is a risk of acute effects is found to be considerably smaller than the area where there is a risk of chronic effects. Figures 9.2-9.4 show the estimated risk quotients calculated as the ratio of the time weighted average of the estimated concentrations and PNEC.
Figure 9.2 Risk quotients for chronic effects of Cocamide DEA in Lillebælt. The red colour indicates RQ ≥ 1. The orange colour indicates RQ between 0.1-1. The green colour indicates RQ ≤ 0.1
Figure 9.3 Risk quotients for chronic effects of 2-Bromo-2-Nitropropane-1,3-Diol in Lillebælt. The red colour indicates RQ ≥ 1. The orange colour indicates RQ between 0.1-1. The green colour indicates RQ ≤ 0.1
Figure 9.4 Risk quotients for chronic effects of Kathon in Lillebælt. The red colour indicates RQ ≥ 1. The orange colour indicates RQ between 0.1-1. The green colour indicates RQ ≤ 0.1 The results of the simulations showed that risk quotients > 1 for chronic effects of Cocamide DEA were found in a considerable segment of Vejle inlet. For 2-Bromo-2-Nitropropane-1,3-Diol and Kathon, risk quotients > 1 for chronic effects were found in the immediate vicinity of the waste water outlet of Vejle waste water treatment plant. Risks of adverse effects of the substances in the aquatic environment were not detected in the remaining waters in the Lillebælt scenario. Estimates of the risk of acute effects showed that risk quotients > 1 was only found for Cocamide DEA in an area in the inner part of Vejle inlet. However, the area was considerably less compared with the estimated risk of chronic effects. Risk quotients > 1 were not found for 2-Bromo-2-Nitropropane-1,3-Diol and Kathon in Lillebælt (data not shown). The inner part of Vejle inlet is characterized by limited change of water compared with the other waste water outlets in Lillebælt. Consequently, it is not surprising that there is a larger probability of effects precisely in this area. The estimates indicate a worst-case situation with an estimated high consumption of liquid hand soaps and a maximum estimated content of the selected substances in the products. 9.7 Summary, effects in the aquatic environmentThe 3 substances selected for simulation of fate and effects in the environment are assessed to give a representative picture of possible environmental impacts in connection with the use of liquid hand soaps. In the light of the simulations carried out in Lillebælt, it can be concluded that the discharge of Cocamide DEA may cause adverse effects (both acute and chronic) in the aquatic environment in areas with waste water discharge when the area is also characterized by a limited exchange of water. Risk of chronic effects of 2-Bromo-2-Nitropropane-1,3-Diol and Kathon in the aquatic environment was found in only a limited area near the waste water outlet. The area is also characterized by a relatively low exchange of water. Risk of acute effects of 2-Bromo-2-Nitropropane-1,3-Diol and Kathon was not detected in Lillebælt. 10 Summary and conclusionThe result of the survey of liquid hand soaps in the summer of 2005 showed that for 6 of the 15 analysed products a content of fragrance chemicals reported as contact allergens was stated on the product label (both on consumer products and on products for occupational use). Furthermore, the survey showed that various preservatives reported as (potential) contact allergens are used in liquid hand soaps. The results of the chemical analyses showed that 9 of the 15 analysed products (no. 5, 6, 7, 8, 15, 21, 50, 34 and 45) contained fragrance chemicals reported as contact allergens in concentrations > 0.01%. The formulation of product 6 has been changed after the analysis were carried out so that the product no longer contains any of the 26 allergen fragrances. Product 7 is now labelled according to the regulation. Product 21 and 8 is no longer on the market. When comparing the content of the most important substances (surfactants, preservatives, and fragrance chemicals) in consumer products and products for occupational use respectively, it is evident that there is no distinctive difference in the substances in the two types of products. Both types of products contain fragrance chemicals and preservatives reported as contact allergens. Based on available data it is not possible to assess if the allergen substances occur more frequently in products used by consumers than in products for occupational use. Exposure calculations showed a very low daily exposure and a high margin of safety for both children and adults when using hand soaps, including products for occupational use. As the assessment concerns substances, which are reported as contact allergens, it can be concluded that fragrance chemicals may be hazardous to human health in particular sensitive consumers and persons with allergy. This is supported by the fact that the fragrance chemicals are subject to an obligation of labelling. According to the Danish legislation on cosmetics, which is based on the EU directive on cosmetics, the concentration of 26 fragrance chemicals reported as contact allergens must be stated on the INCI list of ingredients of the products if they occur in concentrations > 0.01 % in rinse-off products. This regulation enables particularly sensitive consumers to avoid these products and thereby to reduce the cases of allergy. Other fragrance chemicals must be stated on the product label as "parfume" or "parfum". In order to assess the environmental effects of the use of liquid hand soaps, a simulation of the environmental concentration of three selected substances (Cocamide DEA, 2-Bromo-2-Nitropropane-1,3-Diol and Kathon) was performed for Lillebælt. The results of the simulation showed that for Cocamide DEA there was a risk of effects in the aquatic environment in a considerable section of Vejle inlet, which is characterised by a limited water exchange. For 2-Bromo-2-Nitropropane-1,3-Diol and Kathon, the effect was limited to an area in the immediate vicinity of the waste water outlet in Vejle inlet. For other parts of Lillebælt, no risk of effects was predicted. The substances are thus not predicted to cause adverse effects in waters with a regular water flow. 11 References1. Miljøministeriets bekendtgørelse om kosmetiske produkter, nr. 74 af 14. januar 2005, (2005) 2. Informationscenteret for Miljø & Sundhed. Markedsscreening af flydende håndsæbe. http://www.miljoeogsundhed.dk 2005 Mar 4. Available from: http://www.miljoeogsundhed.dk/default.aspx?node=5050. 3. European Commission. Opinion Concerning Fragrance Allergy in Consumers.
A REVIEW OF THE PROBLEM ANALYSIS OF THE
NEED FOR APPROPRIATE CONSUMER INFORMATION AND
IDENTIFICATION OF CONSUMER ALLERGENS. SCCNFP.
1999 Dec 8. Available from:
http://europa.eu.int/comm/health/ph_risk/ 4. European Commission. Opinion concerning Methyleugenol adopted by
the SCCNFP during the 14th plenary meeting of 24 October 2000.
SCCNFP. 2000 Oct 24. Available from:
http://europa.eu.int/comm/health/ph_risk/ 5. Cosmetic Ingredient Review Expert Panel. Final Report on the Safety Assessment of Methyldibromoglutaronitrile. International Journal of Toxicology. 1996;15(2). 6. Larsen JR, Holmberg RD. Kortlægning af kemiske stoffer i forbrugerprodukter nr. 55, 2005. Kortlægning af læbeplejeprodukter med duft, smag m.v. Miljøstyrelsen: 2005. 7. WHO FOOD ADDITIVES SERIES. Cinnamic alcohol and related substances. 1-50. 16-5-2005. 8. Miljøministeriets bekendtgørelse om listen over farlige stoffer nr 923 af 28. september 2005, 923 af 28. september 2005 , (2005) 9. Orientering fra Miljøstyrelsen nr. 8, 2004. Listen over uønskede stoffer
2004. Miljøstyrelsen 2004. Available from:
10. Europa-Parlamentets og Rådets Direktiv 2003/15/EF af 27. februar 2003 om ændring af Rådets direktiv 76/68/EØF om indbyrdes tilnærmelse af medlemsstaternes lovgivning om kosmetiske midler. 11. European Communities, editor. IUCLID [database on the Internet]. European Communities, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [updated 2000]. Available from: http://ecb.jrc.it/esis/. 12. RTECS®: Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health, Cincinnati, Ohio. Thomson MICROMEDEX®, Greenwood Village, Colorado, USA [updated 2004]. Available from: http://csi.micromedex.com. 13. THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS (SCCNFP) INTENDED FOR CONSUMERS. Opinion concerning fragrance allergy in consumers. A review of the problem. Analysis for the need for appropriate consumer information and identification of consumer allergens. http://europa.eu.int/comm/health/ph_risk/committees/sccp/documents/out98_en.pdf 1999 Dec 8 14. Frosch PJ, Johansen JD, White IR, editors. Fragrances: Beneficial and Adverse Effects : Papers From a Thematic Symposium, London 1996. Berlin : Springer: 1998. 15. IFRA (International Fragrance Association) : Code of Practice = IFRA (International Fragrance Association) : Code De Bons = IFRA (International Fragrance Association) : Verfahrenskodex. Geneva: International Frangrance Association; 1999. 16. Rietschel RL, Fowler jr JF, editors. Fisher's Contact Dermatitis. 5th ed. Philadelphia (PA): Williams & Wilkins; 2001. 17. Council of Europe. Chemically-Defined Flavouring Substances. Strasbourg: Council of Europe Publishing; 2000. 18. Hazardous Substances Data Bank (HSDB). National Library of Medicine, Bethesda, Maryland. Thomson MICROMEDEX®, Greenwood Village, Colorado, USA [updated 2004]. Available from: http://csi.micromedex.com. 19. European Food Safety Authority (EFSA). Opinion of the Scientific Panel on Food Additivies, Falvouring, Processing Aids and Materials in Contact with Food (AFC) on a request from the Commission related to Coumarin. Question number EFSA-Q-2003-118. The EFSA Journal. 2004;104:1-36. 20. BioSite. Coumarin. http://www.biosite.dk/leksikon/coumarin.htm 2002. Available from: http://www.biosite.dk/leksikon/coumarin.htm. 21. Miljøstyrelsen. Listen over uønskede stoffer 2004. Orientering fra Miljøstyrelsen nr. 8 2004. Miljøstyrelsen 2004 22. Gangolli S, editor. The Dictionary of Substances and their Effects (DOSE) [database on the Internet]. Royal Society of Chemistry. Cambridge: Royal Society of Chemistry; 1999 [cited 2004 Nov 1]. Available from: http://www.rsc.org/dosesearch. 23. Reynolds JEF, editor. Martindale : the Extra Pharmacopoeia. 31 ed. London: Royal Pharmaceutical Society; 1996. 24. Marzulli FN, Maibach HI, editors. Dermatotoxicology. 4 ed. Washington, D.C. : Hemisphere: 1991. 25. Safety evaluation of certain food additives. Geneva: World Health Organization; Joint FAO/WHO Expert Committee on Food Additives; 1999. (WHO food additives series; 42 26. Geier J, Brasch J, Schnuch A, Lessmann H, Pirker C, Frosch P. Lyral has been included in the patch test standard series in Germany. Contact Dermatitis. 2002;46:295-7. 27. Johansen JD, Frosch P, Svedman C, Andersen KE, Bruze M, Pirker C, et al. Hydroxyisohexyl 3-cyclohexene carboxaldehylde - known as Lyral : quantitative aspects and risk assessment of an important fragrance allergen. Contact Dermatitis. 2003;48(6):310-6. 28. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) 1488/94 on Risk Assessment for Existing Substances and Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market. Part I. 2nd ed. Luxembourg: Office for Official Publications of the European Communities: European Commission, Joint Research Centre, European Chemicals Bureau, Institute for Health and Consumer Protection; 2003. 29. European Commission. The SCCNFP´s Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation. 5th Revision. SCCNFP. 2003 Oct 20. Available from: http://europa.eu.int/comm/health/ph_risk/committees/sccp/sccp_opinions_en.htm. 30. Nielsen E, Thorup I, Schnipper A, et al. Children and the unborn child. Exposure and susceptibility to chemical substances - an evaluation. Miljøstyrelsen, Miljø- og Energiministeriet; 2001. (Environmental Project No. 589 31. Lodén M. Ren, Mjuk Och Vacker - Kemi Och Funktion Hos Kosmetika. Stockholm: Apotekarsocieteten; 2003. 32. Engelund B, Höglund L, Skjødt D. Kortlægning af kemiske stoffer i forbrugerprodukter nr. 43, 2004. Kortlægning af Pletfjernere. Miljøstyrelsen: 2004. 33. Miljøministeriets bekendtgørelse om klassificering, emballering, mærkning salg og opbevaring af kemiske stoffer og produkter, nr. 329 af 16.maj 2002, (2 A.D.) 34. Environmental and Health Assessment of Substances in Household Detergents and Cosmetic Detergent Products. Environmental Project No. 615, 2001. Ministry of Environment and Energy Danish Environmental Protection Agency (2001).; 2001. 615. Environmental Project) 35. Substitution af overflade aktive stoffer i kosmetiske produkter. Arbejdsrapport Nr. 10, 2004. Miljø- og Energiministeriet. Miljøstyrelsen (2004).: 2004. 10. 36. US Environmental Protection Agency (USEPA). ECOTOX Database. http://www.epa.gov/cgi-bin/ecotox_quick_search 2005. Available from: http://www.epa.gov. 37. Punktkilder 2003 – revideret udgave. Orientering fra Miljøstyrelsen nr. 1 2005. Miljø- og Energiministeriet. Miljøstyrelsen (2005).: 2005. 1. 38. Vejledende liste til selvklassificering af farlige stoffer. Miljøstyrelsen, Miljøprojekt nr. 635, 2001 Footnotes[1] I&I = "industry and institutions", which includes industry and companies, child care centres, youth centres, and old people's homes as well as the health sector. [2] Danish statutory order on cosmetics based on the EU Cosmetics legislation Council Directive 76/768/EEC. [3] LD50: exposure dose that can be expected to cause death in 50% of the dosed animals [4] LC50: concentration of a substance in air at which half of the test animals dies [5] TDLo: lowest toxic dose; the lowest dose at which toxic effects have been observed [6] LOAEL: Lowest Observed Adverse Effect Level: the lowest dose or exposure level within a specific test system, where adverse treatment-related findings are observed. [7] NOAEL: No Observed Adverse Effect Level: the highest dose or exposure level within a specific test system, where no adverse treatment-related findings are observed. [8] intraperitoneal (i.p.) exposure: injection of substance in the abdominal cavity [9] NOEL: (No Observed Effect Level): the highest dose or exposure level within a specific test system, where no treatment-related findings are observed.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||