Survey of chemical substances in consumer products no. 78, 2006
Survey and health assessment of chemical substances in massage oils
Contents
- 5.1 Analysis results
- 5.2 Conclusion of analysis results
- 5.3 Selected substances for safety assessment
Enclosure 1. Natural occurrence and application
Summary and conclusions
In the spring of 2005, DTC has assessed 49 typical massage, baby and body oils as well as essential oils for self-mixing. 28 of the products were purchased. The majority of the products is marketed to adults and only baby oils are regarded as meant for children.
Massage, baby and body oils as well as essential oils are in a grey area as to which legislation applies to the products. To determine the existing legislation it is necessary to carry out an overall assessment of several factors for each type of product such as point of sale, target group, claims, labelling, packaging and the general appearance. If the product is assessed neither to be a cosmetic nor a medicine, if for instance it is assessed to be used mainly for well-being, it is assumed that the legislation on chemical substances and products applies and this determines the guideline directives for classification and labelling of the product. Irrespective of the existing legislation, all products are applied to the skin and must not be hazardous to human health when used.
An assessment of all the factors has been made for each of the purchased products., This assessment was made according to EU’s guidance on borderline products. The assessment show that 15 products are covered by the cosmetic legislation. The remaining 13 are covered by the legislation on chemicals substances and products and due to this covered by the rules of classification and labelling. Among other things this means that there are specific rules regarding labelling of sensitising substances.
An evaluation of the purchased 28 products illustrates that the producers of massage- baby- and body oils in most cases follow the cosmetics regulations governing the INCI list of ingredients.
According to the products' list of ingredients, massage, baby and body oils on the Danish market are primarily composed of non-volatile and volatile oils. Other substances are found in very limited amounts. Only a few products are based solely on mineral oils and other additives such as pigments and preservatives.
The result of the chemical analysis of 16 massage, baby and body oils and essential oils (7 massage oils, 2 baby oils, 3 body oils and 4 essential oils) showed that 15 of the products contain one or more sensitizing fragrances. One of the 4 products analysed for methyleugenole contained a concentration of the substance 8 times the legal maximum limit for methyleugenole in a cosmetic leave-on product. One product was analysed for content of Peru balm and several of the subcomponents of Peru balm were detected. This indicates presence of the substance which is unwanted in cosmetic products. Safrol and methylsalicylate were not found in the analysed products.
The EU limit for labelling of 26 fragrances allergens in leave-on cosmetics is 0.001 percentage by weight. A large part of the analysed products (94%) contains one or more of the 26 fragrance allergens in a concentration higher than 0.001 percentage by weight and should therefore be declared on the product.
For chemical products the regulation states that if the product contains more than 0,1 % of a sensitizer the product must be labelled: ”Contains (chemical name). May cause allergy”.
Safety/toxicological profiles were prepared for the substances benzylcinnamate, cinnamal, citral, citronellol and Peru balm, which were all present in one or more of the analysed products. In addition, safety/toxicological profiles from previous surveys have been used for assessment of user exposure.
Exposure scenarios were drawn up for two average models (adult, baby) of the found fragrance allergens (a total of 19) and the user exposure was assessed. The result showed that the largest concentration of fragrance allergens is to be found in products for adults. For the fragrance linalool a safety margin (Mos) below 100 was found which indicates a health risk. In addition, the safety assessment showed that perfumed massage oils for adults should not be used on babies.
Two baby oils were tested. In one of the products the content of d-limonen was so high that the product received a safety margin below 100. Based on this the product is assessed as presenting a health risk to babies. However, as only two baby oils have been analysed the number is too small to conclude that the concentration of fragrance allergens in baby oils is a general health concern.
There is no lower concentration limit concerning the sensitizing effects of the substances. Consequently, several of the products in the survey might present a risk of developing allergy in consumers who are especially sensitive. Likewise there a risk that consumers with perfume allergies might experience irritation, eczema or the likes when using the products.
1 Preface
This survey comprises massage oils to be marketed for physical well-being and to babies.
Massage oils, including baby oils are applied to a bigger or smaller area of the skin and are not intended to be washed off. Massage oils are both products to be applied to the whole body and products to be applied to specific areas of the body, e.g., areas with cellulites and stretch marks. As massage oils are applied to the skin and may have a skin caring effect, the regulations of the cosmetics legislation are generally relevant for these products. If the oil is marketed as a healing and palliative product, the regulations for pharmaceuticals may be the regulations to observe. Massage oils for muscular pains and physical well-being often contain essential oils or plant extracts. For example, cinnamon, peppermint and camphor are well-known heat-providing substances in this type of product, and e.g. lemon oils are often applied because of their fresh fragrance. Erotic massage oils are often perfumed and are marketed to be applied in sexual areas with thin and highly vascularizated skin, with sliding properties, taste or giving a warm feeling. If the product is considered to be neither a cosmetics nor a pharmaceutical, e.g. if it is used only to obtain the feeling of well-being, the legislation on chemical substances and products prevails and give advice on classification and labelling of the product. Irrespective of the regulation, the products are all applied to the skin and must not be hazardous to human health when used.
A risk evaluation of massage oils must take possible content of unwanted components and sensitizing fragrance substances into consideration. Because of the similarity between massage oils and cosmetic products it is relevant to make a survey of the product group and to examine, if the products meet the safety requirements on cosmetics.
Many of the 26 fragrance allergens requiring labelling in accordance with Attachment 3 of the Danish Statutory order of Cosmetics (1) are found in popular fragrances and in natural form in various plants- extracts/oils. High amounts of essential oils in massage oils may therefore be assumed to contain fragrance allergens, which can be a health risk for the consumer.
To clarify which fragrance substances and particularly which oils are applied in massage oils to day, this project carries out a market survey as well as an assessment of the possible health effects the product may have on the user.
Labelling of the products and possible claims will also be treated in the report.
2 Objective
The objective of the project on survey and safety assessment of chemical substances in massage oils is
1. to prepare a survey of the market for massage oils including market share and quantitative amounts of massage oils to be applied for physical well-being in adults and babies.
2. to examine which ingredient (including fragrance substances/active substances and preservatives are applied, and to perform a safety assessment of selected ingredients with regard to target groups and site of application.
3. to examine which types of oils are applied in massage oils.
4. to examine the labelling and possible claims of the products.
The result of the project is an assessment of the user’s exposure to possible hazardous substances when applying massage oils as well as recommendations to precautionary measures.
3 Results of the survey
3.1 Delimination and selection of products
Massage oils are marketed for many different purposes and cover a broad line of products sold in retail stores, chains of shops and on the internet.
As the survey mainly focuses on products for general comfort and to babies, products marketed particularly for erotic purposes and for muscular pains- and soreness are not treated.
With respect to products marketed to babies, the survey focuses on oil and not necessarily only on massage oil. This means that bathing oil is also included in the survey.
3.1.1 Delimination of massage oils in the project
Massage oils in this survey is defined as oil-based products to be applied to the skin to contribute to the well-being of the consumer.
Massage oils may be products for general application to the complete body or products applied to specific areas of the body, e.g., areas with cellulites and stretch marks in the skin.
A number of oils with the designation ‘body oils’ were found in the shops. These oils are marketed as a type of body lotion to be applied to dry skin, e.g. just after bathing. As these products are marketed for the well-being of the consumer, they are assessed to be applied for massage too, and are therefore also included in the survey. Upon request, many shops presented ‘body oils’ as oils suitable for massage. In advertising material it has been seen, that some body oils are also marketed as massage oils.
Essential oils are also included in the survey as they can be used as massage oils when mixed with basic oil.
The Information Centre for Environment & Health has performed a safety assessment of seven massage oils marketed to children. These products are not included in the survey (2).
3.1.2 Type of products
The limit between massage and body oils seems to be very vague. Both massage and body oils can be applied directly to the skin and their ingredients seem to be identical. Essential oils, however, should not be applied directly to the skin, but must be mixed with a basic oil before application. Instructions are given accordingly.
3.1.2.1 Massage oils
Massage oils for physical well-being are marketed as massage oils. They may be pure oils or contain a very complex oil phase based on either vegetable oils or – which is less frequently, mineral oil-based.
3.1.2.2 Baby oils
Oils marketed to babies can be both bathing, body and massage oil. The products can be marketed for physical comfort and for application in a specific area, e.g. for massage of the stomach. The main part of the products with a mineral based oil composition is found within the baby oils.
3.1.2.3 Body oils
Body oils are marketed as body lotions, and sometimes also as combined body and massage oils. Application is recommended all over the body after bath and in areas with dry skin. In accordance with information from the shop assistants, body oils are applied as body lotions, as they penetrate the skin quickly. Therefore body oils are also called ‘dry oils’. Questioned if body oils can be applied for massage, the shop assistants both confirmed and disconfirmed that body oils can be applied for this purpose, but it was mostly confirmed. There are no examples showing that essential oils are sold for mixture in body oils. This category of oils is only sold as ready mixed oils.
3.1.2.4 Essential oils
Essential oils are extracted from the leaves and flowers of the plant and have often a strong fragrance. Essential oils for massage are not to be applied alone. In the shops they are placed next to one or more different basic oils. The purpose is to purchase one or more essential oils, which are added to the basic oil, until a desired fragrance and concentration is obtained.
During the survey also ‘nature identical essential oils’ or synthetically produced oils have been found. They are sold as or together with the pure essential oils. The production costs of synthetic oils are markedly lower than that of ‘pure’ oils, and it is possible to keep down the price for essential oils by using synthetic essential oils with fragrance of even exotic plants such as orchids.
3.1.3 Purchase
3.1.3.1 Purchase
The purpose of purchasing the massage oils is the identify and make a survey of the selection of these products in the Danish market. As a great number of massage oils for physical well-being and oil-based products for babies are marketed, a number of purchasing selection criteria were set up.
Indication of the word ‘massage’ on the product label was the most important criterion. Body oils and products to be combined in different ways for self mixture of massage oils were purchased. This type of products consists typically of one or more basic oils to which one or more pure essential oils can be added. With the exception of the seven products that IMS had already assessed in 2003 all oils marketed to children/babies were purchased.
The ingredients of some products, particularly the body oils, can be found on the home pages of the producers. Consequently, these products were not purchased, but are included in the survey.
Based on the above criteria, supposedly a representative part of massage, baby and body oils for physical well-being in the Danish market in the Spring 2005 has been purchased or surveyed. However, for instance professional masseurs and beauty salons are expected to apply special products that are not available to the ordinary consumer or are high-price products. These products are not included in this survey. The survey gives the impression that there is a quick change in products on the market. This gives reason to believe that there are limited distributed products sold in special shops, such as beauty salons and health shops, which are not included in this survey.
3.1.3.2 Shop visits
A number of different shops were visited. They were: 4 druggists, 4 Department stores, 3 health shops and 1 pharmacy.
3.1.3.3 Internet
In the spring of 2005 search was made on Danish internet pages to find products and product information through this medium. Search criteria such as “massage oil”, “massage”, “oils”, “well-being”, and “baby oil” were used. Visits to a selection of well-established perfume houses were paid both in the department stores and on the internet to illustrate, if they had massage oils in their product assortment. Massage or body oils were only found in the product assortment of 3 well assorted perfume houses. Therefore, it is assessed that there are relatively few suppliers of massage oils on the Danish market and that they are often small.
3.1.4 The products
The survey of the massage oils comprises information collected for 49 products.
Totally 28 products were purchased. Of these 15 massage oils, 6 baby oils, 3 body oils and 4 essential oils in the 12 visited shops. Besides the first mentioned, 21 not purchased products (12 massage oils, 2 baby oils, and 7 body oils) were included in the survey, because their complete composition was found on the internet or in the advertising material.
Part of the products was identified in the shops, on the internet or in advertisements. Among the reasons why these products are not included in the survey are that they were found on the internet and not sold in the retail trade or only in very few shops in Denmark. Some of the products in a product line were not purchased due to high prices and consequently they had a very limited market share. Another reason was that their ingredients had been found and analysed in other purchased products.
The products on the market in the spring of 2005 is a great number of products ranging from simple products with few ingredients, e.g. pure apricot oil to more sophisticated products containing several ingredients with a highly complex oil phase. Some products were colourless and had a faint fragrance and other products had a bright, coloured content of oil and an intense fragrance.
One massage cream was found. This cream has a very complex lipid phase and a melting point above skin temperature. This means that the product is solid at room temperature, but becomes fluid when massaged into the skin. Interviewed masseurs claim that this type of product is often applied for professional massage, because the product is easier to control and will not run down and spoil the clothes of the customer. The masseurs also told that normally they do not apply products with fragrance. The fragrance is too strong to work in for longer periods and clients have different taste with regard to fragrance. Visit at a chiropractor showed that he never uses massage oils or lotion. For this type of treatment roll-on products containing muscle warming ingredients as for instance camphor are applied.
3.1.4.1 Prices
The prices of the products have been registered. The price per liter of the product has been calculated on the basis of the sales price and the indicated volume of content.
The prices vary on a scale from DKK 26.75 for the cheapest product until DKK 310 for the most expensive. Converted to price per litre, this price spread will be DKK 149.75 for the cheapest product and DKK 3100 for the most expensive. Price spread and average prices are shown in table 3-1. The average price per litre product is based on 37 products, of which only 28 were purchased. The price of the remaining 9 not purchased products was found on the internet.
Table 3-1: Lowest and highest prices of the purchased massage oils. Lowest and highest price per litre is based on cost price and volume indication of the product. 37 products are calculated – only 28 were purchased. The price of the remaining 9 not purchased products was found on the internet
| Price | Individual product (DKK) |
Average* (DKK) |
| Lowest price/product | 26.75 | 102.64 |
| Highest price/product | 310.00 | |
| Lowest price/l | 149.75 | 1054.60 |
| Highest price/l | 3100.00 |
The individual massage oils are sold in volumes of between 50 and 200 ml - by far the majority of the products in 100 ml packings. The price calculations in table 3-1 are calculated both per product and per litre.
The price level of the 3 perfume houses having massage- or body lotions in their product assortment was between DKK 200 and 375 per bottle (100 ml).
Essential oils are sold in smaller packagings and products containing 5 and 10 ml were found. The price of essential oils depends on among others how common the plant is as well as the quality of the extraction and if pure or synthetically oil. The price of the products purchased for this project was DKK 32.00 for 10 ml and DKK 45.00 for 5 ml. Essential oils and nature identical fragrance oils are sold in the same type of packaging and are placed together in the shops.
3.1.5 Target groups and marketing
3.1.5.1 Target groups
Of the 49 products in the survey, 8 are for babies and 41 for adults. Three of the 8 products marketed for babies are minerally based and therefore the group containing most minerally based products (37.5% of the products). Essential oils (4 products of the 49) are assessed separately, and all of them are assessed as marketed for adults.
The survey divides the products into 2 target groups, babies and adults. The designation “adults” covers all other target groups than babies, e.g. adults, young people, women and men.
It should be observed that massage and body oils for adults in some cases are mostly intended for women, as the oils are marketed for application to a specific body area, e.g. areas with cellulites, or in specific periods, for instance during pregnancy.
3.1.5.2 Marketing of massage oils in Denmark
It is generally maintained that application of massage oils marketed for general well-being gives the consumer both physical and psychical well being. The asserted effects of the oils are further assessed in section 6.1.2 about claims.
In the literature, the term ‘performance cosmetics’ is found and used among others about products containing many plant oils (3). The term covers products that can be sold at a high market price, often due to trends in the society to the effect that ‘natural’ products are good and safe to apply. This concept can possibly also be used to explain why there are many body oils on the market, even if their ingredients apparently do not differ from typical massage oils. The word “body oil” seems to sell better and for the consumer the use of it is more universal than the word “massage oil”.
3.2 Ingredients
In accordance with the list of ingredients of the products, mainly vegetable oils are contained in massage, baby and body oils on the Danish market. Occurrence of other types of ingredients is very limited. Only few products are solely based on mineral oils and other addititives, such as for instance colourants and preservatives.
Oils and other extracts from plants have been applied throughout the history as or in cosmetic products. In spite of the fact that many are synthesized in the laboratories today, essential oils and extracts from plants are today applied primarily because of their fragrance properties (3).
Fragrant essential oils contain many of the same fragrances allergens evaluated by EU and must be stated on the label, if they are contained in leave on cosmetic products, in a concentration of more than 0.001%.
The EU Scientific Committee of Cosmetics and non-food products (SCCNFP) claimed in 2003 that essential oils applied in cosmetic products must comply with the demands on perfumes with respect to content of fragrance allergens (4). The claim compensates for investigations that indicate the difference in amount of allergens between synthetic fragrances and fragrance substances extracted from natural products. Application of essential oils causes serious problems for an effective quality control. Depending on parameters, such as the quality of the plant, which part of the plant is applied, extraction technique, time of harvest and climate, the content of sensitizing substances in the extracted oils may vary considerably (3), (4).
If the application of essential oils in massage, baby and body oils implies a content of one or more declaration demanding fragrance substances in concentration above 0.001%, they must be declared on the product, if they are cosmetic products. If it is not a cosmetic product, classification and labelling in accordance with the chemicals legislation are demanded. If the product appears to contain a sensitizing substance in a concentration > 0.1%, this must be indicated using the sentence “Contain (name of the sensitizing substance). May effect allergic reaction”, unless other limits for the substance in question have been specified (5).
The content of each individual vegetable and essential oils is registered in the survey. The following section gives a total description of these oils.
3.2.1 Vegetable oils
During the survey of massage, baby, and body oils, the applied oils are divided into vegetable non-volatile and volatile (essential oils) The vegetable non-volatile oils are applied as basic oil in the products.
Table 3-2 The most often applied basic oils (non-volatile oils) in massage, baby and body oils
| Name | Number of products | INCI | CAS no |
| Almond oil | 16 | Prunus amygdalus dulcis oil | 8007-69-0 |
| Jojoba oil | 13 | Buxus chinensis / Simmondsia chinensis oil | -/61789-91-1 |
| Peanut oil | 10 | Arachis hypogaea oil | 8002-03-7 |
| Olive oil | 7 | Olea europaea oil | 8001-25-0 |
| Wheat germ oil | 5 | Triticum vulgare germ oil | 68917-73-7 |
| Soya bean oil | 5 | Glycine soja oil | 8001-22-7 |
| Sesame oil | 4 | Sesamun indicum oil | 8008-74-0 |
| Macadamia nut oil | 3 | Macadamia ternifolia seed oil | |
| Safflower oil | 3 | Carthamus tinctorius seed oil | 8001-23-8 |
| Abricot seed oil | 3 | Prunus armeniaca kernel oil | 72869-69-3 |
| Rice oil | 2 | Oryza sativa germ oil | 90106-37-9 |
| Sunflower oil | 2 | Helianthus annuus seed oil | 8001-21-6 |
| Cocoabutter /extraxt from bark and seed | 2 | Theobroma cacao extract | 84649-99-0 / 8002-31-1 |
| Plantain onagraceae oil | 2 | Oenothera biennis oil | - |
| Avocado oil | 1 | Persea Gratissima oil | 8024-32-6 |
| Maize oil | 1 | Zea mays extract | 84696-06-0 |
| Burdock oil | 1 | Arctium tomentosum | - |
Olive oil, peanut oil and cocoa butter, which in accordance with the survey has been applied as basic oil in several massage, baby and body oils, is in literature described to cause other unwanted effects than allergy, e.g. acne and inflammation in the hair follicle of the skin (comedones) (3).
Table 3-3 shows different essential oils and individual extracts declared on the products contained in this survey. Some of the listed substances can be purchased both as pure essential oils and can be found as ingredients in massage, baby and body oils. In attachment 1 is listed the natural appearance and application of the 26 fragrance substances which the EU considers allergenic and of which (76 %) have been found during the survey.
Table 3-3 Applied essential oils and extracts in massage, baby and body oils (purchased products and products found on the internet)
| Name | Number of products |
INCI | CAS no. |
| Lavender oil | 8 | Lavandula angustifolia oil | 8000-28-0 |
| Birch leaves extract | 7 | Betula alba leaf extract | 84012-15-7 |
| Lemon oil | 7 | Citrus medica limonum oil | 8008-56-8 |
| Marigound | 4 | Calendula officinalis oil | 70892-20-5 |
| Arnica oil | 4 | Arnica montana extract | 68990-11-4 |
| Rose olie (damascener rose) | 4 | Rosa damascena oil | - |
| Rosemary extrakt | 4 | Rosmarinus officinalis extract | 84604-14-8 |
| Camille flower oil | 3 | Chamomilla recutita oil | 8002-66-2 |
| St. John's Wort | 3 | Hypericum Perforatum oil | 68917-49-7 |
| Mandarin oil | 3 | Citrus nobilis oil | 8008-31-9 |
| Orange oil | 2 | Citrus aurantium dulcis oil | 8008-57-9 |
| Geranium oil | 2 | Pelargonium odoratis-simum (geranium) oil | - |
| Peppermint oil | 2 | Mentha piperita | 8006-90-4 |
| Benzoetree extract | 2 | Styrax benzoin extract | 84929-79-3 |
| Aloe Vera | 2 | Aloe Barbadensis | - |
| Nettle | 2 | Urtica urens / Urtica dioica | 90131-83-2 / - |
| Anis oil | 1 | Pimpinella anisum | 84775-42-8 |
| Lime blossom oil | 1 | Tilia cordata oil | 68916-81-4 |
| Patchouli oil | 1 | Pogostemon cablin oil | - |
| Juniper berry oil | 1 | Juniperus communis oil | 73049-62-4 |
| Eucalyptus oil | 1 | Eucalyptus citriodora oil | 223748-96-7 |
| Ginger oil | 1 | Zingiber officinale oil | 8007-08-7 |
| Basile oil | 1 | Ocimum basilicum | 8015-73-4 |
| Palm rose oil | 1 | Cymbopogon martini oil | 84649-81-0 |
| Sage oil | 1 | Salvia sclarea | - |
| Sandalwood oil | 1 | Amyris balsamifera oil | 8015-65-4 |
| Tea Tree oil | 1 | Melaleuca Alternifolia oil | 85085-48-9 |
| Vanilla oil | 1 | Vanilla planifolia | - |
| Ylang ylang oil | 1 | Cananga odorata oil | 8006-81-3 |
| Peru balsam | 1 | Myroxylon Pereirae | 8007-00-9 |
| Sphagnum extract | 1 | - | - |
| Smooth burdock extract | 1 | Arctium lappa extract | 84012-13-5 |
| Fennel olie | 1 | Foeniculum vulgare oil | 8006-84-6 |
| Coffee bean oil | 1 | Coffea arabica oil | - |
| Cardamom oil | 1 | Elettaria cardamomum oil | 8000-66-6 |
| Coming oil | 1 | Carum carvi oil | 8000-42-8 |
| Paddock pipe extract | 1 | Equisetum arvense extract | - |
| Hawthorn oil | 1 | Hippophae rhamnoides oil | 225234-03-7 |
| Black extract | 1 | Prunus spinosa extract | 90105-94-5 |
| Citronella oil, orange grass | 1 | Cymbopogon nardus oil | 8000-29-1 |
| Rose oil (hip seed oil) | 1 | Rosa moschata seed oil | - |
| Rose oil (moss rose) | 1 | Rosa centifolia oil | 84604-12-6 |
| Rose oil (vinegar rose) | 1 | Rosa gallica oil | 84604-13-7 |
| Rose oil (dog rose) | 1 | Rosa canina oil | 84696-47-9 |
On the labelling of 17 products is stated pure essential oils or ‘perfume (essential oils)’. In this case it is not known which essential oils it concerns.
Table 3-3 shows that 5 different rose oils were found, the damascene rose oil as the most popular. The fragrance of all lemon oils is fresh and is applied in many products. Extracts of different species of both lemon and roses were found.
Body oils are marketed more frequently than massage oils with a specific fragrance, e.g. ‘lavender oil’. Table 3-4 illustrates how many body oils the survey has identified and with which fragrance they have been marketed.
Table 3-4 Most frequently applied fragrances in body oils on the Danish market
| Frangrance | Number of products | INCI |
| Lavender | 6 | Lavandula angustifolia |
| Rose | 5 | Rose sp. |
| Lemon | 4 | Citrus sp. |
| Rosemary | 3 | Rosmarinus officinalis |
| Marigold | 3 | Calendula officinalis |
Together with arnica, safflower, mandarin and chamomile oil (table 3-3), the oils in table 3-4 are the most common in massage, baby and body oils on the Danish market in the spring of 2005.
The survey of massage, baby and body oils has identified totally 65 different oils extracted from plants. The survey is assessed to give a realistic impression of how many different sorts of oil this type of product contains. 31 of the 65 different oils only appear once on the labels of the products comprised by the survey.
3.2.2 Other ingredients
Vegetable substances such as extracts, oils and essential oils are practically all ingredients in the surveyed massage oils.
Preservatives are only applied in a very small number of the surveyed products (8%). The most frequently applied preservative is phenoxyethanol (CAS nr. 122-99-6), which has been applied in 3 different products.
The mineral oil paraffinum liquidum (CAS no. 8012-95-1) has been applied in 3 products.
Addition of an antioxidant to avoid rancidity of the product is more common. Tocopherol (CAS no. 10191-41-0) and tocopheryl acetate (CAS no. 7695-91-2) have been added to14 products. Tocopheryl acetate is found naturally in wheat germ oil, which is applied as basic oil in several products. The literature describes the substance as a mild contact allergen, but well-known in cosmetics (6).
3 products have been added ethanol (CAS nr. 64-17-5), and a violet colourant has been applied (CI 60725) in one product.
3.2.2.1 Potential impurities and hazardous substances
Vegetable oils may contain unintended impurities. Impurities in oils and extracts may be pesticide residues, residue concentrations of the applied solvent, metals, microbiological pollution, plant chemicals and metabolites incident to the extraction (7).
Examples of unwanted substances in vegetable oil based massage oils are shown below. Some substances are naturally occurring components or metabolites, and others are pollution from the extraction. Independent of the fact that the different massage, baby and body oils are comprised by the regulations in the EU cosmetics order, it is interesting to examine, if they, or a selection of these substances are found in the products, as they are all applied to the skin and may cause a health risk to the consumers when applied as desired.
Many massage, body and essential oils contain fragrance substances. One or more of the 26 fragrance substances that are assessed by the EU as allergenic are likely to occur in many of the massage oils, as many of the fragrance substances are used very often (8). It is interesting to examine the volume of concentration of these fragrance substances.
The substance safrol is found in rosmary oil extract. The substance is prohibited in concentrations above 100 ppm in cosmetic products, as it has show carcinogenic properties and liver tumours when administered orally. The Council of Europe recommends that cosmetic products for babies do not contain safrol (9).
The substance estragol are found in various spices, for instance anis oil and basilie. The substance is slightly hepatocarcinogenic when administered subcutaneously to young mice. The Council of Europe asks for additional examinations of the substance and its concentrations in cosmetic ingredients (9).
The substance methyleugenole can be found in rose oils. I accordance with the cosmetic order, enclosure 2, it is not permitted in concentrations > 0.0002% in cosmetic leave-on products – or 0.002%, if the massage is placed on an equal footing with perfumed creams (1).
The substance escin can be found in e.g. horse chestnut oil. The Council of Europe recommends that the content of escin in fragrances does not exceed more then 1% (9).
The substance eucalyptus oil is found in eucalyptus oil and causes hypersensitivity reactions (9).
The substance methylsaliclat is the main component of wintergreen, which is the essential oil from the plant Gaultheria procumbes. Methylsalicylat is also a naturally occurring metabolite in extract of birch leaves. The Council of Europe recommends that extract of birch leave is only applied in cosmetic products, if they do not contain methylsalicylat (9).
Peru balsam is a highly allergenic substance that is not allowed cosmetic products (1).
4 Analysis programme
4.1 Analysis programme
The analyses programme has been established with the objective to examine the massage and body oils including the most widely marketed oils for children and essential oil.
4.1.1 Selection of analyses
In order to reach the broadest customer segment, it was endeavoured that selection of the products should be based on an examination of the most widely marketed products and essential oils. The importance of including examination of both products to children and adults was emphasized representing the different product types for body and massage. Therefore, essential oils used in massage oils, products marketed as massage oils, products for baby massage and products marketed as both body- and massage oil or only body oil products were selected.
The selected 16 products were all analysed for the 26 fragrance allergens that were assessed EU (8). Among those, three products were also selected to be analysed for methyleugenole due to its content of rose fragrance, which was indicated on the label.
Besides, other 2 of the 16 products were analysed for content of methylsalicylat, as birch extract was indicated to be one of the fragrance substances. Finally 2 other products were analysed for safrol, due to its content of rosemary, which was indicated to be one of the fragrance substance.
Peru balsam is a mixture of chemical substances, among others vanillin and benzyl cinnamate. In one of the products it was relevant to analyse for content of vanillin in order to further determine the content of Peru balsam.
The following analyses were performed:
26 fragrance substances:
16 products, of these 7 massage oils, 2 baby oils, 3 body oils and 4 essential oils. Hazardous substances in lemon oils are covered by this analyses.
Metyleugenol:
4 products containing rose fragrance, of these 1 massage oil, 1 body oil, 1 oil diluted essential oil and a nature identical fragrance oil.
Methylsalicylate:
4 products containing birch fragrance, of these 1 massage oil, 1 body oil.
Safrol:
2 products containing rosemary, of these 1 body oil and 1 essential oil
Peru balsam:
1 massage oil is analysed for content of Peru balsam.
4.2 Analyses methods
4.2.1 Methyleugenole, methylsalicylate and safrol
A partial sample of the product is extracted with dichlormethane for one hour on a shaking table and stands until next morning. A partial sample of the extract is selected and analysed directly using combined gas chromatography and mass spectrometry. The content is calculated quantitatively.
The analysis uncertainty is 10-15% RSD. The analyses are performed as pure double determinations. The detection limit is 10-20 mg/kg.
4.2.2 Fragrance substances
A partial sample of the product is selected and extracted with water and tert-butylmethylether by shaking, heating, cooling and standing during approx. 16 hrs.
A partial sample of the extract is selected and analysed directly using combined gas chromatography and mass spectrometry (GC/MS). The analyses are performed as pure double determinations. The detection limit is 10 mg/kg. and the analysis uncertainty is 10-15% RSD.
The detection limit of oak moss extract and tree moss extract cannot be determined, because they are natural extracts with many components and not solely one pure substance. As the content of these natural extracts vary an accurate detection limit cannot be calculated. Instead, the limit is indicated as “not established”.
4.2.3 Peru balsam
Peru balsam is a mixture of chemical substances. The mixture is of natural origin and thus he content varies qualitatively and quantitatively. It is therefore difficult to perform a qualitative and quantitative determination of specific marker substances in the mixture.
In relation to this, a demonstration of vanillin and benzoacid has been searched for in the chromatogramme for sample 8 from the analyses for methyleugenole.
5 Chemical analyses
- 5.1 Analysis results
- 5.2 Conclusion of analysis results
- 5.3 Selected substances for safety assessment
5.1 Analysis results
Table 5-1 contains a survey of the results of the analyses for content of methyleugenole, methylsalicylate and safrol and the results are afterwards presented in detail.
Table 5-1. Results of analysis for methyleugenole, methylsalicylate and safrol in selected products indicated as an average (mg/kg) of a double determination)
| Identification no. | Methyleugenole | Methyl salicylate | Safrol |
| 8 | < 10 | - | - |
| 46 | < 10 | - | - |
| 47a | < 10 | - | - |
| 50a | 160 | - | - |
| 4 | - | < 10 | - |
| 32 | - | < 10 | - |
| 45 | - | - | < 20 |
| 48 | - | - | < 20 |
<.: less than the indicated detection limit
a essential oils, of which no. 50 is an diluted essential oil (3 % essential oil)
5.1.1 Methyleugenole
Four products were analysed for methyleugenole, and the results of the analyses are shown above in table 5-1. Double determinations were performed. The unit is mg/kg and the detection limit is 10 mg/kg.
5.1.2 Methylsalicylate
No content of methylsalicylate above the detection limit was demonstrated in the two analysed samples. The samples were analysed in double determination. The result was indicated in mg/kg and the detection limit was 10 mg/kg. The result of the analyses is shown in the above table 5-1.
5.1.3 Safrol
In two products was analysed in double determination for safrol. The analyses did not show content of safrol. Due to a limited amount of sample, the sample material for the analyses was slightly reduced. The results of the analyses is indicated in table 5-1 above.
The results are indicated in mg/kg and the detection limit is 20-70 mg/kg.
5.1.4 Fragrance substances
16 massage, baby, body and essential oils were analysed for 26 specific fragrance substances (Table 5-2). Only one product did not demonstrate at least one of the 26 substances. The total content of all 26 fragrance allergens is indicated on the bottom line of the table. The total content varies from 170 to 700,000 mg corresponding to from 0.017% - 73%. Two of the three products of essential oils represent the two highest contents of 40-50% and 70-72%.
Table 6-1 Results from the analysis of fragrance substances. The unit is mg/kg. The result indicates the average of a double determination
Click here to see the Table5.1.5 Peru balsam
Peru balsam is a mixture of chemical substances. The substance is of natural origin and its content is therefore varied qualitatively and quantitatively. The perfume materials benzyl cinnamate and benzyl benzoate are among the main components and are demonstrated in the amount 245 mg/kg and 1150 mg/kg, see table 5-2. Apart from this, it contains mall amounts of e.g. vanillin. In the chromatogramme of product no. 8 of the methyleugenole analyses, a search to demonstrate vanillin and benzo acid was performed. Vanillin in amounts of 3.6 mg/kg calculated as C8 was detected. Benzo acid was not demonstrated. Due to this, the tested product is very likely to contain Peru balsam as declared on the product.
5.2 Conclusion of analysis results
Seven of the 26 fragrances were not found in any of the 16 analysed massage- and body oils and in the 4 essential oils. In one of the products, the fragrances could not be demonstrated. The remaining 15 products contained from 2 to 12 different of the tested fragrances and the highest total content of the selected essential oils.
Table 5-3 shows the number of fragrance substances found in the products. The target group of massage and body oils is mainly adults, several of them intended for pregnant women (one of these products is tested). Two of the selected products were intended for baby massage and contained relatively few of the 26 fragrance allergens, cf table 5-2 and table 5-3.
Table 5-3 Number of fragrance substances
| Number of fragrance substances | Number of products |
| 0 fragrances | 1 product |
| 2 fragrances | 2* products |
| 3 fragrances | 1 product |
| 4 fragrances | 4* products |
| 5 fragrances | 1 product |
| 6 fragrances | 3 products |
| 7 fragrances | 2 products |
| 11 fragrances | 1 product |
| 12 fragrances | 1 product |
*Of these 1 product intended for babies
The occurrence of fragrance substances was grouped as follows:
The following 7 fragrance substances were not found in any of the analysed products:
Anisyl alkohol, amyl cinnamal, amyl cinnamal alkohol, isoeugenol, methyl heptine carbonate, oak moss and tree moss. The grouping of the remaining 19 fragrance substances is shown in table 5-4.
Table 5-4 Grouping of the 19 demonstrated fragrance substances
| Fragrance substances | Number of products |
| Linalool | 15 |
| d-Limonene | 13 |
| Geraniol | 10 |
| Citronellol | 8 |
| Citral | 7 |
| Eugenol | 6 |
| Benzyl cinnamate | 4 |
| Benzyl alkohol | 3 |
| Benzyl benzoate | 3 |
| Cinnamal | 3 |
| Farnesol | 2 |
| Benzyl salicylate | 2 |
| Cinnamyl alkohol | 2 |
Table 5-4 shows that some of the most applied fragrance substances are linalool, d-limonene, geraniol, citronellol, citral og eugenol. A great variation is seen in the concentrations of both the individual substances and the total content of the 26 fragrance substances. This is due to the testing of pure essential oils, which is also performed. However, several products are seen to have a high content of linalool, d-limonene, geraniol, citronellol, citral, which causes the high total content of fragrance substances. The total content of the 26 tested fragrance substances in weight percentage is shown in table 5-5 below. The content of fragrance substances of most of the selected products (75% of the products) is under 1 %.
Table 5-5 The Total content of the 26 tested fragrance substances in weight percentage.
| Total content of fragrance substances | Number of products | Number of fragrance substances. |
| 0 – 0,1 % | 3 products* | 0 – 6 fragrance substances |
| 0,1 – 0,5 % | 6 products *, ** | 2 – 12 fragrance substances |
| 0,5 – 1 % | 3 products | 4 fragrance substances |
| 1 – 5 % | 2 products ** | 6 - 11 fragrance substances |
| 5 – 50 % | 1 product ** | 6 fragrance substances |
| 51 – 100 % | 1 product ** | 7 fragrance substances |
*of these 1 product intended for babies
’’ of these 1 essential oil
Table 5-6 present a summary of the analysis results in number of products, the measured minimum and maximum values and the maximum value expressed as the weight percentage of the fragrance substances in the product. The products have been grouped in massage oils and body oils and essential oils to visualize possible differences in the concentrations of the individual fragrance substance in types of products
Table 5-6 Summary of analysis results
| Occurrences in the products |
Content in the products (mg/kg) | weight-percentage (max. content) |
|||||
| Massage- and body oils | Essential oils | ||||||
| number | % | Min. | Max. | Min. | Max. | ||
| Anisyl alkohol | 0 | 0 | - | - | - | - | - |
| Amyl cinnamal | 0 | 0 | - | - | - | - | - |
| Amyl cinnamyl alkohol | 0 | 0 | - | - | - | - | - |
| Benzyl alkohol | 3 | 18.75 | 220 | 220 | 46 | 140 | 0.022 |
| Benzyl benzoate | 3 | 18.75 | 1150 | 1150 | 26.5 | 79.5 | 0.115 |
| Benzyl cinnamate | 1 | 6.25 | 245 | 245 | - | - | 0.025 |
| Benzyl salicylate | 2 | 12.5 | 46.5 | 125 | - | - | 0.013 |
| Cinnamyl alkohol | 2 | 12.5 | 45.5 | 45.5 | 13.5 | 13.5 | 0.005 |
| Cinnamal | 3 | 18.75 | 6 | 210 | 14.5 | 14.5 | 0.021 |
| Citral | 7 | 43.75 | 19 | 1750 | 37 | 32500 | 3.250 |
| Citronellol | 8 | 50 | 19.5 | 460 | 100 | 475000 | 47.500 |
| Coumarin | 2 | 12.5 | 34 | 330 | - | - | 0.033 |
| Eugenol | 6 | 37.5 | 22 | 55 | 120 | 225 | 0.023 |
| Farnesol | 2 | 12.5 | 135 | 135 | 76 | 76 | 0.014 |
| Geraniol | 10 | 62.5 | 37 | 1250 | 375 | 230000 | 23.000 |
| Hexylcinnamaldehyde | 2 | 12.5 | - | - | 19.5 | 67.5 | 0.007 |
| Hydroxycitronellal | 1 | 6.25 | - | - | 41 | 41 | 0.004 |
| α-Isomethylionon | 1 | 6.25 | - | - | 79.5 | 79.5 | 0.008 |
| Lillial | 1 | 6.25 | - | - | 22.5 | 22.5 | 0.002 |
| d-limonene | 13 | 81.25 | 39 | 9250 | 220 | 410000 | 41.000 |
| Linalool | 15 | 93.75 | 18.5 | 8050 | 97.5 | 7600 | 0.805 |
| Lyral | 1 | 6.25 | - | - | 21.5 | 21.5 | 0.002 |
| Isoeugenol | 0 | 0 | - | - | - | - | - |
| Methyl heptin carbonate | 0 | 0 | - | - | - | - | - |
| Oak moss | 0 | 0 | - | - | - | - | - |
| Tree moss | 0 | 0 | - | - | - | - | - |
Methyleugenole is not on the list of the 26 fragrance allergens. However, the substance has also been analysed because of its carcinogenic effect (13). The substance was found in one of the tested products (an oil diluted essential oil).
5.3 Selected substances for safety assessment
Selection of fragrance substances for the safety assessment was based on content in the products. The fragrance substances contained in most products were: citral, citronellol, geraniol, d-limonene and linalool.
As the substances d-limonene, geraniol and linalool have been assessed in connection with previous survey projects of the Danish Environmental Protection Agency (13), (14) citral and citronellol were selected to be assessed on safety due to their frequent occurrence. Of other substances found in the products but not previously assessed in survey projects, cinnamal, benzyl cinnamate and Peru balsam were selected to be assessed on safety.
6 Legislation
6.1 Labelling and legislation
Massage oils are in a grey area with respect to legislation. To determine which legislation is prevailing for the product group, it is necessary to make a total evaluation of more factors for the individual products, e.g. where they are sold, target group, objective, claims and labelling as well as type of packaging and appearance.
All massage, baby and body oils are applied to a greater or smaller area of the skin and are not intended to be washed off. Generally, the cosmetics legislation is relevant for all products for the skin. When marketed, the products must be specifically labelled in accordance with the cosmetics legislation, among others with an INCI declaration. If the product has a healing or relieving effect, the regulations for pharmaceuticals must be observed. If the product is assessed to be neither a cosmetics or a pharmaceutical, for instance if it is mainly intended for procuring the feeling of well-being, the legislation on chemical substances and product is prevailing and provides among others guideline directions for classification and labelling of the product.
Definition of cosmetics and chemical substances are given below.
6.1.1.1 Definition of cosmetics and chemical substances
I accordance with the “kosmetikbekendtgørelsen”, the Danish cosmetics statutory order (1), cosmetic products are defined as any substance or preparation intended for contact with different kinds of the surface of the human body. This may be skin, hair of the head or other areas with hair, nails, lips, and exterior sexual organs, or if the product is intended for contact with the teeth and the mucosa of the mouth. The preparation must be applied solely or mainly for the purpose of cleaning and perfuming, changing appearance, correcting body odour, protecting or keeping in good condition the area of application. The enclosure of the Danish cosmetics statutory order contains an indicative list of products that are regarded as cosmetics, e.g. cream, emulsion, lotion, gel and oil for the skin.
The cosmetics statutory order does not comprise preparations that prevent, recognize, relieve, treat and heal illnesses, illness symptoms, pain or changes of the body functions. These products fall within the legislation for pharmaceuticals.
By chemical products are understood both solvents such as solid, liquid and gaseous mixtures of two or more chemical substances. Medicine, food stuffs etc., cosmetics and pesticides constitute an exception due to their different legislation Before being sold, chemical products must be classified to identify the physical-chemical, toxicological and ecotoxicological properties that may cause hazards by normal handling or use. The classification comprises placing in hazard classes, allocation of risk sentences (R-sentences) specifying the hazard of the products. To protect the user, chemical products classified as dangerous must be labelled with hazard symbols and risk sentences as well as security sentences (S-sentences) providing directions of necessary precautions to be taken in account (5).
6.1.2 Claims
The majority of the purchased product has a label with directions for use. On the label is often also described the effect of the oil when applied to the skin. This description is part of the marketing of the product and is in many in the nature of a claim. Emotional expressions are often used, often adjectives, and the consumers are not left in doubt that the product is good for the skin and may also have a relaxing effect and prevents stretch marks in the skin. Among the words on the label of the products, could be for instance: caring, vitalizing, preventing, relaxing, bracing, moisting, stimulating, warming, protects, rejuvenates dry and pale skin, stimulates the blood circulation in the skin, prevents muscular tensions, prevents stretch marks. Table 6-1 exemplifies text of the product labelling. It should be emphasized that not only the labelling of the product decides, whether it is a cosmetics but also a total consideration of several factors on the individual products, for instance where it is sold, target group, claims and labelling as well as type of packaging and other appearance.
In some cases, you may doubt, whether the claimed effect is caused by the massage or the massage oil.
Table 6-1 Examples of labelling of purchased massage, body and baby oils.
| Product ID | Comments |
| 4 | …stimulates the blood circulation in the skin, strengthen its natural functions and keep it smooth and lithe. Massage using the massage oil warms, loosens and prevents muscle tightenings. It also prevents pregnancy stretch marks in the skin. |
| 16 | ...an effective skin care product to be applied to almost any kind of skin problems, e.g. dry skin, skin abrations, flushing and sun burning. The oil also effects lithe and softening of the skin, which fx reducing the risk of stretch marks during pregnancy'. |
| 17 | Especially recommended for cleaning of the diper area, for baby massage and generally for skin care. |
| 18 | Recommende for relaxation, cure of swollenness and colic. |
| 19 | Ante-natal oil is a purely natural, particularly developmed care product to be applied before delivery. Almond oil and wheat germ oil rich of vitamin E – loosen and increase the elastidity in the perineal tissue. Massage imporves the blood circulation, which again increases the litheness in the perineum. |
| 23 | The material of the producer says:…. ‘a soft and nourishing film is creasted on the skin of your baby during and after bathing’….and ‘ the lavender in these mixtures is very calming and may contribute to making the babies relax at the end of the day.’ |
| 24 | The material of the producer says: ‘ Massaging the child’s stomach clockwise may contribute to cure painful, troublesome colic. Apply more oil if demanded and continue the massage, until the child calms down and relaxes. This massage oil is also good for stressed mothers.’ |
| 25 | This mild oil is particularly applicable to thighs and buttocks, where the skin is uneven with small dimples and needs extra care. |
| 30 | Prevents stress marks in pregnancy. Regular massage with the oil on abdomen, thights, seats and breast prevents stretch marks and protects the skin from drying. |
| 31 | Gives freshness and is a good cure against cellulitis. |
| 32 | Birch extract strengthen the body after hard training, where arnika coddles the skin with strenght and warmth. |
| 33 | The warming qualities of the lavender restitute the peace of the body and the mind, qualities that makes this body- and massage oil ideal for the modern life. |
| 34 | For sensitive skin care for sensitive people. Softens and reduces flushing and irritated skin. Applicable in old people’s care |
| 35 | Caring oil for protection and softening of the skin. The beautiful fragrance has an relaxing effect and increases the well being. Particularly well fitted for sensitive skin and skin sensitive to weather changes. |
| 36 | This exclusive body oil is good in stressing/exhausting periods. Rose oil has a nourishing effect on even the most sensitive skin and is particularly suited for babycare and for children and women. |
| 37 | Stimulates lactation. Massage with the oil increases circulation and warms through the breast. |
| 38 | A fantastixwarming and protecting allround body oil. |
| 39 | Effective against cellulitis. Massage the oil into thights, hips and buttocks with rotating movements. The oil penetrates the skin gently, gives the skin a silky gloss and permits the valuable active substances to go deep into the skin. |
| 40 | …with a refreshing fragrance of lemon the skin functions harmonize and and keep the skin fresh and elastics and protects from drying. For every-day application after bath. To be massaged into the warm and humid skin. Calendula has a cleansing effect.. |
| 41 | Cares and protects dry skin. Softens and lithes the skin. Stimulates circulation and harmonizes the functions of the skin. Contain almond oil that protects the skin from drying. Easily absorbed by the skin. Gives a fresh natural lemon fragrance. Relieving effect on children’s eczema. |
| 42 | A mild massage with lavender massage oil cares for the skin and gives relaxation to both body and mind. The sun saturated essential oils of the lavender flower spreads a mild fragrance, which loosens up inner tensions and gives balance. Also good for children with difficulty in falling asleep at night.. |
| 43 | ...Cares and has a nice fragrance. It Den appeals to the body and the mind and maintain the skin elastic and lithe. The oil from Rosa mosqueta, jojoba oil and almond oil coddles og refreshes the skin. The mild fragrance of roses has a calming and harmonizing effect . Is easily absorbed by the skin. |
| 44 | A lovely composition of sesame oil, hawthorn oil and naturally essential oils has an intensely caring and protecting effect to the skin also at environemental strains and after sun bathing. The skin gets vital force and feels lithe and nice. Can be applied to cure athlete's foot. |
Table 6-1 illustrates that the products are labelled very differently with respect to claims/effect. As part of the products is marked as ‘ good for the skin’ and as it is applied just like cosmetics products, the safety effects will be assessed based on the Danish cosmetics statutory order and the chemicals legislation.
Claims for essential oils often emphasise that application of the oil has special physic and psychical advantages. The properties of the essential oils are well defined, but it is difficult to find scientific investigations confirming the claimed effects. Examples of claims on the effects of essential oils are given below in table 6-2.
Table 6-2 Examples of the effect of pure essential oils when applied
| Essential oil | Application |
| Eucalyptus and rosemary | Winter cold and constipation. Enables breathing through the nose. |
| Lavender and Ylang ylang | Nervousness and sleeping problems. Calms down the mind and gives a good night’s sleep. |
| Rosmarin og pebermynte | Lack of concentration. To clear the mind. |
| Appelsin og Ylang ylang | Busyness. Relaxing effect. |
| Jasmin | Warming effect |
| Citron | Stimulating effect |
| Rose | Creates inner balance |
Besides the positive claims indicated in table 6-2 ,marketing folders have warned seriously against wrong application of essential oils, e.g. intake or application of undiluted essential oil.
6.2 Assessment of massage oils
Compliance with the legislation and particularly the labelling of the different product types is described in the following.
6.2.1 Massage and body oils
Only a limited number of the selected products claim to be for massage purposes without also mentioning effects such as softeners, moisteners or other caring effect which massage oils can add to the skin. Of 18 purchased massage and body oils, 4 products did not have any indication of caring effect on the label, but only describes application for massage. However, 3 of these 4 products followed the determinations in the Danish statutory order on cosmetics, as they were labelled with the INCI declaration of ingredients. 2 of the products had indications of one or more of the 26 fragrance allergens. Judged from its presentation (logo and packaging), the last product could be a cosmetics product.
Of the purchased 18 massage- and body oils, 14 products were indicated to have a caring effect to the skin. The product packagings were coloured or uncoloured bottles of plastic or glass, one single product in the form of an ointment. Most bottles had no special eye catcher as for instance gold or silver shining print and gave the impression of being a neutral, almost medicine like drug. All products had the INCI declaration of ingredients.
Of the purchased massage- and body oils, 10 products, all with INCI declarations were tested for content of the 26 fragrance allergens. In its INCI declaration, one of the products indicated content of limonene. However this could not be demonstrated by the test. The results of the remaining 9 tested products showed content of more of the 26 fragrance allergens, however only one was declared on the label.
6.2.2 Baby oils
Of 28 purchased massage oils, 6 products were for baby massage. The products were all composed of a number of fragrance substances . One of these products did not indicate caring effect to the skin and consequently it was not labelled in accordance with the cosmetics regulations. The product contained essential oil and its presentation (logo and packaging) might give the impression of being a cosmetics product. The remaining 5 products, all indicating caring effect can be regarded as cosmetics and was labelled with the INCI declaration of ingredients. One of these product did not contain perfume, the other five did.
Later on, two of the baby oils were tested for content of the 26 fragrance allergens. The test results showed that one of the tested products had not indicated the detected fragrance allergens in its INCI declaration of ingredients, whereas the other product had indicated the current fragrance allergens correctly.
6.2.3 Essential oils
The selected and purchased essential oils were all in small bottles of brown glass with a volume of 5 and 10 ml, some containing an information sheet indicating the application. The packaging, labelling or indications on the labels or on the information sheet did not give the impression that the products were for cosmetic use or should be applied as a pharmaceutical. The selected essential oils were labelled in accordance with the chemicals legislation.
In the survey, 4 essential oils were tested for content of the 26 fragrance allergens. The test results revealed however that the essential oils marketed in concentrated form contained more than 0.1% of one or more fragrance allergens and that the labelling regulations on allergy warning had not been observed, as this was not indicated on the label. If the product contains a sensitizing substance in a concentration > 0.1%, the following must be indicated “contain (name of the sensitizing substance). may cause allergic reaction”, unless other limits have been specified for the substance in question (5).
6.2.4 Evaluation
An assessment of all the factors like where they are sold, target group, objective, claims and labelling as well as type of packaging and appearance
has been made for each of the purchased products., This assessment was made according to EU’s guidance on borderline products. The assessment show that 15 of the 28 purchased products are covered by the cosmetic legislation (1). The remaining 13 are covered by the legislation on chemicals substances and products and due to this also covered by the rules of classification and labelling (5). As stated above this means among other things that there are specific rules regarding labelling of sensitising substances.
7 Safety assessment
Safety assessments of the fragrances benzyl cinnamate, cinnamal, citronellol, citral and Peru balsam have been performed. Acute and chronic risk assessments have been performed, including the ability of the substances to induce allergy.
A NO(A)EL[1] for the critical effect of the substances has been established. The critical effect of the established NOAEL is no allergy, as there is no lower concentration limit showing when a substance induces allergy. The NO(A)EL value is applied in Chapter 8 on exposure scenarios.
7.1 Benzyl cinnamate
Occurrence and application
Benzyl cinnamate is applied as a fix active in heavy oriental fragrancies. The substance is also applied as a flavour additive in foodstuffs. Ingestion of the substance is estimated to be 44 µg/person/d (0,7 µg/kg bw/d) in Europe and 69 µg/person/d (1 µg/kg bw/d) in the USA, part of which is a natural ingredient in foodstuffs (15). Benzyl cinnamate is an ingredient of Peru balsam.
Identification
Benzyl cinnamate is an ester.
| Chemical name | Benzyl cinnamate |
| Synonyms | Benzyl cinnamate, cinnamic acid benzyl esther |
| CAS-No. | 103-41-3 |
| EINECS No. | 203-109-3 |
| Molecular formula | C16H14O2 |
| Molecular structure | 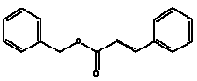 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Not classified. Listed, as a substance which is allergic by skin contact. On the EU list of 26 fragrance allergens. The fragrance is stated on the product label of cosmetics if applied in quantities above 0.01% in leave-on products, and 0.001% in wash-off products. |
Physical-Chemical properties
| Physical state | Crystals (pale yellow) (15) |
| Molecular weight (g/mol) | 238.3 (15) |
| Melting point °C | 37-39 °C (15) |
| Boiling point, °C | 195-200 °C ved 6.7 hPa (15) |
| Vapour pressure (Pa) | No information |
| Octanol/water partition coefficient (log Pow) | No information |
WHO has established a NOEL[2] for benzyl cinnamate of 500 mg/kg bw/d for rat (15)
Acute toxicity
No information was found on the LD50[3] of benzyl cinnamate for neither oral nor dermal exposure.
Local irritation
No information was found on the skin or eye irritating effects of benzyl cinnamate.
Allergy
EU Scientific Committee (SCCNFP) has registered benzyl cinnamate on the list of fragrance allergens. However, not many reports on allergy to the consumers are available. In a study, 6 patients out of 182 (3% showed positive reaction when tested with 8% benzyl cinnamate. In another study, 19 patients out of 103 (18%) with contacting allergy to Peru balsam showed allergic reaction to benzyl cinnamate when tested with 8% benzyl cinnamate. (18).
Long-term, repeated exposure
5 rats of each sex were administered benzyl cinnamate in their diet for19 weeks with a highest dose level of 500 mg/kg bw/d. No effects on body weight, foot intake or blood were found differing from the control group or from the normal area. In this study, NOEL1 was found to be higher than 500 mg/kg bw for benzyl cinnamate (15).,
Benzyl cinnamate was tested in an Ames test and in another bacterium test with a negative result. (15).
No information was found on the effects of benzyl cinnamate by inhalation.
Critical effect
The critical effect of benzyl cinnamate is evaluated to be contactallergy. Due to the allergenic effect of benzyl cinnamate , persons allergic to the substance should avoid skin contact, as there are no lower limit for this side effect.
No lower limit for the contactallergy is known, MoS[4]-calculations are be based on effects other than allergy. The below calculation is based on NOEL from the above study with dosage of rats for 19 weeks.
Tabel 7-1 Summary of data used for the calculation of MoS for benzyl cinnamate
| Toxicological data (animals) | |
| NOEL1, (mg/kg bw/d), intake, rat | 500 |
7.2 Cinnamal
Occurrence and application
Cinnamal is applied as a fragrance in perfumery articles (17) and is naturally occurring in among others cinnamon, blueberries, and cranberries. The substance is also applied as flavour additive in foodstuffs. Intake of the substance was estimated to be 42 µg/kg bw/d in Europe and 991 µg/kg bw/d in the USA. A great part of the applied cinnamal came from natural sources (15).
Identification
Cinnamal is an aldehyde.
| Chemical name | Cinnamale, Cinnamaldehyde |
| Synonyms | 3-Phenyl-2-propenal, Cinnamic aldehyde, cinnamon aldehyde |
| CAS-No. | 104-55-2 |
| EINECS No. | 203-213-9 |
| Molecular formula | C9H8O |
| Molecular structure | 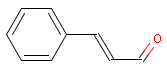 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Not classified Listed, as a substance which is allergic by skin contact. On the EU list of 26 fragrance allergens. The fragrance is stated on the product label of cosmetics if applied in quantities above 0.01% in leave-on products, and 0.001% in wash-off products. |
Physical-chemical properties
| Physical state: | Liquid (18) |
| Molecular weight (g/mol) | 132.2 (19) |
| Melting point °C | -7.5 °C (18) |
| Boiling point, °C | 246 °C (at 1014 hPa) (18) |
| Vapopour pressure (Pa) | 2.25 hPa at 20 °C calculated) (18) |
| Octanol/water partition coefficient (log Pow) | 2.22 at 18 °C (calculated) (18) |
| Water solubility (mg/L) | 1400 (18) |
WHO has established a NOEL[5] for cinnamal of 620 mg/kg bw/d (15).
Acute toxicity
The LD50-values by oral administration of cinnamal in rats was found to be 2200 - 3400 mg/kg bw, by oral administration in mice 3400 mg/kg bw, and in guinea pigs 1160-3400 mg/kg bw (20), (21). The toxic effects were reduced activity (rats), convulsion(s) (mice), and coma (guinea pigs) (21).
WHO has assessed that cinnamal is metabolised to a harmless substance, the main part of which is eliminated quickly through the kidneys.(15).
LD50-values by dermal exposure of rats and rabbits is greater than 2000 mg/kg bw (20).
No data on dermal absorption of cinnamal were found.
Local irritation
Cinnamal was found irritating to skin in guinea pigs and humans. No information on eye irritation was found (20). Undiluted substance on the skin of humans for 48 hours causes serious irritation. (21).
Allergy
The Scientific Committe of EU SCCP has registered Cinnamal on the list of fragrance allergens. The fragrance substances on the list are well known allergens, about which many cases of allergy in the consumers were reported.
Three studies showed that 6 of 20 patients (30%), 7 of 182 patients (3,7%) and 24 of 167 patients (14.44%) reacted allergenic to cinnamal (18)
Cinnamal is part of fragrance mix for diagnostic test, in which the substance accounts for 5.5 – 36% of the reactions on this mixture (5.5% in Italy, 16.9% in Denmark, 21% in Germany, 24% in Hungary and 36% in France)(16). The substance was identified to cause allergenic reactions in humans, of whom between 1% and 30% persons suffered from cosmetics eczema. In 78 European consumers with fragrance eczema, 12.8% reacted positively when tested with 1% cinnamal (18).
IFRA (International Fragrance Association) recommends that cinnamal as a fragrance is exclusively applied together with substances preventing sensibilisation, such as for instance equal portions eugenol or d-limonene. Investigations have shown that mixtures of equal amounts of cinnamal and eugenole or cinnamal and d-limonen are not sensitizing, even if the individual substances separately are sensitizing (22)..
Long-term, repeated exposure
Three studies of 90 days duration with approximately identical results were found. The National Toxicology Program is described as follows: Groups of 10 rats of each sex were daily administered cinnamal in their diet (1.25%) for 13 weeks at doses of 620 mg/kg bw/d. At this concentration, no effects on kidneys, liver, blood were found differing from the control group or from the normal area. In this study, NOEL was found to be 620 mg/kg bw/d. At higher doses (1250 mg/kg bw/d) abdominal mucosal irritation as well as reduced growth was observed (probably caused by the taste) (15).
In a 2-year study, groups of rat (50 of each sex) and mice (50 of each sex) were administered cinnamal in their diet. No effect on kidneys, liver and blood were found differing from the control group or from the normal area at the highest concentrations. NOAEL[6] was therefore the highest exposure dose: 200 mg/kg bw/d for rats and 550 mg/kg bw/d for mice. No signs of carcinogenic effects were observed in neither rats nor mice (23).
Cinnamal was tested for mutegenic (genotixic) properties in Ames’ test and other analyses in cells with conflicting results. The results of the main part of the analyses in multicellular organisms were negative (11), (18). It was not found likely that cinnamal has a genotoxic effect (23).
In a 2-generation study with rats administered 5 mg cinnamal/kg bw there were no effect on growth, development or survival of parents or pups, but the fat content in the liver increased by 20% in the first generation and 22% in the second generation (20).
Female rats administered a daily dose of 5 mg/kg bw from the 7th to the 17th day of gestation were not affected by the dosage, but delayed development of the embryos and effects on the urinary tract of the embryos were observed. Apparently, embryos are more sensitive than dams (20).
No information was found on inhalation of cinnamal.
Critical effect
The critical effect of cinnamal was assessed to be contactallergy. Because of the allergenic potential of cinnamal, humans allergic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
No lower limit for the contactallergy is known. MoS[7]-calculation is based on effects other than allergy. The below calculation is based on NOEL and LOEL[8] from the above study with administration of rats for 13 weeks: NOAEL from the 2-year study with rats of 200 mg/kg bw/d is the highest dose in a study with no toxicity observed.
Table 7-2 Summary of data used for calcualtion of MoS for cinnamal
| Toxicological data (animals) | |
| LD50, (mg/kg bw), oral, rat | 2220 (18,19) |
| NOEL, (mg/kg bw/d), intake (13 weeks), rats | 620 (11) |
| LOEL, (mg/kg bw/d), intake (13 weeks), rats | 1250 (11) |
7.3 Citral
Occurrence and application
Citral is seldomly applied as fragrances in perfumes (22).
Citral has a strong lemon fragrance. The substance is applied as flavour additive in foodstuffs. Intake of the substance is estimated to be 6849 µg/person/d (114 µg/kg bw/d) in and 6990 µg/person/d (117 µg/kg bw/d) in the USA. In the USA, a little more than half of the substance is considered to originate from natural sources. (23).It occcurs in lemon grass in its natural form (19).
JECFA (Joint Expert Committee for Food Additives) has established a group-ADI (Acceptable Daily Intake) (through foodstuffs)) for citral, citronellol, geranyl acetate, linalool og linalyl acetate, of 0-0.5 mg/kg bw (calculated as citral) (25).
Identification
Citral is a terpenaldehyde. The substance is a mixture of the aldehydes geranial 55-70 % (trans-structure – the shown) and neral 35-45 % (cis-structure) (26).
| Chemical name | Citral |
| Synonym | 3,7-Dimethyl-2,6-octadienal |
| CAS-No. | 5392-40-5 |
| EINECS No. | 226-394-6 |
| Molecular formula | C10H16O |
| Molecular structure | 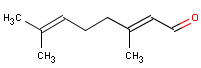 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Xi; R 43 Listed, as the substance is assessed to be allergenic at skin contact. and is 1 of th 26 fragrance allergens.assessed by SCCNFP. Fragrances are declared in cosmetics if applied in quantities above 0.01% in products which are cleaned and 0.001% in products which are not cleaned. |
Physical/Chemical properties
| Physical state | Liquid |
| Molecular weight (g/mol) | 152.3 (19) |
| Melting point °C | < -10 °C (24) |
| Boiling point, °C | 226-228 °C (24) |
| Vapour pressure (Pa) | < 130 Pa ved 100 °C (24) |
| Octanol/water partition coefficient (log Pow) | 2.8 for neral og 3.0 for geranial ved 25 °C (24) |
| Water solubility (mg/L) | 590 mg/L ved 25 °C (24) |
WHO has established a NOEL[9] for citral of 100 mg/kg bw/d for rats (25).
7.3.1.1 Acute toxicity
The LD50-value by oral administration of citral in rats was found to be 4960 mg/kg bw (26)
WHO has found that citral metabolises to harmless substances that are quickly excreted quickly through the kidneys (25), (26)
The dermal LD50-value for rabbits was found to be 2250 mg/kg bw (26).
7.3.1.2 Local irritation
Testing in rabbits showed that citral was found skin irritating, but not eye irritating (26).
7.3.1.3 Allergy
EU Scientific Committe (SCCP) has registered citral on the list of fragrances. The fragrances on the list are well known allergens. Many cases of allergy in consumers have been reported. In two described studies 4 of 228 patients (1.7%) and 19 of 1855 patients (1%) respectively showed allergic reactions to citral (18).
Citral was found strongly sensitizing in guinea pigs. Between 12 and 64% voluntary test persons were sensitized using the Human Maximization Test in humans (18).
Citral belongs to fragrances which should no be used separately but only in mixtures with substances depressing the sensitizing effect of the substance (22). IFRA (International Frangrance Association) recommends that citral is only applied in products together with substances preventing a sensitizing effect, for example 25% d-limonenee, mixed citrus terpenes eller a-pinenes (22).
7.3.1.4 Long-term, repeated exposure
Citral in micro encapsulated form was administered 3 groups of 10 rats of each sex in their daily diet during 14 weeks, the daily dose being 345, 820 and 1785 mg/kg bw for males and 335, 675, and 1130 mg/kg bw for females. All doses showed effects on kidneys in males. In females, the highest dose showed low increase and reduction of the bone marrow. In this study, a NOEL2 was found to be lower than 345 mg/kg bw/d for male rats. In female rats, NOEL was 645 mg/kg bw (25).
A 2-year study group of 50 rats of each sex was exposed through their diet to 0, 50, 10 and 210 mg/kg/bw/d. In the male group was found a dose-dependent increase of the mineralization of the kidneys, which was interpreted as an increase of normally occurring deviation in the rat strain. In this study, NOEL for citral was 100 mg/kg bw/d due to reduced increase in female rats at highest dose. There was no indication that citral is carcinogenic in rats (25).
Many studies with long-term repeated exposure in rats were performed, but the studies referred are the latest and were performed by NTP (National Toxicology Program) in the USA. Their laboratories are considered to be very reliable.
Citral has been tested in an Ames’ test and in a number of other tests with bacteria and mammal cells. It has also been tested for genotoxic properties after administration in living mice. Almost all results were negative (25)
Daily doses of citral of until 1000 mg/kg bw/day before, during and after mating did not effect the fertility of rats. In the high dose analyses, microscopy showed changes in the stomach of the experimental animals. The embryos showed reduced growth in the period of lactation (26).
A concentration of 423 mg citral/ m3 inspirated air resulted in reduced growth and abortation and death among pregnant female rats exposed for 6 hours/d on the 6-15 day of gestation, but was not teratogenetic (26).
Critical effect
The critical effect of citral is assessed to be contactallergy. Because of the allergenic potential of citral, humans allergenic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
No lower limit for the contactallergy is known. MoS[10]-calculation is based on effects other than allergy. The below calculation is based on NOEL for 2 years:
Table 8-3 . Summary of data used for calculation of MoS for citral
| Toxicological data (animals) | |
| LD50, (mg/kg bw), oral, rat | 4960 (24) |
| NOEL, (mg/kg bw/d), intake (2 year), rat | 100 (23) |
| LOEL, (mg/kg bw/d), intak (14 weeks), rat | 345 (23) |
7.4 Citronellol
7.4.1.1 Occurrence and application
Citronellol is applied as perfumery material in perfumes, often in flower fragrancies. The substance is also applied as a flavour additive in foodstuffs. Intake of the substance is estimated to be 370 µg/person/d (6,2 µg/kg bw/d) in Europe and 771 µg/person/d (13 µg/kg bw/d) in the USA, Much of this originates from natural ingredients in foodstuffs (23). Citronellol also occurs in rose- and geranium oil and as gland secretion in alligators. (19).
Identifikation
Citronellol består af en terpenalkohol.
| Chemical name | Citronellol |
| Synonyms | 3,7-Dimethyl-6-octen-1-ol, dl-Citronellol |
| CAS-No. | 106-22-9 |
| EINECS No. | 203-375-0 |
| Molecular formula | C10H20O |
| Molecular structure |  |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (14) |
Not classified Listed, as the substance is assessed to be allergenic at skin contact. The fragrance is stated on the product label of cosmetics if applied in quantities above 0.01% in products which are cleaned and 0.001% in products which are not cleaned. |
Physical-chemical properties
| Physical state: | Liquid |
| Molecular weight (g/mol) | 156.3 (19) |
| Melting point °C | No information |
| Boiling point, °C | No information |
| Vapopour pressure (Pa) | No information |
| Octanol/water partition coefficient (log Pow) | No information |
| Water solubility (mg/L) | No information |
WHO har established a NOEL[11] for citronellol of > 51 mg/kg bw/d for male rats (25).
JEFCA (Joint Expert Committee for Food Additives) has established a group-ADI (Acceptable Daily Intake) of 0-0.5 mg/kg bw/d expressed as citral for the group of terpene-containing flavour additives (citral, geranylacetate, citronellol, linalool and linalyl acetate) (1).
Acute toxicity
LD50-values at oral administration of citronellol in rats were found to be 3450 mg/kg bw (21), (25) and 5000 mg/kg bw (21).
WHO assessed that citronellol most likely metabolises to harmless substances based on data on a corresponding substance (geraniol). The end product is excretred through the urine (25).
The LD50-value of citronellol applied on the skin of rabbits was found to be 2650 mg/kg bw (21).
Local irritation
The effect of citronellol is moderately irritating to the skin of humans for 48 timer, and seriously irritating to the skin of rabbit and guinea pig for 24 hours (21). No information was found on eye irritation.
Allergy
The Scientific Committe of EU SCCP has registered Cinnamal on the list of fragrances. The fragrances on the list are well known allergens, on which, however, not many cases on allergy in consumers are reported. Two out of 20 perfume allergy sufferers (35%) and 2 out of 119 (1.7%) patients suffering from cosmetic allergy showed allergic reactions to citronellol (18).
Long-term, repeated exposure
10 rats of each sex were administered citronellol (mixed with equal parts of linalool) in the diet daily for 12 weeks. The doses were 51 mg/kg bw/d for males and 56 mg/kg bw for females. At this concentration, no effects on kidneys, liver, blood were found differing from the control group or from the normal area. In this study, NOEL for citronellel was found to be higher than 51 and 56 mg/kg bw in male and female rats respectively) (25).
The result of an Ames’ test and another bacterium test with Citronellol was negative (25).
TCLo[12] for citronellol for rats was found to be 1.3 mg/m³ air by inhalation for 4 hours. By repeated inhalation for 4 hours daily for 3 days, TCLo decreased to 0.3 mg/m³ air. In both analyses, the rats showed behavioural changes (21).
Critical effect
The critical effect of citronellol is assessed to be contactallergy. Because of the allergenic potential of citronellol, humans allergic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
No lower limit for the contactallergy is known. MoS[13]-calculation is based on effects other than allergy. The below calculation is based on NOEL from the above study with administration of rats for 12 weeks.
Table 7-4 Summary of data used for calculation of MoS for citronellol
| Toxicological data (animals) | |
| LD50, (mg/kg bw), oral, rat | 3450 (19,23) |
| NOEL, (mg/kg bw/d), intake, male rat | > 51 (23) |
7.5 Peru balsam
Occurence and application
Peru balsam is extracted from the cortex of the tree Myroxolon balsamum pereiae that grows in Central America, e.g. along the coast of El Salvador. Peru balsam is applied as fragrances and as a flavour additive in foodstuffs. Peru balsam is weakly antiseptic, and down through the ages it has been applied in pharmaceuticals for wound treatment and for skin problems, such as eczema and itching. In addition, it has been applied in pharmaceuticals for the treatment of inconveniences due to hemorrhoids (27) (28).
Identification
Peru balsam is mainly composed of 50-60% of a mixture of esthers of cinnamateic acid and benzo acid, with benzyl cinnamate, benzyl benzoate and cinnamyl cinnamate as the main components. Also small amounts of vanillin, eugenol and free cinnamateic acid are contained (19), (27). A great number of other substances have been identified in Peru balsam, but the exact composition is not known (29).
| Chemical name | Peru balsam |
| Synonym | Myroxylon pereirae Klotzsch |
| CAS-No. | 8007-00-9 |
| EINECS No. | 232-352-8 |
| Molecular formula | Not indicated |
| Molecular structure | Not indicated |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (30) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Not classified Peru balsam contains several unwanted fragrances which are assessed to be allergenic by skin contact. Fragrance allergens are stated on the product label of cosmetics, if they are applied in quantities above 0.01% in products, which are cleaned and 0.001 % in products which are not. Peru balsam is prohibited in cosmetic products annex 2 “løbenummer nr 1163” (1). |
Physical-chemical properties
| Physical state | Braunish viscous liquid |
| Molecular weight (g/mol) | No information |
| Melting point °C | No information |
| Boiling point, °C | No information |
| Vapopour pressure (Pa) | No information |
| Octanol/water partition coefficient (log Pow) | No information |
| Water solubility (mg/L) | Insoluble in water |
Acute toxicity
LD50-values of Peru balsam orally administered to rats were found to be > 5.000 mg/kg bw, and dermally applied to rabbits > 10,000 mg/kg bw (21).
No data for absorption of Peru balsam through the skin were found.
Local irritation
Peru balsam applied to the skin in concentrations of 25% caused moderate irritation in children and mild reaction in women. Peru balsam in concentrations of 25% showed moderate irritation of the skin in tests with rabbits (21).
Allergy
Contact with Peru balsam caused allergy in both children and adults. In a study of 101 children below 15 years 24% of the children showed allergic reaction to Peru balsam, and of 2000 examined adults, 6% showed allergic reaction to the substance. Nettle rash by contact with Peru balsam is not unusual (19). Peru balsam har shown phototoxic reaction (32).
The Scientific Committe of EU SCCP has registered the main components in Peru balsam, benzyl cinnamate and benzyl benzoate on the list of fragrances. The fragrances on the list are well known allergens, of which however not many cases on allergy in consumers are reported. In 103 cases of allergy caused by Peru balsam, 12% of the patients showed positive reaction to benzyl benzoate and 18% of the patients positive reaction to benzyl cinnamate (18). Patients with cronic ezcema and allergy caused by Peru balsam, showed improvement by avoiding foodstuffs containing balsam components in e.g. foodstuffs spiced with cinnamon, lemon fruits and vanilla. (19).
Caused by the risk of allergy, IFRA has prohibited Peru balsam as an fragrance substance in cosmetic (22). The substance is today prohibited in cosmetic products (1).
Long-term, repeated exposure
No information on mutagenic, carcinogenic and reprotoxic effects in Peru balsam was found.
No information on effects after inhalation of Peru balsam has been found.
Critical effect
The main components of Peru balsam is benzyl cinnamate, benzyl benzoate and cinnamyl cinnamate. According to EU, the substances are assessed to be allergenic. Because of the allergenic potential of Peru balsam, humans allergic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
Tabel 7-5 Summary of data used for calculation of MoS for Peru balsam.
| Toxicological data (animal) | |
| LD50, (mg/kg bw), oral, rat | >5000 (21) |
8 User exposure
The assessment of exposure to fragrance substances in massage and body oils is based on the analysed content of fragrance substances in selected oils on the Danish market (Chapter 6) and is performed in accordance with the principles in the EU Technical Document (TGD) (33) and SCCNFP’s guidelines (34). The internal body dose (Systemic Exposure Dose, SED) is estimated in a worst case scenario for 2 model persons by applying standard parameters from TGD. The safety risk of exposure to fragrance substances in massage, baby, body and essential oils is assessed by calculating the MoS (Margin of Safety). The calculation is based on NO(A)EL, possibly LOEL of the toxicological profiles prepared in this survey project and previous survey projects (13), (14) and the estimated SED of the exposure assessment.
8.1 Exposure assessment
Fragrance substances in massage and body oils can be absorbed through the skin. The fragrance substances are volatile at room- and skin temperature and can be inhaled after evaporation from the greased areas of the skin. Therefore, it is relevant to include calculation of inhalation in the dose estimation. However, it has not been possible to procure information about the effect of fragrance substances by inhalation. Owing to this, assessment of the systemic dose (SED) is only performed by dermal exposure and absorption through the skin. A worst-case scenario assuming that the total amount of fragrance substances in the applied oil is absorbed through the skin is used to estimate the dose. Therefore, calculation of absorption by inhalation makes no different.
The EU has assessed 26 fragrance allergens. 19 of them were found in the analysed massage, baby, body oils and essential oils. In the massage, baby and body oils were found 14 different fragrance substances and in the essential oils 16. The daily exposure of these 19 fragrance substances has been calculated for two model persons, an adult of 60 kg and a baby of 5 kg (< 1 year). The daily exposure was calculated for the highest measured content of fragrance substances in massage- or body oils. A separate scenario for the essential oils was set up, because they are mixed with a basic oil in the proportion 1:10, before application.
The following calculation is used as worst-case scenario with dermal absorption as the only exposure route for massage, baby and body oils.
Weight of person, adult 60 kg
Weight of person, baby < 1 year (35) 5 kg
Number of applications per day (33) 1
Applied amount per application for adult (33) 10 g
Applied amount per application for baby 5 g
Material in massage oils
(Example: Benzyl benzoate, cf. Table 6-2 or 6-6): 1150 mg/kg
Absorption through the skin 100%
It is assessed that the amount of massage, baby or body oil, which is applied to a baby, does not exceed 50% of the amount applied to adults. The estimate is based on the body area of babies (~ 0.5 m²), which is considerably smaller than that of adults (~ 1.8 m²). As a smooth layer is applied to the body, the body area is decisive for the amount of applied oil (36).
Daily exposure, benzyl benzoate, adult:
![]()
Daily exposure, benzylbenzoate, baby (< 1 year):
![]()
The daily dose (SED) of the 14 fragrance substances in massage and body oils is calculated as mg per kg bodyweight per day (mg/bw/day) for the two model persons. The calculation is based on the maximum content of fragrance substances found in the oils in products for adults (chapter 6). The results are shown in table 8-1-
Table 8-1 Daily dose of two model persons of fragrance substances found in selected massage and body oils on the Danish market in the spring of 2005
| Fragrance substances | Max. content in the products (mg/kg) | Daily dose, adult, 60 kg (SED) (mg/kg bw/d) |
Daily dose, baby, 5 kg, (SED) (mg/kg bw/d) |
| Benzyl alkohol | 220 | 0.037 | 0.22 |
| Benzyl benzoate | 1150 | 0.19 | 1.15 |
| Benzyl cinnamate | 245 | 0.041 | 0.25 |
| Benzyl salicylate | 125 | 0.021 | 0.13 |
| Cinnamyl alkohol | 45,5 | 0.0076 | 0.046 |
| Cinnamal | 210 | 0.035 | 0.21 |
| Citral | 1750 | 0.29 | 1.8 |
| Citronellol | 460 | 0.077 | 0.46 |
| Coumarin | 330 | 0.055 | 0.33 |
| Eugenol | 55 | 0.0092 | 0.055 |
| Farnesol | 135 | 0.023 | 0.14 |
| Geraniol | 1250 | 0.21 | 1.25 |
| d-Limonene | 9250 | 1.54 | 9.25 |
| Linalool | 8050 | 1.34 | 8.05 |
The daily dose (SED) of the 16 fragrance substances in essential oils is calculated as mg per kg bodyweight per day (mg/kg/day) for the two model persons. The calculation is based on the highest content of fragrance substances found in the oils, cf. chapter 6. Before application, the user will mix the essential oil with a basic oil. According to the instructions that often follow the purchased essential oils, 6 drops (» 1 ml) are mixed with approx. 10 ml basic oil before application, which means that the essential oil is diluted approx. 10 times. The results can be seen in table 8-2.
Table 8-1 Daily dose of two model persons of fragrance substances found in selected essential oils mixed with basic oils on the Danish market in the spring of 2005
| Fragrance substance | Max. content in the products (mg/kg) | Max. content in the mixed products (mg/kg) | Daily dose, Adult, 60 kg (SED) (mg/kg bw/d) |
Daily dose, Baby, 5 kg. (SED) (mg/kg bw/d) |
| Benzyl alkohol | 140 | 14 | 0.0023 | 0.014 |
| Benzyl benzoate | 79.5 | 7.95 | 0.0013 | 0.0080 |
| Cinnamyl alkohol | 13.5 | 1.35 | 0.00023 | 0.0014 |
| Cinnamal | 14.5 | 1.45 | 0.00024 | 0.0015 |
| Citral | 32500 | 3250 | 0.54 | 3.3 |
| Citronellol | 47500 | 4750 | 0.79 | 4.8 |
| Eugenol | 225 | 22.5 | 0.0038 | 0.023 |
| Farnesol | 76 | 7.6 | 0.0013 | 0.0076 |
| Geraniol | 23000 | 2300 | 0.38 | 2.3 |
| Hexylcinnam aldehyde |
67.5 | 6.75 | 0.0011 | 0.0068 |
| Hydroxycitronellal | 41 | 4.1 | 0.00068 | 0.0041 |
| α-Isomethylionon | 79.5 | 7.95 | 0.0013 | 0.008 |
| Lillial | 22.5 | 2.25 | 0. 00038 | 0.0023 |
| d-Limonene | 41000 | 4100 | 0.68 | 4.1 |
| Linalool | 7600 | 760 | 0.13 | 0.76 |
| Lyral | 21.5 | 2.15 | 0.00036 | 0.0022 |
8.2 Safety assessment of selected fragrances
Normally, a cosmetic product with a margin of safety (MoS) of more than 100 is considered to be a product exposing the user to an acceptable (minimum) safety risk. When calculating the MoS, a safety factor of 10 for extrapolation of data from animals to humans and a safety factor of 10 for particularly sensitive human individuals are taken into account.
Based on the demonstrated NO(A)EL values in the project and previous surveys, a margin of safety (MoS) is calculated for the highest concentrations of fragrance substances found in massage, baby and body oils as well as in mixed essential oils (table 8-3 respectively 8-4). As there are no lower limit when a substance causes allergy, these safety calculations are not based on the critical effect of many fragrance substances: Allergy.
Table 8-3 Safety assessment of the exposure of two persons to 14 fragrance substances found in selected massage and body oils on the Danish market in the spring of 2005 calculated as the margin of safety (MoS)
| Perfumery material | NO(A)EL (mg/kg bw/d) |
Daily dose, adult, 60 kg (SED) (mg/kg bw/d) |
Daily dose, baby, 5 kg, (SED) (mg/kg bw/d) |
MoS[14] (Adult/Baby)[15] |
| Benzyl alkohol | 5 1) * | 0.037 | 0.22 | 135 / 23 |
| Benzyl benzoate | 595 1) | 0.19 | 1.15 | 3130 / 517 |
| Benzyl cinnamate | 500 2) | 0.041 | 0.25 | 12,200 / 2,000 |
| Benzyl salicylat | - | 0.021 | 0.13 | - |
| Cinnamyl alkohol | - | 0.0076 | 0.046 | - |
| Cinnamal | 620 2) | 0.035 | 0.21 | 17,700 / 2,900 |
| Citral | 100 2) | 0.29 | 1.75 | 350 / 50 |
| Citronellol | 50 | 0.077 | 0.46 | 650 / 110 |
| Coumarin | 10 3) | 0.055 | 0.33 | 182 / 30 |
| Eugenol | 79.3 4) | 0.0092 | 0.055 | 8,620 / 1,440 |
| Farnesol | - | 0.023 | 0.14 | - |
| Geraniol | 78.3 4) | 0.21 | 1.25 | 373 / 63 |
| d-Limonene | 250 1) | 1.54 | 9.25 | 162 / 27 |
| Linalool | 50 4) | 1.34 | 8.05 | 40 / 6 |
| 1) Survey of stain removers (14) 2) Chapter 8 3) Survey of hand soap (37) 4) Survey of lip care, (13) * Based on acceptable daily intake (ADI) |
||||
Table 8-4 Safety evaluation of the exposure of two model persons to 16 fragrances found in selected essential oils on the Danish market in the spring of 2005 calculated as margin of safety (MoS). The safety is assessed for essential oils mixed in basic oil in the proportion 1:10
| Perfumery material | NO(A)EL (mg/kg bw/d) |
Daily dose, adult, 60 kg (SED) (mg/kg bw/d) |
Daily dose, baby, 5 kg, (SED) (mg/kg bw/d) |
MoS (Adult/Baby) |
| Benzyl alkohol | 5 1) * | 0.0023 | 0.014 | 2,174 / 357 |
| Benzyl benzoate | 595 1) | 0.0013 | 0.0080 | > 74,000 (children) |
| Cinnamyl alkohol | - | 0.00023 | 0.0014 | - |
| Cinnamal | 620 2) | 0.00024 | 0.0015 | > 400,00 (children) |
| Citral | 100 2) | 0.54 | 3.3 | 185 / 65 |
| Citronellol | 50 2) | 0.79 | 4.8 | 60 / 10 |
| Eugenol | 79.3 3) | 0.0038 | 0.023 | 20,600 / 3,450 |
| Farnesol | - | 0.0013 | 0.0076 | - |
| Geraniol | 78.3 3) | 0.38 | 2.3 | 206 / 34 |
| Hexylcinnamaldehyde | - | 0.0011 | 0.0068 | - |
| Hydroxycitronellal | - | 0.00068 | 0.0041 | - |
| α-Isomethylionon | - | 0.0013 | 0.008 | - |
| Lillial | - | 0. 00038 | 0.0023 | - |
| d-Limonene | 250 1) | 0.68 | 4.1 | 368 / 61 |
| Linalool | 50 3) | 0.13 | 0.76 | 385 / 66 |
| Lyral | - | 0.00036 | 0.0022 | - |
| 1) Survey of stain removers (10) 2) Chapter 8 3) Survey of hand soap (34) 4) Survey of lip care, (9) * Based on acceptable daily intake (ADI) |
||||
The calculated margin of safety (MoS) is for several of the found fragrance substances greater than 100, which indicates that the safety risk when applying the product is acceptable.
For other fragrance substances the calculated safety margin is less than 100 indicating that the product may be hazardous to health.. The concentrations of linalool found in massage and body oils for adults are so high that the safety margin is below 100. When essential oils mixed with basic oil are applied, citronellol is seen to cause a safety margin below 100.
The maximum concentrations of fragrance substances are all found in products marketed to adults. There is only a very small probability that these oils are applied to babies, but to be safe, exposure to babies was also assessed. If applied to babies, the substances benzyl alcohol, citral, citronellol, coumarin, geraniol, d-limonene and linalool showed a safety limit below 100. Consequently it is assessed that there may be a health risk for babies if fragranced massage and body oils as well as basic oils mixed with essential oils intended for adults are to be applied to babies.
Two baby oils were tested for content of fragrance substances. The content of d-limonene of one of the products (no. 17) was so high that the safety limit was only 50 compared to the critical effect (liver damages) of d-limonene. d-Limonene was not declared on the product. The other product (no. 18) contained two fragrance substances; both in low concentrations. As only two baby oils were analyses, the number of products is not sufficient to conclude in general that the concentration of fragrance substances in baby oils may be hazardous to health.
Of the mentioned fragrance substances, the safety limit of linalool and citronellol for adults was calculated. Linalool has been assessed previously in the Danish EPA’s survey project (13). The NOEL value for linalool in this project is indicated to be 50 mg/kg bodyweight/day with liver damage as critical effect. Compared to the calculated daily exposure (massage or body oil) a MoS is calculated to be 6 for children and 40 for adults. Linanool is observed to be quickly absorbed through the skin by massage with an oil containing linalool, but is also seen to be excreted again from the body through the urine (13). SCCNFP has listed linalool as a perfumery material causing allergy, but the number of reports referred to about allergy in consumers is limited. 1 and 3 cases of contact allergy from two investigations of 119 and 75 patients respectively have been reported corresponding to 0.8% and 5% of the patients with cosmetics eczema (13).
Citronellol has been assessed in Chapter 7.4 above. A NOEL of >51 mg/kg/bodyweight/day was indicated, which compared with the calculated daily exposure (mixed essential oil) resulted in a MoS of 10 for children and 60 for adults, see table 8-4.
At a NOEL value of d-limonene of 250 mg/kg bodyweight/day by liver damages, a low MoS for d-limonene has been calculated for babies. SCCNFP has listed d-limonene as a perfumery material causing allergy. However, not many cases of allergy in the consumers were reported. 1 and 3 cases of contact dermatitis from two investigations of 119 and 75 patients respectively have been reported corresponding to 0.8% and 5% of patients with cosmetics eczema (13).
In accordance with Danish legislation, Peru balsam is prohibited in cosmetic product (1). This is caused by the fact that many investigations have proved occurrence of allergy in the substance (31). Consequently, we have assessed that the content of Peru balsam in massage-and body oils, irrespective of the amount, is not appropriate, because of the oil being in contact with the skin.
Due to the carcinogenic effect of methyleugenol, SCCNFP has established a limit for the content of methyleugenol in leave-on cosmetic products of 0.0002 weight% (10). Methyleugenol containing 160 mg/kg (0.016 weight%) was found in one of the analysed essential oils. Mixed in a basic oil in a ratio of 1:10, this corresponds to approx. 16 mg/kg (0.0016 weight%). This means that the content of methyleugenol in the product is approx. 8 times above the legal maximum limit for methylgeugenol in leave-on cosmetic products. The content of methyleugenol is therefore assessed to be hazardous to health when contained in the product.
SCCNFP has assessed 26 fragrances as allergens by skin contact. As there is no “zero effect level” for this effect, it is essential to emphasize that persons with perfume allergies or especially sensitive skin should avoid skin contact with these substances (8). There is reason to be aware of the content of perfume in the products since massage and body oils or essential oils for use in self mixed massage oils should are in fact applied to a great area of the skin.
9 Summary and conclusions
Massage, baby and body oils as well as essential oils are in a grey area as to which legislation applies to the products. To determine the existing legislation it is necessary to carry out an overall assessment of several factors for each type of product such as target group, claims, objective, labelling, packaging and the general appearance.
An assessment of all the factors has been made for each of the purchased products., This assessment was made according to EU’s guidance on borderline products. The assessment show that 15 products are covered by the cosmetic legislation. The remaining 13 are covered by the legislation on chemicals substances and products and due to this covered by the rules of classification and labelling. Among other things this means that there are specific rules regarding labelling of sensitising substances.
An evaluation of the purchased 28 products illustrates that the producers of massage- baby- and body oils in most cases follow the cosmetics regulations governing the INCI list of ingredients.
The tested essential oils were also assessed not to comply with the regulations on labelling of chemical substances and products. The test results illustrated that the essential oils marketed in concentrated form contained more than 0.1% of one or more fragrance allergens. Because of insufficient allergy warning on the label, the labelling regulations had not been observed.
According to the products' list of ingredients, massage, baby and body oils on the Danish market are primarily composed of non-volatile and volatile oils. Other substances are found in very limited amounts. Only a few products are based solely on mineral oils and other additives such as pigments and preservatives.
The result of the chemical analysis of 16 massage, baby and body oils and essential oils (7 massage oils, 2 baby oils, 3 body oils and 4 essential oils) showed that 15 of the products contain one or more of the fragrance allergens. One of the 4 products analysed for methyleugenole contained a concentration of the substance 8 times the legal maximum limit for methyleugenole in a cosmetic leave-on product. One product was analysed for content of Peru balsam and several of the subcomponents of Peru balsam were detected. This indicates presence of the substance which is unwanted in cosmetic products. safrol and methylsalicylate were not found in the analysed products.
The limit of declaration for the 26 fragrance allergens in leave on products assessed by the EU is 0.001 weight percentage. In 94% of the analysed products, 1 or more of the 26 fragrance allergens are contained in concentrations of 0.001 weight percentage and are to be stated on the label.
For chemical products the regulation among other things states that if the product contains more than 0,1 % of a sensitizer the product must be labelled: ”Contains (chemical name). May cause allergy”.
Safety/toxicological profiles were prepared for the substances benzyl cinnamate, cinnamal, citral, citronellol and Peru balsam, which were all present in one or more of the analysed products. In addition, safety/toxicological profiles from previous surveys have been used for assessment of user exposure.
Exposure scenarios were drawn up for two average models (adult, baby) of the found fragrance allergens (a total of 19) and the user exposure was assessed. The result showed that the largest concentrations of fragrance allergens are found in products for adults. For the fragrance linalool a margin of safety (MoS) below 100 was found which indicates a health risk. In addition, the safety assessment showed that perfumed massage and body oils as well as basic oils mixed with essential oils for adults should not be used on babies.
Two baby oils were tested. In one of the products the content of d-limonene was so high that the safety margin of the product was determined to be below 100. Based on this the product is assessed to present a health risk to babies. However, as only two baby oils have been analysed the number is too small to conclude that the concentration of fragrance allergens in baby oils is a general health concern.
SCCNFP has assessed the 26 fragrance substances to be allergenic by skin contact. As this effect has no “zero effect level”, it is important to emphasize that for persons with sensitive skin or perfume allergy skin, contact with these substances should be avoided. Likewise these persons should avoid using the massage, baby and body oils and basic oils mixed with essential oils containing the 26 fragrance substances as they are in fact applied to a large area of the skin. These allergenic substances must be stated on the product label of cosmetics products, in order for the consumer to have the possibility not to choose products containing unwanted fragrance substances.
10 References
1. Miljøministeriets bekendtgørelse om kosmetiske produkter, nr. 74 af 14. januar 2005. Now replaced by Miljøminsteriets bekendtgørelse nr. 422 af 4. maj 2006.
2. Informationscenteret for Miljø og Sundhed. Vurdering af massageolier til børn. Jan 2003. Available from: http://www.miljoeogsundhed.dk/default.aspx?node=4370.
3. Lovell CR. Plants and the Skin. London : Blackwell Scientific Publications: 1993.
4. Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers (SCCNFP). Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers Concerning Essential Oils. SCCNFP/0673/03. 2003
5. Miljøministeriets bekendtgørelse om klassificering, emballering, mærkning salg og opbevaring af kemiske stoffer og produkter, nr. 329 af 16. maj 2002.
6. Cosmetic Ingredient Review. Final Report on the Safety Assessment of Triticum vulgare germ oil. CTFA Scientific/ Regulatory Reference CD-ROM; 2000
7. Patri G, Silano V. Plant Preparations Used As Ingredients of Cosmetic Products = Préparations De Plantes Utilisées En Tant Que Matières Premières Dans Les Produits Cosmétiques. Strasbourg: Council of Europe;Conseil de l'Europe; 1989.
8. European Commission. Opinion Concerning Fragrance Allergy in Consumers. SCCNFP. 8. Dec1999. Available from: http://europa.eu.int/comm/health/
ph_risk/committees/sccp/documents/out98_en.pdf.
9. Patri F, Silano V. Plants in Cosmetics : Plants and Plant Preparations Used As Ingredients for Cosmetic Products. Vol.1 = Les Plantes Dans Les Cosmétiques : Plantes Et Préparations à Base De Plantes Utilisées En Tant Qu'Ingédients Dans Les Produits Cosmétiques. Vol.1. Strasbourg : Council of Europe Publishing; 2002. Health Protection of the Consumer;Protection De La Santé Du Consommateur).
10. European Commission. Opinion concerning Methyleugenol adopted by the SCCNFP during the 14th plenary meeting of 24 October 2000. SCCNFP. 2000 Oct 24. Available from: http://europa.eu.int/comm/health/
ph_risk/committees/sccp/sccp_opinions_en.htm.
11. Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers (SCCNFP). Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers Concerning An update of the intial list of perfumery materials which must not form part of fragrance compounds used in cosmetic products. 2003
12. European Commission. Kommissionens Direktiv 2005/42/EF af 20. juni 2005 om ændring med henblik på tilpasning til den tekniske udvikling af bilag II, IV og VI til Rådets direktiv 76/768/EØF vedrørende kosmetiske midler. Den Europæiske Unions Tidende 2005
13. Larsen JR, Holmberg RD. Survey of chemical substances in consumer products No. 55 2005. Survey of lip care products with fragrance and flavour. Danish EPA 2005.
14. Engelund B, Hùglund L, Skjødt D. Survey of chemical substances in consumer products no. 43 2003.Mapping of stain removers. Danish EPA: 2004.
15. WHO FOOD ADDITIVES SERIES. Cinnamic alcohol and related substances. 1-50. 5-16-2005.
16. List of Undesirable Substances 2004, Environmental Review no. 15 2004. Danish EPA
17. ChemFinder on-line database. 2005.
18. THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS (SCCNFP) INTENDED FOR CONSUMERS. Opinion concerning fragrance allergy in consumers. A review of the problem. Analysis for the need for appropriate consumer information and identification of consumer allergens; 1999. http://europa.eu.int/comm/health/
ph_risk/committees/sccp/documents/out98_en.pdf
19. Rietschel RL, Fowler jr JF, editors. Fisher's Contact Dermatitis. 5th ed. Philadelphia (PA): Williams & Wilkins; 2001.
20. European Communities, editor. IUCLID [database on the Internet]. European Communities, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau. [updated 2000]. Available from: http://ecb.jrc.it/esis/.
21. RTECS®: Registry of Toxic Effects of Chemical Substances. National Institute for Occupational Safety and Health, Cincinnati, Ohio. Thomson MICROMEDEX®, Greenwood Village, Colorado, USA [updated 2004]. Available from: http://csi.micromedex.com.
22. IFRA (International Fragrance Association) : Code of Practice = IFRA (International Fragrance Association) : Code De Bons = IFRA (International Fragrance Association): Verfahrenskodex. Geneva: International Frangrance Association; 1999.
23. Bickers D. A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients. Calow PGHHJMRAESJHSIGSRLTH. Food and Chemical Toxicology 43, 799-836. 2005.
24. Frosch PJ, Johansen JD, White IR, editors. Fragrances : Beneficial and Adverse Effects : Papers From a Thematic Symposium, London 1996. Berlin : Springer: 1998.
25. WHO FOOD ADDITIVES SERIES: 52. Aliphatic branched-chain saturated and unsaturated alcohols, aldehydes, acids, and related esters. 1-37. 2004.
26. OECD SIDS. Citral, CAS No:5392-40-5. 1-113. 2001. UNEP Publications.
27. O'Neil MJ, et al., editors. The Merck Index : an Encyclopedia of Chemicals, Drugs, and Biologicals. 13 ed. Whitehouse Station, N.J.: Merck & Co; 2001.
28. Reynolds JEF, editor. Martindale : the Extra Pharmacopoeia. 31 ed. London: Royal Pharmaceutical Society; 1996.
29. de Groot AC, Frosch PJ. Adverse reactions to fragrances. Contact Dermatitis. 1996;36(1997):57-86.
30. Miljøministeriets bekendtgørelse om listen over farlige stoffer nr 923 af 28. september 2005, 923 af 28. september 2005 , (2005)
31. European Commission. AN UPDATE OF THE INITIAL LIST OF PERFUMERY MATERIALS WHICH MUST NOT FORM PART OF FRAGRANCE COMPOUNDS USED IN COSMETIC PRODUCTS. SCCNFP. 2003 Dec 9. Available from: http://europa.eu.int/comm/health/
ph_risk/committees/sccp/documents/out251_en.pdf.
32. de Groot AC, Weyland JW, Nater JP. Unwanted Effects of Cosmetics and Drugs Used in Dermatology. 3 ed. Amsterdam: Elsevier: 1994.
33. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) 1488/94 on Risk Assessment for Existing Substances and Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market. Part I. 2nd ed. Luxembourg: Office for Official Publications of the European Communities: European Commission, Joint Research Centre, European Chemicals Bureau, Institute for Health and Consumer Protection; 2003.
34. European Commission. The SCCNFP´s Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation. 5th Revision. SCCNFP. 2003 Oct 20. Available from: http://europa.eu.int/comm/health/
ph_risk/committees/sccp/sccp_opinions_en.htm.
35. Nielsen E, Thorup I, Schnipper A, et al. Children and the unborn child. Exposure and susceptibility to chemical substances - an evaluation.Miljøstyrelsen, Miljø- og Energiministeriet; 2001. (Environmental Project No. 589
36. Lentner C, editor. Geigy Scientific Tables: - 1: Units of Measurement, Body Fluids, Composition of the Body, Nutrition. 8 ed. Basle: CIBA-GEIGY; 1981.
37. Larsen JR. Kortlægning af kemiske stoffer i forbrugerprodukter nr. XX. Kortlægning af håndsæber. Miljøstyrelsen; 2005.
Enclosure 1. Natural occurrence and application
Natural occurrence and application of the 26 fragrances which EU has appointed allergenic.
| Number Alphabethically |
Cas no. | Fragrance substance | Application in fragrance substance (1, 2) |
Naturally occurring (2, 3, 4) |
| 1 | 105-13-5 | Anisyl alcohol (anise alcohol) |
Flower fragrance in drinks and confectionary. |
Tomato, aniseed, honey, vanilla |
| 2 | 122-40-7 | Amyl cinnamal | Soy boean | |
| 3 | 101-85-9 | Amylcinnamyl alcohol | No data. | |
| 4 | 100-51-6 | Benzyl alcohol | Slight sweet fragrance, Solvent, starting material for synthesis of benzylesters. | Apple juice, fruits |
| 5 | 120-51-4 | Benzyl benzoate | Fixative, modifier in tongue. Flower fragrance. | Main component in Peru balsam. cranberry |
| 6 | 103-41-3 | Benzylcinnamate | Fixative. In tongue, oriental frangrances. | No data. |
| 7 | 118-58-1 | Benzyl salicylate | Fixative. In flowers/spicy fragrances and i aromas. blomster/krydrede dufte og i aromaer. | Cranberry, clove |
| 8 | 127-51-5 | 3-methyl-4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-3-buten-2-on | ”Highly valued fragrance material”. In flowers and “fantasy” fragrances. | No data. |
| 9 | 104-54-1 | Cinnamyl alcohol | In many flower fragrances (lilac, hyacinth, lily of the valley). Cinnamon notes. As ”round off”. | Blueberry, cranberry |
| 10 | 104-55-2 | Cinnamal (cinnamic aldehyde) |
Blueberry, cranberry. | |
| 11 | 5392-40-5 | Citral | Strong lemon fragrance. | Orange juice, lemon olie, lemon grass |
| 12 | 106-22-9 | Citronellol | Rose fragrance, extended use, often in lemon fragrances. | Rose, geranium, blackberry, fruits. |
| 13 | 91-64-5 | Coumarin | “Spicy green notes”. In parfumes for soaps and as “brightener”. | |
| 14 | 97-53-0 | Eugenol | Clove fragrance, ”oriental”, ”spicy” fragrances”. | Clove and cinnamon, strawberry, fruits, nutmeg |
| 15 | 4602-84-0 | Farnesol | In flower fragrances. Fixative, deodorizing. |
Grape fruit juice. |
| 16 | 106-24-1 | Geraniol | Flower/rose fragrance. May emphasize lemon fragrance. Extended use. | Rose, geranium, citronella, apple juice, fruits. |
| 17 | 101-86-0 | Hexylcinnamaldehyd | Jasmine fragrance. In flower fragrances. | Rice, boiled. |
| 18 | 107-75-5 | Hydroxycitronellal | In many flower fragrances (lime, lily of the valley, caprifoil, lilly, woodbine, lilje, cyclamen). | Synthetic |
| 19 | 97-54-1 | Isoeugenol | In flower fragrances (pinks, cloves). “oriental”, “spicy”. | Beer, rum, coffee, nutmeg |
| 20 | 80-54-6 | Lillial (tradename) 2-(4-tert-butylbenzyl) propionaldehyd |
In flower fragrances (cyclamen, lily of the valley). Extended use. | Synthetic |
| 21 | 5989-27-5 | d-limonene | Lemon fragrance. From the peel of lemon fruits. | Orange juice, fruits, , frugter, celery, vegetables. |
| 22 | 78-70-6 | Linalool | In flower fragrances. Extended use. | Freesia, lily of the valley, lavender, orange juice, carrot |
| 23 | 31906-04-4 | Lyral (tradename) Hydroxymethylpentyl-cyclohexencarboxal dehydr. |
In flower fragrances, lily of the valley. | Synthetic |
| 24 | 111-12-6 | Methyl heptin carbonat | Melon fragrance | Synthetic |
| 25 | 90028-68-5 | Oakmoss | Dry, sweet, leather. Base note. Fixative. |
Moss (Evernia prunastri)on oaktree. |
| 26 | 90028-67-4 | Treemoss | Moss on spruce and pine (Evernia barbata og Evernia furfuraceae) |
1. Bauer K, Garbe D, Surburg H. Common fragrance and flavor materials: preparation, properties and uses. 4th ed. Weinheim: Wiley-VCH; 2001.
2. Secondini O. Handbook of perfumes and flavors. New York (NY): Chemical Publishing; 1990.
3. Council of Europe. Chemically-defined flavouring substances. Strasbourg: Council of Europe Publishing; 2000.
4. Sell, CS. Discovery and Design of Novel Molecules. A Fragrant Introduction to Terpenoid Chemistry. The Royal Society of Chemistry; 2003
Enclosure 2. Analysis results
Table 1 Results from the analysis for fragrances. The unit is mg/kg. A and B show the double determinations
Click here to see the TableTable 2 Results from the analysis for methyleugenol. The unit is mg/kg. A and B show the double determinations
| 46 | 8 | 47 | 50 | |||||
| A | B | A | B | A | B | A | B | |
| Methyleugenol | < 10 | < 10 | < 10 | < 10 | < 10 | < 10 | 160 | 160 |
<.: Less than the indicated detection limit
Table 3 Results from the analysis for methylsalicylate. The unit is mg/kg. A and B show the double determinations
| 4 | 32 | |||
| A | B | A | B | |
| Methylsalicylat | < 10 | < 10 | < 10 | < 10 |
<.: Less than the indicated detection limit
Tabel 4 Results from the analysis for safrol. The unit is mg/kg. A and B show the double determinations
| 45 | 48 | |||
| A | B | A | B | |
| Safrol | <20 | <20 | <20 | <20 |
<.: Less than the indicated detection limit
Footnotes
[1] No Observed (Adverse) Effect Level
[2] NOEL: (No Observed Effect Level), exposure dose with no observed effect on the experimental animals.
[3] LD50: The dose, where lethality occurs in 50% of the experimental animals
[4] MoS: Margin of Safety
[5] NOEL: (No Observed Effect Level), the highest exposure dose with no observed effect on the experimental animals.
[6] NOAEL: (No Observed Adverse Effect Level), the highest exposure dose observed without adverse effects in the experimental animals.
[7] MoS: Margin of Safety
[8] LOEL: (Lowest Observed Effect Level), the lowest exposure dose observed with effects in the experimental animals.
[9] NOEL: (No Observed Effect Level), the highest exposure dose observed without adverse effects in the experimental animals
[10] MoS: Margin of Safety
[11] NOEL: (No Observed Effect Level), the highest exposure dose observed without adverse effects in the experimental animals
[12] TCLo: Lowest toxic concentration
[13] MoS: Margin of Safety
[14] Margin of Safety
[15] Systemic dose of exposure
Version 1.0 October 2006, © Danish Environmental Protection Agency