|
Survey of liquid hand soaps, including health and environmental assessments 6 Health assessment
6.1 Selection of substances for health assessmentWhen selecting the substances for health assessment, particular emphasis has been placed on the risk of developing allergy when considering the product content of allergen substances. Selection of fragrance chemicals for health assessment has been carried out based on the occurrence of the substances in the products and the result of the chemical analysis. The most frequently used fragrance chemicals in the products were in descending order: Linalool, Geraniol, Citronellol, Hexylcinnamaldehyde, and Lilial. Table 6.1 Content and occurrence of substances in the hand soaps
Linalool and Geraniol were assessed in connection with a previous survey for the Danish Environmental Protection Agency (6). When comparing the information on content and occurrence of substances in hand soaps, cf. table 5.6, with the previous assessments the following substances have been selected for health assessment:
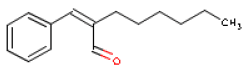
Methyldibromoglutaronitril will not be assessed as the substance was not detected in the products. Toxicological profiles of the selected fragrance chemicals have been drawn up below. Information on each substance has been found in toxicological reference books and databases as well as in scientific articles. Based on the published data a NOAEL/NOEL or LOAEL has been identified for the user exposure in chapter 7. 6.2 HexylcinnamaldehydeOccurrence and useHexylcinnamaldehyde is used as a fragrance ingredient in perfumes, often in flowery perfumes. Occur naturally in for instance boiled rice. Hexylcinnamaldehyde is the main component of jasmine fragrances. The consumption of the substance is estimated at 87 μg/person/day or 1 μg/kg body weight/day in Europe and at 11 μg/person/day or 0.2 μg/kg body weight/day in the USA (7). IdentificationHexylcinnamaldehyde is an aldehyde.
Physical-chemical properties (11)
Acute toxicityLD50 [3] values have been found to be 3100 and 4650 mg/kg body weight in rats orally exposed to Hexylcinnamaldehyde (11,12). By oral exposure of mice LD50 is approx. 2300 mg/kg body weight (12). Observed toxic effects were dose dependent and included somnolence (generally reduced activity) and effects on lungs (respiratory depression) (12). LD50 is stated as > 3000 mg/kg body weight in rabbit skin exposed to Hexylcinnamaldehyde (11). The body weight was found to be affected in rat experiments in which the animals breathed in a concentration of 2.12 mg Hexylcinnamaldehyde/L air (nominal concentration was 5.00 mg/l). At a microscopical examination 14 days after exposure enlarged bronchial lymph nodes were found, sometimes accompanied by pulmonary congestion or with many grey-green pinpoint foci in the lungs. LC50 [4] was determined at > 5 mg/L (11). Local irritationExposure to undiluted Hexylcinnamaldehyde on shaved rabbit skin for 24 hours in doses of 0.1 g and 0.5 g caused moderate to severe irritation (11,12). On guinea pig skin 0.1 g exposure to undiluted Hexylcinnamaldehyde for 24 hours caused severe irritation (12). Skin irritation or sensitization were not observed in humans, to whom 12-12.5% Hexylcinnamaldehyde in Ethanol or Petrolatum was applied repeatedly (11). AllergyHexylcinnamaldehyde is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens but less frequently reported as contact allergens. In 3 studies with 20, 119 and 179 patients respectively with cosmetics eczema, 1,1 and 7 cases of contact allergy were reported, which corresponds to 5, 0.8 and 3.9% of the patients (13). Hexylcinnamaldehyde is one of the positive control substances in OECD's guideline for animal sensitization tests (13,14). Repeated exposureNo reports on the effect of repeated oral exposure.Several reports on dermal experiments with mice. Exposure of 750 mg/kg body weight/day for 3 days caused skin sensitization. Exposure of 1800 mg/kg body weight/day for 3 days caused skin sensitization and skin inflammation. TDLo [5] in these two studies is 750 and 1800 mg/kg body weight respectively (12). Reduced growth in females, effects on the gastrointestinal tract and blood effects at the lowest dose were observed in groups of 15 rats of each sex, which were exposed dermally to 125, 250, 500 and 1000 mg Hexylcinnamaldehyde/kg body weight/day for 90 days. Irritation of the gastrointestinal tract, increased liver and kidney weight, and microscopical changes in these organs were observed at higher doses. At 1000 mg/kg body weight/day the mortality exceeded 50% (8/15). In the light of this a LOAEL [6] of 125 mg/kg body weight/day was determined (11). Hexylcinnamaldehyde is tested negative (did not cause mutations) in Ames test in doses up to 3.6 mg/plate with or without metabolic activation with S-9 (11). The substance did not cause chromosome changes in vivo in a Drosophila melanogaster test at doses of 2163 mg/L (10 mmol) (11) or in the mouse bone marrow chromosome aberration test at 657 mg/kg body weight (7). Critical effects The critical effect of Hexylcinnamaldehyde is contactallergy. Because of the sensitizing effects of Hexylcinnamaldehyde, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.2 NOAEL used for calculation of MoS for Hexylcinnamaldehyde
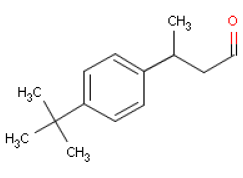
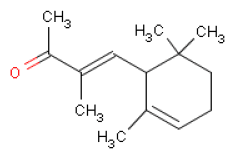
6.3 LilialOccurrence and useLilial is the trade name of a synthetically made substance that is used widely as a fragrance ingredient in perfumes, often in flowery perfumes (cyclamen, lily of the valley). IdentificationLilial is an aldehyde.
Physical-chemical properties
Acute toxicityLD50 values have been found to be 1390 mg/kg body weight in rats orally exposed to Lilial (11,12), which means the substance is hazardous to human health. LD50 values at intraperitoneal (i.p.) [8] exposure of mice is approx. 700 mg/kg body weight. Observed toxic effects included somnolence, generally reduced activity, and respiratory depression (11,12). At skin exposure of rats the LD50 value is stated at > 2000 mg/kg body weight (11) and >5000 mg/kg body weight respectively. In rats Lilial may be absorbed through the skin (19% of a dose during 120 hours) and is secreted mainly through the kidneys. The substance could not be detected in the blood 30 minutes after application of approx. 0.2 g Lilial on the skin (9 cm2). The highest concentration in the blood was measured after 60 minutes at 484 ng/mL, and after 6 hours the blood concentration went down quickly (11). Local irritationUndiluted Lilial on shaved rabbit skin for 24 hours caused moderate irritation (11). Skin irritation or sensitizing of human skin was not observed after repeated application of first 5% and later 4% Lilial in vaseline (11). Undiluted Lilial in rabbit eyes did not cause irritation (11). AllergyLilial is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens but less frequently reported as contact allergens. Two and five cases respectively of contact dermatitis have been reported in two studies of 167 og 179 patients with cosmetics eczema, which corresponds to 1.2% and 2.8% of the patients (13). In a number of guinea pig tests for the sensitizing potential of Lilial, the substance was found to be sensitizing in 5/8 studies (11). In human studies Lilial caused contact allergy in one out of 8 studies (11). Lilial did not cause photo sensitization in guinea pig studies (11). Repeated exposure Oral exposure (by stomach tube) of groups of 8 male rats of 25, 50, 100, 200 and 400 mg Lilial/kg body weight/day in sunflower oil for 5 days caused effects on the testicles, epididymes, and the sperm ducts. A NOEL [9] of 25 mg/kg body weight/day was found for this effect (11). No effects observed after oral dosing of male mice, male guinea pigs, and male rhesus monkeys with 100 mg Lilial/kg body weight/day for 5 days, nor on testicles (11). Oral exposure of pregnant female rabbits with 7020 mg/kg body weight/day on gestation day 7-19 caused changes in the skeletal muscles of the foetuses (12). No effects observed in dogs after doses of up to 200 mg/kg body weight/day (in gelatine capsules) for up to 90 days (11). In two studies male rats and male mice were exposed dermally to Lilial. A dose of 2000 mg/kg body weight/day was applied to the rat skin for 5 days causing effects on testicles, epididymes, and the sperm ducts. In the mice study, a dose of 750 mg/kg body weight/day was applied to the mice skin causing skin sensitization (12). A NOAEL of 25 mg/kg body weight/day has been determined based on a 90-day oral exposure study with rats, in which reduced choline and acetylcholinesterase in the plasma was observed but not in the brain or in the red blood cells/erythrocytes. Moreover, male rats had disorders in the spermatozoon formation (11). Lilial was tested negative (did not cause mutations) in Ames' test (11). No available data for inhalation of Lilial. Critical effects The critical effect of Lilial is contactallergy. Because of the sensitizing effects of Lilial, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.3 NOAEL used for calculation of MoS for Lilial
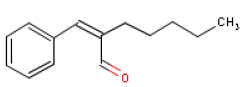
6.4 AmylcinnamalOccurrence and useAmylcinnamal is used as a fragrance ingredient in perfumes. Amylcinnamal occur naturally in for instance soy beans. Amylcinnamal is a synthetically made substance and has a jasminelike odour (16). Consumption of the substance is estimated at 25 μg/person/day or 0.42 μg/kg body weight/day in Europe and at 23 μg/person/day or 0.38 μg/kg body weight/day in the USA (7). Amylcinnamal is one of the constituents of Fragrance Mix (FM), a perfume blend used in dermal clinics for diagnosing contact allergy to fragrances. Identification Amylcinnamal is an aldehyde (17).
Physical-chemical properties (18)
WHO has determined a NOAEL for Amylcinnamal of 290 mg/kg body weight/day for male rats and 320 mg/kg body weight/day for female rats (7). Acute toxicityLD50 values have been found at 3730 mg/kg body weight/day in rats orally exposed to Amylcinnamal (12). Observed toxic effects included effects on sense organs, somnolence and generally depressed activity (12). No available data for the toxicity of Amylcinnamal after dermal exposure. Local irritation100 mg undiluted Amylcinnamal on shaved rabbit skin caused severe irritation. A 5% solution on guinea pig skin for 2 weeks caused mild irritation, while undiluted Amylcinnamal on guinea pigs for 24 hours caused moderate irritation (12). Irritation and sensitization of human skin not observed after repeated application of 20% Amylcinnamal (14). AllergyAmylcinnamal is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens and for which many reports on user allergy are available. Five cases of contact allergy are reported from two studies of 1072 and 167 patients with cosmetics eczema, which corresponds to 0.47% and 3% of the patients. The patients were exposed to 1% and 5% Amylcinnamal in Vaseline. Of 179 patients with putative cosmetics allergy, 7 (3.9 %) reacted positively when tested with 10% Amylcinnamal in vaseline (13). Amylcinnamal is a known allergen. It is part of the Fragrance Mix for diagnostic testing. The substance is responsible for 2-3% of the reactions of this blend (1.9% in Italy, 2.3% in Denmark and 2.5% in France) (13). Amylcinnamal is identified as the cause of allergic reactions in persons with fragrance cosmetic allergy. In 78 European consumers with fragrance eczema, 2.6% reacted positively when tested with 2% Amylcinnamal (13). Repeated exposureOral exposure of rats with 500 mg/kg body weight/day for 64 days caused enzyme changes in the liver and increased the liver weight (7). Groups of 15 rats were exposed through the feed to 0, 80, 400 and 4000 mg Amylcinnamal/kg feed for 14 days. In the highest dose group a statistically significant increased liver weight in male and female rats was observed, including an increased kidney weight in male rats in proportion to the body weight. Microscopical changes in livers and kidneys were not observed in the animals. Based on this a NOAEL of 290 (320) mg/kg body weight/day for male rats (female rats) was determined (7). Effects concerning growth, consumption, blood or clinical chemistry were not observed after oral exposure with Amylcinnamal through the feed of 15 rats of each sex for 90 days corresponding to 6.1 mg and 6.6 mg/kg body weight/day for male and female rats. Moreover, no toxic effects of the microscopical examination were observed after termination of the study. NOAEL was determined at 6.1 mg/kg body weight/day for male rats and 6.6 mg/kg body weight/day for female rats (7). Amylcinnamal was tested negative (did not cause mutations) in Ames' test in concentrations up 1000 μg/plate with or without metabolic activation with S-9 mixture. The substance did not cause chromosome changes in vivo in a Drosophila melanogaster test at concentrations of up to 2023 mg/L (10 mmol/L) or in the mouse bone marrow chromosome aberration test when exposed to 1213 mg/kg body weight/day (7). No available data for inhalation of Amylcinnamal. Critical effects The critical effect of Amylcinnamal is contactallergy. Because of its sensitizing effects, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.4 NOAEL used for calculation of MoS for Amylcinnamal
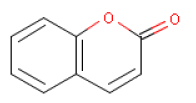
6.5 CoumarinOccurrence and useCoumarin has a pleasant odour and is used as a fragrance ingredient in perfumes and as a flavouring agent in food. The substance occurs naturally in tonka beans (seeds of Dipteris odorata) and as an ingredient in essential oils, e.g. cassia leaf oil (Chinese cinnamon, Cassia fistula) (up to 83.300 mg/kg), cinnamon leaf oil (40.600 mg/kg), cinnamon bark oil (7000 mg/kg), and in lavender and peppermint oil (7000 mg/kg) (19). Coumarin is a lactone produced by enzymatic hydrolysis of the glucoside melilotoside. Coumarin glucosides occur in plants such as woodruff (Asperula odorata), rue (Ruta graveolens), and in lovage (Levisticum officinale). Melilotoside occurs in plants such as golden melilot (Melilotus altissima) and meadow melilot (Melilotus arvensis) (20). IdentificationCoumarin is a e.
Physical-chemical properties (22)
EFSA, the European Food Safety Authority, states a NOAEL for Coumarin of 10 mg/kg bodyweight/day based on the liver toxicity of the most sensitive species, dog. A safety factor of 10 allowing for variation between the species and another safety factor of 10 for variation between individuals gives a tolerable daily intake (TDI) of 0-0.1 mg coumarin/kg body weight/day (19). Acute toxicityLD50 oral, mice, rat, guinea pig: 196-680 mg/kg body weight/day (22). Coumarin has been used in the treatment of lymphoedema. From this it is known that most humans tolerate single doses of at least 400 mg. However, a few, 17 out of 2173 patients in a clinical-toxicological study, suffered injuries on the liver after repeated dosing resulting in increased liver enzyme figures (23). No available data for acute toxicity after dermal exposure. Local irritationNo available data. AllergyCoumarin belongs to the group of fragrance chemicals which in 1999 were reported most frequently as the cause of contact allergy (13). Coumarin is not considered a photoallergen. It is only coumarin derivatives which are found to be photoallergens (24). Repeated exposureSeventeen out of 2173 patients, of which the majority received oral doses of 100 mg Coumarin daily for a month followed by 50 mg daily for two years, had increased liver enzyme figures. None of the patients had permanent injuries on the liver. In five of the 17 patients, who continued to take Coumarin, the liver enzyme figures fell to a normal level. In five studies, supported by the Lymphedema Association in Australia, 1106 patients received 400 mg Coumarin daily for an average of 14.6 months resulting in two cases of liver damage (23). Both the European Food Safety Authority (EFSA) as well as IARC have previously concluded that Coumarin is carcinogenic in rats and possibly in mice. However, in 2004 EFSA's scientific panel for food additives etc. concluded that it must be a non-genotoxic mechanism as a covalent linkage of Coumarin to DNA could not be proved in rat livers and rat kidneys (19). Critical effects The critical effect of consumption and absorption is liver toxicity. The critical effect of skin contact is contact allergy. Table 6.5 NOAEL used for calculation of MoS for Coumarin
6.6 α-IsomethyliononOccurrence and useα-Isomethylionon is used as a fragrance ingredient in perfumes, often in flowery perfumes. In addition, it is used as a flavour additive in foods. The consumption of the substance is estimated at 0.09 μg/kg body weight/day in Europe and at 0.02 μg/kg body weight/day in the USA (25). No available data on natural occurrence. Identificationα-Isomethylionon is a cyclohexanol ring with a ketone side chain.
Physical-chemical properties
WHO states a NOEL for α-Isomethylionon of < 4 mg/kg body weight/day (25). Acute toxicityLD50 values have been found to be > 5000 mg/kg body weight/day at oral exposure of rats with a mixing of Methyl-α-ionon and α-Isomethylionon. Based on data for a similar substance, β-ionon, WHO estimates that most likely α-Isomethylionon is transformed into harmless substances in the body (25). No available data for absorption of α-Isomethylionon through the skin or for inhalation of α-Isomethylionon. Local irritationNo available data concerning the irritating effect of -Isomethylionon on skin and eyes. Allergy-Isomethylionon is included on the EU scientific committee SCCNFP's list of fragrance chemicals, which are known allergens but less frequently reported as contact allergens. Three patient studies of allergy to cosmetics are described in which 2 of 179 patients (1.1 %), one of 75 patients (1.3 %) and one of 119 patients (0.8 %) showed allergic reactions to -Isomethylionon (13). Repeated exposure-Isomethylionon was administered daily to groups of 15 rats of each sex in their feed for 90 days in doses of 4 mg/kg body weight/day. No effects reported on kidneys, livers or the blood that differed from the control group or were outside normal. A NOEL was stated to be more than 4 mg/kg body weight/day (25). No available data on mutagenic effects for α-Isomethylionon. An analogous substance, Methyl--ionon, was not mutagenic in Ames' test and in tests with Drosophila melanogaster (25). Critical effects The critical effect of -Isomethylionon is allergy. Because of the sensitizing effects of α-Isomethylionon, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.6 Summary of data used for calculation of MoS for α-Isomethylionon
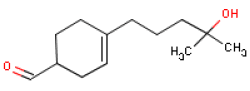
6.7 Lyral®Occurrence and useLyral® with the chemical name 4-(4-Hydroxy-4-methyl pentyl)-3-cyclohexene-1-carboxaldehyde is used as a fragrance ingredient in perfumes. Lyral® is also described as a blend of 3- and 4-(4-Hydroxy-4-methyl pentyl)-3-cyclohexene-1-carboxaldehyde. Lyral® is not a natural substance but is a synthetic fragrance chemical. IdentificationLyral® is an aldehyde.
Physical-chemical properties (12)
Acute toxicityAt oral exposures of rats to Lyral®, LD50 have been found to be 3250 mg/kg body weight. Observed toxic effects were lacrimation, strong somnolence and tremor (12). At exposure of Lyral® to rabbit skin, LD50 is 11300 mgL/kg body weight. Observed toxic effects were somnolence and changes in the structure or function of the salivary glands (12). No available data concerning inhalation of Lyral®. Local irritationThe substance is indicated to be mildly irritating and patch tests on humans have seldom shown irritation. An irritation test of 0.5 ml Lyral® on shaved rabbit skin for 4 hours showed a mild reaction (12). A study of Lyral®, 5% in petrolatum, on humans showed irritation of the skin in 4 out of 3245 patients (0.1 %) from dermal clinics (26). Application of 100 mg in rabbit eyes for an unspecified period of time caused mild irritation of the eyes (12). AllergyThe EU scientific committee SCCNFP has listed Lyral® on the list of fragrance chemicals which are known allergens and are most frequently reported as contact allergens. Lyral® has been identified as the cause of contact allergy in 2-3% of patients with eczema who were examined by lap tests. Today the substance is included in standard lap tests in many dermal clinics (27). There are several reports on Lyral® studies on patients from dermal clinics. One study of 106 patients showed a positive reaction in 3 (2.8 %) patients with Lyral®, 5% in petrolatum, and in 1 (0.9 %) patient with Lyral® 1% Clinical relevance has not been clearly demonstrated but was probably relevant in 2 patients. The last of the patients with a positive reaction may have had a skin irritation reaction (3). A study of 1855 patients with eczema who were tested with a screening series of 11 fragrance chemicals showed positive reactions in 50 (2.7%) patients with Lyral®, 5% in petrolatum, of which probably 2/3 were relevant (3). A study from 2003 has shown that Lyral® in dilutions from 6 ppm to 6% caused allergic reactions in almost all persons who were sensitized with Lyral®, and that a reduction in the use of the fragrance chemical was necessary to avoid contact allergy (27). Today, IFRA has limited the use of Lyral® to 1.5% in both leave-on products and rinse-off products, including household products such as detergents and cleaning materials (15). Repeated exposureNo available data on repeated exposure at oral administration of Lyral®. No available data on mutagenic effects, carcinogenic effects or reproductive effects of Lyral®. Critical effects The critical effect of Lyral® is contactallergy. Because of the sensitizing effects of Lyral®, persons who are allergic to the substance should avoid skin contact as there is no lower limit for its side-effects. Table 6.7 NOAEL used for calculation of MoS for Lyral®
Footnotes[3] LD50: exposure dose that can be expected to cause death in 50% of the dosed animals [4] LC50: concentration of a substance in air at which half of the test animals dies [5] TDLo: lowest toxic dose; the lowest dose at which toxic effects have been observed [6] LOAEL: Lowest Observed Adverse Effect Level: the lowest dose or exposure level within a specific test system, where adverse treatment-related findings are observed. [7] NOAEL: No Observed Adverse Effect Level: the highest dose or exposure level within a specific test system, where no adverse treatment-related findings are observed. [8] intraperitoneal (i.p.) exposure: injection of substance in the abdominal cavity [9] NOEL: (No Observed Effect Level): the highest dose or exposure level within a specific test system, where no treatment-related findings are observed.
|