Survey and health assesment of chemicals substances in sex toys
7 Health risk assessment
7.1 Introduction
In this section, potential health effects from identified and selected substances are assessed. The focus of the assessment is aimed towards adults only.
For each of the identified and quantified substances information of the substances’ identity as well as chemical and physical properties are presented. This will include data on material state, melting point, boiling point, octanol/water partition coefficient, vapour pressure and solubility.
A search in the open literature has been performed. Focus has been on the ability of skin absorption and effects by oral intake. The most important test results, the effects, and circumstances are presented. The aim has been to find data for NOAEL/LOAEL (No or Low Observed Adverse Effect Levels) for the selected substances or other relevant data if available.
Based on NOAEL or similar data and the amount of the substances it can be assessed whether the substance may cause a negative health effect from the use of the tested products.
7.2 Method
It is assumed that the substances can be absorbed in the body by oral intake and by penetration through skin and mucous membranes.
From the information on the products no special directions regarding the recommended use was given for all products. In order to assess all products and compare these, the exposed area is related to relative product size. The exposure period is based on normal use and realistic worst case per day (Table 7.1).
Regarding exposure, one common scenario has been selected.
A scenario, where product no. 2, 8, 11, 15 (Vibrators) is used in the vagina/orally and product no. 14 (Gag) is used in the mouth. It is assumed that use in vagina and mouth will be comparable to oral use. No. 10 (Artificial vagina) is used in skin contact. The exposed area and time of use depends on product as given in Table 7.1. A bodyweight of 70 kg is assumed. It is assumed that 100 percent of the substances are absorbed to the body. The exposed area in cm² is shown in the table for products and the time of exposure for normal use and worst case use.
Table 7.1 Data for products and time of exposure
| Product | 2 vibrator | 8 vibrator | 11 vibrator | 15 vibrator | 14 gag ³ | 10 Artificial vagina |
| Area of exposure (cm²) | 163 | 120 | 168 | 164 | 38 | Assumed to correspond to 150 cm² |
| Time of exposure normal use (Hours/day) ¹ | 0.0357 | 0.0357 | 0.0357 | 0.0357 | 0.033 | 0.0357 |
| Time of exposure worst case (Hours/day)² | 1 | 1 | 1 | 1 | 0.033 | 1 |
1 For normal use, product no. 2, 8, 11, 15 and 10 is used 52 times a year for 15 minutes; Product no. 14 is used 1 hour 12 times a year
2 For worst case, use product no. 2, 8, 11, 15 and 10 is used 1 hour each day; product 14 is used 1 hour 12 times a year
3 For product no. 14 the same time of exposure was assumed in normal and worst case.
The exposure scenarios are defined according to the EU's Technical Guidance Document (TGD, 2003).
The uptake is calculated as:
The exposure from the scenario is calculated by:
Intake per day per kg b.w. = [ M × A × H × F] / b.w. {equation 1}
| b.w.: | Body weight (kg) |
| M: | Migrated amount of substance (mg/cm²×h) |
| A: | Exposed skin area (cm²) |
| H: | Time of exposure per day (hours) |
| F: | Fraction absorbed |
Equation 1 can be reduced to:
Intake per day per kg body weight (mg/kg)
= M × A × F × H × 0.014 (mg/kg) {equation 2}
by using 70 kg of average body weight.
The variable M in equation is measured in the migration experiments (chapter 6). For the products 2, 8, 11, 14, 15 which is used orally the variable F is assumed to be 100 percent (TGD, 2003). For product 10 the fraction absorbed is assumed to be somewhere between skin contact and oral use. Based on a worst case scenario F is set to 100%.
In some cases the migrated amount is not available, but only analysis of content by extraction with solvent.
The dependence between migration and concentration is dependent on characteristics of the product, the chemical substance and the simulant contact medium (etc artificial sweat) and the exact dependence can only be found from experiments.
In some cases the migration of substances from materials may be explained by using Ficks law J=-D ×dc/dx where
D is diffusion coefficient of the substance
J is the flux (mole of substance per time unit)
dc/dx is the concentration difference of the substance over the diffusion distance
From Ficks law a linear relation between concentration and flux can be expected for some products.
Therefore, in order to obtain an indication of the migration for products where only the content has been measured, it is assumed that there is a linear depence between migration and concentration.
In case the migration is known for a comparable product M(2), an indicative migration can be estimated for the product M(1) as
M(1)=M(2) × C(2)/C(1) × T(2)/T(1) × A(2)/A(1)
where
M: Migrated amount of substance (mg/cm² *h)
C : Content (mg/g)
A: Exposure area (cm²)
T: Time of use (h)
There will be a considerable uncertainty in the estimate especially as the material characteristics can be different and therefore the estimate must only be used as a crude estimate of the migration.
Assessment of risk
In the health risk assessment the calculated absorption is compared with NOAEL or a similar value. As NOAEL typically is based on examinations on animals and for different periods, an uncertainty factor is used (typically a factor of 10) to bring the values at a comparable level.
An uncertainty factory of 10 is used for extrapolation between species (interspecies) and a factor 10 to protect particularly sensitive species i.e. children (intraspecies). If data are inferior or based on LOAEL, additional uncertainty factors can be used (typically a factor of 10).
In the health risk assessment, NOAEL is compared to the calculated absorption. The relation between NOAEL and the exposure (the absorption of the substance) is defined as MOS (Margin of Safety). If the data are valid a MOS of 100 will be satisfactory, whereas inferior data will require further safety factors. The overall uncertainty factor is the total product of the individual uncertainty factors.
7.3 Selected substances
The substances described in the following subsections are selected as the most significant substances for potential health risks from using these products.
7.3.1 DEHP
7.3.1.1 Identity
| Name | Bis (2-ethylhexyl)phthalate |
| CAS-number | 117-81-7 |
| EINECS number | 204-211-0 |
| Molecular formula | C24H38O4 |
| Molecular structure | 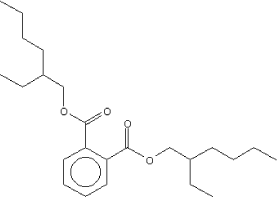 |
| Molecular weight | 390.56 |
| Synonyms | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester Bis(2-ethylhexyl) phthalate DEHP Di(2-ethylhexyl) phthalate Di-(2-ethylhexyl) phthalate Di-2-ethylhexyl phthalate Di-2-ethylhexylphthalate Di-sec-octyl phthalate Diethylhexyl phthalate Ethyl hexyl phthalate Octyl phthalate Phthalic acid, bis(2-ethylhexyl) ester |
The substance is a colourless, oily liquid. It has a boiling point of 230°C (Clayton, 1981-1982) and a melting point of -55°C (Lide, 1995-1996).
The substance is more soluble in organic solvents than in water. The solubility in water according to (Yalkowsky, 1992) is 0.285 mg/l at 24°C.
The partition coefficient Log KOW is determined to be 7.6 (Debruijin, 1989).
Vapour pressure is determined to be 7.23×10-8 mm Hg at 25°C (Daubert, 1989).
The substance has a slight odour (NIOSH, 1994).
7.3.1.2 Detected quantities
DEHP has been detected in quantitative analysis for phthalates in 8 of the 15 products. In sample no. 8, 702 mg/g has been determined and in the products 2, 3, 4, 9, 11, 14 and 15, the concentration was between 0.73 mg/g and 610 mg/g (0.07 to 61 % w/w).
In migration tests, DEHP has been detected in 5 out of 6 products with the highest value for product no. 8 (6 µg/dm²). The migration for product no.14, 15 was on the same level. The migration for product no.8 increased to 40 µg/dm² when water based lubricant was used and to 5480 µg/dm² when oil based lubricant was used. It is assumed that the increase is caused by a somewhat higher solubility of DEHP in the water based lubricant than in the extraction media (artificial perspiration solution) as the cream may have some content of emulsifiers etc. The solubility in oil based cream is much higher than in water as DEHP has a low solubility in water of 285 µg/l and a Log KOW of 7.6.
7.3.1.3 Function of substance
The function of the substance is as plasticizer.
7.3.1.4 Classifications and TLV’s
Bis(2-ethylhexyl)phthalate is included in the List of dangerous substances and classified as:
| Repr.Cat. 2;R60-61 | May impair fertility and may cause harm to the unborn child |
The Danish threshold limit value is 3 mg/m³ (The Danish Working Environment Service, 2005).
7.3.1.5 Health Effects
DEHP is in the process of being evaluated by EU in the Programme on existing chemical substances. Sweden is the rapporteur country. The risk assessment report is not yet finalised, but a draft can be found at the ECB homepage.
Data regarding health effects is included in IUCLID. The following is based on the data sheet, databases in TOXNET and the risk evaluation above.
Acute toxicity
Test for acute toxicity in animals shows that DEHP is not acute toxic.
LD50 Mouse oral >30,000 mg/kg (WHO, 1992 )
LD50 Rat oral ca. 25,000 mg/kg (WHO, 1992 )
Sub-chronic toxicity
DEHP has been shown to be a weak irritant to mammalian skin when administered topically or intradermally (0.2 mL of an emulsion of 100 g/L)
(WHO, 1992).
Data for fertility showed a statistically significant reduction of sperm for rats in all doses >250 mg/kg/day in a 15 day test. Based on this the following No Adverse Observed Effect Level was found: NOAEL<250 mg/kg/day (IUCLID).
Chronic toxicity
DEHP is classified as A3 Confirmed animal carcinogen with unknown relevance to humans (American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 2005).
Studies for carcinogenity in animals have been found in the dataset for DEHP (IUCLID).
A 2 year study was performed with continuous feeding of 50 male and 50 female Fischer 344 rats with either 400 or 800 mg/kg/d DEHP for 103 weeks. Incidents of hepatocellular carcinomas and neoplastic nodules was 3/50 (male) and 0/50 (female) in controls, 6/49 (male) and 6/49 (female) in rats exposed for 400 mg/kg/d and 12/49 (male), 13/50 (female) in rats exposed for 800 mg/kg/d. An increased level of hepatocellular tumours was also present at the two dose levels. Similar results were found for B6C3F1 mouse with doses of 375 mg/kg/d and 750 mg/kg/day for 103 weeks. (IUCLID)
According to (IARC, 2000), the mechanism by which DEHP increases the incidence of hepatocellular tumours in rats and mice is not relevant to humans and therefore the overall evaluation was: Bis (2-ethylhexyl)phthalate is not classifiable as to its carcinogenicity to humans (Group 3).
Studies on fertility effects are also reported in the data set for DEHP (IUCLID):
Effects on fertility of mouse was shown with 200 mg/kg/day oral feed and no effect was observed with 20 mg/kg/day for 105 day period experiment giving a value of NOAEL parental of 20 mg/kg/day.
A 78 day two generation test of rats with oral feed of DEHP gave a value of NOAEL parental = 95 mg/kg/day and NOAEL offspring F1 of 48 mg/kg/day.
Tests for teratogen effects on CD-1 mouse with oral feed of DEHP gave a value of NOAEL maternal toxicity = 91 mg/kg/d and NOAEL teratogen = 44 mg/kg/d from day 0-17 of gestation.
The Reference Dosis for Chronic oral exposure RfD = 0.02 mg/kg/day. (IRIS)
This value is based on a study with guinea pigs which seems to be more sensitive than rats (Carpenter, 1953). In the study, male and female guinea pigs were fed diets containing DEHP for a period of 1 year with dietary levels corresponding to 64, 19 or 0 mg/kg bw/day based on measured food consumption. No treatment-related effects were observed on mortality, body weight, kidney weight, or gross pathology and histopathology of kidney, liver, lung, spleen, or testes. Statistically significant increases in relative liver weights were observed in both groups of treated females (64 and 19 mg/kg bw/day). A LOAEL value for guinea pigs is therefore determined to be 19 mg/kg/day.
In the determination of RfD value factors of 10 each were used for interspecies variation and for protection of sensitive human subpopulations. An additional factor of 10 was used since the guinea pig exposure was longer than subchronic but less than lifetime, and because, while the RfD is set on a LOAEL, the effect observed was considered to be minimally adverse.
In the risk assessment on bis(2-ethylhexyl) phthalate (Risk assessment, 2003), a 3 generation rat guideline study is reported.
Testicular as well as developmental toxicity was found with increased incidences of small testes, epididymes, and seminal vesicles, as well as cases of minimal testes atrophy. The toxicity was aggravated by exposure during the gestational/pup-period. LOAEL was estimated to 14 mg/kg/day and NOAEL 4.8 mg/kg/day. (Wolfe, 2003). Other effects reported (Risk assessment, 2003) are kidney effects in a two year oral study in rats with NOAEL= 29 mg/kg/day.
Summary
Values for teratogenicity are given for short term studies with animals. The lowest value of NOAEL was 44 mg/kg b.w. per day based on oral feeding.
Values for fertility showed a lowest value of NOAEL 20 mg/kg/day based on oral feeding.
In the new draft for risk assessment on DEHP the value of NOAEL is 4.8 mg/kg/day for testicular and developmental effects.
Kidney effects at NOAEL of 29 mg/kg/day have also been reported.
Values for carcinogenicity for mouse and rats showed effects at approximately 400 mg/kg/day.
Values for increase in liver weight was observed in guinea pigs with a value of LOAEL =19 mg/kg/day.
7.3.1.6 Exposure scenarios
The maximum value found in the migration experiments for product 8 was 5480 µg/dm²*h corresponding to 0.055mg/cm²*h for oil based lubricant cream. With an area A = approx. 120 cm² for vibrators (no. 2,3,4,8,11,15), an area of 38 cm² for gag (no.14), and assuming 50% absorption, values for oral uptake has been calculated as shown in Table 7.2.
The value for absorption is based on data for oral absorption in adults (Risk assessment, 2003, chapter 4.1.1)
Table 7.2 Calculated and estimated intake for products
| Product no | Content (mg/g) | Migration artificial sweat (µg/dm²) | Migration oil based lubricant (µg/dm²) | Internal dose normal use (mg/kg b.w.) |
Internal dose worst case (mg/kg b.w.) |
| 2 | 0.73 | 1 | Not analysed | <0.02 1,2 | |
| 3 | 610 | Not analysed | Not analysed | <0.05 ¹ | |
| 4 | 363 | Not analysed | Not analysed | <0.05 ¹ | |
| 8 | 702 | 6 | 5480 | 0.0017 | 0.047 |
| 9 | 265 | Not analysed | Not analysed | Low | |
| 11 | 3.5 | <0.5 | Not analysed | <0.005 1,2 | |
| 14 | 176 | 6 | Not analysed | <0.0005 ³ | |
| 15 | 200 | 5 | Not analysed | <0.05 1,2 |
1 Estimate based on content, area and migration results for product no.8 in oil based lubricant
2 Estimate supplemented with migration results of this product in artificial sweat
3 Estimate based on content area and migration results for product no.8. It is assumed that migration is significantly lower when used in the mouth than in the products which may be used with oil based lubricant.
Based on the content and the migration results, the products 3, 4, 15 are assumed to give an intake within the same range as product no.8. For product no. 2, 11, 14 the intake is expected to be lower.
7.3.1.7 Assessment
DEHP is a substance that may cause reprotoxic effects including fertility or teratogenic effects in humans. Indications for other long term effects have not been found.
DEHP has been detected in 8 out of 15 samples with the concentrations from 0.07 to 70%.
Based on the NOAEL data for teratogenicity a margin of safety (MOS) is 26000 for normal use and 934 for worst case use.
Using the new data for testicular and developmental effects in (Risk assessment, 2003) MOS is 4.8/0.047 = 100 in the worst case scenario (max. use). In the risk evaluation p.296 a safety factor of 250 is recommended for pregnant women and breastfeeding women, whereas the safety factor is 100 for other adults.
Table 7.3 Estimate of margin of safety for products
| Product no. | MOS (Normal use) | MOS (worst case use) |
| 8 (oil based lubricant) | 2850 | 100 |
| 3,4,15 (oil based lubricant) | Estimate :approx. 2850 | Estimate:approx. 100 |
| 2 (oil based lubricant) | Estimate:>16000 | Estimate:>600 |
| 11 (oil based lubricant) | Estimate:>32000 | Estimate:>1200 |
| 14 | Estimate >9500 | Estimate:>9500 |
It is concluded that by worst case use of product no. 8 there is a minor risk of developmental health effects from DEHP for pregnant and breastfeeding women if oil based gliding cream is used as MOS = 100 is lower than the safety factory of 250. As to the comparable products 3, 4, and 15, the uncertainty when calculating the internal dose (thus also MOS) is higher, as the calculation of the internal dose is based on migration in oil based lubricant for product no. 8. It is estimated that the internal dose for products nos. 3, 4, and 15 will be at the same level or lower than for product no. 8 due to the lower concentration in the products and because they are made of comparable materials. Just as for product no. 8, these products contain a minor risk of developmental health effects from DEHP for pregnant and breastfeeding women if oilbased lubricant is used and in worst case scenario. For product no. 2 and 11 MOS is higher than the safety factory, why there will be no risk at maximum use.
For adults (not pregnant or breastfeeding) the health risk is less as the NOAEL values according to the risk evaluation (Risk assessment, 2003) are higher and because the safety factor used for comparison is 100. For this group it is estimated that the products 3, 4, and 15 carry no risk within the uncertainty in calculation of the internal dose.
Product no.14 (gag) is not used as frequently as the other products in worst case use and is further used without oil based lubricant. It is not known, how much the content of substances in the saliva will increase migration in comparison to artificial sweat. It is assessed that product no. 14 involves no risk.
Product no.9 (fetish) is used with skin contact and here the migration will be much lower than for vibrators which are used in vagina/anally and with or without oil based lubricant. Therefore, there is no risk with DEHP for product no. 9.
7.3.2 DNOP
7.3.2.1 Identity
| Name | Dioctyl phthalate |
| CAS-number | 117-84-0 |
| EINECS number | 204-214-7 |
| Molecular formula | C24H38O4 |
Molecular structure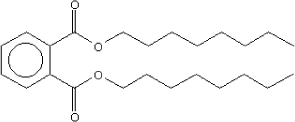 |
|
| Molecular weight | 390.56 |
| Synonyms | 1,2-Benzenedicarboxylic acid, dioctyl ester Di-n-octyl phthalate Dioctyl phthalate Phthalic acid, dioctyl ester |
The substance is a Colourless, oily liquid. It has a boiling point of 220°C (Callahan, 1979) and a melting point of -25°C. (Callahan, 1979)
The substance is more soluble in organic solvents than in water. The solubility in water according to (Wolfe, 1980) is 3 mg/l at 25°C. The value is high when compared with DEHP.
The partition coefficient Log KOW is determined to be 8.1. (Ellington, 1996)
Vapour pressure is determined to be 7.6×10-6 mm Hg at 25°C. (Perwak, 1981)
7.3.2.2 Detected quantities
DNOP has been detected in quantitative analysis for phthalates in 2 of the 15 products. In sample no. 11, 239 mg/g has been analysed and in sample no. 13, 161 mg/g.
In migration tests, DNOP has been detected from product no.11 with a value of 8 µg/dm².
7.3.2.3 Function of substance
The function of the substance is as plasticizer.
7.3.2.4 Classifications and TLV’s
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
7.3.2.5 Health Effects
The following is based on databases in TOXNET.
Acute toxicity
Test for acute toxicity in animals shows that DNOP is not acute toxic.
LD50 Mouse oral 13,000 mg/kg (International Labour Office, 1983)
LD50 Rat oral ca. 30,000 mg/kg (International Labour Office, 1983).
DNOP has shown no irritating effects at normal temperatures of use.
DNOP produces no ill effects at normal temp but may give off irritating vapour at high temperature. (Prager, 1996)
Sub-chronic toxicity
Published data shows only effects at high concentrations:
Young male Wistar rats were fed diets containing 2% di-n-octyl phthalate for one week. An increase in absolute and relative liver weights was observed. This was also observed in similar tests with other phthalates like di-n-butyl phthalate. Rats treated with di-n-octyl phthalate had decreased zinc concentrations in the tests. (USEPA/ECAO, 1980)
Studies show that there is a high cumulative toxicity as expected from the high octanol/water ratio:
The results of a study of the cumulative toxicity of different esters for the mouse revealed that di-n-octylphthalate is the compound with the highest cumulative toxicity among the eight substances tested. Results for the di-n-octyl ester were: LD50 (acute) 67.18 ml/kg; LD50 for 12 week study (different doses 5 days/week) was 3.09 ml/kg with a factor of cumulative toxicity of acute to chronic of 21.74.
(International Labour Office, 1983)
Chronic toxicity
Test for reproductive toxicity has shown no effects of DNOP even at high concentrations.
In a continuous breeding protocol CD-1 mice where given diets with DNOP in concentration 0, 1.25, 2.5 and 5% in a 7 day period prior to and during a 98 day cohabitation period. There was no apparent effect on reproductive function in the animals exposed to di-n-octyl phthalate at dose levels sufficient to cause a significant increase in liver weight. A comparison of seven phthalate esters tested using this continuous breeding protocol indicates the relative order of reproductive toxicity as diethylhexyl, dihexyl, dipentyl, dibutyl, dipropyl; diethyl and dioctyl are nontoxic. (Heindel, 1989)
Di-N-octylphthalate (DNOP) was tested using the RACB protocol in Swiss CD-1 mice as part of a structure-activity evaluation of a variety of phthalates. Body weights, food and water consumptions, and clinical signs in a dose-range-finding study were used to set doses for the main study of 0.0, 1.25%, 2.5% and 5% in feed (1.8, 3.6 and 7.5 g DNOP/kg bw/day).
There was no effect of DNOP exposure on the number of litters/pair, the mean number of live pups/litter, proportion born alive, live pup weight adjusted for litter size or days to deliver each litter. In the F1 mice, growth and viability were unaffected by DNOP consumption. Reproduction was also unaffected. The same proportion of treated and control mice mated, and bore live litters.
Epididymal sperm concentration and motility were unchanged by DNOP exposure at 5%. The single finding of a slight reduction in F1 seminal vesicle weight is interesting in light of current concerns about second-generation reproductive toxicity, but needs confirmation. Overall, these data show that, at doses that induced significant hepatomegaly, DNOP was without any adverse reproductive effect in Swiss mice.
(Department of Health,1985)
Summary
Studies have shown that there are no reprotoxic effects even at doses of 7500 mg DNOP/kg bw/day in animals.
No evidence for carcinogenity was found.
Studies show a high cumulative toxicity with a factor of 22 from acute effects to chronic effects.
Based on the lowest LD50 value for mice =13000 mg/kg and studies of cumulative toxicity, chronic toxicity effects is expected at less than 13000/22 = 600 mg/kg in mice.
From the studies the lowest values for chronic effects will probably be effects on the liver like seen for DEHP. The information in the studied data has not been sufficient to find a NOAEL for chronic liver effects. However, from the similarity of DEHP and DNOP the value may be in the same range.
7.3.2.6 Exposure scenarios
When comparing with DEHP a similar migration in oil based cream is expected.
From this a worst case uptake of approx. 0.05 mg/kg b.w. is expected.
7.3.2.7 Assessment
DNOP is a plasticizer which has shown less reprotoxic effects in studies than DEHP.
As with DEHP and other phthalates there will be effects on the liver weight.
Based on the estimated LOAEL value for mice for liver effects of 600 mg/kg, a margin of safety (MOS) = 12000 can be calculated for the worst case.
The NOAEL value for liver effects is expected to be lower and may be based on a level comparable to DEHP i.e. 20 mg/kg/day.
Table 7.4 Estimates margin of safety for products
| Product no. | MOS (normal use) | MOS (worst case use) |
| 11,13 (oil based lubricant) | Estimate:>5600 | Etimate:400 |
Within the considerable uncertainty when estimating the internal dose and NOAEL for liver effects from the comparable substance DEHP, MOS will be >400, and it is thus concluded that there will be no risk in using products nos. 11 and 13 in the worst case scenario and if used with oil based lubricant.
7.3.3 Cyclohexanone
7.3.3.1 Identity
| Name | Cyclohexanone |
| CAS-number | 108-94-1 |
| EINECS number | 203-631-1 |
| Molecular formula | C6H10O |
Molecular structure |
|
| Molecular weight | 98.14 |
| Synonyms | Cyclohexyl ketone Ketohexamethylene |
The substance is an oily liquid. It has a boiling point of 155.6°C (Budavari, 1989) and a melting point of -31°C (Lide, 1994-1995).
The substance is soluble in water and polar media like ethanol, acetone and ethyl ether. The solubility in water is 50 g/l at 30°C.
(Lide, 1994-1995).
The partition coefficient Log KOW is determined to be 0.81 (Hansch, 1995).
Vapour pressure is determined to be 5 mm Hg at 26.4°C (The Merck Index, 1983).
The substance has an odour with a reminiscent of peppermint and acetone (Budavari, 1989).
7.3.3.2 Detected quantities
The substance is detected by headspace analysis in 9 of the 15 products. In sample no. 3 , the highest value of 58 µg was found in headspace after 180 min. Sample no 2 and 8 gave comparable levels in headspace (51 and 39 µg) where the other samples gave values at least 40 times lower.
Extraction with dichloromethane showed cyclohexanone in 6 samples with values in sample 2,3,8,15 from 0.5 to 2.5 mg/g sample.
In migration tests cyclohexanone is detected in 3 of 6 products. The highest migration was for product no.2 with 1320 µg/dm², followed by product no.8 with 1000 µg/dm² and no.15 with 382 µg/dm².
7.3.3.3 Function of substance
The function of the substance is as solvent in the production process.
7.3.3.4 Classifications and TLV’s
Cyclohexanone is included in the list of dangerous substances and classified as:
| R10 | Flammable |
| Xn;R20 | Harmful by inhalation |
The Danish threshold limit value is 40 mg/m³. The substance is marked H for penetration through skin.
7.3.3.5 Health Effects
Data regarding health effects is included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Test for acute toxicity on animals for cyclohexanone shows a low acute oral toxicity:
LD50 Mouse oral 1600-3200 mg/kg (IUCLID)
LD50 Rat oral ca. 1300-2700 mg/kg (IUCLID)
LC50 Rat inhalation 10-32 mg/l, 4 hours (IUCLID)
According to (Farm Chemicals Handbook 87, 1987) vapours of cyclohexanone may irritate mucous membranes and contact with the liquid may produce dermatitis in sensitive individuals.
Allergic contact dermatitis to cyclohexanone resin has been reported (IARC, 1989).
Chronic toxicity
Cyclohexanone is classified as A3 Confirmed animal carcinogen with unknown relevance to humans (American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 2005).
Studies reported in IUCLID for teratogeniticity (inhalation) did not show any effect on mice in the range up to 1400 ppm (5.6 mg/l).
A two-generation study with 6 hours daily exposure by inhalation showed effects on fertility. No effects were observed for1000 ppm (4.1 mg/l) for the first generation and for 500 ppm (2mg/l) for the second generation.
The reference dose for chronic oral exposure RfD=5 mg/kg b.w./day (IRIS).
The RfD value is based on a chronic bioassay study with cyclohexanone which was conducted with F344 rats and B6C3F1 mice. Cyclohexanone was administered as a solution in the drinking water. Rats were dosed at 3300 or 6500 ppm levels, male mice at 6500 or 13,000 ppm, and female mice at 6500, 13,000 or 25,000 ppm levels. Each treatment group consisted of 52 animals/sexes (males and females) both mice and rats. Survival and weight gain were similar to the controls in both sexes of either species treated with the lowest dosage of cyclohexanone, but weight gain was depressed at all of the higher doses. Female mice treated with both the higher doses (13,000 or 25,000 ppm) and male mice treated with the high dose (13,000 ppm) exhibited increased mortality as compared with controls. 50% of the females treated with 25,000 ppm cyclohexanone survived beyond 1 year. Based on these effects, the 3300 ppm level dose of cyclohexanone (converted to 462 mg/kg/day) in rats is considered as the NOAEL value, whereas the high dose (6500 ppm or 910 mg/kg/day) that causes decreased body weight gain was considered the LOAEL in rats. (Lijinsky, 1986). In the determination of RfD an uncertainty factor of 100 was applied; 10 for interspecies extrapolation and 10 for intraspecies variability among the human population.
Summary
Cyclohexanone is an animal carcinogen with unknown relevance to humans.
There is some data on effects on fertility by inhalation.
It may produce allergic contact dermatitis in sensitive individuals.
The NOAEL for chronic effects seen as decreased body weight gain by intake with drinking water has been estimated to 462 mg/kg/day in rats.
7.3.3.6 Exposure scenarios
The maximum value found in the migration experiments for product no. 2 was 1320 ug/dm2 *h, i.e. M = 0.013 mg/cm²*h. With an area A =163 cm² and assuming 100% uptake values for intake has been calculated as shown in Table 7.5.
Table 7.5 Calculated and estimated intake for products
| Product no | Extraction Dichloromethane (mg/g) |
Migration artificial sweat (µg/dm²) |
Intake normal use (mg/kg b.w.) |
Intake worst case (mg/kg b.w.) |
| 2 | 2.02 | 1320 | 0.001 | 0.03 |
| 3 | 2.5 | Not analysed | 0.001 ¹ | 0.03 ¹ |
| 8 | 0.51 | 1001 | 0.001 | 0.02 |
| 15 | 1.5 | 382 | 0.0003 | 0.009 |
1 Based on extraction in dichloromethane
All other products are expected to give much lower values based on headspace analysis.
7.3.3.7 Assessment
Based on the data for chronic oral exposure with a NOAEL of 462 mg/kg/day a marginal of safety (MOS) is more than 420000 for normal use and 15000 for worst case use.
The ratio between RfD and the calculated intake is 162 in the worst case scenario.
Use of either water or oil based gliding cream with better solubility characteristics for substances with low water solubility is not expected to increase the migration significantly of cyclohexanone as log KOW =0.81 and cyclohexanone therefore should have a good solubility (50 g/l) in the used migration medium.
It is concluded that there is no health effects for cyclohexanone based on the data for chronic oral exposure and the observed migration. However, it must be mentioned that the compound in 2005 has been classified as a confirmed animal carcinogen with unknown relevance for humans and that the compound may produce allergic contact dermatitis in sensitive individuals.
7.3.4 2-Ethylhexanoic acid
7.3.4.1 Identity
| Name | 2-ethylhexanoic acid |
| CAS-number | 149-57-5 |
| EINECS number | 205-743-6 |
| Molecular formula | C8H16O2 |
Molecular structure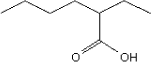 |
|
| Molecular weight | 144.22 |
| Synonyms | 2-Ethylhexanoic acid 2-Ethylhexoic acid Ethyl hexanoic acid, 2- Hexanoic acid, 2-ethyl- |
The substance is a clear liquid. It has a boiling point of 228°C (Lide, 1995-1996).
The substance is more soluble in organic solvents than in water. It is soluble in ethyl ether, carbon tetrachloride and slightly soluble in ethanol. The solubility in water is 1.4 g/l at 25°C (Ashford, 1994).
The partition coefficient Log KOW is determined to be 2.64 (Hansch, 1995).
Vapour pressure is determined to be 0.03 mm Hg at 20°C (Flick, 1991).
The substance has a mild odour (Flick, 1991).
7.3.4.2 Detected quantities
2-Ethylhexanoic acid was detected in headspace from product 1 and 4.
The substance is detected in 9 of the 15 products when extracted with dichloromethane. Sample no. 4 showed the highest value with 14.1 mg/g sample. The samples 1, 3, 8, 11, 12, 13, 14 and 15 gave values from 0.16 to 3.1 mg/g.
In migration tests 2-ethylhexanoicacid was found in 3 of 6 products. The highest migration was for product no.11 with 423 µg/dm² followed by product no.8 with 220 µg/dm² and no.15 with 113 µg/dm².
7.3.4.3 Function of substance
The function of the substance is as stabiliser for PVC products.
7.3.4.4 Classifications and TLV’s
2-Ethylhexanoic acid is included in the list of dangerous substances and classified as:
Repr.cat.3;R63 Possible risk of harm to unborn child
No Danish threshold limit value for the substance has been found.
7.3.4.5 Health Effects
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Tests for acute toxicity on animals show that 2-ethylhexanoic acid has a low acute toxicity by ingestion.
- LD50 Rat oral 1,600-3,000 mg/kg (Clayton, 1993-1994)
- LD50 Rabbit oral 1,300 mg/kg (Clayton, 1993-1994)
The pure substance is harmful if swallowed, inhaled or absorbed through the skin and is extremely destructive to tissues of mucous membranes and upper respiratory tract, eyes, and skin. (Prager, 1996)
Some results on rabbits in the IUCLID data set show the component is irritating, other not.
Subchronic toxicity
Data in HSDB and IUCLID report teratogenic effects of 2-ethylhexanoic acid.
Results with continuous administration in drinking water for Wistar rats up to day 20 of gestation show skeletal malfunctions in offspring like clubfoot, absence of fibula etc. for doses from 100 mg/kg/day and above. The number of affected foetuses was control: 2.4%, 100 mg/kg/day: 4.9%, 300 mg/kg/day: 8.9% and 600 mg/kg/day: 15.3%. The NOAEL for teratogenic effects was set to 100 mg/kg/day.
The developmental toxicity of 2-ethylhexanoic acid was studied in animals treated by gavage with doses 0, 100, 250, 500 mg/kg bw/day on gestation day 6-15 for rats and with doses 0, 25, 125, 250 mg/kg bw/day on gestation day 6-18 for rabbits. The results suggest that 2-ethylhexanoic acid induces developmental toxicity in rats only at doses that cause maternal toxicity. 2-Ethylhexanoic acid causes maternal toxicity in rabbits without affecting foetal development. The no observable effect levels for maternal and developmental toxicity in rats are 250 and 100 mg/kg, respectively. The no observable effect levels for maternal and developmental toxicity in rabbits are 25 mg/kg and 250 mg/kg or more.
(Hendrickx, 1993)
Data is also reported in IUCLID for fertility effects for rats with 100, 300 or 600 mg/kg/day added in drinking water with a premating exposure of 10 weeks for male and 2 weeks for female. The result was a value of NOAEL parental = 300 mg/kg/day and NOAEL offspring = 100 mg/kg/day.
No data was found for carcinogenic or sensitising effects.
Summary
2-Ethylhexanoic acid is a substance that may cause reprotoxic effects including fertility or teratogenic effects in humans. Indications for other long term effects have not been found.
Values for teratogenic effects in rats gave NOAEL = 100 mg/kg bw/day.
Values for fertility effects in rats gave NOAEL = 100 mg/kg/day whereas values for developmental toxicity in rabbits was NOAEL = 25 mg/kg b.w. per day.
7.3.4.6 Exposure scenarios
The maximum value found in the migration experiments for product 11 was 423 ug/dm2 *h i.e. M=0.00423mg/cm²*h. With an area A =168 cm² and assuming 100% uptake values for intake has been calculated as shown in Table 7.6.
Table 7.6 Calculated and estimated intake for products
| Product no | Extraction Dichloromethane (mg/g) |
Migration artificial sweat (µg/dm²) |
Intake normal use (mg/kg b.w.) |
Intake worst case (mg/kg b.w.) |
| 1 | 3.1 | Not analysed | ||
| 3 | 2.4 | Not analysed | ||
| 4 | 14.1 | Not analysed | 0.0036 ¹ | 0.1 ¹ |
| 8 | 0.5 | 220 | ||
| 11 | 1.47 | 423 | 0.00036 | 0.01 |
| 12 | 1.12 | Not analysed | ||
| 13 | 0.55 | Not analysed | ||
| 14 | 0.16 | |||
| 15 | 1.5 | 113 |
1 Based on extraction in dichloromethane
When looking at the results of analysis by extraction with dichlorometane, a 10 times higher concentration is found in product 4 (14.1 mg/g) than in product no.11 (1.47 mg/g). This may lead to 10 times increased migration results for product no.4 if analysed.
7.3.4.7 Assessment
Based on a subchronic study with a NOAEL = 25 mg/kg/day for developmental toxicity in rabbits and the highest measured migration of product no. 11, the margin of safety (MOS) is more than 69000 for normal use and 2500 for worst case use.
For product no.4 the migration may be up to 10 times higher which gives a value of MOS of 250.
Use of either water or oil based gliding cream with better solubility characteristics for substances with low water solubility is only expected to increase the migration of 2-ethylhexanoic acid a little as log KOW = 2.64 and the substance therefore have some solubility (1.4 g/l) in the used migration medium.
Table 7.7 Estimates margin of safety for products
| Product no. | MOS (Normal use) | MOS (worst case use) |
| 4 | 6900 | 250 |
| 11 | 69000 | 2500 |
Based on the data above and the uncertainty regarding a possible increased migration in oil based lubricant, as well as a safety factor of 1000 for data from a subchronic study it is concluded that there may be a minor risk of developmental effects from 2-ethylhexanoic acid for product no. 4 by worst case use and by use of oil based lubricant, and no risk involved with the products 1, 3, 8, 11, 12, 13, 14 and 15.
7.3.5 3,3'-Oxydipropiononitril
7.3.5.1 Identity
| Name | 3,3'-Oxydipropiononitril |
| CAS-number | 1656-48-0 |
| EINECS number | 216-750-9 |
| Molecular formula | C6H8 N2O |
| Molecular structure |
|
| Molecular weight | 124.14 |
| Synonyms | Propanenitrile, 3,3'-oxybis- Propionitrile, 3,3'-oxydi- 2-Cyanoethyl Ether |
The substance is a colourless liquid. It has a boiling point of 143°C. (Safety datasheet, 2000)
The solubility in water is high with 900 g/100 ml water indicating a negative Log KOW (Safety datasheet, 2000)
7.3.5.2 Detected quantities
The substance is detected in migration experiments with product 14 at a value of 50 µg/dm².
7.3.5.3 Function of substance
The function of the substance is not known.
7.3.5.4 Classifications and TLV’s
3,3'-Oxydipropiononitrile is not included in the list of dangerous substances
and there is no Danish threshold limit value.
7.3.5.5 Health Effects
Data regarding health effects is very limited with no information in HSDB and IUCLID. There is some limited data in ChemID.
Acute toxicity
Test for acute toxicity on animals shows that 3,3'-Oxydipropiononitril
is not acute toxic.
LD50 Rat oral 2800 mg/kg (AMA Archives, 1954)
LDlo rabbit skin 4200 mg/kg (National Technical Information Service)
A draize test on rabbit has shown mild irritant effects on eye and skin at 500 mg/24 h (Marhold, 1986).
Sub-chronic toxicity
No tests were found.
Chronic toxicity
No tests were found.
Summary
Very limited data exists for this compound with data for acute toxicity of older date. Data suggests that the substance is irritating for eyes and skin.
7.3.5.6 Exposure scenarios
The maximum value found in the migration experiments for product 14 was 50 ug/dm2 *h i.e. M=0.0005 mg/cm²*h. With an area A =38 cm² and assuming 100% uptake the following worst case value can be calculated:
Table 7.8 Calculated intake
| Product no | Migration artificial sweat (µg/dm²) | Intake worst case (mg/kg b.w.) |
| 14 | 50 | 0.009 |
7.3.5.7 Assessment
Very limited data exists showing a low acute toxicity and some irritating effects.
Based on the acute toxic values any chronic effects in humans is expected a factor 1000 lower which means that NOAEL may be around 2 mg/kg.
Based on this the margin of safety (MOS) is 221000.
The migration is not expected to increase significantly when using water or oil based cream as the substance is very water soluble.
It is concluded that there is no health effects of the substance based on the present knowledge.
7.3.6 Phenol
7.3.6.1 Identity
| Name | Phenol |
| CAS-number | 108-95-2 |
| EINECS number | 203-632-7 |
| Molecular formula | C6H5OH |
Molecular structure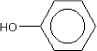 |
|
| Molecular weight | 94.1 |
| Synonyms | Carbolic acid Hydroxybenzene Phenyl alcohol |
The substance is colourless acicular crystals or white, crystalline mass.
It has a boiling point of 181.8°C and a melting point of 40.9°C (Kirk-Othmer, 1996).
The substance is very soluble in alcohol, chloroform, ether, glycerol, carbon disulfide, petrolatum, volatile and fixed oils, and aqueous alkali hydroxides. The solubility in water is 82.8 g/l at 25°C (Southworth, 1986).
The partition coefficient Log KOW is determined to be 1.46 (Hansch, 1995).
Vapour pressure is determined to be 0.03 mm Hg at 25°C (Lide, 2002-2003)
The substance has a mild odour (Flick, 1991)
7.3.6.2 Detected quantities
The substance is detected in headspace in 6 of the 15 products with the highest concentration in product no.11. Results of extraction with dichloromethane gave a value of 3.5 mg/g for product 11 with values for product no. 3, 4, 8, 13 and 15 larger than 0.19 mg/g.
In migration tests, phenol has been detected in 5 out of 6 products with the highest value for product no.11 (866 µg/dm²) followed by product no 15 and 8.
7.3.6.3 Function of substance
The function of the substance is not known but it may be a residue from the production process.
7.3.6.4 Classifications and TLV’s
Phenol is included in the list of dangerous substances and classified as:
| T;R23/24/25 | Toxic by inhalation, in contact with skin and if swallowed |
| Xn;R48,20/21/22 | Harmful: danger of serious damage to health by prolonged exposure through inhalation, in contact with skin and if swallowed |
| C;R34 | Causes burns |
| Muta. cat.3; R68 | Possible risk of irreversible effects |
The Danish threshold limit value is 4 mg/m³ and it is marked with H for penetrable through skin.
7.3.6.5 Health Effects
Data regarding health effects is included in IUCLID. The following is based on the data sheet and databases in TOXNET and (Nilsson, 2004).
Acute toxicity
Phenol is classified as toxic by skin contact and by ingestion and the substances is also classified corrosive (R34).
Phenol is toxic with a lethal dose of 50-500 mg/kg for humans. Some persons can be hypersensitive with serious effects or death caused by exposure to even lower doses.
Tests for acute toxicity on animals shows:
LD50 Rat oral 530 mg/kg (O'Neil, 2001)
LD50 Rat dermal 669 mg/kg (Lewis, 1996)
LD50 Mouse oral 270 mg/kg (Lewis, 1996)
LD50 Mouse i.v. 112 mg/kg (Lewis, 1996)
LD50 Cat oral 100 mg/kg (Verschueren, 1983)
Chronic toxicity
Phenol is included in the IUCLID database from 2000. From the data set comes the following information. Tests show that phenol is not a sensitizer. In a 28-days test with mice were shown that oral intake cause effects on red blood cells and on the level of antibodies in the blood. LOAEL was estimated to 1.8 mg/kg body weight.
Phenol is not recognised as a carcinogen (IARC, group3) based on insufficient evidence for both humans and animals (IARC, 1999)).
In a test with rats (Argus Research Laboratories, 1997) the effects on the development of the offspring was analysed. A NOAEL of 60 mg/kg per day was estimated. A benchmark dose level (BMDL) of 93 mg/kg was calculated.
Another developmental and reproductive toxicity study gave NOAEL = 70 mg/kg/day for male rats and 93 mg/kg/day for female rats
Based on these studies, the BMDL value and by including a safety factor of 300 the reference dose was estimated to:
RfD = 0.3 mg/kg/day (IRIS database).
Summary
Phenol produces developmental effects in offspring from rats with a NOAEL value of 60 mg/kg/day.
The lowest effect level however is on blood cells and antibodies with LOAEL = 1.8mg/kg/day for mice.
7.3.6.6 Exposure scenarios
The maximum value found in the migration experiments for product 11 was 866 ug/dm2 *h i.e. M=0.00866mg/cm²*h. With an area A =168 cm² and assuming 100% uptake values for intake has been calculated as shown in Table 7.9
Table 7.9 Calculated and estimated intake for products
| Product no | Extraction Dichloromethane (mg/g) |
Migration artificial sweat (µg/dm²) |
Intake normal use (mg/kg b.w.) |
Intake worst case (mg/kg b.w.) |
| 2 | 0.02 | 26 | ||
| 3 | 0.7 | Not analysed | 0.004 ¹ | |
| 4 | 0.9 | Not analysed | 0.005 ¹ | |
| 8 | 0.19 | 182 | 0.004 | |
| 11 | 3.5 | 866 | 0.0007 | 0.021 |
| 13 | 1.06 | Not analysed | 0.006 ¹ | |
| 14 | 0.07 | 55 | ||
| 15 | 0.7 | 264 | 0.006 |
1 Based on extraction in dichloromethane
The migration from the products 8,15 was approximately 25% of this value.
Based on extraction in dichloromethane, the products 3,4,8,13,15 are assumed to give an intake comparable within a decade.
7.3.6.7 Assessment
Phenol is a substance which has shown developmental and immunotoxic effects in animals and is classified as having a risk of irreversible effects (mutagenic) in humans.
Based on the data for LOAEL on effects on blood cells and antibodies in mice a margin of safety (MOS) is more than 2700 for normal use and 96 for worst case use.
The ratio between RfD and the calculated intake is 14 in the worst case.
Use of either water or oil based gliding cream with better solubility characteristics for substances with low water solubility is only expected to increase the migration of phenol a little as log KOW = 1.46 and the substance therefore have some solubility (83 g/l) in the used migration medium.
Table 7.10 Estimates margin of safety for products
| Product no. | MOS (normal use) | MOS (worst case use) |
| 11 | 2700 | 96 |
| 3,4,8,13,15 | >9450 | >280 |
It is concluded that there are minor health risk effects from phenol by max. use of product no. 11 and a possible minor risk with the products 3, 4, 8, 13, and 15, considering the uncertainty in connection with estimating intake and migration in the relevant migration liquid.
7.3.7 Carbon disulfide
7.3.7.1 Identity
| Name | Carbon disulphide |
| CAS-number | 75-15-0 |
| EINECS number | 204-843-6 |
| Molecular formula | CS2 |
| Molecular structure |
|
| Molecular weight | 76.14 |
| Synonyms | Carbon bisulphide Dithiocarbonic anhydride |
The substance is a clear, colourless or faintly yellow liquid. It has a boiling point of 46°C (Lide, 1995-1996) and a melting point of -111.5°C (Lide, 1995-1996).
The substance is more soluble in organic solvents than in water. The solubility in water according to (Yalkowsky, 1992) is 2860 mg/l at 25°C.
The partition coefficient Log KOW is determined to be 1.94 (Hansch, 1995).
Vapour pressure is determined to be 359 mm Hg at 25°C (Yaws, 1994).
The odour of carbon disulphide depends on grade. Commercial grades have a foul smelling whereas pure distillates have a sweet, pleasing and etherial odour (Budavari, 1996).
7.3.7.2 Detected quantities
Carbon disulphide is detected in 7 of the 15 products in headspace with the highest concentration from product no.5 (458 ng). In all the products 3, 4, 5, 6, 7, 9 and 14 the concentration in headspace was larger than 110 ng.
In migration experiments carbon disulphide was only detected for product no.14 with 500 µg/dm².
7.3.7.3 Function of substance
The function of the substance is a pyrolysis product from dithiocarbamate based sulphur accelerators used in the vulcanisation of rubber.
7.3.7.4 Classifications and TLV’s
Carbon disulphide is included in the list of dangerous substances and classified as:
| F;R11 | Highly flammable |
| Xi;R36/38 | Irritating to eyes and skin |
| T;R48/23 | Toxic: Danger of serious damage to health by prolonged exposure through inhalation |
| Repr. Cat. 3;R62-63 | Possible risk of impaired fertility and of harm to the unborn child |
The Danish threshold limit value is 15 mg/m³ and it is marked with H which means that the substance can be absorbed through the skin.
7.3.7.5 Health Effects
Data regarding health effects is included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Test for acute toxicity on animals shows that carbon disulphide is not acute toxic.
- LD50 Mouse oral >2,780 mg/kg
- LD50 Rat oral 3,188 mg/kg
- LD50 Rat inhalation 27 g/m³, 2 hr (Lewis, 1996)
The substance is severely irritating to eyes, skin and mucous membranes and skin sensitization may occur (Sittig, 1985).
Chronic toxicity
The substance is a potent nerve toxin. Chronic long-term exposures may result in elevated blood cholesterol, retinopathy, peripheral neuropathy, decreased glucose tolerance, reduced serum thyroxine levels, and parkinsonism. (Ellenhorn, 1988)
Long-term exposure to levels in excess of 20 ppm may result in atherogenic and diabetogenic changes (Ellenhorn, 1988).
Adverse effects of carbon disulfide exposure on reproductive function have been reported in exposed workers, with significantly lower sperm counts and more abnormal spermatozoa than in unexposed control subjects.
(Rom, 1992)
Studies on reproductive effects include a study on rats and rabbits which showed no effect when the animal was exposed to 20 ppm or 40 ppm which was estimated to correspond to oral doses of 5 or 10 mg/kg for rats and 11 or 22 mg/kg for rabbits (Hardin, 1981).
The reference dosis for chronic oral exposure RfD = 0.1 mg/kg/day (IRIS).
The value is based on a NCTR-NTP oral study (Jones-Price, 1984 a,b).
In which was observed 25 mg/kg/day in rabbits as an FEL (foetal resorption). Foetal malformations in this study were not observed in rats at the lowest level (100 mg/kg/day) of carbon disulphide exposure. The data from this study also suggest that the rabbit foetus is more sensitive than the rat foetus to carbon disulphide induced toxicity. These data were supplemented with an epidemiologic study by (Johnson, 1983) to support a NOAEL of 11 mg/kg for foetal toxicity/malformations from carbon disulphide oral exposure. By using an uncertainty factor of 100 (10 for interspecies and 10 for intraspecies variation) the RfD value was derived.
Summary
Carbon disulphide is irritating to eye and skin.
Chronic effects of carbon disulphide include possible risk of impaired fertility and of harm to the unborn child and long term nerve toxic effects
7.3.7.6 Exposure scenarios
The maximum value found in the migration experiments for product 14 was 500 ug/dm2 *h i.e. M=0.005mg/cm²*h. With an area A =38 cm² and assuming 100% uptake values for intake has been calculated in Table 7.11
For other products with vagina/anal use, carbon disulphide was also found in headspace. The exposed area was 4 times higher for product no. 3, 4 and 7. As these products are used more frequently in worst case, the intake shown in Table 7.11 has been calculated by assuming the same migration as for product no.14.
For the product no. 5, 6 and 9 the uptake will be through skin. The area of the products is large and the products are applied in a way where carbon disulphide cannot evaporate easily.
Assuming a worst case scenario with a whole body suit of area 20000 cm²and 100% penetration through skin, values for intake has been calculated in Table 7.11. As time of exposure for worst case use was assumed a use of 7 hours each week (1 hour/day) and for normal use 3 hours each month.
Table 7.11 Calculated and estimated intake for products
| Product no | Headspace analysis (ng) | Migration artificial sweat (µg/dm²) | Intake normal use (mg/kg b.w.) |
Intake worst case (mg/kg b.w.) |
| 3 (vibrator) | 111 | Not analysed | 0.00036 ¹ | 0.01 ¹ |
| 4 (vibrator) | 121 | Not analysed | 0.00036 ¹ | 0.01 ¹ |
| 5 (fetish) | 458 | Not analysed | ||
| 6 (fetish) | 405 | Not analysed | ||
| 7 (vibrator) | 179 | Not analysed | 0.00036 ¹ | 0.01 ¹ |
| 9 (fetish) | 455 | Not analysed | ||
| 14 (gag) | 138 | 500 | 0.00009 | 0.00009 |
| Fetish whole body coverage | 0.14 ² | 1.4 ² |
1 Estimate based on headspace analyses and migration results from product no.14.
2 Estimate based on headspace analyses of product no 5,6,9,14 and migration results from product no.14
7.3.7.7 Assessment
Carbon disulphide is a substance that may cause reprotoxic effects and nerve toxic effects. Indications for other long term effects have not been found.
Carbon disulphide has been detected in migration experiments from 1 sample (Gag).
Based on the data for foetal toxicity a margin of safety (MOS) is 121000 and a ratio between RfD and the calculated intake is 1100.
For the products 3, 4, 7 (vibrators) the margin of safety can be estimated to MOS= 963 in the worst case and the ratio between RfD and the calculated intake is 9.
For use of fetish products, a calculation has been made for a whole body coverage and by assuming an intake through skin comparable to oral uptake.
This gives MOS =8 for a worst case scenario with a use of 1 hour each day and RfD=0.07.
Table 7.12 Estimates margin of safety for products
| Product no. | MOS (Normal use) | MOS (worst case use) |
| 3,4,7 | 27000 | 963 |
| 14 | 121000 | 121000 |
| Fetish products like no.5,6,9 with whole body coverage | 80 | 8 |
It is concluded that there is no health risk with exposure of the given levels of carbon disulphide for product no.14. Regarding products 3, 4, and 7 there may be a minor risk by max. use, when the uncertainty regarding estimation of intake based on migration from a quite different material (no. 14) and the uncertainty regarding migration in the actual migration fluids are considered.
For fetish products like no. 5,6,9 the estimations suggest there is probably a risk of health effects, especially if the products are used for longer periods covering a large part of the body.
7.3.8 Tetrahydrofuran
7.3.8.1 Identity
| Name | Tetrahydrofuran |
| CAS-number | 109-99-9 |
| EINECS number | 203-726-8 |
| Molecular formula | C4H8O |
| Molecular structure |
|
| Molecular weight | 72.11 |
| Synonyms | Tetramethylene oxide 1,4-Epoxybutane Butane, 1,4-epoxy- Butane, alpha,delta-oxide Butylene oxide Cyclotetramethylene oxide Diethylene oxide Furan, tetrahydro- Furanidine Oxacyclopentane Oxolane |
The substance is a colourless, mobile liquid. It has a boiling point of 65°C (Lide, 2000) and a melting point of --108.3°C (Lide, 2000).
The substance has a solubility of 30% in water 25°C (International Labour Office, 1983)
The partition coefficient Log KOW is determined to be 0.46 (Hansch, 1995).
Vapour pressure is determined to be 162 mm Hg at 25°C (Daubert, 1989).
The substance has an ether like odour (Budavari, 1996).
7.3.8.2 Detected quantities
The substance is detected in 8 of the 15 products in headspace with the highest concentration for product no.8 (5384 ng) and product 2,3,15 at comparable levels. The rest of the products gave more than a decade lower headspace concentrations.
In migration experiments tetrahydrofuran was detected in product 8,15 with 12 µg/dm² in both products.
7.3.8.3 Function of substance
The function of the substance is a solvent for PVC.
7.3.8.4 Classifications and TLV’s
Tetrahydrofuran is included in the list of dangerous substances and classified as:
| F;R11-19 | Highly flammable; May form explosive peroxides |
| Xi;R36/37 | Irritating to eyes and respiratory system |
The Danish threshold limit value is 148 mg/m³.
7.3.8.5 Health Effects
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Test for acute toxicity on animals shows that tetrahydrofuran is not acute toxic.
- LD50 Rat oral 1,650 mg/kg
- LD50 Mouse ip >1,900 mg/kg (Lewis, 1996)
- LC50 Rat inhalation =21,000 ppm/ 3hr
Tetrahydrofuran is a strong irritant to skin and mucous membranes (Gosselin, 1984).
Chronic toxicity
Tetrahydrofuran is classified as A3 Confirmed animal carcinogen with unknown relevance to humans (American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 2005).
Tetrahydrofuran can cause dermatitis on prolonged exposure (Mackison, 1981).
Two inhalation studies for 105 weeks with rats and mouse with doses 0.6, 1.8 and 5.4 mg/l (200, 600, and 1800 ppm) is reported in the IUCLID data set. It was found that there was some evidence for carcinogenic activity in the two high doses for male rats (increased renal tubule epithelial adenoma and carcinomas). For female mouse, the incidents of hepatocellular neoplasms (adenoma and carcinoma) was significantly greater (85%) for high doses than in control groups and was evaluated as clear evidence. Based on this the NOAEL level for mice, rats is around 600 ppm (1.8 mg/l).
In a two generation study on Wistar rats, developmental toxicity in the form of reduced pup growth and delayed eye opening were noted at tetrahydrofuran doses administered with water at 782 mg/kg/day but not at 305 mg/kg/day (IUCLID).
Summary
New evaluations of results from 2005 have classified tetrahydrofuran as a confirmed carcinogen for animals with unknown relevance to humans.
Indications for other long term effects have not been found.
Data for carcinogenic effects by inhalation giving a NOAEL value around 600 ppm.
No data was found for oral uptake. Assuming a 100% uptake of tetrahydrofuran by inhalation and orally, the NOAEL can be roughly estimated as NOAEL (oral) = NOAEL (inhalation) * LD50 (oral)/LC50( inhalation) =600*1650/21000=47 mg/kg/day.
7.3.8.6 Exposure scenarios
The maximum value found in the migration experiments for product 8 and 15 was 12 µg/dm2 *h i.e. M=0.00012 mg/cm²*h. With an area A =164 cm² and assuming 100% uptake values for intake has been calculated in Table 7.13
Table 7.13 Calculated and estimated intake for products
| Product no | Headspace analysis (ng) |
Migration artificial sweat (µg/dm²) |
Intake normal use (µg/kg b.w.) |
Intake max. use (µg/kg b.w.) |
| 2 | 2163 | <0.5 | <0.001 | <0.03 |
| 3 | 4521 | Not analysed | 0.01 | 0.3 |
| 8 | 5384 | 12 | 0.01 | 0.28 |
| 15 | 2330 | 12 | 0.01 | 0.28 |
Based on the head space analyses and migration analyses, the product 3 is assumed to give an intake in the same range as for no. 8 and 15 whereas no. 4, 5, 6 and 7 would be at least a decade lower.
7.3.8.7 Assessment
Based on the estimated NOAEL of 47 mg/kg/day the margin of safety (MOS) is 4.7 mill. for normal use and 167000 for worst case use.
Use of either water or oil based gliding cream with better solubility characteristics for substances with low water solubility is only expected to increase the migration of tetrahydrofuran a little as log KOW = 0.46 and the substance therefore have some solubility (300 g/l) in the used migration medium.
Table 7.14 Estimates margin of safety for products
| Product no. | MOS (Normal use) | MOS (worst case use) |
| 3,8,15 | 4700000 | 167000 |
It is concluded that there is no health risk of carcinogenic effects with the observed concentration of tetrahydrofurane.
However, it must be mentioned that the compound may produce allergic dermatitis in prolonged exposure.
7.3.9 Trimethyltin chloride
7.3.9.1 Identity
| Name | Trimethyltin chloride |
| CAS-number | 1066-45-1 |
| EINECS number | 213-917-8 |
| Molecular formula | C3H9ClSn |
| Molecular structure |
|
| Molecular weight | 199.26 |
| Synonyms | Stannane, chlorotrimethyl- Trimethylchlorostannane Trimethylchlorotin Trimethylstannyl chloride |
The substance consists of colourless needles. It has a boiling point of 154-156°C (Kirk-Othmer Encyclopedia of Chemical Technology, 1997) and a melting point of 37.5°C (Kirk-Othmer Encyclopedia of Chemical Technology, 1997).
The substance is soluble in chloroform and other organic solvents (Lide, 1994) and is miscible with water (Kirk-Othmer Encyclopedia of Chemical Technology, 1997).
7.3.9.2 Detected quantities
Trimethyltin chloride was only detected in sample no. 2 with a value of 0.04mg/kg
The migration experiment confirmed this with a detected amount of 99 µg/dm².
7.3.9.3 Function of substance
The function of the substance is probably as a PVC stabilizer as triethyltin chloride is used for this purpose
7.3.9.4 Classifications and TLV’s
Trimethyltin chloride is not included in the list of dangerous substances and no Danish threshold limit value exists.
7.3.9.5 Health Effects
Data regarding health effects is not included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Trimethyltin chloride is highly neurotoxic according to the literature (Seiler, 1988).
Test for acute toxicity on animals shows that trimethyltin chloride is acute toxic.
- LD50 Mouse intravenous >1,8 mg/kg (Lewis, 1996)
- LD50 Rat oral ca. 12 mg/kg (Lewis, 1996)
- LD50 Rat ip. 7.45 mg/kg (Lewis, 1996)
From the values it can be seen that trimethyltin chloride should have a classification for acute toxicity as Tx/R28 if included on the hazardous substance list.
Intoxication incidents in a chemical company where 6 workers where exposed to trimethyl chloride showed a number of neurotoxic symptoms 2-3 days after exposure including loss of hearing, difficulties in finding right words etc. The symptoms grew worse with one death caused by cerebral edema, pulmonary edema and kidney failure. The intoxication resulted in permanent damage for two patients (Seiler, 1988).
Sub-chronic toxicity
The neurotoxic effects of trimethyltin chloride have been studied in a number of references.
Comparison of tri-organotin compounds showed that trimethyltin chloride and triethyltinchloride was neurotoxic whereas tri-n-propyl chloride and n-butyltin chloride induced a dose related decrement in weights of thymus and spleen. Tri-n-propyltin chloride and triphenyltin chloride were immunotoxic
(Snoeij, 1985).
In another study, effects of trimetyltin chloride were studied in mice and rats. The effect on rats with a dose of 7.5 mg/kg was much less than on mice with a dose of 3 mg/kg. Generative changes in brain stem neurons and spinal cord were observed in all mice 3-5 days after the dosage (Chang, 1983).
Trimethyltin chloride was given to Syrian hamsters, gerbils and marmosets and changes in the brain were studied from day 1 to 7 weeks later. The signs of poisoning included whole-body tremors and prostration. Within the marmoset brain, trimethyltin chloride was found to be uniformly distributed, similar to that in the rat. In all three species, signs of poisoning included whole-body tremors and prostration, while death might occur in 3-4 days. In Marmosets ataxia, agitation, aggression and occasional fits were also observed. Bilateral symmetrical neuronal necrosis and chromatolysis were seen in the majority, which involved the hippocampus, pyriform cortex, amygdaloid nucleus, neocortex, various brain stem nuclei and in marmosets the retina. The lethal dose for the three species was around 3 mg/kg which is lower than for rats. This is explained by a partial binding of trimethyltin chloride to haemoglobin in rats which is not found for the studied species and human haemoglobin. Therefore, the lethal dose for humans is expected to be around 3 mg/kg. The dose required to produce neuronal damage will be less (Brown, 1984).
Trimethyltin chloride treatment produced severe and permanent damage in the central nerve system which was characterised by neuronal necrosis rather than intramyelinic edema. Neuronal degeneration and necrosis was observed in rats treated with 15 ppm trimethyltin acetate in the diet for 2 weeks. Similar results were reported in rats orally exposed to trimethyltin chloride in a single dose at 10 mg/kg, in multiple doses at 4 mg/kg/week for 4 weeks and at 1 mg/kg/day for 2 weeks.
(Boyer, 1989)
The data indicates that trimethyltin chloride accumulates which is also found in tests of the clearance half-time in brain and blood concentration which has been estimated to at least 16 days (Friberg, 1986).
Developmental effects were studied in THA rats with 0, 5, and 7 mg/kg single dose injected on gestational day 12. In Sidman avoidance test, the avoidance rate of the treated offspring rats was lower when compared to the controls. This result suggests that prenatal trimethyltin chloride administration disrupts learning ability (Miyake, 1989).
A 56 days' study (The U.S. Department for Health and Human Services, 2005) states a LOAEL value of 0.05 mg/kg regarding significant drop in the learning ability of rats with oral intake through drinking water. Further it states a LOAEL of 0.8 mg/kg for neurological effects on rats by continuous oral intake over 25 days.
For tributylinchloride the same reference determines a NOAEL for developmental effect of 0.025 mg/kg and chronic effect also of 0.025 mg/kg for rats.
Chronic toxicity
Trimethyltin chloride is classified as A4; Not classifiable as a human carcinogen (American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices, 2005).
Summary
Trimethyltin chloride is a substance that may cause neurotoxic effects in animals and humans. Indications for other long term effects have not been found.
Values are only given for acute toxic effects in short term studies with animals. The lowest value was 1.8 mg/kg b.w. per day for LD50 intravenous in mouse.
Data for LD50 (oral) in a single dose are low with values around 2-3 mg/kg for the most sensitive animals (mouse and hamsters). The human LD59 (oral) is expected to be in the same range.
Neuronal degeneration and necrosis was observed at multiple oral doses of 1 mg/kg/day.
Data for long-term chronic effects (neurotoxic) by long-term continuous exposure are not found.
A subchronic study shows significant neurological effects on rats with LOAEL of 0.8 mg/kg and another study shows effects on the learning ability with LOAEL = 0.05 mg/kg on rats.
Trimethyltin chloride will accumulates in the body at daily exposure as the clearance half-time is approximately 16 days.
A NOAEL value for neurotoxic effects has not been found.
7.3.9.6 Exposure scenarios
The maximum value found in the migration experiments for product 2 was 99 µg/dm2 *h i.e. M=0.00099mg/cm²*h. With an area A =163 cm² and assuming 100% uptake the following intake has been calculated as seen in Table 7.15.
Table 7.15 Calculated and estimated intake for products
| Product no | Extraction Dichloromethane (mg/g) |
Migration artificial sweat (µg/dm²) |
Intake normal use (mg/kg b.w.) |
Intake worst case (mg/kg b.w.) |
| 2 | 0.04 | 99 | 0.000082 | 0.002 |
7.3.9.7 Assessment
The margin of safety (MOS) of the expected LD50 value for humans is 24000 for normal use and 867 for worst case use.
Based on the subchronic LOAEL value 0.05 mg/kg for learning ability effect on rats, a MOS of 0.05/0.002=25 at maximum use and MOS=610 at normal use can be calculated.
For the subchronic LOAEL value for neurological effects on rats, the safety margins were calculated to MOS=400 at maximum use and MOS=9800 at normal use.
A uncertainty factor of 1000-10000 may be expected at extrapolation to humans originating from a factor 10 for interspecies extrapolation, 10 for intraspecies variation in humans and a factor 10-100, as it is both a LOAEL value and a subchronic study. A NOAEL value of 0.025 mg/kg for the related substance trimethyltin chloride indicates that a safety factor of 1000 is satisfactory.
It is not known whether use of oil based or water based cream will increase the migration which will further increase the risk.
Table 7.16 Estimates margin of safety for products
| Product no. | MOS (Normal use) | MOS (worst case use) |
| 2 | 10 ¹ | 0.36 ¹ |
1 NOAEL =0.8 µg/kg/day is estimated from LD50 as explained above
It is concluded that for product no.2 there is a health risk of developmental effects (learning ability) on progeny and a minor risk of neurological effect on adults by worst case use. By normal use there may be a risk of developmental effecs within the uncertainty factor.
7.3.10 Other components
Toluene was found in a number of products.
The health effects of toluene has been assessed in (Nilsson, 2004) and only the data necessary for assessment is shown here.
7.3.10.1 Identity
| Name | Toluene |
| CAS-number | 108-88-3 |
| EINECS number | 203-625-9 |
| Molecular formula | C7H8 |
Molecular structure |
|
| Molecular weight | 92.14 |
| Synonyms | Benzene, methyl- |
The boiling point of toluene id 110.6°C.
Toluene is more soluble in organic solvents than water with a partition coefficient Log KOW of 2.73 and solubility in water of 526 mg/l, 25°C.
7.3.10.2 Detected quantities
Toluene is detected in 14 of the 15 products in headspace. The highest concentration is for product no.4. Migration in dichloromethane shows 2.1 mg/g from sample 4, 1.3 mg/g from no 15, 0.3 mg/g from no.3 and 0.26 mg/g from sample no.2. The rest is much lower.
In migration experiments toluene was detected in 3 of 6 products with 54 µg/dm² for product 15 and comparable levels for product no. 2 and 8.
7.3.10.3 Function of substance
The function of the substance is as solvent in the production process.
7.3.10.4 Classifications and TLV’s
Toluene is included in the list of dangerous substances:
| F; R11 | Highly flammable |
| Repr. Cat.3; R63 | Possible risk of harm to unborn child |
| Xn; R48/20-R65 | Harmful: danger of serious damage to health by prolonged exposure through inhalation, may cause lung damage if swallowed |
| Xi; R38 - R67 | Irritating to skin, vapours may cause drowsiness and dizziness |
7.3.10.5 Health Effects
A reference dose for chronic oral exposure has been calculated in IRIS with the newest revision in September 2005.
The value is based on a 13 week rat gavage study with changes in liver and kidney weight as critical effect giving a LOAEL of 446 mg/kg/day. A benchmark approach gave BMD=238 mg/kg/day. Based on this value and an uncertainty factor of 3000 the following value calculated (IRIS):
RfD= 0.08 mg/kg/day.
7.3.10.6 Exposure scenarios
The maximum value found in the migration experiments for product 15 was 54 µg/cm2 *h i.e. M=0.00054 mg/cm²*h. With an area A =164 cm² and assuming 100% uptake the following intake has been calculated as seen in Table 7.17.
For the products 5, 6 and 9 the uptake will be through skin. The area of the products is large and the products are applied in a way where toluene cannot evaporate easily. Products 5 and 6 gave a concentration in headspace which was 30 lower than for product no. 15.
Assuming a worst case scenario with a whole body suit of area 20000 cm²and 100% penetration through skin, values for intake has been calculated in Table 7.17. As time of exposure for worst case use was assumed a use of 7 hours each week (1 hour/day) and for normal use 3 hours each month.
Table 7.17 Calculated and estimated intake for products
| Product no | Head space analysis (ng) | Extraction Dichloromethane (mg/g) | Migration artificial sweat (µg/dm²) | Intake normal use (mg/kg b.w.) |
Intake worst case (mg/kg b.w.) |
| 2 (vibrator) | 28121 | 0.26 | 22 | 0.00002 | 0.0006 |
| 3 (vibrator) | 27505 | 0.3 | Not analysed | <0.000045¹ | <0.0012¹ |
| 4 (vibrator) | 165332 | 2.1 | Not analysed | <0.00009¹ | <0.0024¹ |
| 5 (fetish) | 1016 | Not analysed | |||
| 6 (fetish) | 705 | Not analysed | |||
| 8 (vibrator) | 15821 | 0.04 | 38 | 0.00003 | 0.0009 |
| 9 (fetish) | 147 | Not analysed | |||
| 12 (vibrator) | 1745 | 0.02 | Not analysed | ||
| 14 (gag) | 80 | 0.03 | |||
| 15 (vibrator) | 29320 | 1.3 | 54 | 0.000045 | 0.0012 |
| Fetish whole body coverage | 0.0005 | 0.005 |
1 Based on headspace data and data for extraction in dichloromethane
7.3.10.7 Assessment
Based on the bench mark value of 238 mg/kg/day the margin of safety MOS can be calculated to 188000 for product 15.
The ratio between RfD and the calculated intake is 63 in the worst case.
However in product no.4, the migration seems to be the double from extraction in dichloromethane giving MOS=90000 in the worst case.
For use of fetish products a calculation has been made for whole body coverage and by assuming an intake through skin comparable to oral uptake
This gives MOS =46000 with a ratio between RfD and the calculated intake of 15 for worst case use.
Use of either water or oil based gliding cream with better solubility characteristics for substances with low water solubility is only expected to increase the migration of tetrahydrofuran to a minor extent as log KOW =2.73 and the substance therefore have some solubility (0.5 g/l) in the used migration medium.
Table 7.18 Estimates margin of safety for products
| Product no. | MOS (Normal use) | MOS (worst case use) |
| 4 | 2521000 | 90000 |
| Fetish products like no.5,6,9 with whole body coverage | 460000 | 46000 |
It is concluded that there is no health effects from toluene.
7.4 Overall Assessment
7.4.1 Substances
In Table 7.19 the found health effects of the evaluated substances are shown
Table 7.19 Effects of evaluated substances
| Substance | Irritating and sensitizing effects | Reprotoxic effects | Carcinogenic effects | Mutagenic effects | Neurotoxic effects |
| DEHP | R60-61 (may impair fertility/ cause harm to unborn child) | ||||
| DNOP | |||||
| Cyclohexanone | Irritating to mucous membranes /risk of contact dermatitis in sensitive individuals | A3 confirmed animal carcinogen-unknown relevance in humans | |||
| 2-Ethylhexanoic acid | R63 possible harm to unborn child | ||||
| 3,3'oxydipro- piononitrile |
|||||
| Phenol | R68 possible risk of irreversible effects | ||||
| Carbon disulphide | R36/R38 Irritating to eyes and skin Other: irritating to mucous membranes | R62-63 possible risk of impaired fertility/ harm to unborn child | |||
| Tetrahydrofuran | R36/37 Irritating to eyes and respiratory system Other: irritating to skin and mucous membranes | A3 confirmed animal carcinogen-unknown relevance in humans | |||
| Trimethyltin chloride | Produces permanent neurotoxic effects. Expected classification as Tx;R28 | ||||
| Toluene | R38 Irritating to skin | R63 possible risk of harm to unborn child |
In the following an overview of the evaluations of the substances in section 7.3 is given. Data in the table are given for the sample with the highest determined concentration of the actual substance.
Table 7.20: Toxic effects for selected substances in sex toys
| Substance | Max Uptake mg per kg b.w. |
NOAEL mg/kg b.w. per day |
MOS (worst case use) | RfD/MaxUptake | Remarks |
| DEHP | 0.094 | 4.8 | 100 | 0.4 | Pregnant/breastfeeding women, max. use, oil based lubricant, developmental/testicular health effects. Minor risk (products nos. 3,4,8,15. |
| DNOP | 0.1 | 19 | 200 | No value | No risk of health effect, but MOS is subject to uncertainty as it is based on data for DEHP (Estimate based on similarities. |
| Cyclohexanone | 0.03 | 462 | 15000 | 162 | No risk of health effects |
| 2-Ethylhexanoic acid | 0.1 | 25 | 250 | No value | Possible minor risk of health effects for product no.4 by max use, oil based lubricant. |
| 3,3'oxydipro- piononitrile |
0.009 | 2 | 221000 | No value | No risk of health effect based on very few data. |
| Phenol | 0.02 | 2 | 96 | 14 | Minor risk of health effects (product no. 11) by max. use. Possible minor risk (products 3, 4, 8, 13, and 15) by max. use. |
| Carbon disulphide | 0.01 (vibrator) | 11 | 963 (vibrator), 8(fetish) | 9 (vibrator), 0.07 (fetish) | Possible minor risk of health effects for product no.3,4,7 and probably a risk by products 5,6,9 when a large part of the body is covered and by max. use. |
| Tetrahydrofuran | 0.28 | 47 | 167000 | No value | No risk of health effects |
| Trimethyltin chloride | 0.002 | 0.0008 | 0.36 | Risk of developmental effects and minor risk of neurological effects on adults by max. use. Minor risk of developmental effect by normal use. | |
| Toluene | 0.0024 | 238 | 90000 (vibrator) 46000 (fetish) | 63 | No risk of health effects |
7.4.2 Products
Table 7.21 contains the assessment of the health effect of each product.
Table 7.21: Identified health effects for products
| Product no | Type | Health effect Normal use ³ |
Health effect use Max. use ³ |
| 1 | Dildo | None | None |
| 2 | Dildo | Minor risk pregnant/breast feeding (trimethyltin chloride) | Risk for pregnant/breastfeeding, minor risk other adults (trimethyltin chloride) |
| 3 | Dildo | None | Minor risk for pregnant/breastfeeding (DEHP)² , possible minor risk (phenol, carbon disulphide) |
| 4 | Dildo | None | Minor risk for pregnant/breastfeeding (DEHP)² , possible minor risk (phenol, carbon disulphide, 2-ethylhexane acid) |
| 5 | Dress | None¹ | Possible minor risk ¹ |
| 6 | Gloves | None ¹ | None ¹ |
| 7 | Dildo | None | Possible minor risk (carbon disulphide) |
| 8 | Dildo | None | Minor risk for pregnant/breastfeeding (DEHP) ², possible minor risk (phenol) |
| 9 | Patent leather top | None ¹ | None¹ |
| 10 | Art. Vagina | None | None |
| 11 | Dildo | None | Minor risk (phenol) |
| 12 | Dildo | None | None |
| 13 | Dildo | None | Possible minor risk (phenol) |
| 14 | Gag | None | None |
| 15 | Dildo | None | Minor risk for pregnant/breastfeeding (DEHP) ², possible minor risk (phenol) |
| 16 | Dildo | None | None |
1: Calculations indicate a risk of reprotoxic health effects from carbon disulphide exposure when using closed bodysuits for prolonged periods. No risk when using items which cover only a small part of the body, products No. 6, 9. There may be a minor risk with product No. 5 by max. use.
2: The risk is dependant on the use of lubricant cream; however, the risk is reduced by use of water-based lubricant cream.
3. Normal use of dildos and artificial vaginas has been determined to be once a week for 15 minutes. Max. use 1 hour per day. Gag is used for 1 hour per month by normal and by max. use. Fetish products (Nos. 5, 6, 7) are used for 3 h/month by normal use and 7 h/week by max. use.
As to the dildos it should be mentioned that the migration of DEHP in waterbased lubricant cream was 100 lower than in oil based lubricant cream, however, 8 times higher than in synthetic sweat. Thus the waterbased lubricant reduces the risk of health effects of substances as DEHP and DNOP with a very low degree of water solubility. The conditions applying to vaginal, anal and oral are expected to a certain degree to differ from the synthetic sweat, and expelled fluids as e.g. saliva will presumably increase the migration of substances with a low degree of water solubility such as DEHP.
Overall, only 7 products, nos. 1, 6, 9, 10, 12, 14 and 16, contained no health risks in worst case use but product no. 16 contains cadmium above the allowed level and is therefore not allowed on the European market.
Product no. 2 should not be used by pregnant/breastfeeding women due to its content of trimethyltin chloride which may cause irreversible neurotoxic effects (brain damage) to progeny. Additionally, there is a minor risk of neurotoxic effects to adults in worst case.
The other products, apart from product no. 2, involve no health risks by normal use.
Version 1.0 September 2006, © Danish Environmental Protection Agency