Survey and health assessment of chemical substances in massage oils
7 Safety assessment
Safety assessments of the fragrances benzyl cinnamate, cinnamal, citronellol, citral and Peru balsam have been performed. Acute and chronic risk assessments have been performed, including the ability of the substances to induce allergy.
A NO(A)EL[1] for the critical effect of the substances has been established. The critical effect of the established NOAEL is no allergy, as there is no lower concentration limit showing when a substance induces allergy. The NO(A)EL value is applied in Chapter 8 on exposure scenarios.
7.1 Benzyl cinnamate
Occurrence and application
Benzyl cinnamate is applied as a fix active in heavy oriental fragrancies. The substance is also applied as a flavour additive in foodstuffs. Ingestion of the substance is estimated to be 44 µg/person/d (0,7 µg/kg bw/d) in Europe and 69 µg/person/d (1 µg/kg bw/d) in the USA, part of which is a natural ingredient in foodstuffs (15). Benzyl cinnamate is an ingredient of Peru balsam.
Identification
Benzyl cinnamate is an ester.
| Chemical name | Benzyl cinnamate |
| Synonyms | Benzyl cinnamate, cinnamic acid benzyl esther |
| CAS-No. | 103-41-3 |
| EINECS No. | 203-109-3 |
| Molecular formula | C16H14O2 |
| Molecular structure | 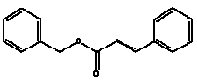 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Not classified. Listed, as a substance which is allergic by skin contact. On the EU list of 26 fragrance allergens. The fragrance is stated on the product label of cosmetics if applied in quantities above 0.01% in leave-on products, and 0.001% in wash-off products. |
Physical-Chemical properties
| Physical state | Crystals (pale yellow) (15) |
| Molecular weight (g/mol) | 238.3 (15) |
| Melting point °C | 37-39 °C (15) |
| Boiling point, °C | 195-200 °C ved 6.7 hPa (15) |
| Vapour pressure (Pa) | No information |
| Octanol/water partition coefficient (log Pow) | No information |
WHO has established a NOEL[2] for benzyl cinnamate of 500 mg/kg bw/d for rat (15)
Acute toxicity
No information was found on the LD50[3] of benzyl cinnamate for neither oral nor dermal exposure.
Local irritation
No information was found on the skin or eye irritating effects of benzyl cinnamate.
Allergy
EU Scientific Committee (SCCNFP) has registered benzyl cinnamate on the list of fragrance allergens. However, not many reports on allergy to the consumers are available. In a study, 6 patients out of 182 (3% showed positive reaction when tested with 8% benzyl cinnamate. In another study, 19 patients out of 103 (18%) with contacting allergy to Peru balsam showed allergic reaction to benzyl cinnamate when tested with 8% benzyl cinnamate. (18).
Long-term, repeated exposure
5 rats of each sex were administered benzyl cinnamate in their diet for19 weeks with a highest dose level of 500 mg/kg bw/d. No effects on body weight, foot intake or blood were found differing from the control group or from the normal area. In this study, NOEL1 was found to be higher than 500 mg/kg bw for benzyl cinnamate (15).,
Benzyl cinnamate was tested in an Ames test and in another bacterium test with a negative result. (15).
No information was found on the effects of benzyl cinnamate by inhalation.
Critical effect
The critical effect of benzyl cinnamate is evaluated to be contactallergy. Due to the allergenic effect of benzyl cinnamate , persons allergic to the substance should avoid skin contact, as there are no lower limit for this side effect.
No lower limit for the contactallergy is known, MoS[4]-calculations are be based on effects other than allergy. The below calculation is based on NOEL from the above study with dosage of rats for 19 weeks.
Tabel 7-1 Summary of data used for the calculation of MoS for benzyl cinnamate
| Toxicological data (animals) | |
| NOEL1, (mg/kg bw/d), intake, rat | 500 |
7.2 Cinnamal
Occurrence and application
Cinnamal is applied as a fragrance in perfumery articles (17) and is naturally occurring in among others cinnamon, blueberries, and cranberries. The substance is also applied as flavour additive in foodstuffs. Intake of the substance was estimated to be 42 µg/kg bw/d in Europe and 991 µg/kg bw/d in the USA. A great part of the applied cinnamal came from natural sources (15).
Identification
Cinnamal is an aldehyde.
| Chemical name | Cinnamale, Cinnamaldehyde |
| Synonyms | 3-Phenyl-2-propenal, Cinnamic aldehyde, cinnamon aldehyde |
| CAS-No. | 104-55-2 |
| EINECS No. | 203-213-9 |
| Molecular formula | C9H8O |
| Molecular structure | 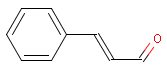 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Not classified Listed, as a substance which is allergic by skin contact. On the EU list of 26 fragrance allergens. The fragrance is stated on the product label of cosmetics if applied in quantities above 0.01% in leave-on products, and 0.001% in wash-off products. |
Physical-chemical properties
| Physical state: | Liquid (18) |
| Molecular weight (g/mol) | 132.2 (19) |
| Melting point °C | -7.5 °C (18) |
| Boiling point, °C | 246 °C (at 1014 hPa) (18) |
| Vapopour pressure (Pa) | 2.25 hPa at 20 °C calculated) (18) |
| Octanol/water partition coefficient (log Pow) | 2.22 at 18 °C (calculated) (18) |
| Water solubility (mg/L) | 1400 (18) |
WHO has established a NOEL[5] for cinnamal of 620 mg/kg bw/d (15).
Acute toxicity
The LD50-values by oral administration of cinnamal in rats was found to be 2200 - 3400 mg/kg bw, by oral administration in mice 3400 mg/kg bw, and in guinea pigs 1160-3400 mg/kg bw (20), (21). The toxic effects were reduced activity (rats), convulsion(s) (mice), and coma (guinea pigs) (21).
WHO has assessed that cinnamal is metabolised to a harmless substance, the main part of which is eliminated quickly through the kidneys.(15).
LD50-values by dermal exposure of rats and rabbits is greater than 2000 mg/kg bw (20).
No data on dermal absorption of cinnamal were found.
Local irritation
Cinnamal was found irritating to skin in guinea pigs and humans. No information on eye irritation was found (20). Undiluted substance on the skin of humans for 48 hours causes serious irritation. (21).
Allergy
The Scientific Committe of EU SCCP has registered Cinnamal on the list of fragrance allergens. The fragrance substances on the list are well known allergens, about which many cases of allergy in the consumers were reported.
Three studies showed that 6 of 20 patients (30%), 7 of 182 patients (3,7%) and 24 of 167 patients (14.44%) reacted allergenic to cinnamal (18)
Cinnamal is part of fragrance mix for diagnostic test, in which the substance accounts for 5.5 – 36% of the reactions on this mixture (5.5% in Italy, 16.9% in Denmark, 21% in Germany, 24% in Hungary and 36% in France)(16). The substance was identified to cause allergenic reactions in humans, of whom between 1% and 30% persons suffered from cosmetics eczema. In 78 European consumers with fragrance eczema, 12.8% reacted positively when tested with 1% cinnamal (18).
IFRA (International Fragrance Association) recommends that cinnamal as a fragrance is exclusively applied together with substances preventing sensibilisation, such as for instance equal portions eugenol or d-limonene. Investigations have shown that mixtures of equal amounts of cinnamal and eugenole or cinnamal and d-limonen are not sensitizing, even if the individual substances separately are sensitizing (22)..
Long-term, repeated exposure
Three studies of 90 days duration with approximately identical results were found. The National Toxicology Program is described as follows: Groups of 10 rats of each sex were daily administered cinnamal in their diet (1.25%) for 13 weeks at doses of 620 mg/kg bw/d. At this concentration, no effects on kidneys, liver, blood were found differing from the control group or from the normal area. In this study, NOEL was found to be 620 mg/kg bw/d. At higher doses (1250 mg/kg bw/d) abdominal mucosal irritation as well as reduced growth was observed (probably caused by the taste) (15).
In a 2-year study, groups of rat (50 of each sex) and mice (50 of each sex) were administered cinnamal in their diet. No effect on kidneys, liver and blood were found differing from the control group or from the normal area at the highest concentrations. NOAEL[6] was therefore the highest exposure dose: 200 mg/kg bw/d for rats and 550 mg/kg bw/d for mice. No signs of carcinogenic effects were observed in neither rats nor mice (23).
Cinnamal was tested for mutegenic (genotixic) properties in Ames’ test and other analyses in cells with conflicting results. The results of the main part of the analyses in multicellular organisms were negative (11), (18). It was not found likely that cinnamal has a genotoxic effect (23).
In a 2-generation study with rats administered 5 mg cinnamal/kg bw there were no effect on growth, development or survival of parents or pups, but the fat content in the liver increased by 20% in the first generation and 22% in the second generation (20).
Female rats administered a daily dose of 5 mg/kg bw from the 7th to the 17th day of gestation were not affected by the dosage, but delayed development of the embryos and effects on the urinary tract of the embryos were observed. Apparently, embryos are more sensitive than dams (20).
No information was found on inhalation of cinnamal.
Critical effect
The critical effect of cinnamal was assessed to be contactallergy. Because of the allergenic potential of cinnamal, humans allergic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
No lower limit for the contactallergy is known. MoS[7]-calculation is based on effects other than allergy. The below calculation is based on NOEL and LOEL[8] from the above study with administration of rats for 13 weeks: NOAEL from the 2-year study with rats of 200 mg/kg bw/d is the highest dose in a study with no toxicity observed.
Table 7-2 Summary of data used for calcualtion of MoS for cinnamal
| Toxicological data (animals) | |
| LD50, (mg/kg bw), oral, rat | 2220 (18,19) |
| NOEL, (mg/kg bw/d), intake (13 weeks), rats | 620 (11) |
| LOEL, (mg/kg bw/d), intake (13 weeks), rats | 1250 (11) |
7.3 Citral
Occurrence and application
Citral is seldomly applied as fragrances in perfumes (22).
Citral has a strong lemon fragrance. The substance is applied as flavour additive in foodstuffs. Intake of the substance is estimated to be 6849 µg/person/d (114 µg/kg bw/d) in and 6990 µg/person/d (117 µg/kg bw/d) in the USA. In the USA, a little more than half of the substance is considered to originate from natural sources. (23).It occcurs in lemon grass in its natural form (19).
JECFA (Joint Expert Committee for Food Additives) has established a group-ADI (Acceptable Daily Intake) (through foodstuffs)) for citral, citronellol, geranyl acetate, linalool og linalyl acetate, of 0-0.5 mg/kg bw (calculated as citral) (25).
Identification
Citral is a terpenaldehyde. The substance is a mixture of the aldehydes geranial 55-70 % (trans-structure – the shown) and neral 35-45 % (cis-structure) (26).
| Chemical name | Citral |
| Synonym | 3,7-Dimethyl-2,6-octadienal |
| CAS-No. | 5392-40-5 |
| EINECS No. | 226-394-6 |
| Molecular formula | C10H16O |
| Molecular structure | 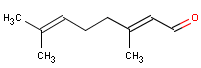 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Xi; R 43 Listed, as the substance is assessed to be allergenic at skin contact. and is 1 of th 26 fragrance allergens.assessed by SCCNFP. Fragrances are declared in cosmetics if applied in quantities above 0.01% in products which are cleaned and 0.001% in products which are not cleaned. |
Physical/Chemical properties
| Physical state | Liquid |
| Molecular weight (g/mol) | 152.3 (19) |
| Melting point °C | < -10 °C (24) |
| Boiling point, °C | 226-228 °C (24) |
| Vapour pressure (Pa) | < 130 Pa ved 100 °C (24) |
| Octanol/water partition coefficient (log Pow) | 2.8 for neral og 3.0 for geranial ved 25 °C (24) |
| Water solubility (mg/L) | 590 mg/L ved 25 °C (24) |
WHO has established a NOEL[9] for citral of 100 mg/kg bw/d for rats (25).
7.3.1.1 Acute toxicity
The LD50-value by oral administration of citral in rats was found to be 4960 mg/kg bw (26)
WHO has found that citral metabolises to harmless substances that are quickly excreted quickly through the kidneys (25), (26)
The dermal LD50-value for rabbits was found to be 2250 mg/kg bw (26).
7.3.1.2 Local irritation
Testing in rabbits showed that citral was found skin irritating, but not eye irritating (26).
7.3.1.3 Allergy
EU Scientific Committe (SCCP) has registered citral on the list of fragrances. The fragrances on the list are well known allergens. Many cases of allergy in consumers have been reported. In two described studies 4 of 228 patients (1.7%) and 19 of 1855 patients (1%) respectively showed allergic reactions to citral (18).
Citral was found strongly sensitizing in guinea pigs. Between 12 and 64% voluntary test persons were sensitized using the Human Maximization Test in humans (18).
Citral belongs to fragrances which should no be used separately but only in mixtures with substances depressing the sensitizing effect of the substance (22). IFRA (International Frangrance Association) recommends that citral is only applied in products together with substances preventing a sensitizing effect, for example 25% d-limonenee, mixed citrus terpenes eller a-pinenes (22).
7.3.1.4 Long-term, repeated exposure
Citral in micro encapsulated form was administered 3 groups of 10 rats of each sex in their daily diet during 14 weeks, the daily dose being 345, 820 and 1785 mg/kg bw for males and 335, 675, and 1130 mg/kg bw for females. All doses showed effects on kidneys in males. In females, the highest dose showed low increase and reduction of the bone marrow. In this study, a NOEL2 was found to be lower than 345 mg/kg bw/d for male rats. In female rats, NOEL was 645 mg/kg bw (25).
A 2-year study group of 50 rats of each sex was exposed through their diet to 0, 50, 10 and 210 mg/kg/bw/d. In the male group was found a dose-dependent increase of the mineralization of the kidneys, which was interpreted as an increase of normally occurring deviation in the rat strain. In this study, NOEL for citral was 100 mg/kg bw/d due to reduced increase in female rats at highest dose. There was no indication that citral is carcinogenic in rats (25).
Many studies with long-term repeated exposure in rats were performed, but the studies referred are the latest and were performed by NTP (National Toxicology Program) in the USA. Their laboratories are considered to be very reliable.
Citral has been tested in an Ames’ test and in a number of other tests with bacteria and mammal cells. It has also been tested for genotoxic properties after administration in living mice. Almost all results were negative (25)
Daily doses of citral of until 1000 mg/kg bw/day before, during and after mating did not effect the fertility of rats. In the high dose analyses, microscopy showed changes in the stomach of the experimental animals. The embryos showed reduced growth in the period of lactation (26).
A concentration of 423 mg citral/ m3 inspirated air resulted in reduced growth and abortation and death among pregnant female rats exposed for 6 hours/d on the 6-15 day of gestation, but was not teratogenetic (26).
Critical effect
The critical effect of citral is assessed to be contactallergy. Because of the allergenic potential of citral, humans allergenic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
No lower limit for the contactallergy is known. MoS[10]-calculation is based on effects other than allergy. The below calculation is based on NOEL for 2 years:
Table 8-3 . Summary of data used for calculation of MoS for citral
| Toxicological data (animals) | |
| LD50, (mg/kg bw), oral, rat | 4960 (24) |
| NOEL, (mg/kg bw/d), intake (2 year), rat | 100 (23) |
| LOEL, (mg/kg bw/d), intak (14 weeks), rat | 345 (23) |
7.4 Citronellol
7.4.1.1 Occurrence and application
Citronellol is applied as perfumery material in perfumes, often in flower fragrancies. The substance is also applied as a flavour additive in foodstuffs. Intake of the substance is estimated to be 370 µg/person/d (6,2 µg/kg bw/d) in Europe and 771 µg/person/d (13 µg/kg bw/d) in the USA, Much of this originates from natural ingredients in foodstuffs (23). Citronellol also occurs in rose- and geranium oil and as gland secretion in alligators. (19).
Identifikation
Citronellol består af en terpenalkohol.
| Chemical name | Citronellol |
| Synonyms | 3,7-Dimethyl-6-octen-1-ol, dl-Citronellol |
| CAS-No. | 106-22-9 |
| EINECS No. | 203-375-0 |
| Molecular formula | C10H20O |
| Molecular structure | 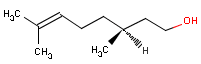 |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (12) List of Undesirable Substances 2004 (16) Cosmetics (14) |
Not classified Listed, as the substance is assessed to be allergenic at skin contact. The fragrance is stated on the product label of cosmetics if applied in quantities above 0.01% in products which are cleaned and 0.001% in products which are not cleaned. |
Physical-chemical properties
| Physical state: | Liquid |
| Molecular weight (g/mol) | 156.3 (19) |
| Melting point °C | No information |
| Boiling point, °C | No information |
| Vapopour pressure (Pa) | No information |
| Octanol/water partition coefficient (log Pow) | No information |
| Water solubility (mg/L) | No information |
WHO har established a NOEL[11] for citronellol of > 51 mg/kg bw/d for male rats (25).
JEFCA (Joint Expert Committee for Food Additives) has established a group-ADI (Acceptable Daily Intake) of 0-0.5 mg/kg bw/d expressed as citral for the group of terpene-containing flavour additives (citral, geranylacetate, citronellol, linalool and linalyl acetate) (1).
Acute toxicity
LD50-values at oral administration of citronellol in rats were found to be 3450 mg/kg bw (21), (25) and 5000 mg/kg bw (21).
WHO assessed that citronellol most likely metabolises to harmless substances based on data on a corresponding substance (geraniol). The end product is excretred through the urine (25).
The LD50-value of citronellol applied on the skin of rabbits was found to be 2650 mg/kg bw (21).
Local irritation
The effect of citronellol is moderately irritating to the skin of humans for 48 timer, and seriously irritating to the skin of rabbit and guinea pig for 24 hours (21). No information was found on eye irritation.
Allergy
The Scientific Committe of EU SCCP has registered Cinnamal on the list of fragrances. The fragrances on the list are well known allergens, on which, however, not many cases on allergy in consumers are reported. Two out of 20 perfume allergy sufferers (35%) and 2 out of 119 (1.7%) patients suffering from cosmetic allergy showed allergic reactions to citronellol (18).
Long-term, repeated exposure
10 rats of each sex were administered citronellol (mixed with equal parts of linalool) in the diet daily for 12 weeks. The doses were 51 mg/kg bw/d for males and 56 mg/kg bw for females. At this concentration, no effects on kidneys, liver, blood were found differing from the control group or from the normal area. In this study, NOEL for citronellel was found to be higher than 51 and 56 mg/kg bw in male and female rats respectively) (25).
The result of an Ames’ test and another bacterium test with Citronellol was negative (25).
TCLo[12] for citronellol for rats was found to be 1.3 mg/m³ air by inhalation for 4 hours. By repeated inhalation for 4 hours daily for 3 days, TCLo decreased to 0.3 mg/m³ air. In both analyses, the rats showed behavioural changes (21).
Critical effect
The critical effect of citronellol is assessed to be contactallergy. Because of the allergenic potential of citronellol, humans allergic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
No lower limit for the contactallergy is known. MoS[13]-calculation is based on effects other than allergy. The below calculation is based on NOEL from the above study with administration of rats for 12 weeks.
Table 7-4 Summary of data used for calculation of MoS for citronellol
| Toxicological data (animals) | |
| LD50, (mg/kg bw), oral, rat | 3450 (19,23) |
| NOEL, (mg/kg bw/d), intake, male rat | > 51 (23) |
7.5 Peru balsam
Occurence and application
Peru balsam is extracted from the cortex of the tree Myroxolon balsamum pereiae that grows in Central America, e.g. along the coast of El Salvador. Peru balsam is applied as fragrances and as a flavour additive in foodstuffs. Peru balsam is weakly antiseptic, and down through the ages it has been applied in pharmaceuticals for wound treatment and for skin problems, such as eczema and itching. In addition, it has been applied in pharmaceuticals for the treatment of inconveniences due to hemorrhoids (27) (28).
Identification
Peru balsam is mainly composed of 50-60% of a mixture of esthers of cinnamateic acid and benzo acid, with benzyl cinnamate, benzyl benzoate and cinnamyl cinnamate as the main components. Also small amounts of vanillin, eugenol and free cinnamateic acid are contained (19), (27). A great number of other substances have been identified in Peru balsam, but the exact composition is not known (29).
| Chemical name | Peru balsam |
| Synonym | Myroxylon pereirae Klotzsch |
| CAS-No. | 8007-00-9 |
| EINECS No. | 232-352-8 |
| Molecular formula | Not indicated |
| Molecular structure | Not indicated |
| Legislation: Classification in accordance with the list of hazardous substances (Gov. order 439 of 3 June 2002) (30) List of Undesirable Substances 2004 (16) Cosmetics (1) |
Not classified Peru balsam contains several unwanted fragrances which are assessed to be allergenic by skin contact. Fragrance allergens are stated on the product label of cosmetics, if they are applied in quantities above 0.01% in products, which are cleaned and 0.001 % in products which are not. Peru balsam is prohibited in cosmetic products annex 2 “løbenummer nr 1163” (1). |
Physical-chemical properties
| Physical state | Braunish viscous liquid |
| Molecular weight (g/mol) | No information |
| Melting point °C | No information |
| Boiling point, °C | No information |
| Vapopour pressure (Pa) | No information |
| Octanol/water partition coefficient (log Pow) | No information |
| Water solubility (mg/L) | Insoluble in water |
Acute toxicity
LD50-values of Peru balsam orally administered to rats were found to be > 5.000 mg/kg bw, and dermally applied to rabbits > 10,000 mg/kg bw (21).
No data for absorption of Peru balsam through the skin were found.
Local irritation
Peru balsam applied to the skin in concentrations of 25% caused moderate irritation in children and mild reaction in women. Peru balsam in concentrations of 25% showed moderate irritation of the skin in tests with rabbits (21).
Allergy
Contact with Peru balsam caused allergy in both children and adults. In a study of 101 children below 15 years 24% of the children showed allergic reaction to Peru balsam, and of 2000 examined adults, 6% showed allergic reaction to the substance. Nettle rash by contact with Peru balsam is not unusual (19). Peru balsam har shown phototoxic reaction (32).
The Scientific Committe of EU SCCP has registered the main components in Peru balsam, benzyl cinnamate and benzyl benzoate on the list of fragrances. The fragrances on the list are well known allergens, of which however not many cases on allergy in consumers are reported. In 103 cases of allergy caused by Peru balsam, 12% of the patients showed positive reaction to benzyl benzoate and 18% of the patients positive reaction to benzyl cinnamate (18). Patients with cronic ezcema and allergy caused by Peru balsam, showed improvement by avoiding foodstuffs containing balsam components in e.g. foodstuffs spiced with cinnamon, lemon fruits and vanilla. (19).
Caused by the risk of allergy, IFRA has prohibited Peru balsam as an fragrance substance in cosmetic (22). The substance is today prohibited in cosmetic products (1).
Long-term, repeated exposure
No information on mutagenic, carcinogenic and reprotoxic effects in Peru balsam was found.
No information on effects after inhalation of Peru balsam has been found.
Critical effect
The main components of Peru balsam is benzyl cinnamate, benzyl benzoate and cinnamyl cinnamate. According to EU, the substances are assessed to be allergenic. Because of the allergenic potential of Peru balsam, humans allergic to the substance should avoid skin contact, as there is no lower limit for this adverse effect.
Tabel 7-5 Summary of data used for calculation of MoS for Peru balsam.
| Toxicological data (animal) | |
| LD50, (mg/kg bw), oral, rat | >5000 (21) |
Footnotes
[1] No Observed (Adverse) Effect Level
[2] NOEL: (No Observed Effect Level), exposure dose with no observed effect on the experimental animals.
[3] LD50: The dose, where lethality occurs in 50% of the experimental animals
[4] MoS: Margin of Safety
[5] NOEL: (No Observed Effect Level), the highest exposure dose with no observed effect on the experimental animals.
[6] NOAEL: (No Observed Adverse Effect Level), the highest exposure dose observed without adverse effects in the experimental animals.
[7] MoS: Margin of Safety
[8] LOEL: (Lowest Observed Effect Level), the lowest exposure dose observed with effects in the experimental animals.
[9] NOEL: (No Observed Effect Level), the highest exposure dose observed without adverse effects in the experimental animals
[10] MoS: Margin of Safety
[11] NOEL: (No Observed Effect Level), the highest exposure dose observed without adverse effects in the experimental animals
[12] TCLo: Lowest toxic concentration
[13] MoS: Margin of Safety
Version 1.0 October 2006, © Danish Environmental Protection Agency