Survey of Chemical Substances in Consumer Products, No. 90, 2008
Survey, emission and health assessment of chemical substances in baby products
Contents
2 Survey of content of chemical substances - chemical analyses
- 2.1 Analysis programme
- 2.2 Methods
- 2.2.1 Analysis for content of azo-colourants
- 2.2.2 Analysis for content of disperse colourants
- 2.2.3 Analysis of the content of selected organic tin compounds
- 2.2.4 Analysis of the content of selected organic phosphorous compounds
- 2.2.5 Analysis of content of chlorinated benzenes and toluenes
- 2.2.6 Analysis for content of chlorinated phenols and OPP
- 2.2.7 Analysis for content of selected phthalates
- 2.2.8 Analysis for content of formaldehyde
- 2.2.9 Screening analyse by GC-MS
- 2.2.10 Analysis for the content of Br, P, Cl, Sn, b, F by EDXRF
- 2.2.11 Migration test in artificial sweat and artificial saliva, respectively
- 2.2.12 Analysis of volatile organic components (VOC) by headspace analysis
- 2.2.13 Analysis of isocyantes with HPLC
- 2.3 Analysis results divided according to sample types
- 2.4 Analysis results for specific analyses according to analysis type
- 2.4.1 Analysis results for arylamines
- 2.4.2 Analysis results for disperse colourants
- 2.4.3 Analysis results for P-containing flame retardants [µg/g]
- 2.4.4 EDXRF analysis - results presented as % (w/w)
- 2.4.5 Analysis results for organic tin compounds
- 2.4.6 Chlorinated benzenes and toluenes
- 2.4.7 Analysis results for chlorinated phenols and OPP
- 2.4.8 Analysis results for phthalates, mg/g
- 2.4.9 Formaldehyde
- 2.4.10 Migration test in sweat of selected products
- 2.4.11 Migration test in saliva for selected products
- 2.4.12 Migration test in sweat and saliva for PS-pellets
- 2.4.13 Emission test of volatile organic components - PS-pellets
- 2.5 Selection of chemical substances for evaluation
- 3.1 Introduction
- 3.2 Method
- 3.3 Selected substances
- 3.3.1 Hexabromocyclododecane (HBCD)
- 3.3.2 Toluene 2,4-diisocyanate (TDI)
- 3.3.3 2-Ethylhexanoic acid (2-EHA)
- 3.3.4 Acetophenone
- 3.3.5 1,1,2,2-Tetrachloroethane
- 3.3.6 Formaldehyde
- 3.3.7 Styrene
- 3.3.8 2-bromo-4,6-dinitroaniline (BDNA)
- 3.3.9 Hexaethylene glycol dimethyl ether
- 3.3.10 Tetrapropylene glycol monomethyl ether
- 3.3.11 DEHP
- 3.3.12 DINP
- 3.3.13 Xylene
- 3.4 Overall Assessment
- 4.1.1 Hexabromocyclododecane (HBCD)
- 4.1.2 Toluene 2,4-diisocyanate (TDI)
- 4.1.3 2-Ethylhexanoic acid (2-EHA)
- 4.1.4 Acetophenone
- 4.1.5 Formaldehyde
- 4.1.6 1,1,2,2-Tetrachloroethane
- 4.1.7 Styrene
- 4.1.8 2-Bromo-4,6-dinitro-benzenamine (BDNA)
- 4.1.9 Hexaethylene glycol dimethyl ether
- 4.1.10 Tetrapropylene glycol monomethyl ether
- 4.2 Overall Assessment
Preface
The project ”Survey, emissions and health assessment of chemical substances in baby products” has been performed during April 2005 to December 2005.
This report presents the results of the project including survey of products and consumption, chemical analyses, and health evaluation of a number of selected products and substances.
The purpose of the projects was to illustrate the extent and content of substances hazardous to health and environment in products to babies and to elucidate if health problems exist to the consumer from the use of the products.
The project is performed by the Danish Technological Institute, Division of Materials. Project responsible for the Danish Technological Institute was chemo technician Eva Pedersen and Cand. Arch. Kathe Tønning.
The survey has been prepared by BSc Anette Drøjdahl Lomholt from the Danish Technological Institute Building Division, Textile, Section of EcoTex and Indoor Climate.
Responsible for the laboratory analyses and migration studies has been the manager of the laboratory section Nils Bernth and chemo technician Eva Pedersen, Chemistry and Water Technology, and the Head of section Paul Lyck Hansen for quality assurance.
For screening and assessment of health effects (consumer exposure), risk and environmental evaluation, Lic.Techn Bjørn Malmgren-Hansen, PhD Per Woin and MSc Lise Møller were attached as experts and MSc Ole Christian for quality assurance.
The project was followed by a reference group consisting of:
Lea Frimann Hansen, Danish Environmental Protection Agency (Chairman)
Shima Dobel, Danish Environmental Protection Agency
Ole Christian Hansen, Danish Technological Institute (until August 1, 2005)
Eva Pedersen, Danish Technological Institute.
The Project is financed by the Danish Environmental Protection Agency.
Summary and conclusions
Several baby products are intended to more or less to get in direct contact with baby skin or be in close contact to the skin. Moistening in the form of water, saliva, sweat or urine may cause that substances contained in the products, which can be released to these liquids, may get into contact with the skin or mouth of the baby.
Besides uptake of chemical substances through the skin and from the baby sucking on the material exposure may also take place by inhaling of gasses slowly released/evaporating from the baby product of dusty particles and fibres released during use.
The products included in the examination are primarily baby products intended for at children at the age of 0 to 1 year. Baby products have been deliberately selected that based on information from retailers are sold to a reasonable extent.
The project primarily concerned products of textile or plastic with an upholstery or padding. In addition also baby products of flexible foam material were included.
Baby clothing, bed linen (bolster case), shaped plastic objects (bath tubs, chamber pots, comforters, and baby plates), baby cutlery, and toys, wooden beds (cots) and baby care agents/remedies were not included in the project.
Survey
In the survey, the following activities are included:
- Contact to retailers
- Search on the Internet
- Use of questionnaires
- Evaluation of the material from the different types of baby products relating to the content of health hazardous substances.
Consumption of baby products
As several of the larger enterprises/retailers with specialty in baby and child products have not presented detailed and specific information it was not possible directly to estimate the actual consumption of the individual types of baby products.
Nor Statistics Denmark presented a possibility to estimate the consumption of baby products, therefore the volume of trade of the different product types and trademarks must be based on rough estimates.
Some of the products may due to their durability be used for more than one child. In return, supplementary products per child may exist in e.g. child care centres and day-care facilities and perhaps also at grand parents.
Tthe number of purchased new perambulators per year is evaluated to be considerably lower than the number of newly born, which is approx. 65,000 per year, i.e. approx. 25-40,000 perambulators per year.
For other less expensive and less durable baby products the number of pieces per year is evaluated to be closer to the number of new-borns at an estimate of 40-60,000 pieces.
In co-operation with the Danish Environmental Protection Agency, the following types of baby products are selected for a further examination of their content of chemical substances and colorants harmful or of concern to the health:
- Pillows for baby feeding (product no. 4, 5).
- Baby carriers (product no. 9, 10).
- Nursing pillows/ cushions with different covers and stuffing (product no. 7, 8).
- Baby mattresses with stuffing of foam, to beds (product no. 6, 13).
- Aprons to perambulators (product no. 11, 12).
- Disposable foam washcloths (product no. 1, 2, 3).
Each selected baby product is represented by 2 different articles or trade marks, for the disposable foam wash cloths, however, 3 different articles. Thus, a total of 13 products have been selected for further examination. The products are presented in Table 0.1.
Chemical analyses
The 13 different products were selected and divided into subsamples for chemical analysis.The products were analysed for i.a. colourants, organic tin compounds, flame retarders and were screened for phthalates and organic compounds. Additionally, migration tests were carried out in saliva and sweat.
Assessment related to ecolabelling
Several of the products contain compounds that exceed the limit values to acquire ecolabels. Only one of the examined products carries an ecolabel: product no. 10 which is EcoTex labelled.
Health assessment
The following conclusions could be drawn from the study:
- All analysed baby products contain measurable quantities of more than one compound classified as hazardous to health and/or environment
- No substances with hazardous health risks were found in the products.
- The analysed disposable foam wash cloths contain 2 substances with carcinogenic, sensitizing and reproduction toxic effects.
- The analysed pillows for baby feeding emit formaldehyde which is probably sensitizing and in higher concentrations carcinogenic by inhalation and may cause sensitisation by skin contact. The assessment shows that the worst case migration to skin may contribute significantly to the acceptable daily intake in this case it is assumed that 100% of the formaldehyde is absorbed via the skin
- The analysed nursing pads contain low concentrations of substances with carcinogenic, sensitizing and reproduction toxic effects which may have a possible health risk in some cases.
- The analysed baby mattresses contain low concentrations of substances with a possible reproduction toxic effect in some cases.
- There are uncertainties in the assessment of the absorbed amount of 2-ethylhexane acid in the products which have not been subjected to migration tests. This means that within this uncertainty there may be a slight health risk with two of the disposable foam wash cloths, one mattress, and one of the nursing pillows.
- One product contained compounds (phthalates) forbidden by Danish and EU-legislation to occur in certain childcare products above a set amount. The excess has been reported to the Danish Environmental Protection Agency.
- Some of the products contain several substances with chronic effects which are also found in a number of other products in the society. The effect of such multi-source exposure must be of major concern as babies are a sensitive part of the population and should be protected from chemical exposure.
In Table 0.1 is shown the evaluated substances.
Table 0.1 Health effects of evaluated substances
| Substance | CAS no. | Health effects |
| Hexabromocyclo-dodecane (HBCD) | 25637-99-4 | Developmental neurotoxic effects |
| Toluene 2,4-diisocyanate (TDI) | 584-84-9 | Carc3, R40 (carcinogen) R42/R43 (sensitization) |
| 2-Ethylhexanoic acid (2-EHA) | 149-57-5 | Rep.3;R63 possible harm to unborn child |
| Acetophenone | 98-86-2 | Irritating |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | Very toxic by inhalation and in skin contact |
| Formaldehyde | 50-00-0 | Carc3, R40 (carcinogen) R43 (sensitization) |
| Styrene | 100-42-5 | Irritating |
| 2-Bromo-4,6-dinitroaniline (BDNA) | 1817-73-8 | Few data |
| Hexaethylene glycol dimethyl ether | 1072-40-8 | Few data |
| Tetrapropylene glycol monomethyl ether | 20324-34-9 | Few data |
From analysis data of the emitted substances in Table 0.1from the different products a margin of safety (MOS) was calculated when possible and the health risks was assessed.
For the individual products the overall result was:
Product no. 4 (baby feeding pillow) and 5 (baby feeding pillow) contribute with a significant part of the acceptable daily intake of formaldehyde.
DEHP was found in product 11 (apron for perambulator) with no risk found in the assessment, but with a considerable uncertainty in the estimate regarding migration conditions.
In product no. 8 (nursing pillow), the content of phthalates in the product or parts of the product was above the allowed limit of 0.05 wt% stipulated in Statutory Order no. 151 of 15/03/1999, banning phthalates in toys for children aged 0 – 3 and in certain childcare articles. The excess has been reported to the Danish Environmental Protection Agency.
For the products 9 (baby carrier) and 12 (apron for perambulator) no possible risk of health effects was found and no phthalates with content above allowed limits was present.
Environmental assessment
The classification of the substances regarding the environment is given in the Table 0.2 below.
Table 0.2 Overview for environmental classification
| Substance | CAS No. | Classification |
| Hexabromocyclododecane (HBCD) | 25637-99-4 | N;R50-53 |
| Toluene 2,4-diisocyanate (TDI) | 584-84-9 | R52-53 |
| 2-Ethylhexanoic acid (2-EHA) | 149-57-5 | Lack of data (Possibly R52/53) |
| Acetophenone | 98-86-2 | N.C. |
| Formaldehyde | 50-00-0 | N.C. |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | N;R51-53 |
| Styrene | 100-42-5 | N.C.* |
| 2-Bromo-4,6-dinitroaniline (BDNA) | 1817-73-8 | N;R51/53** (Possibly release of potentially hazardous aromatic amines) |
| Hexaethylene glycol dimethyl ether | 1072-40-8 | No data |
| Tetrapropylene glycol monomethyl ether | 20324-34-9 | No data |
N.C.: Not classified as dangerous to the aquatic environment (N-CLASS 2005).
*: Proposal (EU Risk Assessment Report on styrene 2000).
**: Proposal (Danish EPA, Advisory list of self classification 2005).
The three substances hexabromocyclododecane (HBCD), 1,1,2,2-tetrachloroethane and toluene 2,4-diisocyanate (TDI) are very toxic, toxic or harmful to aquatic organisms, respectively and may all cause long-term adverse effects in the aquatic environment. The discharge and exposure of these substances to the aquatic environment should therefore be reduced or prevented.
Furthermore, the possible environmental effects of the substances 2-ethylhexanoic acid (2-EHA), 2-bromo-4,6-dinitroaniline (BDNA), hexaethylene glycol dimethyl ether and tetrapropylene glycol monomethyl ether are not known due to lack of data. The discharge and exposure of these substances to the aquatic environment should therefore be minimized until the possible environmental effects are known.
It may be assumed in general, that the direct waste water discharge of substances from the daily use and washing of these types of products, except for the disposable washcloths, is minor compared to other kinds of e.g. textiles, which are washed more regularly. The wash cloths contain 2-EHA among other substances, which may be discharged to the waste water system through continued use of new cloths. As HBCD and the phthalates are substances with low vapour pressure and a high log Kow value, it is most likely that the majority of the content will remain in the products during the time use of their lifecycle unless the products get in contact with organic solvents or are heated to higher temperatures for longer periods. Nevertheless, the release of these substances to the environment will most likely proceed in small amounts as long as products contain these substances.
Sammenfatning og konklusioner
Mange babyprodukter er beregnet til mere eller mindre at komme i direkte kontakt med babyens hud eller befinde sig tæt på huden. Befugtning (i form af vand, spyt, sved eller urin) kan medføre, at indholdsstoffer, der frigives til disse væsker, vil kunne komme i kontakt med barnets hud eller mund.
Foruden optagelse af kemiske stoffer gennem huden og ved at en baby sutter på materialet, kan påvirkning også ske ved indånding af luftarter, der langsomt afdamper fra babyproduktet, eller af støvlignende partikler og fiberfnug, der afgives under brug.
De produkter, der indgår i undersøgelsen, er primært babyprodukter, som er beregnet til børn i alderen 0-1 år. Der er bevidst søgt babyprodukter, som på basis af oplysninger fra forhandlere sælges i rimeligt omfang.
Projektet har primært drejet sig om produkter af tekstil eller plast med polstring eller fyld. Derudover indgår også babyprodukter af fleksibelt skummateriale.
Babytøj, sengelinned (dynebetræk), formede plastgenstande (badekar, potter, sutter, børnetallerkener), børnebestik samt legetøj, træsenge og babyplejemidler er ikke omfattet af projektet.
Kortlægning
I kortlægningen indgår følgende aktiviteter:
- Kontakt til detailhandel
- Søgning på Internet
- Anvendelse af spørgeskema
- Vurdering af materialerne i de forskellige typer babyprodukter med hensyn til indhold af sundhedsskadelige stoffer.
Forbrug af babyprodukter
Da flere af de større virksomheder med speciale i baby- og børneprodukter ikke har givet detaljerede og specifikke oplysninger, er det ikke umiddelbart muligt at anslå det faktiske forbrug af de enkelte typer af babyprodukter.
Heller ikke via Danmarks Statistik er det muligt at få tal for babyprodukter. Omsætningen af produkttyper og varemærker må derfor vurderes skønsmæssigt.
Nogle produkter vil holdbarhedsmæssigt kunne anvendes til mere end ét barn, til gengæld vil der være ekstra produkter pr. barn hos fx børneinstitutioner og dagplejere og evt. også hos bedsteforældre.
Det vurderes, at antal nyindkøbte barnevogne pr. år vil ligge væsentlig lavere end antallet af nyfødte, som er ca. 65.000 pr år, skønsmæssigt 25-40.000 barnevogne pr. år.
For andre, knap så dyre og knap så holdbare, babyprodukter vil antal stk. pr. år anslået ligge tættere på antallet af nyfødte, skønsmæssigt i størrelsesordenen 40-60.000 stk.
I samarbejde med Miljøstyrelsen er følgende typer babyprodukter udvalgt til nærmere undersøgelser for indhold af sundhedsskadelige eller betænkelige kemikalier og farvestoffer:
- Ammepuder (produkt nr. 4 og 5)
- Bæreseler (produkt nr. 9 og 10)
- Puslepuder/-hynder med forskellige betræk og forskelligt fyld (produkt nr. 7 og 8)
- Madrasser med fyld af skum, til senge (produkt nr. 6 og 13)
- Forlædere til barnevogne (produkt nr. 11 og12)
- Engangsskumvaskeklude (produkt nr. 1, 2 og 3).
Hvert udvalgt babyprodukt er repræsenteret ved 2 forskellige artikler/varemærker, for engangsskumvaskekludene dog 3 forskellige. Der er således i alt udvalgt 13 produkter til nærmere undersøgelse.
Kemiske analyser
De 13 udvalgte produkter blev opdelt i delprøver til den kemiske analyse. Der blev bl.a. analyseret for farvestoffer, organiske tinforbindelser, flammehæmmere, screenet for organiske forbindelser og ftalater. Herudover er der foretaget migrationsforsøg til spyt og sved.
Vurdering i relation til miljømærker
Flere af produkterne indeholder stoffer, der overskrider grænseværdierne for opnåelse af miljømærker. Kun et enkelt af de undersøgte produkter bærer miljømærke, nemlig produkt nr. 10, som er Øko-Tex mærket.
Sundhedsmæssig vurdering
Følgende konklusioner kan udledes af undersøgelsen:
- Alle de undersøgte babyprodukter indeholder målelige mængder af mere end ét stof, der er klassificeret som farligt for sundhed og/eller miljø.
- Der er ikke fundet stoffer, som udgør nogen væsentlig sundhedsmæssig risiko i produkterne.
- De undersøgte engangsskumvaskeklude indeholder 2 stoffer med kræftfremkaldende, reproduktionstoksiske og sensibiliserende effekter.
- De undersøgte ammepuder afgiver formaldehyd, som kan være sensibiliserende og i højere koncentrationer kræftfremkaldende ved indånding. Vurderingen viser, at “worst case” migration til huden – hvor man antager at 100% af stoffet optaget gennem huden - kan bidrage væsentligt til det acceptable daglige indtag (ADI).
- De undersøgte puslepuder indeholder stoffer med kræftfremkaldende, sensibiliserende og reproduktionstoksiske effekter i lave koncentrationer, som i visse tilfælde kan udgøre en sundhedsmæssig risiko.
- De undersøgte babymadrasser indeholder lave koncentrationer af stoffer med en mulig reproduktionstoksisk effekt i visse tilfælde.
- Der er en betragtelig usikkerhed i estimeringen af optaget mængde af 2-ethylhexansyre for de produkter, hvor der ikke er foretaget migrationsforsøg. Dette betyder, at der inden for usikkerheden kan være en lille sundhedsmæssig risiko ved brug af to af engangsskumvaskekludene, samt en madras og en af puslepuderne.
- Et produkt indeholder blødgørere (phthalater) i mængder som er forbudt ved dansk lovgivning og lovgivning i EU for babyprodukter. Kemikalieinspektionen har behandlet sagen.
- Nogle af produkterne indeholder flere stoffer med kroniske effekter, som også findes i andre produkter i samfundet. Effekten af en sådan eksponering fra flere forskellige kilder bør vække bekymring, da babyer er en følsom del af befolkningen og derfor bør beskyttes mod kemisk eksponering.
I Tabel 0.1 er gengivet, hvilke stoffer der er vurderet.
Tabel 0.1 De vurderede stoffers sundhedseffekter
| Stof | CAS-nr. | Sundhedseffekter |
| Hexabromcyclododecan (HBCD) | 25637-99-4 | Udviklings- og neurotoksiske effekter |
| Toluen- 2,4-diisocyanat (TDI) | 584-84-9 | Carc.3;R40 (mulig kræftfremkaldende) R42/R43 (mulig sensibiliserende) |
| 2-Ethylhexansyre (2-EHA) | 149-57-5 | Rep.3;R63 Mulig skade på barnet under graviditet |
| Acetophenon | 98-86-2 | Lokalirriterende |
| 1,1,2,2-Tetrachlorethan | 79-34-5 | Meget giftig ved indånding og ved hudkontakt |
| Formaldehyd | 50-00-0 | Carc3, R40 (mulig kræftfremkaldende) R43 (mulig sensibiliserende) |
| Styren | 100-42-5 | Lokalirriterende |
| 2-Brom-4,6-dinitroanilin (BDNA) | 1817-73-8 | Få data |
| Hexaethylen-glycol dimethyl ether | 1072-40-8 | Få data |
| Tetrapropylen glycol monomethyl ether | 20324-34-9 | Få data |
Fra analysedata af de frigjorte (emitterede) stoffer i Tabel 0.1 fra de forskellige produkter, er der, hvor det er muligt, beregnet en sikkerhedsmargin (MOS), og den sundhedsmæssige risiko er vurderet.
For de enkelte produkter var det samlede resultat:
Produkt nr. 4 (ammepude) og produkt nr. 5 (ammepude) bidrager med en betydelig andel af den acceptable daglige indtagelse af formaldehyd.
DEHP blev fundet i produkt 11 (forlæder til barnevogn) uden, at der blev vurderet at være en sundhedsmæssig risiko, men med en betydelig usikkerhed i estimatet på grund af migrationsbetingelserne.
I produkt nr. 8 (puslepude) var indholdet af phthalater i produktet eller dele af produktet over den tilladte mængde på 0,05 vægt % i Bekendtgørelse nr. 151 af 15/03/1999 om forbud mod phthalater i legetøj til børn i alderen 0-3 år samt i visse småbørnsartikler mv. Overskridelsen er indberettet til Miljøstyrelsen, og Kemikalieinspektionen har behandlet sagen.
For produkterne 9 (bæresele) og 12 (forlæder til barnevogn) blev der ikke fundet nogen sundhedsmæssig risiko og ingen phthalater over den tilladte mængde.
Miljømæssig vurdering
Stoffernes miljøfareklassificering er vist i Tabel 0.2.
Tabel 0.2 Oversigt over miljøfareklassifikation
| Stof | CAS-nr. | Miljøfareklassifikation |
| Hexabromcyclododecan (HBCD) | 25637-99-4 | N;R50-53 |
| Toluen-2,4-diisocyanat (TDI) | 584-84-9 | R52-53 |
| 2-Ethylhexansyre (2-EHA) | 149-57-5 | Mangler data (Muligvis R52/53) |
| Acetophenon | 98-86-2 | N.C. |
| Formaldehyd | 50-00-0 | N.C. |
| 1,1,2,2-Tetrachlorethan | 79-34-5 | N;R51-53 |
| Styren | 100-42-5 | N.C.* |
| 2-Brom-4,6-dinitroanilin (BDNA) | 1817-73-8 | N;R51/53** (Mulig frigivelse af potentielt farlige aromatiske aminer) |
| Hexaethylenglycol dimethylether | 1072-40-8 | Ingen data |
| Tetrapropylenglycol monomethylether | 20324-34-9 | Ingen data |
N.C.: Ikke miljøfareklassificeret (Not classified, N-CLASS 2005).
*: Forslag (EU Risk Assessment Report on styrene, 2000).
**: Forslag (Miljøstyrelsen, Vejledende liste til selvklassifikation, 2005).
De tre stoffer hexabromcyclododecan (HBCD), 1,1,2,2-tetrachlorethan og toluen-2,4-diisocyanat (TDI) er henholdsvis meget giftige, giftige eller skadelige for organismer, der lever i vand og kan alle forårsage uønskede langtidsvirkninger i vandmiljøet. Udledning og eksponering af disse stoffer til vandmiljøet bør derfor reduceres eller forhindres.
Derudover er de mulige miljømæssige effekter af stofferne 2-ethylhexansyre (2-EHA), 2-brom-4,6-dinitroanilin (BDNA), hexaethylenglycol dimethylether og tetrapropylenglycol monomethylether, som følge af manglende data, ikke fuldt klarlagt. Udslip og udledninger til vandmiljøet af disse stoffer bør derfor minimeres, indtil de mulige miljøeffekter er kendte.
Det kan generelt antages, at udledning til spildevand af stofferne fra en daglig anvendelse og vask af disse typer af produkter, med undtagelse af engangsskumvaskekludene, er langt mindre omfattende end fra andre former for tekstiler, som bliver vasket mere regelmæssigt. Engangsskumvaskekludene indeholder 2-EHA blandt andre stoffer, som kan blive udledt til kloaksystemet ved brug af nye engangsskumvaskeklude. Fordi HBCD og phthalaterne er stoffer med et lavt damptryk og en høj log Kow værdi, er det mest sandsynligt, at størsteparten af indholdet vil blive i produkterne i deres anvendelsestid, med mindre produktet kommer i kontakt med organiske opløsningsmidler eller opvarmes til højere temperaturer gennem længere tid. Ikke desto mindre vil frigivelsen af disse stoffer til miljøet sandsynligvis fortsætte i små mængder, så længe produkterne indeholder dem.
1 Survey
1.1 Purpose
The purpose of the survey is to monitor which baby products that exist on the Danish market and to have a test related basis for evaluating if these products contain chemicals including colourants that are of health concern.
The survey on which products in the category baby products that exist on the market and what materials the products are made of is a prerequisite to select and purchase products for a further chemical examination and to evaluate the health risk in relation to the use of the products.
1.2 Delimitation
The registration of baby products concerns articles intended for babies up to 1 year of age.
The registration has primarily concerned articles of textile and plastic with padding or stuffing.
In addition, also baby products of flexible foam materials are included.
The following product types were not included in the registration: Baby clothing, bed linen (bolster case), shaped plastic objects (bath tubs, chamber pots, comforters, baby plates), baby cutlery, and toys, wooden beds (cots) and babycare agents/remedies.
1.3 Procedure
The survey is performed by:
- Visits to retailers where the commodity supply is inspected and leaflets collected
- Search on the Internet
- Gathering information from retailers, importers and producers by sending out questionnaires and supplementary contact by telephone or e-mail.
Information has been retrieved about the type of baby products which are sold by retailers and through the Internet. In addition inquiries were made on which materials these baby products were made of to evaluate the health risk and further to acquire a basis to select and purchase baby products for a further chemical examination.
1.4 Implementation
1.4.1 Visits to retailers
Retailers / ware houses in the vicinities of Copenhagen were visited. The visited stores have been specialty stores for baby and child equipment, furniture department stores with sections for baby and toddler equipment, and super markets (convenience stores) with departments for baby and child equipment.
At the same time leaflets are collected when possible. This was performed in order to get information on materials in the individual baby products.
1.4.2 Internet search
Search is made on www.krak.dk under Trade for the groups ”babyartikler” (baby articles) and ”barnevogne” (perambulators) and via Google using the search word ”babyudstyr” (babycare articles). The results of the searches were studied and based on company profile information the most relevant companies were selected. If a company or enterprise homepage existed they were visited also.
1.4.3 Obtaining information by questionnaire
A questionnaire was sent to a series of retailers, importers, and producers. Eighteen selected enterprises found by Internet search or from our general knowledge among others by advertisements and articles on studies on consumer products. The questionnaire is prepared in Danish and English, respectively, to make it possible also to obtain responses from foreign producers.
1.4.3.1 Preparation of the questionnaire study
The questionnaire is prepared based on the general experience and knowledge in the field of the Danish Technological Institute combined with information obtained from visits in the retail stores including obtained leaflets and information found on the Internet.
The questionnaires contain the following questions:
- Background information
- Company name
- Addresses
- Contact person
- Telephone no.
- Baby product type
- Commodity name/Manufacturer
- Design name
- Produced amount – pieces per year
- Imported amount – pieces per year
- Sold amount – pieces per year
- Cover material (with following tick off possibilities)
- Cotton
- Plastic covered textile
- Plastic film
- Wool
- Synthetic (state fibre/plastic type)
- Other
- Stuffing/padding material (with following tick off possibilities)
- PU–foam (polyurethane)
- Granulated foamNatural latex foam
- Polystyrene pellets
- Hollow plastic pellets (state plastic type)
- Polyester fibre fill
- Cotton fibre fill
- Wool fibre fill
- Feather/down
- Other
- Impregnations (with following tick off possibilities)
- Flame retardants
- Antibacterial
- Other
- Contents of chemicals (state perhaps if the product is Eco-Tex-labelled or in any other way environment labelled).
Questionnaires are sent to a total of 18 enterprises. 11 companies have responded totally or partially on the questionnaire at a different degree of detail to the given information.
The questionnaire has been responded to by 4 nationwide department stores with departments for baby products, just as 6 importers and producers with a relatively limited selection of goods have presented information. One importer has referred to his mother company which is the producer.
On the contrary, several of the larger baby equipment enterprises that are specialty dealers, importers or producers of baby and children articles have either not responded with information or – as was the case for 2 enterprises – responded with more general information.
One of the larger players on the market has announced that it required too large resources to respond to the questionnaire and that the requested information were not available. This was elaborated by telephone contact.
Another larger player has contacted the Danish Technological Institute and stated that it was a very labour-intensive task and that all their products observed ”all environment requirements and EU standards including Eco-Tex”.
At the same time this company expressed great discontent as the project report would not state the names of the design, dealer or company for the baby products to be examined. According to the company a ”bad” result for an anonymous product in the consumers eyes recoil on all designs of the same type. (The company has not presented any information).
1.5 Results of the survey
Acquaintance was made with a long series of articles or products intended for babies up to 1 year of age.
Below is presented the results of the registration of type of baby products, the products material contents and the registered consumption distributed on product types.
1.5.1 Registration of baby products on the market
Following baby products are registered:
- Nursing pads/ -cushions/ -pillows
- Carrycots/ perambulator upholstery
- Bunting / carrier-/sleeping bags /play rugs (that is not toy/CE-labelled), including combined products
- Baby carrier/ baby slings
- Pillow for baby feeding
- Baby chairs and loose chair covers/-cushions, cradle seats, sedan chairs, baby bouncers
- Bed frames and head protection articles for perambulators
- Baby mattresses and –mattress pads
- Baby duvets and –pillows
- Sack chair/support pillows
- Child car seats
- Padded baby seats and baby supports to cycle trailers and
walk/running trolleys - Cycle seats
- Wet-sheets
- Disposable wash cloths.
1.5.2 Material composition
Based on information from labels, tags and leaflets, and from our evaluation based on material knowledge to textiles, the individual composition of materials in the baby products are registered as follows:
Nursing pads/-cushions/-pillows
Cover of plastic film or textile (of cotton or in cases cotton/polyester) with a plastic surface or similar surface treatment, also covers of nylon fabrics. The plastic may be PVC, polyurethane (PU), acrylic or other. The cover often has a printed pattern.
Stuffing of foam (polyurethane foam, PU) or of polyester fibre fill.
Carrycots/perambulator upholsteries
Cover/exterior fabrics are textile often with a plastic treatment either on the outside or inside, or with a water-repellent impregnation. The fabric is most often polyester but may also be cotton or cotton/polyester. The surface treatment may be polyurethane (PU) of the type FR (= containing a flame retardant product).
Upholstery or stuffing of polyurethane foam (PU) and/or polyester fibre fill.
Bunting/ carry-/sleeping bags
Cover/exterior of polyester, polyester/cotton or cotton.
Interior fabric (lining) of polyester/cotton, cotton or polyester fleece (furry fabric).
Stuffing/interior of polyester fibre fill, wool fibre fill or down/feathers.
Baby carriers
Exterior fabric of cotton, cotton/polyester, polyester.
Stuffing/upholstery of polyester fibre fill, in case polyurethane foam (PU).
Pillows for feeding baby
Exterior of cotton or cotton /polyester. Often with printed pattern.
Stuffing of polystyrene pellets with flame retardants. Some with fibre fill (probably polyester), PU-foam granulate or plastic flakes.
Baby chairs and loose chair linings/cushions, cradle seats, sedan chairs, baby bounders
Exterior fabric of cotton or cotton/polyester.
Stuffing of polyester fibre fill.
Bed edges and head protection in perambulators
Exterior fabric of cotton or cotton/polyester.
Stuffing of polyester fibre fill, in case PU-foam.
Baby mattresses and mattress pads
Cover of cotton and cotton/polyester.
Stuffing of polyurethane foam (PU), polyester fibre fill, latex foam, PU-foam with flame retardant, flax felt glued on PU-foam.
Baby eiderdowns/duvets and pillows
Cover of cotton or cotton /polyester.
Stuffing of polyester fibre or down/feathers.
Also eiderdowns and pillows with interior heat regulating non-woven membrane, Outlast®.
Baby car seat
Covers of cotton, polyester and polyester/cotton.
The upholstery is most often padding with polyester fibre fill. Cotton wool may occur. Also polyurethane foam (PU) may occur.
The padded cover is mounted on a plastic shell chair.
Padded baby seats and baby supports to cycle trailers and walking/running trolleys
Cover of polyester fabric with PVC-lining, polyester micro fibre fabric and polyester fleece (furry fabric).
Stuffing/upholstery of polyurethane foam (PU) with content of flame retardant substance and polyester fibre fill.
Wet-sheets
Upper side of cotton frotte with plastic cover on the backside.
Foam wash cloths
Flexible foam of polyurethane (PU).
1.5.3 Consumption divided into product types
Several of the nationwide department stores have reported sold amounts of their baby products, just as a series of minor producers have reported produced number of a few baby products. This information is presented in Table 1.1.
Table 1.1 Number of sold units reported in the questionnaire responses and from telephone calls
| Dealers | Products | Number per year |
| Specialty stores (retail + Internet) | Cycle trailer | Not reported |
| Cycle trailer + walking-/running trolley | Not reported | |
| Baby seat to car | Not reported | |
| Baby support | Not reported | |
| Bunting to car | Not reported | |
| Strap to car | Not reported | |
| Producer | Perambulator | 2.000 |
| Department store | Auto seat | Not reported |
| Importer | Pillow for feeding baby | 125 ca. |
| Department store | Bunting | 1.000 |
| Nursing pillow | 10.000 | |
| Pillow for feeding baby | 6.000 | |
| Cradle seat | 5.000 | |
| Baby carrier | 3.000 | |
| Carrycot/ bunting/ play rug | 2.000 | |
| Department store | Nursing cushion | Not reported |
| Pillow for feeding baby | Not reported | |
| Eiderdown/ pillow | 725/846 | |
| Carrying-/sleeping bag | 1.000 | |
| Department store | Mattress pad | 2.330 |
| Spring mattress | 2.786 | |
| Foam mattress | 3.009 | |
| Nursing table with nursing pillow | 555 | |
| Nursing pillow | 5.013 | |
| Bed lining | 3.249 | |
| Bed side protection | 131 | |
| Cushion/pillow | 4.235 | |
| Eiderdown / duvet | 1.538 | |
| Pillow | 1.997 | |
| Specialty store | Perambulator | 10-15.000 estimated |
| Nursing cushion | 15-30.000 estimated | |
| Importer | Perambulator | 2.630 |
| Twin perambulator | 594 | |
| Carrycot | 2.210 | |
| Bunting | 100 | |
| Running trolley | 20 |
Since several of the larger enterprises which specialises in baby and childcare products have not responded with detailed specific information it was not immediate possible to estimate the actual consumption of the individual types of baby products and of the individual trade marks.
Nor via the Statistics Denmark is it possible to obtain a number of baby products either as a collected group or as individual types. However, based on information on the number of births are estimated approximately 65,000 newborns per year.
The trade of product types and trade marks is therefore estimated from general market knowledge and catalogues, supplemented with there information received from the questionnaires and the number of yearly newborns
Some of the products may due to their durability be used for more than one child. In return, supplementary products per child will exist in e.g. child care centres and day-care facilities and perhaps also at grand parents.
The number of purchased new perambulators per year is evaluated to be considerably lower than the number of newly born at an estimate of 25-40,000 perambulators per year. This is caused by the perambulators being relatively expensive products and that most perambulators have a durability that causes them to be used for at least 2 children in total.
For other less expensive and less durable baby products the number of pieces per year is estimated to be closer to the number of new-borns at an estimate of 40-60,000 pieces.
1.5.4 Evaluation of materials
The materials are evaluated based on the Danish Technological Institute’s knowledge and experience of textiles, plastics and foam, and other upholstery and stuffing ,materials. The evaluation is performed with consideration of the content of chemical substances known or under reasoned suspicion to be harmful to health.
Products with stuffing of polyester fibre fill do not present the same risk of harmful substances as stuffing of polyurethane foam (PU) or polystyrene pellets. Polyurethane foam and polystyrene pellets may contain flame retardants (in case brome based preparations) and may also release volatile organic compounds that may be harmful.
Polyester fibre fill may contain the heavy metal antimony which is used as catalyst.
Furthermore the polyurethane foam (PU) can not be precluded to contain the organic tin compounds dibutyltin (DBT) and tributyltin (TBT) added as antibacterial treatment.
Products with a cover of plastic or plastic treated surface may contain phthalates (PVC plasticizers). It has also been found that such products contain the biological active substance triclosane (bacterial growth inhibitor). Besides that also flame retardant substances may sometimes be present.
Cover of fabric with a printed pattern and/or has an impregnation or surface treatment may contain formaldehyde.
Both coloured and printed fabrics may contain heavy metals and harmful amines from azocolourants and pigments.
Coloured polyester fabrics may contain allergenic dispersion colourants.
All cover fabrics may contain residues of chlorinated phenols and OPP (ortho-phenyl phenol).
Cover fabrics of textile may be impregnated with flame retardants but it is more likely for baby products unless a specific declaration of contents is available.
Cover fabrics may also be impregnated with PFOS compounds as water repellent treatment.
1.5.5 Criteria for selection of baby products for analyses
Babies will be exposed to effects from a possible content of harmful chemicals and colorants in products by uptake through the skin, by direct contact, by sucking on the products or by inhaling the volatiles or released particles (dust) from the material itself.
Essential factors in the selection of products have been: The time a baby may be expected to be exposed to the product and the exposed surface area of the baby in contact with the product during use and how certain it will be that the baby sucks on it.
Knowledge of materials has also been included in the selection.
Furthermore, the Danish Environmental Protection Agency has emphasised that for each type of product selected, an article in both the less expensive as well as the expensive end of the scale of prices was selected.
1.5.6 Selected products
In co-operation with the Danish Environmental Protection Agency, the following types of baby products are selected for a further examination of their content of chemical substances and colorants harmful or of concern to the health:
- Pillows for baby feeding
- Baby carriers
- Nursing pillows/ cushions with different covers and stuffing
- Baby mattresses with stuffing of foam, to beds
- Aprons to perambulators
- Disposable foam wash cloths.
Each selected baby product is represented by 2 different articles or trade marks, for the disposable foam wash cloths, however, 3 different articles. Thus, a total of 13 products have been selected for further examination. The products are presented in Table 1.2.
Table 1.2 Summary of selected baby products
| Product no. | Product type |
| 1 | Disposable foam wash cloth |
| 2 | Disposable foam wash cloth |
| 3 | Disposable foam wash cloth |
| 4 | Pillow for baby feeding |
| 5 | Pillow for baby feeding |
| 6 | Baby mattress |
| 7 | Nursing pillow |
| 8 | Nursing pillow |
| 9 | Baby carrier |
| 10 | Baby carrier |
| 11 | Apron for perambulator |
| 12 | Apron for perambulator |
| 13 | Baby mattress |
2 Survey of content of chemical substances – chemical analyses
- 2.1 Analysis programme
- 2.2 Methods
- 2.2.1 Analysis for content of azo-colourants
- 2.2.2 Analysis for content of disperse colourants
- 2.2.3 Analysis of the content of selected organic tin compounds
- 2.2.4 Analysis of the content of selected organic phosphorous compounds
- 2.2.5 Analysis of content of chlorinated benzenes and toluenes
- 2.2.6 Analysis for content of chlorinated phenols and OPP
- 2.2.7 Analysis for content of selected phthalates
- 2.2.8 Analysis for content of formaldehyde
- 2.2.9 Screening analyse by GC-MS
- 2.2.10 Analysis for the content of Br, P, Cl, Sn, b, F by EDXRF
- 2.2.11 Migration test in artificial sweat and artificial saliva, respectively
- 2.2.12 Analysis of volatile organic components (VOC) by headspace analysis
- 2.2.13 Analysis of isocyantes with HPLC
- 2.3 Analysis results divided according to sample types
- 2.4 Analysis results for specific analyses according to analysis type
- 2.4.1 Analysis results for arylamines
- 2.4.2 Analysis results for disperse colourants
- 2.4.3 Analysis results for P-containing flame retardants [µg/g]
- 2.4.4 EDXRF analysis - results presented as % (w/w)
- 2.4.5 Analysis results for organic tin compounds
- 2.4.6 Chlorinated benzenes and toluenes
- 2.4.7 Analysis results for chlorinated phenols and OPP
- 2.4.8 Analysis results for phthalates, mg/g
- 2.4.9 Formaldehyde
- 2.4.10 Migration test in sweat of selected products
- 2.4.11 Migration test in saliva for selected products
- 2.4.12 Migration test in sweat and saliva for PS-pellets
- 2.4.13 Emission test of volatile organic components - PS-pellets
- 2.5 Selection of chemical substances for evaluation
To ensure optimal clearness, the chapter concerning the chemical analyses has been subdivided according to types of products.
- Foam wash cloths (disposable wash cloths)
- Pillows for feeding the baby
- Mattresses
- Nursing pillows
- Baby carriers
- Apron for perambulators.
2.1 Analysis programme
Some of the examined products are composed of several elements and where considered relevant these elements are examined individually as the different materials calls for different analysis method.
2.1.1 Foam materials
Under this material designation belongs the following products or parts of products:
- Foam wash cloths
- Foam mattresses
- Foam in nursing pillows
- Foam in baby carriers.
For the detection of possible content of flame retardants, organic tin compounds, tin and antimony for all foam materials are performed a screening analysis by EDXRF to measure chromium, chlorine, phosphorous, tin and antimony.
Products where tin is determined are supplemented with a specific analysis for the content of organic tin compounds.
For all foam materials, a screening analysis by GC-MS for the contents of volatile organic components is preformed. By this determination, a further specific analysis for the content of phosphorous flame retardants is performed.
A specific analysis for certain isocyanates (MDI and TDI) has been performed on the three types of foam wash cloths using the HPLC method.
2.1.2 Textiles
Under this material designation belongs the following part of products:
- Cover on foam mattresses
- Cover on pillows for feeding the baby
- Cover on nursing pillows
- Textiles on baby carriers.
Textiles of cotton are examined for their content of formaldehyde. Coloured textiles are examined contents of azo-colourants and disperse colourants.
Synthetic fabrics are examined for contents of chlorinated phenols – tetrachlorophenol (TeCP) and pentachlorophenol (PCP), - 2-phenylphenol (OPP), for the content of chlorinated benzenes and chlorinated toluenes.
For selected textiles a screening analysis by GC-MS for the content of volatile organic components (VOC) has been performed.
For the detection of possible content of flame retardants, surface treated textiles are analysed by an EDXRF-screening to measure bromine, chlorine and phosphorous. In this context a further measurements for the contents of fluorine and sulphur was performed to the identification of potential content of PFOS compounds.
2.1.3 Polymer materials
Under this material designation is included the following part of products:
- Cover of nursing pillows
- Labels, buckles, etc.
All polymer materials including textiles surface treated with polymer materials are examined for contents of phthalate based plasticizers.
For selected products, a screening analysis by GC-MS for contents of volatile organic compounds has been performed.
2.1.4 Polystyrene pellets
Under this material designation is included the following part of products:
- Stuffing material in pillows for feeding the baby.
To determine possible content of flame retardants the polystyrene pellets have been examined by an EDXRF screening analysis to measure for bromine, chlorine and phosphorous.
A screening analysis by GC-MS for the content of volatile organic compounds has been performed.
2.1.5 Summary of performed analyses
Table 2.1 and Table 2.3 summarise which analyses that have been performed on the different materials and subsamples
Table 2.1 Summary of analysis parameters related to materials
| Components | Foam | Textiles | Polymers | PS-pellets |
| Azocolourants | X | |||
| Disperse colourants | X | |||
| Flame retardants - EDXRF Br, P, Sb, Cl, Sn |
X | X | ||
| Fluorine – EDXRF | X | |||
| Organic tin compounds | X | |||
| Selected organic P-compounds Flame retardants |
X | |||
| Chlorinated benzenes and toluenes | X | |||
| Phenols | X | |||
| Phthalates | X | X | ||
| Formaldehyde | X | |||
| Screening - organic components | X | X | X | X |
| Emission of VOC | X | |||
| Migration test in saliva | X | X | ||
| Migration test in sweat | X | X | X | X |
| Isocyanates - HPLC | X |
Summary of selected baby products and the analysed subsamples is presented in Table 2.2.
Table 2.2 Summary of selected baby products and the analysed subsamples
| Sample mrk | Product type | Subsample – Material |
| 1 | Disposable foam wash cloth | PU foam |
| 2 | Disposable foam wash cloth | PU foam |
| 3 | Disposable foam wash cloth | PU foam |
| 4 | Pillow for baby feeding | A - Outer cover, cotton with printing B - Inner cover, nylon or polyester C - Stuffing, polystyrene (PS) pellets |
| 5 | Pillow for baby feeding | A - Outer cover, cotton with printing B - Inner cover, non-woven fliseline C - Stuffing, PS pellets |
| 6 | Mattress with cover | A - Cover, cotton B - Foam |
| 7 | Nursing pillow | A - Cover, surface treated textile B - Foam |
| 8 | Nursing pillow | A - Plastic cover, coloured B - Foam C - Plastic underfelt |
| 9 | Baby carrier | A - Strap (reins), textile B - Inner strap, textile C - Straps D - Foam |
| 10 | Baby carrier | A - Outer textile, uniform coloured B - Inner textile with printing C - Short PES-strap D - Grey plastic badge E - Grey printing F - Printed instruction G - Metal buttons H - Foam I - Metal part of buckle |
| 11 | Apron for perambulator | A - Outer cover, PES with plastic cover B - Inner cover, PES / nylon C - Metal buttons D - Foam in wind shield E - Foam in the apron itself |
| 12 | Apron for perambulator | A - Outer cover, PES with plastic cover B - Inner cover C - Plastic parts D - Foam in the wind shield E - Fibre fill (PES) in the apron |
| 13 | Mattress with cover | A - Cover B - Foam C - Foam D - Fibre fill |
The analysis programme for the selected baby products was established based on the composition of the materials, knowledge to the material character and experience from published and corresponding studies. Summary of the analysis programme is presented in Table 2.3.
Table 2.3 Summary of analysis programme for baby products distributed in subsamples
| Components | Subsamples for the analysis programme |
| Azocolourants | 9A, 9B, 9C, 10A, 10B, 10C, 11A, 12A |
| Disperse colourants | 9A, 9B, 9C, 10C, 11A, 12A |
| Flame retardants - EDXRF Br, P, Sb, Cl, Sn |
1, 2, 3, 4C, 5C, 6B, 7B, 8B, 9D, 10H, 11A, 11D, 11E, 12A, 12D, 13B, 13C |
| Fluorine – EDXRF | 11A, 12A |
| Organic tin compounds | 1, 2, 3, 5C, 6B, 7B, 8B, 9D, 10H, 11D, 11E, 12A, 12D, 13B |
| Selected organic P-compounds Flame retardants |
1, 2, 3, 4A, 4C, 5A, 5C, 6A, 6B, 7A, 7B, 8A, 8B, 11A, 11D, 11E, 12A, 12B, 12D, 12E, 13A, 13B, 13C, 13D |
| Organic carriers (chlorinated benzenes and toluenes) | 9A, 9B, 9C, 11A, 12A |
| Phenols | 4A, 5A, 7A, 11A, 12A, 12B |
| Phthalates | 7A, 8A, 8B, 8C, 10D, 10E, 10F, 11A, 12A, 12C, 13C |
| Formaldehyde | 4A, 5A, 6A, 7A, 10B, 11B, 12B, 13B |
| Screening – organic components | 1, 2, 3, 4A, 4C, 5A, 5C, 6A, 6B, 7A, 7B, 8A, 8B, 11A, 11D, 11E, 12A, 12B, 12D, 12E, 13A, 13B, 13C, 13D |
| Migration test in saliva | 4C, 5C, 11A, 12A |
| Migration test in sweat | 4A, 4C, 5A, 5C, 7A, 8A, 13B, 13C |
| Emission of VOC | 4C, 5C |
| Isocyanates- HPLC | 1, 2, 3 |
2.2 Methods
A representative amount of sample or a represenative area of the tested materials were removed for analysis. The analyses were performed by double determination according to the following methods.
2.2.1 Analysis for content of azo-colourants
The analysis was performed according to DS/EN 14362-1. A weighed amount of sample, approx. 1 g, was extracted in a buffer solution at 70ºC for 30 minutes after the addition of internal standards of aniline-d4 and naphthaline-d8. Then a solution of sodium dithionite was added and the sample was left for 30 minutes at 70ºC. The concentration was increased on SPE-filter followed by extraction using MTBE. The extract was analysed by gaschromatography-mass spectrometry (GC-MS in scan mode).
Quantification performed against external standards of each of the analytes.
A summary of the azo-colourants screened for is presented in Table 2.4.
The detection limit are for all components 5 µg/g.
Table 2.4 Summary of azo-colourants
| Selecte¨’d components | CAS no. |
| 4-Aminobiphenyl | 92-67-1 |
| Benzidine | 92-87-5 |
| 4-Chlor-o-toluidine | 95-69-2 |
| 2-Naphthylamine | 91-59-8 |
| o-Aminoazotoluene | 97-56-3 |
| 2-Amino-4-nitrotoluene | 99-55-8 |
| 4-Chloroaniline | 106-47-8 |
| 2,4-Diaminoanisol | 615-05-4 |
| 4,4’-Diaminodiphenylmethane | 101-77-9 |
| 3,3’- Dichlorobenzidine | 91-94-1 |
| 3,3’- Dimethoxybenzidine | 119-90-4 |
| 3,3’- Dimethylbenzidine | 119-93-7 |
| 3,3’- Dimethyl-4,4’-diaminodiphenylmethane | 838-88-0 |
| p-Cresidine | 120-71-8 |
| 4,4’-Methylene-bis-(2-chloraniline) | 101-14-4 |
| 4,4’-Oxydianiline (4,4’-Diaminodiphenylether) | 101-80-4 |
| 4,4-Thiodianiline (4,4’-Diaminodiphenylsulfide) | 139-65-1 |
| o-Toluidine | 95-53-4 |
| 2,4-Toluenediamine (2,4-Diaminotoluene) | 95-80-7 |
| 2,4,5-Trimethylaniline | 137-17-7 |
| o-Anisidine | 90-04-0 |
| 2,4-Xylidine + 2,6-Xylidine | 95-68-1 / 87-62-7 |
2.2.2 Analysis for content of disperse colourants
A weighted amount of sample, 1 to 5 g, was extracted in acetone by ASE-extraction or Soxhlet extraction. The extracts were analysed for their content of disperse colourants by TLC using different solvents.
Determinations were performed against external standards of each of the analytes.
Table 2.5 presents the disperse colourants that were screened for.
Table 2.5 List of measured disperse colourants
| Disperse colourants | CAS no. |
| Disperse Blue 1 | 2475-45-8 |
| Disperse Blue 3 | 2475-46-9 |
| Disperse Blue 7 | 3179-90-6 |
| Disperse Blue 26 | 3860-63-7 |
| Disperse Blue 35 | 12222-75-2 |
| Disperse Blue 102 | 12222-97-8 |
| Disperse Blue 106 | 12223-01-7 |
| Disperse Blue 124 | 61951-51-7 |
| Disperse Orange 1 | 2581-69-3 |
| Disperse Orange 3 | 730-40-5 |
| Disperse Orange 37 | 12223-33-5 |
| Disperse Orange 76 | 12223-33-5 |
| Disperse Red 1 | 2872-52-8 |
| Disperse Red 11 | 2872-48-2 |
| Disperse Red 17 | 3179-89-3 |
| Disperse Yellow 1 | 119-15-3 |
| Disperse Yellow 3 | 2832-40-8 |
| Disperse Yellow 9 | 6373-73-5 |
| Disperse Yellow 39 | 12236-29-2 |
| Disperse Yellow 49 | 54824-37-2 |
2.2.3 Analysis of the content of selected organic tin compounds
A weighed amount of sample, 0.5 to 1 g, was extracted in 70 ml acid methanol by ultrasound for 2 hours. After pH adjustment with sodium hydroxide a solution of sodium tetraethylborate was added. After 1 hour reaction at mechanical shaking the extract was transferred to a SPE-filter which was then extracted by isooctane. The extract was analysed by gaschromatography-mass spectrometry (GC-MS in scan mode).
Quantification was performed against external standards of each of the analytes (selected organic tin compounds).
Table 2.6 presents the organic tin compounds that were screened for. The detection limit for the used analysis method was 0.02 µg/g.
Table 2.6 List of organic tin compounds
| Component | CAS no. |
| Monobutyltin (MBT) | 78763-54-9 |
| Dibutyltin (DBT) | 1002-53-5 |
| Tributyltin (TBT) | 688-73-3 |
| Tetrabutyltin (TeBT) | 1461-25-2 |
| Monooctyltin (MOT) | 3091-25-6 |
| Dioctyltin (DOT) | 15231-44-4 |
| Tri-cyclohexyltin | 3091-32-5 |
| Triphenyltin | 668-34-8 |
2.2.4 Analysis of the content of selected organic phosphorous compounds
A weighed amount of sample, 0.5 to 2 g, was extracted in 25 ml dichloromethane for 2 hours by mechanical shaking. The extract was analysed by gaschromatography-mass spectrometry (GC-MS in scan mode).
Quantification was performed against external standards of each of the analytes.
Table 2.7 presents the organic P-compounds screened for. The detection limit of the used analysis method is 1 µg/g.
Table 2.7 List of P-compounds screened for
| Selected components | CAS no. |
| Tributylphosphate (TBP) | 126-73-8 |
| Triphenylphosphate (TPP) | 115-86-6 |
| Tris(2-chlorethyl)phosphate (TCEP) | 115-96-8 |
| Tris(2-ethylhexyl)phosphate (TEHP) | 78-42-2 |
| Tris(2-butoxyethyl)phosphate (TBEP) | 78-51-3 |
2.2.5 Analysis of content of chlorinated benzenes and toluenes
A weighed amount of sample, approx. 2 g, was extracted in 40 ml acetone added internal standards of C13-labelled hexachlorobenzene for 2 hours by mechanical shaking. The extracts were concentrated and analysed by gaschromatography-mass spectrometry (GC-MS in scan mode).
Quantification was performed against external standards of each of the analytes.
In connection to the performed determinations, dichloro-, trichloro-, tetrachloro-, pentachloro-, hexachlorobenzenes, and chloro-, dichloro-, trichloro-, tetrachloro-and pentachlorotoluenes were analysed for.
The detection limit for the used analysis method is 0.05 µg/g.
2.2.6 Analysis for content of chlorinated phenols and OPP
A weighed amount of sample, approx. 5 g, was added internal standards of C13-labelled pentachlorophenol and extracted in a solution of K2CO3 by ultrasonic extraction. Derivatization by acetic acid anhydride followed by extraction by hexane. The extract was concentrated and analysed by gaschromatography-mass spectrometry (GC-MS in SIM mode).
Quantification was performed against external standards of each of the analytes.
Table 2.8 presents the chlorinated phenols and OPP screened for and the detection limit for the used analytical method.
Table 2.8 Detection limit for chlorinated phenols and OPP
| Selected components | CAS no. | Detektion limit, µg/g |
| o-Phenylphenol (OPP) | 90-43-7 | 0.01 |
| 2,3,5,6-Tetrachlorophenol (TeCP) | 935-95-5 | 0.02 |
| Pentachlorophenol (PCP) | 87-86-5 | 0.02 |
2.2.7 Analysis for content of selected phthalates
A weighed amount of sample, 0.1 to 1 g, was extracted in 20 ml dichloromethane added internal standards of BBP-d4 and DEHP-d4 by mechanical shaking for 2 hours. The extract was analysed by gaschromatography-mass spectrometry (GC-MS in scan mode).
Quantification was performed against external standards of each of the analytes.
Table 2.9 presents the phthalates screened for and the detection limits of the used analytical methods.
Table 2.9 Detection limit of determined phthalates
| Selected components | CAS no. | Detektion limit, mg/g |
| Dimethylphthalate (DMP) | 131-11-3 | 0.020 |
| Diethylphthalate (DEP) | 84-66-2 | 0.020 |
| Diisobutylphthalate (DIBP) | 84-69-5 | 0.020 |
| Dibutylphthalate (DBP) | 84-74-2 | 0.020 |
| Butylbenzylphthalate (BBP) | 85-68-7 | 0.020 |
| Di-(2-ethylhexyl)-phthalate (DEHP) | 117-81-7 | 0.020 |
| Di-n-octylphthalate (DNOP) | 117-84-0 | 0.020 |
| Di-iso-nonylphthalate (DINP) | 28553-12-0 | 0.050 |
| Di-isodecylphthalate (DIDP) | 26761-40-0 | 0.050 |
2.2.8 Analysis for content of formaldehyde
The content of formaldehyde was determined according to standard method ISO 14184-1:1998 (spectrometry). The detection limit of the used method is 20 µg/g.
%RSD = 3.
2.2.9 Screening analyse by GC-MS
A weighed amount of sample, 0.5 to 3 g, was extracted in 25 ml dichloromethane added internal standards of ethylbenzene-d10, chlorobenzene-d5, pyrene-d10 and DEHP-d4 by mechanical shaking for 2 hours. The extract was analysed by gaschromatography-mass spectrometry (GC-MS in scan mode).
The components were identified by comparison of the relevant mass spectres with mass spectres in the NIST library.
Semi-quantitative determination was performed against the internal standards and selected analytes.
2.2.10 Analysis for the content of Br, P, Cl, Sn, b, F by EDXRF
Analysis by x-ray technique (energy-dispersive X-ray fluorescence (EDXRF)
Philips PW2400/UNIQuant ver. 4.51). The detection limit for the used analytical method is 0.001 %w/w.
2.2.11 Migration test in artificial sweat and artificial saliva, respectively
Selected samples were examined for migration of organic compounds to simulated sweat and saliva.
A representative area of material was removed and extracted in artificial sweat and artificial saliva (saliva solution), respectively, in an end-over-end shaker in an incubator at 37ºC for 4 hours. The extract was decanted to a separatory funnel, added internal standards of toluene-d8, ethylbenzene-d10, naphthalene-d8 and DEHP-d4 and then extracted by dichloromethane. The resulting extracts were analysed by gaschromatography-mass spectrometry (GC-MS in scan mode).
Quantitative determination was performed against internal standards and selected analytes.
The procentual relative standard deviation based on duplicate analyses was estimated to 10-15% for the reported analytes in the analyzed material.
Equipment used for the above mentioned GC-MS analyses.
A HP gaschromatograph 5890 with a HP mass spectrometer 5972 was used.
Analysis parameters
| GC/MS-instrument | HP 5890 gaschromatograph with HP 5972 mass spectrometer |
| MS-parameter | Scan mode 29-500 m/z, solvent delay: 0.1 min. |
| GC-parameter | Column: CP Sil 8CB Low bleed MS 30 m x 0.25 mm, film: 0.5 µm Oven prog.: Depends of solvent and analysis Carrier: Helium |
2.2.12 Analysis of volatile organic components (VOC) by headspace analysis
A weighed amount/area of sample was transferred to a 5 L glass container (emission chamber). Air samples were extracted by a membrane air pump and collected on a solid adsorbent (Tenax TA). The Tenax filters were exposed during 3 succeeding time periods of 60 minutes.
The Tenax filters were then analysed by thermic desorption combined with gas chromatography-mass spectrometry (ATD/GC-MS in scan mode).
Components were identified by comparison of the respective mass spectres with the mass spectres from the NIST library.
The amount of detected components was determined against external standards of toluene.
Equipment
A Perkin-Elmer TurboMass Mass spectrometer with Perkin-Elmer ATD 400 was used for the analysis.
Analysis parameters
| GC/MS-instrument | Perkin Elmer Turbomass |
| MS-parameter | Scan mode 29-500 m/z, solvent delay: 0,1 min |
| GC-parameter | Oven prog.: 35ºC for 3 min., 10ºC/min. to 260ºC hold in 5 min. Carrier gas: Helium Column: CP Sil 8CB Low bleed MS 30 m x 0.25 mm, film: 0.5 µm |
| ATD-parameter | ATD-column: Tenax TA Desorption temp.: 290ºC |
2.2.13 Analysis of isocyantes with HPLC
A sample of approximately 3 gram was extracted with 130 ml dichloromethane. The concentration of the extract was increased and the content of isocyanates was derivatized by piperazin.
The following quantitative analysis was made by liquid chromatography with HPLC combined with fluorescence detection.
2.3 Analysis results divided according to sample types
The analysis results for the performed determinations are divided into the individual product types. For the specific analyses an empty box means that the analysis in question was not performed for the concerned sample. For the screenings of volatile organic components, an empty box means that the substance in question was not detected in the concerned sample.
2.3.1 Foam wash cloths
The analysis results for the measurements performed on foam wash cloths are summarised in Table 2.10 and Table 2.11.
Table 2.10 Specific analyses – foam wash cloths
| Component | Sample 1 µg/g |
Sample 2 µg/g |
Sample 3 µg/g |
Det.limit µg/g |
| Bromine (Br) | < 10 | < 10 | < 10 | 10 |
| Phosphorous (P) | < 10 | < 10 | < 10 | 10 |
| Chlorine (Cl) | 26 | < 10 | < 10 | 10 |
| Tin (Sn) | 299 | 17 | 437 | 10 |
| Antimony (Sb) | < 10 | < 10 | < 10 | 10 |
| Selected organic tin compounds* | < 0.02 | < 0.02 | < 0.02 | 0.02 |
| Selected organic phosphorous compounds* | < 1 | < 1 | < 1 | 1 |
| Tri-(2-chloroethyl)-phosphate (TCEP)* | < 1 | < 1 | < 1 | 1 |
*The analysis uncertainty is between 5-10% for these compounds
Table 2.11 Screening for volatile organic compounds – foam wash cloths
| Component* | CAS no. | Sample 1 µg/g |
Sample 2 µg/g |
Sample 3 µg/g |
Det.limit µg/g |
| 1-Methoxy-4-(2-phenylethenyl)-benzene | 1142-15-0 | 22 | i.p. | i.p. | 5-20 |
| 2,4-Diisocyanato-1-methylbenzene | 584-84-9 | + | + | + | |
| 2-Ethylhexanoic acid | 149-57-5 | 241 | 55 | 424 | |
| 2-Methyl-1,3-benzenediamine | 823-40-5 | 43 | i.p. | 10 | |
| 5-Methylbenzimidazolone | 5400-75-9 | i.p. | 142 | 47 | |
| Anethol | 104-46-1 | i.p. | i.p. | 38 | |
| N-Methylmorpholine | 109-02-4 | 19 | i.p. | i.p. | |
| p,p-Dioctyldiphenylamine | 101-67-7 | 61 | 48 | i.p. |
”+” Indicates that a content of the component has been found in connection with the used analysis method.
i.p. = Not detected
*The analysis uncertainty is between 10-25% for these compounds
Table 2.12 Specific analysis for selected isocyanates – foam wash cloths
| Component | CAS no. | Sample 1 µg/g |
Sample 2 µg/g |
Sample 3 µg/g |
Det.limit µg/g |
| 4,4-Methylenediphyl diisocyanate (MDI) | 101-68-8 | <5 | <5 | <5 | 5 |
| Toluen 2,4-diisocyanate (TDI) |
584-84-9 | <5 | <5 | <5 | 5 |
| Toluen 2,6-diisocyanate (TDI) |
91-08-7 | <5 | <5 | <5 | 5 |
2.3.2 Pillows for feeding the baby
The analysis results for measurements performed on pillows for feeding the baby is summarised in Table 2.13 and Table 2.14.
Table 2.13 Specific analyses – Pillows for feeding the baby
| Component | CAS no. | Sample 4 µg/g |
Sample 5 µg/g |
Det.limit µg/g |
| Cover – Sample 4A, 5A | ||||
| Formaldehyde | 50-00-0 | 26 | 65 | 20 |
| 2-Phenylphenol (OPP)* | 90-43-7 | 0.02 | 0.03 | 0.01 |
| Tetrachlorophenol (TeCP)* | 935-95-5 | < 0.02 | < 0.02 | 0.02 |
| Pentachlorophenol (PCP)* | 87-86-5 | < 0.02 | < 0.02 | 0.02 |
| Polystyrene pellets – Sample 4C, 5C | ||||
| Bromine (Br) | 4,480 | 3,670 | 10 | |
| Phosphorous (P) | 102 | < 10 | 10 | |
| Chlorine (Cl) | < 10 | < 10 | 10 | |
| Tin (Sn) | < 10 | 57 | 10 | |
| Antimony (Sb) | < 10 | < 10 | 10 | |
| Selected organic tin compounds* | Not analysed | < 0.02 | 0.02 | |
| Selected organic phosphorous compounds* | < 1 | < 1 | 1 | |
| Tri(2-chlorethyl)phosphate (TCEP)* | 115-96-8 | < 1 | < 1 | 1 |
*The analysis uncertainty is between 5-10% for these compounds
Table 2.14 Screening for volatile organic compounds – pillows for feeding baby
| Component | CAS no. | Sample 4 µg/g |
Sample 5 µg/g |
Det.limit µg/g |
| Cover –Sample 4A, 5A | ||||
| Styrene | 100-42-5 | i.p. | 9 | 5-20 |
| Diethylene glycol | 111-46-6 | i.p. | 127 | |
| Dipropylene glycol | 110-98-5 | i.p. | 19 | |
| Triethylene glycol | 112-27-6 | i.p. | 12 | |
| N,N-Dimethyl-1-dodecanamine | 112-18-5 | i.p. | 9 | |
| n-Hexadecanoic acid | 57-10-3 | i.p. | 35 | |
| t-Sitosterol | 83-47-6 | i.p. | 51 | |
| Polystyrene pellets – Sample 4C, 5C | ||||
| Styrene | 100-42-5 | 315 | 679 | 5-20 |
| Tetrachloroethane | 79-34-5 | 278 | 493 | |
| 2-Ethyl-1-hexanol | 104-76-7 | 196 | ||
| Acetophenone | 98-86-2 | 1,010 | 1,572 | |
| Dimethyl benzene methanol | 617-94-7 | 125 | i.p. | |
| Benzoic acid phenylester | 93-99-2 | 45 | i.p. | |
| p-Propionotoluidide | 2759-55-9 | 48 | i.p. | |
| Hexabromocyclododecane | 3194-55-6 | 457 | 433 | |
| 1,2-Diphenylethanone | 451-40-1 | 220 | i.p. | |
| Cyclooctacosane | 297-24-5 | 245 | i.p. | |
| Cumyl alcohol | 617-94-7 | i.p. | 321 | |
| 1,3-Diphenyl propane | 1081-75-0 | i.p. | 78 | |
| 2-Phenylbutyrophenone | 16282-16-9 | i.p. | 191 |
i.p. = Not detected
*The analysis uncertainty is between 10-25% for these compounds
2.3.3 Baby mattresses
The analysis results for measurements performed on mattresses is summarised in Table 2.15 and Table 2.16.
Table 2.15 Specific analyses – baby mattresses
| Component | CAS no. | Sample 6 µg/g |
Sample 13 µg/g |
Det.limit µg/g |
|
| Cover – Sample 6A, 13A | |||||
| Formaldehyde | 50-00-0 | < 20 | i.a. | 20 | |
| Foam – Sample 6B, 13B+C | 13B | 13C | |||
| Bromine (Br) | < 10 | < 10 | < 10 | 10 | |
| Phosphorus (P) | < 10 | 63 | < 10 | ||
| Chlorine (Cl) | 62 | 388 | 232 | ||
| Tin (Sn) | 421 | 402 | 15 | ||
| Antimony (Sb) | < 10 | < 10 | < 10 | ||
| Selected organic tin components* | < 0.02 | < 0.02 | < 0.02 | 0.02 | |
| Tri(2-chlorethyl)phosphate (TCEP) | 115-96-8 | < 1 | < 1 | < 1 | 1 |
| Other selected organic phosphorous compounds* | < 1 | < 1 | < 1 | 1 | |
i.a. = Not analysed
*The analysis uncertainty is between 5-10% for these compounds
Table 2.16 Screening for volatile organic components – baby mattresses
| Component * | CAS no. | Sample 6 µg/g |
Sample 13 µg/g |
Det.limit µg/g |
|
| Cover – Sample 6A, 13A | 6A | 13A | |||
| Styrene | 100-42-5 | 20 | i.p. | 5-20 | |
| 2-Ethyl-1-hexanol | 104-76-7 | 18 | i.p. | ||
| Nonanal | 124-19-6 | 25 | i.p. | ||
| Nonanoic acid | 112-05-0 | 13 | i.p. | ||
| Hexadecanoic acid | 57-10-3 | i.p. | 41 | ||
| Heptadecanoic acid | 506-12-7 | i.p. | 8 | ||
| Octadecanoic acid | 57-11-4 | i.p. | 39 | ||
| Foam – Sample 6B, 13B+C | 6B | 13B | 13C | ||
| 2-Ethyl hexanoic acid | 149-57-5 | 30 | 66 | i.p. | 5-20 |
| 2,4-Diisocyanato-1-methylbenzene | 584-84-9 | + | + | i.p. | |
| 5-Methylbenzimidazolone | 5400-75-9 | 32 | i.p. | i.p. | |
| m-Styryl (E)-anisol | 14064-41-6 | 33 | 92 | i.p. | |
| 3-benzyloxy-1,2-diacetyl-1,2-propanediol | 13754-10-4 | 61 | i.p. | ||
| 1-Methyl-1H-benzimidazol-2-ol | 1849-01-0 | i.p. | 66 | i.p. | |
| p,p-Dioctyldiphenylamine | 101-67-7 | i.p. | 77 | i.p. | |
| Dipropylene glycol | 110-98-5 | i.p. | i.p. | 56 | |
| Triethylene glycol monomethyl ether | 112-35-6 | i.p. | i.p. | 42 | |
| Tripropylene glycol | 1638-16-0 | i.p. | i.p. | 117 | |
| Diphenylamine | 122-39-4 | i.p. | i.p. | 10 | |
| Dimethoxytetraethylene glycol | 143-24-8 | i.p. | i.p. | 135 | |
| Diphenylmethan diisocyanate | 101-68-8 | i.p. | i.p. | 22 | |
| Hexaethylen glycol dimethyl ether | 1072-40-8 | i.p. | i.p. | 2582** | |
| Tetrapropylene glycol, monomethyl ether | 20324-34-9 | i.p. | i.p. | 715*** | |
*The analysis uncertainty is between 10-25% for these compounds
**2582: Sum of 3 isomer peaks of 484+976+1122 µg/g, respectively
*** 715: Sum of 2 isomer peaks of 622+93 µg/g, respectively
”+” Indicates that a content of the component has been found in connection with the used analysis method.
i.p. = Not detected
2.3.4 Nursing pillows
The analysis results of the measurements performed on mattresses are summarised in Table 2.17 and Table 2.18.
Table 2.17 Specific analyses – nursing pillows
| Component | CAS no. | Sample 7 µg/g |
Sample 8 µg/g |
Det.limit µg/g |
|
| Cover – Sample 7A, 8A+C | 8A | 8C | |||
| Formaldehyde | 50-00-0 | 100 | i.a. | i.a. | 20 |
| 2-Phenylphenol (OPP)* | 90-43-7 | < 0.01 | i.a. | i.a. | 0.01 |
| Tetrachlorophenol (TeCP)* | 935-95-5 | < 0.02 | i.a. | i.a. | 0.02 |
| Pentachlorophenol (PCP)* | 87-86-5 | < 0.02 | i.a. | i.a. | 0.02 |
| Phthalates - DINP* | i.a. | 144.000 | <50 | 50 | |
| Phthalates - DIBP + DBP* | i.a. | <20 | 70 | 20 | |
| Phthalates - DINP + DIDeP* | i.a. | <50 | 220.000 | 50 | |
| Triphenylphosphate* | 115-86-6 | 5 | 30 | i.a. | 1 |
| Selected organic phosphorous compounds | < 1 | < 1 | i.a. | 1 | |
| Foam – Sample 7B, 8B | 7B | 8B | |||
| Bromine (Br) | < 10 | < 10 | 10 | ||
| Phosphorous (P) | < 10 | < 10 | |||
| Chlorine (Cl) | 28 | 160 | |||
| Tin (Sn) | 341 | 252 | |||
| Antimony (Sb) | <10 | < 10 | |||
| Selected organic tin compounds* | < 0.02 | < 0.02 | 0.02 | ||
| Tri(2-chlorethyl)phosphate (TCEP)* | 115-96-8 | < 1 | < 1 | 1 | |
| Other selected organic phosphorous compounds* | < 1 | < 1 | 1 | ||
| Phthalates - DINP* | 3800 | 50 | |||
*The analysis uncertainty is between 5-10% for these compounds
i.a. = not analysed
Table 2.18 Screening for volatile organic compounds – nursing pillows
| Component * | CAS no. | Sample 7 µg/g |
Sample 8 µg/g |
Det.limit µg/g |
| Cover – Sample 7A, 8A | ||||
| Styrene | 100-42-5 | 14 | i.p. | 2-10 |
| 2,2'-oxybis|N,N-dimethyl]-ethanamine | 3033-62-3 | 31 | i.p. | |
| Nonanal | 124-19-6 | 12 | i.p. | |
| 3-methylglytaconic acid, diethylester | 8 | i.p. | ||
| Dodecanol | 112-53-8 | 25 | i.p. | |
| N,N-Dimethyl-1-dodecanamine | 112-18-5 | 13 | i.p. | |
| Tridecanol | 112-70-9 | 14 | i.p. | |
| N,N-Dimethyl-1-tetradecanamine | 112-75-4 | 15 | i.p. | |
| Ethylene glycol monododecyl ether | 4536-30-5 | 23 | i.p. | |
| Diethylene glycol monododecyl ether | 3055-93-4 | 33 | i.p. | |
| Heptadecanol | 1454-85-9 | 16 | i.p. | |
| Triethylene glycol monododecyl ether | 3055-94-5 | 41 | i.p. | |
| Tetraethylene glycol monododecyl ether | 5274-68-0 | 87 | i.p. | |
| Pentaethylene glycol monododecyl ether | 3055-95-6 | 34 | i.p. | |
| Eicosanol | 629-96-9 | 74 | i.p. | |
| 2-Ethylhexanoic acid | 149-57-5 | i.p. | 21 | |
| p-tert-butylbenzoic acid | 98-73-7 | i.p. | 41 | |
| Octadecanoic acid | 112-80-1 | i.p. | 85 | |
| Triphenylester phosphoric acid | 101-02-0 | i.p. | 63 | |
| Foam – Sample 7B, 8B | ||||
| Styrene | 100-42-5 | 79 | 28 | 5-20 |
| 2-Ethylhexanoic acid | 149-57-5 | 81 | 495 | |
| 2,4-Diisocyanato-1-methylbenzene | 584-84-9 | + | + | |
| 5-Methylbenzimidazolone | 5400-75-9 | 20 | 65 | |
| N-methyl-4-(methylthio)-2-(2,2-dimethylpropylidene)amino-butanamide | 97443-86-2 | 28 | ||
| Phenol | 108-95-2 | i.p. | 30 | |
| Butylated hydroxytoluene (BHT) | 128-37-0 | i.p. | 21 | |
| 4-Nonylphenol | 104-40-5 | i.p. | 51 | |
| Triphenylphosphate | 115-86-6 | i.p. | 49 |
*The analysis uncertainty is between 10-25% for these compounds
”+” Indicates that a content of the component has been found in connection with the used analysis method.
i.p. = Not detected
2.3.5 Baby carriers
Table 2.19 Specific analyses – baby carriers
| Component | Sample 9 µg/g |
Sample 10 µg/g |
Det.limit µg/g |
| Textile – Sample 9A+B+C, 10A+B+C | |||
| Azo-colourants – Sample A, B, C* | < 5 | < 5 | 5 |
| Disperse colourants * | Orange 37 (sample 9A) | i.a. | 50 |
| Chlorinated benzenes and toluenes** | < 0.05 | i.a. | 0.05 |
| Foam – Sample 9D, 10H | |||
| Bromine (Br) | < 10 | < 10 | 10 |
| Phosphorous (P) | < 10 | < 10 | |
| Chlorine (Cl) | 110 | 49 | |
| Tin (Sn) | 468 | 381 | |
| Antimony (Sb) | < 10 | < 10 | |
| Selected organic tin compounds | < 0.02 | < 0.02 | 002 |
| Plastic badge/-print – Sample 10D+E+F | |||
| Phthalates – DEP** | i.a. | 350 (D) 60 (F) |
20 |
| Phthalates – DIBP** | i.a. | 120 (D) 760 (E) 25 (F) |
20 |
*The analysis uncertainty is between 10-25% for these compounds
**The analysis uncertainty is 5-10 for these compounds
i.a. = Not analysed
2.3.6 Apron for perambulators
Table 2.20 Specific analyses – apron for perambulators
| Component | Sample 11 µg/g |
Sample 12 µg/g |
Det.lim µg/g |
||
| Cover – Sample 11A+B, 12A+B | 11A | 11B | 12A | 12B | |
| Azo-colourants* | < 5 | i.a. | < 5 | i.a. | 5 |
| Disperse colourants* | i.a. | i.a. | i.a. | i.a. | 50 |
| Chlorinated benzenes and toluenes** | < 0.05 | < 0.05 | 0.05 | ||
| 2-Phenylphenol (OPP) | 0.01 | i.a. | 0.01 | < 0.01 | 0.01 |
| Tetrachlorophenol (TeCP) | < 0.02 | i.a. | < 0.02 | < 0.02 | 0.02 |
| Pentachlorophenol (PCP) | < 0.02 | i.a. | < 0.02 | < 0.02 | 0.02 |
| Phthalates – DEHP | 40 | i.a. | < 20 | ||
| Formaldehyde | < 20 | i.p. | < 20 | 20 | |
| Bromine (Br) (inner side / outer side)*** | 1220 / 1220 | i.a. | 1310 / 1320 | i.a. | 10 |
| Phosphorous (P) (inner side / outer side)*** | < 10 / 15 | i.a. | 64 / < 10 | i.a. | |
| Chlorine (Cl) (inner side / outer side)*** | 1600 / 721 | i.a. | 756 / 513 | i.a. | |
| Tin (Sn) (inner side / outer side)*** | < 10 / < 10 | i.a. | < 10 / 41 | i.a. | |
| Antimony (Sb) (inner side / outer side)*** | 88 / 122 | i.a. | 130 / 114 | i.a. | |
| Selected organic tin compounds** | < 0.02 | i.a. | < 0.02 | < 0.02 | 0.02 |
| Tri(2-chlorethyl)phosphate (TCEP)** | < 1 | i.a. | < 1 | < 1 | 1 |
| Tributylphosphate** | 2 | i.a. | < 1 | < 1 | 1 |
| Triphenylphosphate** | 0.4 | i.a. | < 1 | < 1 | 0.2-1 |
| Other selected organic phosphorous compounds** | < 1 | i.a. | < 1 | < 1 | 1 |
| Foam – Sample 11D+E, 12D+E | 11 D | 11 E | 12D | 12E | |
| Bromine (Br) | < 10 | < 10 | < 10 | i.p. | 10 |
| Phosphorous (P) | < 10 | < 10 | < 10 | i.p. | |
| Chlorine (Cl) | 106 | 22 | 72 | i.p. | |
| Tin (Sn) | 258 | 337 | 658 | i.p. | |
| Antimony (Sb) | < 10 | 28 | < 10 | i.p. | |
| Selected organic tin compounds** | < 0.02 | < 0.02 | < 0.02 | i.p. | 0.02 |
| Tri(2-chloroetyl)phosphate (TCEP)** | < 1 | < 1 | < 1 | < 1 | 1 |
| Triphenylphosphate | 5 | < 1 | 1 | ||
| Other selected organic phosphorous compounds** | < 1 | < 1 | < 1 | < 1 | 1 |
| Plastic parts - Sample 12C | 12C | ||||
| Phthalates | i.p | i.p | < 20 | 20 | |
*The analysis uncertainty is between 10-25% for these compounds
**The analysis uncertainty is 5-10 for these compounds
*** Analyses have been performed on both inner and outer side. Both results are stated.
i.p. = Not detected
i.a. = Not analysed
Table 2.21 Screening for volatile organic components – apron to perambulators
| Component * | CAS no. | Sample 11 µg/g |
Sample12 µg/g |
Det.lim µg/g |
||
| Cover – Sample 11A, 12A+B | 11A | 12A | 12B | 2-10 | ||
| 1H,1H,2H,2H-Perfluorooctan-1-ol | 647-42-7 | 6 | i.p. | i.p. | ||
| Benzaldehyde | 100-52-7 | 4 | i.p. | i.p. | ||
| 2-Ethyl-1-hexanol | 104-76-7 | 5 | i.p. | i.p. | ||
| Nonanal | 124-19-6 | 8 | i.p. | i.p. | ||
| 2-Dibutylaminoethylamine | 3529-09-7 | 6 | i.p. | i.p. | ||
| Cyclododecane | 294-62-2 | 16 | i.p. | i.p. | ||
| Tributylphosphate | 126-73-8 | 8 | i.p. | i.p. | ||
| 2-Chloro-4,6-dinitro-benzenamine | 3531-19-9 | i.p. | 9 | i.p. | ||
| 2-Bromo-4,6-dinitro-benzenamine | 1817-73-8 | 31 | 108 | i.p. | ||
| 2-(2-Bromo-1-methylethyl)-isoindole-1,3-dione | 106363-58-0 | i.p. | 112 | i.p. | ||
| 1-Bromo-3,5-dinitrobenzene | 18242-39-2 | i.p. | 7 | i.p. | ||
| N-Acetonyl-phthalimide | 3416-57-7 | i.p. | 136 | i.p. | ||
| 2,6-Dichloro-4-nitroaniline | 99-30-9 | i.p. | 8 | i.p. | ||
| Hexadecanoic acid | 57-10-3 | i.p. | i.p. | 16 | ||
| Foam – Sample 11D+E, 12D+E | 11D | 11E | 12D | 12E | ||
| Dioctyldiphenylamine | 101-67-7 | 30 | i.p. | i.p. | i.p. | |
| 2,4-Diisocyanato-1-methylbenzene | 584-84-9 | i.p. | + | i.p. | i.p. | |
| N,N-Dimethyl-N'-phenyl-methanimidamide | 1783-25-1 | i.p. | 36 | i.p. | i.p. | |
| 5-Methylbenzimidazolone | 5400-75-9 | i.p. | i.p. | 52 | i.p. | |
*The analysis uncertainty is between 10-25% for these compounds
”+” Indicates that a content of the component has been found in connection with the used analysis method.
i.p. = Not detected
2.4 Analysis results for specific analyses according to analysis type
2.4.1 Analysis results for arylamines
The samples marked 9A, iB, 9C, 10A, 10B, 10C, and 12A: Content of selected arylamines above the detection limit of 5 µm/g was not detected.
2.4.2 Analysis results for disperse colourants
Table 2.22 Results for disperse colourants
| Sample mrk. | Detected colourants* |
| 9A | Disperse orange 37 |
| 9B | None detected |
| 9C | None detected |
| 10C | None detected |
| 11A | None detected |
| 12A | None detected |
*The detection limit for disperse colourants is 50 µg/g and the analysis uncertainty is 10-20%
2.4.3 Analysis results for P-containing flame retardants [µg/g]
Table 2.23 Results for P-containing flame retardants
| Selected component | CAS no. | Sample 7A | Sample 8A | Sample 8B | Sample 11A | Sample 12D |
| Tributylphosphate (TBP) | 126-73-8 | < 1 | < 1 | < 1 | 2 | < 1 |
| Triphenylphosphate (TPP) | 115-86-6 | 5 | 30 | 40 | < 1 | 5 |
| Tri(2-chlorethyl)phosphate (TCEP) | 115-96-8 | < 1 | < 1 | < 1 | < 1 | < 1 |
| Tri(2-ethylhexyl)phosphate (TEHP) | 78-42-2 | < 1 | < 1 | < 1 | < 1 | < 1 |
| Tri(2-butoxyethyl)phosphate (TBEP) | 78-51-3 | < 1 | < 1 | < 1 | < 1 | < 1 |
| For samples mrk. 1, 2, 3, 4A, 4C, 5A, 5C, 6A, 6B, 7B, 11D, 11E, 12A, 12B, 12E, 13A, 13B, 13C, 13D the content of the listed components are below the detection limit (1 µg/g) | ||||||
*The detection limit for r P-containing flame retardants is 1 µg/g and the analysis uncertainty is 5-10%
2.4.4 EDXRF analysis – results presented as % (w/w)
Table 2.24 Results EDXRF analysis
| Sample mrk. | P | Cl | Br | Sn | Sb | F |
| 11E | < 0.001 | 0.0022 | < 0.001 | 0.0337 | 0.0028 | i.a. |
| 13/Green glue | 0.0017 | 4.13 | 0.0019 | 0.0144 | < 0.001 | i.a. |
| 1 | < 0.001 | 0.0026 | < 0.001 | 0.0299 | < 0.001 | i.a. |
| 2 | < 0.001 | < 0.001 | < 0.001 | 0.017 | < 0.001 | i.a. |
| 3 | < 0.001 | < 0.001 | < 0.001 | 0.0437 | < 0.001 | i.a. |
| 4C | 0.0102 | < 0.001 | 0.448 | < 0.001 | < 0.001 | i.a. |
| 5C | < 0.001 | < 0.001 | 0.367 | 0.0057 | < 0001 | i.a. |
| 6B | < 0.001 | 0.0062 | < 0.001 | 0.0421 | < 0.001 | i.a. |
| 7B | < 0.001 | 0.0028 | < 0.001 | 0.0341 | < 0.001 | i.a. |
| 8B | < 0.001 | 0.016 | < 0.001 | 0.0252 | < 0.001 | i.a. |
| 9D | < 0.001 | 0.011 | < 0.001 | 0.0468 | < 0.001 | i.a. |
| 10H | < 0.001 | 0.0049 | < 0.001 | 0.0331 | < 0.001 | i.a. |
| 11A / outer side | < 0.001 | 0.16 | 0.122 | < 0.001 | 0.0088 | 1.2 |
| 11A / inner side | 0.0015 | 0.0721 | 0.122 | < 0.001 | 0.0122 | 0.2 |
| 11D | < 0.001 | 0.0106 | < 0.001 | 0.0258 | < 0.001 | i.a. |
| 12A / outer side | 0.00064 | 0.0756 | 0.131 | < 0.001 | 0.013 | 0.6 |
| 12A / inner side | < 0.001 | 0.0513 | 0.132 | 0.0041 | 0.0114 | 0.3 |
| 12D | < 0.001 | 0.0072 | < 0.001 | 0.0658 | < 0.001 | i.a. |
| 13C / white | < 0.001 | 0.0232 | < 0.001 | 0.0015 | < 0.001 | i.a. |
| 13B / yellow | 0.0063 | 0.0388 | < 0.001 | 0.0402 | < 0.001 | i.a. |
’*’ means that the sample has not been analysed for content of fluor (F).
i.a. = Not analysed
The detection limit is 0.001 % (w/w)
2.4.5 Analysis results for organic tin compounds
Samples marked 1, 2, 3, 5C, 6B, 7B, 8B, 9D, 10H, 11D, 11E, 12A, 12D and 13B: There was not detected any content of selected organic tin compound above the detection limit of 0.02 µg/g.
2.4.6 Chlorinated benzenes and toluenes
Samples marked 9A, 9B, 9C, 11A and 12A: There was not detected any content of chlorinated benzenes and toluenes above the detection limit of 0.05 µg/g.
2.4.7 Analysis results for chlorinated phenols and OPP
Table 2.25 Results for chlorinated phenols and OPP
| o-Phenylphenol (OPP) | 2,3,5,6-Tetrachlorophenol (TeCP) | Pentachlorophenol (PCP) | |
| CAS no. | 90-43-7 | 935-95-5 | 87-86-5 |
| Sample mrk. | µg/g | µg/g | µg/g |
| 4A | 0.02 | < 0.02 | < 0.02 |
| 5A | 0.03 | < 0.02 | < 0.02 |
| 7A | < 0.01 | < 0.02 | < 0.02 |
| 11A | 0.01 | < 0.02 | < 0.02 |
| 12A | 0.01 | < 0.02 | < 0.02 |
| 12B | < 0.01 | < 0.02 | < 0.02 |
| Detection limit | 0.01 | 0.02 | 0.02 |
The analysis uncertainty is 5-10% for all compounds
2.4.8 Analysis results for phthalates, mg/g
Table 2.26 Results for phthalates
| Sample mrk. | DEP | DIBP | DEHP | DINP | Other phthalates |
| 7A | < 0.02 | < 0.02 | < 0.02 | < 0.05 | < 0.05 |
| 8A | < 0.02 | < 0.02 | < 0.02 | 143 / 145 mg/g | < 0.05 |
| 8B | < 0.02 | < 0.02 | < 0.02 | 3.8 mg/g | < 0.05 |
| 8C | < 0.02 | 0.07 mg/g incl. DBP |
< 0.02 | 210 / 230 mg/g incl. DIDeP |
< 0.05 |
| 10D | 0.34 / 0.36 mg/g | 0.11 / 0.12 mg/g | < 0.02 | < 0.05 | < 0.05 |
| 10E | < 0.02 | 0.76 / 0.04 mg/g | < 0.02 | < 0.05 | < 0.05 |
| 10F | 0.06 / 0.06 mg/g | 0.03 / 0.02 mg/g | < 0.02 | < 0.05 | < 0.05 |
| 11A | < 0.02 | < 0.02 | 0.04 / 0.04 mg/g | < 0.05 | < 0.05 |
| 12A | < 0.02 | < 0.02 | < 0.02 | < 0.05 | < 0.05 |
| 12C | < 0.02 | < 0.02 | < 0.02 | < 0.05 | < 0.05 |
| 13C | < 0.02 | < 0.02 | < 0.02 | < 0.05 | Diundecylphthalat CAS nr. 3648-20-2 4.4 mg/g |
Values separated by oblique stroke indicate results of single determinations.
The analysis uncertain is 5-10% for phthalates
2.4.9 Formaldehyde
Table 2.27 results for formaldehyde
| Sample mrk. | µg/g |
| 4A | 26 |
| 5A | 65 |
| 6A | <20 |
| 7A | 100 |
| 10B | <20 |
| 11B | <20 |
| 12B | <20 |
| 13B | <20 |
The detection limit for formaldehyde is 20 µg/g and the uncertainty is 5-10%
2.4.10 Migration test in sweat of selected products
Samples 4A, 5A, 7A, 8A, 13B, and 13C have been selected for migration test in sweat, as these products are normally in direct skin contact. Further, 4C and 5C (PS-pellets) were selected, see table 2.33.
The results of performed migration tests in sweat for selected samples are summarised in Table 2.28, Table 2.29, Table 2.30, and Table 2.31.
The analysis uncertainty of the migration tests is 5-15%.
Table 2.28 Results of performed migration tests in sweat for selected subsamples
| Sample mrk. | Weight of subsample g |
Area of subsample cm² |
Styrene µg total |
1,1,2,2-Tetrachloro-ethane µg total |
Aceto-phenone µg total |
| 4A – sweat | 3.49 | 200 | < 0.5 | < 0.5 | 1.0 |
| 4A – sweat | 3.71 | 200 | < 0.5 | < 0.5 | 1.0 |
| 5A – sweat | 3.65 | 210 | < 0.5 | < 0.5 | 2.0 |
| 5A – sweat | 3.53 | 247 | < 0.5 | < 0.5 | 1.9 |
| 7A – sweat | 4.38 | 196 | < 0.5 | < 0.5 | 0.6 |
| 7A – sweat | 4.38 | 196 | < 0.5 | < 0.5 | 0.4 |
| CAS no. | 100-42-5 | 79-34-5 | 98-86-2 | ||
| Detection limit | 0.5 | 0.5 | 0.2 |
Table 2.29 Results of migration test performed in sweat for selected subsamples
| Sample mrk. | Weight of subsample g | Area of subsample cm² |
Formaldehyde ¹ µg total |
| 4A – sweat | 3.49 | 200 | 74 |
| 5A – sweat | 3.65 | 210 | 376 |
| 7A – sweat | 4.38 | 196 | 70 |
>¹ measured with HPLC
Table 2.30 Results of migration test performed in sweat for selected subsamples
| Sample mrk. | Weight of subsample g |
Area of subsample cm² |
DINP µg total |
| 8A – sweat | 4.64 | 200 | 4.8 |
| 8A – sweat | 4.78 | 200 | 6.6 |
| 13C – sweat | 5.03 | 150 | < 2 |
| 13C – sweat | 4.85 | 150 | < 2 |
| CAS no. | 28553-12-0 | ||
| Detection limit | 2 |
Table 2.31 Results of migration test in saliva for selected subsamples
| Sample mrk. | Weight of subsample g |
Area of subsample cm² |
2-Ethylhexanoic acid µg total |
Toluene-2,4-diisocyanate µg total |
| 13B – saliva | 5.97 | 260 | 64 | + |
| 13B – saliva | 5.96 | 260 | 55 | + |
| CAS no. | 149-57-5 | 584-84-9 | ||
| Det. limit | 0.5 | 0.5 |
”+” Indicates that a content of the component has been found in connection with the used analysis method.
2.4.11 Migration test in saliva for selected products
Samples 11A and 12A were selected for migration test in saliva, as babies are often in direct mouth contact with these materials. Further samples 4C and 5C (PS pellets) were selected, see Table 2.33.
The results for the performed migration test in saliva for selected products are summarised in Table 2.32.
The uncertainty of the migration tests is 5-15%.
Table 2.32 Results of migration test in saliva for selected products
| Sample mrk. | Weight of subsample g |
Area of subsample cm² |
2-bromo-4,6-dinitrobenzenamine µg total |
DEHP µg total |
| 11A – saliva | 3.80 | 200 | < 0.5 | 0.49 |
| 11A – saliva | 3.45 | 200 | < 0.5 | 0.48 |
| 12A – saliva | 4.46 | 200 | < 0.5 | Not determined |
| 12A – saliva | 4.39 | 200 | < 0.5 | Not determined |
| CAS no. | 1817-73-8 | 117-81-7 | ||
| Det. limit | 0.5 | 0.2 |
2.4.12 Migration test in sweat and saliva for PS-pellets
The results of the migration test in sweat and saliva for polystyrene (PS) pellets are summarised in Table 2.33.
The analysis uncertainty is 5-15%.
Table 2.33 Results of migration test in sweat and saliva for PS-pellets
| Sample mrk. | Weight of subsample g |
Styrene µg/g |
1,1,2,2-Tetrachloroethane µg/g |
Acetophenone µg/g |
| 4C – sweat | 1.02 | < 0.5 | < 0.5 | 1.3 |
| 4C – sweat | 1.04 | < 0.5 | < 0.5 | 1.4 |
| 4C – saliva | 1.04 | < 0.5 | < 0.5 | 1.6 |
| 5C – sweat | 1.02 | < 0.5 | < 0.5 | 3.0 |
| 5C – sweat | 1.06 | < 0.5 | < 0.5 | 5.9 |
| 5C – saliva | 1.14 | < 0.5 | < 0.5 | 2.3 |
| CAS no. | 100-42-5 | 79-34-5 | 98-86-2 | |
| Det. limit | 0.5 | 0.5 | 0.2 |
Sample 4C: 1.0 g of sample corresponds to approx. 80 ml. Total weight of PS-pellets in the baby feeding pillow: 350-400 g.
Sample 5C: 1.0 g sample corresponds to approx. 80 ml. Total weight of PS-pellets in the baby feeding pillow: 350-400 g.
2.4.13 Emission test of volatile organic components – PS-pellets
The results of the performed emission test of VOC for PS-pellets are summarised in Table 2.34.
The analysis uncertainty of the tests is 5-15%.
Table 2.34 Results of performed emission test for volatile organic components in PS-pellets
| Sample mrk. | Xylenes µg/m³ air |
Styrene µg/m³ air |
1,1,2,2-Tetrachloroethane µg/m³ air |
Acetophenone µg/m³ air |
| 4C – 1. exp. | 0.37 | 0.30 | < 0.1 | < 0.1 |
| 4C – 2. exp. | 0.22 | 0.17 | < 0.1 | < 0.1 |
| 4C – 3. exp. | 0.23 | 0.20 | < 0.1 | < 0.1 |
| 5C – 1. exp. | 0.39 | 0.77 | < 0.1 | 0.20 |
| 5C – 2. exp. | 0.39 | 0.67 | < 0.1 | 0.29 |
| 5C – 3. exp. | 0.40 | 0.69 | < 0.1 | 0.41 |
| CAS no. | 100-42-5 | 79-34-5 | 98-86-2 | |
| Det. limit | 0.1 | 0.1 | 0.1 | 0.1 |
Sample 4C: 3.0 g sample corresponds to 250 ml. Total weight of PS-pellets: 350-400 g.
Sample 5C: 3.1 g sample corresponds to 250 ml. Total weight of PS-pellets: 350-400 g.
Exposure in 5 l climate chamber:
| 1st exposure period | 0-1 hour in climate chamber | 1 hour exposure. |
| 2nd exposure period | 1-2 hours in climate chamber | 1 hour exposure. |
| 3rd exposure period | 2-3 hours in climate chamber | 1 hour exposure. |
The air change was once every second hour
2.5 Selection of chemical substances for evaluation
Out of the identified substances and based on classification and occurrence was selected a number of substances for further evaluation of a possible health risk to consumers. The consumer is in this context defined as children at the age of 0 to 1 year.
In co-operation with the Danish Environmental Protection Agency 10 substances for health and environmental assessment are selected. The substances are presented in Table 2.35.
Table 2.35 Summary of selected substances
| Component | CAS no. | Concentration µg/g (ppm) From screening |
Sample mrk. |
| 2,4-Diisocyanato-1-methylbenzene, (Toluene-2,4-diisocyanate), TX;R26 XI;R36/37/38 Carc.cat.3;R40 R42/43 R52/53 |
584-84-9 | + | 1 |
| + | 2 | ||
| + | 4 | ||
| + | 6B | ||
| + | 8B | ||
| + | 11E | ||
| + | 7B | ||
| + | 13B | ||
| 2-Bromo-4,6-dinitro-benzenamine, | 1817-73-8 | 31 | 11A |
| 108 | 12A | ||
| 2-Ethylhexanoic acid, Rep.car.3;R63 |
149-57-5 | 30 | 6B |
| 241 | 1 | ||
| 55 | 2 | ||
| 424 | 3 | ||
| 66 | 13B | ||
| 81 | 7B | ||
| 21 | 8A | ||
| 495 | 8B | ||
| Acetophenone, Xn;R22 Xi;R36 |
98-86-2 | 1010 | 4C |
| 1572 | 5C | ||
| Hexabromocyclododecane (HBCD), N;R50/53 (EU RAR draft), |
3194-55-6 | 457 | 4C |
| 433 | 5C | ||
| Hexaethylene glycol dimethyl ether, | 1072-40-8 | 484 | 13C |
| 976 | 13C | ||
| 1122 | 13C | ||
| Styrene, R10 Xn;20- Xi;R36/38 , Metabolite Styreneoxide: Carc.cat.2;R45 |
100-42-5 | 315 | 4C |
| 9 | 5A | ||
| 679 | 5C | ||
| 20 | 6A | ||
| 14 | 7A | ||
| 79 | 7B | ||
| 28 | 8B | ||
| 1,1,2,2-Tetrachloroethane, TX;R26/27 N;R51/53 |
79-34-5 | 278 | 4C |
| 493 | 5C | ||
| Tetrapropylene glycol, monomethyl ether, | 20324-34-9 | 622 | 13C |
| 93 | 13C | ||
| Formaldehyde, T;R23/24/25 C;R34 CARC3;R40 R43 |
50-00-0 | 26 | 4A |
| 65 | 5A | ||
| 100 | 7A |
”+” Indicates that a content of the component has been found in connection with the used analysis method.
3 Health Assessment
- 3.1 Introduction
- 3.2 Method
- 3.3 Selected substances
- 3.3.1 Hexabromocyclododecane (HBCD)
- 3.3.2 Toluene 2,4-diisocyanate (TDI)
- 3.3.3 2-Ethylhexanoic acid (2-EHA)
- 3.3.4 Acetophenone
- 3.3.5 1,1,2,2-Tetrachloroethane
- 3.3.6 Formaldehyde
- 3.3.7 Styrene
- 3.3.8 2-bromo-4,6-dinitroaniline (BDNA)
- 3.3.9 Hexaethylene glycol dimethyl ether
- 3.3.10 Tetrapropylene glycol monomethyl ether
- 3.3.11 DEHP
- 3.3.12 DINP
- 3.3.13 Xylene
- 3.4 Overall Assessment
3.1 Introduction
In this section, potential health effects from identified and selected substances are assessed. The focus of the assessment is primarily aimed towards children aged 0 to 1 year.
For each of the identified and quantified substances, information of the substances identity as well as chemical and physical properties is presented. This will include data on material state, melting point, boiling point, vapour pressure and solubility.
A search in the open literature and in recently published research papers has been performed. Focus has been on the ability of absorption of the substance through skin, lung and the gastrointestinal tract. The most important test results, the effects and circumstances are presented. The aim was to find data for NOAEL/LOAEL (No or low observed adverse effect levels) for the selected substances or other relevant data if available.
Based on NOAEL or similar data and the amount of the substances the margin of safety (MOS) can be calculated, and it can be assessed whether the substance may cause a negative health effect for the tested products. However, it is very important to note that for most compounds it is impossible to calculate a true margin of safety because essential information on toxicokinetics, biotransformation, and molecular interaction with biomolecules are lacking. Therefore, much care must be taken when MOS values are used for decision making on xenobiotic chemicals, especially when it is a question of protecting children and young adults.
Further it is assessed from the gathered data whether the substances may cause a negative environment effect for the tested products.
3.2 Method
It is assumed that the substances can be absorbed in the body or may act negatively on external and internal surfaces of an organism.
Regarding exposure the following scenarios are assessed.
- Products with uptake through skin
As worst case, the baby is assumed to have a naked body with skin contact with the product in an area of 15×30 = 450 cm². This area roughly corresponds to the back of a 8 months old baby (measured out) and has been estimated as a realistic worst case exposure area when using nursing pillows, mattresses, etc. If no values for uptake through skin is available 100% uptake is assumed if Log KOW < 4 and 10% uptake if Log KOW > 4 of the substance. The body weight of a baby is set at 5 kg based on the range 3-10 kg the first year. The time of use is dependent on product and shown in Table 3.1.
- Products with oral uptake
As worst case, the baby is assumed to lick on an area of contact with the product in an area of 5 cm × 5 cm =25 cm² from which the migrated substance is transferred. As the uptake is orally 100% uptake is assumed.
The body weight of a baby is set at 5 kg based on the range 3-10 kg the first year. The time of use is dependent on product and shown in Table 3.1.
- Products with uptake through air
For some products with contents of polystyrene pellets, specific volatile components may be emitted and inhaled. For these products it is assumed that the chemical substance is emitted from the total weight of the product to a volume of approximately 1 m³ as the baby will be positioned in close connection with the product. The analysis of emitted volatile components is performed in a laboratory climate chamber of 5 litres with a 3 gram sample. The total weight of PS pellets in the product is approximately 400 gram which gives the same concentration of emitted components from a climate chamber of 0.7 m³.
Table 3.1 Time of use
| Product no. | Type | Time of use, skin contact (h/d) ¹ | Time of use, oral contact (h/d) ¹ |
| 1 | Disposable foam wash cloth | 0.2 | 0.2 |
| 2 | Disposable foam wash cloth | 0.2 | 0.2 |
| 3 | Disposable foam wash cloth | 0.2 | 0.2 |
| 4 | Pillow for feeding baby | 3 | 0.2 |
| 5 | Pillow for feeding baby | 3 | 0.2 |
| 6 | Baby mattress | 3 | 3 |
| 7 | Nursing pad | 1 | 0.2 |
| 8 | Nursing pad | 1 | 0.2 |
| 9 | Baby carrier | 1 (summer) | 1 |
| 10 | Baby carrier | 1 (summer) | 1 |
| 11 | Apron to perambulator | None | 3 |
| 12 | Apron to perambulator | None | 3 |
| 13 | Baby mattress | 3 | 3 |
1 The time of use is a worst case estimate based on interviews with a number of parents
The exposure scenarios are defined according to the EU's Technical Guidance Document (TGD, 2003).
The exposure from scenario 1 is calculated by:
Uptake per day per kg b.w. = [ M × A × H × F] / b.w. {1}
| M: | Migrated amount of substance (mg/cm²×h) |
| A: | Exposed skin area (cm²) |
| H: | Time of exposure per day (hours) |
| F: | Fraction absorbed |
| b.w.: | Body weight (kg) |
F: Fraction of absorbed substance. If no specific values for F is found then the default values is used: F = 1, i.e. 100 % if Log KOW < 4 and F = 0.1, i.e.10% if Log KOW > 4.
By inserting the bodyweight of 5 kg, the equation can then be reduced to:
Uptake per day per kg b.w. = 0.2 × M × A × H × F
In some cases the migrated amount is not available, but only analysis of content by extraction with solvent.
The dependence between migration and concentration is dependent on characteristics of the product, the chemical substance and the simulant contact medium (e.g. artificial sweat) and the exact dependence can only be found from experiments.
In some cases the migration of substances from materials may be explained by using Ficks law J=-D ×dc/dx where
D is diffusion coefficient of the substance
J is the flux (mole of substance per time unit)
dc/dx is the concentration difference of the substance over the diffusion distance
From Ficks law a linear relation between concentration and flux can be expected for some products.
Therefore, in order to obtain an indication of the migration for products where only the content has been measured, it is assumed that there is a linear dependency between migration and concentration.
In case the migration is known for a comparable product M(2), an indicative migration can be estimated for the product M(1) as
M(1)=M(2) × C(2)/C(1) × T(2)/T(1) × A(2)/A(1)
where
M: Migrated amount of substance (mg/cm²/h)
C: Content (mg/g)
A: Exposure area (cm²)
T: Time of use (h)
There will be a considerable uncertainty in the estimate especially as the material characteristics can be different and therefore the estimate must only be used as a crude estimate of the migration.
Evaluation of risk
In the evaluation of health risks the calculated intake has to be compared with the NOAEL or similar values.
Because NOAEL typically is based on studies with animals and different durations uncertainty factors are used to make the value comparable. The uncertainty factors are based on an uncertainty factor of 10 for extrapolation between species (interspecies) and a factor of 10 meant to protect sensitive individuals like children (intraspecies). If the data is of less quality or based on LOAEL an additional uncertainty factor may be applied (typically 10).
In the evaluation of health risks, NOAEL is compared with the calculated uptake. The ratio between the NOAEL and the exposure (substance uptake) is defined as the margin of safety (MOS: Margin of safety). If the data for animals is of sufficient valid the margin of safety factor of 100 may be considered sufficient. But are data inadequate further addition of uncertainty factors may be necessary.
3.3 Selected substances
The substances described in the following are selected as the most important substances for the potential health risks of using these products. The selected substances for health and environmental assessment are:
- Hexabromocyclododecane (HBCD or HBCDD)
- Toluene 2,4-diisocyanate (TDI)
- 2-ethylhexanoic acid (2-EHA)
- Acetophenone
- Formaldehyde
- 1,1,2,2-Tetrachloroethane
- Styrene
- 2-Bromo-4,6-dinitroaniline (BDNA)
- Hexaethylene glycol dimethyl ether,
- Tetrapropylene glycol, monomethyl ether
Apart from the selected substances, the health risk from DEHP, DINP and xylene has been assessed.
3.3.1 Hexabromocyclododecane (HBCD)
3.3.1.1 Identity
| Name | 1,2,5,6,9,10-Hexabromocyclododecane |
| CAS-number | 3194-55-6, 25637-99-4 |
| EINECS number | 221-695-9, 247-148-4 |
| Molecular formula | C12H18Br6 |
Molecular structure |
|
| Molecular weight | 641.70 |
| Synonyms | Cyclododecane, 1,2,5,6,9,10-hexabromo- Hexabromocyclododecane HBCD |
Hexabromocyclododecane are presented by two different CAS numbers. CAS no. 25637-99-4 (EINECS no. 247-148-4) describes a mixture of mainly three stereoisomers. CAS no. 3194-55-6 (EINECS no. 221-695-9) is 1,2,5,6,9,10-hexabromocyclododecane. The properties of the substances are assumed to be comparable if no other data are available.
The substance consists of a white odourless solid. It has documented melting points between 170 and 190ºC depending on the relative composition of the three stereo-isomers. A boiling point has not been documented since the compound starts to decompose just above the melting point (EC Draft RAR 2003).
The substance has a very low solubility in water. Several values have been reported and evaluated in the EC risk assessment that concluded on a water solubility of 0.0034 mg/L in pure water at 25°C (EC Draft RAR 2003). HBCD has a high solubility in ketone, chlorinated and aromatic solvents (HSDB 6110).
The partition coefficient Log Kow has been estimated to be 7.74 and the Henry’s Law Constant 4.60 E-5 atm-m³/mole at 25°C (Syracuse Research Corporation 2005).
The vapour pressure of HBCD is low. Thus the vapour pressure is stated to 6.3x10-5 Pa in the EU risk assessment and 4.7 x E-7 mm Hg at 21°C (HSDB 6110).
This means that HBCD will evaporate only slowly from aquatic surfaces. However, since HBCD can be predicted to adsorb to suspended matter and in the aquatic environment eventually end up in sediment, evaporation of HBCD is not a probable exposure route (EC Draft RAR 2003).
There are however some uncertainties in the reported physico-chemical properties of HBCD, since the commercial HBCD is composed of three isomers and also some impurities, this, should, have no influence on the assessment.
3.3.1.2 Function of substance
HBCD is as an additive-type flame retardant and is thus added to the plastics materials without reacting with the plastic polymer. HBCD is used in extruded and expanded polystyrene foam. Other applications include crystal and high-impact polystyrene, SAN (Styrene-AcryloNitrile) resins, adhesives, and coatings (HSDB 6110).
3.3.1.3 Classification and TLV
This chemical substance is not classified in the Annex I of Directive 67/548/EEC.
It is a high production volume (HPV) chemical and it is a prioritised substance within the Programme of Existing Substances in the EU. It is currently undergoing a comprehensive EU risk assessment with Sweden as rapporteur country (EC Draft RAR 2003).
Threshold limit value (TLV) was not found.
3.3.1.4 Detected quantities
The substance is detected in 2 products.
In sample number 4C is found 457 µg/gram
In sample number 5C is found 433 µg/gram
Note: The chemical is thermal instable and therefore it is likely that decomposition occurs in both the injector inlet of the chromatographic device as well as in the detection chamber (EC Draft RAR 2003). The determinations may therefore be underestimated.
3.3.1.5 Health Effects
Very few official data on health effects on humans exist. Skin irritation and sensitisation test on guinea pigs have been performed resulting in conclusion of HBCD being a mild skin allergen (HSDB 6110).
Consumers are exposed to HBCD from products containing the chemical, e.g. polymers. The flame retardant is physically bound within the polymer matrix, however it is not chemically bound and may therefore migrate out of the matrix. Therefore, release of HBCD from the surface of the product and to atmosphere from plastic products may be a potential way of exposure.
In the EU report, no measured data on consumers’ exposure have been submitted by the industry. The available mathematical models for consumer exposure are not applicable for the calculation of consumer exposure to HBCD. Products, e.g. textiles, may contain up to 25% HBCD (EC Draft RAR 2003).
Acute toxicity
Two acute dermal toxicity studies have been reported, both with negative results (no animals died) (EC Draft RAR 2003).
Acute oral toxicity studies also showed no toxic or gross pathological changes after high dosing (10 g/kg).
Some acute studies have reported different kinds of sublethal effects; diarrhoea, body weight reduction, depressed activity, increasing apathy and trembling (EC Draft RAR 2003).
Inhalation studies have also confirmed the low acute toxicity of HBCD.
Sub-chronic toxicity
Two 28-day studies both demonstrate a rather low order of toxicity of HBCD upon repeated administration. The increase in liver weights was not accompanied by any pathological findings, and it might reflect a reversible adaptive change characterised by induction of the microsomal enzymes and proliferation of the endoplasmic reticulum. When the exposure is for a limited period of time, like in this case, the hypertrophy is reversible but if it continues for a longer period of time, exposure may exceed the metabolic capacity of the liver and necrosis occurs. (Newberne, 1982).
Two 90-days and one lifetime (18 month) studies on rats and mice support the theory that the liver and the thyroid hormone system are the target for HBCD toxicity in mammals. LOAEL based on these studies of 100 mg/kg/day have been deduced (EC Draft RAR 2003).
Two ordinary developmental toxicity studies have failed to demonstrate any fetotoxicity, teratogenic potential, or adverse effects from HBCD on development of rats.
Few studies on mutagenicy and carcinogenity have been performed and none have reported positive results for HBCD.
Recent studies has indicated that HBCD may cause developmental neurotoxic effects as illustrated by statistically significant changes in spontaneous behaviour, learning and memory defects, and reduced number of nicotine receptors. An indicative LOAEL of 0.9 mg/kg b.w/day can be deduced from these studies (Darnerud 2003, Eriksson et al 2002). The neurotoxicological potential of HBCD has also been confirmed in a study where it was demonstrated that HBCD inhibit plasma membrane uptake of the neurotransmitters dopamine, glutamate and GABA. The response occurred at concentration levels similar to what previously have been found for PCBs and Ecstasy (Mariussen and Fonnum 2003).
Summary
HBCD shows similar chemical and physical properties as well-known persistent organic pollutants and at the same time at a first glance seems to lack toxic action, as were the case with PCBs back in the 1950-ties.
Relevant toxicity studies are lacking for the substance. However, the few studies that have been published so far are enough for being the basis for an immediate strict regulation of the compound. Effects on hormone systems and behaviour at low concentration levels indicate that infants definitely should be protected from ever possible risk of exposure.
3.3.1.6 Exposure scenarios
Human exposure of different populations and subpopulations by multiple exposure routes is possible. These are workers, consumers, and humans exposed to HBCD via the environment (via food, drinking water and air). Worker and consumer exposure are mainly via the dermal and inhalation routes, whereas exposure via the environment occurs via the oral route.
HBCD was not found in the migration experiments.
3.3.1.7 Assessment
HBCD has a high log KOW value and a low indicative LOAEL of 0.9 mg/kg/day. The substance has only been analysed in PS pellets covered by outer layers in product 4, and5 (pillows for feeding the baby). The substance is solid at the temperatures of use and has a low solubility in water. Therefore, it is rather unlikely that a high diffusion through the outer cover layers will occur even if the pillow gets wet accidentally.
As no data was found in the migration analysis for HCBD a margin of safety can not be calculated.
3.3.2 Toluene 2,4-diisocyanate (TDI)
3.3.2.1 Identity
| Name | Toluene 2,4-diisocyanate |
| CAS-number | 584-84-9 |
| EINECS number | 209-544-5 |
| Molecular formula | C9H6N2O2 |
| Molecular structure |  |
| Molecular weight | 174.16 |
| Melting point | 20.5ºC at 760 mmHg |
| Boiling point | 251ºC at 760 mmHg |
| Log Kow (octanol/water) | 3.74 (estimated, esc.syrres.com) |
| Synonyms | 2,4-Diisocyanato-1-methylbenzene (9CI) 2,4-Toluene diisocyanate 4-Methyl-m-phenylene diisocyanate Benzene, 2,4-diisocyanato-1-methyl- Isocyanic acid, 4-methyl-m-phenylene ester (8CI) |
Technical toluene diisocyanate is a mixture of 2.4- and 2.6-isomers (80:20). The pure substance is a colourless to light yellow clear liquid or crystals. It is a quite reactive compound used in the manufacture of polyurethane foams and other elastomers.
The compound has a sharp and pungent odour (Budavari 1996). It is miscible with alcohol, ether, acetone, benzene, carbon tetrachloride, chlorobenzene, diglycol monomethyl ether, kerosene, olive oil, as well as soluble in ethyl acetate (Budavari 1996). The vapour pressure is stated to 1.07 Pa (0.008 mm Hg) at 20°C (Boublik et al 1984).
Along with the estimated octanol-water partitioning coefficient log Kow 3.74, an estimate of water solubility of 37.6 mg/L, as well as a Henry’s law constant of 1.11 E-5 atm-m³/mole, is also reported (esc.syrres.com). However, as TDI is highly reactive and reacts with water with evolution of carbon dioxide (Budavari 1996), these estimates should not be regarded as particularly valid.
3.3.2.2 Function of substance
The substance is a chemical intermediate used in the preparation of polyurethane foams, elastomers and coatings.
3.3.2.3 Classification and TLV
TDI is classified in the List of dangerous substances (Miljøministeriet 2005):
| Tx;R26 | Very toxic by inhalation |
| Xi;R36/37/38 | Irritating to eyes, respiratory system and skin |
| Carc.cat.3;R40 | Limited evidence of carcinogenic effects |
| R42/43 | May cause sensitization by inhalation and skin contact |
| R52/53, | Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
The classification is depending on the concentration in the product as:
Conc.>=20%: Tx;R26 Xi;R36/37/38 Carc3;R40 R42/43
7%<=conc.<20%: Tx;R26 Carc3;R40 R42/43
1%<=conc.<7%: T;R23 Carc3;R40 R42/43
0.1%<=conc.<1%: Xn;R20 R42. (MSDS 584-84-9)
The threshold limit value (Occupational exposure limits, OEL) in Denmark is 0.035 mg/m³ corresponding to 0.005 ppm. The substance is marked with K, which means that the substance is included in the list of substances considered carcinogenic (Arbejdstilsynet 2005).
TDI is a high production volume chemical.
3.3.2.4 Detected quantities
Foam based polyurethane products may in principle emit small amounts isocyanates or the corresponding diamines (Kortlægning nr.28, 2003). TDI was quantitatively analysed by the GC/MS screening of the products 1,2,3,6B,7B,8B,11E,13B but this results is regarded as reaction products from the heating in the GC column. By using HPLC for analysis of foam wash cloths the content was below the detection limit of 5 µg/g.
3.3.2.5 Health effects
In the safety data sheet MSDS the substance is described as a potent skin irritant and allergen.
Potential Health Effects stated are:
Eye: Causes severe eye irritation. Lachrymator (substance which increases the flow of tears).
Skin: Causes severe skin irritation and eczema. May be harmful if absorbed through the skin. May cause sensitization by skin contact.
Ingestion: May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation: Causes respiratory tract irritation. May cause severe irritation of the respiratory tract with sore throat, coughing, shortness of breath and delayed lung oedema. Toxic if inhaled.
Chronic: Repeated exposure may cause allergic respiratory reaction (asthma).
May cause allergic skin reaction in some individuals. Limited evidence of a carcinogenic effect.
Acute toxicity
Exposure to levels as low as 0.014 mg/m³ (0.002 ppm) can result in chronic loss of pulmonary function. A more acute, asthmatic type of bronchitis is not uncommon (IARC 1979).
Acute skin absorption tests on rabbits produced severe irritation but failed to kill, even with very high doses (16 g/kg b.w) (IARC 1979). Another reported acute value is LD50 for rat oral at 3060 mg/kg (EHC 75). For mouse with oral intake LD50 1950 mg/kg was reported (Lewis 1996).
Men complained of neurological symptoms after single exposure to TDI. Effects were euphoria, ataxia, and loss of consciousness; headache, difficulty in concentration, poor memory and confusion. Four year after the exposure incident personality changes, irritability and depression was still noted (HSDB 874).
Sub-chronic toxicity
There is inadequate evidence for the carcinogenicity of TDI in humans. There is sufficient evidence for the carcinogenicity of TDI in experimental animals. Overall evaluation: Toluene diisocyanates are possibly carcinogenic to humans (Group 2B) (IARC 1999, HSDB 874).
Groups of mice and rats in a sub-chronic study were feed corn oil spiked with TDI in different concentrations. The mice in the experiment were administrated 120 or 240 mg/kg. No treatment related tumour was seen in male mice. However, the female mice got two different tumour types; 0% in control, 10% in low-dose, and high dose animals gave a positive trend (p=0.01) (IARC 1986). In the rat test the males were administrated 30 or 60 mg/kg b.w and females 60 or 120 mg/kg b.w of TDI. Treatment related effects were seen in males at low-dose (IARC 1986).
Summary
A broad range of toxic effects is reported for TDIs in the literature. The majority of reported endpoints and observed harm are directly related to TDIs inherent property of being an aggressive and highly reactive compound. Therefore, it is also natural that populations subjected to occupational exposure are at highest risk. However, because of the compounds high toxicity and possibility to induce damage to epithelium, small residues of free compound in/on products that can come in contact with infants should not be ignored.
The long-term effects on brain functions mentioned above must naturally be taken into account when evaluating potential health effects in children.
3.3.2.6 Exposure scenarios
There is no available data for migration of TDI. The content in the foam wash cloths was below 5µg/g. If a worst case assumption is made where the extractable amount of isocyanate corresponds to the detection limit, the maximum extractable amount can be calculated to 5µg*2,34 g /351 cm² =0,033 µg/cm² as a foam washing cloth weighs 2,34 g and has an area of approx. 18,5*19=351 cm².
Based on an extractable content of 0,033 µg/cm², 6 times daily use, an area of exposure of 450 cm² and 100 % absorption, the uptake in a worst case scenario can be calculated to
Uptake = 6*0,033*450*1*0,2 = 17,8 µg/kg/day.
3.3.2.7 Assessment
Based on a LOAEL of 30 mg/kg/day for carcinogenic effects in rats and the highest calculated uptake, the margin of safety (MOS) is 1700 for foam wash cloths
Table 3.2 Estimated margin of safety for products
| Product no. | MOS |
| 1,2,3 | >1700 |
As the data is based on a subchronic study and LOAEL, the uncertainty factor for risk evaluation is assumed to be at least 1000.
It is concluded that there is no risk to the health for consumers from toluene 2,4-diisocyanate by using foam wash cloths based on an assumption that all TDI present at a concentration corresponding to the detection limed can be extracted and absorbed.
It must be mentioned that no quantitative measurements has been made for the other foam containing products. A content of extractable isocyanates will be a source for allergic reactions.
3.3.3 2-Ethylhexanoic acid (2-EHA)
3.3.3.1 Identity
| Name | 2-ethylhexanoic acid |
| CAS-number | 149-57-5 |
| EINECS number | 205-743-6 |
| Molecular formula | C18H16O2 |
| Molecular structure |  |
| Molecular weight | 144.22 |
| Synonyms | 2-Ethylhexanoic acid 2-Ethylhexoic acid Ethyl hexanoic acid, 2- Hexanoic acid, 2-ethyl- |
The substance is a clear liquid. It has a boiling point of 228ºC (Lide, 1995-1996).
The substance is more soluble in organic solvents than in water. It is soluble in ethyl ether, carbon tetrachloride and slightly soluble in ethanol. The solubility in water according to (Ashford, 1994) is 1.4 g/l at 25ºC.
The octanol/water partition coefficient Log KOW is determined to be 2.64 (Hansch, 1995).
Vapour pressure is determined to be 4Pa (0.03 mmHg) at 20ºC (Flick, 1991).
The substance has a mild odour (Flick, 1991).
3.3.3.2 Function of substance
The function of the substance is as stabiliser for PVC products. The substance may also be regarded as a residue from PU production since the salt of 2-EHA and tin, stannous octoate, is the most popular catalyst in PU production. The fact that the substance in this study is found in PU samples and that the presence of tin also is verified in these samples indicate an origin from the catalyst.
3.3.3.3 Classification and TLV
2-Ethylhexanoic acid is included in the List of dangerous substances (corresponding to Annex I of Directive 57/548/EC) and classified as:
Repr.cat.3;R63 Possible risk of harm to unborn child
No Danish threshold limit value for the substance has been found.
3.3.3.4 Detected quantities
The substance is detected in 8 of the samples when extracted with dichloromethane and in 1 in migration tests.
Table 3.3 Detected quantities
| Product no. | Type | Content, µg/g |
Area migration cm² |
Migrated amount µg/cm² |
| 1 | Disposable foam wash cloth | 241 | - | - |
| 2 | Disposable foam wash cloth | 55 | - | - |
| 3 | Disposable foam wash cloth | 424 | - | - |
| 6B | Baby mattress | 30 | - | - |
| 7B | Nursing pad | 81 | - | - |
| 8A | Nursing pad | 21 | - | - |
| 8B | Nursing pad | 495 | - | - |
| 13B | Baby mattress | 66 | 260 | 0.25 |
”-” No analysis
3.3.3.5 Health effects
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Tests for acute toxicity on animals show that 2-ethylhexanoic acid has a low acute toxicity by ingestion.
- LD50 Rat oral 1,600-3,000 mg/kg (Clayton and Clayton 1993-1994)
- LD50 Rabbit oral 1,300 mg/kg (Clayton and Clayton 1993-1994)
The pure substance is harmful if swallowed, inhaled or absorbed through the skin and is extremely destructive to tissues of mucous membranes and upper respiratory tract, eyes, and skin (Prager, 1996).
Some results on rabbits in the IUCLID data set show the component is irritating, other not.
Subchronic toxicity
Data in HSDB and IUCLID report teratogenic effects of 2-ethylhexanoic acid.
Results with continuous administration in drinking water for Wistar rats up to day 20 of gestation shows skeletal malfunctions in offspring like clubfoot, absence of fibula etc. for doses from 100 mg/kg/day and above. The number of affected foetuses was control: 2.4%,100 mg/kg/day: 4.9%, 300 mg/kg/day: 8.9% and 600 mg/kg/day: 15.3%. The NOAEL for teratogenic effects was set to 100 mg/kg/day.
The developmental toxicity of 2-ethylhexanoic acid was studied in animals treated by gavage with doses 0, 100, 250, 500 mg/kg b.w/day on gestation day 6-15 for rats and with doses 0, 25, 125, 250 mg/kg b.w/day on gestation day 6-18 for rabbits. The results suggest that 2-ethylhexanoic acid induces developmental toxicity in rats only at doses that cause maternal toxicity. 2-Ethylhexanoic acid causes maternal toxicity in rabbits without affecting foetal development. The no observable effect levels for maternal and developmental toxicity in rats are 250 and 100 mg/kg, respectively. The no observable effect levels for maternal and developmental toxicity in rabbits are 25 mg/kg and 250 mg/kg or more. (Hendrickx, 1993)
Data is also reported in IUCLID for fertility effects for rats with 100, 300 or 600 mg/kg/day added in drinking water with a premating exposure of 10 weeks for male and 2 weeks for female. The result was a value of NOAEL parental =300 mg/kg/day and NOAEL offspring =100 mg/kg/day.
No data was found for carcinogenic or sensitizing effects.
Summary
2-Ethylhexanoic acid is a substance that may cause reproduction toxic effects including fertility or teratogenic effects in humans. Indications for other long term effects have not been found.
Values for teratogenic effects in rats gave NOAEL=100 mg/kg b.w/day.
Values for fertility effects in rats gave NOAEL=100 mg/kg/day whereas values for developmental toxicity in rabbits was NOAEL=25 mg/kg b.w. per day.
3.3.3.6 Exposure scenarios
From the highest value of the migration results for product 13B (foam in mattress) of 0.25 µg/cm² (4 hour experiment), a use of 3 hours, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.25*450*(3/4)*1*0.2=16.6 µg/kg/day.
This will require that the mattress is wetted somewhat in order to make contact between skin, outer layer of the mattress and the foam containing the substance. This is most likely when the baby is sweating (during summer).
For product 3 (wash cloth), the content is 6 times as high as in product 13, but the time of use is 15 times less indicating a 2.5 times lower uptake:
Uptake=(424/66)*0.25*450*(0.2/4)*1*0.2=7 µg/kg/day. Similar estimates are shown for product no.1,2 and the other products in Table 3.4.
Table 3.4 Calculated and estimated uptake for products
| Product no | Content (mg/g) | Migration (µg/cm²) | Uptake worst case (µg/kg b.w.) |
| 1 | 241 | Not analysed | 4² |
| 2 | 55 | Not analysed | 0.9 2, |
| 3 | 424 | Not analysed | 7 2,, |
| 6B | 30 | Not analysed | 7.5 ² |
| 7B | 81 | Not analysed | 6.8 ² |
| 8A | 21 | Not analysed | 1.8 ² |
| 8B | 495 | Not analysed | 0 ¹ |
| 13B | 66 | 0.25 | 16.6 |
1: Insignificant uptake as the foam has plastic cover
2: Estimate based on assuming migration of product 13B
3.3.3.7 Assessment
Based on a NOAEL of 25 mg/kg/day for maternal and developmental toxicity in rabbits and the highest calculated uptake, the margin of safety (MOS) is 1504 for product 13.
In the estimate it is assumed that the observed effects will also have an effect on a baby.
Table 3.5 Estimates margin of safety for products
| Product no. | MOS |
| 1 | 6200 |
| 2 | 27000 |
| 3 | 3513 |
| 6B,7B | 3300-3700 |
| 8A | >14000 |
| 13B | 1504 |
As the data are based on a subchronic study, the uncertainty factor for risk evaluation is assumed to be at least 1000 for product 13B. For the products 1,2,3,6,7,8 there is an uncertainty regarding the estimated migration, as the materials may not be of the same physical characteristic and thereby the diffusion coefficient of the substance may be different. It is likely that the uncertainty in the estimation is less than a factor 10.
It is concluded that there is no health risk for the consumer from 2-ethylhexanoic acid for the product no. 13. Based on analysis results from extraction in dichloro methane and assuming similar migrations as for product 13 which introduces an additional uncertainty factor (<10), it is concluded that there is a possible minor risk for products nos.1, 3, 6 and 7. A real assessment of the risk will require a migration analysis.
For product 8A there is no risk of health effects.
3.3.4 Acetophenone
3.3.4.1 Identity
| Name | Acetophenone |
| CAS-number | 98-86-2 |
| EINECS number | 202-708-7 |
| Molecular formula | C8H8O |
| Molecular structure |  |
| Molecular weight | 120.15 |
| Synonyms | 1-phenylethanone Acetophenon Benzoyl methide Ethanone, 1-phenyl- Ketone, methyl phenyl- Methyl phenyl ketone Phenyl methyl ketone 1-Phenyl-1-ethanone |
Liquid. Forms laminar crystals at low temperature.
Orange blossom or jasmine-like odour. Bitter, aromatic flavour.
Boiling point: 202°C and melting point 20.5°C. Vapour Pressure: 53 Pa (0.397 mmHg) at 25° C. Henry's Law constant= 1.04 E-5 atm-cu m/mole.
Log Kow is 1.58. Slightly soluble in concentrated sulphuric acid; freely soluble in alcohol, chloroform, ether, acetone, benzene, fatty oils, and glycerol. The water solubility is 6.13 mg/L at 25°C.
3.3.4.2 Function of substance
Acetophenone has several different uses: In perfumery to impart an orange-blossom-like odour; catalyst for the polymerization of olefins; in organic syntheses, esp. as photosensitizer; flavouring agent in non-alcoholic beverages, ice cream, candy, baked goods, gelatines and puddings, chewing gum; fragrance ingredient in soaps, detergents, creams, lotions, perfumes.
Solvent for synthesis of pharmaceuticals, rubber chemicals, dyestuffs and corrosion inhibitors (HSDB 969).
3.3.4.3 Classification and TLV
This chemical substance is classified in the Annex I of Directive 67/548/EEC and in the Danish List of hazardous compounds as:
Xn;R22 Harmful if swallowed.
Xi;R36 Irritating to eyes.
Occupational exposure to acetophenone may occur through inhalation and dermal contact with this compound at workplaces where acetophenone is produced or used. The European regulation recommends (as well as the Danish Working Environment Authorities) a TLV-TWA of 49 mg/m³ (10 ppm, 8-hr) (Arbejdstilsynet 2005).
3.3.4.4 Detected quantities
In the screening with dichloromethane the substance was detected in two products.
Table 3.6 Detected quantities
| Product no. | Type | Content in µg/g |
Area migration (cm²) |
Migrated amount µg/cm² |
Migrated amount µg/g |
VOC µg/m³ |
| 4A | Pillow for feeding baby | 200 | 0.005 | - | - | |
| 5A | Pillow for feeding baby | 210 | 0.01 | - | - | |
| 7A | Pillow for nursing | 196 | 0.003 | - | - | |
| 4C | Pillow for feeding baby | 1010 | - | - | 1.6 | - |
| 5C | Pillow for feeding baby | 1572 | - | - | 5.9 | 0.41 |
“-“ No analysis
3.3.4.5 Health effects
Acute toxicity
Acetophenone had a moderate to low acute oral toxicity in laboratory animals and a low dermal toxicity in guinea-pigs. Central nervous system depression occurred in laboratory animals exposed orally and by injection.
Acetophenone was a skin irritant in rabbits and guinea-pigs. It was a severe eye irritant in rabbits. No skin- sensitizing potential was demonstrated when solutions of acetophenone were tested on guinea-pigs.
The highest toxicity value reported in HSDB (2005) is 200 mg/kg (LD50, mouse).
Only effects on human beings have been examined as result of its use as hypnotic or sedative, and with fairly high dosage there appears to be a slightly depressant action on pulse and slight but continuous decrease of haemoglobin.
Among healthy subjects no effects of any kind were perceptible following ingestion of 0.1-0.3 g, but with 0.45-0.6 g micturition (urination frequency) was increased, pulse weakened and slowed after 5-6 hr, and there was slight but continuous decrease of haemoglobin, returning to normal when dosage ceased.
Sub-chronic toxicity
Acetophenone levels ranging from 1-102 mg/kg/day failed to cause any reduction in body weight or any histopathologic abnormalities in the liver, kidney, spleen, or testis when incorporated in the diet of Sherman rats for 30 days. Acetophenone in the diet of male and female Osborne-Mendel rats at levels 1,000, 2,500, or 10,000 ppm (0.1, 0.25, 1.0%) for 17 weeks found no toxic effects on body weight, haematological indices (red and white blood cell counts, haemoglobin, and hematocrit), nor histopathological abnormalities of the liver, kidney, spleen, heart, testis, muscle, or bone marrow.
Application of 480 mg/kg of acetophenone to the skin of pregnant rats on days 10-15 of pregnancy did not cause any change in the gestation period, size of litter, weight of the offspring, time for appearance of teeth or hair, opening of the eyes, or appearance of reflexes.
There was no evidence of mutagenicity in Ames bacterial tests.
In USEPAs integrated information system (IRIS 2005) oral reference dose (RfD) have been calculated to 0.14 mg/kg bw/day, including an uncertainty factor of 3000. NOAEL was 10,000 ppm or 423 mg/kg/day in a supporting study (Hagan, 1967)
Single dose oral LD50 values for rats range from 0.9-3.2 g/kg bw indicating that the subchronic NOAEL defined by (Hagan,1967) may be close to the threshold for toxicity.
Summary
Thresholds for toxic responses to acetophenone seem in general to be high. If any risk to exposure other than occupational should be regarded, it should be the one that may occur in the scenarios depicted in this investigation. Some references indicate that acetophenone might have irritating properties on both skin and lungs. Therefore, there may be a potential risk for the establishment of chronically diseases as asthma and allergies in sensitive individuals.
3.3.4.6 Exposure scenarios
From the highest value of the migration results for product 5A of 0.01 µg/cm² (4 hour experiment), a use of 3 hours, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.01*450*(3/4)*1*0.2=0.65 µg/kg/day.
The internal PS pellets contain a considerable amount of acetophenone, but the amount can only be extracted if the outer and inner cover layers are penetrable by water. Assuming that this would be possible and that 450 cm² was wetted in a depth of 1 cm this corresponds to 450 cm³ which is approximately 6 g of PS pellets or 36 µg/450 cm² =0.08 µg/cm² (80 cm³ of PS pellets weighs 1 g)
In this case the uptake would be
Uptake=0.08*450*(3/4)*1*0.2=5.4 µg/kg/day.
From the area of exposure and exposure time, the oral uptake will be a factor 270 less.
Table 3.7 Calculated and estimated uptake for products
| Product no | Migrated amount (µg/cm²) |
Migration (µg/g) |
Uptake worst case (µg/kg b.w.) |
| 4A | 0.005 | Not analysed | 0.3 |
| 5A | 0.01 | Not analysed | 0.65 |
| 7A | 0.003 | Not analysed | 0.2 |
| 4C | Not analysed | 1.6 | 1.5 ¹ |
| 5C | Not analysed | 5.9 | 5.4 ¹ |
1 Assuming wetting of in 1 cm depth of 450 cm² of PS pellets
3.3.4.7 Assessment
Based on a NOAEL of 423 mg/kg/day and the highest calculated uptake by skin contact, the margin of safety (MOS) is 658500 for product 5A and MOS =78000 for product 5C.
The corresponding ratio between RfD and uptake is 217 for product 5A and 26 for product 5C. The last is a very unlikely scenario and it is assessed that there is no health effects from oral uptake or skin contact from acetophenone for the tested products.
Table 3.8 Estimates margin of safety for products
| Product no. | MOS |
| 4A, 5A, 7A | >658000 |
| 4C,5C | >78000 |
Regarding uptake by air the ratio between the TLV of 49000 µg/m³ and the concentration of acetophenone measured in climate chamber of 0.41 µg/m³ is 119500. Based on this it is assessed that there is no health effects from intake by air for acetophenone for the tested products.
3.3.5 1,1,2,2-Tetrachloroethane
Identification
| Name | 1,1,2,2-Tetrachlorethane |
| CAS no. | 79-34-5, 25322-20-7 (tetrachloro-ethane) |
| EINECS no. | 201-197-8 |
| Molecular formula | C2H2Cl4 |
| Molecular structure | 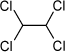 |
| Molecular weight | 167.85 g/mol |
| Synonyms | Sym-tetrachloroethane |
| acetylene tetrachloride |
The melting point of the substance is –43.8ºC. The boiling point is 146ºC (Budavari 1989). The vapour pressure is 800 Pa at 25ºC (Howard 1990), 1200 Pa at 30ºC (9 mmHg) (Flick 1985: HSDB). The water solubility is 2860 mg/l at 25ºC (1 g/350 ml, Budavari 1989). The partitioning coefficient log Kow has experimentally been determined to 2.39 (Hansch et al. 1995).
3.3.5.1 Function of substance
1,1,2,2-Tetrachloroethane is produced by chlorination of ethylene, ethane or 1,2-dichlorethane. 1,1,2,2-Tetrachloroethane is used as solvent for a wide range of substances, but the use is decreasing due to the high toxicity of the substance and the emergence of suitable alternatives.
The source of the recoveries might be the use in the production of polymers or the use as solvent in adhesives. Thus, 1,1,2,2-tetrachloroethane can be a residue from the use in the production process, but it can also be an accidental by-product from the production of another substance, which is used in the production.
3.3.5.2 Classification and TLV
1,1,2,2-Tetrachloroethane, CAS no. 79-34-5, is classified (Miljøministeriet 2005):
| Tx;R26/27 | Toxic. Very toxic by inhalation and in contact with skin |
| N;R51/53 | Dangerous for the environment. Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
Classification Tx;R26/27 N;R51/53 Tx;R26/27 N;R52/53 T;R23/24 N;R52/53 T;R23/24 Xn;R20/21 |
Concentration Conc.>=25% 7% <= conc.<25% 2.5%<= conc.<7% 1%<= conc.<2.5% 0.1%<= conc.<1% |
The threshold limit value is 7 mg/m³ and the substance is marked H which means that the substance can be absorbed through the skin (Arbejdstilsynet, 2005).
3.3.5.3 Detected quantities
In the screening with dichloromethane 1,1,2,2-tetrachloroethane was detected in 2 products (278 µg/g in 4C and 493 µg/g in 5C). The content of 1,1,2,2-tetrachloroethane is analysed in migration studies by sweat test of pillows for feeding the baby (4A, 5A) and a nursing pillow (7A) and in sweat and saliva tests of PS pellets in pillows for feeding the baby (4C, 5C), and by emission measurements of PS pellets in pillows for feeding the baby (4C, 5C). No concentrations of 1,1,2,2-tetrachloroethane were determined above the detection limit in the analysed products. The detection limit is 0.5 µg corresponding to 0.0025 µg/cm² for the products 4A, 5A and 7A.
3.3.5.4 Health effects
The acute toxicity of 1,1,2,2-tetrachloroethane is slight to moderate.
The data below is partly base don Survey report no. 42 (Miljøstyrelsen 2004).
Acute toxicity:
| Acute oral, rat | LD50 | 800 mg/kg | NIOSH 1997 |
| Acute oral, rat | LD50 | 200 mg/kg | HSDB 2003 |
| Acute oral, human | TDLO* | 30 mg/kg | NIOSH |
*: Lowest observed dose with toxic effect
Based on the results of principally limited short-term and subchronic studies, the liver appears to be the most sensitive target organ.
1,1,2,2-Tetrachloroethane is found to be hepathotoxic and nephrotoxic. In a rat study with short-time oral exposure of 1,1,2,2-tetrachloroethane, hepathotoxicity, nephrotoxicity, effects on testes etc. were observed at the lowest dose level. Thus, LOAEL was 8 mg/kg b.w/day (Hassauer et al. 1993).
The acceptable daily intake (ADI) for short-time oral absorption of 8 µg/kg b.w/day is based on a LOAEL for rats of 8 mg/kg b.w/day and a safety factor of 1000 (Hassauer et al. 1993).
In a rat inhalation study, based on immunotoxicity a NOAEL of 2 mg/m³ was observed. The reference has recalculated the exposure to a NOAEL of 60 µg/kg/day assuming an absorption from inhalation of 50% (Hassauer et al. 1993).
ADI: 0.6 µg/kg bw/day is based on absorption via inhalation (NOAEL 60 µg/kg/day from 2 mg/m³, and a safety factor of 100) (Hassauer et al. 1993).
Chronic toxicity
In a long-term oral exposure of 1,1,2,2-tetrachloroethane to rats, hepathotoxicity, nephrotoxicity, effects on testes etc. were observed. NOAEL was 3.2 mg/kg bw/day (Hassauer et al. 1993).
ADI for long-time oral absorption of 0.3 µg/kg bw/day (based on a NOAEL for rats of 3.2 mg/kg bw/day and a safety factor of 10000).
Long-time oral intake of tetrachloroetane resulted in an increased number of liver-tumours in mice. It has not been possible to repeat the results in other species of animal. The exposure for 78 weeks for 0, 142 or 284 mg/kg bw/day is used in an American model (Multistage model) for the evaluation of its carcinogenic potency. The potency, which resulted in 5% increase of liver-tumours (TD0.05), was between 5.8 and 28 mg/kg bw/day (CICAD 1998).
3.3.5.5 Bioavailability
1,1,2,2-Tetrachloroethane is readily absorbed via the skin (MSDS, HSDB).
References suggest an absorption between 70 and 100% after oral exposure. In an experiment with 1.5 mg/kg for rats and mice, 41% was recovered in the exhaled air, 23% in urine and 4% in faeces for rats, for mice the figures were 51%, 22% and 6%, respectively (ATSDS 1996). An absorption of 100% is used in the evaluation.
3.3.5.6 Exposure
In the worst case is assumed that the migration corresponds to the detection limit or 0.0025 µg/cm² (4 hour experiment) for the products 4A, 5A, 7A. Based on this a maximum uptake is calculated to:
Uptake=0.0025*450*(3/4)*1*0.2=0.17 µg/kg/day by skin adsorption
From exposure area and time is found that maximum oral uptake is 270 times less.
3.3.5.7 Assessment
Based on NOAEL 3.2 mg/kg/day and the maximum uptake using the detection level is determined a margin of safety (MOS) of 19000 by absorption through skin while the ratio between ADI and maximum uptake is 1.9.
From this it is concluded that no health risk by absorption through the skin exists for the products 4, 5, 7.
Oral uptake is assessed not to pose any health risk as MOS is 5200000.
The assessment of intake via inhalation can be estimated from the ratio between the occupational threshold limit value (7000 µg/m³) and the concentration from the measurement in climate chamber (<0.1 µg/m³). From this the ratio is >70000. Based on this is assessed that no health effects is expected by inhalation of tetrachloroethane from the tested products.
3.3.6 Formaldehyde
3.3.6.1 Identity
| Name | Formaldehyde |
| CAS-number | 50-00-0 |
| EINECS number | 200-001-8 |
| Molecular formula | CH2O |
| Molecular structure | 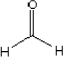 |
| Molecular weight | 30.03 |
| Synonyms | Formalin Methanal |
The substance is a gas. It has a melting point of -92ºC and a boiling point of -19°C (Budavari 1996)
The substance has a high water solubility of 40,000 mg/l at 20 °C
(Pickrell,1983). At high concentrations the substance polymerises.
The partition coefficient log KOW is determined to be 0.35 (Hansch,1995).
Vapour pressure is determined to be 518 kPa (3890 mmHg) at 25ºC (Boublik, 1984).
The substance has a pungent, suffocating odour (NIOSH, 1997).
3.3.6.2 Function of substance
The presence of formaldehyde is probably a reaction by-product which arises from used additives during the production process of textiles
3.3.6.3 Classification and TLV
Formaldehyde is included in the List of dangerous substances and classified as:
| Carc. Cat. 3;R40 | Limited evidence of a carcinogenic effect |
| T;R23/24/25 | Toxic by inhalation, in contact with skin and if swallowed |
| C;R34 | Causes burns |
| R43 | May cause sensitisation by skin contact |
At concentrations in the interval 1-5 % formaldehyde is classified Carc. Cat.3;R40 and R43 and at concentrations 0.2-1% the classification is R43.
The Danish threshold limit value for the working environment is for the substance 0.4 mg/m³ and marked with H for penetrable through skin and K for being considered carcinogenic. For indoor climate there is a Danish norm value of 0.15 mg/m³ (Arbejdstilsynet, 2005) which is close to WHOs guidance value of 0.1 mg/m³. Sensitive persons react with mucous membrane and eye irritation from 0.06 mg/m³.
3.3.6.4 Detected quantities
Formaldehyde was detected in the following products
Table 3.9 Detected quantities
| Product no. | Type | Content µg/g |
Area migration cm² |
Migrated amount µg/cm² |
| 4A | Pillow for feeding baby | 26 | 200 | 0.37 |
| 5A | Pillow for feeding baby | 65 | 219 | 1.7 |
| 7A | Pillow for nursing | 100 | 196 | 0.36 |
3.3.6.5 Health effects
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET and Survey no. 39 (Eggert and Hansen 2004)..
Acute toxicity
Tests for acute toxicity on animals shows:
- LD50 Rat oral 600 mg/kg (IUCLID)
- LD50 Rat oral 100 mg/kg (Lewis,1996)
- LD50 mouse oral 42 mg/kg (IUCLID)
- LC50 Rat inhalation 480 mg/m³ (Tomlin, 1994)
- Formaldehyde is irritating to skin, eyes and mucous membranes and causes allergic sensitisation. (Thomsen,1990) and (Tomlin, 1994)
Chronic toxicity
Formaldehyde is a probably human carcinogen with limited evidence in humans and sufficient evidence in animals. The evidence include an increased amount of nasal squamous cell carcinomas in long term inhalation studies on rats and mice supported with in vitro genotoxicity data. Formaldehyde is placed in group 2A by IARC (IARC, 1995)
Formaldehyde is revaluated and in the draft (IARC, 2005) it has been concluded that there is sufficient evidence for nasopharyngeal carcinoma in humans for placing formaldehyde in group 1.
The reference dose for chronic oral exposure, RfD, is 0.2 mg/kg/day. The value is based on a 2-year experiment with Wistar rats where formaldehyde was administered daily with drinking water. The LOAEL for weight gain and histopathy was 82 mg/kg/day whereas NOAEL was 15 mg/kg/day. By using an uncertainty factor of 100 for inter- and intraspecies differences, a RfD value of 0.2 mg/kg/day was obtained.
Summary
Formaldehyde causes allergic sensitisation and is a probable human carcinogen. The NOAEL value is 15 mg/kg/day.
3.3.6.6 Exposure scenarios
From the highest value of the migration results for product 5A of 1.7 µg/cm² (4 hour experiment), a use of 3 hours, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=1.7*450*(3/4)*1*0.2 = 115 µg/kg/day. Uptake for 5A and 7A is shown in Table 3.10.
Table 3.10 Calculated and estimated uptake for products
| Product no | Migrated amount (µg/cm²) |
Uptake worst case (µg/kg b.w.) |
| 4A | 0.37 | 25 |
| 5A | 1.7 | 115 |
| 7A | 0.36 | 24 |
3.3.6.7 Assessment
Based on a NOAEL of 15 mg/kg/day for effects on weight gain and histopathy on rats and the highest calculated uptake, the margin of safety (MOS) is 128 for product 5.
The ratio between RfD and the calculated uptake is 1.3 for product 5 and 6 for product 4, 7.
Table 3.11 Estimates margin of safety for products
| Product no. | MOS |
| 4A | 584 |
| 5A | 128 |
| 7A | 604 |
It is concluded that there is a no potential risk to the health of the consumer from products no. 4, 5, and 7. However, it must be noted that the formaldehyde exposure is close to the acceptable daily intake and that the uptake therefore may contribute significantly to the other sources for formaldehyde found in homes (wood sources, electronics etc.). It is assumed that formaldehyde has an absorbtion of 100% and this is not very likely, but it can not be rejected that formaldehyde in the pillow could have a sentisising effect.
3.3.7 Styrene
3.3.7.1 Identity
| Name | Styrene |
| CAS-number | 100-42-5 |
| EINECS number | 202-851-5 |
| Molecular formula | C8H8 |
| Molecular structure | 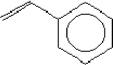 |
| Molecular weight | 104.15 |
| Synonyms | Ethenylbenzene Vinyl benzene |
The substance is a colourless to yellowish oily liquid. It has a melting point of -31ºC and a boiling point of 145°C (Lide, 2000).
The substance is less soluble in water than organic solvents with a water solubility of 310 mg/l at 25°C (Yalkowsky, 1990). At high concentrations the substance polymerises.
The partition coefficient Log KOW is determined to be 2.95 (Hansch, 1995).
Vapour pressure is determined to be 850 Pa (6.4 mmHg) at 25ºC (Chao, 1983).
The substance has a sweet and pleasant odour if pure, but usually contains aldehydes that have a typical penetrating smell, sharp, sweet, and unpleasant odour (Verschueren, 2001).
3.3.7.2 Function of substance
The presence of styrene is not intended but is a monomer residue from the production process.
3.3.7.3 Classification and TLV
Styrene is included in the List of dangerous substances and classified as:
| R10 | Flammable |
| Xn;R20; | Harmful by inhalation |
| Xi;R36/R38 | Irritating to eyes and skin |
The threshold limit value for the working environment is 105 mg/m³ (25 ppm) with notation LHK. L means that the threshold limit value is a ceiling value which at no time must be exceeded. H means that the substance is penetrable to the skin. K means that the substance is adopted on the list of substances that may be carcinogenic (Arbejdstilsynet 2005).
3.3.7.4 Detected quantities
In the screening with dichloromethane the styrene was detected in 4 products
Table 3.12 Detected quantities
| Product no. | Type | Content in µg/g |
Area migration (cm²) |
Migrated amount µg/cm² |
Migrated amount µg/g |
VOC µg/m³ |
| 4A | Pillow for feeding baby | 200 | <0.0025 | <0.5 | - | |
| 5A | Pillow for feeding baby | 9 | 210 | <0.0024 | <0.5 | - |
| 7A | Pillow for nursing | 14 | 196 | <0.0025 | <0.5 | - |
| 4C | Pillow for feeding baby | 315 | 200 | - | <0.5 | 0,3 (max) |
| 5C | Pillow for feeding baby | 679 | 210 | - | <0.5 | 0,3 (max) |
| 6A | Cover for mattress | 20 | - | - | - | - |
| 7B | Pillow for nursing | 79 | - | - | - | - |
| 8B | Pillow for nursing | 28 | - | - | - | - |
“-“ No analysis
3.3.7.5 Health effects
The substance is under evaluation in the EU Risk Assessment Programme for Existing Substances but the assessment is not yet finalised.
Data regarding health effects are included in IUCLID. The following is based on the data sheet and databases in TOXNET.
Acute toxicity
Tests for acute toxicity on animals shows:
- LD50 Rat oral 1000 mg/kg (Verschueren, 1983)
- LD50 Mouse oral 316 mg/kg (Lewis, 1996)
- LD50 Rat inhalation 24000 mg/m³ (Lewis, 1996)
Acute exposure to high concentrations of styrene may produce irritation of the mucous membranes of the upper respiratory tract, nose and mouth
(Environment Canada, 1981).
Chronic toxicity
There is limited evidence in animals and limited evidence in humans for carcinogenity of styrene.
Data from both laboratory (in vitro and in vivo) and human studies indicate that styrene exposure can result in DNA damage in individuals who possess the capacity to activate styrene metabolically to styrene-7,8-oxide.
The lung tumours in mice probably develop as a result of in-situ formation of styrene 7, 8-oxide which causes cytotoxicity and increased cell proliferation, but the roles of circulating styrene 7,8-oxide and of DNA adducts cannot be discounted. Based on metabolic considerations, it is likely that the proposed mechanism involving metabolism of styrene to styrene 7,8-oxide in mouse Clara cells is not operative in human lungs to a biologically significant extent. However, based on the observations in human workers regarding blood styrene 7,8-oxide, DNA adducts and chromosomal damage, it cannot be excluded that this and other mechanisms are important for other organs (IARC, 2002).
Studies of reproductive effects on rats where styrene was administered on day 6-15 of gestation showed effects like decreased maternal and foetal body weight and increased foetal resorption at 400 mg/kg/day but not at 250 mg/kg/day (Srivastava, 1990).
An embryotoxic study showed a toxicity of the metabolite styrene oxide of 0.038 umol/ml, 1 umol/ml of styrene and 1.56 umol/ml of benzene (Brown-Woodman, 1994).
The reference dose for chronic oral exposure RfD is 0.2 mg/kg/day.
The value is based on a subchronic study where beagle dogs were gavaged with 0, 200, 400, 600 mg/kg/day of styrene in peanut oil for 560 days. Effects where found on red blood cells and liver from at 400 mg/kg/day but not at 200 mg/kg/day. From this NOAEL = 200 mg/kg/day. An uncertainty factor of 1000 was used reflecting a factor of 10 for intraspecies, 10 for interspecies and a factor of 10 for extrapolation of subchronic to chronic effects (IRIS).
Summary
There is limited evidence for carcinogenity of styrene.
A study showed reproductive effects on rats.
The NOAEL value=200 mg/kg/day based on effects on red blood cells and liver on beagle dogs.
3.3.7.6 Exposure scenarios
Styrene was not detected in the migration measurements but from the highest value based on the detection limit for product 5A a maximum migration of 0.0025 µg/cm² (4 hour experiment)can be calculated. Using 3 hours of exposure time, an area of 450 cm² of exposure and 100% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.0025*450*(3/4)*1*0.2 = 0.17 µg/kg/day.
The internal PS pellets contain a considerable amount of styrene, but the amount can only be extracted for dermal contact if the outer and inner cover layers are penetrable by water. Assuming that this would be possible and that 450 cm² was wetted in a depth of 1 cm this corresponds to 450 cm³ which is approximately 6 g of PS pellets or 3 µg/450 cm² =0.0067 µg/cm². In this case the uptake would be
Uptake=0.0067*450*(3/4)*1*0.2 = 0.45 µg/kg/day.
From the area of exposure and exposure time, the oral uptake will be a factor 270 less.
3.3.7.7 Assessment
Based on a NOAEL of 200 mg/kg/day for effects on red blood cells and liver of beagle dogs and the highest calculated uptake which is based on the detection limit, the margin of safety (MOS) is approximately 1200000 for product 4A, 5A, 7A
For product 4C, 5C and 7C MOS =452000.
Table 3.13 Estimates margin of safety for products
| Product no. | MOS |
| 4A,5A,7A | 1,200,000 |
| 4C,5C,7C | 452,000 |
It is concluded that there is no health effect from styrene by skin contact and oral uptake.
Regarding uptake by air via inhalation the ratio between the TLV of 105000 µg/m³ and the concentration of styrene measured in climate chamber of 0.77 µg/m³ is 136000. Based on this it is assessed that there is no health risk from intake by air of styrene for the tested products.
3.3.8 2-bromo-4,6-dinitroaniline (BDNA)
3.3.8.1 Identity
| Name | 2-bromo-4,6-dinitroaniline |
| CAS-number | 1817-73-8 |
| EINECS number | 217-329-2 |
| Molecular formula | C6H4BrN3O4 |
| Molecular structure | 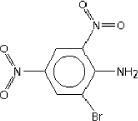 |
| Molecular weight | 262.03 |
| Melting point | 153.5 (C°) |
| Boiling point | Sublimes before reaching BP (C°) |
| Log Kow (octanol-water) | 2.73 (estimate) |
| Synonyms | 2-Bromo-4,6-dinitroaniline Aniline, 2-bromo-4,6-dinitro- Benzenamine, 2-bromo-4,6-dinitro |
It is in its commercial formulation a yellow-orange powder (HSDB 5453).
The substance is very soluble in hot alcohol and acetone, soluble in hot acetic acid.
The water solubility is estimated to 92 mg/l.
3.3.8.2 Function of the substance
The substance is used as a chemical intermediate for Azo derivatives and Disperse Violet 7 and 24.
3.3.8.3 Classification and TLV
This chemical substance is not classified in the Annex I of Directive 67/548/EEC, and not listed in any priority list of existing chemicals (Council Regulation (EEC) No 793/93).
The substance is classified in the Danish Advisory List for self-classification of hazardous substances as:
| N;R51/53 | Dangerous for the environment. Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
No other classification or TLVs are reported except for those in MSDS (cf. Health effects).
It is a high production volume chemical according to US-EPA based on that it is a major intermediate for disperse azo colorants.
3.3.8.4 Detected quantities
Results of extraction with dichloromethane.
Table 3.14 Detected quantities
| Product no. | Type | Content in µg/g | Area migration (cm²) c | Migrated amount µg/cm² |
| 11A | Apron to perambulator | 31 | 200 | 0.0025 |
| 12A | Apron to perambulator | 108 | 200 | 0.0025 |
3.3.8.5 Health effects
In the MSDS for the compound some warnings for occupational handling of the product is described: It may be harmful by inhalation and causes mild eye irritation. It may cause irritation of the digestive tract and cause headache.
It is, however also stated that the toxicological properties of the compound have not been fully investigated. The MSDS state the Hazard symbol: Xn (Harmful) and Risk Phrases R20/21 (Harmful by inhalation and in contact with skin).
Acute toxicity
Oral, rat LD50= 4100 mg/kg (MSDS 1817-73-8).
Acute oral toxicity was evaluated in single male or female rats administered 6-bromo-2,4-dinitroaniline (the less common isomer in the technical grade formulation) at levels of 0.28, 0.62, 1.4, 3.2, 7.1, or 10.7 g/kg body weight. Mortality after 12 days was induced at 7.1 g/kg (female) and 10.7 g/kg (male). However, relevant sublethal endpoints were discovered at lower dosing (3.2 g/kg) and before death. Observations included central depression and lowered response to painful stimuli, brownish urine during the first 24 hours post-dosing and marked loss of body weight. Necropsy of decedents revealed yellowish-brown discoloration of all internal organs, oedema of the liver, hyperaemia of the lungs and contraction of the ventricles of the heart (EPA/OTS 1983).
The disposition and metabolism of BDNA were investigated in rats after a single intravenous dose of either 1, 10, or 100 micromoles per kilogram (micromole/kg) BDNA. Animals were sampled for blood, liver, muscle, skin, kidney, and adipose tissue after different times of metabolism. No signs of toxicity were noted at any dose. About 46 to 62 percent of the BDNA was excreted in the urine and from 33 to 43 percent in the faeces. Relative amounts did not depend on dose. BDNA was found in all major tissues and was more or less evenly distributed except for the organs involved in clearance, metabolism, and excretion. After 72 hours the amount of BDNA remaining in the body was negligible. The authors conclude that BDNA is readily absorbed from the gastrointestinal tract and that rapid metabolism and excretion prevent its accumulation (Chopade and Matthews 1986).
Chronic toxicity
The mutagenicity of 2-bromo-4,6-dinitroaniline (BDNA) has been evaluated in some Salmonella test strains TA98, TA100, TA1535, TA1537 and TA1538 (Ames Test). BDNA was tested for mutagenicity at concentrations ranging from 10-1000 ug/plate using the plate incorporation method. BDNA caused a positive response in all of the bacterial test strains except TA1535, both in the presence and absence of metabolic activation.
In vivo studies have also confirmed evidence of cytotoxicity at each tested concentration (HSDB 5453).
Summary
Because no available chronic studies on the compound were found it is difficult to define a NOAEL value for further risk assessment. The only values found is the LD50 value of 4.1 mg/kg and the value for sublethal endpoint of 3.2 mg/kg bw as there were pronounced detrimental responses at this level.
3.3.8.6 Exposure scenarios
In the migration experiments the substance was below the detection limit of 0.5 µg for product 11A, 12A. This corresponds to 0.5/200=0.0025 µg/cm² (4 hour experiment).
Assuming oral intake with an area of 25 cm² the maximum uptake can be calculated to:
Uptake=0.002*25*(3/4)*1*0.2=0.009375 µg/kg/day.
3.3.8.7 Assessment
Based on the value for sublethal endpoint of 3.2 g/kg and assuming a safety factor of 10000 gives a value of NOAEL of 0.3 mg/kg/day.
Based on this estimate of NOAEL a margin of safety (MOS) of 32000 can be calculated. Thus there is no indication of health effects. But data are seriously missing to perform an actual and reasonable assessment.
3.3.9 Hexaethylene glycol dimethyl ether
3.3.9.1 Identity
| Name | Hexaethylene glycol dimethyl ether |
| CAS-number | 1072-40-8 |
| EINECS number | 214-006-8 |
| Molecular formula | C14H30O7 |
| Molecular structure | |
| Molecular weight | 310.4 |
| Synonyms | 2,5,8,11,14,17,20-heptaoxahenicosane (EINECS name) |
3.3.9.2 Use and function of substance
The substance is assumed to be part of non-reacted poly glycol ether isomers from the main polyurethane foam material in the mattress, in where the breathable structure of flexible polyether foam allows good air circulation (Bayer, 2005).
Other hexaethylene glycols (CAS no. 2615-15-8) are reported to be ingredients in personal care products such as lip liner and toothpastes (National Institute of Health, United States, 2005).
3.3.9.3 Classification and TLV
Hexaethylene glycol dimethyl ether is not included in the List of dangerous substances or the Advisory list of self-classification and there is no Danish threshold limit value.
The Risk phrases for few other different Ethylene glycol ether substances were stated.
Hexaethylene glycol monodecyl ether (CAS no. 3055-96-7, C24H50O7):
R36 R37 R38 R41
Pentaethylene glycol monodecyl ether (CAS no. 3055-95-6, C22H46O6):
R36 R37 R38 R41
Triethylene glycol monodecyl ether (CAS no. 3055-94-5, C18H38O4):
R36 R37 R38 R41
These ethylene glycol ethers are irritating to eyes, the respiratory system and to skin and cause risk of serious damage to the eye (Oxford University, 2005).
3.3.9.4 Detected quantities
The substance is extracted with dichloromethane in the product 13C and measured in a semi quantitative concentration of 2582 µg/g.
3.3.9.5 Health Effects
No data regarding health effects has been found for the substance.
Summary
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
3.3.9.6 Exposure scenarios
Hexaethylene glycol dimethyl ether was not found in the migration experiments.
3.3.9.7 Assessment
Due to the limited data available no assessment is suggested for hexaethylene glycol dimethyl ether.
3.3.10 Tetrapropylene glycol monomethyl ether
3.3.10.1 Identity
| Name | Tetrapropylene glycol monomethyl ether |
| CAS-number | 20324-34-9 |
| EINECS number | |
| Molecular formula | C13H28O5 |
| Molecular structure | 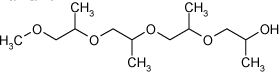 |
| Molecular weight | 264.37 |
| Synonyms | 4,7,10-Trimethyl-2,5,8,11-tetraoxatetradecan-13-ol |
3.3.10.2 Use and function of substance
The substance is assumed to be part of non-reacted poly glycol ether isomers from the main polyurethane foam material in the mattress, in where the breathable structure of flexible polyether foam allows good air circulation (Bayer, 2005).
3.3.10.3 Classification and TLV
Tetrapropylene glycol monomethyl ether is not included in the list of dangerous substances or self classification and there is no Danish threshold limit value.
3.3.10.4 Detected quantities
The substance is extracted with dichloromethane in the product 13C and measured in a semi quantitative concentration of 715 µg/g g divided in two peaks of 622 µg/g and 93 µg/g. Tetrapropylene glycol monomethyl ether is suggested by the NIST chemical identification program as the best match of these peaks.
3.3.10.5 Health effects
No data regarding health effects has been found for the substance.
Summary
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
3.3.10.6 Exposure scenarios
Tetrapropylene glycol monomethyl ether was not found in the migration experiments.
3.3.10.7 Assessment
Due to the limited data available no assessment is suggested for tetrapropylene glycol monomethyl ether.
3.3.11 DEHP
DEHP is not among the substances selected for assessment, but based on the measured concentrations the health effect is evaluated in the following.
The data physico -chemical and toxicological properties for DEHP in this section is based on data in the project (Kortlægning nr. 77, 2006).
3.3.11.1 Identity
| Name | Bis (2-ethylhexyl)phthalate |
| CAS-number | 117-81-7 |
| EINECS number | 204-211-0 |
| Molecular formula | C24H38O4 |
| Molecular structure |  |
| Molecular weight | 390.56 |
| Synonyms | 1,2-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester Bis(2-ethylhexyl) phthalate DEHP Octyl phthalate Phthalic acid, bis(2-ethylhexyl) ester |
The substance is a colourless, oily liquid. It has a boiling point of 230ºC (Clayton, 1981-1982) and a melting point of -55ºC (Lide, 1995-1996).
The substance is more soluble in organic solvents than in water. The solubility in water according to (Yalkowsky, 1992) is 0.285 mg/l at 24ºC.
The partition coefficient Log KOW is determined to be 7.6 (Debruijin, 1989).
Vapour pressure is determined to be 9.6´10-6 Pa (7.23 X10-8 mmHg) at 25ºC (Daubert, 1989).
The substance has a slight odour (NIOSH, 1994).
3.3.11.2 Function of substance
The function of the substance is as plasticizer.
3.3.11.3 Classification and TLV
Bis(2-ethylhexyl)phthalate is included in the List of dangerous substances and classified as:
| Repr.Cat. 2;R60-61 | May impair fertility and may cause harm to the unborn child |
The Danish occupational threshold limit value is 3 mg/m³ (Arbejdstilsynet, 2005).
3.3.11.4 Detected quantities
DEHP has been detected in analysis for phthalates in one of the products, which is 11A a front cover (apron) to a perambulator. DEHP has been detected in semi quantitative analysis of 0.04 mg/g and in migration tests with artificial sweat the concentration was between 0.48 µg/g (in 3.45 g product) and 0.49 µg/g (in 3.80 g product).
3.3.11.5 Health Effects
DEHP is in the process of being evaluated by EU in the Programme on existing chemical substances. Germany is the rapporteur country. The risk assessment report is not yet finalised, but a draft can be found at the ECB homepage (ecb.jrc.it).
Data regarding health effects is included in IUCLID. The following is based on the data sheet, databases in TOXNET and the EU risk assessment above.
Acute toxicity
Tests for acute toxicity on animals show that DEHP is not acute toxic.
LD50 Mouse oral >30,000 mg/kg (WHO, 1992 )
LD50 Rat oral ca. 25,000 mg/kg (WHO, 1992 )
Sub-chronic toxicity
DEHP has been shown to be a weak irritant to mammalian skin when administered topically or intradermally (0.2 mL of an emulsion of 100 g/L) (WHO, 1992).
Chronic toxicity
DEHP is classified as A3 Confirmed animal carcinogen with unknown relevance to humans (ACGIH, 2005).
Studies for carcinogenity in mouse and rats have been found in the dataset for DEHP (IUCLID) with values for effects at approximately 400 mg/kg/day.
The Reference Dose for Chronic oral exposure RfD = 0.02 mg/kg/day. (IRIS)
In the risk assessment on bis(2-ethylhexyl) phthalate (Risk assessment, 2003), a 3 generation rat guideline study is reported. Testicular as well as developmental toxicity was found with increased incidences of small testes, epididymes, and seminal vesicles, as well as cases of minimal testes atrophy. The toxicity was aggravated by exposure during the gestational/pup-period. LOAEL was estimated to 14 mg/kg/day and NOAEL 4.8 mg/kg/day. (Wolfe, 2003)
Summary
Values for carcinogenity for mouse and rats showed effects at approximately 400 mg/kg/day.
In the new draft for risk assessment on DEHP the value of NOAEL is 4.8 mg/kg/day for testicular and developmental effects.
3.3.11.6 Exposure scenarios
There has been no migration experiment for DEHP in the present product. However, in (Kortlægning nr. 77, 2006) was found a migration of 0.01µg/cm² (1 hour experiment) for a product with a concentration of 730 µg/g. As a worst case it is assumed that the migration is the same from product no.11 which have a lower concentration of DEHP=40µg/g.
From this the following maximum oral uptake can be estimated:
Uptake=0.01*25*3*1*0.2=0.15 µg/kg/day
3.3.11.7 Assessment
Based on a NOAEL of 4.8 mg/kg/day and the estimated maximum uptake, the margin of safety (MOS) is 32000 for product 13
The ratio between the RfD value and the uptake is a factor 133.
From these data it is concluded that there seems to be no health risk for DEHP by oral uptake for product 11 (apron for perambulator). However from (Kortlægning nr. 77, 2006), it must be mentioned that the uptake is very dependent on the actual migration conditions, as DEHP has a high solubility in organic solvents but not in water. Therefore the migration may be somewhat different in saliva than under the conditions in (Kortlægnin nr. 77, 2006).
3.3.12 DINP
DINP is not among the substances selected for assessment, but based on the measured concentrations the health risk is evaluated in the following.
3.3.12.1 Identity
| Name | Diisononylphthalate |
| CAS-number | 28553-12-0 |
| EINECS number | 249-079-5 |
| Molecular formula | C26H42O4 |
| Molecular structure |  |
| Log KOW | 8.8 (EU risk assessment on DINP, 2003). |
3.3.12.2 Detected quantities
DINP has been found in analysis for product 8A with 144 mg/g (14.4%), in product 8C with 220 mg/g (incl. DIDeP) and in migration tests for product 8A with 0.033 µg/cm² as the highest value.
3.3.12.3 Health effects
A NOAEL of 88 mg/kg/day is found for effects on the liver and kidneys in rats based on a chronic/carcinogenic study. For reproductive organs NOAEL =276 mg/kg/day based on a mouse study (EU risk assessment of DINP, ECB 2003). A lower value of NOAEL 15 mg/kg/day has, however, been suggested in the EU by the scientific committee CSTEE, which is used here.
3.3.12.4 Exposure scenarios
From the highest value of the migration results for product 8A of 0.033 µg/cm² (4 hour experiment), a use of 1 hour, an area of 450 cm² of exposure and 10% absorption, corresponding to scenario 1, the uptake in worst case can be calculated to
Uptake=0.033*450*(1/4)*0.1*0.2=0.0743µg/kg/day.
The oral uptake will be 9 times less if 100% absorption is assumed by oral contact.
3.3.12.5 Assessment
Based on a NOAEL of 15 mg/kg/day and the estimated maximum uptake, the margin of safety (MOS) is 202800 for product 8A.
From these data it is concluded that there is no health risk for DINP by skin contact or oral uptake for product 8A. However, from (Kortlægning nr. 77, 2006), it must be mentioned that the uptake is very dependent on the actual migration conditions, as phthalates has a high solubility in organic solvents but not in water. In (Kortlægning nr. 77, 2006) the migration for DEHP was increased a factor 8 in water based cream and a factor 1000 in oil based cream. Therefore the concentration on the skin of DINP released from the product may be somewhat higher if creams, moisturizers etc. is used on the baby.
Assuming worst case and a factor 1000 increase of migration in oil based media the uptake will be 74 µg/kg which gives a margin of safety (MOS) of 203. As the estimate is based on data for another product and another phthalate an additional uncertainty factor of a factor of 10 for uptake is applied leading to a combined uncertainty of 10000.
It is concluded that there is a possible minor risk from DINP exposure for product 8A if the baby is in direct contact between the plastic layer and skin and oil based cream or moisturizers is used.
It must also be mentioned that the content of DINP phthalate of 14.4 % is a factor 288 above the allowed limit value of 0.05 wt% for use of phthalates in products for children.
3.3.13 Xylene
Xylene has been measured for product 4C, 5C in climate chamber.
The highest concentration was for product 5C with 0.4 µg/m³.
Regarding uptake by air the ratio between the TLV (109 mg/m³) and the concentration of xylene measured in climate chamber is 272500. Based on this it is assessed that there is no health effects from intake by air for xylene for the tested products.
3.4 Overall Assessment
The health assessment focuses on:
1) What kind of chemical exposure risks that may be found in certain consumer products aimed for use with babies
2) Risks for potential health effects on infants during their critical stages of development
The following conclusions could be drawn from the study:
- All studied baby products contain measurable quantities of more than one compounds classified as hazardous to health and/or environment
- Disposable foam washing cloths contain 2 substances with carcinogenic, sensitizing and reproduction toxic effects. There may be a minor risk of presence of 2-ethylhexanoic acid but a real assessment will require a migration analysis.
- Pillows for baby feeding emit formaldehyde which is carcinogenic and sensitizing. The assessment shows that the worst case migration to skin may contribute significantly to the acceptable daily intake
- Nursing pads contain substances with carcinogenic, sensitizing and reproduction toxic effects which may have a possible health risk in some cases.
- Baby mattresses contain substances with a possible reproduction toxic effect in some cases.
- Some products contain compounds (phthalates) forbidden by Danish and EU-legislation to occur in baby products.
- Some of the products contain several substances with chronic effects which are also found in a number of other products in the society. The effect of such multi-source exposure must be of major concern as babies are a sensitive part of the population and should be protected from chemical exposure.
In the following is shown the results of evaluation of the substances.
Table 3.15 and Table 3.16 show results of health assessments for the selected substances based on the highest uptake found either by uptake by skin or by oral uptake.
Table 3.15 Results of health assessment for selected substances
| Substance | CAS no. | Measured concentrations (sample: µg/g) |
Calculated and estimated uptake for products (sample: µg/kg b.w. per day) |
NOAEL (mg/kg b.w. per day) |
Health effects |
| Hexabromocyclo- dodecane (HBCD) |
25637-99-4 | 4C: 457 5C: 433 |
Not possible (no migration data) | LOAEL approx 0.9 | Developmental, neurotoxic effects |
| Toluene 2,4-diisocyanate (TDI) | 584-84-9 | 1,2,3: <5 | 1,2,3<18 | LOAEL =30 | Carc3, R40 (carcinogen) R42/R43 (sensitization) |
| 2-Ethylhexanoic acid (2-EHA) | 149-57-5 | 1: 241 2: 55 3: 424 6B: 30 7B: 81 8A: 21 13B: 66 |
1: 4 ¹, 2: 0.9 ¹ 3: 7 ¹ 6B: 7.5 ¹ 7B: 6.8 ¹ 8A: 1.8 ¹ 13B: 16.6 |
25 | R63 possible harm to unborn child |
| Acetophenone | 98-86-2 | 4C :1010 5C: 1572 |
4A :0.3 5A :0.65 7A: 0.2 4C: 1.5 ² 5C: 5.4 ² |
423 | Irritating |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | 4C: 278 5C: 493 |
4A,5A,7A<0.17 | 3.2 | Very toxic by skin contact |
| Formaldehyde | 50-00-0 | 4A: 26 5A: 65 7A: 100 |
4A: 25 5A: 115 7A: 24 |
15 | Carc3, R40 (carcinogen) R43 (sensitization) |
| Styrene | 100-42-5 | 4A: none 5A: 9 7A: 14 5C: 679 6A: 20 7B: 79 8B: 28 |
4A,5A,7A <0.17 4C,5C,7C <0.45 |
200 | Irritating |
| 2-Bromo-4,6-dinitroaniline (BDNA) | 1817-73-8 | 11A : 31 12A: 108 |
11A, 12A< 0.009375 |
0.3 ³ | Few data |
| Hexaethylene glycol Dimethyl ether | 1072-40-8 | 13C:2582 | Not possible: Not found in migration experiments |
No data found | Few data |
| Tetrapropylene glycol monomethyl ether | 20324-34-9 | 13C:715 | Not possible: Not found in migration experiments |
No data found | Few data |
1: The estimate has a considerable uncertainty as it is based on migration data for product 13
2: Based on wetting of PS pellets in 1cm depth in an area of 450 cm²
3: Estimate
Table 3.16 Results of health assessment for selected substances continued
| Substance | CAS nr. | MOS ² | Uncertainty factor |
| Hexabromocyclododecane (HBCD) | 25637-99-4 | Not possible | |
| Toluene 2,4-diisocyanate (TDI) | 584-84-9 | 1,2,3: >1700 | 1,2,3 1000 |
| 2-Ethylhexanoic acid (2-EHA) | 149-57-5 | 1: 6200 2: 27000 3: 3513 6B,7B 3300-3700 8A:>14000 13B :1500 |
1,2,3,6,7,8: 10000 13: 1000 |
| Acetophenone | 98-86-2 | 4A,5A,7A>658000 4C,5C >78000 |
4,5,7 : 3000 |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | 4A,5A,7A>19000 | 10000 |
| Formaldehyde | 50-00-0 | 4A: 584 5A: 128 7A: 604 |
100 |
| Styrene | 100-42-5 | 4A,5A,7A >1200000 4C,5C,7C>452000 |
1000 |
| 2-Bromo-4,6-dinitroaniline (BDNA) | 1817-73-8 | 11A,12A>32000 | 10000 |
| Hexaethylene glycol Dimethyl ether | 1072-40-8 | Not possible | |
| Tetrapropylene glycol monomethyl ether | 20324-34-9 | Not possible |
1: The uncertainty is a combination of uncertainty on the NOAEL value and an additional uncertainty on uptake as described in chapter 3.2.
4: Italic type means that MOS <Uncertainty factor which means that there is a possible risk of health effect
Table 3.17 states the results of additional health assessments based on the highest uptake found either by uptake via skin or by oral uptake.
Table 3.17 Results of health assessment for additional substances
| Substance | CAS no. | Measured concentrations (sample: µg/g) |
Calculated and estimated uptake for products (sample: µg/kg b.w. per day) | NOAEL (mg/kg b.w. per day) |
MOS 4 | Uncertainty factor |
| DEHP | 117-81-7 | 11A: 40 | 0.15 1, 2 | 4.8 | 32000 | 10000 |
| DINP | 584-84-9 | 8A: 144000 8C 220000 |
8A: 0.0743 ² 8A:74.3 1,3 |
15 | 8A: 203000 ² 8A:203 ³ |
10000 |
1: Based on a worst case estimate of the migration from another product with higher content of DEHP
2: In artificial sweat. The MOS value is expected to decrease significantly (1-3 decades) if moisturizers and creams are used on baby
3: Estimate for oil like creams and moisturizers
4: Bold means that MOS <Uncertainty factor which means that there is a possible risk of health effect
The main results for each assessed substance is:
- Hexabromocyclododecane (HBCD)
HBCD shows similar chemical and physical properties as well-known persistent organic pollutants and at the same time at a first glance seems to lack toxic action, as were the case with PCBs back in the 1950-ties.
Relevant toxicity studies are lacking for the substance. However, the few studies that have been published so far are enough for being the basis for an immediate strict regulation of the compound. Effects on hormone systems and behaviour at low concentration levels indicate that infants definitely should be protected from ever possible risk of exposure.
HCBD was detected in PS pellets inside product 4,5 but not found in migration tests.
It is rather unlikely that the pillow’s content of the substance will migrate to the surface even if the pillow for feeding babies gets wet.
- Toluene 2,4-diisocyanate (TDI)
TDI was not found quantitatively in foam wash cloths (products no. 1,2,3)
The evaluation is based on NOAEL of 30 mg/kg/day for carcinogenic effects in rats in a subchronic study and an uncertainty factor for NOAEL of 1000, and a worst case assumption where a content corresponding to the detection limit is absorbed through the skin.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects by skin contact:
- There is no risk for products no. 1,2 and 3.
- Data for other foam based products is insufficient for a risk evaluation.
- 2-Ethylhexanoic acid (2-EHA)
2-EHA was found in migration tests or estimated for product 1,2,3,6,7,8,13.
The evaluation is based on a NOAEL of 25 mg/kg/day for maternal and developmental toxicity in a subchronic study on rabbits and an uncertainty factor for NOAEL of 1000.
There is an additional uncertainty factor of 10 on uptake for products nos. 1,2,3,6,7,8.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects by skin contact:
- There is no risk the product no. 13
- There is a possible minor risk for products nos. 1,3, 6 and 7 within the uncertainty in estimating the amount of uptake
In the estimate it is assumed that the observed effects will also have an effect on a baby.
- Acetophenone
Acetophenone was found in migration tests for product 4,5,7.
The evaluation is based on a NOAEL of 423 mg/kg/day and an uncertainty factor for NOAEL of 3000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for acetophenone for the tested products by oral uptake or skin contact.
Regarding intake by air the ratio between the occupational threshold limit value and the concentration of acetophenone measured in climate chamber is 119500. Based on this it is assessed that
- there is no health effects from intake by air for acetophenone for the tested products.
- 1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloromethane was found in analysis for product 4,5,7, but in migration tests the concentrations was below detection limits. This limit is used in a worst-case estimate.
The evaluation is based on a NOAEL of 3.2 mg/kg/day and an uncertainty factor for NOAEL of 10000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for acetophenone for the tested products by oral uptake or skin contact
Regarding uptake by air the ratio between the occupational threshold limit value and the concentration of tetrachloroethane measured in climate chamber is 70000. Based on this it is assessed that
- there is no health effect from uptake by air for tetrachloroethane for the tested products.
- Formaldehyde
Formaldehyde was found in migration tests for product 4,5,7.
The evaluation is based on a NOAEL of 15 mg/kg/day for effects on weight gain and histopathy on rats and an uncertainty factor of 100.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for formaldehyde for the tested products by oral uptake or skin contact
- The estimated formaldehyde exposure is very close to the acceptable daily intake for product 5 and 1/6 of the uptake for product 4 and 7 and therefore formaldehyde may contribute significantly to other sources of formaldehyde in the home, when it is assumed that 100% of the formaldehyde is absorbed via the skin.
- Styrene
Styrene was found in analysis for product 4,5,7, but in migration tests the concentrations was below detection limits. This limit is used in a worst-case estimate.
The evaluation is based on a NOAEL of 200 mg/kg/day for effects on red blood cells and liver of beagle dogs and an uncertainty factor of 1000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for styrene for the tested products by oral uptake or skin contact
Regarding intake by air the ratio between the occupational threshold limit value and the concentration of styrene measured in climate chamber is 136000. Based on this it is assessed that
- there are no health effects from intake by air for styrene for the tested products.
- 2-Bromo-4,6-dinitroaniline (BDNA)
BDNA was found in analysis for product 11,12 but in migration tests the concentrations was below detection limits. This limit is used in a worst case estimate.
Because no available chronic studies on the compound was found, an estimate of NOAEL was made based on a value for sublethal end point of 3.2 mg/kg bw and an uncertainty factor of 10000.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for BDNA for the tested products by oral uptake or skin contact
- Hexaethylene glycol dimethyl ether
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
Hexaethylene glycol dimethyl ether was not found in the migration experiments.
Due to the limited data available no assessment is suggested for hexaethylene glycol dimethyl ether.
- Tetrapropylene glycol monomethyl ether
No data has been found for this compound but data from other ethylene glycol ethers indicate that these substances can damage eyes and be irritating for eyes and skin.
Tetrapropylene glycol monomethyl ether was not found in the migration experiments.
Due to the limited data available no assessment is suggested for tetrapropylene glycol monomethyl ether.
- DEHP
DEHP was detected in product 11.
The evaluation is based on a NOAEL of 4.8 mg/kg/day and a combined uncertainty factor for NOAEL and the uncertainties in estimating the uptake of 10000. The assessment is based on migration data for another product.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is no risk for DEHP for the tested products by oral uptake or skin contact
There is a considerable uncertainty in the estimate and the migration conditions may be somewhat different by oral uptake for a baby than in the in the migration conditions used for the assessment.
- DINP
DINP was found in migration tests for product 8.
The evaluation is based on a NOAEL of 15 mg/kg/day for effects on liver and kidneys in rats in a chronic/carcinogenic study and a combined uncertainty factor for NOAEL and the uncertainties in estimating the uptake of 10000. The assessment is based on migration data for another product and another phthalate.
By comparing the estimated MOS with the uncertainties the following is concluded for health effects:
- There is a possible risk for DINP for the tested product by skin contact if oil based cream or moisturizer is used on the baby’s skin
Apart from this it must be mentioned that
- the content of DINP in product 8 is above the allowed value for childcare articles for children aged 0-3 years as mentioned later.
- Xylene
Regarding intake by air the ratio between the occupational threshold limit value and the concentration of xylene measured in climate chamber is 272500. Based on this it is assessed that
- there are no health effects from intake by air for xylene for the tested products.
3.4.1 Products
In the following are shown products and the components with identified risk for health effects.
- Product no.1, 2, 3 Disposable foam washing cloths
For the products 1 and 3 there may be a minor risk by skin exposure to 2-ethylhexanoic acid within the uncertainty in estimating the uptake amount.
The risk can be minimised by washing the baby with fresh water and drying with a towel after each use of the foam washing cloths.
- Product no 4, 5 Pillow for feeding baby
For these products the estimated formaldehyde exposure is very close to the acceptable daily intake for product 5 and 1/6 of the uptake for product 4
Therefore formaldehyde may contribute significantly to other sources of formaldehyde exposure in the home. The risk assessment assumes that 100% of the formaldehyde is absorbed via the skin.
- Product no.7, 8 Nursing pads
For product no.7
- the calculated uptake of formaldehyde is 1/6 of the acceptable daily intake and therefore the product is a significant source for formaldehyde exposure.
- Within the uncerntainties there is a possible risk for 2-ethyl-hexanoic acid.
For product no.8
- the content of the phthalate DINP above the allowed limit value of 0.05 wt%
- There is a possible risk for DINP for the tested product by skin contact if oil based cream or moisturizers are used on the baby’s skin.
- Product no. 6 and 13 Baby mattress
For product 6 there may be a minor risk by skin exposure to 2-ethylhexanoic acid within the uncertainty in estimating the uptake amount.
For product no.13 it was not possible to evaluate the health effects from the found content of hexaethylene glycol dimethyl ether and tetrapropylene monomethyl ether.
The content of phthalates was above the allowed limit value of 0.05 wt% in the foam part of product 13.
- Product no. 9, 10 Baby carriers
No risk of health effects was found in the assessment.
- Product no.11, 12 Apron to perambulator
No risk of health effects was found for product 12.
In product 11, the phthalate DEHP was found and although the assessment showed no risk there is a considerable uncertainty in the estimate as the migration is based on an estimate and not the actual migration conditions for oral uptake.
In summation, possible health risks were found for product no.1, 2,3,6,7,8.
Product no. 4 and 5 contributes with a significant part of the acceptable daily intake of formaldehyde
DEHP was found in product 11 with no risk found in the assessment, but with a considerable uncertainty in the estimate regarding migration conditions.
In product no.8,10,13 the content of phthalates in the product or parts of the product was above the allowed limit of 0.05 wt%.
For the products 9,12 , no possible risk of health effects was found and no phthalates with content above allowed limits was present.
3.4.1.1 Alert report to the Danish Environmental Protection Agency during investigation
As it is forbidden to produce, import and sell toys and childcare articles for children aged 0-3 if the products contain more than 0,05 weight percent phthalates, the measured content above this level has been reported to the Danish EPA - referring to "Statutory order no. 151 of March 15. 1999. Banning phthalates in toys for children aged 0-3 and in certain childcare articles etc."
The following has been reported:
The content of DINP:
144 mg/g (14,4%) in the product 8A (pillow for nursing).
3.8 mg/g (0,38%) in the product 8B (foam part of pillow for nursing).
The content of DIBP:
0.76 mg/g (0,076%) in product 10E (Mark on a carrying sling).
The content of DINP+DIDeP:
220 mg/g (22%) in product 8C (Plastic underlayer of a pillow for nursing).
The content of Diundecylphthalat:
4.4 mg/g (0,44%) in product 13C (white foam part of a mattress).
Only the pillow for nursing was assessed as an infringement. The Danish EPA has handled the case and the nursing pillow is no longer on the Danish marked.
4 Environment assessment
- 4.1.1 Hexabromocyclododecane (HBCD)
- 4.1.2 Toluene 2,4-diisocyanate (TDI)
- 4.1.3 2-Ethylhexanoic acid (2-EHA)
- 4.1.4 Acetophenone
- 4.1.5 Formaldehyde
- 4.1.6 1,1,2,2-Tetrachloroethane
- 4.1.7 Styrene
- 4.1.8 2-Bromo-4,6-dinitro-benzenamine (BDNA)
- 4.1.9 Hexaethylene glycol dimethyl ether
- 4.1.10 Tetrapropylene glycol monomethyl ether
- 4.2 Overall Assessment
4.1.1 Hexabromocyclododecane (HBCD)
Hexabromocyclododecane, CAS no. 25637-99-4, is not classified regarding to the environment. The proposed classification for the environment is N;R50/53 (EC Draft RAR 2003). This means that the substance is Dangerous to the environment, very toxic to aquatic organisms and may cause long-term adverse effects in the aquatic environment.
Due to the use of HBCD and occurrence in products the environment will still be exposed through different productions and products.
Hexabromocyclododecane (HBCD) is a brominated flame retardant that is extensively used especially in Europe. Additive flame retardants as HBCD, contrary to reactive flame retardants, are not chemically incorporated in the material. This implies that they rather easily can leak out of the material during the entire lifetime of the product, causing a diffuse contamination to the environment (Hutzinger and Thoma, 1987, Alaee and Wenning 2002; Remberger et al. 2004).
HBCD has been found in environmental samples from different parts of the world, e.g. Japan (water, sediment and fish samples collected in 1987; Watanabe and Sakai, 2003), UK and the Netherlands (river sediment and sewage sludge; EC Draft RAR 2003) and Norway (cod; EC Draft RAR 2003).
In Sweden, high levels of HBCD was found in river sediment and fish (pike) collected in 1995 (Sellström et al., 1998).
HBCD has also been detected in Swedish air (Bergander et al., 1995), municipal sewage sludge (Nylund et al., 2002), and recently also in eggs collected in 1987-1999 from the wild populations of peregrine falcon (Falco peregrinus) breeding in Sweden (Lindberg et al., 2004).
Furthermore, the substance was found in a variety of samples, including air, water, sediment and fish collected both close to point sources and in remote regions in the Swedish environment (Remberger et al., 2004).
As is the case for many other persistent organic pollutants, the organisms in the Baltic Sea seem to be particularly at risk for HBCD exposure. The substance has been found in guillemot (Uria aalge) eggs collected at Stora Karlsö in the Baltic Sea proper from year 1969 to 2001, with concentrations approximately doubled during the study period (Sellström et al., 2003).
More so, HBCD was the most abundant BRF in Baltic Sea herring (Clupea harengus), salmon (Salmo salar) and guillemot sampled in year 2000, while the levels in grey seal (Halichoerus grypu) muscle were at least as high as those of the major PBDE congener (BDE-47) (EC Draft RAR 2003).
HBCD is a prioritised substance within the Programme of Existing Substances in the EU and the EU risk assessment draft is currently being prepared by the Swedish Chemical Inspectorate (EC Draft RAR 2003).
HBCD is lipophilic, with a water solubility of 3.4 µg/l and log Kow 5.6 (EC Draft RAR 2003).
Since HBCD is not readily biodegradable and has a high affinity to accumulate in biota (the bioconcentration factor for fish is determined to 18 100) (EC Draft RAR 2003) it is potentially harmful in the environment.
Ecotoxicity data is available for a few species of phyto- and zooplankton in single-species tests (EC Draft RAR 2003). The concentration resulting in a 50% population growth reduction (EC50) in three marine microalgae was 9.3 µg/l for Skeletonema costatum, 50 µg/l for Thalassiosira pseudonana, while Chlorella sp. was not inhibited to 50% by as much as 1.5 mg/l of HBCD (Walsh et al., 1987).
In an acute toxicity test for Daphnia magna the no observed effect concentration (NOEC) was determined to1 mg/l (EC Draft RAR 2003).
However, in a life-cycle toxicity test with D. magna (21d), the toxicity of the substance was much higher, with LOEC (lowest observed effect concentration) determined to 5.6 µg/l (Drottar and Krueger, 1998).
This kind of single species toxicity tests on plankton can give an idea of the range of concentrations where direct toxic effect may occur for specific aquatic organisms.
A further step in the risk assessment, and in the understanding of the mechanisms behind change in the whole ecosystem, can be taken with model ecosystem experiments.
In enclosures, often referred to as micro- or mesocosms, assemblages of organism groups (plankton communities in the present study) coexist and interact at conditions similar to those in natural ecosystems.
There are many strong arguments in favour of model ecosystem studies, the most obvious being that they more closely represent the real world than single species laboratory tests do.
The direct toxic effect can be determined on several species simultaneously, which is of great importance since even closely related species can differ significantly in toxic response towards a specific substance (see e.g. the algal toxicity data for HBCD presented above).
In addition, secondary effects, i.e. the effect that direct toxicity on one species can have on connected species, can be assessed.
Theoretically, secondary indirect effects could be detected at lower concentrations than primary toxic effects.
A decrease in grazing capability by zooplankton due to direct toxic effect could, for instance, be detected as an increase in phytoplankton abundance at lower concentrations than what causes mortality (i.e. visible change) to the grazers.
4.1.1.1 Concluding remarks
A quite recent founding has concluded that HBCD has the potential to induce profound changes in the composition of natural plankton communities at low (ppb) concentrations (Pirzadeh, P. Gustafsson, K and Woin P. 2004).
Depending on the inherent properties, showing PBT risks, in combination with indications from recent independent research results (EC Draft RAR 2003, Gustafsson 2004), a general warning flag has to be hoist up.
There seems to be high risks for both the environment and human health on the long term scale, and therefore, in agreement with the precautionary principle the substance could be considered undesirable.
4.1.2 Toluene 2,4-diisocyanate (TDI)
Toluene 2,4-diisocyanate, CAS no. 584-84-9, is classified "R52/53" for the environment. This means that the substance is harmful to aquatic organisms and may cause long-term adverse effects in the aquatic environment.
Due to the use of Toluene 2,4-diisocyanate and occurrence in products the environment will still be exposed through different productions and use of products.
4.1.3 2-Ethylhexanoic acid (2-EHA)
2-Ethylhexanoic acid, CAS no. 149-57-5, is not classified regarding to the environment. 2-ethylhexanoic acid is marked in the N-CLASS database as N.C. (not classified as dangerous to the aquatic environment) assessed in 1995-96 but with the remark “no data found” (N-CLASS, 2005).
2-Ethylhexanoic acid is marked as WGK=1 (Weakly water polluting) in Germany (Iuclid, 2005).
As Log Kow is 2.64-2.81 and the substance is more soluble in organic solvents than in water it would be relevant to consider BCF values if they were available.
The available data on biodegradation is very limited and does not refer to the Standard OECD methods for ready biodegradability (Iuclid, 2005).
The test result show that the lowest L/EC50 is about 40 mg/l (Iuclid, 2005 and US EPA, 2005).
If 2-ethylhexanoic acid is not readily biodegradable, the substance would be in the range for R52/53.
Due to the use of 2-ethylhexanoic acid as a stabiliser for PVC products and occurrence as a rest product from the PU production the environment will still be exposed through different productions and uses.
4.1.4 Acetophenone
Acetophenone, CAS no. 98-86-2, is not classified regarding to the environment. Log Kow is 1.58 and L/EC50 is above 100 mg/l (US EPA, 2005). Acetophenone is marked in the N-CLASS database as N.C. (not classified as dangerous to the aquatic environment) (N-CLASS, 2005).
4.1.5 Formaldehyde
Formaldehyde, CAS no. 50-00-0 is not classified regarding to the environment. Formaldehyde is marked in the N-CLASS database as N.C. (not classified as dangerous to the aquatic environment) (N-CLASS, 2005).
4.1.6 1,1,2,2-Tetrachloroethane
1,1,2,2-Tetrachloroethane, CAS no. 79-34-5, is classified "N;R51/53" for the environment. This means that the substance is toxic to aquatic organisms and may cause long-term adverse effects in the aquatic environment.
The use of tetrachloroethane is decreasing, but due to its wide use and occurrence in products the environment will still be exposed through different productions and products.
4.1.7 Styrene
Styrene, CAS no. 100-42-5, is currently not classified for the environment.
Data on the acute toxicity values for fish, daphnia and algae all lie between 1 and 10 mg/l, which is the range for R51. Styrene is readily biodegradable but has a log Kow value of approximately 3, so it may accumulate in organisms. The available data leave styrene on the borderline for classification, but it is concluded that styrene will not accumulate in aquatic organisms and that R53 is therefore not appropriate. The proposal is that styrene is not classified as dangerous to the environment (EU Risk Assessment Report, 2000. Styrene).
4.1.8 2-Bromo-4,6-dinitro-benzenamine (BDNA)
2-Bromo-4,6-dinitro-benzenamine, CAS no. 1817-73-8, is not classified regarding to the environment. Log Kow is 2.73. No data regarding environmental effects has been found for the substance.
BDNA is used as a chemical intermediate for azo dyestuff production. The environment will be exposed through productions and use of mainly textile products. In a sediment-water system the reduction of BDNA and formation of 3-bromo-5-nitro-1,2-diaminobenzene among others was reported (Weber, E.J., Rebecca, L.A., 1995).
The reduction of BDNA in natural sediments can result in subsequent release of potentially hazardous aromatic amines to the water column, which may affect the environment as well.
4.1.9 Hexaethylene glycol dimethyl ether
Hexaethylene glycol dimethyl ether, CAS no. 1072-40-8, is not classified regarding to the environment. No data regarding environmental effects has been found for the substance.
4.1.10 Tetrapropylene glycol monomethyl ether
Tetrapropylene glycol monomethyl ether, CAS no. 20324-34-9, is not classified regarding to the environment No data regarding environmental effects has been found for the substance.
4.2 Overall Assessment
The classification of the substances regarding the environmental part is given in the Table 4.1 below.
Table 4.1 Overview for environmental classification
| Substance | CAS No. | Classification |
| Hexabromocyclododecane (HBCD) | 25637-99-4 | N;R50-53 |
| Toluene 2,4-diisocyanate (TDI) | 584-84-9 | R52-53 |
| 2-Ethylhexanoic acid (2-EHA) | 149-57-5 | Lack of data (Possibly R52/53) |
| Acetophenone | 98-86-2 | N.C. |
| Formaldehyde | 50-00-0 | N.C. |
| 1,1,2,2-Tetrachloroethane | 79-34-5 | N;R51-53 |
| Styrene | 100-42-5 | N.C.* |
| 2-Bromo-4,6-dinitroaniline (BDNA) | 1817-73-8 | N;R51/53** (Possibly release of potentially hazardous aromatic amines) |
| Hexaethylene glycol dimethyl ether | 1072-40-8 | No data |
| Tetrapropylene glycol monomethyl ether | 20324-34-9 | No data |
N.C.: Not classified as dangerous to the aquatic environment (N-CLASS 2005).
*: Proposal (EU Risk Assessment Report 2000).
**: Proposal (Danish EPA, List of self classification 2005).
The three substances hexabromocyclododecane (HBCD), 1,1,2,2-tetrachloroethane and toluene 2,4-diisocyanate (TDI) are very toxic, toxic or harmful to aquatic organisms, respectively and may all cause long-term adverse effects in the aquatic environment. The discharge and exposure of these substances to the aquatic environment should therefore be reduced or prevented.
Furthermore the possible environmental effects of the substances 2-ethylhexanoic acid (2-EHA), 2-bromo-4,6-dinitroaniline (BDNA), hexaethylene glycol dimethyl ether and tetrapropylene glycol monomethyl ether is not known due to lack of data. The discharge and exposure of these substances to the aquatic environment should therefore be minimized until the possible environmental effects are known.
It may be assumed in general, that the direct waste water discharge of substances from the daily use and washing of these types of products, except for the washing cloths, is minor compared to other kinds of e.g. textiles, which are washed more regularly.
The washing cloths contain 2-EHA among other substances, which may be discharged to the waste water system through continued use of new cloths (see table 2.5).
As HBCD and the phthalates are substances with low vapour pressure and a high log Kow value, it is most likely that the majority of the content will remain in the products during the use time of their lifecycle unless the products come in contact with organic solvents or are heated to higher temperatures for longer periods. Nevertheless, the distribution of these substances to the environment will most likely proceed in small amounts as long as products contain these substances.
5 References
ACGIH (1991). Documentation of the Threshold Limit Values and Biological Exposure Indices. 6th Edition. Vol. 2. American Conference of Governmental Industrial Hygienists Inc., Cincinnati (OH), 1063-1066.
ACGIH, 2001, American Conference of Governmental Industrial Hygienists. Documentation of Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 2001. Cincinnati, OH. 2001.
ACGIH, 2003, American Conference of Governmental Industrial Hygienists. TLV's BEIs: Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 2003. Cincinnati, OH. 2003.
ACGIH, 2005 American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinatti, OH, 2005, p. 17.
Alaee M, Wenning RJ. 2002. The significance of brominated flame retardants in the environment: current understanding, issues and challenges. Chemosphere 46: 579-582.
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991.
American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinatti, OH, pp.22-56, (2005).
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984.
Arbejdstilsynet, 2005. Grænseværdier for stoffer og materialer. At-vejledning – C.0.1. Arbejdstilsynet, København, April 2005.
(Limit values for substances and materials. WEA guide - C.0.1. Danish Working Environment Authorities, Copenhagen, April 2005).
Ashford, R.D. Ashford's Dictionary of Industrial Chemicals. London, England: Wavelength Publications Ltd., 1994.
ATSDR (1996): Toxicological profile for 1,1,2,2-tetrachloroethane. US Department of Health and Human Services. Agency for Toxic Substances and Disease Registry, Georgia, USA.
Bayer, material Science, 2005. Online: http://www.pu.bayer.com/db/pu/pu_cms_internet.nsf/id/polyether-foams_en
Bergander L, Kierkegaard A, Sellström U, Wideqvist U, de Wit and C. Are brominated flame retardants present in ambient air? 1995; Poster, 6th Nordic Symposium on Organic Pollutants, Smygehuk, September 17-20, 1995.
Boublik, T., Fried, V., and Hala, E., 1984. The Vapour Pressures of Pure Substances. Second Revised Edition. Amsterdam: Elsevier, 1984., p. 697.
Brown-Woodman PD et al; Reprod Toxicol 8 (2): 121-35 (1994).
Budavari, S. (ed.). 1989. The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989.
Budavari, S. (ed.). 1996. The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck and Co., Inc., 1996.
C.0.1 (2005) At-vejledning: Grænseværdier for stoffer og materialer.
Carpenter, C.P., C.S. Weil and H.F. Smyth. Chronic oral toxicity of di(2-ethylhexyl)phthalate for rats and guinea pigs. Arch. Indust. Hyg. Occup. Med. 8: pp.219-226. (1953).
Chao J et al; J Phys Chem Ref Data 12: 1033-63 (1983).
Chopade HM and Matthews HB.1986: Disposition And Metabolism Of 2-Bromo-4,6-dinitroaniline In The Male F344 Rat. Journal of Toxicology and Environmental Health, 17: 37-50.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons., p. 2344, (1981-1982).
Clayton, G.D., F.E. Clayton (eds.) 1993-1994. Patty's Industrial Hygiene and Toxicology. Volumes 2A, 2B, 2C, 2D, 2E, 2F: Toxicology. 4th ed. New York, NY: John Wiley & Sons Inc., 1993.
Darnerud, P.O. 2003. Toxic effects of brominated flame retardants in man and in wildlife. Environmental International, 29, 841-853.
Daubert, T.E., R.P. Danner. Physical and Thermodynamic Properties of Pure Chemicals Data Compilation. Washington, D.C.: Taylor and Francis,., (1989).
Debruijin J et al; J Environ Toxicol Chem 8: 499-512 (1989).
Drottar, K. and Krueger, H. 1998. Hexabromocyclododecane(HBCD): Flow-through life-cycle toxicity test with the cladocerna (Daphnia magna). Project No.: 439A-108. Wildlife International, Ltd. Easton, MD, USA.
EC Draft RAR 2003. Draft Risk assessment report on Hexabromocyclododecane CAS# 25637-99-4, R044_0308_ENV_HH.DOC, European Chemicals Bureau, Ispra, Italy.
EHC 75. (1987). Toluene Diisocyanates. Environmental Health Criteria 75 The International Programme on Chemical Safety (IPCS) World Health Organization, Geneva.
Environment Canada; Tech Info for Problem Spills: Styrene (Draft) p.71 (1981).
Eriksson P, Viberg H, Fischer C, Wallin M and Fredriksson A. 2002. A comparison on developmental neurotoxic effects of hexabromocyclododecan, 2,2´,4,4´,5,5´-hexabromodiphenyl ether (PBDE 153) AND 2,2´,4,4´,5,5´-hexachlorobiphenyl (PCB 153). Abstract no 488. In DIOXIN 2002. 2002.
EPA/OTS, 1983. Doc #878213970: 6-Bromo-2,4-dinitroaniline determination of the approximate lethal oral dose (ALD) by the method of Deichmann and Leblan.
EU Risk Assessment Report, 2000. Styrene. Final Draft, Jan. 2000 (Environment Section only).
EU Risk Assessment Report, 2003. Risk-Assessment Report Vol.35 on: 1,2-Benzenedicarboxylic acid, di-C8-10-branched alkyl esters, C9-rich, CAS#: 68515-48-0, EINECS#: 271-090-9 and: di-"isononyl" phthalate (DINP), CAS#: 28553-12-0, EINECS#: 249-079-5. Publication: EUR 20784 EN. European Chemicals Bureau, Ispra, Italy .
Flick EW 1991. Industrial Solvents Handbook, 4th ed Noyes Data Corp, Park Ridge, NJ p. 690 (1991).
Gustafsson, K. (2004). Uptake and toxicity of brominated flame retardants and pesticides: Studies on littoral organisms and model communities. http://urn.kb.se/resolve?urn=urn:nbn:se:su:diva-158 (2004-09-01).
Hagan, E.C., W.H. Hansen, O.G. Fitzhugh, et al. 1967. Food flavorings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol. 5: 141-157.
Hansch C et al 1995. Exploring QSAR. Hydrophobic, Electronic, and Steric Constants. ACS Profess Ref Book. Heller SR (consult ed) Washington, DC: Amer Chem Soc p. 49 (1995).
Hansch C, Leo A, Hoekman D (1995): Exploring QSAR - Hydrophobic, Electronic, and Steric Constants. Washington, DC: American Chemical Society.
Hassauer M, Kalberlah F, Offmanns J, Schneider K (1993): Basisdaten Toxicologie für umweltrelevante Stoffe zur Gefahrenbeurteilung bei Atlasten. UBA Berichte 4/93. Umweltbundesamt, Berlin.
Hendrickx AG et al 1993. Fundam and Appl Toxicol 20 (2): 199-209 (1993).
HSDB #123. US National Library of Medicine's Hazardous Substance Database.2005. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.htm.
HSDB #6110. US National Library of Medicine's Hazardous Substance Database.2005. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.htm.
HSDB #874. US National Library of Medicine's Hazardous Substance Database.2005. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.htm.
HSDB #969. US National Library of Medicine's Hazardous Substance Database.2005. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?HSDB.htm.
HSDB 2003: Hazardous Substances Databank, a database of the National Library of Medicine´s TOXNET system (http://toxnet.nlm.nih.gov).
HSDB 2005, the National Library of Medicine's Hazardous Substance Database, 03/28/2005.
Hutzinger O and Thoma H. 1987. Polybrominated dibenzo-p-dioxins and dibenzofurans – The flame retardant issue. Meeting on chlorinated dioxins and related compounds held at the sixth international symposium, Fukuoka, Japan, september 16-19, 1986. Chemosphere 16 (8-9) : 1877-1888.
IARC 1979. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work), p. V19 310 (1979).
IARC 1986. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work), p. V39 302 (1986).
IARC 1986. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work), p. V39 303.
IARC 1986. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work), p. V39 302 (1986).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work), p. V62 336 (1995).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work), p. V77 p.41 (2000).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)V82 (2002) (p. 437).
IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work)V88 (2005) (in preparation).
IRIS. Database under TOXNET: http://toxnet.nlm.nih.gov/.
Iuclid, 2005. Database available online from European Chemicals Bureau: http://ecb.jrc.it.
Kortlægning af kemiske stoffer i ørepropper, Kortlægning af kemiske stoffer i forbrugerprodukter nr.28, 2003.
Kortlægning og afgivelse af kemiske stoffer i røgelse, Kortlægning af kemiske stoffer I forbrugerprodukter nr.39, 2004.
Kortlægning og sundhedsmæssig vurdering af kemiske stoffer i sexlegetøj.
Kortlægning af kemiske stoffer i forbrugerprodukter nr. 77, 2006.
Lewis, R.J. 1996. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996.
Lide, D.R. (ed.). CRC Handbook of Chemistry and Physics. 76th ed. Boca Raton, FL: CRC Press Inc., pp. 3-189, (1995-1996).
Lide, DR (ed.). CRC Handbook of Chemistry and Physics. 81st Edition. CRC Press LLC, Boca Raton: FL 2000, p. 3-46.
Lindberg, Peter; Sellstrom, Ulla; Haggberg, Lisbeth, et al.
Higher brominated diphenyl ethers and hexabromocyclododecane found in eggs of peregrine falcons (Falco peregrinus) breeding in Sweden.
Environmental Science & Technology 38 (1): 93-96 January 1, 2004.
Mariussen, E. and Fonnum, F. 2003. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochemistry International 43.
Miljøministeriet (2005): Bekendtgørelse om listen over farlige stoffer. Bekendtgørelse nr. 923 af 28. september 2005. Miljøministeriet, København.
Miljøstyrelsen, 2004: Kortlægning af kemiske stoffer i tandbørster. Kortlægning af kemiske stoffer i forbrugerprodukter nr. 42, 2004”. Udarbejdet af Teknologisk Institut for Miljøstyrelsen.
(Survey of chemical substances in toothbrushes. Survey of chemical substances in consumer products no. 42, 2004. Danish Environment Protection Agency, Copenhagen).
MSDS 584-84-9. Provided from http://www.chemexper.com/.
National Institute of Health, United States, 2005. Household Products Database. Online: http://hpd.nlm.nih.gov/cgibin/household/search?queryx=2615-15-8&tbl=TblChemicals&prodcat=all
N-CLASS, 2005. Online database on environmental classification. Data on acetophenone assessed in 1997. http://apps.kemi.se/nclass/SubstanceInfo.asp?id=28062
Newberne PM 1982.Assessment of the Hepatocarcinogenic Potential of Chemicals: Response of the Liver. In Toxicology of the Liver. Edited by Plaa G and Hewitt WR 1982; pp pp 265. Raven Press, New York.
NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 94-116. Washington, D.C.: U.S. Government Printing Office,., p. 118, (June 1994).
NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 148.
Nylund K, Haglund M, Berggren D, Kierkegaard A, Allan A, Asplund L, "WC and C. Bromerade flamskyddsmedel i avloppsslam - analyser från 50 reningsverk i Sverige. 2002; 5188, pp 44 pp. Naturvårdsverket, Stockholm.
Oxford University, 2005. Online: http://ptcl.chem.ox.ac.uk/MSDS/cas3.html.
http://ptcl.chem.ox.ac.uk/MSDS/HE/hexaethylene_glycol_monododecyl_ether.html.
Pickrell JA et al; Environ Sci Technol 17: 753-7 (1983).
Pirzadeh, P. Gustafsson, K. and Woin, P. 2004. Effects of the brominated flame retardant HBCDD on the structure of natural brackish water plankton communities. SETAC conference in Prague 2004, Abstract nr.: WEPO7/014.
Prager, J.C. 1996. Environmental Contaminant Reference Databook Volume 2. New York, NY: Van Nostrand Reinhold, 1996.
Remberger, M., Sternbeck, J., Palm, A., Kaj, L., Strömberg, K., Brorström-Lundén, E. 2004. The environmental occurrence of hexabromocyclododecane in Sweden. Chemosphere 54, p. 9-21.
Risk assessment on bis(2-ethylhexyl) phthalate, CAS-No.: 117-81-7, EINECS-No.: 204-211-0. Consolidated Final Report: September 2003.
CSTEE, Scientific committee on Toxicity, Ecotoxicity and Environment. Opinion on the risk assessment of Cas-No.; 28553-12-0 from 27th CSTEE meeting on 30 October 2001.
Srivastava S et al; J Environ Biol 11 (1): 73-7 (1990).
Sellström U, Bignert A, Kierkegaard A, Haggberg L, De Wit CA, Olsson M, Jansson B. 2003. Temporal trend studies on tetra-and pentabrominated diphenyl ethers and hexabromocyclododecane in guillemot egg from the Baltic Sea. Environmental Science & Technology 37: 5496-5501.
Sellström U, Kierkegaard A, de Wit C, Jansson B. 1998. Polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from a Swedish river. Environmental Toxicology and Chemistry 17: 1065.
Syracuse Research Corporation 2005. PhysProp estimation. http://www.syrres.com/esc/physdemo.htm.
TGD 2003. Technical Guidance Document on Risk Assessment. European Commission, ISBN 92-827-8011-2.
Thomsen KG (1990): Allergi- og overfølsomhedfremkaldende stoffer i arbejdsmiljøet. AMI raport nr. 34/1990. Arbejdsmiljøinstituttet, Arbejdstilsynet).
Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 525.
US EPA, 2005. Online Ecotox database. http://www.epa.gov/ecotox/.
Verreault, J. Gabrielsen, G.W. Chu, S. Muir, D.C.G. Andersen, M. Hamaed, A. and Letcher, R.J. 2005. Flame retardants and methoxylated and hydroxylated polybrominated diphenyl ethers in two norwegian arctic top predators: Glaucous Gull and Polar Bears. Environ. Sci. Technol. 39, 6021-6028.
Verschueren, K. Handbook of Environmental Data of Organic Chemicals. 2nd ed. New York, NY: Van Nostrand Reinhold Co., 1983., p. 105.
Verschueren, K. Handbook of Environmental Data on Organic Chemicals. Volumes 1-2. 4th ed. John Wiley & Sons. New York, NY . 2001, p. 1899.
Walsh, G.E., Yoder, M.J., McLaughlin, L.L. 1987. Responses of Marine Unicellular Algae to Brominated Organic Compounds in Six Growth Media. Ecotoxicology and Environmental Safety 14, 215-222.
Watanabe I, Sakai S. 2003. Environmental release and behavior of brominated flame retardants. Environment International 29: 665-682.
Weast, R.C. (ed.). Handbook of Chemistry and Physics. 60th ed. Boca Raton, Florida: CRC Press Inc., 1979., p. C-113.
Weber, E.J., Rebecca, L.A., 1995. Chemical- and Sediment-Mediated Reduction of Azo Dye Disperse Blue 79. Environ. Sci. Technol. 1995, 29, 1163-1170.
WHO; Environ Health Criteria 131: Diethylhexyl Phthalate pp.58-62 (1992).
Wolfe et al., Multigeneration reproduction toxicity study in rats (unaudited draft): Diethylhexylphtalate: Multigenerational reproductive assessment by continuous breeding when administered to Sprague-Dawley rats in the diet. TherImmune Research Corporation (Gaithersburg, Maryland), TRC Study No 7244-200. (2003).
Yalkowsky SH, Dannenfelser RM; AQUASOL dATAbASE of Aqueous Solubility. 5th ed. Tucson, AZ: Univ of Arizona, College of Pharmacy (1990).
Yalkowsky SH, Dannenfelser RM; Aquasol Database of Aqueous Solubility. Version 5. College of Pharmacy, Univ of Ariz - Tucson, AZ. PC Version (1992).
Version 1.0 March 2008, © Danish Environmental Protection Agency