Survey and health assessment of chemical substances in hobby products for children
4 Health Assessment
- 4.1 Introduction
- 4.2 Method
- 4.3 Selected substances
- 4.3.1 Aniline
- 4.3.2 p-chloroaniline
- 4.3.3 N-methylaniline
- 4.3.4 C.I. Pigment Red 3
- 4.3.5 N,N-Dimethylacetamide
- 4.3.6 BIS(2-Ethylhexyl) Adipate
- 4.3.7 P-Anisidine
- 4.3.8 2-Ethoxy Ethanol
- 4.3.9 Citral
- 4.3.10 2-Ethylhexyl acrylate
- 4.3.11 Other substances: Formaldehyde
- 4.3.12 Inhalation
- 4.3.13 Overall conclusion on the health risk assessment
4.1 Introduction
This chapter assesses the potential health effects of the identified substances. The assessment focuses on children of nursery and school age.
Information is available for each of the quantified substances on their identity and their chemical and physical properties. The data comprises structure, melting point, boiling point, density, vapour pressure and solubility.
The available literature in the field has been reviewed and focus has been put on ability to absorption via the skin and effects of oral intake. The most important test results and effects are presented. The object has been to find data on NOAEL/LOAEL (No or Low Observed Adverse Effect Levels) for the selected substances or other relevant available data.
Based on the NOAEL or similar data and the amount of substance the child is exposed to, the safety margin can be calculated (MOS), enabling us to assess whether the substance has a potential adverse health effect when using the tested products.
4.2 Method
4.2.1 Exposure paths
At the preliminary health screening hazardous substances were found in marker pens, gel pens, glitter glue, and acrylic paint, but not in shrink plastic.
It is assumed that the substances are absorbed in the body by oral intake via the mucous membranes in the mouth, when children are mouthing the objects or by penetration of the skin. Substances with a high vapour pressure may be absorbed through inhalation and via the lungs by e.g. evaporation from drawings etc.
4.2.2 Exposure scenarios
The absorbed amount will depend on the children’s use of the products.
The following is based on interviews with parents and known practice in nurseries/schools.
Regarding marker pens and gel pens it is well-known that children like to paint on their skin, to suck on the pens and even use the pens as lipstick. The paint may also be rubbed off from the drawings to the skin.
When using marker pens and gel pens a relatively limited amount of substances are slowly transferred to the paper.
Glitter glue may be applied to the skin, when the children squeeze out the glue or they touch their creations before the glue is dry (the glue dries up slowly). Children also tend to suck on objects, using them as lipsticks, etc. Larger areas of paper may rapidly be covered by glue and evaporation of the glue will occur.
Acrylic paint can be transferred directly to the skin partly during the creation process partly during the drying and indirectly orally, when children are sucking their fingers or the paint brushes. Relatively large areas will be covered by the paints and evaporation will occur.
There is no information in TGD (2003) regarding the transferred amount, therefore, we have set up some realistic exposure scenarios based on interviews with parents.
Exposure scenarios
Marker pens and gel pens
Skin contact
It is assumed that an area corresponding to two child palms of 5*5 cm (50 cm²) is painted, and in worst case this happens once a day. Substances are assumed to be absorbed according to log KOW. The amount being transferred to paper is determined by painting a square on paper, weighing the applied amount and calculating weight per exposure area (see Table 4.1). The amount is determined to 0.05 g for 50 cm².
Oral intake
It is assumed that the amount of oral intake corresponds to the amount absorbed via the skin (0.05 g/day).
Respiratory passages
The amount is assumed to be limited. For diffusible substances an estimate is made of the instant evaporation of all transferred substances to the local zone (1,5 m³) and to a typical children’s room with a volume of 18 m³. The values are compared to the occupational exposure limit; OEL.
Glitter glue
Skin contact
Larger amounts may be applied to the skin. As worst case it is assumed that children transfer 3 ml = 3 g to the skin, equivalent to the weight of a densely painted area of 50 cm² (see Table 4.1).
Oral intake
The worst case is assumed similar to the amount transferred by skin contact (3 g).
Inhalation
For diffusible substances an estimate was made of instant evaporation of all transferred substance to the local zone (1,5 m³) and to a typical children’s room with a volume of 18 m³. The values are compared to the threshold limit value (TLV).
Acrylic paint
Skin contact
The amount of acrylic paint applied to the skin is larger than for marker pens. As worst case is assumed an amount corresponding to a densely painted area of 50 cm² (see Table 4.1). The amount is determined to 1.25 g.
Oral intake
Worst case corresponding to the amount for skin contact. (1.25 g).
Inhalation
For volatile substances an estimate is made of instant evaporation of all transferred substance to the local zone (1,5 m³) and to a typical children’s room with a volume of 18 m³. The values are compared to occupational exposure limit; OEL.
Table 4.1 Results of tests with densely colouring/painting of 100 cm² paper
| No. | Type | Weight (g/100 cm²) |
| 17 | Marker pen | 0.108 |
| 45 | Market pen | 0.134 |
| 4 | Gel pen | 0.195 |
| 54 | Acrylic paint | 2.760 |
| 55 | Acrylic paint | 3.059 |
| 61 | Acrylic paint | 1.785 |
| 26 | Glitter glue | 5.550 |
| 29 | Glitter glue | 6.186 |
| g | Set value | |
| Marker pen | 0.121 | 0.1 |
| Gel pen | 0.195 | 0.2 |
| Acrylic paint | 2.535 | 2.5 |
| Glitter glue | 5.868 | 6 |
Weight
The weight of the exposed children is for worst case scenario determined to 15 kg, corresponding to a 3-year old child.
It is estimated that all children will get in contact with the products.
Exposure scenarios are defined according to EU’s Technical Guidance Document (TGD, 2003).
Intake of a substance through skin or orally is calculated as:
I = Q * M* F/BW
Where:
I Intake per day per kg body weight
Q Concentration of substance (mg substance/gram sample)
M Intake amount (gram per day)
F Fraction of substance absorbed
BW Body weight (kg)
If no data are available for skin absorption, 100% absorption is assumed (F = 1), if the substance log KOW is < 4, and 10 % absorption (F = 0,1), if log KOW is < -1 and log KOW > 4.
If no data are available for absorption through the mucous membranes in the mouth (orally), absorption is assumed to be 100 % i.e. F = 1.
Risk assessment
In the health risk assessment the calculated exposure, i.e. absorption shall be compared to NOAEL or similar values. As NOAEL is typically based on animal tests, the margin of safety (MOS) is calculated by dividing NOAEL in mg/kg b.w by the intake.
If the data for animal are based on a high quality chronic long-term study the safety margin in the risk assessment will typically be 10. The safety factors used for derivation of a NOAEL for humans are often based on animal tests with e.g. mice or rats. For instance a factor 10 is used for extrapolation between species (different species) and a factor 10 is used for protecting sensitive individuals within the species such as children. If the data are based on LOAEL or a subchronic study an additional safety factor is being added (typically 10). The total safety factor is the combined product of the individual safety factors.
In the assessment of health effects MOS is not used for sensitizing effects as they have no lower concentration limit.
4.3 Selected substances
The substances described in the following have been identified as being the most important in terms of health risk by use.
4.3.1 Aniline
4.3.1.1 Identity
| Name | Aniline |
| CAS-no. | 62-53-3 |
| EINECS-no. | 200-539-3 |
| Molecular formula | C6H7N |
| Substance composition | 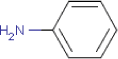 |
| Molecular weight (g/mol) | 93.13 |
| Synonyms | Benzenamine, phenylamine |
| Description | The substance is an oil liquid, colourless in its pure state. |
| Boiling point | 184.1 ºC (Lide, D.R., 1998-1999) |
| Melting point | -6 ºC (Lide, D.R., 1998-1999) |
| Solubility | 36,000 mg/l, 25 ºC, water (Yalkowski SH, 1992) Miscible with ethanol, ethyl ether, acetone (Lide, D.R, 1998-1999) |
| Distribution coefficient Log Kow |
0.9 (Hansch, 1995) |
| Vapour pressure | 0.49 mm Hg at 25 ºC (Daubert, T.E., 1989) |
| Odour | Aromatic amine-like odour (NIOSH, 1997) |
4.3.1.2 Detected amounts
The substance was detected in product no. 45 (green colour) in a concentration of 0.22 mg/gram (0.022 %) and in product no. 25 (pink) with 0.11 mg/kg.
4.3.1.3 Function
Aniline is used for synthesis of a number of chemicals, i.a. rubber accelerators, colorants, herbicides, pesticides, pharmaceutical substances (HDSB) and (EC 2004).
4.3.1.4 Classification and limit values
The substance is included in the List of Dangerous Substances (Miljøministeriet, 2005) and is classified as:
| T;R23/24/25 | Toxic by inhalation, skin contact and if swallowed |
| R48/23/24/25 | Serious health effects by long-term exposure through inhalation, skin contact and if swallowed |
| Carc 3;R40 | Potential carcinogenic effect |
| Xi;R41 | Risk of serious eye injury |
| R43 | Allergic contact dermatitis |
| Mut3;R68 | Risk of permanent health damage |
| N;R50 | Very toxic for aquatic organisms. |
The threshold limit value for occupational health and safety is 1 ppm, corresponding to 4 mg/m³ with an HK note, which means that the substance can be absorbed through the skin and is included in the list of carcinogenic substances (AT 2005).
The B-value, indicating the maximum concentration acceptable in the environment, is perhaps a better measure in this connection. The B-value is 0.08 mg/m³ (see B-value Guideline, EPA 2002).
4.3.1.5 Health effects
We have retrieved data regarding health effects in TOXNET and related databases. The substance has its own fact sheet in IUCLID and an EU risk assessment is worked out (EC 2004).
Acute toxicity
The substance is toxic.
Acute toxicity if swallowed based on animal tests indicate that the substance is on the verge of being toxic (LD50 rat close to 200 mg/kg):
- LD50 rat, oral 250 mg/kg (Lewis, R.J.,1996)
- LD50 dog 195 mg/kg (Lewis, R.J., 1996 )
- LD50 rat skin 1,400 mg/kg (Lewis, R.J.,1996)
- LC50 mouse inhalation 175 ppm, 7 hours (Lewis, R.J., 1996)
The substance oxidizes iron II to iron III in haemoglobin, thus forming methaemoglobin, whereby the oxygen transport in the blood is reduced.
More accidents have been reported about human exposure to aniline. Thus, an oral intake of 60 ml or 876 mg/kg is fatal (Janik-Kurylcio et al., 1973).
In the EU risk assessment(EU risk assessment, 2004) aniline is from an overall assessment of a number of data for animals and humans classified toxic with risk phrases R23,R24,R25 (see also comments about the methemoglobin forming effects of the substance group under p-anisidine).
Aniline is strongly irritating to eyes (Lewis, R.J., 1996).Aniline is easily absorbed orally, by skin contact and by inhalation. Data in the EU risk assessment (EU risk assessment, 2004) indicate a skin absorption of more than 38 %.
Subchronic toxicity
Aniline has a sensitizing effect demonstrated on hamsters (Goodwin et al., 1981) and in patch tests, where about 5-9 % reacted positively on aniline (Meneghini et al., 1963) and (Angelini et al., 1975).
Repeated exposure to aniline will have a hemotoxic effect.
In a 14-day inhalation test with rats a LOAEC of 17 ppm was demonstrated (EPA, 1981).
Chronic toxicity
A 103-week test with repeated addition of aniline in the rat feed showed hemotoxic effects at levels as low as LOAEL = 7 mg/kg/day (CIIT, 1982). The test also established tumours in 39 % of the rats at a dosage of 72 mg/kg/day, 1.1 % tumours at 22 mg/kg/day and 0 % at 7 mg/kg/day. In the tests a NOAEL of 21 mg/kg/day was calculated for development toxicity.
The values for the hemotoxic effect at repeated exposure is in (EU risk assessment, aniline, 2004) used for calculating a safety factor of 107 at dermal absorption of 7 mg/kg/day.
For an adult a critical exposure level is calculated to 7/107 = 0.065 mg/kg/day or 5 mg/person/day (70 kg/person).
Assuming 100 % absorption via the skin, orally and by inhalation a critical level has been calculated for inhalation of 0.5 mg/m³ for 8 hours light work with an air consumption of 10 m³ and a body weight of 70 kg.
The values for the detected tumours have been used for calculating a safety factor for carcinogenic effects in humans (EU risk assessment, 2004). A multistage model is used which indicates a risk level of 9.1·10-4 at 1 mg aniline/kg/day for rats. The model is linear at low concentrations.
Based on the model above, the risk level, where an acceptable low effect exists, (the critical level) has been preset to 1·10-4, equivalent to 0.11 mg/kg/day for rats. A factor of 10 is assumed for interpolation between species and further a correction for exposure time has been made. Thus an adult, who is exposed to the substance during working hours, will have a correction factor of (75 years * 52 weeks * 7 days)/(40 years * 48 weeks * 5 days) = 2.84. The critical exposure level for skin absorption is then 0.11/10 * 2.84 = 0.03 mg/kg/day or 2 mg/person/day.
Regarding development toxicity the (EU risk assessment, 2004) uses a safety factor of 10 for interpolation between species, by which a critical exposure level is calculated to 2.1 mg/kg/day.
Summary
A hemotoxic effect has been established in rats with a LOAEL = 7 mg/kg/day for aniline.
The substance may be carcinogenic (R40) and sensitizing.
At a risk level of 10-4 for carcinogenic effect the critical exposure level by skin absorption is 0.03 mg/kg/day.
4.3.1.6 Exposure scenarios
The maximum content in a marker pen is 0.22 mg per gram.
The exposed area is assumed to be 50 cm² and it is further assumed that the aniline is absorbed before it is washed off after e.g. 1 hour which is realistic with log KOW = 0.9. Tests have proven that the amount of ink transferred to 50 cm² is 0.05 gram. Afterwards the maximum intake is calculated on assumption of 100 % skin absorption.
Intake, skin = 0.22 mg/g * 0.05 g/15 kg = 0.00073 mg/kg b.w./day.
Oral intake is assumed to be the same whether the child is sucking his fingers or sucking on a pen.
4.3.1.7 Assessment
Based on the LOAEL for hemotoxic effect on rats a safety margin of MOS = 7/0.00073 = 9500 is found, which is almost 100 times above the safety factor of 107, which is stipulated in (EU risk assessment, 2004) for skin contact.
As to the carcinogenic effect the intake amount = 0.03/0.00073 = 40 times below the critical exposure level for carcinogenic effects with a risk factor of 10-4.
The concentration of aniline in a marker pen is 0.022 %. The ink from the marker pen will be applied to the skin in the same way as creams and other cosmetics. Compared to the Statutory Order (Statutory Order On Cosmetics Products, 2006), the substance should be declared on a product label (required for > 0.01 % for substances to be washed off and >0,001 % for substances which cannot be washed off). Aniline shall be labelled with R43, “Allergic contact dermatitis”, it is therefore assessed that if the marker pen comes in contact with skin it can cause risk of senbilisation.
4.3.2 p-chloroaniline
4.3.2.1 Identity
| Name | p-chloroaniline |
| CAS-no. | 106-47-8 |
| EINECS-no. | 203-401-0 |
| Molecular formula | C6H6ClN |
| Substance composition | 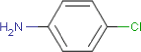 |
| Molecular weight | 127,57 g/mol |
| Synonyms | 4-chloraniline 1-amino-4-chlorobenzen p-chlorphenylamine |
| Description | Consists of colourless crystals |
| Boiling point | 232 ºC (O’Neil, M.J. (ed.), 2001) |
| Melting point | 72,5 ºC (O’Neil, M.J. (ed.), 2001) |
| Solubility | 3.900 mg/l, 25 ºC (Kilzer L et al; 1979). The substance is soluble in alcohol, ether, acetone, carbon disulphide (O’Neil, M.J. (ed.), 2001) |
| Distribution coefficient Log Kow | 1,83 (Hansch, C., Leo, A., D. Hoekman, 1995) |
| Vapour pressure | 0,071 mm Hg at 25 ºC (Daubert, T.E., R.P. Danner, 1989) |
| Odour | Vague, sweet characteristic amine odour (U.S. Coast Guard, Department of Transportation, 1984-5) |
4.3.2.2 Detected amounts
The substance was detected in 2 acrylic paint products (nos. 6 and 55). The amount is quantified in one product (no. 55) to 0.37 mg/g.
4.3.2.3 Function
The substances are used in the production of colorants, agrochemical (pesticides) and in medicine. The content is assumed to be remains from colorant production.
4.3.2.4 Classification and limit values
The substance is included in the List of Dangerous Substances (EPA, 2005) and is classified as:
| T;R23/24/25 | Toxic by inhalation, skin contact and if swallowed Carc.cat.2;R45 Carcinogenic |
| R43 | Allergic contact dermatitis |
| N;R50-53 | Very toxic for aquatic organisms May cause undesired long term effects to the water environment |
There is no limit value for occupational health and safety.
4.3.2.5 Health effects
Data regarding health effects have been retrieved in TOXNET and related databases.
Acute toxicity
The substance is toxic:
- LD50 rat, oral 200-480 mg/kg (IARC, 1972-present)
- LD50 mouse, oral 100 mg/kg (Lewis, R.J. 1996)
- LD50 cat, dermal 239 mg/kg (Lewis, R.J. 1996)
According to (CICAD, 2003) studies of rats, mice and cats have established that the substance is a stronger methaemoglobin former than aniline. The haemoglobin binding index is thus 569 at a concentration of 0.6 mmol/kg in rats against a factor 22 at 0.47 mmol aniline/kg.
Cyanosis and methemoglobinaemia have been reported for premature babies poisoned by p-chloroaniline in connection with an incubator. The incubator was equipped with a humidifier with chlorhexidine solution, which may decompose to p-chloroaniline when heated. A concentration of methaemoglobin between 6.5 and 45.5 % was found against a normal value below 2.3 % and a fatal concentration over 70 % (CICAD, 2003).
(See also comments on methemoglobin forming effect of the substance group under p-anisidine).
The substance is classified as an eye irritant (International Labour Office, ILO 1983).
Subchronic toxicity
Tests on guinea pigs show that p-chloroaniline can be classified as a skin sensitizing substance (CICAD, 2003).
Chronic toxicity
A 103-week test with repeated addition of aniline in the rat feed showed hemotoxic effects (increased methaemoglobin level, impact on number of reticulytes etc.) by all dosage levels including the lowest value of 2 mg/kg/day (CICAD, 2003).
The substance appeared to be carcinogenic in a number of tests. A large number of tumours were detected by a dosage of 18 mg/kg in 103 weeks (36 out of 50 male rats), by 6 mg/kg it is 3 out of 50, by 2 mg/kg 1 tumour and no one in controls (CICAD, 2003). Females are less responsive than males.
For mice a significant increase was observed in the number of hemangiosacoma at 0 (2 out of 20), 2.5 mg/kg (9 out of 50), and 5 mg/kg (14 out of 50) for male mice in 78-week test with 13 following weeks for observation. Same for hepatocellular carcinomas at 0 (3 out of 50), 3 mg/kg (7 out of 49), 10 mg/kg (11 out of 50) and 30 mg/kg (17 out of 50) in a 103-week feeding test (IARC, 1972- present).
A comparison of the two chronic 103-week rat tests indicates that the carcinogenic effect of p-chloroaniline is higher than aniline.
In (CICAD, 2003) it is stated that the available screening tests indicate a possible mutagenicity of p-chloroaniline.
A reference dose exists for chronic oral exposure of p-chloroaniline based on a 78-week feeding test with rats and a LOAEL of 12.5 mg/kg. A safety factor of 3000 gives an RfD of 0.004 mg/kg/day (IRIS, 1995).
Summary
Chronic data for hemotoxic effect of p-chloroaniline in rats gives LOAEL = 2 mg/kg/day.
RfD for chronic oral exposure is 0.004 mg/kg/day.
The substance has sensitizing effect.
The substance is carcinogenic (R45) in rats and mice. Carcinogenic effect in % level is observed already by 2 mg/kg/day.
4.3.2.6 Exposure scenarios
The maximum content in acrylic paint no. 55 is 0.37 mg per gram.
The exposed area is assumed to be 50 cm² and it is further assumed that all p-chloroaniline is absorbed before washing off after e.g. 1 hour which is realistic with log KOW = 1.8. Tests have proven that the amount of ink transferred to 50 cm² is 1.25 gram.
Intake, skin = 0.37 mg/g * 1.25 g/15 kg = 0.031 mg/kg b.w./day.
It is assumed that the max. oral intake by e.g. finger sucking or sucking a paintbrush is 1 ml or approx. 1 gram, corresponding to max. skin absorption.
4.3.2.7 Assessment
Based on the LOAEL for hemotoxic effect on rats the safety margin will be 2/0.031= 65.
The margin is rather low as it is based on LOAEL, therefore, apart from a factor 100, a factor 10 for extrapolation from LOAEL to NOAEL should be added.
This is confirmed by the RfD = 0.004 mg, which is by 8 times below the calculated intake.
Additionally, there may be carcinogenic effects, which are observed in rats and mice already by a dose of 2 mg/kg/day. These effects have no lower limit but decrease with degree of concentration. The critical exposure level for carcinogenic effects is expected to be the same - or even below the level for aniline, where the critical exposure level is 0.03 mg/kg at a risk level of 10-4. The level is exceeded in acrylic paint no. 55 as the absorbed amount is 0.031 mg/kg/day and there is thus a significant risk of a carcinogenic effect af the absorbed quantity of acrylic paint. Product no. 55 is no longer sold.
The paint will be applied to the skin in the same way as creams and other cosmetics. When compared to the Statutory Order (Statutory Order on Cosmetics Products, 2006), the substance should be labelled with declaration of contents (required for > 0.01 % for substances to be washed off and >0,001 % for substances which cannot be washed off). P-chloroaniline shall be labelled with R43, “Allergic contact dermatitis”, it is therefore assessed that if the marker pen comes in contact with skin it can cause risk of senbilisation.
4.3.3 N-methylaniline
4.3.3.1 Identity
| Name | N-methylaniline |
| CAS-no. | 100-61-8 |
| EINECS-no. | 202-870-9 |
| Molecular formula | C7H9N |
| Substance composition | 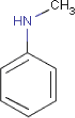 |
| Molecular weight | 107.15 g/mol |
| Synonyms | Methylphenylamine Aniline, N-methyl |
| Description | Colourless or faint yellow liquid |
| Boiling point | 196,25 ºC (Riddick, J.A. et al., 1985) |
| Melting point | -57 ºC (Riddick, J.A. et.al., 1985) |
| Solubility | 5.624 mg/l water at 25 ºC (Yalkowsky SH, Dannenfelser RM, 1992). Soluble in ethanol, ether and carbon tetrachloride (Lide, D.R. (ed.), 1994-1995) |
| Distribution coefficient Log Kow | 1.66 (Hansch, C. and A. Leo, 1987) |
| Vapour pressure | 0.453 mm Hg at 25 ºC (Daubert, T.E., R.P. Danner, 1989) |
| Odour | Vague ammonia-like odour (NIOSH, 1994) |
4.3.3.2 Detected amounts
The substance was detected in 4 marker pens in products nos. 10 and 45. 0.44 mg/g is quantified in no. 10, orange, and 0.99 mg/g in no. 45, green.
4.3.3.3 Function
The substance is used as chemical intermediate and solvent (HDSB).
4.3.3.4 Classification and limit values
The substance is included in the List of Dangerous Substances (EPA, 2005) and classified as:
| T;R23/24/25 | Toxic by inhalation, skin contact and if swallowed |
| R33 | May be accumulated in the body by repeated use |
| N;R50-53 | Very toxic for aquatic organisms; May cause long-term damage to the water environment. |
The threshold limit value for occupational health and safety is 0.5 ppm, equivalent to 2.25 mg/m³ with a note H, which means it can be absorbed through the skin.
4.3.3.5 Health effects
Data on health effects have been retrieved in TOXNET and related databases.
Acute toxicity
- LD50 rat, oral 951 mg/kg (National technical, 1982)
- LD50 rabbit, skin 1,77 mL/kg (American Industrial, 1962)
- LDlo, cat, intravenously 24 mg/kg (American conference, 1991)
The data for rats indicate that the substance is hazardous to health, but it is classified as toxic. Its tendency to form haemoglobin may, however, justify this classification. (Please also see comment under p-anisidine).
Human poisoning has not been reported, but the clinical toxilogical effect is expected to comparable with aniline poisoning, hereunder methaeglobinaemi with signs of cyanosis. Like aniline the substance oxidizes iron II to iron III in haemoglobin, thus forming methaemoglobin, whereby the oxygen transport in the blood is reduced (American conference, 1991).
Subchronic toxicity
Data covering this substance are sparse. Based on its chemical structure the effects may be expected to be in-between those of N,N-dimethylaniline and aniline.
Data for N,N-dimethylaniline (CAS-no.121-69-5) from a 13-week feeding test with rats (10 males, 10 females) indicate a LOAEL of 31 mg/kg. Similar tests have been made on mice, indicating NOAEL=32 mg/kg (IUCLID dataset N,N-dimetylaniline, 2000).
Chronic toxicity
Data are sparse. Based on its chemical structure the effects may be expected to be in-between those of N,N-dimethylaniline and aniline.
A 2-year study has been carried out for N,N-dimethylaniline in rats with doses up to 30 mg/kg. A positive trend was observed for cancer cells in male rat spleens and it was noted that both rats and mice would be able to resist much higher doses.
As the toxilogical properties of N-methylaniline are estimated to be in-between aniline and N,N-dimethylaniline, it may have a potential carcinogenic effect.
Summary
The substance has hemotoxic effects. No data are available on the substance but we know that the LOAEL for rats is 31 mg/kg/day for N,N-dimethylanilind and 7 mg/kg/day for aniline.
LOAEL for N-methyl-aniline is estimated to be between these values and is thus preset to 15 mg/kg/day.
From the data for aniline (potential carcinogenic effect in humans (R40)), and N,N-dimethylaniline (carcinogenic effect in spleens in male rats), it is estimated that N-methylaniline may also have a carcinogenic effect.
4.3.3.6 Exposure scenarios
The maximum content in marker pen was 0.99 mg per gram.
The exposed area is assumed to be 50 cm² and it is further assumed that the aniline is absorbed before being washed off after e.g. 1 hour which is realistic with log KOW = 1.66. Tests have proven that the amount of ink transferred to 50 cm2 is determined to 0.05 g.
Intake, skin = 0.99 mg/g * 0.05 g/15 kg = 0.0033 mg/kg b.w./day.
It is assumed that the max. oral intake by e.g. finger sucking or sucking a marker will be the same.
4.3.3.7 Assessment
With an estimated LOAEL for hemotoxic effect of 15 mg/kg/day the MOS is calculated to 15/0.0033 = 4545, thus there will be no risk of hemotoxic effects.
The substance may be carcinogenic when comparing to the data from the related substances aniline and N,N-dimethylaniline.
4.3.4 C.I. Pigment Red 3
4.3.4.1 Identity
| Name | C.I. Pigment Red 3 |
| CAS-no. | 2425-85-6 |
| EINECS-no. | 219-372-2 |
| Molecular formula | C17H13N3O3 |
| Substance composition | 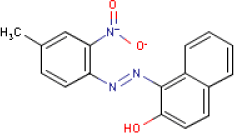 |
| Molecular weight | 307,33 g/mol |
| Synonyms | Toluidine red CI 12120 1-((4-Methyl-2-nitrophenyl)azo)-2-naphthalenol 2-Naphthalenol, 1-((4-methyl-2-nitrophenyl)azo)- |
| Description | Solid substance |
| Boiling point | Unknown |
| Melting point | 270-272 ºC (MSDS, 1996) |
| Solubility | Insoluble (Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason, 1976), estimated to 0.05 mg/l of (US-EPA 2003) |
| Distribution coefficient Log Kow | 6.45 estimated (US EPA, 2003) |
| Vapour pressure | Very small (solid substance) |
| Odour | Not known |
4.3.4.2 Detected amounts
The substance has been detected in product no. 54 in a concentration of 104 mg/g.
4.3.4.3 Function
The substance is a colorant.
4.3.4.4 Classification and limit values
This substance is not classified according to Directive 67/548/EEC, Annex I, and is thus not included in the List of Dangerous Substances (EPA 2005).
4.3.4.5 Health effects
Data on health effects have been found in TOXNET and related databases.
Acute toxicity
Results from feedings tests indicate a very low acute toxicity on rats and mice. LD50 for rats is thus expected to be significantly over the 6,500 mg/kg based on the subchronic data below.
Subchronic toxicity
A 2-week feeding test on rats and mice showed no deaths even with up to 100,000 ppm in the feed.
At a 13-week feeding test with 10 mice and 10 rats with up to 50,000 ppm of the substance no deaths were reported. On the assumption that a rat of 200 g eats 15 g feed, 100.000 ppm correspond to a LDlo of over 6,500 mg/kg.
Chronic toxicity
IARC has classified the substance under group 3, covering substances with inconclusive evidence of carcinogenic effect in humans and reduced effect in experimental animals (IARC, 1972-present).
In a 2-year feeding test on mice and rats some evidence of carcinogenic effects was seen (Toxicology, 1992). Thus there was a positive trend in the number of hepatocellular tumours in female rats (0 ppm: 0/50, 6.000 ppm: 0/50, 12,500 ppm: 1/50 og 25,000 ppm: 10/50).
Tubular tumours in the renal cortex showed positive trends (0 ppm: 0/50, 12500 ppm: 0/50, 25000 ppm: 0/50 og 50000 ppm: 6/50) for male mice just like follicular tumours in the thyroid glad with (0 ppm: 0/50, 12500 ppm: 0/49, 25000 ppm: 1/50 og 50000 ppm: 5/50).
The tests showed no indication of toxic effects.
The test also showed a weight loss of more than 10 % in rates with doses of 12500 and 25000 ppm and in mice with doses of 50000 ppm.
With a conversion factor of 15, the 12.500 ppm for rats correspond to a LOAEL of approx. 830 mg/kg, based on the assumption that a rat of 200 g eats 15 g per day.
Summary
Subchronic feeding tests with rats show that the substance with an LDlo of approx. 3,200 mg/kg is not very toxic.
There is some evidence of carcinogenic effect in rats and mice but not sufficient evidence to prove carcinogenic effect in humans. Carcinogenic effects in the range of 2 % of the experimental animals have been observed at approx. 830 mg/kg for rats, and further the weight loss was over 10 %.
4.3.4.6 Exposure scenarios
The maximum content in a sample is 104 mg per gram.
The exposed area is assumed to be 50 cm² and it is further assumed that 10% pigment Red 3 is absorbed before washing off after e.g. 1 hour which is realistic with log KOW = 6.5. Tests have proven that the amount of acrylic paint transferred to 50 cm2 is determined to 1.25 g. Subsequently, the maximum intake is calculated assuming 100 % oral absorption.
Intake, skin = 104 mg/g * 1.25 g/15 kg/10 = 0.87 mg/kg b.w./day.
It is assumed that the max. oral intake by e.g. finger sucking or sucking a paint brush is 1 ml or approx. 1 gram, corresponding to 10 times the maximum skin absorption.
Intake, oral = 104 mg/g * 1.25 g/15 kg = 8.7 mg/kg b.w./day.
4.3.4.7 Assessment
At a 2-year feeding tests with rats a weight loss of more than 10% was observed at a dose of 830 mg/kg. At the same concentration carcinogenic effects were observed in approx. 2% of the rats.
In the absence of NOAEL for effects, the lowest concentrations where such effects were observed were used.
Based on this, a MOS = 958 by skin absorption is calculated and MOS = 95 by oral intake.
It is estimated that there will be no risk of toxic effects by skin absorption, however, a minor risk by oral intake, as MOS = 100.
Potential carcinogenic risk would exist, if data from rats could be transferred to humans.
4.3.5 N,N-Dimethylacetamide
4.3.5.1 Identity
| Name | N,N-Dimethylacetamide |
| CAS-no. | 127-19-5 |
| EINECS-no. | 204-826-4 |
| Molecular formula | C4H9NO |
| Substance composition | 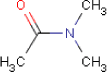 |
| Molecular weight | 87.12 g/mol |
| Synonyms | Acetamide, N,N-dimethyl- dimethylacetamide Acetic acid, dimethylamide |
| Description | Colourless liquid (NIOSH, 1997) |
| Boiling point | 163-165 ºC (Budavari, S. (ed.), 1996) |
| Melting point | -18.59 ºC (Lide, DR (ed.), 2000) |
| Solubility | Soluble in benzene, alcohol, acetone, ether (Lide, DR (ed.), 2000) |
| Distribution coefficient Log Kow | -0.77 (Hansch, C., Leo, A., D. Hoekman, 1995) |
| Vapour pressure | 2 mm Hg at 25 ºC (Daubert, T.E., R.P. Danner, 1989) |
| Odour | Weak ammonia-like or fishlike odour (NIOSH, 1997) |
4.3.5.2 Detected amounts
The substance was detected in 2 marker pens in the colours red and light green in product no. 17 in concentrations 0.22 and 0.4 mg/g.
4.3.5.3 Function
The substance is used as solvent in industrial applications (BASF 2006).
4.3.5.4 Classification and limit values
The substance is included in the List of Dangerous Substances (EPA 2005) and is classified as:
| Rep2;R61 | May harm the unborn child during pregnancy |
| Xn;R20/21 | Harmful by inhalation and skin contact |
The threshold limit value for occupational health and safety is 10 ppm, equivalent to 35 mg/m³ with a note H, which means that the substance can be absorbed through the skin.
B-value is 0.1 mg/m³ (EPA 2002).
4.3.5.5 Health effects
Data on health effects were retrieved in TOXNET and in related databases. The substance is found in IUCLID and is further described in (Survey no. 42, 2004).
Acute toxicity
Low acute toxicity:
- LD50 rat, oral 4.390 mg/kg (Prager, J.C., 1995)
- LD50 rabbit, dermal 2.240 mg/kg (Lewis, R.J, 1996)
- LC50 rat, inhalation, 1 time 2.475 ppm (Snyder R., 1990)
Subchronic toxicity
Data for oral dosing of the substance in pregnant rats show a NOEL for maternal toxicity of 65 mg/kg/day and shows also 65 mg/kg/day for teratogenic effect.
Chronic toxicity
At a 90-day feeding test a NOEL of 200 ppm (Kennedy, 1986) was determined based on liver effects.
In a 2-year inhalation study with rats exposed to the substance 5 days/week, 6 hours a day, changes occurred in absolute and relative liver weight, at 100 ppm various liver defects followed, but not by a dose of 25 ppm (0.09 mg/l), which is the NOAEL value for inhalation (IUCLID N,N-dimethylacetamide, 2000).
Summary
Liver effects were demonstrated at 2000 ppm.
By an assumed rat weight of 200 g and a feed consumption of 20 gram per day this corresponds to NOEL = 20 mg/kg/day.
From an inhalation study with NOAEL = 0.09 mg/l it is possible similarly to calculate a NOAEL = 0.8 * (6 * 60) * 0.09 = 26 mg/kg/day, by a respiration of 0.8 l/min/kg and 6 hours exposure/day.
4.3.5.6 Exposure scenarios
The maximum content in a marker pen was 0.4 mg per gram.
The exposed area is assumed to be 50 cm² and it is further assumed that N,N-dimetylacetamide is absorbed before the ink is washed off after e.g. 1 hour which is realistic with log KOW = 0.77. Based on tests, the amount of acrylic paint transferred to 50 cm2 is determined to 0.05 g.
Intake, skin = 0.4 mg/g * 0.05 g/15 kg = 0.00133 mg/kg k.v./day.
It is assumed that the max. oral intake by e.g. finger sucking or sucking a marker pen is the same.
4.3.5.7 Assessment
A NOEL of 20 mg/kg/day is set for liver effects in rats.
Based on this value the MOS is 20/0.00133 = 15000.
We have therefore assessed that there are no health effects in connection with exposure to N,N-dimethylacetamide in the stated amounts.
4.3.6 BIS(2-Ethylhexyl) Adipate
4.3.6.1 Identity
| Name | BIS(2-Ethylhexyl) Adipate |
| CAS-no. | 103-23-1 |
| EINECS-no. | 203-090-1 |
| Molecular formula | C22H42O4 |
Substance composition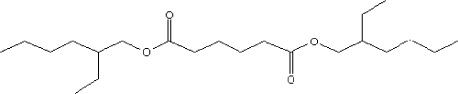 |
|
| Molecular weight | 370,57 (Lide, D.R. (ed.), 1998-1999) |
| Synonyms | |
| Description | Faintly coloured oily liquid (Lewis, R.J., Sr (Ed.), 1997) |
| Boiling point | 214 ºC (Lide, D.R. (ed.), 1998-1999) |
| Melting point | -67.8 ºC (Lide, D.R. (ed.), 1998-1999) |
| Solubility | Soluble in ethanol, ethyl ether, acetone and acetic acid (Lide, D.R. (ed.), 1998-1999) In water 0.78 mg/l at 22 ºC (Felder JD et al, 1986) |
| Distribution coefficient Log Kow | 8.1 (Verschueren, K, 1996) |
| Vapour pressure | 8.5 x 10-7 mm Hg at 20 ºC (Felder JD et al, 1986) |
| Odour | Vague aromatic odour (Lefaux, R, 1968) |
4.3.6.2 Detected amounts
The substance has been detected in product no. 10 in the colours purple and orange in concentrations of 0.32 and 0.35 mg/g.
4.3.6.3 Function
The substance is a plasticizer. The concentrations are rather low and therefore the plasticizer is not considered to have any technical function, its presence is probably due to a contamination either in connection with the production of the ink liquid or due to migration of the plastic in the marker pen.
4.3.6.4 Classification and limit values
The chemical substance is not in the List of Dangerous Substances (EPA, 2005), which means that it is not classified according to directive 67/548/EEC, Annex I.
4.3.6.5 Health effects
Data on health effects were retrieved in TOXNET and related databases. The substance is specified in IUCLID.
Acute toxicity
Very low level of acute toxicity:
- LD50 rat, oral 5.610-20.000 mg/kg (IUCLID dataset bis (2-ethylhexyl)adipate, 2000)
- LD50 mice, oral 15.000 mg/kg (IUCLID dataset bis (2-ethylhexyl)adipate, 2000)
- LD50 guinea pig, oral 12.900 mg/kg (IUCLID dataset bis (2-ethylhexyl)adipate, 2000)
The substance is classified as an eye irritant (U.S. Coast Guard, 1984-5).
Subchronic toxicity
In a 19-week one generation study with doses of 0, 28, 170 and 1080 mg/kg/day in 15 male and 30 female rats a reduction in body weight of progeny and increased liver weight of males and females were found with a calculated LOAEL of 1080 mg/kg/day and NOAEL = 170 mg/kg/day.
A 91-day study of rats and mice established reduced body weight when dosing 700 and 1500 mg/kg/day, but not at 400 mg/kg/day.
The tests have been used for estimating the RfD = 0.6 mg/kg/day with a factor 10 for both species uncertainty and for species variations, and a factor 3 for lack of reliable data from a multigeneration study (IRIS, Bis-2-ethyl hexyl) adipate, 1989).
A study with development toxicity demonstrated minor effects on skeletons at 170 mg/kg and 1080 mg/kg, but not at 28 mg/kg/day, where NOAEL for teratogenic effect is set to 28 mg/kg/day (IUCLID dataset bis (2-ethylhexyl) adipate 2000).
The mentioned data for development toxicity have been used for calculation of TDI (tolerable daily intake) of 0.3 mg/kg/day (OECD SIDS, 2000).
Chronic toxicity
A 2-year study with 50 male and 50 female rats and a similar number of mice with resp. 12.500 ppm and 25.000 ppm demonstrated statistically significant hepatocellular carcinoma and adenoma in female mice, these cannot however be related to effects in humans (DHHS/NTP, 1982).
IARC has classified the substance in group 3 (Cannot be classified with regard to carcinogenic effect in humans) (IARC, 1972-present).
Summary
The substance causes increased liver weight with an estimated NOAEL of 170 mg/kg in a subchronic study with rats.
A teratogenic effect has been established for rats with a LOAEL = 170 mg/kg/day and NOAEL = 28 mg/kg/day.
The substance cannot be classified with regard to carcinogenic effect in humans.
4.3.6.6 Exposure scenarios
The maximum content in a marker pen was 0.35 g per gram.
The area is assumed to be 50 cm² and it is further assumed that 10 % of bis (2-ethylhexyl) adipate absorbed before the ink is washed off, e.g. after 1 hour, based on the high value of log Kow= 8.1. Based on tests the amount of ink transferred to 50 cm² determined to be 0.05 gram. Based on this the maximum intake is calculated assuming 100 % oral absorption.
Intake, skin = 0,35 mg/g * 0.05 g/15 kg/10 = 0.00012 mg/kg b.w./day.
The oral intake is assumed to be by 10 times higher due to 100 % absorption e.g. when sucking fingers or on the marker pen.
4.3.6.7 Assessment
From the effect on liver weight of rats, the MOS is = 170/0.00012 = 1.45 millions by skin absorption and 145,000 by oral intake. It can thus be concluded that there are no health effects connected with intake of the substance, it should however be noted that possible carcinogenic effects in humans have not been investigated in detail.
4.3.7 P-Anisidine
4.3.7.1 Identity
| Name | P-Anisidine |
| CAS-no. | 104-94-9 |
| EINECS-no. | 203-254-2 |
| Molecular formula | C7H9NO |
| Substance composition | 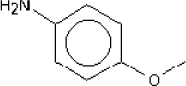 |
| Molecular weight | 123,15 g/mol |
| Synonyms | 4-anisidine Aniline, 4-methoxy- benzenamine, 4-methoxy- |
| Description | White crystals (Gerhartz, W. (exec ed.), 1985) |
| Boiling point | 243 ºC (Lide, DR (ed.), 2000) |
| Melting point | 57.2 ºC (Lide, DR (ed.), 2000) |
| Solubility | Soluble in benzene, solubility very good in ether and ethanol (Lide, DR (ed.), 2000) In water 21,000 mg/l 20 ºC (Verschueren, K., 2001) |
| Distribution coefficient Log Kow | 0.95 (Hansch, C., Leo, A., D. Hoekman, 1995) |
| Vapour pressure | 3.0 x 10-2 mm Hg at 20 ºC (Verschueren, K., 2001) |
| Odour | Amine-like odour (NIOSH, 1997) |
4.3.7.2 Detected amounts
The substance is detected in marker pen no. 57 in pink in a concentration of 0.12 mg/g. Further, the related substance N-methyl para-anisidine was present in a concentration of 0.36 mg/g.
4.3.7.3 Function
p-Anisidine is an intermediate used for production of colorants.
4.3.7.4 Classification and limit values
p-Anisidine is specified in the List of Dangerous Substances and is classified as:
| Tx;R26/27/28 | Very toxic by inhalation, skin contact and if swallowed |
| R33 | Accumulated in the body by repeated use |
| N;R50 | Very toxic for aquatic organisms |
For comparison the isomer o-anisidine (CAS-no. 90-04-0) is classified as:
| T;R23/24/25 | Toxic by inhalation, skin contact and if swallowed |
| Carc.Cat.2;R45 | May be carcinogenic |
| Mut.Cat.3;R68 | Risk of permanent damage to health |
and N-methyl p-anisidine (CAS-no. 5961-59-1):
| Mut3;R40 Carc.3;R40 | May be carcinogenic |
| R43 | Allergenic by skin contact |
The threshold limit value for occupational health and safety for p-anisidine is 0.1 ppm, equivalent to 0.5 mg/m³ with a note H, which means that the substance can be absorbed through the skin (AT 2005).
4.3.7.5 Health effects
Data on health effects were retrieved from TOXNET and other relevant databases. The substance is not listed in IUCLID, but EUCLID data exist for the related substance o-anisidine CAS-no. 90-04-0.
Acute toxicity
Data for p-anisidine specify:
- LD50 rat, oral 1400 mg/kg (Lewis, R.J. 1996)
- LD50 rat skin 3200 mg/kg (Lewis, R.J. 1996)
- LD50 mouse, oral 1300 mg/kg (Prosolenko, 1976)
Just like aniline the substances oxidizes iron II to iron III in haemoglobin, thus forming methaemoglobin, whereby the oxygen transport in the blood is reduced.
Workers which were exposed to 0.4 ppm for 3.5 hours per day for 6 months thus developed anaemia and were chronically poisoned. (American conference, 1991).
The acute toxicity for rats classifies p-anisidine as hazardous to health. Studies with cats establish that it develops anaemia by intravenous dosing. Thus the methaemoglobin went up from 1.1 to 11.5 % by an intravenous dosing of only 7.7 mg/kg for cats, whereas the level in mice rose from 0.66 to 4.8 % by one single dose. Therefore the substance is classified toxic with R23/R24/R25 in (EU risk evaluation o-anisidine 2002).
We have found no reason to classify p-anisidine as Tx (very toxic). A classification would be expected to be the same as for o-anisidine, but data on the substance are sparse.
Subchronic toxicity
The substance is mild sensitizing and may cause contact allergy (Lewis, R.J, 1996).
The substance was not mutagenic in S.typhimurium and there were no morphological changes in young guinea pig cells (IARC, 1972-present).
No relevant data found.
Data exist on the related substance o-anisidine, where a 28-day experiment with rats with daily doses of 0, 16, 80 and 400 mg/kg showed a NOAEL of 16 mg/kg/day, as the dosage of 80 mg/kg developed yellow urine and faint haemolytic anaemia.
A comparison with anilines shows a hemotoxic effect of 7 mg/kg against 2 mg/kg for p-chloro aniline. It is assumed that NOAEL for p-anisidine for hemotoxic effects is at the same level as o-anisidine, being 16 mg/kg/day.
Chronic toxicity
A 103-week test with rats and mice with 55 of each gender could not demonstrate a certain carcinogenic effect of the substance in concentrations of up to 0.6 % for rats and 1 % for mice. (DHEW/NCI, 1978).
It should be noted that the related substance CAS-no. 90-04-4 has been found carcinogenic in a 2-year study with rat and mice, with doses of 0, 5000 and 10,000 ppm (1 %) o-anisidine in the rat feed and 0, 2500, 5000 ppm in the mice feed. There was a strong statistical significance by doses higher than or equivalent to 5,000 ppm with finds in the bladder. Also in rats there was a significant increase of cancer cells in the liver at the highest concentration of LOAEL for rats of 256 mg/kg/day (EU risk evaluation o-anisidine, 2002).
Summary
The substance is hemotoxic with a NOAEL of 16 mg/kg/day based on the o-anisidine values.
No carcinogenic effect of p-anisidine was found, but the isomer o-anisidine is carcinogenic in rats at doses of 256 mg/kg/day and is classified with an R45. Another related substance N-methyl-p-anisidine is potential carcinogenic (R40).
4.3.7.6 Exposure scenarios
The maximum content in ink was 0.12 mg per gram.
The exposed area is assumed to be 50 cm² and it is further assumed that all anisidine is absorbed before washing off, e.g. after 1 hour, which is realistic with log KOW = 0.95. Based on tests, the amount of ink transferred to 50 cm² was determined to 0.05 gram.
Intake, skin = 0.12 mg/g * 0.05 g/15 kg = 0.0004 mg/kg b.w./day.
The oral intake is assumed to be same for finger sucking as for sucking on the pen.
4.3.7.7 Assessment
With a NOAEL for hemotoxic effect of 16 mg/kg/day for o-anisidine, MOS can be calculated to = 16/0.0004 = 40,000 thus it is assessed to have no hemotoxic effects.
The substance is estimated potential carcinogenic compared to data on the isomer o-anisidine and the related substance N-methyl-p-anisidine.
4.3.8 2-Ethoxy Ethanol
4.3.8.1 Identity
| Name | 2-Ethoxy ethanol |
| CAS-no. | 110-80-5 |
| EINECS-no. | 203-804-1 |
| Molecular formula | C4H10O2 |
| Substance composition | |
| Molecular weight | 90.12 g/mol |
| Synonyms | Glycol monoethyl ether Ethylene glycol monoethyl ether Ethanol, 2-ethoxy- |
| Description | Colourless liquid (NIOSH, 2001) |
| Boiling point | 135.6 ºC (Lewis, R.J., Sr (Ed.), 1997) |
| Melting point | -70 ºC (O’Neil, M.J. (ed.), 2001) |
| Solubility | Miscible in all ratios with acetone, benzene, tetrachloride, ethyl ether, methanol, and water (Flick, E.W. (ed.), 1991) |
| Distribution coefficient Log Kow |
-0.32 (Hansch, C., Leo, A., D. Hoekman, 1995) |
| Vapour pressure | 5.31 mm Hg at 25 ºC (Daubert, T.E., R.P. Danner, 1989) |
| Odour | Faint, pleasant ether-like odour (NIOSH, 2001) |
4.3.8.2 Detected amounts
The substance is detected in marker pen no. 25 in a pink and red colour in concentrations of 19 and 7.4 mg/gram.
4.3.8.3 Function
The substance is used as solvent in paint, ink and lacquer and also for increasing the stability of emulsions with colorants.
4.3.8.4 Classification and limit values
The substance is included in List of Dangerous Substances and (Miljøministeriet, 2005) classified as:
| R10 | Flammable |
| Rep. cat.2;R60 | May be harmful to reproduction |
| R61 | May harm the unborn child during pregnancy |
| Xn;R20/21/22 | Dangerous by inhalation, skin contact, and if swallowed |
The threshold limit value for occupational health and safety is 5 ppm, equivalent to 18.5 mg/m³ with a note H, which means that the substance can be absorbed through the skin (AT 2005).
B-value is 0.2 mg/m³ (Miljøstyrelsen 2002).
4.3.8.5 Health effects
Data on health effects have been retrieved in TOXNET and related databases. The substance is found in IUCLID. Description is additionally based on (Kortlægning no. 60, 2005).
Acute toxicity
The substance is hazardous to health according to the below values:
- LD50 rat, oral 1.746 mg/kg (IUCLID dataset 2-ethoxyethanol, 2000)
- LD50 mouse, oral 1.519 mg/kg (IUCLID dataset 2-ethoxyethanol, 2000)
- LD50 rabbit, dermal 3.300 mg/kg (IUCLID dataset 2-ethoxyethanol, 2000)
- LC50, 3 hours, rat, inhalation 19.700 mg/m³ (IUCLID dataset 2-ethoxyethanol, 2000)
Subchronic toxicity
In a 13-week study the substance was fed orally to dogs in concentrations of 0, 46, 93 and 186 mg/kg/day. Based on testicle oedema, reduction in haemoglobin concentration hematocrit values NOAEL was determined to 93 mg/kg/day (IUCLID dataset 2-ethoxyethanol, 2000).
In a 13-week inhalation study the rats were exposed to 0, 92, 380, 1485 mg/m³ for 6 hours/week, 5 hours a day. At the highest concentration we established reduction in the hypophysis weight of males and drop of the number of leucocytes in females; thus a NOAEL was determined to 103 ppm (380 mg/m³) (Barbee et al. 1984).
Additionally, there was a drop in fertility by 300 mg/kg/day for male rats (IUCLID dataset 2-ethoxyethanol, 2000) and drop in fertility in mice of both genders were observed at LOAEL = 1500 mg/kg/day and NOAEL = 750 mg/kg/day.
Chronic toxicity
No data found.
A reference value for chronic inhalation for humans was calculated on the 13-week inhalation test to be RfC = 0.2 mg/m³ (IRIS, 1991).
Summary
Data relevant for children are NOAEL = 93 mg/kg based on effect by oral dosing of dogs.
For rats a NOAEL= 380 mg/m³ was determined based on effects by inhalation.
On the assumption of 100 % absorption, a respiration of 0.8 l/min/kg, 6 hours daily exposure, the inhalation test corresponds to a NOAEL of 109 mg/kg/day, which is the same level as NOAEL for dogs.
4.3.8.6 Exposure scenarios
Max. Content in marker pen was 19 mg per gram.
The exposed area is assumed to be 50 cm², and it is further assumed that all 2-ethoxyethanol is absorbed before the ink is washed off, e.g. after 1 hour, which is realistic with a log Kow = -0.32. Based on tests the amount of ink transferred to 50 cm² is determined to 0.05 gram.
Intake, skin = 19 mg/g * 0.05 g/15 kg = 0.063 mg/kg b.w./day.
The oral intake is assumed to be the same whether the child is finger sucking or sucking on a marker pen.
4.3.8.7 Assessment
Based on NOAEL = 93 mg/kg/day for effects by oral dosing of dogs the safety margin is MOS = 1468. Being a subchronic study a safety factor of 1000 is appropriate. It is assessed that there are no health effects of 2-ethoxyethanol by the absorbed amounts.
4.3.9 Citral
4.3.9.1 Identity
| Name | Citral |
| CAS-no. | 5392-40-5 |
| EINECS-no. | 226-394-6 |
| Molecular formula | C10H16O |
| Substance composition | 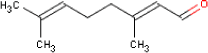 |
| Molecular weight | 152,23 g/mol |
| Synonyms | 2,6-Octadienal, 3,7-dimethyl- 3,7-Dimethyl-trans-2,6-octadienal |
| Description | The substance is liquid at room temperature |
| Boiling point | 226-228 ºC (Chemicals Inspection and Testing Institute; 1992) |
| Melting point | < -10 ºC (Chemicals Inspection and Testing Institute; 1992) |
| Solubility | In 5 volume parts 60 % alcohol in all ratio benzyl benzoate, diethyl phthalate, glycerol, propylene glucol, mineral oil, 95 % alcohol. (Lewis, R.J., Sr (Ed.), 1993) In water 1.34X10³ mg/l at 37 ºC (Yalkowsky SH, Dannenfelser RM, 1992) |
| Distribution coefficient Log Kow | 2,76 (IUCLID citral, 2000) |
| Vapour pressure | < 1hPa at 50 ºC (IUCLID citral, 2000) |
| Odour | Strong lemon like odour (Fenaroli’s Handbook of Flavor Ingredients, 1975) |
4.3.9.2 Detected amount
The substance is detected in marker pen no. 16 in orange and yellow colours at concentrations of 0.3 and 0.7 mg/gram.
4.3.9.3 Function
The substance is a basic material for production of pheromones and is used directly as pheromone at a level of 50 ppm (IUCLID citral, 2000).
4.3.9.4 Classification and limit values
The substance is specified in the List of Danish Substances (Miljøministeriet, 2005) and classified as:
| Xi;R38 | Irritating to skin |
| R43 | May be allergenic by skin contact. |
4.3.9.5 Health effects
Data on health effects were retrieved in TOXNET and related databases. The substance is included in IUCLID.
Acute toxicity
The substance is not toxic as it appears from below:
- LD50 rat, oral 4960 mg/kg (IUCLID Citral, 2000)
- LD50 mouse, oral 6000 mg/kg (IUCLID Citral, 2000)
- LD50 rabbit, skin 2250 mg/kg (IUCLID Citral, 2000)
- LD50 mouse, intravenous 460 mg/kg (IUCLID Citral, 2000)
Citral is a skin irritant (IUCLID Citral, 2000). In a test of 19 oily perfumes and 20 synthetic perfumes on 50 male volunteers it appeared to be the most skin irritant substance (Motoyoshi K et al, 1979).
Subchronic toxicity
The substance is sensitizing. In a patch test on 680 persons, 16 persons reacted positive equivalent to 2.3 % (IUCLID Citral, 2000).
In tests with repeated feeding of 5 male and 5 female rats five days per day during a fortnights with concentrations of 570, 1140, 2280 mg/kg only a minimal influence of epithelium cells was observed at the highest concentration. Based on this the NOAEL was determined to 1140 mg/kg/day (IUCLID Citral, 2000).
In another 13-week feeding test with rats no effects were observed even at the highest concentration of 833 mg/kg/day, of which NOAEL> = 833 mg/kg/day (IUCLID Citral, 2000).
A 46-week feeding test with rats demonstrated effects in the stomach at 1000 mg/kg/day, but not at 200 mg/kg/day. The NOAEL was thus determined to 200 mg/kg/day (OECD SIDS, 2001).
Test for mutagenicity (AMES test on salmonella and test on cells from guinea pigs) showed no sign hereof (IUCLID citral, 2000).
The developmental toxic effects of citral were analysed in tests with rats fed with citral and maize oil for a period of 6-15 days during pregnancy. The concentration varied between 60 mg/kg/day and 1000 mg/kg/day. NOAEL for toxic effect on the mother animal was 125 mg/kg/day. Developmental toxic effects were observed in all concentrations hereunder reduced weight increase and a higher level of minor abnormalities in the skeleton of foetus than observed in the control group. Based on the LOAEL for development toxicity is determined to 60 mg/kg/day. Teratogenic effects were not observed even at 1000 mg/kg/day. (IUCLID citral, 2000).
Chronic toxicity
No relevant data found.
Summary
Citral is irritant and sensitizing to skin.
Repeated feeding of rats shows effects at 1000 mg/kg/day with a NOAEL of 200 mg/kg/day.
For development toxicity the LOAEL = 60 mg/kg/day for rats, whereas the toxic effect for the mother animal is observed at 125 mg/kg/day, which has not been assessed relevant for children and juveniles.
4.3.9.6 Exposure scenarios
The maximum content in a marker pen was 0.7 mg per gram.
The exposed area is assumed to be 50 cm², and it is further assumed that citral is absorbed before the ink is washed off, e.g. after one hour, which is realistic with Log Kow = 2.76 and the low vapour pressure. The Amount of ink transferred to 50 cm² is determined to 0.05 gram.
Intake, skin = 0.7 mg/g * 0.05 g/15 kg = 0.00233 mg/kg b.w./day.
The oral intake is assumed to be same whether the child is finger sucking or sucking on a marker pen.
4.3.9.7 Assessment
With a NOAEL of 200 mg/kg and a MOS of 86,000 is calculated. It is assessed that there are no toxic effects for children and juveniles by the ingested concentration.
If expecting mothers (who are not the target of the assessment) were exposed in the same way, the MOS is 26000 for development toxicity in the foetus. This safety margin is sufficient to avoid developmental toxic effects.
The substance is highly allergenic with a concentration in a marker pen of 0.07 %. The ink will be in skin contact in the same ways as creams and other cosmetics. When comparing with (Statutory Order No. on Cosmetic Products, 2005), the product should be labelled with substance name (required for > 0.01 % for substances, to be washed off, and > 0.001 % for substances, which are not washed off). Citral shall be labelled with R43, “Allergic contact dermatitis”, it is therefore assessed that if the marker pen comes in contact with skin it can cause risk of senbilisation.
4.3.10 2-Ethylhexyl acrylate
4.3.10.1 Identity
| Name | 2-Ethylhexyl acrylate |
| CAS-no. | 103-11-7 |
| EINECS-no. | 203-080-7 |
| Molecular formula | C11H20O2 |
| Substance composition | 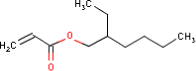 |
| Molecular weight | 184,28 |
| Synonyms | 2-Propenoic acid, 2-ethylhexyl ester Acrylic acid, 2-ethylhexyl ester |
| Description | Colourless liquid (Lide, DR (ed.), 2000) |
| Boiling point | 214-218 ºC (Lewis, R.J, 1997) |
| Melting point | -90 ºC (Lide DR, 2000) |
| Solubility | 100 mg/l, 25 ºC (Chemicals inspection, 1992) |
| Distribution coefficient Log Kow | 4.09 (US EPA,2003) |
| Vapour pressure | 0.178 mm Hg at 25 ºC (Daubert,1989) |
| Odour | Pleasant (Clayton,1993-94) |
4.3.10.2 Detected amounts
The substances were found in acrylic paint nos. 60 and 61. In product no. 61 a content of 0.35 mg/gram was quantified.
4.3.10.3 Function
The substance is used for production of plastics, coatings and waterbased paints (Lewis R.J, 1997). The detected concentrations are small and are presumed to be residues from the production.
4.3.10.4 Classification and limit values
The substance is specified in the List of Dangerous Substances (Miljøministeriet, 2005) and is classified as:
| Xi;R37/38 | Respiratory and skin irritation |
| R43 | May be allergenic by skin contact |
No Danish limit value for occupational health and safety exists for this substance.
B-value is 0.01 mg/m³ (Miljøstyrelsen 2002).
4.3.10.5 Health effects
Data on health effects were retrieved in TOXNET and related databases. The substance is specified in IUCLID. An EU Health Risk Assessment exist (EU:2005).
Acute toxicity
The substance is not acutely toxic:
- LD50 rat, oral 5600 mg/kg (IUCLID dataset, 2-ethylhexylacrylate, 2000)
- LD50 mouse, oral 4400 mg/kg (IUCLID dataset, 2-ethylhexylacrylate, 2000)
- LD50 rat, dermal 12000 mg/kg (IUCLID dataset, 2-ethylhexylacrylate, 2000)
- LD50 rabbit, dermal 7540 mg/kg (IUCLID dataset, 2-ethylhexylacrylate, 2000)
The substance is strongly irritant to skin (IUCLID dataset, 2-ethylhexylacrylate, 2000) and (Lewis, R.J., 1996).
Subchronic toxicity
The substance is strongly sensitizing in tests on guinea pigs. (EU Risk assessment 2-ethylhexyl acrylate, 2005). Further there are results of positive patch tests in humans – however in at limited number, e.g. 5 volunteers, who all reacted positive on 5 % substance in olive oil. A number of acrylates are generally known as allergenic.
From data of repeated inhalation of the substance in rats for 90 days a NOAEC was found of 10 ppm (0.075 mg/l) for local effects in the air pipe and a NOAEC of 30 ppm for systemic effects (lethargy, ptosis and reduced body weight).
A number of tests verified that the substance is not in vivo mutagenic.
In an inhalation test where pregnant rats from the 6th to 20th day of the pregnancy inhaled up to 100 ppm of the substance, a NOAEC for toxicity in the mother animal of 75 ppm (0,56 mg/l) was found. Development toxicity was not observed even at the highest concentration of 100 ppm.
In (EU Risk assessment 2-ethylhexyl acrylate, 2005) data from the inhalation study is used for calculation of NOAEL for systemic effects by skin exposure using the following data: weight rat = 250 g, inhalation 0.8 l/min/kg, daily exposure 6 hours. The result is a NOAEL of 66 mg/kg/day for oral intake or skin absorption. In (EU Risk assessment 2-ethylhexyl acrylate, 2005) the following safety factors are valid for humans: Subchronic to chronic: 2, species variation: 4 and a factor 3 for variation within the species, giving a safety factor of 24.
No long-term studies exist on carcinogenity by oral dosing. Test with application on the skin of mice resulted in tumours, which is ascribed to a non genotoxic mechanism originating from skin irritation with skin lesions.
Tests with the hydrolysis product acrylic acid, which has no oral carcinogenic effect, and the negative mutagenic test, the substance has been assed carcinogenic in guinea pigs (EU Risk assessment 2-ethylhexyl acrylate, 2005).
Chronic toxicity
No data found.
Summary
NOAEL for systemic effect is estimated to 66 mg/kg/day.
The substance is an irritant.
4.3.10.6 Exposure scenarios
Maximum content in a sample of acrylic paint is 0.35 mg per gram.
The exposed area is set to 50 cm² and it is assumed that 10 % of the 2-ethylhexyl acrylate is absorbed before the paint is washed off, e.g. after 1 hour, based on Log Kow= 4,09. The amount of acrylic paint transferred to 50 cm² is determined to 1.25 gram.
Intake, skin = 0.35 mg/g * 1.25 g/15 kg/10 = 0.00292 mg/kg b.w./day.
It is assumed that a maximum oral intake by finger sucking or sucking on brushes is 1 ml or approx. 1 gram, equivalent to approx. 10 * the maximum skin absorption or 0.0292 mg/kg/day.
4.3.10.7 Assessment
Based on NOAEL = 66 mg/kg/day for systemic effects in rats is calculated MOS = 66/0.00292 = 22628 by skin absorption and MOS = 66/0.0292 = 2263 by oral intake.
These values are more than 180 times higher than the safety factor of 24 from (EU Risk assessment 2-ethylhexyl acrylate, 2005). From this it is assessed that there are no health effects at the assumed doses.
2-Ethylhexyl acrylate shall be labelled with R43, “Allergic contact dermatitis”, it is therefore assessed that if the paint comes in contact with skin it can cause risk of senbilisation.
4.3.11 Other substances: Formaldehyde
Below data retrieved from (Kortlægning, babyprodukter)
| Name | Formaldehyde |
| CAS-no. | 50-00-0 |
| EINECS-no. | 200-001-8 |
| Molecular formula | CH2O |
| Substance composition | 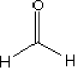 |
| Molecular weight | 30,03 |
| Synonyms | Formalin Methanal |
| Description | The substance is a gas |
| Boiling point | -19 ºC |
| Melting point | -92 ºC |
| Solubility | 40,000 mg/l, 25 ºC |
| Distribution coefficient Log Kow | 0.35 |
| Vapour pressure | 3890 mm Hg at 25 ºC |
4.3.11.1 Detected amounts
The substance is detected in 10 glitter glue products. Concentrations above 0.01 mg/gram were detected in products no. 15: 0.059, no. 26: 0.043, no. 29: 0.013, no.33: 0,063 and no.50: 0.111 mg/gram.
4.3.11.2 Classification and limit value
Formaldehyde is included in the List of Dangerous Substances and is classified as:
| Carc.3;R40 | Potential carcinogenic effect |
| T;R23/24/25 | Toxic by inhalation, skin contact and by if swallowed |
| C;R34 | May cause burns |
| R43 | May be allergenic by skin contact |
In concentrations of 1-5 % formaldehyde is classified Carc.3;R40 og R43, and in concentrations 0.2-1 % it is classified by an R43.
The Danish limit value for occupational health and safety for this substance is 0.4 mg/m³ with a note H for skin permeability and K for carcinogenic effect. The rated value for indoor climate is determined to 0.15 mg/m³ (Arbejdstilsynet, 2005 (Danish Working Environment Authority)), which is close to the threshold limit value recommended by WHO.
The B-value is 0.01 mg/m³ (Miljøstyrelsen 2002).
4.3.11.3 Health effects
Reference dose for chronic oral exposure, RfD, is 0.2 mg/kg/day. The value is based on a 2-year study with Wistar rats, which were given formaldehyde daily in the water. LOAEL for weight increase and histopathology was 82 mg/kg/day, where the NOAEL was 15 mg/kg/day. By an uncertainty factor of 100 for inter- and intraspecies difference, the RfD value would be 0.2 mg/kg/day.
4.3.11.4 Exposure scenarios
The maximum content in glitter glue is 0.11 mg per gram.
The exposed area is assumed to be 50 cm², and it is further assumed that 100 % formaldehyde is absorbed before the glue is washed off, e.g. after 1 hour based on Log Kow = 0,35. It should be mentioned that some formaldehyde will evaporate due to the high vapour pressure. Based on tests the amount of glitter glue transferred to 50 cm² is determined to 3 gram.
Intake, skin = 0.11 mg/g * 3 g/15 kg = 0.022 mg/kg b.w./day.
It is assumed that the maximum oral intake will be the same whether the child is sucking fingers or sucking on objects like glitter glue.
4.3.11.5 Assessment
Based on NOAEL = 15 mg/kg/day the safety margin can be calculated to MOS = 15/0,022 = 676 by skin absorption or by oral intake. This corresponds to 9 times below the critical reference dose. There is thus no health effect by intake of the assumed dose.
The substance will give an increased risk of carcinogenic and allergenic effect. Further it contributes to other known sources for formaldehyde in Danish homes, e.g. chipboards, electronic products etc.
4.3.12 Inhalation
To assess the evaporations into the indoor climate it is necessary to carry tests out in climate chambers, which has not been effected in this project.
To be able to assess the risk of inhalation of hazardous substances a number of worst case scenarios have been estimated, where it is assumed that all substances are evaporated instantaneously to a local area of 1.5 m³, which is assumed to be the child’s inhalation zone. Alternatively, the maximum substance concentration is estimated in a closed child’s room with recirculation of 3 * 3 * 2 m = 18 m³.
4.3.12.1 Formaldehyde
Formaldehyde is found in glitter glue, which is used in amounts of approx. 6 g/100 cm² in concentrations of up to 0.11 mg/g.
The substance has a very low occupational threshold limit value (TLV) of 0.4 mg/m³ and a recommended value for indoor climate of 0.15 mg/m³.
To obtain the TLV in a local area of 1.5 m³ the formaldehyde from 5.4 g glue must evaporate instantaneously, which is not realistic, as glitter glue dries up slowly.
In a closed child’s room of 18 m³, the formaldehyde concentration of 6 g glitter glue will be maximum reach 0.04 mg/m³, which is 10 times below the TLV and 4 times below the recommended indoor climate value.
Our assessment is that there are no health problems connected with inhalation of glue, but in very small closed rooms, the use of one or more tubes of glitter glue may lead to formaldehyde concentrations which contribute considerably to other formaldehyde sources. It is therefore recommended that the room is ventilated properly when working with large volumes of glitter glue.
4.3.12.2 2-ethoxyethanol
2-ethoxyethanol is found in marker pens, which is used in amounts up to 0.1 g/100 cm² in concentrations up to 19 mg/g.
The TLV of the substance is 18.5 mg/m³
To reach the limit value required for a local area of 1.5 m³ all the ethoxyethanol in 1.5 g ink shall evaporate instantaneously, which is not realistic at a vapour pressure of approx. 5 mm Hg.
In a closed child’s room of 18 m³ without air circulation the ethoxyethanol concentration on 4 densely painted A4 papers correspond to 2.4 gram ethoxyethanol. The concentration of the spread ethoxyethanol will maximum be 2.5 mg/m³ in the room which is 7 times below the TLV.
The result of the assessment is that there are no health problems connected with inhalation when using marker pens.
4.3.13 Overall conclusion on the health risk assessment
In the analysed products we have found a number of harmful substances. For 11 of these substances we have prepared exposure scenarios and health risk assessments based on scenario with two painted child palms.
The established health effects of the analysed substances appear from Table 4.2 and Fejl! Henvisningskilde ikke fundet..
Table 4.2 Effects of assessed substances
| Substance | Irritant and sensitizing effects | Reprotoxic effects | Carcinogenic effects | Mutagenic effects |
| Aniline | R41 risk of serious eye damage R43 sensitizing | Carc3;R40 Possible carcinogenic effect | Mut3;R68 Risk of permanent damage to health | |
| P-chloroaniline | R43 sensitizing | Carc.cat.2;R45 Carcinogenic | ||
| N-methyl aniline | Potential carcinogenic based on data for N,N-dimethylaniline and aniline | |||
| C.I.Pigment red 3 | Potential carcinogenic based on animal tests | |||
| N,N-dimethylacetamide | Rep.2;R61 harmful to unborn child | |||
| Bis(2-ethylhexyl)adipate | Teratogenic in rats | |||
| P-anisidine | Potential carcinogenic based on data for o-anisidine and N-methyl-p-anisidine | |||
| 2-Ethoxy ethanol | Rep.cat.2:R60 May be harmful to reproduction with R61 harmful to unborn child | |||
| Citral | R38 skin irritation R43 sensitizing |
|||
| 2-Ethylhexyl acrylate | R37/38 respiratory and skin irritation R43 sensitizing | |||
| Formaldehyde | R34 Causes burns R43 sensitizing |
Carc 3;R40 Potential carcinogenic effect |
There are also other substances in the products that have health effects, but a health assessment is not made. In the products investigated in the report there has been found:
- 12 allergenic substances
- 7 substances with potential or proven carcinogenic effect
- 3 substances with mutagenic effect
- 3 substances with reprotoxic effect
Table 4.3 shows the results of the assessment of their toxic effect.
Table 4.3 Toxic effects of selected substances
| Substance | Max.Intake mg per kg b.w. |
NOAEL mg/kg b.w. per day |
MOS (worst case) | RfD/max. Absorption | Comments |
| Aniline | 0,00073 | 7 | 9500 | No hemotoxic risk – risk of sensitizing and carcinogenic effects | |
| p-chloroaniline | 0,031 | 2 (LOAEL) | 65 | 0,004 | Risk of hemotoxic effect, RfD exceeded by 8 times. Carcinogenic and allergenic risk. |
| N-methyl aniline | 0,0033 | 15 (LOAEL) | 4545 | No hemotoxic risk, but risk of carcinogenic effects | |
| C.I. pigment red 3 | 8,7 | 830 (LOAEL) | 95 | No risk of toxic effects by skin absorption, minor risk by oral intake, potential risk of carcinogenic effects | |
| N,N-dimethylacetamide | 0,00133 | 20 | 15000 | No risk | |
| Bis(2-ethylhexyl)adipate | 0,0012 | 170 | 145000 | No risk, but carcinogenic effects not thoroughly investigated | |
| p-anisidine | 0,0004 | 16 | 40000 | No hemotoxic risk, but possible risk of carcinogenic effects | |
| 2-ethoxy ethanol | 19 | 93 | 1468 | No risk | |
| Citral | 0,0023 | 200 | 86000 | No risk of toxic effects but may be allergenic | |
| 2-ethylhexyl acrylate | 0,029 | 66 | 2263 | No risk of toxic effects but may be allergenic | |
| Formaldehyde | 0,022 | 15 | 676 | 0,2 | No risk of toxic effects but carcinogenic and allergenic risk |
Table 4.4 lists products with toxic effects and CMR or allergenic effects detected in the qualitative or the quantitative analyses.
Table 4.4 Toxilogical effects
| Product no. | Type | Toxic effect based on health assessment | Allergenes | Possible carcinogenic substances |
| 2 | Gel pen | Yes | ||
| 3 | Marker pen | Yes | ||
| 5 | Marker pen ³ | |||
| 6 | Acrylic paint | Possible hemotoxic effect see no.55 | Yes | Yes |
| 10 | Marker pen | Maybe ³ | ||
| 15 | Glitter glue | Yes 1 | Yes 1 | |
| 16 | Aroma pen | Yes (3 pheromones in the EU list 4, Plus another allergenic substance) | ||
| 17 | Marker pen 5 | |||
| 25 | Marker pen 5 | Yes | ||
| 26 | Glitter glue | Yes 1 | Yes 1, 2 | |
| 29 | Glitter glue | Yes 1 | Yes 1 | |
| 33 | Glitter glue | Yes 1 | Yes 1 | |
| 45 | Marker pen | Yes | Yes | |
| 46 | Marker pen | Yes | ||
| 50 | Glitter glue | Yes 1 | Yes 1 | |
| 54 | Acrylic paint | Minor risk by oral intake | Yes (contains azocolorant) | |
| 55 | Acrylic paint | Hemotoxic worst case- scenario exceeds limit value (RfD) by 8 times | Yes (2 substances) | Yes |
| 57 | Marker pen | Yes (2 substances) | Yes | |
| 58 | Marker pen | Yes (2 substances) | Yes | |
| 59 | Marker pen | Yes | ||
| 60 | Acrylic paint | Yes (1 substance) | ||
| 61 | Acrylic paint | Yes (1 substance) |
1 More than 0.01mg/gram formaldehyde
2 Contains also the mutagenic substance phenol
3 Contains phthalate plasticizer
4 More than 0.1 mg/gram allergenic pheromone
5 Contains reprotoxic substance
Hazardous substances were found in 10 marker pen products, 5 glitter glue products, 4 acrylic paint products and in one gel pen.
The analysed aroma pen contained the allergenic substances d-limonene, benzylalcohol and citral in concentration of between 0.01 and 0.1 weight percentage.
An estimate of maximum evaporation of formaldehyde in a small child’s room shows that the formaldehyde content in 6 gram (ca. 6 ml) glitter glue can maximum contribute with up to 25 % of the recommended indoor climate concentration in a room of 3 * 3 * 2 m. It is therefore not recommended to work with large amounts of glitter (several tubes) in small rooms with inferior air circulation.
Generally, the content of heavy metals in the analysed products is low. Thus no contents of lead over 21 ppm and no cadmium was found. The remaining heavy metals probably come from colorants, glitter particles or trace amounts of the substances in the colourants.
The following products contained neither CMR nor allergenic substances:
Acrylic paint: nos. 7, 8, 9, 18, 34, 35, 36, 40, 41, 48, 49, 51, and 56
Marker pen: nos. 1, 11, 12, 22, 30, 31, 32, 39, 42, 43, 47, 62, 63, and 64
Gel pen: nos. 4 and 44
Glitter glue: nos. 13, 14, 23, 28, 38 and 53 – however, formaldehyde was quantified with concentrations < 0.01 mg/g in nos. 13, 23, 28, 38, and 53
Version 1.0 May 2008, © Danish Environmental Protection Agency