Survey of Chemical Substances in Consumer Products, No. 94, 2008
Survey and health assessment of chemical substances in jewelleries
Contents
- 3.1 The market for jewelleries in Denmark
- 3.2 Purchase of metal jewelleries
- 3.3 Purchase of textile necklaces
- 3.4 Results from the screening
- 3.4.1 The uncertainty of the XRF screening
- 3.4.2 Test for a content of Arsenic and Barium
- 3.4.3 Content of heavy metal in the metal jewelleries
- 3.4.4 Content of heavy metal in relation to price/gram
- 3.4.5 Content of heavy metal in relation to the country of origin
- 3.4.6 Content of heavy metal in the different product types
- 3.4.7 Content of heavy metal in the different types of jewellery parts
- 3.4.8 Content of heavy metal in the overall product categories gold, silver and non-precious metal
- 3.4.9 Content of heavy metal in the specified product categories
- 3.4.10 Content of heavy metal in relation to shop type
- 4.1 Selection of metal jewellery for migration analysis
- 4.1.1 Focus on jewellery parts with low content of Pb and Cd
- 4.1.2 Even distribution of the overall categories gold, silver and non-precious metal
- 4.1.3 Even distribution in the specified product categories
- 4.1.4 Conditional distribution among types of jewellery parts and jewellery types.
- 4.1.5 Selected jewellery parts for migration test
- 4.2 Analysis method
- 4.3 Analysis results
5 Benzidine analysis of textile necklaces
- 5.1 Results of the screening
- 5.2 Selection of textile necklaces for benzidine analysis
- 5.3 Analysis method
- 5.4 Results of the analysis
6 Health assessment of lead, cadmium, copper and nickel
- 6.1 Lead
- 6.1.1 Occurrence and use
- 6.1.2 Identification
- 6.1.3 Physical and chemical properties
- 6.1.4 Oral absorption
- 6.1.5 Dermal absorption
- 6.1.6 Distribution
- 6.1.7 Acute toxicity
- 6.1.8 Local irritation and allergy
- 6.1.9 Long-lasting, repeated effect and gene-damaging effects
- 6.1.10 Tolerable daily intake - TDI
- 6.2 Cadmium
- 6.2.1 Occurrence and use
- 6.2.2 Identification
- 6.2.3 Physical and chemical properties
- 6.2.4 Oral absorption
- 6.2.5 Dermal absorption
- 6.2.6 Distribution
- 6.2.7 Acute toxicity
- 6.2.8 Local irritation and allergy
- 6.2.9 Long-lasting, repeated effect and gene-damaging effects
- 6.2.10 Tolerable daily intake - TDI
- 6.3 Copper
- 6.3.1 Occurrence and use
- 6.3.2 Identification
- 6.3.3 Physical and chemical properties
- 6.3.4 Oral absorption
- 6.3.5 Dermal absorption
- 6.3.6 Distribution
- 6.3.7 Local irritation and allergy
- 6.3.8 Acute toxicity
- 6.3.9 Long-lasting, repeated effect and gene-damaging effects
- 6.3.10 Tolerable daily intake - TDI
- 6.4 Nickel
- 6.4.1 Occurrence and use
- 6.4.2 Identification
- 6.4.3 Physical and chemical properties
- 6.4.4 Oral absorption
- 6.4.5 Dermal absorption
- 6.4.6 Distribution
- 6.4.7 Local irritation and allergy
- 6.4.8 Acute toxicity
- 6.4.9 Long-lasting, repeated effect and gene-damaging effects
- 6.4.10 Tolerable daily intake - TDI
7 Exposure scenarios and risk assessment
- 7.1 Assumptions and uncertainties related to the exposure calculations
- 7.2 Dermal exposure
- 7.3 Oral exposure
- 7.4 Total health risk by using jewelleries
Appendix A: Extracts from the database of jewelleries
Appendix B: Note on ”Heavy metal in precious metals jewelleries”
Appendix C: DST categorization of jewelleries
Appendix D: Amount of jewelleries on the market in Denmark
Appendix G: The requirements of the Toys Statutory Order on maximum release of heavy metals
Appendix H: Samples where duplicate determinations varied
Appendix I: Results from screening of textile necklaces
Appendix J: Results from the migration analysis calculated in relation to areal jewellery
Appendix K: Selected jewellery parts for migration test
Appendix L: Correlation between migration and content of metal
Preface
This project ”Survey and health assessment of chemical substances in jewelleries” is carried out by FORCE Technology for the Danish Environmental Protection Agency. The completed analyses are carried out by FORCE Technology, the Department for Chemical Analysis.
The purpose of the project was to map the Danish market for jewelleries and based on this, to select a number of jewelleries for screening for content of heavy metal as well as analysis for release of heavy metals. Furthermore, the purpose was to analyse a number of textile necklaces for content of benzidine, which is banned in the EU.
The project was completed over a period of eight months (April to November) in 2007.
A number of offences have been found in the project. All offences have been reported to the Danish Environmental Protection Agency.
The report has been written with the purpose of presenting the results of the health assessment of lead in an international context.
Summary and conclusion
Background and purpose
Jewelleries are available in countless variants, including a large number of metal jewelleries with and without a content of precious metal. These jewelleries can potentially contain and release problematic substances as for instance heavy metals. A number of examinations from abroad have proved a problem with the content of large amounts of lead in so-called cheap jewelleries. The seriousness of the problem is confirmed by a 4 year old boy’s death who by accident had swallowed a heart-shaped piece of jewellery containing above 99% lead. The incident lead to a voluntary recall of 300,000 pieces of the jewellery in question (Berg et al., 2006). Furthermore, it has turned out that the textile part of a necklace imported from Turkey contained the substance benzidine which is banned in the EU.
Thus, this project has had the purpose of providing an overview of whether metal jewelleries with a problematic content of heavy metals are available on the Danish market as well as whether this content can be released in an amount that causes health related problems for humans. The purpose was also to clarify whether there was a relation between among other things price (quality), country of origin and content/release of heavy metal. Another element in the project was to examine whether textile necklaces containing benzidine are available on the Danish market.
The survey
The project is carried out by FORCE Technology, the Department for Applied Environmental Assessment, who has been responsible for the survey, the selection of products for analysis as well as the health assessment. The analyses of the jewelleries have been completed by FORCE Technology, the Department for Chemical Analysis.
Metal jewelleries
The survey and purchase of jewelleries were completed in the period April to July 2007. In total 170 pieces of metal jewelleries were purchased, divided between the product types rings, necklaces, bracelets, earrings, piercing jewelleries and ankle chains. These product types were selected as they all are products getting into contact with the skin. All the purchases took place in the area of Copenhagen. The main part of the jewelleries was bought in the following types of shops: 10 kroner’s shops, shops with owners of different ethnic background, jewellery shops, department stores, supermarkets and clothes shops, while a small part was bought in internet shops.
All metal jewelleries were screened for a content of Pb, Hg, Cd, Se, Cr, Sb, As, Ba by use of a XRF device. As metal jewelleries often consist of different metal parts (with a different content of metal and thereby release) each piece of jewellery was screened on 3 different parts as a maximum. Thus, in total 318 jewellery parts were screened.
Based on the screening results 25 jewellery parts were selected for a migration test for release of As, Ba, Cd, Cr, Cu, Hg, Ni, Pb, Sb and Se. The used analysis method was “Migration to artificial sweat” according to DS/EN 1811:2000. The jewellery parts were selected in such a way that they covered the different product types (rings, necklaces, bracelets etc.) and the different product categories (silver-coated, golden-like, non-precious metal etc.) as broadly as possible; however, with the primary criterion that they represented a part of the piece of jewellery being in contact with the skin. As far as possible, the attempt was to select jewellery parts representing the entire concentration span related to the content of lead and cadmium; however, focus was primarily on the low part of the spectrum, i.e. for lead between 100 and 2000 ppm and for cadmium between 75 and 2000 ppm.
Textile necklaces
In total 62 textile necklaces were purchased. The main part of the textile necklaces was purchased at the same time as the metal jewelleries. During the entire purchasing phase focus was on purchasing textile necklaces of different types and colours. Furthermore it was attempted to buy textile necklaces “targeting” children as well as adults.
The 62 pieces of textile necklaces were screened for a content of azo-dye which through reduction may form aromatic amines as for instance benzidine. The screening consisted of lowering the textile necklaces into water (40 degrees) for a period of 4 hours. Coloured extracts in the water are an indication of a possible content of azo-dyes. Hereafter, 10 textile necklaces representing different colourings were selected for a quantitative analysis for a content of benzidine. The analysis method being used was EN 14364-1:2003.
Results and main conclusions
Conclusions from the screening
The most important conclusions from the screening of the metal jewelleries were:
- 58% of all examined jewelleries contained lead in a concentration above 100 ppm. The maximum content of lead in a jewellery part was 69.6 %.
- 24% of all examined jewelleries contained cadmium in a concentration above 75 ppm. The maximum content of cadmium in a jewellery part was 29.15%.
- 2% of all the examined jewelleries exceeded a content of 100 ppm of mercury.
- 25% of all examined jewelleries turned out to contain nickel in a volume percentage above 1. The maximum content of nickel in a jewellery part was 95%.
- Furthermore, a tendency was proved that especially charms, and particularly catches, contain lead and cadmium. Generally, there was no relation between the types of jewelleries (bracelets, earrings etc.) and the content of lead and cadmium.
- Based of the present data material it cannot be concluded whether jewelleries bought from a certain country have a larger probability of containing heavy metals. However, it can be mentioned that for the 37 jewelleries which are known to originate from China 30% has turned out to contain above 100 ppm lead while 24% has turned out to contain above 75 ppm of cadmium.
- No relation was found between shop type and purchase of jewelleries with a high content of heavy metals.
- There seems to be a greater chance of a large content of Pb in the cheaper metal jewelleries (0 – approx. 10 kr. per gram), while for Cd there does not seem to be a direct relation.
- The results did not indicate a relation between content of heavy metal and the product category (i.e. gold coated, silver-like, non-precious metal etc.). Thus, it cannot be assumed that jewelleries of for instance gold have a larger probability of containing lead or cadmium than jewelleries made of silver or non-precious metal.
- Furthermore, there was no relation between the three superior product categories (gold, silver and non-precious metal) and content of lead and cadmium.
Conclusions from the migration analysis
The jewelleries for the migration test were selected based on an even distribution among the selection criteria, i.e. content of heavy metal, coating, product type and jewellery part.
The results from the migration analysis for artificial sweat showed that lead, cadmium, nickel and copper migrated in a concentration above the detection limit.
Based on the test arrangement used in this project it is not possible to determine whether nickel migrates above 0.2 mg/cm²/week or 0.5 µg /cm2 /week in a period of at least two years at normal use; in other words, whether the products comply with the nickel statutory order.
The results from the migration analysis did not show any direct relation between migration and content of the metal, i.e. it cannot be assumed that a high concentration of the metal in the jewellery causes a high migration. However, it shall be noted that nearly no migration of lead has taken place for jewelleries where the content of lead is below approximately 1%. Similar tendency is not seen for the other metals.
As only relatively few migration analyses have been performed it is not possible to determine whether the coating (for instance gold coated or silver-like) has any influence on the migration.
It shall be noted that the result from the sweat test are used for the oral exposure calculations. However, the pH value in the sweat test is 1.5 higher than it would have been in a saliva test. This may indicate that a lesser amount of metal compounds are formed in the sweat test than what would have been formed in a saliva test. In other words this means that the oral exposure in this study is underestimated to a certain degree. However, it is assumed based on a study concerning formation of metal chlorides in seawater (Strandesen et al., 2007) that this difference (in formation of metal chlorides) is in the order of magnitude of 3-4% and therefore is not expected to have significant influence on the results.
Furthermore, it shall be noted that the exposure calculations are associated with some uncertainty as the calculations are based on the assumption that it is primarily inorganic metal compounds (especially metal chlorides) that are formed in sweat (and saliva) that are in contact with the jewelleries. In other words, oral and dermal absorption rates are primarily based on studies concerning inorganic metal compounds. This assumption is based on a study by Menné (1994) (quoted from ATSDR (2005a)) which claims that nickel alloys being in contact with the skin form nickel chlorides. The assumption is likewise based on the Danish Environmental Protection Agency’s risk assessment of nickel from 2005 in which they state that corrosion of metal in sweat primarily is dependent on the chloride and oxygen content.
Finally, it turned out that 2 of the 10 analysed textile necklaces had a content of benzidine of 100 and 1200 mg/kg respectively and thus had a content of benzidine exceeding the legally recognized amount.
Conclusions from the health and risk assessment
Health assessments for lead, cadmium, nickel and copper were performed and followed by exposure calculations for all samples showing migration above the detection limit.
Dermal exposure
Wearing the jewelleries examined in this project does not result in problems related to exposure of lead through the skin (though a number of jewelleries is close to exceed the TDI value). However, many of the examined jewelleries cause problems related to cadmium exposure through the skin while in a few cases there are also problems related to nickel exposure through the skin. The copper content in the jewelleries gave no cause for problems.
Average background exposure
The results show that for nearly all the jewelleries from which cadmium migrated in an amount above the detection limit there is a potential health risk for children (who are already exposed to an average background exposure) by wearing the jewelleries for 16 and 24 hours a day respectively. For two of the jewelleries the total tolerable daily dose is even exceeded by a factor 10. In other words, children wearing these jewelleries get 10 times the tolerable intake of cadmium a day. However, it shall be mentioned that the tolerable daily intake (TDI) states what a person may get daily through an entire lifetime without experiencing health related effects. Thus, for a shorter period the TDI value can be exceeded without this causing any effects - if in a corresponding period later in life an equally lesser amount is taken in. For adults the TDI value related to cadmium for two of the jewelleries is exceeded when the jewelleries are worn for 24 hours – and a single one is close to exceed the TDI value for nickel.
Maximum background exposure
When looking at the population group being exposed to the maximum background exposure the picture looks more serious. Here the results show that the 5% of Danish children being exposed to the maximum background exposure of cadmium already have exceeded the limit of tolerable daily intake of cadmium – without including the contribution from wearing jewelleries. For the 5% of adults being exposed to the maximum background exposure of cadmium the TDI value is not exceeded in advance but when the contribution from wearing the jewelleries is added it turns out that the TDI value is exceeded for four of the jewelleries when being worn for 24 hours.
Oral exposure
Furthermore, the results show that potential health risks are generated relating to cadmium, nickel and lead when a person sucks the jewelleries for two hours. However, most definitely for lead and cadmium as nearly all jewelleries (showing migration of the metal in question) exceed the TDI value related to lead and cadmium (both children and adults). This applies to humans being exposed to an average background exposure. None of the jewelleries gave cause to problems related to copper.
The results show that the lowest content of lead giving cause for health problems by sucking the jewelleries for two hours is a content of lead of 1.77%, i.e. somewhat above the requirements of the lead statutory order on a maximum content of 0.01% of lead. At the same time it is seen that none of the jewelleries containing a maximum of 100 ppm of lead caused health problems.
Furthermore, the calculations have shown that for a single piece of jewellery it turns out that children (being exposed to an average background exposure of lead) can suck the charm for one minute only before the daily tolerable intake is exceeded.
Overall conclusion
Based on the findings of this project it cannot be excluded that by wearing or sucking some of the metal jewelleries examined in this project potential health risks related to especially cadmium and nickel arise. This is valid for people exposed to the background exposure found in Denmark. For lead there are primarily problems when sucking the jewelleries. None of the examined jewelleries gave problems related to copper.
The health related risks associated with the three metals are effects on the kidneys (cadmium), increased mortality risk on foetuses (nickel) and reduced IQ at children (lead).
If the jewelleries comply with the legal demand related to content of lead, the results in this project indicate that no health related risks arise by wearing or sucking the jewelleries.
The jewelleries containing mercury were not examined further in the migration analysis. Therefore, it cannot be excluded that, as for lead and cadmium, there is a health risk by wearing or sucking the jewelleries.
However, there are a number of reservations being decisive whether a health problem will occur. Among other things, the jewelleries have to be worn/sucked every day for a long period as a short exceeding of the tolerable daily intake does not necessarily result in effects hazardous to health - unless an amount equal to the tolerable daily is taken in every day for the rest of the person’s life. In addition, it is assumed in the calculations that the migration of metals from the jewelleries is constant during time. This will decrease in the course of time.
Finally it is in the project concluded that there exist textile necklaces on the Danish market, which contain benzidine in amounts exceeding the allowable.
Sammenfatning og konklusioner
Baggrund og formål
Smykker findes i et utal af varianter, heriblandt en lang række metalsmykker med og uden indhold af ædelmetal. Disse smykker kan potentielt indeholde og afgive problematiske stoffer som f.eks. tungmetaller. En række undersøgelser fra udlandet har påvist et problem med indhold af store mængder bly i såkaldte billige smykker. Alvorligheden af problemet bekræftes af et nyligt dødsfald af en 4-årig dreng, som ved et uheld havde slugt et hjerteformet smykke, der indeholdt over 99% bly. Episoden ledte til en frivillig tilbagekaldelse af 300.000 eksemplarer af det nævnte smykke (Berg et al., 2006). Yderligere har det vist sig, at tekstildelen i en halskæde importeret fra Tyrkiet indeholdt det i EU forbudte stof benzidin
Dette projekt har således haft til formål at give et overblik over, hvorvidt der findes metalsmykker på det danske marked med et problematisk indhold af tungmetaller, samt hvorvidt dette indhold kan afgives i en koncentration, der giver anledning til sundhedsmæssige problemer for mennesker. Formålet var tillige at afklare, hvorvidt der var en sammenhæng mellem bl.a. pris (kvalitet), oprindelsesland og indhold/afgivelse af tungmetal. Et andet element i projektet var at undersøge, hvorvidt der eksisterer tekstilkæder på det danske marked indeholdende benzidin.
Undersøgelsen
Projektet er udført af FORCE Technology, Afdelingen for Anvendt Miljøvurdering, der har stået for kortlægningen, udvælgelsen af produkter til analyse, samt den sundhedsmæssige vurdering. Analyserne af produkternes indholdsstoffer er blevet foretaget FORCE Technology, Afdelingen for Kemisk Analyse.
Metalsmykker
Kortlægningen og indkøb af smykker blev gennemført i perioden april til juli 2007. Der blev i alt indkøbt 170 metalsmykker fordelt på produkttyperne ringe, halskæder, armbånd, øreringe, piercingsmykker og ankelkæder. Disse produkttyper blev valgt, idet de alle er produkter, der kommer i kontakt med huden. Alle indkøb blev foretaget i Københavnsområdet. Hovedparten af smykkerne blev købt i følgende butikstyper: 10 kr's butikstyper, butikker med ejere af anden etnisk baggrund, smykkebutikker, stormagasiner, supermarkeder og tøjbutikker, mens en mindre del blev købt i internetbutikker.
Samtlige metalsmykker blev screenet for indhold af Pb, Hg, Cd, Se, Cr, Sb, As, Ba ved brug af et XRF-apparat. Idet metalsmykker ofte består af forskellige metaldele (med forskelligt metalindhold og dermed afgivelse), blev hvert enkelt smykke screenet på maksimalt 3 forskellige dele. Der blev således i alt screenet 318 delsmykker.
På basis af screeningsresultaterne blev der udvalgt 25 delsmykker til migrationstest for afgivelse af As, Ba, Cd, Cr, Cu, Hg, Ni, Pb, Sb og Se. Analysemetoden, der blev anvendt, var "Migration til kunstig sved" efter DS/EN 1811:2000. Delsmykkerne blev udvalgt således, at de dækkede de forskellige produkttyper (ringe, halskæder, armbånd, mv.) og de forskellige produktkategorier (sølvbelagt, guldlignende, uægte metal, mv.) så bredt som muligt, dog med det primære kriterium, at de repræsenterede en del af smykket, som havde kontakt til huden. Det blev så vidt muligt forsøgt at udvælge smykkedele som repræsenterede hele koncentrationsspændet relateret til indhold af bly og cadmium, dog blev fokus lagt på den lave ende af spektret, dvs. for bly mellem 100 og 2000 ppm og for cadmium mellem 75 og 2000 ppm.
Tekstilkædesmykker
Der blev i alt indkøbt 62 tekstilkædesmykker. Hovedparten af tekstilkædesmykkerne blev indkøbt samtidig med metalsmykkerne. Der var gennem hele indkøbsfasen fokus på at indkøbe tekstilkædesmykker af forskellig art og farve, samt både til børn og voksne.
De 62 tekstilkædesmykker blev screenet for indhold af azofarvestof, der ved reduktion kan danne aromatiske aminer som bl.a. benzidin. Screeningen bestod i, at tekstilkæderne blev nedsænket i vand (40 grader) i 4 timer. Farvede ekstrakter i vandet er en indikation af muligt indhold af azofarvestoffer. Herefter blev 10 tekstilkæder, repræsenterende forskellige farveudslag, udvalgt til kvantitativ analyse for indhold af benzidin. Analysemetoden, der blev anvendt var EN 14364-1:2003.
Resultater og hovedkonklusioner
Konklusioner fra screeningen
De vigtigste konklusioner fra screeningen af metalsmykkerne var:
- 58 % af alle undersøgte smykker indeholdt bly i en koncentration på over 100 ppm. Det maksimale indhold af bly i et delsmykke var på 69,6 %.
- 24 % af alle undersøgte smykker indeholdt cadmium i en koncentration over 75 ppm. Det maksimale indhold af cadmium i et delsmykke var på 29,15 %.
- 2 % af de undersøgte smykker overskred et indhold på 75 ppm kviksølv.
- 25 % af alle undersøgte smykker viste sig at indeholde nikkel i en volumenprocent over 1. Det maksimale indhold af nikkel i et delsmykke var på 95 %.
- Herudover blev der påvist en tendens til at specielt vedhæng, og især låse, indeholder bly og cadmium. Generelt var der ingen sammenhæng mellem smykketyperne (armbånd, øreringe, osv.) og indhold af bly og cadmium.
- Det kan på grundlag af nærværende datamateriale ikke konkluderes om smykker indkøbt fra et bestemt land har større sandsynlighed for at indeholde tungmetaller. Dog kan nævnes, at for de 37 smykker, der vides at stamme fra Kina, har 30% vist sig at indeholde over 100 ppm bly, mens 24% har vist sig at indeholde over 75 ppm cadmium.
- Der blev ikke fundet nogen sammenhæng mellem butikstype og indkøb af smykker med et højt indhold af tungmetaller.
- Der lader til at være en større chance for et stort indhold af Pb i de billigere metalsmykker (0 - ca. 10 kr. pr. gram), mens der for Cd ikke ser ud til at være en umiddelbar sammenhæng.
- Der fandtes ingen sammenhæng mellem indhold af tungmetal og produktkategorien (dvs. guldbelagte, sølvlignende, uægte metalsmykker, mv.). Det kan således ikke antages, at smykker af f.eks. guld har større sandsynlighed for at indeholde bly eller cadmium end smykker af sølv eller uægte metal.
- Der var ligeledes heller ingen sammenhæng mellem de tre overordnede produktkategorier (guld, sølv og uægte metal) og indhold af bly og cadmium.
Konklusioner fra migrationsanalysen
Smykkerne til migrationstesten blev valgt ud fra en ligelig fordeling blandt udvælgelseskriterierne, dvs. indhold af tungmetal, belægning, produkttype samt delsmykketype.
Resultaterne fra migrationsanalysen til kunstig sved viste, at bly, cadmium, nikkel og kobber migrerede i en koncentration over detektionsgrænsen.
Det er ikke muligt ud fra den anvendte forsøgopstilling at afgøre om nikkel migrerer over 0,2 ?g/cm2/uge eller d 0,5 µg /cm2 /uge i en periode på mindst to år ved normal anvendelse. Med andre ord om produkterne overholder nikkelbekendgørelsen.
Resultaterne fra migrationsanalysen viste ingen umiddelbar sammenhæng mellem migration og indhold af metallet, dvs. man kan ikke antage, at en høj koncentration af metallet i smykket medfører en høj migration. Det skal dog bemærkes, at for smykker, hvor blyindholdet ligger under ca. 1 %, er der stort set ingen migration af bly fundet sted. Lignende tendens ses ikke for de andre metaller
Da der kun er udført relativt få migrationsanalyser er det ikke muligt at sige noget om, hvorvidt belægningen (f.eks. guldbelagt eller "sølvlignende") har indflydelse på migrationen.
Det skal bemærkes, at resultaterne fra svedtesten er anvendt til de orale eksponeringsberegninger. pH værdien i svedtesten er imidlertid 1,5 højere end den ville være i en spyttest, hvilket kan betyde, at der dannes lidt færre metalforbindelser i svedtesten end der ville være dannet i en spyttest. Dette betyder med andre ord, at den orale eksponering er undervurderet til en vis grad. Det antages dog, på baggrund af et studie vedrørende dannelse af metalchlorider i havvand (Strandesen et al., 2007), at denne forskel (i dannelse af metalchlorider) ligger i størrelsesordenen 3-4 % og derfor ikke forventes at have afgørende indflydelse på resultaterne.
Det skal ligeledes bemærkes, at eksponeringsberegningerne er behæftet med en vis usikkerhed, idet beregningerne er baseret på antagelsen om, at det primært er uorganiske metalforbindelser (især metalchlorider), der dannes i sved (og spyt), der har kontakt med smykkerne. Dvs. orale og dermale optagelsesrater er primært baseret på studier vedrørende uorganiske metalforbindelser. Denne antagelse er baseret på et studie af Menné (1994) citeret fra ATSDR (2005a), der hævder at nikkellegeringer, der er i kontakt med huden, danner nikkelchlorider, samt på Miljøstyrelsens risikovurdering af nikkel fra 2005, der hævder at korrosion af metal i sved primært er afhængig af chlorid- og oxygenindholdet.
Endelig viste det sig, at 2 af de 10 analyserede tekstilkæder havde et indhold af benzidin på henholdsvis 100 og 1200 mg/kg, og dermed havde et indhold af benzidin, der overskrider det tilladte.
Konklusioner fra sundheds- og risikovurderingen
Der blev udarbejdet en sundhedsvurdering for bly, cadmium, nikkel og kobber, hvorefter der blev lavet eksponeringsberegninger for samtlige prøver, der viste migration over detektionsgrænsen.
Dermal eksponering
Når man bærer smykkerne undersøgt i dette projekt, er der ingen af dem, der giver problemer relateret til blyeksponering via huden (dog ligger en del smykker tæt på at overskride TDI værdien). Imidlertid giver mange af de undersøgte smykker problemer relateret til cadmiumeksponering via huden, mens der i enkelte tilfælde også er problemer relateret til nikkeleksponering via huden. Kobberindholdet i smykkerne gav ikke anledning til problemer.
Gennemsnitlig baggrundseksponering
Resultaterne viser, at for stort set alle de smykker, hvorfra cadmium migrerede i en mængde over det målbare, er der en potentiel sundhedsrisiko for børn (som i forvejen er udsat for en gennemsnitlig baggrundseksponering) ved at bære smykkerne i hhv. 16 og 24 timer om dagen. For to af smykkerne overskrides den samlede tolerable daglige dosis endda med en faktor 10. Dvs. børn, der bærer disse smykker får 10 gange den tolerable dosis cadmium om dagen. Her skal det dog nævnes, at den daglige tolerable dosis (TDI) er et udtryk for hvad man dagligt må få gennem et helt livsforløb, uden at der opstår sundhedsskadelige effekter. Man kan således godt i en kortere periode overskride TDI værdien, uden at dette får nogle effekter, såfremt man i en tilsvarende periode senere i livet indtager mindre end TDI værdien. For voksne overskrides TDI værdien relateret til cadmium for to af smykkerne, når de bæres i 24 timer - og et enkelt overskrider TDI værdien for nikkel.
Maksimal baggrundseksponering
Når man ser på befolkningsgruppen, der er udsat for den maksimale baggrundseksponering ser billedet mere alvorligt ud. Her viser resultaterne, at de 5% danske børn, som udsættes for den maksimale baggrundseksponering af cadmium, allerede har overskredet grænsen for tolerabelt dagligt indtag af cadmium - uden at der er medtaget bidraget fra smykker. For de 5% voksne, der udsættes for den maksimale baggrundseksponering af cadmium, er TDI værdien ikke oveskredet på forhånd, men når der lægges bidraget fra smykkerne til, viser det sig at TDI værdien overskrides for fire af smykkerne, når de bæres i 24 timer.
Oral eksponering
Resultaterne viser desuden, at når der suttes på smykkerne i to timer, opstår der potentielle sundhedsmæssige risici relateret til både cadmium, nikkel og bly. Dog mest udtalt for bly og cadmium, idet næsten samtlige smykker (der viste migration af det pågældende metal) overskrider TDI værdien relateret til bly og cadmium (både børn og voksne). Dette er gældende for mennesker, der er udsat for en gennemsnitlig baggrundseksponering. Ingen af smykkerne gav anledning til problemer relateret til kobber.
Resultaterne viser, at det laveste indhold af bly, som giver anledning til sundhedsmæssige problemer ved at sutte på smykkerne i to timer, er et blyindhold på 1,77%, dvs. en del over blybekendtgørelsens krav om maksimalt indhold af 0,01% bly. Samtidig ses, at ingen af de smykker, som indeholdt maksimalt 100 ppm bly, gav anledning til sundhedsmæssige problemer.
Beregningerne har desuden vist, at for et enkelt smykke viser det sig, at børn (der er udsat for en gennemsnitlig baggrundseksponering af bly) maksimalt kan sutte på vedhænget i 1 min. før det daglige tolerable indtag er overskredet.
Overordnet konklusion
Alt i alt viser det sig, at det ikke kan udelukkes at der for nogle af de undersøgte smykker i Danmark er potentielle sundhedsrisici relateret til især cadmium og nikkel ved at bære og/eller sutte på disse metalsmykker. For bly er der primært problemer, når der suttes på smykkerne. Ingen af de undersøgte smykker gav problemer relateret til kobber.
De sundhedsmæssige risici, der er forbundet med de tre metaller er for cadmiums vedkommende effekter på nyrerne, mens det for nikkel er øget risiko for fosterdød. For bly er den sundhedsmæssige risiko nedsat IQ hos børn.
Hvis smykkerne overholder det lovlige indhold af bly indikerer resultaterne i dette projekt, at der ikke vil opstå sundhedsmæssige problemer ved at bære eller sutte på smykkerne.
Smykkerne der indeholdt kviksølv blev ikke undersøgt i migrationsanalysen. Det kan derfor ikke udelukkes at der ligesom for bly og cadmium er en sundhedsrisiko ved at bære eller sutte på smykkerne.
Der er imidlertid en række forbehold, der er afgørende for, om der opstår et sundhedsmæssigt problem. Bl.a. skal smykkerne bæres/suttes på hver dag over en lang periode, idet en kortvarig overskridelse af den tolerable daglige dosis ikke nødvendigvis resulterer i sundhedsskadelige effekter, medmindre man resten af livet hver dag indtager den tolerable daglige dosis. Dertil kommer at der i beregningerne er antaget, at migrationen af metaller fra smykkerne er konstant over tid. Denne vil med tiden aftage.
Endelig kan det i projektet konkluderes, at der findes tekstilhalskæder på det danske marked, der indeholder benzidin i en mængde, der overskrider det tilladte.
1 Preface and purpose
Jewelleries are found in a countless number of variants, including a large number of metal jewelleries with and without a content of precious metal. These jewelleries can potentially contain and release problematic substances such as for instance heavy metals.
A number of studies from abroad have proved a problem with the content of large amounts of lead in cheap imported jewelleries. The seriousness of this problem is confirmed by a recent death of a 4-year-old boy who by accident had swallowed a heart-shaped piece of jewellery containing more than 99% of lead. The incident led to a voluntary recall of 300,000 pieces of the mentioned piece of jewellery (Berg et al., 2006).
Furthermore, it has turned out that the textile part in a necklace imported from Turkey contained the substance benzidine, which is banned in the EU.
Thus, this project has the purpose to:
- Give an overview whether there are metal jewelleries on the Danish market with a problematic content of heavy metals.
- Clarify which types of heavy metals which in this case are present in problematic amounts in metal jewelleries in Denmark.
- Clarify whether there is a relation between price (quality), country of origin, jewellery part, jewellery type and category as well as content/release of heavy metal.
- Assess possible health consequences by using jewelleries containing heavy metals in problematic amounts.
- Give an indication whether there are textile necklaces on the Danish market containing benzidine.
2 Legislation in the area
In the following relevant legislation regarding use of heavy metals and benzidine is shortly described.
2.1 Limitation of use of certain heavy metals
For the heavy metals lead, mercury, nickel and cadmium there is legislation on limitation of the use of these substances. The following legislation is relevant:
- Statutory order on ban on import and sale of products containing lead. Stat.Ord. no. 1082 of 13.09.2007. (Taking effect as from 1 November 2007 and replacing Stat.Ord. no. 1012 of 13.11.2000).
- Statutory order on ban on import, sale and export of mercury and mercury-containing products. Stat.Ord. 627 of 01.07.2003.
- Statutory order on ban on sale, import and production of cadmium-containing products. Stat.Ord. 1199 of 23.12.1992.
- Statutory order on ban on import and sale of certain nickel-containing products. Stat.Ord. no. 24 of 14.01.2000.
- Statutory order on change of statutory order on ban on import and sale of certain nickel-containing products. Stat.Ord. no. 789 of 12.08.2005.
- Law on control of works of precious metal etc. Law no. 308 of 17.05.1995.
According to these statutory orders it is banned to import and sell products, including jewelleries, containing more than 100 ppm (mg/kg) of mercury or lead in the homogeneous single parts of the product. Furthermore, it applies that import, sale and production of products in which cadmium forms a part as surface treatment (cadmium plating), dye pigment or plastic stabilizer with more than 75 ppm in the homogeneous single parts of the product is banned.
According to the Nickel Statutory Order (Stat.Ord. no. 24 of 14.01.2000 including the supplement Stat.Ord. no. 789 of 12.08.2005) it applies that “Nickel must not form a part of studs which are inserted into pierced ears and other pierced parts of the body unless the nickel release from such studs are less than 0.2 mg/cm²/week (migration limit)” – and that “Nickel must not form a part of products which are intended to get into direct and long contact with the skin if the nickel release from these parts which get into direct and long contact with the skin is larger than 0.5 mg/cm²/week for a period of at least two years at normal use”.
The law of control with works of precious metal etc. deals with among other things the requirements to the content of precious metals in goods, including jewelleries being sold as goods of precious metals. However, an inquiry (see Appendix B) among companies acting in the Danish precious metal industry indicated that there does not seem to be a problem with the content of undesirable heavy metals in products related to the precious metal industry, thus there is no focus on these types of jewelleries in this project.
Finally, the Toys Statutory Order can be mentioned which includes the requirements that toys for children may only release a certain amount of among others lead, mercury, cadmium, selenium, chromium, barium, arsenic and antimony (see Appendix G). Jewelleries addressing children and possessing a “play function” must be labelled with the CE label. CE labels indicate that the jewellery fulfils all the valid rules within the toys area. Even if a few of the purchased jewelleries looked like jewelleries addressing children it was not possible to find jewelleries labelled with the CE label during the purchasing phase.
2.2 Limitation of use of benzidine
The following legislation is relevant regarding textile necklaces containing benzidine:
- Statutory Order on ban on import, sale and use of certain azo-dyes. Stat.Ord. no. 755 of 15.08.2003.
According to this Statutory Order applies that use of azo-dyes is banned in textile and leather goods which can get into direct contact with skin or oral cavity for a longer period at humans if these azo-dyes at reductive decomposition of one or several azo-groups can release one or several of the aromatic amines, which are listed in Appendix F of this report, in concentrations above 30 ppm in the finished good or in the dyed parts of this. Benzidine is one of the aromatic amines mentioned in Appendix F.
According to the Statutory Order it is not forbidden to sell a product containing benzidine as long as not more than 30 ppm of benzidine is released. Furthermore, it applies that import and sale of the above-mentioned textile and leather goods are banned if they are dyed with the azo-dyes mentioned in Appendix E of this report.
3 Survey and purchase
- 3.1 The market for jewelleries in Denmark
- 3.2 Purchase of metal jewelleries
- 3.3 Purchase of textile necklaces
- 3.4 Results from the screening
- 3.4.1 The uncertainty of the XRF screening
- 3.4.2 Test for a content of Arsenic and Barium
- 3.4.3 Content of heavy metal in the metal jewelleries
- 3.4.4 Content of heavy metal in relation to price/gram
- 3.4.5 Content of heavy metal in relation to the country of origin
- 3.4.6 Content of heavy metal in the different product types
- 3.4.7 Content of heavy metal in the different types of jewellery parts
- 3.4.8 Content of heavy metal in the overall product categories gold, silver and non-precious metal
- 3.4.9 Content of heavy metal in the specified product categories
- 3.4.10 Content of heavy metal in relation to shop type
3.1 The market for jewelleries in Denmark
Jewelleries on the market in Denmark are in statistical respect divided into a number of groups as described in Appendix C. Largely, it is distinguished between jewelleries of precious metals (in this project also named precious metal jewelleries) and bijouterie. According to the legislation jewelleries of precious metals must contain at least 333 per thousand gold, 800 per thousand silver, 850 per thousand platinum and 500 per thousand palladium. Bijouterie covers on the whole all other types of jewelleries consisting of metals, precious metals, plastic, wood etc. In practice, it is an open question how the specific differentiation between the product groups within bijouterie is made.
An extract from Statistics Denmark (see Appendix D) shows that in 2005 the turnover was approx. 706 tonnes of bijouterie on the Danish market of which metal-bijouterie with and without precious metal represented approx. 358 and 312 tonnes respectively. Non-metallic bijouterie which includes plastics and textiles jewelleries represented approx. 36 tonnes. It was not possible to get data from Statistics Denmark which specifically deals with sale and production of the different types of non-metallic bijouterie.
Far the main part of the bijouterie is imported from Asia, primarily China, but other countries such as Hong Kong, South Korea and Taiwan also represent a relatively large part of the total import as stated in the table below (see Appendix D for more details).
Table 3-1: Import of jewelleries from abroad in percent.
| Import from | Precious metal jewelleries | Bijouterie – precious metal | Bijouterie – non-metal | Bijouterie – without precious metal |
| USA | 4.2% | 5.5% | 0.6% | 0.4% |
| Indonesia | 0.4% | 0.3% | 0.9% | 0.3% |
| China | 53.7% | 87.9% | 37.8% | 54.0% |
| South Korea | 2.5% | 2.9% | 0.6% | 2.1% |
| Japan | 3.7% | 0.0% | 0.0% | 0.0% |
| Taiwan | 0.0% | 1.3% | 1.1% | 1.1% |
| Hong Kong | 10.4% | 1.8% | 5.4% | 6.6% |
| Total | 21.912 kg | 385,322 kg | 380,337 kg | 282,229 kg |
The market for precious metal jewelleries is not that transparent as companies with below 10 employees (which is typical for goldsmith's and silversmith’s workshops) have no obligation to report the amount of produced products to Statistics Denmark.
In the project no complete survey of the market for jewelleries in Denmark was performed. The primary attempt was to cover the market by purchasing jewelleries from typical players in the retail trade as well as through the Internet. The main part of the jewelleries is bought in the retail trade, however, primarily in shop types like clothes shops, supermarkets, 10 kroner’s shops, jewellery shops etc. In the following sections there is a detailed description telling how the purchases of metal jewelleries as well as textile necklaces have been carried out and how the purchased jewelleries are classified.
3.1.1 Division of jewelleries into jewellery parts
The primary purpose of this project is to identify whether there is a problematic content of heavy metals in metal jewelleries (hereafter mentioned as jewelleries) in Denmark. However, jewelleries typically consist of several different metal parts (catch, chain, charm etc.) which each may have a different content of heavy metal. Therefore, in this project it is decided to analyse a maximum of 3 parts of each purchased piece of jewellery.
In the project, these different parts of the jewelleries are mentioned as “jewellery parts” as illustrated in the figure below. In the project a total of 170 pieces of jewelleries were purchased. These pieces of jewelleries were each scanned on a maximum of three different parts which has resulted in 318 jewellery parts.
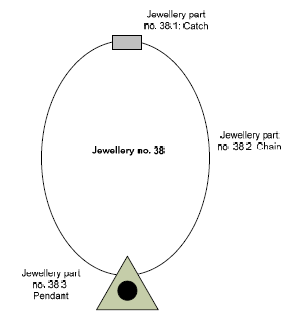
Figure 3-1: Illustration of the definition of a jewellery part.
3.1.2 Categorization of jewellery parts
The categorization of the jewelleries is made on each jewellery part as jewelleries as mentioned often consist of several different metal parts, each with a unique appearance (gold, silver etc.) which makes an overall categorization of the jewellery as a whole impossible.
The jewellery parts in this project are categorized according to the following product categories:
Table 3-2: Description of product categories used to classify the jewellery parts.
| Product category | Content of precious metal (%) | Comment regarding the classification |
| Genuine gold | Au > 33 | |
| Genuine silver | Ag > 80 | If the jewellery part contains > 80% of silver but is coated with gold, the jewellery part is classified as ”gold coated”. |
| Gold coated | Au: 0-33 | If the content of gold is very low and the jewellery part visually does not seem to contain gold it is not classified as ”gold coated”. |
| Silver coated | Ag: 0-20 | If the jewellery part contains both gold and silver it is classified according to what it visually seems to contain. |
| Silver alloy | Ag: 20-80 | |
| Golden-like | Au: 0 | |
| Silver-like | Ag: 0 | |
| Clearly non-precious metal | Au: 0 Ag: 0 |
Here an assessment has taken place whether the jewellery part clearly seems not to contain precious metal. In a few cases, a jewellery part may be classified as ”clearly non-precious metal” even if it contains a small amount of gold or silver. |
Alloyed jewelleries consist of metal which is ”melted together” while the category ”coated jewelleries” includes jewelleries where precious metals lie as a separate metal layer on the surface. There are no jewelleries of the type “gold alloy” as on the whole genuine gold jewelleries always are alloyed jewelleries, i.e. the jewelleries which are defined as “genuine gold” here are in practice alloyed jewelleries.
Furthermore, the jewellery parts are generally divided into ”gold”, ”silver” and “clearly non-precious metal” since it for the consumer is possible visually to distinguish between these types of goods. In other words, with this division the consumer will have the possibility of deselecting a product if a possible relation between a problematic content of heavy metal and type of jewellery (gold, silver or non-precious metal) is proved.
According to content of precious metal, the categorization of precious metal has taken place on basis of analysis results from a XRF screening of the different jewellery parts by means of X-rays; however, it shall be mentioned that the results imply a relatively large uncertainty factor, as described in section 3.4.1.
The contents of precious metal in percentage which is the basis of the categorization of the jewellery parts in the groups “coated” and “alloyed” are solely based on experiences from the Danish assay office. Thus, this division cannot be regarded as an “official” categorization in terms of “alloyed” and “coated” jewelleries.
The reason that gold and silver jewellery parts are divided into “coated”, “alloyed” and “-like” respectively is due to the fact that a possible problematic content of heavy metals might be released differently, depending on whether the product is coated with precious metals, alloyed with precious metals or does not contain precious metals at all. The Danish assay office states that coatings of especially precious metals can to a certain degree prevent heavy metals from being released and thus reduce a potential health risk.
In a few cases, a small content of gold or silver (< 1%) is ignored during the categorization as the uncertainty of the XRF measurements are relatively large when the content appears to be below one percent. In other words, where the XRF result indicates a content of gold of for instance 0.08% a jewellery part can prove to contain no gold at all.
Finally, the categories ”genuine silver” and “genuine gold” are included even if these jewelleries are not in focus in this survey. The reason why is that a few of the purchased jewelleries turned out to contain jewellery parts which in fact had a content of precious metal which fulfils the legal requirement for precious metal jewelleries.
3.2 Purchase of metal jewelleries
In total, 170 metal jewelleries are purchased, divided into the product types rings, necklaces, bracelets, earrings, piercing jewelleries and ankle chains. These product types are selected as they all are products getting into contact with the skin.
The purchases were made in three phases with duration of 1-2 days during a total period of about 1.5 months. In the periods between the purchases the purchased jewelleries were screened for a content of heavy metals and then categorized. Based on this knowledge the strategy for the following purchases was made.
The primary elements which were assessed after each purchase/screening with regard to the next purchase were as follows:
- Proportionally equal distribution of price per gram.
- Proportionally equal distribution between the product types (rings, necklaces etc.).
- Proportionally equal distribution between the product categories (gold, silver etc.).
- A representative distribution in relation to country of origin.
- Reasonable distribution of purchases in the different types of shops.
3.2.1 Price per weight unit
During the purchases it was important to get a representative selection of products which were different with regard to price and quality. The basic measurements used to describe the relation between price and quality is price per weight unit (gram) as the type and price of the raw materials may be expected to be a substantial element in this situation. However, it shall be mentioned that it is difficult to define quality in relation to jewelleries as also a certain degree of subjective aesthetics is included in the term. For instance, a high degree of processing (e.g. in filigree jewelleries) can be a quality, which influences the price without the used raw materials necessarily being expensive.
The table below shows the purchase of jewelleries divided into the different relations regarding price/gram.
Table 3-3: The division of jewelleries in the different relations regarding price/gram.
| Kr./gram | Number of jewelleries |
| < 2 | 32 |
| 2.00 – 3.00 | 31 |
| 3.00 – 5.50 | 33 |
| 5.5 – 10.00 | 37 |
| > 10 | 37 |
| Total | 170 |
3.2.2 Product types
As far as possible it was attempted to purchase an equal division of jewelleries in the different product types. However, it was judged that the use of products like piercing jewelleries and ankle chains are not as widespread as for instance necklaces, rings, bracelets and earrings. Thus, the purchase of piercing jewelleries and ankle chains was not in the same proportion as the remaining product types.
Table 3-4: The division of purchased jewelleries in the different product types
| Product type | Number of jewelleries |
| Others | 5 |
| Ankle chain | 6 |
| Bracelet | 43 |
| Necklace | 51 |
| Piercing | 3 |
| Ring | 28 |
| Earring | 34 |
| Total | 170 |
The product type ”Others” covers for instance toe rings etc.
3.2.3 Product categories
Furthermore, the purchase of the jewelleries was attempted to be equally divided on the different product categories (gold coated; silver-like etc.). However, in the purchase situation it was not possible to clarify whether the jewellery in fact contained precious metal, neither if it was alloyed or coated. Even if the purchases were made in several phases and the jewelleries were categorized continuously it was necessary with a more pragmatic approach to the purchase situation in the form of some overall product categories. This meant that focus was on purchasing a representative selection of jewelleries in the overall product categories “gold jewelleries”, “silver jewelleries” and “clearly non-precious jewelleries”. In other words, the overall product category “gold jewelleries” included jewelleries coated with gold as well as golden-like jewelleries. The categorization of the jewelleries has, as mentioned earlier, taken place on each part of the jewellery (with a maximum of three parts) as jewelleries as mentioned can consist of a silver part as well as a gold part. In the table below the distribution of the purchases in relation to the overall product category is shown.
Table 3-5: Division of purchases in relation to overall product category.
| Overall product category | Number of jewellery parts |
| Gold jewelleries | 131 |
| Silver jewelleries | 160 |
| Non-precious metal jewelleries | 27 |
| Total | 318 |
3.2.4 Purchase in relation to country of origin
As one of the purposes with the project was to clarify whether there is a relation between content/release of heavy metal and country of origin it was attempted, if possible, in the purchase situation to get information about the country of origin of the jewellery. However, it turned out to be difficult to get information regarding country of origin as most of the shop assistants had no possibility of replying to this. However, at a number of shops it was possible to get a telephone number to their supplier, head office etc. This contact information is registered in the database. Subsequently, it was tried to contact the supplier by phone to get information about importer/producer as well as country of origin. However, it was not possible to get this information in all cases and within the time frame of the project it was not possible to contact all shops. The table below represents the procured information.
Table 3-6: Division of purchased jewelleries in relation to country of origin
| Country of origin | Number of jewelleries |
| The shop does not know | 95 |
| England | 3 |
| Holland | 1 |
| India | 4 |
| China | 37 |
| South Korea | 1 |
| Thailand | 2 |
| Investigated but cannot be informed | 9 |
| The East | 18 |
| Total | 170 |
The definition ”The shop does not know” represents the shops which have not been contacted by phone and which in the purchase situation were not able to inform country of origin. The definition “Investigated but cannot be informed” represents situations where phone calls to the supplier have not resulted in an identification of the country of origin.
In total, information regarding country of origin was procured for 66 out of the 170 purchased jewelleries corresponding to 39%.
As it has not been possible in the main part of the purchase situation to get information about country of origin it has been difficult to purchase an equal distribution of jewelleries representing different countries. As the results immediately indicate the main part of the purchased jewelleries originates from Asia, especially China. However, this is not surprising as information from Statistics Denmark shows that the main part of bijouterie is imported from Asia, primarily China (see Table 3-1 and Appendix D).
However, it can be mentioned that the jewelleries are selected and purchased at random which means that with the large amount of purchased jewelleries there ought to be a representative distribution of the jewelleries as to their country of origin.
3.2.5 Where are the purchases made?
Purchase of the jewelleries is primarily made in retail trade (156 pcs.), however, supplemented by a small purchase via the Internet (14 pcs.). The purchases were made in the area of Copenhagen as it was assessed that jewelleries sold in this area cover a representative selection of the jewelleries being sold on national basis. Jewelleries were purchased in the following areas:
- Town centre Copenhagen: Strøget, Købmagergade incl. several side streets.
- Frederiksberg: Frederiksberg Center, Falkoner Allé, Godthåbsvej and Gl. Kongevej.
- Nørrebro: Nørrebrogade, Elmegade and Ravnsborggade.
- Vesterbro: Istedgade, Vesterbrogade and Fisketorvet.
- Lyngby: Lyngby Storcenter and Magasin.
- Kgs. Nytorv: Magasin.
- Strøget: Illum.
- Brønshøj/Husum: Frederikssundsvej.
The main part of the jewelleries was purchased in shops as it was assessed that only a small part of the population buys jewelleries via the Internet. Jewelleries were purchased in the following shop types:
- 10 kr’s shop types
- Other type of shops
- Shops with owners of different ethnic background
- Internet shops
- Jewellery shops
- Department stores
- Supermarkets
- Clothes shops
The category ”Other type of shops” covers among other shops booksellers, toyshops, children’s wear shops and different non-definable shops.
3.3 Purchase of textile necklaces
In total 62 textile necklaces were purchased. The main part of the textile necklaces were purchased at the same time as the metal jewelleries which means that the above-mentioned shop types also represent the shops where the textile necklaces were purchased. However, it was tried to purchase a little more of textile necklaces in children’s wear shops and toyshops as the notified textile necklace with a content of benzidine was targeted children. Furthermore, during the purchase phase there was a focus on purchase of textile necklaces of different kind and colour.
3.4 Results from the screening
All 170 pieces of metal jewelleries were screened for a content of metals by means of a XRF device. In each case it was assessed how many different parts of the jewellery it was relevant to screen; however, a maximum of 3 different parts of the jewellery were screened. In total 318 screenings of jewellery parts were made.
3.4.1 The uncertainty of the XRF screening
Results reached through a XRF screening indicate first and foremost which elements being present as well as their approximately proportional distribution with regard to amount. Thus the result does not represent a reliable value for the exact content of the metal in percentages.
The content of for instance lead in the screening will be determined with a much greater reliability if for instance the content of lead is located on the outside of the jewellery than if the content of lead is placed in the middle of the jewellery. The reason being, that the XRF device uses X-rays to indicate a content of metal. These X-rays have more difficulties in penetrating into the middle of the jewellery and out from the jewellery (especially if it is coated with precious metals) which is the reason for the larger uncertainty of the result if the searched metal is in the middle of the jewellery (here the volume of the jewellery also has an influence). When using this type of measurements alone there is no possibility of immediately assessing where in the jewellery a possible content of heavy metals is located.
Another reason why it is not possible to describe the exact uncertainty of the completed XRF measurements is that the jewelleries vary a lot in shape and construction in relation to known reference samples. The XRF device shows in percentages a content of metal in the surface which the device immediately can “see”. Thus, to achieve a “perfect” screening the sample should consist of a perfect homogeneous surface which is not the case with the different jewellery parts.
At the semi-quantitative screening, which is the one being carried out in this project, a positive result implies that there is a content of the element in question; i.e. a positive quantitative determination. A strong signal implies that the jewellery contains relatively large amounts of the element in question.
It is judged that the results from the XRF screening are usable to form a reasonable impression of which jewelleries ought to be selected for further test for release of problematic heavy metals.
3.4.2 Test for a content of Arsenic and Barium
The used XRF device was not calibrated to test for a content of As and Ba and therefore a test for a content of these elements was made through a visual examination of the spectrum which came out for each XRF scanning.
The method consisted in defining the wavelength which As and Ba respectively represented and thereafter in interpreting visually whether there was a “top” on level with the respective “wavelength”. Despite the visual character of the method it can still indicate a possible content of these two metals with an acceptable certainty.
However, all the screenings showed no sign of a content of either As or Ba which was not expected anyway as normally these two metals are not used in the production of jewelleries.
3.4.3 Content of heavy metal in the metal jewelleries
The 170 pieces of metal jewelleries were tested for a content of the following metals: Au, Ag, Cu, Sb, Pb, Se, Cr, Cd, Hg, Sn, Al, Mo, Nb, Zr, Bi, W, Zn, Ni, Co, Fe, Mn, V, Ti, In, Pt, Pd, As, Ba.
The following eight metals were judged to be the most interesting in terms of potential health risks related to migration to the skin as well as ingestion through the mouth: Pb, Hg, Cd, Se, Cr, Sb, As, Ba.
Appendix A shows the content of the above-mentioned 8 metals as well as gold, silver and copper in all the 318 screenings (corresponding to all the 170 pieces of jewelleries).
The screening revealed that approx. 58% of all examined jewelleries contained lead in a concentration above 100 ppm.
As regards the content of cadmium the screening showed that approx. 24% of all the examined jewelleries contained cadmium in a concentration above 75 ppm. However, based on the XRF screening it is not possible to conclude whether the content of cadmium is found in the form of a cadmium plating of the surface or whether it is found in the middle of the jewellery.
Regarding mercury, 4 pieces of jewelleries, corresponding to 2% of the examined jewelleries, exceeded the maximally allowed content of 75 ppm Hg.
Table 3-7: Content of Pb, Cd, Hg and Ni respectively in the examined jewelleries/jewellery parts.
| Content of metal | Number of jewelleries | In percentage of total number of jewelleries | Number of jewellery parts | In percentage of total number of jewellery parts |
| Pb content above 100 ppm | 98 | 58% | 143 | 45% |
| Cd content above 75 ppm | 41 | 24% | 49 | 15% |
| Hg content above 100 ppm | 4 | 2% | 4 | 1% |
| Ni content above 1% | 42 | 25% | 72 | 23% |
| Total | 170 | 318 |
The completed tables presenting the content of Ni, Cd, Pb, Hg, Sb and Cr respectively in all the jewellery parts (sorted in descending concentration) are enclosed in Appendix A. No content of Se was found in the jewelleries.
The maximum content of lead of 69.6% was found in a gold coated catch (jewellery part no. 95.3). Furthermore, 47 jewellery parts were found to contain above 10% of lead. Of these 47 jewellery parts, 16 (corresponding to 34%) were catches.
For cadmium, the maximum content in a jewellery part was 29.2% and in total 9 jewellery parts had content above 1% cadmium.
40 jewellery parts contained above 10% of nickel while two jewellery parts contained 93 and 95% of nickel respectively. Up to 25% of all examined jewelleries turned out to contain nickel in a volume percentage above 1. However, the legislation regarding nickel does not ban the use of nickel in jewelleries but there are requirements on the amount of nickel which maximally may migrate from the jewellery.
3.4.4 Content of heavy metal in relation to price/gram
The tables and figures below are included with the purpose of showing whether there is a relation between the quality of the jewelleries (price per gram) and the content of heavy metals. The tables show the content of Pb and Cd respectively in relation to the quality of the jewellery (price per gram of jewellery – i.e. the price of the jewellery divided by the weight of the jewellery).
Table 3-8: Content of Pb in relation to kr/gram
| Kr/gram | < 0.01 Pb (%) |
0.01 – 1 Pb (%) |
1 – 5 Pb (%) |
5 - 10 Pb (%) |
> 10 Pb (%) |
Number of jewelleries |
| < 2 | 48% | 20% | 3% | 3% | 25% | 32 |
| 2.00 – 3.00 | 60% | 13% | 10% | 6% | 11% | 31 |
| 3.00 – 5.50 | 38% | 27% | 5% | 8% | 22% | 33 |
| 5.5 – 10.00 | 56% | 18% | 12% | 4% | 10% | 37 |
| > 10 | 70% | 22% | 2% | 0% | 6% | 37 |
| Total | 170 | |||||
Table 3-9: Content of Cd in relation to kr/gram
| Kr/gram | < 0.0075 Cd (%) |
0.0075 – 1 Cd (%) |
1 – 5 Cd (%) |
5 - 10 Cd (%) |
> 10 Cd (%) |
Number of jewelleries |
| < 2 | 88% | 10% | 2% | 0% | 0% | 32 |
| 2.00 – 3.00 | 86% | 13% | 0% | 0% | 2% | 31 |
| 3.00 – 5.50 | 76% | 22% | 2% | 0% | 0% | 33 |
| 5.5 – 10.00 | 93% | 6% | 1% | 0% | 0% | 37 |
| > 10 | 80% | 13% | 3% | 5% | 0% | 37 |
| Total | 170 | |||||
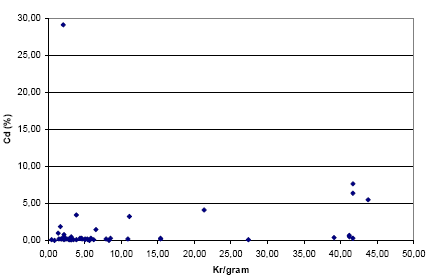
The figures below show the distribution in relation to kr/gram of the jewellery parts which were found to contain Cd and Pb respectively. Immediately, there seems to be a greater chance of a large content of Pb in the cheaper metal jewelleries (0 – approx. 10 kr. per gram) while for Cd there does not seem to be an immediate relation.
Figure 3-2: The distribution of jewellery parts containing Pb in relation to kr/gram
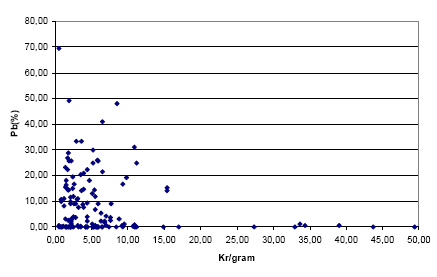
NB. A screening result for a single jewellery part with a kr/gram ratio of 66.64 (Pb content of 0.11%) is not included here. The reason for this is to create a more clearly visual presentation of the remaining data.
Figure 3-3: The distribution of jewellery parts containing Cd in relation to kr/gram
3.4.5 Content of heavy metal in relation to the country of origin
In the table below the content of Pb and Cd in jewelleries from the different countries is seen. Only data from the jewelleries where it has been possible to get information about the country of origin are included (i.e. 66 jewelleries out of the in total 170 purchased jewelleries).
Table 3-10 Content of Pb and Cd in relation to country of origin
| Country of origin | Number of jewelleries | % of jewelleries containing > 75 ppm Cd | % of jewelleries containing > 100 ppm Pb |
| The East | 18 | 22% | 56% |
| Thailand | 2 | 0% | 0% |
| South Korea | 1 | 0% | 0% |
| China | 37 | 24% | 30% |
| Holland | 1 | 100% | 100% |
| India | 4 | 0% | 0% |
| England | 3 | 67% | 67% |
Due to the limited amount of information regarding country of origin it is no possible to conclude anything about the probability of finding jewellery containing a certain amount of Pb and Cd in the respective countries. The reason for this is that there are not jewelleries enough from most of the countries to give a representative overview over the probability of a possible content of heavy metal. However, with a reasonable statistical certainty it can be said that for the 37 jewelleries being known to originate from China 30% has turned out to contain above 100 ppm of lead while 24% has turned out to contain above 75 ppm cadmium.
3.4.6 Content of heavy metal in the different product types
The XRF screening showed an even distribution of content of lead as well as cadmium among the different product types. Thus, it cannot be concluded that some of the product types have a larger probability of containing lead or cadmium than other product types. However, it is seen that none of the purchased ankle chains had a content of cadmium but the number of purchased ankle chains (6 pcs.) is not high enough to assume with certainty that in general ankle chains do not contain cadmium.
Table 3-11: Content of Pb and Cd in relation to product type
| Product type | Number of jewelleries | % with > 100 ppm Pb | % with > 75 ppm Cd |
| Other | 5 | 20% | 20% |
| Ankle chain | 6 | 33% | 0% |
| Bracelet | 43 | 51% | 23% |
| Necklace | 51 | 80% | 35% |
| Piercing | 3 | 67% | 33% |
| Ring | 28 | 50% | 18% |
| Earring | 34 | 47% | 24% |
| Total | 170 | ||
3.4.7 Content of heavy metal in the different types of jewellery parts
As shown in the tables below 70% of the examined charms (belonging to the product types earrings, necklaces and bracelets) has turned out to contain above 100 ppm of lead. Equally is that 78% of the examined charms (belonging to the product types earrings, necklaces and bracelets) has a content of cadmium above 75 ppm.
Furthermore, it has turned out that for the ”jewellery part type” catches belonging to the product types bracelets and necklaces 14% contained cadmium in a concentration above 75 ppm while 20% turned out to contain a minimum of 100 ppm lead.
From this it can be concluded that the tendency is that especially charms but also partly catches contain lead and cadmium. The jewellery part which turned out to contain the largest amount of lead (approx. 70%) was a catch. Furthermore, 15 other catches turned out to contain above 15% of lead (see Appendix A).
Table 3-12: Distribution of the content of heavy metal in relation to type jewellery part belonging to earrings
| Type jewellery part belonging to earrings | % jewellery parts with > 0.0075% Cd | % jewellery parts with > 0.01% Pb |
| Ear studs | 16.7% | 30% |
| Charm | 83.3% | 70% |
Table 3-13: Distribution of the content of heavy metal in relation to type jewellery part belonging to bracelet
| Type jewellery part belonging to bracelet | % jewellery parts with > 0.0075% Cd | % jewellery parts with > 0.01% Pb |
| Catch | 16.7% | 13.3% |
| Charm | 83.3% | 86.7% |
Table 3-14: Distribution of the content of heavy metal in relation to type jewellery part belonging to necklaces
| Type jewellery part belonging to necklaces | % jewellery parts with > 0.0075% Cd | % jewellery parts with > 0.01% Pb |
| Charm | 80% | 53.6% |
| Chain | 10% | 20.3% |
| Catch | 10% | 26.1% |
3.4.8 Content of heavy metal in the overall product categories gold, silver and non-precious metal
The results of the XRF screening showed that there does not seem to be a direct relation between the content of heavy metal and the overall product category (gold, silver or non-precious metal) (see Table 3-15). Thus it cannot be assumed that jewelleries of for instance gold have a greater possibility of containing lead or cadmium than jewelleries of silver or non-precious metal. Here it shall be mentioned that the overall product category “gold” covers jewelleries which contain gold as well as jewelleries looking like gold. Correspondingly for the overall product category “silver”.
Table 3-15: Content of Pb and Cd in relation to overall product category
| Overall product category | Percentage jewellery parts with > 0.01% Pb | Percentage jewellery parts with > 0.0075 % Cd | Total number of jewellery parts |
| Gold | 39.7% | 15.2% | 131 |
| Silver | 47.5% | 15.6% | 160 |
| Non-precious metal | 55.6% | 14.8% | 27 |
3.4.9 Content of heavy metal in the specified product categories
As it is seen from the tables below there does not seem to be a significant difference of the content of lead in the specified product categories, i.e. it cannot directly be concluded that for instance precious metal-coated jewelleries contain less lead than precious metal-alike jewelleries. The same applies to cadmium.
Table 3-16: Number of jewellery parts with a content of Pb in the different product categories.
| Product category | < 0.01 Pb (%) |
0.01 – 1 Pb (%) |
1 – 5 Pb (%) |
5 - 10 Pb (%) |
> 10 Pb (%) |
Number of jewellery parts | % jewellery parts with Pb > 0.01% |
| Genuine gold | - | - | - | - | - | 3 | 0 |
| Genuine silver | - | 3 | - | - | - | 11 | 27 |
| Clearly non-precious metal | 12 | 8 | - | 2 | 5 | 27 | 56 |
| Gold coated | 53 | 18 | 7 | 4 | 18 | 100 | 47 |
| Silver coated | 25 | 8 | 2 | - | 8 | 43 | 42 |
| Silver alloy | 5 | - | 1 | - | 1 | 7 | 29 |
| Golden-like | 23 | 4 | 1 | - | - | 28 | 18 |
| Silver-like | 45 | 22 | 9 | 8 | 15 | 99 | 55 |
Table 3-17: Number of jewellery parts with a content of Cd in the different product categories.
| Product category | < 0.0075 Cd ( %) |
0.0075 - 1 Cd (%) |
1 – 5 Cd(%) |
5 - 10 Cd (%) |
> 10 Cd (%) |
Number of jewellery parts | % jewellery parts with Cd > 0.0075% |
| Genuine gold | - | - | - | - | - | 3 | 0% |
| Genuine silver | - | - | - | 1 | - | 11 | 9% |
| Clearly non-precious metal | - | 2 | 1 | - | 1 | 27 | 15% |
| Gold coated | 80 | 17 | 1 | 2 | - | 100 | 20% |
| Silver coated | 37 | 5 | 1 | - | - | 43 | 14% |
| Silver alloy | 6 | - | 1 | - | - | 7 | 14% |
| Golden-like | 28 | - | - | - | - | 28 | 0% |
| Silver-like | 82 | 16 | 1 | - | - | 99 | 17% |
3.4.10 Content of heavy metal in relation to shop type
The result from the screening did not show a clear relation between shop type and content of heavy metal. In other words, it cannot be directly concluded that there is a larger possibility of buying jewelleries with a content of lead and cadmium above 100 ppm and 75 ppm respectively in certain shop types.
It is calculated for each shop type how large a part of the purchased jewelleries that contained above 100 ppm lead and 75 ppm cadmium respectively (se the table below). However, it shall be mentioned here that the average does not take into account the uncertainty related to the fact, that in some of the shop types only a very few pieces of jewelleries were purchased.
Table 3-18: Percentage of jewelleries with above 100 ppm lead and 75 ppm cadmium respectively, seen in relation to shop type
| Shop type | Number of purchased jewelleries | % with > 100 ppm Pb | % with > 75 ppm Cd |
| 10 kr's shop | 5 | 60% | 20% |
| Other type of shop | 39 | 67% | 23% |
| Shop with owners of different ethical background | 5 | 40% | 0% |
| Internet shop | 14 | 50% | 14% |
| Jewellery shop | 27 | 59% | 30% |
| Department store | 7 | 43% | 29% |
| Supermarket | 3 | 100% | 0% |
| Clothes shop | 70 | 53% | 26% |
4 Migration analysis
- 4.1 Selection of metal jewellery for migration analysis
- 4.1.1 Focus on jewellery parts with low content of Pb and Cd
- 4.1.2 Even distribution of the overall categories gold, silver and non-precious metal
- 4.1.3 Even distribution in the specified product categories
- 4.1.4 Conditional distribution among types of jewellery parts and jewellery types.
- 4.1.5 Selected jewellery parts for migration test
- 4.2 Analysis method
- 4.3 Analysis results
4.1 Selection of metal jewellery for migration analysis
In total 25 jewellery parts were selected for migration analysis of metals. The reason for selecting jewellery parts and not jewellery was that jewellery as mentioned earlier consists of several separate parts with each their content (and thereby release) of metals.
The jewellery parts were selected in such a way that they covered the product types as widely as possible, yet with the primary criterion that they represented a part of the jewellery that had contact with the skin. I.e. a jewellery part as for instance a pendant belonging to an earring was not selected.
Below is a short description of the background related to the selection criteria.
4.1.1 Focus on jewellery parts with low content of Pb and Cd
The selection of jewellery for migration analysis was done among the jewellery parts that had a content of lead and cadmium respectively varying from approximately 100 ppm to the maximum measured values. The reason for this focus on jewellery that contained primarily these two metals was that it thereby became possible to select enough jewellery to illustrate a possible correlation between migration and content.
In total 15 jewellery parts were selected among the jewellery parts that contained more than 100 ppm lead and 10 jewellery parts among those that contained more than 75 ppm cadmium.
The selected jewellery parts represent the entire concentration span though focus was on the low end of the spectrum i.e. for lead between 100 and 2000 ppm and for cadmium between 75 and 2000 ppm. The reason for this was a desire to clarify how big a violation of the law it would take before a health risk could occur.
4.1.2 Even distribution of the overall categories gold, silver and non-precious metal
Since the results from the XRF-screening showed no apparent correlation between the content of heavy metals and the overall product categories: gold, silver and non-precious metal (see Table 3-15), jewellery parts were chosen that, as far as possible, were distributed evenly in the overall categories.
4.1.3 Even distribution in the specified product categories
As the screening results (Table 3-16 and Table 3-17) indicated that there was not a directly significant difference on the content of lead and cadmium in relation to the specific product categories (gold coated, silver-like etc.) jewellery parts were selected that, as far as possible, was distributed evenly among the product categories. Yet no jewellery parts were included from the category “genuine silver” because these were not in the project focus.
4.1.4 Conditional distribution among types of jewellery parts and jewellery types.
The screening results indicated that a relatively large percentage of the analysed pendants had a content of lead and cadmium respectively (see Table 3-12, Table 3-13 and Table 3-14). 70% of the analysed pendants (belonging to the product types earrings, necklaces and bracelets) showed a content of more than 100 ppm lead while for cadmium 78% of the analysed pendants had a cadmium content of more than 75 ppm. Thus, during the selection it was attempted to choose more pendants than e.g. rings and bracelets.
In addition it was attempted as far as possible to get a representative segment of the different product types (bracelet, necklaces etc.). Yet there were limited choices among the separate product types (i.e. piercing jewellery and ankle chains), which meant that a majority of the selected jewellery parts belonged to other product types (especially necklaces).
4.1.5 Selected jewellery parts for migration test
During the selection of jewellery parts for migration test it was as a main rule attempted to select jewellery parts that were distributed evenly among the selection criteria (content of heavy metals, coating, product type and type of jewellery part).
Yet in relation to the selection criterion ”content of heavy metal” a proportional larger amount of jewellery parts were selected among those that had a relatively low content of heavy metal. The reason for this was to clarify whether there could be a health related problem related to jewellery that had a content of lead and cadmium respectively close to the permitted limit.
Likewise relatively more jewellery parts of the pendant type were selected because 80% of these according to the XRF-screening contained heavy metal.
It must be pointed out that it was not possible to make a 100 % even distribution due to the limited number of purchased jewellery. In appendix K it is demonstrated how the chosen jewellery parts meet the selection criteria.
4.2 Analysis method
The 25 jewellery parts were analysed for migration of the following metals: As, Ba, Cd, Cr, Cu, Hg, Ni, Pb, Sb and Se.
The method used was ”Migration to artificial sweat” according to DS/EN 1811:2000.
The solution for artificial sweat consisted of:
1.0 g urea, 5.0 g NaCl and 0.940 µl lactic acid dissolved in 1 litre of demineralised water. pH was set to 6.5 and the extraction was made at 40 degrees.
Sample pieces (2 g) with a variable area was placed in 25 ml artificial sweat and placed at 40 °C for 4 hours. Afterwards the water phase was decanted from the sample pieces. Subsequently the water phase was analysed for content of emitted metals by optic emission spectrometry (ICP-OES). The identified contents were given in relation to sample weight (µg extracted metal per gram sample) as well as in relation to the surface of the sample (µg extracted metal per cm²).
After cutting of the jewellery part, the exposed side was covered with wax in order to make sure that migration only happened from the parts of the sample which were coated or alloyed with precious metals.
As detection limit was used what resembles 5 times the detection limit for the ICP-OES analysis.
Duplicate determination was performed on all tests. Yet two jewellery parts (both ear pins) were so small, that sample pieces on 2 x 2 g could not be extracted. In these two cases a smaller sample weight was used and the detection limit was estimated to be the actual sample weight.
In some cases the duplicate determinations were not in concordance. In these cases the analysis of metals was repeated using the ICP-OES, though without any deviation from already found results.
The measurement of the area on the samples took place by individual measurement and following calculation. As the jewellery varies a lot in their design it can be difficult to measure the area precisely and therefore the measurement of the area took place with some approximation.
4.3 Analysis results
The results for migration to artificial sweat calculated in relation to sample weight (µg extracted metal per gram sample) are presented in Table 4-1. The values represent the average value for the duplicate determinations. In cases where the two determinations deviate significantly from each other both values are presented.
Table 4-1: Results from migration analysis (artificial sweat) of metals from 25 jewellery parts. The values are calculated as metal release per gram jewellery part. Blank fields indicate samples where the migration did not exceed the detection limit (The detection limit varied between 0.25 – 2.5 depending on the metal and is given in appendix H).
| Jewellery part no. | Description of jewellery part | As | Ba | Cd | Cr | Cu | Hg | Ni | Pb | Sb | Se |
| Unit: µg/g | |||||||||||
| 169.1 | Pendant | 0.2/11 | 0.5/23 | 17 | |||||||
| 164.1 | Heart backside | 2.6 | 2/4 | ||||||||
| 152.2 | Matt ring | 0.46 | |||||||||
| 138.1 | Pendant from behind | 2.4 | 0.57 | 150/210 | |||||||
| 136.2 | Disc | 1.2 | 0.81 | 100 | |||||||
| 130.1* | Needle | 25/1300 | |||||||||
| 125.1 | Fingerring | 0.86 | 0.73 | 0.5/29 | 93/540 | ||||||
| 107.1 | Chain with balls | 53 | |||||||||
| 101.3 | Cornet | 16/34 | |||||||||
| 101.1 | Backside of heart | 1.5 | 73 | ||||||||
| 99.2* | Non-twisted part | 150/220 | |||||||||
| 99.1 | Twisted rings | 160 | |||||||||
| 95.3 | Catch | 3.3 | 250 | ||||||||
| 91.1 | Big ball | 26/160 | 90/210 | ||||||||
| 91.3 | Chain | 16/28 | 13 | ||||||||
| 88.1 | Ring without stone | 0.6/16 | 2.3 | 2 / 5 | |||||||
| 70.3 | Catch | 1.5 | 140 | ||||||||
| 70.1 | Pendant | 0.31 | 0.50 | ||||||||
| 68.1 | Ring | 3.8 | 0.89 | 140 | |||||||
| 62.1 | Pendant | 3.0 | 0.67 | 39 | |||||||
| 60.1 | Bracelet | 3.4 | |||||||||
| 56.1 | Pendant backside | 2.1 | 23/64 | ||||||||
| 38.1 | Jewellery without stone | 12 | 0.59 | 220 | |||||||
| 26.2 | Catch | 0.44 | |||||||||
| 6.1 | Angel | 21 | 0.30 | 6/160 | |||||||
NB. Samples marked with * have been performed with an adjusted detection limit due to reduced sample size.
Results for migration to artificial sweat calculated in relation to sample area (µg extracted metal per cm²) are presented in appendix J.
Figure 4-1: Migration of respectively Cd, Cu, Ni and Pb in relation to the content of the respective metals (according to the XRF-screening) in the jewellery.
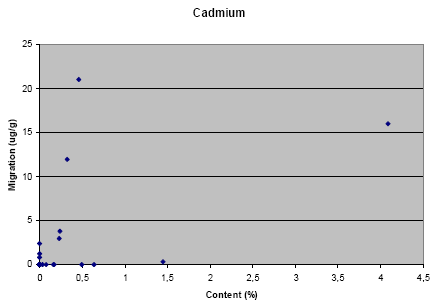
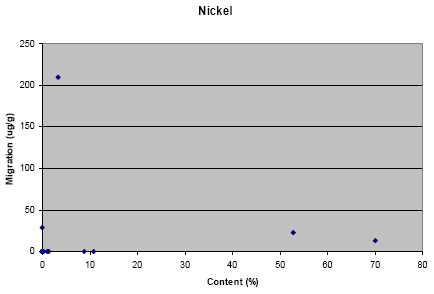
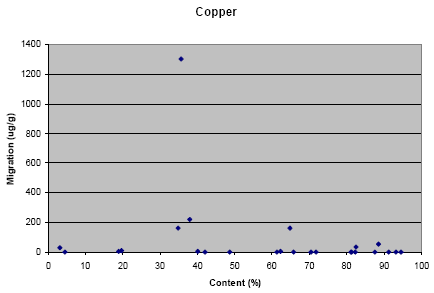
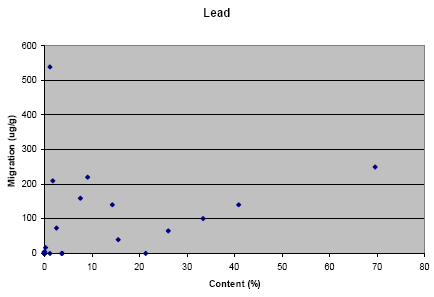
NB: In samples where the duplicate determination deviated significantly the highest value has been used.
As it is shown in Figure 4-1 there is no immediate relation between the migration and the content of the different metals. i.e. it is not possible to assume that a high concentration of the metal in jewellery gives a high migration. It must be noted though that for jewellery where the content of lead is less than approximately 1% hardly no migration has occurred. A similar tendency was not observed for the other metals (see appendix L).
There are too few pieces of jewellery in the migration analysis for it to be possible to conclude any relation between the jewellery parts, the jewellery type and the jewellery categories.
5 Benzidine analysis of textile necklaces
- 5.1 Results of the screening
- 5.2 Selection of textile necklaces for benzidine analysis
- 5.3 Analysis method
- 5.4 Results of the analysis
All together 62 textile necklaces were bought. The necklaces were subsequently screened in order to identify a potential content of easily soluble dyes. Azo-dyes are characterized by being easily soluble. The screening was performed by lowering the textile necklaces in water (40 degrees) for a period of 4 hours. Coloured extracts in the water are an indication of a possible content of azo-dyes which can form aromatic amines (among these benzidine) by degradation. During the test none of the textile necklaces were destroyed, since only a part of the textile necklace was lowered in the water. Though, in a few cases, it could not be avoided that the pendant as well was in contact with the water.
5.1 Results of the screening
The results of the screening test can be seen in Appendix I which contains the following information related to each textile necklace:
- Colouring of the water (1= yes; 0 = no)
- Shade of colour
- Degree of colouring (1 = weak; 2 = medium; 3 = strong)
55 of the 62 examined textile necklaces resulted in colouring of the water. Of these 27, which corresponds to approximately 44% of the examined textile necklaces, showed a strong colouring.
| Degree of colouring | Number of textile necklaces |
| No colouring | 7 |
| Week colouring | 15 |
| Medium colouring | 13 |
| Strong colouring | 27 |
| Total | 62 |
In a few cases the relatively small piece of necklace string was able to dye the water pitch-black during the 4 hour period (se figure below).

The distribution of the colourings according to number of necklaces can be seen below:
| Colouring | Number of textile necklaces |
| Turquoise | 2 |
| Black | 6 |
| Red/brown | 1 |
| Red | 8 |
| Orange | 1 |
| Dark green | 1 |
| Purple | 1 |
| Yellow | 16 |
| Green | 5 |
| Brown, blurred | 1 |
| Brown | 5 |
| Blue | 8 |
| Total | 55 |
5.2 Selection of textile necklaces for benzidine analysis
No obvious selection criteria were available, since one cannot rank a strong colouring alongside a bigger ”chance” of finding azo-dyes.
Furthermore, azo-dyes come in a variety of colours, thus a selection based on a certain shade of colour, is likewise not a reliable selection method.
Thus, the only selection criterion was to select textile necklaces which represented different shades of colours.
The 10 selected textile necklaces are presented in the table below.
Table 5-1: textile necklaces selected for benzidine analysis
| Textile necklace no. | Shade of colour detected during the screening |
| T6 | Black |
| T7 | Turquoise |
| T24 | Blue |
| T25 | Orange |
| T40 | Red |
| T44 | Black |
| T53 | Brown |
| T55 | Yellow |
| T59 | Red |
| T62 | Green |
5.3 Analysis method
The analysis for potential content of benzidine produced by reduction of one or more azo-dyes was conducted according to EN 14362-1:2003: extraction of colour in citric acid buffer, reduction by natrium dithionite, purification on column (Merck Extralut), extraction from column by methyl tert-butyl ether, concentration by means of heating and re-dissolvation in solvent (MTBE). Subsequently the extract was analysed for content of benzidine by GCMS. Duplicate determinations were performed for all samples besides sample no. T24, which was so small (180 mg) that duplicate determination was not possible. The detection limit related to sample no. T24 is thus larger (2 mg/kg). Five of the samples did not include enough textile string to perform duplicate determination based on extraction from 1g (as the standard prescribes), thus duplicate determinations were performed by extracting from approximately 0.5 g.
5.4 Results of the analysis
Two of the ten textile necklaces had a content of benzidine of 100 and 1200 mg/kg respectively, thus far above the permitted content of 30 ppm. The results can be seen in the table below. For samples marked with ”≤1 mg/kg” no signal for benzidine was observed.
Table 5-2: Results of the benzidine analysis of the 10 textile necklaces.
| Textile necklace no. | Benzidine (mg/kg) |
| T6 | ≤1 |
| T7 | ≤1 |
| T24 | ≤2 |
| T25 | ≤1 |
| T40 | ≤1 |
| T44 | ≤1 |
| T53 | 100 |
| T55 | ≤1 |
| T59 | 1200 |
| T62 | ≤1 |
The concentration of benzidine was determined by external calibration based on a solution with 1000 mg/l. This was done because the two samples, which showed a content of benzidine, had a content well above 10 mg/l.
6 Health assessment of lead, cadmium, copper and nickel
- 6.1 Lead
- 6.1.1 Occurrence and use
- 6.1.2 Identification
- 6.1.3 Physical and chemical properties
- 6.1.4 Oral absorption
- 6.1.5 Dermal absorption
- 6.1.6 Distribution
- 6.1.7 Acute toxicity
- 6.1.8 Local irritation and allergy
- 6.1.9 Long-lasting, repeated effect and gene-damaging effects
- 6.1.10 Tolerable daily intake - TDI
- 6.2 Cadmium
- 6.2.1 Occurrence and use
- 6.2.2 Identification
- 6.2.3 Physical and chemical properties
- 6.2.4 Oral absorption
- 6.2.5 Dermal absorption
- 6.2.6 Distribution
- 6.2.7 Acute toxicity
- 6.2.8 Local irritation and allergy
- 6.2.9 Long-lasting, repeated effect and gene-damaging effects
- 6.2.10 Tolerable daily intake - TDI
- 6.3 Copper
- 6.3.1 Occurrence and use
- 6.3.2 Identification
- 6.3.3 Physical and chemical properties
- 6.3.4 Oral absorption
- 6.3.5 Dermal absorption
- 6.3.6 Distribution
- 6.3.7 Local irritation and allergy
- 6.3.8 Acute toxicity
- 6.3.9 Long-lasting, repeated effect and gene-damaging effects
- 6.3.10 Tolerable daily intake - TDI
- 6.4 Nickel
- 6.4.1 Occurrence and use
- 6.4.2 Identification
- 6.4.3 Physical and chemical properties
- 6.4.4 Oral absorption
- 6.4.5 Dermal absorption
- 6.4.6 Distribution
- 6.4.7 Local irritation and allergy
- 6.4.8 Acute toxicity
- 6.4.9 Long-lasting, repeated effect and gene-damaging effects
- 6.4.10 Tolerable daily intake - TDI
The selection of metals for health assessment was based on the results from the migration analysis. Thus, only the 4 metals which migrated in a concentration above the detection limit were selected for exposure and health assessment.
The chosen metals are presented in the table below.
Table 6-1: Selected metals for exposure and health assessment.
| Selected metal | CAS No. | Chemical name | Number of samples (out of a total of 25) which showed migration |
| Pb | 7439-92-1 | Lead | 14 |
| Cd | 7440-43-9 | Cadmium | 10 |
| Ni | 7440-02-0 | Nickel | 5 |
| Cu | 7440-50-8 | Copper | 25 |
In the following health assessment it is assumed that metal in contact with sweat primarily forms metal chlorides. Thus, TDI values, dermal and oral absorption rates are, as far as possible, based on information related to metal chlorides (or the metal itself) – or alternatively studies regarding inorganic metal compounds.
The argumentation for this assumption is partly based on a study by Menné (1994) quoted from ATSDR (2005a), which claims that nickel alloys that are in contact with the skin, form nickel chlorides. The argumentation is likewise based on a risk assessment of nickel performed by the Danish Environmental Protection Agency in 2005 (Andersen et al., 2005a). According to this risk assessment a corrosion of metal surfaces takes place when the metal surfaces are in close contact to skin/sweat. The corrosion is caused by the constituents of sweat. The primary constituents in sweat are chlorides (average value: 0.44-1.44 g/L), natrium (0.33-1.28 g/L), kalium (0.29-0.39 g/L), urea (0.26-1.22 g/L), ammonia (0.06-0.11 g/L), amino acids (0.48-1.4 g/L) and lactic acid (0.4-3.6 g/L). The corrosion of metal in sweat is according to the risk assessment primarily dependent on the chloride and oxygen content, which in part supports the assumption of metal chlorides being the primary component formed in sweat.
The solution used for simulating saliva contains, besides the constituents mentioned above, also natrium sulphate. However, natrium sulphate is not expected to form organic compounds, thus it is assumed that the primary metal compound of interest (which will form in saliva, which is in contact with metal jewellery) likewise is metal chloride.
However, it is possible that other forms of metal compounds can be formed in sweat (or saliva), compounds that might have a significant impact on the total absorption of metals. However, it is not possible within the scope of this project to clarify exactly which kind of metal compounds that can be formed (besides metal chlorides), since the literature on this subject is very limited. In theory, studies (including laboratories tests) should be performed in order to clarify exactly which compounds are formed.
A massive amount of information is available regarding the health effects associated with the four metals. Thus, it is stressed that the health assessments carried out below are based on extracts of the most relevant available information for this project.
The primary goal of the health assessments here is to locate a NOAEL (No Observed Adverse Effect Level) or TDI (Tolerable Daily Intake) value related to the critical effect of the metal. The term “critical effect” relates to the health effect that occurs at the lowest exposure of the substance. NOAEL or TDI values related to this critical value are used subsequently along with an exposure calculation in order to clarify whether the amount of metal that humans are exposed to by carrying/sucking jewelleries constitutes a health risk. During the exposure calculation the identified dermal and oral absorption rates related to the four metals are used. Here it should be mentioned, that it is assumed that metal absorbed through the skin causes the same toxic effects in the body, as metal ingested through the mouth.
6.1 Lead
6.1.1 Occurrence and use
Lead is a heavy and soft metal which exists naturally in the earth’s crust, however, in the form of lead compounds. Lead is corrosion-resistant, easy to mould, acid-resistant, chemically stable in water, air and soil, and can be combined with other metals in the form of alloys. Due to these qualities lead and lead alloys are used worldwide for among other things pipes, batteries, ammunition, cables and panels for protection against X-raying. The primary use of lead today is in batteries for use in cars and other vehicles (ATSDR, 2005).
In addition to this lead is also used in the production of jewelleries. The reason for this is probably that lead is a relatively cheap metal, which besides being corrosion-resistant, is easy to mould. Furthermore, a content of lead can help make the piece of jewellery heavier and thereby increase the similarity to precious jewellery. Lead weighs almost the same as gold. The use of lead in jewellery could also be caused by the fact that lead can affect the surface of the jewellery in such a way that it looks a bit like precious metal.
Prior to the commencement of the statutory order regarding lead the lead consumption was estimated. In Denmark the total consumption of lead (in finished products) in 2000 was estimated to be between 14,900 and 19,000 tonnes. The consumption was divided between metallic lead (91%), chemical compounds (9%) and “supporting substance” in other goods (0.06%) (Lassen et al., 2004). Worldwide approx. 7 mio. tonnes of lead are produced each year, of which half originates from recycled metal waste[1]. According to WHO (2003) more than 80% of the daily intake of lead originates from food, soil and dust.
6.1.2 Identification
| Chemical name | Lead |
| Synonyms | Pigment metal Metallic lead Plumbum |
| CAS-No. | 7439-92-1 |
| EINECS No. | 231-100-4 |
| Gross formula | Pb |
| Molecular structure | Pb |
| The list of dangerous substances (Stat.Ord. 923, 2005) The list of unwanted substances (Briefing from the Danish EPA, no. 8, 2004) Danish EPA - Self classification (Environmental project no. 635, 2001) |
No, however 23 lead compounds are on the list. Among those: Lead acetate: REP1: R61 R33; May cause harm to the unborn child; Danger of cumulative effects. CARC3: R40: Possible risk of cancer. XN: R48/22: Harmful: danger of serious damage to health by prolonged exposure if swallowed. REP3: R62: Possible risk of impaired fertility. N: R50/53: Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. Lead compounds (not otherwise mentioned on the list): REP1: R61: May cause harm to the unborn child. XN: R20/22: Harmful by inhalation and if swallowed. REP3: R62: Possible risk of impaired fertility. N: R59/53: Dangerous for the ozone layer; may cause long-term adverse effects in the aquatic environment. Yes (lead and lead compounds) No |
6.1.3 Physical and chemical properties
| Physical state | Silver-bluish metal | Chemfinder |
| Molecular weight (g/mol) | 207.2 | Chemfinder |
| Melting point | 327.43 °C | Chemfinder |
| Boiling point | 1740 °C | Laurson et al. (2003) |
| Vapour pressure | 236 Pa at 1000 °C | Laurson et al. (2003) |
| Octanol/water partition coefficient (log POW) | Not relevant | |
| Solubility | Insoluble | Chemfinder |
6.1.4 Oral absorption
Lead taken up through food or beverages is absorbed differently depending on when the person last had a meal. Experiments have shown that adults who had just eaten a meal only absorbed 6% of the ingested amount of lead, while adults who had not eaten for a period of 24 hours absorbed between 60 and 80% of the ingested amount of lead. Typically, children absorb 50% of the ingested amount of lead (ATSDR, 2005).
For use in this project an oral absorption rate of lead compounds is set at 50% - based on the value measured for children. This value is assumed to be a representative value for oral absorption in adults as well, since this value lies between the value for lead absorption with prior food ingestion (6%) and lead absorption without prior food ingestion (60-80%) at adults.
6.1.5 Dermal absorption
Dermal absorption of inorganic lead compounds is generally assumed to be lower than absorption via inhalation or food. Experiments with application of cosmetic products containing 203Pb-marked lead acetate (0.12 mg Pb in 0.1 ml or 0.18 mg Pb in 0.1 g lotion) on 8 adults for a period of 12 hours showed absorption of = 0.3%, based on203Pb measurements in urine and blood in the entire body. It was assumed that by normal use (of the lotions) only 0.06% would be absorbed (Moore et al., 1980 in ATSDR, 2005).
Other experiments (3 non-specified persons, period of 24 hours) with dermal exposure of 5 mg Pb as lead nitrate or lead acetate resulted in less than 1% absorption. The same study showed no absorption of lead carbonate (ATSDR, 2005).
Results from animal experiments have shown similar low absorption rates (lead naphthenate: 0.17%; lead nitrate: 0.03%; lead stearate: 0.006%; lead sulphate: 0.0006%, lead oxide: 0.005% and lead-powder: 0.002%) (Bress and Bidanset, 1991 in ATSDR, 2005).
Based on the above mentioned information a dermal absorption rate of lead (in sweat) of 0.06% is used in this project.
6.1.6 Distribution
Following oral absorption the lead is transported via the red blood cells (where the lead is bound to haemoglobin) from the intestines to the different organs (WHO, 2003). At first the lead ends up in organs such as liver, kidney, lungs, brain, spleen, muscles and heart. After several weeks most of the lead ends up in bones and teeth (ATSDR, 2005). The half-life of lead in blood and “soft tissue” is in adults 20-30 days (IARC, 2006), while it in bones is approx. 30 years (Baars et al., 2001).
In adults approx. 94% of the total amount of accumulated lead ends up in bones and teeth, while in children 73% of the accumulated lead ends up in bones (ATSDR, 2005). Lead can be released from the bones in situations where the person suffers from calcium deficiency or osteoporosis (WHO, 2000). Lead is easily transferred to foetuses during pregnancy (Baars et al., 2001). Inorganic lead is not converted in the body. Unabsorbed lead, which is absorbed via the food, is released through the faeces, while absorbed lead, which is not retained, is released via the kidneys (WHO, 2003).
Children, who ingest more than 5 µg lead per kilo body weight per day, will retain (netto) 32% of the intake, while children who ingest less than 4 µg lead per kilo body weight per day will excrete more than what is taken in (WHO, 2003).
6.1.7 Acute toxicity
Lead affects pretty much all organs in the body, and serious lead poisoning can cause death. This was confirmed by the death of a 4-year old boy, who by accident had swallowed a heart-shaped metal charm (that came along free of charge by purchasing a pair of shoes). The metal charm showed to contain 99% of lead. At the time of death the boy had a blood lead concentration (in short PbB) of 180 µg/dL (Berg et al., 2006).
Obvious signs of acute lead poisoning involve dullness, restlessness, irritation, poor power of concentration, headache, vibrations in muscles, stomach cramps, kidney injuries, hallucinations and loss of memory. These effects can occur at PbB levels of 100-120 µg/dL in adults and 80-100 µg/dL in children (WHO, 2003).
Though, health effects are generally not observed after single doses and no LD50 value related to lead and lead compounds (in humans) are located in the literature (WHO, 2000).
The lowest observed values related to acute deathly oral doses are found in animal studies using lead acetate, lead chlorate, lead nitrate, lead oelate, lead oxide and lead sulphate. The result varied from 300 to 4000 mg per kilo bodyweight. The large span in the result was due to varying absorption of the different lead salts as well as differences in the exposure (WHO, 2000).
6.1.8 Local irritation and allergy
According to IUCLID (2000) no data is available regarding the ability of lead to cause local irritation. Nonetheless, lead(II) oxide (PbO) is known to be moderate skin irritating at exposure levels of 100 mg over a period of 24 hours (IUCLID, 2000a).
According to IUCLID (2000) no data is apparently available regarding the potential allergic qualities related to lead.
6.1.9 Long-lasting, repeated effect and gene-damaging effects
Lead is a chronic accumulative poison. Signs of chronic lead poisoning includes among other things tiredness, sleeplessness, irritation, headache, pains in the joints and problems related to the stomach- and intestinal system. These effects can occur in adults having a PbB between 50-80 µg/dL (WHO, 2003). Lead poisoning in children can additionally cause reduced growth and delayed sexual maturation (ATSDR, 2005).
A number of studies exist, which deal with health related effects of lead and lead compounds. Some of these studies are described in ATSDR (2005) and WHO (2003). Below a segment of these is presented, though focusing on studies which have shown health related effects at the lowest measured concentration of lead in the body.
Neurological effects have been reported in workers having a PbB at 40-80 µg/dL. The neurological effects included among other things uneasiness, forgetfulness, irritation, dullness, headache, tiredness, impotence, decreased libido, dizziness and weakness (ATSDR, 2005).
The kidney function seems to be the biological function, which according to ATSDR (2005) is affected at the lowest measured PbB. Two studies have shown effects related to this function at PbB < 10 µg/dL. Another typical symptom related to chronic lead poisoning is anaemia. At a blood lead concentration < 10 µg/dL an enzyme involved in the synthesis of red blood cells has been seen inhibited. Decreased neurological activity has been reported in children and elderly people with a PbB < 10 µg/dL and according to WHO (2003) a study (from 1987) examining 500 schoolchildren (age 6-9 years) showed a small, but significant correlation between PbB and reduced intelligence test, reading and speaking abilities. The dose-response relationship was between 5.6 – 22.1 µg/dL. However, another similar study has not been able to reproduce the results (WHO, 2003).
Regarding reproductive effects a relation between PbB> 20 µg/dL and an increased chance of abortion and stillborn babies have been proven (WHO, 2000). EPA (1986a) quoted from ATSDR (2005) has furthermore identified a LOAEL value of 60-100 µg/dL related to colic in children as a result of lead poisoning.
Generally inorganic lead compounds are according to IARC (2006) “potentially cancer-causing in humans” (Group 2A), while organic lead compounds are not classified as to their cancer-causing ability in humans.
6.1.10 Tolerable daily intake - TDI
Baars et al. (2001) has through a review of new literature since 1991 not been able to find arguments for altering the TDI value of 25 µg/kg bw/week (based on effects at a PbB at 10 µg/dL), which in 1995 was found still to be valid by WHO (1995) – both regarding adults as well as children. Baars et al. (2001) calculated, based on this TDI value, a tolerable daily intake (TDI) of lead to be 3.6 µg/kg bw/day.
ENHIS (European Environment and Health Information system) presents on their homepage² (updated 14 January 2008) likewise a TDI value related to lead of 25 µg/kg bw/week.
However, WHO (2003) describes a number of studies, which indicate a possible correlation between reduced IQ and a PbB of < 10 µg/dL (5.6 µg/dL). It is, however, not possible based on these studies to calculate a new NOAEL value, since there is not enough information available. However, to take into consideration these new results, which indicate that the TDI value of 3.6 µg/kg bw/day (which is based on effects at a PbB of 10 µg/dL), might be too high, it is chosen to divide the TDI value by two. Thus, a TDI value of 1.8 µg/kg bw/day emerges, which in this project is chosen as the valid TDI value.
Adjustment of TDI values due to background exposure
TDI values shall be seen as the total amount of substance a human can tolerate to take in (ingest) on a daily basis (throughout an entire lifetime) without experiencing health related effects, cf.[2]. However, when dealing with tolerable daily intake of lead, it is important to take into consideration the amount of lead that the population already is exposed to (for instance lead from water, food and air).
Background exposure of lead via food and beverages in Denmark
According to a report published by the Danish Veterinary and Food Administration (Fromberg et al., 2005) the background exposure from food etc. in Denmark is in average 19 µg/day. The numbers in the report are based on studies regarding content of metals in 96 foods in Denmark during the period 1998-2003 (Fromberg et al., 2005), as well as numbers regarding the average food intake in Denmark during the period 2000-2002 (Andersen et al., 2002). The numbers given by Fromberg et al. (2005) include exposure via beverages and tap water.
A recalculation of the average value of 19 µg/day to intake per kilo bodyweight (by the use of TGD’s reference weight related to women (60 kg), since women are assumed to be those, who most often wear jewellery) gives an average daily intake of lead for adults of (19/60) 0.317 µg/kg bw/day. Fromberg et al. (2005) also states numbers for the average intake of lead by children in the age 4-6 years. According to these numbers a child of 4-6 years take in – in average – 9.7 µg lead/day. According to netdoktor.dk[3] a child of 5 years weighs 19 kg (both girls and boys). However, netdoktor.dk informs that children, since the study of which the numbers were based upon was performed, have gotten heavier. It is therefore assumed that a 5 year old child in Denmark weighs 20 kg. This number also correlates with the reference weight, which according to the TGD is supposed to be used for children. With these data in mind, the average background exposure of lead (through food) in children in Denmark is (9.7/20) 0.485 µg/kg bw/day.
Fromberg et al. (2005) also states the background exposure in terms of 95-percentiles (i.e. a value for the maximum level of which 95% of the population is exposed to). If these values are used a background exposure for adults of (31/60) 0.517 µg/kg bw/day is achieved. For children the background exposure would be (15.4/20) 0.77 µg/kg bw/day.
Background exposure of lead via air in Denmark
According to the report “The Danish Air Quality Monitoring Programme” from 2006 (performed by the National Environmental Research Institute) (Kemp et al., 2007) the highest measured average value for content of lead in the air in Denmark was 9.1 ng/ m³ (value measured in Copenhagen, on the street “H.C. Andersen’s Boulevard”). In order to choose a conservative approach this value is used to estimate the amount of lead, that humans in Denmark are assumed to inhale through the air.
According to the TGD an adult inhales 18 m³ of air per 24 hours, while a child of 20 kg (5 years) inhales 11 m³ per 24 hours. Thus, an adult (60 kg) inhales (18m³×9.1ng/m³/1000) 0.1638 µg lead per day. Converted into per kilo bodyweight (60 kg) the number is 0.003 µg/kg bw/dag. And a child (20 kg) would take in (11m³×9.1ng/m³/1000) 0.1001 µg lead per day, which converted into per kilo bodyweight gives a figure of 0.005 µg/kg bw/day related to intake of lead through the air.
Total background exposure
Since the TDI value, as mentioned earlier, represents the maximum amount of substance a person can take in per day (through their entire life) without causing health related effects, the TDI value is deducted the above mentioned values for background exposure. Thereby a number “Margin to TDI value” arises. This number represents the “extra amount” of lead, which a human can have on a daily basis (besides what they are exposed to via food, beverages and air) without experiencing health related effects. During the risk assessment this value is compared with what humans are exposed to by wearing or sucking the jewelleries, which are examined in this project. If the exposure exceeds this “Margin to TDI value” there will be a health related risk by wearing and/or sucking the jewelleries.
Table 6-2: Background exposure of lead in Denmark and “Margin to TDI value” (µg/kg bw/day).
| Background exposure | Children (4-6 years) | Adults | ||
| Average | 95-percentile | Average | 95-percentile | |
| Food and beverages | 0.485 | 0.77 | 0.317 | 0.517 |
| Air* | 0.005 | 0.005 | 0.003 | 0.003 |
| Total background exposure (food, beverages, air) | 0.49 | 0.78 | 0.32 | 0.52 |
| Margin to TDI value (1.8 – total background exposure) | 1.31 | 1.02 | 1.48 | 1.28 |
NB: The 95-percentile relates to the numbers representing background exposure via food and beverages (including tap water). * The value represents an average value from the monitoring station in Denmark, which showed the highest average in 2006.
If people live in surroundings where they are exposed to higher background levels than the ones mentioned above, the health risk by using the examined jewelleries in this project will be underestimated.
6.2 Cadmium
6.2.1 Occurrence and use
Cadmium is a soft silver-white metal that occurs naturally in the surface of the earth. It is often found as cadmium oxide, cadmium sulphite and cadmium carbonate in zinc, lead and copper mineral veins. Furthermore, cadmium is found as cadmium chloride and cadmium sulphite compounds. Both of them are water-soluble (ATSDR, 1999).
Cadmium, cadmium alloys and cadmium compounds are used worldwide in a long range of products. Five main categories are nickel-cadmium batteries, pigments primarily used in plastic, ceramics and glass, stabiliser in PVC, surface cover on steel, few non-iron metals and components in various alloys (ATSDR, 1999). Furthermore, cadmium is often used as solder metals because the content of cadmium promotes the solder properties – the solder metal floats well and easily into the small cracks when there is a content of cadmium. Furthermore cadmium can be a naturally found metal when silver is extracted and this is why it traditionally/historically occurs in silver alloys from some parts of the world. Because of this jewelleries made of so-called ”Indian silver” often have a high content of cadmium. Cadmium is also cheaper than precious metals.
The use of cadmium in Denmark in 1996 was estimated to be 43–71 tons (Environmental project no. 557, 2000).
6.2.2 Identification
| Chemical name | Cadmium |
| Synonyms | Colloid cadmium |
| CAS-No. | 7440-43-9 |
| EINECS No. | 231-152-8 |
| Gross formula | Cd |
| Molecular structure | Cd |
| The list of dangerous substances (Stat.Ord. 923, 2005) The list of unwanted substances (Briefing from the Danish EPA no. 8, 2004) Danish EPA self-classification (Environmental project no. 635, 2001) |
Yes. (cadmium, not stabilized) CARC2;R45: can promote cancer. F;R17: Spontaneously flammable in air. TX;R26: Very toxic by inhalation. T;R48/23/25: Toxic: Danger of serious damage to health by prolonged exposure. REP3;R62-63: Possible risk of impaired fertility; Possible risk of harm to the unburn child. MUT3;R68: Possible risk of irreversible effects. N;R50/53: Very toxic to aquatic organisms; May cause long-term adverse effects in the aquatic environment. (some cadmium compounds are also to be found on the list). Yes (cadmium and cadmium compounds) No |
6.2.3 Physical and chemical properties
| Physical state | Silver-white blank metal with a blue note | Chemfinder |
| Molecular weight (g/mol) | 112.41 | Chemfinder |
| Melting point | 320.9 °C | Chemfinder |
| Boiling point | 765 °C | Chemfinder |
| Vapour pressure | 1 mm Hg at 394 °C | ATSDR (1999) |
| Octanol/water partition coefficient (log POW) | Not relevant | |
| Solubility | Insoluble | Chemfinder |
6.2.4 Oral absorption
An experiment with absorption of cadmium through food (presumably single dosages) showed an absorption of 6% after 20 days. The experiment was performed on 5 adults (Rahola et al., 1973 in ATSDR, 1999). Similar results were achieved in an experiment with 14 adults who absorbed 4.6% cadmium in average from a cadmium chloride solution (administered together with food) after 1-2 weeks where a faecal marker was extracted (McLellan et al., 1978 in ATSDR, 1999). According to WHO (2004) an experiment has been reported (from 1987), where 3-7% cadmium has been absorbed in healthy adults while 15-20% was absorbed in humans suffering from iron deficiency.
As the experiment above indicates the content of iron in the body affects the absorption of cadmium. Experiments using humans with low iron deposits have shown an absorption of 8.9% while humans with adequate iron deposits had an absorption of 2.3% (Flanagen et al., 1978 in ATSDR, 1999).
The EU risk assessment of cadmium from 2007 concludes that the oral absorption of CdO/Cd metal generally is lower than 5%, but only when iron deposits are adequate. When iron deposits are low (typically for women including pregnant women), the oral absorption can rise to 5-10%.
Based on the information above an oral absorption of cadmium for use in this project is set at 8.9%. This value has been chosen because it cannot be ruled out that a part of the Danish population suffers from iron deficiency, among these especially women (who often are those wearing jewellery). The value of 8.9% has been chosen as opposed to the 10% mentioned as maximum absorption in the EU risk assessment, because the experiments that the 10% are based on are not described in details. In addition, the value of 8.9% represents an experiment performed with cadmium chloride, which is seen as relevant for this project.
6.2.5 Dermal absorption
A study (Wester et al., 1992) investigated dermal absorption of cadmium by using in vitro skin cells from humans. Radioactive cadmium (109CdCl2) was poured over the skin for a period of 16 hours. The result was that 0.1-0.6% of the cadmium was absorbed into the plasma.
Experiments with dermal cadmium absorption in animals showed an absorption (measured in liver and kidneys) of 0.4–0.61% two weeks after ended treatment. The experiment involved a rabbit that was dosed with CdCl2 on the skin using a 1% aqueous solution (6.1 mg Cd) or 2% ointment (12.2 mg Cd). The area used on the rabbit consisted of a 10 cm² shaved area. The rabbit was treated 5 times during a period of 3 weeks. Only the content in the kidneys and liver was measured which means that the total skin absorption could have been higher (ATSDR, 1999).
A similar experiment with a hairless mouse, which was treated with CdCl2 on the skin using a 2% ointment (containing 0.61 mg Cd) showed an absorption of cadmium in kidneys and liver of between 0.2 and 0.87% (ATSDR, 1999).
A study described in EU's risk assessment of cadmium from 2007 shows a dermal absorption of cadmium through human skin of 0.6% (absorption into plasma). This study describes the highest measured value for dermal absorption through human skin (sofar identified in this project) and is therefore chosen as valid for dermal absorption of cadmium in this project.
6.2.6 Distribution
Most of the absorbed cadmium ends up in liver and kidneys and stays there for several years. A small amount of the absorbed cadmium will, though, leave the body slowly through the urine and faeces (ATSDR, 1999). The way cadmium is absorbed does not influence the distribution in the body in a degree worth mentioning.
Absorbed cadmium is transported to other parts of the body through the blood. Cadmium will in the body bind to metal-lothioneine where after it will be released to the urine through the kidneys. From the urine it will be re-absorbed. After the re-absorption the bonding to metal-lothioneine is broken and the free cadmium stimulates the production of metal-lothioneine, which again bind cadmium in the kidney cells – thereby preventing the toxic effect related to the free cadmium ion. If the production of metal lothioneine cannot follow the result is damage on the kidney cells, which can be detected by an increased release of proteins (with a low molecular weight) in the urine (Friberg et al., 1986 in WHO, 2004).
In humans the average cadmium concentration in liver and kidneys is equal to nil at birth but increases gradually to around 40-50 mg/kg (w/w) in the kidneys at the age of 50-60 and 1-2 mg/kg (w/w) in the liver at the age of 20-25 (Baars et al., 2001). After ”normal” exposure of cadmium from background levels approximately 50% of the body’s total amount of cadmium is found in the kidneys, approximately 15% in the liver and around 20% in the muscles. Half life periods for cadmium in kidneys and liver are estimated to 6-38 years and 4-19 years respectively (Baars et al., 2001).
6.2.7 Acute toxicity
Cadmium compounds have a moderate acute toxic effect but oral absorption of large amounts of cadmium gives a massive loss of liquid, liquid accumulations, extensive destruction of organs and finally death (Buckler et al., 1986; Wisniewska-Knypl et al., 1971 in ATSDR, 1999). According to a study by Buckler et al. (1986) a 17 year old girl died (unknown weight) 30 hours after having absorbed 150 grams of cadmium chloride.
Oral LD50 values for mice and rats lie between 60 and 5000 mg/kg bw. The most significant effects are desquamation of the tissue in the stomach-intestine canal, destruction of the mucous membrane in the stomach-intestine canal and nutrition disruptions in the liver, heart and kidneys (Krajnc et al., 1987 in WHO, 2004). The lowest acute oral LD50 value resulting in death (for two rats) in a study with 20 rats, was 15.3 mg/kg (Borzelleca et al., 1989 in ATSDR, 1999).
6.2.8 Local irritation and allergy
Cadmium chloride can induce sting and first degree burns on the skin at short time exposure (HSDB).
According to ATSDR (1999) dermal exposure of cadmium does not seem to have any effect on the immune system. Routine ”plaster-tests” among dermatitis and patients with Eczema showed skin irritation at exposure with a 3% cadmium chloride solution. However no signs of allergenic reactions were found at an exposure with a 1% solution on humans who had not been exposed to cadmium previously (Rudzki et al., 1988; Wahlberg, 1977 in ATSDR, 1999).
A study with guinea pigs showed no signs of allergenic reactions after intra dermal or topical exposure of cadmium chloride in concentrations up to 0.5% (ATSDR, 1999).
6.2.9 Long-lasting, repeated effect and gene-damaging effects
Exposure of cadmium/cadmium compounds includes a number of health damaging effects as described in ATSDR (1999). Several studies have indicated that oral absorption of cadmium in high concentrations induces serious irritation of the ingestion system. The typical symptoms include nausea, vomiting, saliva secretion, stomach pains, cramps and diarrhoea. There are no precise values for the dosage available but a content of 16 mg/L cadmium in lemonade has shown to induce stomach problems for children. If an absorption of 0.15 litre and a body weight of 35 kg are assumed, a dosage promoting vomiting is 0.07 mg/kg (ATSDR, 1999).
Several studies have indicated that the kidneys are the organ that is most sensitive to long-term oral exposure of cadmium. The critical (irreversible) effect is kidney damage characterized by enhanced excretion of proteins with low molecular weight in the urine. A study has shown effects at a concentration of cadmium of 50 µg/g wet weight in the renal cortex. The study indicated in a similar way that the critical concentration can be lower for the general population than for people working with cadmium (Buchet et al., 1990 in ATSDR, 1999).
According to Baars et al. (2001) new experiments indicate that the lowest cadmium concentration in the kidneys, which induces kidney damage at approximately 4% of the general population is about 50 mg/kg. This is a level that can be expected to be reached after 40-50 years absorption of 50 µg cadmium per day (corresponding to 1 µg/kg bw/day). This value was established by, among others, the WHO in 1991 as an oral human-toxicological MPR value (maximum acceptable risk) for cadmium, based on kidney damage as the most sensitive effect after oral absorption of cadmium.
However, Baars et al. (2001) claimed that because this oral absorption of 1 µg/kg bw/day results in effects in 4% of the population a TDI value should be set lower. Thus, they used an additional safety factor of 2 and reached a TDI value of 0.5 µg/kg bw/day (Baars et al., 2001).
However, ATSDR (1999) reported an even lower TDI value of 0.2 µg/kg/day related to chronic effects on the kidneys (abnormal concentration in the urine of ß2-microglobuline). The study underlying this value is a study of Nogawa et al. (1989) which includes 1850 cadmium-exposed humans and 294 non-exposed humans. The study showed a NOAEL value of 0.0021 mg/kg/day. ATSDR (1999) used a safety factor of 10 for variation between humans and reached a TDI value of 0.2 µg/kg bw/day.
According to IARC (1997a) cadmium and cadmium compounds are carcinogenic for humans.
6.2.10 Tolerable daily intake - TDI
ATSDR (1999) states an TDI value of 0.2 µg/kg bw/day, which is the lowest value reported in ATSDR.
However, FAO/WHO´s food committee JECFA has as late as 2005 re-assessed cadmium and established a PTWI (provisory tolerable weekly intake) of 0.007 mg/kg bw, which corresponds to 1 µg/kg bw/day. This value is also given at ENHIS’s (European Environment and Health Information System) homepage (updated 14 January 2008).
The value is also used by the food institute in Denmark. The Chemical Agency in Sweden ”Kemi” also uses this PTWI value but states that the value represents an “effect level” and judge that it should be considered to lower the value by using an additional safety factor.
Due to this an additional safety factor of 2 is used in this project resulting in a TDI value of 0.5 µg/kg bw/day.
Adjustment of TDI values due to background exposure
As already mentioned TDI values shall be seen as the amount of substance that humans can tolerate to take in on a daily basis (throughout their entire life) without causing health related effects. It is here important to take into consideration the amount of cadmium that the population already is exposed to (via food, smoking, water and air).
Background exposure of cadmium via food and beverages in Denmark
According to Fromberg et al. (2005) the background exposure of cadmium from food and beverages in Denmark is in average 10 µg/day for adults. If this value is converted to absorption per kilo body weight (based on women’s weight according to TGD (60kg)) an average absorption of cadmium for adults will be (10/60) 0.167 µg/kg bw/day. Children are exposed to 7.7 µg cadmium in average per day according to Fromberg et al. (2005), which converted will result in a background exposure of (7.7/20) 0.385 µg/kg bw/day.
Fromberg et al. (2005) also states the background exposure in 95-percentiles (i.e. a value for the maximum level of which 95% of the population is exposed to). If these values are used a background exposure is obtained for adults of (17/60) 0.283 µg/kg bw/day and for children of (11.9/20) 0.595 µg/kg bw/day.
Background exposure of cadmium via air in Denmark
According to the report ”The Danish Air Quality Monitoring Programme” from 2006 (prepared by National Environmental Research Institute) (Kemp et al., 2007) the highest measured average value for the content of cadmium in the air in Denmark was <2.4 ng/m³ (the value was measured on “Banegårdsgade” in Aarhus). In order to choose a conservative approach this value is used to estimate the amount of cadmium, which an adult in Denmark is expected to inhale via the air.
According to the TDG an adult inhales 18 m³ air during a period of 24 hours, while a 5 year old child (20 kg) inhales 11 m³ during the 24 hours. This means that an adult human (60 kg) absorbs (18m³×2.4ng/m³/1000) 0.0432 µg cadmium per day, which corresponds to 0.001 µg/kg bw/day. Children (20 kg) absorb (11 m³×2.4ng/m³/1000) 0.0264 µg cadmium per day, which corresponds to an absorption of cadmium through the air of 0.001 µg/kg bw/day for children.
Background exposure of cadmium via smoking
According to Baars et al. (2001) a human smoking 20 cigarettes a day absorbs 1-2 µg cadmium a day. Converted into absorption per kilo body weight this corresponds to (2/60) 0.033 µg cadmium/kg bw/day. The data is from 1992 but is assumed still to be valid because the content of cadmium in cigarettes is not assumed to have changed significantly since 1992. Children of 4-6 years of age are not expected to smoke.
Total background exposure
Because the TDI value as mentioned previously represents the amount of substance that a human as maximum can take in per day (during a life time) without causing health related effects, the TDI value is subtracted the above mentioned data for background exposure. Thereby a number is reached (”Margin to TDI value”) representing the ”extra amount” of cadmium that a human can take in on a daily basis (besides what a human is exposed to through food, beverages, air (and smoking)) without experiencing health damaging effects. This value is compared in the risk assessment with the amount that humans are exposed to by wearing or sucking the jewellery examined in this project.
Table 6-3: Background exposure of cadmium in Denmark and “Margin to TDI value” (µg/kg bw/day).
| Background exposure | Children (4-6 years) | Adults | ||
| Average | 95-percentile | Average | 95- percentile |
|
| Food and beverage | 0.385 | 0.595 | 0.167 | 0.283 |
| Air* | 0.001 | 0.001 | 0.001 | 0.001 |
| Total background exposure (food, beverages, air) | 0.39 | 0.60 | 0.17 | 0.28 |
| Margin to TDI value (0.5 – total background exposure) | 0.11 | -0.1 | 0.33 | 0.22 |
NB: The 95-percentile relates to the data for background exposure via food and beverages (including tap water). *The value for air is an average from the monitoring station in Denmark that has shown the highest average. For smokers the total background exposure must be added a value of 0.033 (based on a consumption of 20 cigarettes per day).
As it is shown in the table it seems that the 5% of the Danish children, which are being exposed to the highest background exposure, absorbs an amount of cadmium on a daily basis that is higher than the tolerable daily dosage.
In addition it must be mentioned that adult smokers absorb 0.033 µg cadmium extra per kilo body weight per day. Because this exposure is of own choosing it is not included in the calculations of exposure.
If people live in areas where they are exposed to higher background concentrations than those described above, the health risk when using the jewelleries examined in this project will be underestimated.
6.3 Copper
6.3.1 Occurrence and use
Copper is a reddish metal, which naturally occur in stone, soil, water, sediment and (in low concentrations) in the air. Furthermore, copper is found in a number of different minerals like, among others, chalcocite (Cu2S), malachite (CuCO3*Cu(OH)2) and chalcopyrite (CuFeS2). Copper is naturally found in all plants and animals and is an essential element (in low concentrations) for all living beings (ATSDR, 2004).
Copper is a much used metal, primarily due to its properties as a durable, flexible, malleable metal which can conduct electricity and heat. It is primarily used as a metal in alloys (such as bronze and brass). A minor amount of copper is also used as a constituent in the production of copper compounds, primarily copper sulphate (ATSDR, 2004).
The use of copper is distributed throughout the industry sector as follows: construction (39%), electrical products (28%), transport equipment (11%), industrial machinery and equipment (11%) and consumer products (11%). The 10 most important markets for copper and copper alloys in 1986 were piping, building cables, telecommunication, power stations, equipment for use in plants, air-condition, electrical and non-electrical equipment for the car industry, electronics for the industry and industrial valves and fittings (ATSDR, 2004).
Copper compounds are used in the agriculture as fungicides, algicides, insecticides and pesticides. Furthermore, copper sulphate is used in the industry during production of azo-dyes and textile dyes as well as during refining of petroleum (ATSDR, 2004).
Finally, during many thousands of years copper has been used for production of jewelleries. One of the reasons why copper is used in jewelleries is that copper is the only other metal except from gold which naturally gives a red or yellow colour in alloys. Other reasons are that it is very resistant to corrosion and easy to prepare for plates, threads and similar as well as it is reasonably easily accessible and relatively harmless to work with.
6.3.2 Identification
| Chemical name | Copper |
| Synonyms | Pigment metal Raney copper CuTEA |
| CAS-No. | 7440-50-8 |
| EINECS No. | 231-159-6 |
| Gross formula | Cu |
| Molecular structure | Cu |
| The list of dangerous substances (Stat. Ord. 923, 2005) The list of unwanted substances (Briefing from the Danish EPA no. 8, 2004) The Danish EPA’s self classification (Environmental project no. 635, 2001) |
No. However, 31 copper compounds are on the list, including copper(I)chloride with the classification XN; R22 (Dangerous if swallowed) and N;R50/53 (Very toxic for aquatic organisms; may cause long-term adverse effects in the aquatic environment). Yes (copper and copper compounds) No |
6.3.3 Physical and chemical properties
| Physical state | Reddish brown, flexible and malleable metal | (WHO, 1998) |
| Molar weight (g/mol) | 63.546 | Chemfinder |
| Melting point | 1083 °C | Chemfinder |
| Boiling point | 2595 °C | Chemfinder |
| Vapour pressure | 20 hPa at 1875 °C | IUCLID (2000b) |
| Octanol water distribution coefficient (log POW) | Not relevant | |
| Water solubility | Insoluble (0.01 g/100 mL) | Chemfinder |
6.3.4 Oral absorption
Copper absorption has been examined in 11 young men who had copper administered through the food in different concentrations. The apparent absorption varied inversely by the intake via the food (ranking from 67% at 0.38 mg/day to 12% at 7.53 mg/day). However, a study by Turnland et al. (1998) showed a real absorption of up to 77% (WHO, 2004a). Real absorption shall be regarded as the part of the copper which is absorbed in the organs, i.e. the amount ingested deducted the amount excreted via faeces/urine. Turnland et al. carried out another study in 2005 regarding oral absorption of copper. This study is described in the Copper Industry’s draft for a risk assessment of copper in 2006 but did not show higher values than 77%.
However, several factors can influence the absorption of copper. These factors include the amount of copper in the food, content of other metals (such as zinc, iron and cadmium) and age (ATSDR, 2004). The amount of accumulated copper does not seem to influence the absorption of further copper amounts in humans. Also, there does not seem to be a difference between the absorption in men and women (ATSDR, 2004).
Based on the information above the oral absorption of copper in this project is assumed to be 77%.
6.3.5 Dermal absorption
According to the Copper Industry’s draft for a risk assessment of copper from 2006 (Cross et al., 2006) the available data show that the metal copper and copper compounds can be absorbed through the skin.
According to the risk assessment (Cross et al., 2006), two non-published studies by Roper (2003) and Cage (2003) give the best data regarding dermal absorption of copper in humans. Based on these studies the risk assessment states that a dermal absorption factor of 0.3% applies for insoluble copper compounds (i.e. including copper chloride). This value derives from the highest value measured for copper in receptor fluid added with a value for copper retained in the skin and rounded up to follow a conservative approach. Thus, in these figures they include copper absorbed in the skin as OEDC orders that this has to be included. According to the risk assessment there is no evidence for dermal absorption of copper being larger for soluble compounds than for insoluble compounds. Thus, they recommend using the value of 0.3% for both types of compounds.
Based on this information the dermal absorption of copper in this project is assumed to be 0.3%.
According to Baars et al. (2001) copper can penetrate the skin when it is added in association with salicylic acid or phenyl butazone. However, the rate and scope of the dermal absorption in these cases are not known and therefore the dermal absorption rate of 0.3% is maintained. However, salicylic acid is known to be an ingredient in many skin care products[4] while phenyl butazone is primarily used for treatment of pains (in among others horses) [5].
6.3.6 Distribution
Copper is an essential element. According to WHO (1996) referred in Baars et al. (2001) a daily intake of 20 to 80 µg/kg bw is necessary.
Regarding oral intake of copper the absorption of copper will primarily take place from the stomach and the small intestine. However, different copper compounds will be absorbed from different places (ATSDR, 2004). Immediately after intake of copper an increase in the concentration of copper in the blood follows. Thereafter the copper is transported to (and ends in) liver and kidneys. From the liver the copper can be transported to other tissues (ATSDR, 2004).
The half-life of copper in the different organs is 3.9 and 21 days (liver), 5.4 and 35 days (kidneys) and 23 and 662 days (the heart) respectively. The first value represents ceruloplasmine bound to copper (ATSDR, 2004) while the identity of the other is not clearly stated in the reference.
Copper is primarily excreted via the bile. Normally between 0.5 and 3% of the daily intake of copper will be excreted through the urine (ATSDR, 2004).
6.3.7 Local irritation and allergy
Copper and copper salts can generate allergic reactions by contact with the skin in sensitive individuals. Symptoms includes itch, flush, swelling and vesiculation. Studies have identified a sensitivity reaction at exposure of 0.5 – 5% of copper sulphate in water or petroleum during 24-48 hours (WHO, 1998).
In a few individuals exposure to copper has shown to cause pruritus dermatitis which is itching without visible changes of the skin (ATSDR, 2004). A study has reported a case where a woman had pruritus on her ring finger and wrist as a result of the content of copper in her ring and wrist watch (Saltzer and Wilson, 1968 in ATSDR, 2004). Furthermore, allergic reactions have been observed in individuals after a test with a copper coin and/or a copper sulphate solution (Barranco, 1972; Saltzer and Wilson, 1968 in ATSDR, 2004).
6.3.8 Acute toxicity
Acute toxicity as a result of ingestion of copper is rare in humans. However, it can occur through consumption of water containing copper or by intentional/accidental intake of large amounts of copper salts.
The acute lethal dose for adults is between 4 and 400 mg copper(II) ions per kg bodyweight. These values are based on data from suicide attempts as well as unintended intake of high amounts of copper (WHO, 2004a). Symptoms as a result of intake of large amounts of copper include vomiting, apathy, acute haematological anaemia, kidney and liver injuries, neurotoxicity, increased blood pressure and breathing. In some cases, coma and death follow (ATSDR, 2004).
Studies have shown that 13 out of 53 humans died following an intake of copper in amounts ranging from 6 to 637 mg/kg (copper sulphate). Death, presumable as a result of among other things failure in the central nervous system and kidney injuries, has also been reported in humans who had consumed water containing > 100 mg of copper sulphate per litre (Akintonwa et al., 1989 in ATSDR, 2004).
6.3.9 Long-lasting, repeated effect and gene-damaging effects
Generally, the toxicological effect arises at a structural change/weakening of the sites to which metals bind themselves or when copper binds itself to macro-molecules and enzymes. Furthermore, copper can react with peroxide and create radicals, which can cause damages to the cells. Toxic damages can also be generated by metal-lothioneine becoming saturated with copper (Baars et al., 2001). Lack of copper leads to effects, which are just as critical as the toxic effects related to intake of too much copper.
Several studies have examined possible liver damages in new-borns as a result of copper exposure through drinking water. A NOAEL value of 0.315 mg Cu/kg/day was identified following a study regarding intake of copper sulphate from drinking water (during a period of 9 months) (Olivares et al., 1998 in ATSDR, 2004).
A LOAEL value of 4.2 mg Cu/kg bw/day has also been reported in relation to reduced bodyweight in mice after chronic oral exposure of copper gluconate (ATSDR, 1990 in Baars et al. (2001)).
6.3.10 Tolerable daily intake - TDI
A TDI value of 10 µg/kg/day, suggested by ATSDR (2004), is significantly below the recommended daily dose for intake of copper (20-80 µg/kg/day) and therefore this value is not used.
Vermeire et al. (1991), however, suggest a TDI value for copper of 140 µg/kg bw/day. Data from a RIVM report[6] confirms a tolerable daily intake of 140 µg/kg bw/day. To evaluate whether the value stated by Vermeire et al. (1991) still is valid, Baars et al. (2001) have reviewed literature published since 1991(including ATSDR, 1990; IPCS, 1998; WHO, 1996; WHO, 1998). Among other things, they found the above LOAEL value of 4.2 mg Cu/kg bw/day. Baars et al. (2001) converted this value into a TDI value of 4 µg/kg bw/day. Here they used a safety factor of 1000 (10 for going from LOAEL to NOAEL, 10 for extrapolating experimental results from animal studies to humans and 10 for accounting for variation between humans). However, this TDI value turned out to be far below the minimum requirement for daily intake of copper (20 – 80 µg/kg bw/day). Therefore, Baars et al. (2001) recommended to use the TDI value (140 µg/kg bw/day) suggested by Vermeire et al. (1991).
Based on the above information a TDI value for copper of 140 µg/kg bw/day is assumed for use in this project.
Adjustment of TDI values due to background exposure
As mentioned earlier, TDI values shall be regarded as the amount of substance which a human can tolerate to take in on a daily basis during a life time without experiencing health related effects. Therefore, it is important to take into account the amount of copper which each individual already takes in via air, food and water.
Background exposure of copper via food and beverages in Denmark
According to a report from the Danish Environmental Protection Agency (2000) [7], the normal Danish intake of copper among adults (60 kg) is 2.9 mg/day, corresponding to 48.3 µg/kg/day. According to Fromberg et al. (2005) 2 year old children (15 kg) ingest 59% of the adults’ food consumption which recalculated corresponds to a background exposure of (0.59×2.9/15) 114.1 µg/kg/day. Here it is assumed that the value stated in the report from the Danish Environmental Protection Agency deals with an exposure from food as well as water.
Background exposure of copper via the air in Denmark
According to the report ”The Danish Air Quality Monitoring Programme” from 2006 (prepared by the National Environmental Research Institute) (Kemp et al., 2007) the highest measured average value for a content of copper in the air in Denmark is 50.5 ng/m³ (the value measured on “Jagtvej” in Copenhagen). In order to choose a conservative approach this value is used to estimate which amount of copper an adult in Denmark inhales via the air.
According to the TDG an adult inhales 18 m³ air a day, while a 5 year old child (20 kg) inhales 11 m³ a day. I.e. an adult (60 kg) inhales (18m³×50.5ng/m³/1000) 0.909 µg copper per day. Converted into per kilo bodyweight (60 kg) this corresponds to 0.015 µg/kg bw/day. For children (20 kg) applies that they inhale (11 m³×50.5 ng/m³/1000) 0.556 µg copper per day which corresponds to 0.028 µg/kg bw/day.
Total background exposure
Because the TDI value as mentioned previously represents the amount of substance that a human as maximum can take in per day (during a life time) without causing health related effects, the TDI value is subtracted the above mentioned data for background exposure. Thereby a number is reached (”Margin to TDI value”) representing the ”extra amount” of copper that a human can take in on a daily basis (besides what a human is exposed to through food, beverages and air) without experiencing health damaging effects. This value is compared in the risk assessment with the amount that humans are exposed to by wearing or sucking the jewellery examined in this project.
Table 6-4: Background exposure of copper in Denmark as well as “Margin to TDI value” (µg/kg bw/day).
| Background exposure | Children (4-6 years) | Adults |
| Average | Average | |
| Food and drink** | 114.1 | 48.3 |
| Air* | 0.028 | 0.015 |
| Total background exposure (food, beverages, air) | 114.13 | 48.32 |
| Margin to the TDI value (140 – total background exposure) | 25.9 | 91.7 |
NB: * The value related to air is an average value from the monitoring station in Denmark which has shown the highest average. ** The value for copper consumption from food and beverages in children is based on the intake by 2 year old children (15 kg).
If people live in areas where they are exposed to a higher background concentration than the one mentioned above, the health risk by using the examined jewelleries in this project will be underestimated.
6.4 Nickel
6.4.1 Occurrence and use
Nickel occurs in nature primarily as oxide or sulphide compounds. The earths crust consists of 6% nickel, but nickel is also found in meteorites and on the sea bed as minerals (ATSDR, 2005a).
Pure nickel is a hard, silver-white metal, which is easy to mould. Furthermore it is ferromagnetic and a good conductor of heat and electricity. Nickel is often used in alloys with iron, copper, chromium or zinc. Here it is often used to help increase among other things the hardness and strength of the metal (ATSDR, 2005a).
The different alloys are used in different situations. Copper/nickel alloys are for instance used in coins, pipe laying, maritime equipments, petrochemical equipment, heat exchangers, pumps and electrodes for welding, while nickel/chromium alloys typically are used for heating elements. Furthermore, large amounts of nickel/iron alloys are used for producing steel alloys, corrosion-resistant steel and cast iron (ATSDR, 2005a).
Other nickel compounds include chlorine, sulphur and oxygen. Many of these nickel compounds are soluble in water and are used for among other things nickel coating, colouring of ceramics and for batteries (ATSDR, 2005a).
Finally, nickel is used in jewelleries. In this context nickel is frequently used as “sub-coating” for golden surfaces, since this results in a more shining gold coating. Nickel also forms diffusions barriers which prevent the metal from the parent material to diffuse to the surface, and thereby deteriorates the look of the jewellery.
6.4.2 Identification
| Chemical name | Nickel |
| Synonyms | Raney nickel Metallic nickel |
| CAS-No. | 7440-02-0 |
| EINECS No. | 231-111-4 |
| Gross formula | Ni |
| Molecular structure | Ni |
| The list of dangerous substances (Stat.Ord. 923, 2005) The list of unwanted substances (Briefing from the Danish EPA no. 8, 2004) Danish EPA self-classification (Environmental project no. 635, 2001) |
Yes. XN; R40-43: Limited evidence of a carcinogenic effect; Risk of serious damage to eyes; May cause sensitization by inhalation and skin contact. Yes (nickel, nickel compounds and nickel oxide) No |
6.4.3 Physical and chemical properties
| Physical state | Hard bright silver-white metal | ATSDR (2005a) |
| Molecular weight (g/mol) | 58.6934 | Chemfinder |
| Melting point | 1455 °C | Chemfinder |
| Boiling point | 2730 °C | Chemfinder |
| Vapour pressure | 1 mm Hg by 1810°C | ATSDR (2005a) |
| Octanol-water partition coefficient (log POW) | Not relevant | |
| Solubility | Insoluble | Chemfinder |
6.4.4 Oral absorption
The Danish Environmental and Protection Agency has in 2006 performed a risk assessment of nickel and nickel compounds (Andersen et al., 2006), in which they conclude that based on a number of studies, an oral absorption value of 30% should be used for risk assessments. This value refers to oral absorption of the following nickel compounds: nickel sulphate, nickel chloride, nickel nitrate and nickel carbonate in fasting people. For non-fasting people a value of 5% is recommended.
A study has shown that the bioavailability of nickel is reduced when nickel is given along with full-cream milk, coffee, tea or orange juice (ATSDR, 2005a). Other studies have shown a maximum nickel uptake of between 11.07 and 37.42%. Here nickel (12 µg/kg) was administered 4 hours after ingestion of scrambled eggs. The lowest absorption values (2.83-5.27%) were measured in people who had nickel administered along with a meal (Nielsen et al., 1999 in ATSDR, 2005a) (it was not specified which kind of nickel compound that was used in the study). Another study indicates that nickel absorption reduces with age (Hindsen et al., 1994 in ATSDR, 2005a).
Animal studies have shown that different nickel compounds are absorbed differently. The following absorption values were measured in a study using rats. Nickel oxide (0.01%), metallic nickel (0.09%), black nickel oxide (0.04%), nickel sulphide (0.47%), nickel sulphate (11.12%), nickel chloride (9.8%) and nickel nitrate (33.8%). Generally, the absorption was higher for soluble nickel compounds (ATSDR, 2005a).
In this project it is assumed that the oral absorption of nickel is 30%. The line of reasoning is that the source, which provides this value, is a comprehensive report from 2006, which has made a thorough review of a number of studies. Thus, it is assumed to represent the latest knowledge in the area. Furthermore, the value refers to nickel compounds which are relevant for this project (among these nickel chloride). The value is valid for fasting people, but in order to stick to a conservative approach the value is used anyway.
6.4.5 Dermal absorption
The risk assessment of nickel from 2005 (Andersen et al., 2005a – draft) concludes that absorption of nickel through the skin can occur but that a large proportion of the added dose remains on the skin. They state that limited data is available regarding exactly which fraction that is absorbed, but apply a value of 0.2% regarding dermal absorption of nickel for use in risk assessments. The value is based on an in vivo study in humans (Hostýnek et al. 2001 in Andersen et al., 2005a - draft).
Andersen et al. (2006), however, concludes that for use in risk assessments a value of 2% should be applied, related to dermal absorption of nickel following exposure of nickel chloride. Regarding exposure of nickel metal they recommend a value of 0.2% (based on a study of Hostýnek et al. 2001).
For use in this project a value of 2% is applied in terms of dermal absorption of nickel chloride, since this metal compound exists in sweat that is in contact with jewellery.
6.4.6 Distribution
Nickel is bound to serum proteins in the blood and is transported around the body. Nickel is subsequently concentrated in kidneys, liver and lungs as well as the lymph nodes (Baars et al., 2001).
A study examining individuals who were not on a daily basis exposed to nickel through work showed that the highest concentration of nickel was found in the lungs, followed by the thyroid gland, the suprarenal glands, the kidneys, the heart, the liver, the brain, the spleen and the pancreas (ATSDR, 2005a). A study has furthermore showed that nickel can cross the placenta, which was confirmed by increasing concentrations of nickel in mice foetuses whose mothers have been exposed to nickel during the pregnancy (ATSDR, 2005a).
6.4.7 Local irritation and allergy
Nickel can cause skin allergy and fulfils the criteria for being classified with the following risk phrase: R43: May cause sensitization by skin contact. According to Andersen et al. (2006) it is the Ni2+ ion, which is responsible for the immunological effects of nickel.
Available data shows that as long as a migration rate of 0.5 µg Ni/cm²/uge is not exceeded, there is no risk of causing skin allergy in non-sensitive individuals in a large part of the population (who are exposed to nickel and nickel alloys for an extended period of time) (Andersen et al., 2005a – draft).
According to the risk assessment of nickel from 2005 (Andersen et al., 2005a – draft) there is no simple correlation between the content of nickel and the release of nickel (related to a study of coins). Likewise they mention that when one is to evaluate the risk of sensitization due to nickel, it is the concentration of nickel ions per cm² skin that is interesting and not the total dose of nickel on the skin.
Contact dermatitis caused by nickel allergy is a well-known phenomenon. Contact dermatitis was found in 15.5% of 75,000 individuals who participated in a test with nickel sulphate (Uter et al., 2003 in ATSDR, 2005a), which demonstrates that it is a common reaction (ATSDR, 2005a). The majority of the cases of nickel allergy is caused by skin contact with metallic products such as earrings, jewelleries and buttons (European Environmental Contact Dermatitis Group, 1990 in Andersen et al., 2006).
A study of school children in the age of 7-12 years showed that among children with pierced ears 30.8% had nickel allergy while among the children who did not have pierced ears only 16.3% were allergic to nickel (Dotterud and Falk, 1994 in ATSDR, 2005a).
The majority of nickel tests are made using nickel sulphate since this substance is less irritating than nickel chloride. However, nickel alloys which are in contact with sweat form nickel chloride. Thus it is more relevant to perform nickel studies using nickel chloride (Menné, 1994 in ATSDR, 2005a). Menne and Calvin (1993) quoted from ATSDR (2005a) examined skin reactions related to different nickel chloride concentrations in 51 sensitive and 16 non-sensitive people. At a concentration of 0.01% no reaction was observed. At a concentration of 0.1% reactions in 4 out of the 51 people were observed.
All in all it is concluded in the EU risk assessment of nickel and nickel compounds from 2006 (Andersen et al., 2006) that there is not enough data available regarding dermal exposure of nickel chloride to determine which dose that triggers a reaction.
6.4.8 Acute toxicity
There is no indication of nickel being an essential element (as for instance copper). Thus the body does not require nickel.
Regarding acute nickel poisoning nickel carbonyl, a fugitive fluid of Ni(CO)4, is the most critical. The effects of acute nickel carbonyl poisoning include headache, dizziness, nausea, vomiting, sleeplessness and irritation followed by symptoms which resemble pneumonia (WHO, 1991).
A study (Daldrup et al., 1983 in ATSDR, 2005a) has reported a death of a 2 year old child following ingestion of 570 mg Ni/kg (crude estimate) in the form of nickel sulphate crystals. Four hours after ingestion heart failure occurred and the child died 8 hours after ingestion.
Studies have indicated that soluble nickel compounds are more toxic than less-soluble nickel compounds. Oral LD50values related to nickel sulphate of respectively 46 mg Ni/kg (female rats) and 39 mg Ni/kg (male rats) have been reported (Mastromatteo, 1986 in ATSDR, 2005a). Likewise, oral LD50 values related to nickel acetate of 116 (female rats) and 136 mg Ni/kg (male rats) respectively have been reported (Haro et al., 1968 in ATSDR, 2005a).
Oral LD50values for less-soluble nickel oxides and –subsulphides have been reported to >3.930 and > 3.665 mg Ni/kg respectively (Mastromatteo, 1986 in ATSDR, 2005a).
According to ATSDR (2005a) the lowest value reported (related to oral ingestion) is 39 mg/kg/day. This value is derived from a study in which a male rat ingested nickel sulphate (Mastromatteo, 1986 in ATSDR, 2005a). The lowest value reported in terms of systemic effects caused by acute exposure is a NOEAL value of 0.014 mg/kg/day. This value is reported in relation to dermatitis in nickel-sensitive humans. Nickel was administered in the form of nickel sulphate (ATSDR, 2005a).
6.4.9 Long-lasting, repeated effect and gene-damaging effects
Chronic effects caused by nickel exposure include among other things sinusitis and asthma. Extremely high risk of lung cancer has furthermore been reported amongst workers in nickel refineries. The workers were exposed to nickel subsulphides, nickel oxides and possible nickel sulphate (WHO, 1991).
The lowest NOAEL value reported related to medium long and long exposure of nickel to humans is according to ATSDR (2005a) 20 µg/kg/day. This value is reported in relation to a study in which 8 nickel sensitive humans gradually ingested increasing doses of nickel sulphate (in tap water) over a period of 91-178 days. At the reported value no individuals showed signs of health related effects on the skin (Santucci et al., 1994 in ATSDR, 2005a).
The lowest NOAEL value related to chronic exposure is according to IRIS (1996) related to a study by Ambrose et al. (1976). In this study a NOAEL value of 5000 µg/kg/day related to reduction in bodyweight in rats was reported (the study was completed over a period of 2 years). Nickel was administered as nickel sulphate
Nickel compounds are according to IARC (1997) carcinogenic in humans, while metallic nickel is potential carcinogenic in humans.
Baars et al. (2001) was not able to find new relevant data (after 1990) regarding toxicity caused by oral exposure of nickel or nickel compounds in humans or animals. Thus, they concluded that the TDI value of 50 µg/kg bw/day suggested by Vermerie et al. (1991) (and CEPA (1993), WHO (1996) and ATSDR (1997) in Baars et al., 2001) still is valid.
The study from which the TDI value of 50 µg/kg bw/day was derived is a study performed by Ambrose et al. in 1976. The study found, as described above, a NOAEL value of 5000 µg Ni/kg bw. The study included a 2-year study of rats and the effects observed were reduced bodyweight and higher heart/bodyweight ratio. A later study by American Biogenics Corp. (1988) likewise found a NOAEL value of 5000 µg/kg/day.
The EU risk assessment of nickel sulphate from 2005 (Andersen et al., 2005b – draft) arrives at two NOAEL values for use in risk assessment. One is a NOAEL value of 2200 µg/kg bw/day for oral administration of nickel – derived from a chronic cancer study. The effects at high exposure level were reduced survival in female rats and reduced bodyweight in both sexes. The other NOAEL value is based on a two-generation study of rats (exposed to nickel sulphate) and is related to effects (increased perinatal mortality in the offspring) during the development process. The NOAEL value for the dam was 1100 µg Ni/kg bw/day. Regarding the offspring (dosed after the perinatal period), a NOAEL value of 2200 µg Ni/kg bw/day was identified (Larsen and Tyle, 2008 - draft), since no effect was found in the offspring.
WHO (2007) uses a TDI value of 11 µg/kg/day. This value is calculated based on a NOAEL value of 1100 µg/kg bw/day and by use of a safety factor of 100 (10 for variation between species and 10 for variation within species).
Based on the above mentioned information a NOAEL value of 1100 µg/kg bw/day is assumed to be valid for use in this project.
6.4.10 Tolerable daily intake - TDI
Nickel allergy is already regulated since the release of nickel from products which are meant to be in long-lasting contact with the skin (such as jewellery) must not exceed 0.5 µg /cm² /week[8]. Likewise piercing jewellery must not release more than 0.2 µg/cm² /week[9]. This value is set at such a low level that skin allergy caused by dermal nickel exposure should not occur.
The risk assessment of nickel sulphate from 2005 (Andersen et al., 2005b) gives a NOAEL value of 1100 µg/kg/day related to damaging effects on the foetus. This NOAEL value is converted to a TDI value of 4.4 µg/kg/day by using a safety factor of 250 since the risk assessment states the use of a total safety factor of 200-300. The total safety factor is derived from the combination of a factor of 10 used for extrapolating the results from animal studies to humans; a factor of 10 for variation between humans and finally a factor of 2-3 in order to take into consideration the severity of the effects in question (death of foetuses).
For use in this project a TDI value related to nickel exposure (in women) of 4.4 µg/kg/day is used.
Regarding children a supplement to the EU risk assessment from 2008 has judged that the best basis for establishing a tolerable dose for children is a NOAEL value of 2200 µg/kg/day based on a two-generation study- and dosage of the offspring. By using a safety factor of 100 a TDI value of 22 µg/kg/day for children is achieved (Larsen and Tyle, 2008 – draft). The value related to children is thus higher which means that they are less sensitive than pregnant women.
Adjustment of TDI due to background exposure
As for the other metals humans are also exposed to nickel via other sources such as food, water and air. Thus, it is necessary to take into consideration the background exposure in order to identify the amount of nickel which humans in Denmark can tolerate to get “additionally” per day, without causing health related effects.
Background exposure of nickel via food and beverages in Denmark
According to a report from the Danish Veterinary and Food Administration (Fromberg et al., 2005) the background exposure of nickel from food in Denmark is in average 109 µg/day which corresponds to (109/60) 1.817 µg/kg bw/day. Fromberg et al. (2005) does not state a number related to children (4-6 years) regarding nickel exposure from food. However, Fromberg et al. (2005) states that 2 year old children (15 kg) take in 59% of the amount of food which adults consume. Based on these numbers an average background exposure of nickel via food and beverages in children of ((0.59x109)/15) 4.288 µg/kg bw/day is achieved.
Fromberg et al. (2005) also states a background exposure in terms of 95 percentiles (i.e. a value representing the maximum amount that 95% of the population is exposed to). If these values are used a background exposure for adults of (197/60) 3.283 µg/kg bw/day is achieved. For children the value is ((0.59x197)/15) 7.749 µg/kg bw/day.
Background exposure of nickel via air in Denmark
According to the report “The Danish Air Quality Monitoring Programme” from 2006 (prepared by the National Environmental Research Institute) (Kemp et al., 2007) the highest measured average value for content of nickel in air in Denmark was 5 ng/m³ (value measured in Aarhus, on the street “Banegårdsgade”). In order to choose a conservative approach this value is used to estimate the amount of nickel that an adult in Denmark inhales.
According to the TGD an adult inhales 18 m³ air per day, while a 5-year-old-child (20 kg) inhales 11 m³ per day. Thus, an adult (60 kg) inhales (18m³x5ng/m³/1000) 0.09 µg nickel per day which corresponds to 0.002 µg/kg bw/day. Children (20 kg) inhale (11 m³x5ng/m³/1000) 0.055 µg nickel per day which corresponds to an intake of cadmium via the air of 0.003 µg/kg bw/day.
Total background exposure
As the TDI value, as mentioned earlier, represents the maximum amount of substance that a person can tolerate to take in per day (throughout his/her entire lifetime) without experiencing health related effects, the TDI value is subtracted the above mentioned figures representing background exposure. Hereby a number is attained which represents the “extra addition” of nickel that humans can have on a daily basis (beside what they are exposed to via food, beverages and air) without causing health related effects. In the exposure scenarios this value is then compared with the amount of nickel that humans are exposed to by wearing or sucking the jewellery examined in this project.
Table 6-5: Background exposure of nickel and the “Margin to TDI value” (µg/kg bw/day).
| Background exposure | Children (4-6 years) | Adults | ||
| Average | 95-percentile | Average | 95-percentile | |
| Food and beverages** | 4.288 | 7.749 | 1.817 | 3.283 |
| Air* | 0.003 | 0.003 | 0.002 | 0.002 |
| Total background exposure (food, beverages, air) | 4.29 | 7.75 | 1.82 | 3.29 |
| Margin to TDI value (4.4 (adults) 22 (children) – total background exposure) | 17.71 | 14.25 | 2.58 | 1.11 |
NB: The 95 percentile relates to the numbers regarding background exposure via food and beverages (including tap water). * The value related to air is an average value from the monitoring station in Denmark which has shown the highest average in 2006. ** The value representing background exposure via food (for children) is based on 2 year olds (15 kg).
If people live in areas where they are exposed to a higher background concentration than the above mentioned, the health risk by using the examined jewelleries in this project would be underestimated.
7 Exposure scenarios and risk assessment
- 7.1 Assumptions and uncertainties related to the exposure calculations
- 7.2 Dermal exposure
- 7.3 Oral exposure
- 7.4 Total health risk by using jewelleries
Jewelleries are used by a large part of the population and they are often in direct contact with the skin for many hours at the time – in some cases even 24 hours a day. This means that possible heavy metals/problematic substances in the jewelleries can penetrate the skin and induce toxic effects.
Another form of exposure can arise by sucking the jewelleries. Some people (probably mostly children) have a tendency to suck their pendants on the necklace and thereby absorb the migrating heavy metals/problematic substances directly.
Furthermore, there can be a risk for children, as well as for adults, that small jewelleries by accident can be swallowed. The seriousness of this is confirmed by a recent death of a 4 year old boy. The boy had by accident swallowed a heart-shaped jewellery containing more than 99% lead. The episode let to a voluntarily recall of 300,000 examples of the mentioned jewellery (Berg et al., 2006).
During the migration analysis the amount of heavy metal that can migrate to sweat and thereby potentially be absorbed via the skin is found. Migration tests for eight chosen heavy metals in 25 different jewellery parts have been performed.
Yet only 4 metals (Cu, Pb, Ni and Cd) showed to migrate in a concentration above the detection limit. For these 4 metals a health and risk assessment has been performed in which the TDI values plus the oral and dermal absorption rates for the metals deduced in chapter 6 are used.
For use in the following risk assessment the data below are applied:
Table 7-1: TDI values plus dermal and oral absorption rates for the 4 metals.
| Metal | TDI (µg/kg bw/day) | Dermal absorption (%) | Oral absorption (%) |
| Lead | 1.8 | 0.06 | 50 |
| Cadmium | 0.5 | 0.6 | 8.9 |
| Copper | 140 | 0.3 | 77 |
| Nickel | 4.4 (adults) 22 (children) |
2 | 30 |
Table 7-2: Margin to TDI values for the 4 metals (µg/kg bw/day).
| Metal | Children (4-6 years) | Adults | ||
| Average | 95-percentil | Average | 95-percentil | |
| Lead | 1.31 | 1.02 | 1.48 | 1.28 |
| Cadmium | 0.11 | -0.1 | 0.33 | 0.22 |
| Copper | 25.9 | - | 91.7 | - |
| Nickel | 17.71 | 14.25 | 2.58 | 1.11 |
7.1 Assumptions and uncertainties related to the exposure calculations
7.1.1 Two exposure periods – 16 and 24 hours
During the calculations of exposure through the skin it is conservatively presumed that the jewelleries are in direct contact with the skin during all the time they are worn, e.g. there are for instance no textile between the jewellery and the skin. Some jewellery is worn 24 hours a day, for instance rings and piercings, whereas other jewelleries such as necklaces, bracelets etc. in general are presumed to be removed when the person sleeps. Thus, two different periods of use are applied (16 and 24 hours respectively) depending on whether the jewelleries are removed during the night or not.
7.1.2 Migration analysis for sweat is presumed also to apply saliva
The migration analysis is performed to artificial sweat because this is the type of exposure that most frequently will occur. The results from the migration analysis to artificial sweat are also presumed to be able to be used in the oral exposure calculation because the solutions for artificial sweat and saliva do not deviate significantly from each other.
Migration analysis to sweat:
1.0 g urea ((NH2)2CO), 5.0 g NaCl and 0.940 µl lactic acid (C3H6O3) dissolved in 1 litre demineralised water. Set the pH to 6.5. Extraction/migration at 40 degrees.
Migration analysis to saliva:
4.5 g NaCl + 0.3 g KCl + 0.3 g Na2SO4 + 0.4 g NH4Cl + 3.0 g lactic acid (C3H6O3) + 0.2 g urea ((NH2)2CO) dissolved in 1 litre demineralised water. pH is set to 5.0 with 2 N NaOH. Extraction/migration at 37 degrees.
The solutions consist both primarily of salt water which means that one can expect that the surface tension is approximately the same. Furthermore, metals are positively charged in aqueous solutions and therefore will not form complexes with sodium/potassium, thus the difference in concentration of these has no importance. The temperature in both solutions is approximately the same and the concentration of chloride ions, which is presumed to be the component that primarily forms metal compounds, is also in approximately the same dimensions.
There is approximately 5 times as much urea in the sweat solution as in the saliva solution, but because the urea is not expected to form complexes with the metal it is presumed that this difference is of no importance. Likewise there is much more lactic acid in the saliva solution than in the sweat solution but because lactic acid is not expected to form complex compounds with the metal it is presumed to be of no importance.
Because the pH value in the saliva solution is lower than in the sweat solution (5 versus 6.5) it is presumed that more metal chloride compounds are formed in the saliva solution. This assumption is supported by a study made by Strandesen et al. (2007) where a model is developed to simulate the formation of metal compounds in different aqueous system. This study shows that for salt water with a pH value of 5 3-4% more lead chloride compounds are formed than in salt water with a pH value of 6.5. This means that by using the results from the sweat test in the oral exposure calculations the exposure is underestimated to a certain degree. However, this small difference of 3-4% is not expected to have crucial influence on the results and will probably lie within the measurement uncertainty.
7.1.3 Inorganic metal compounds
It is presumed that it is primarily inorganic metal compounds (as metal chlorides) that are being formed in sweat having contact with the jewelleries, which is the reason why oral and dermal absorption rates as far as possible are based on information concerning relevant inorganic metal compounds. The background for the assumption is found partly in an article by Menné (1994) quoted from ATSDR (2005a) who claims that nickel alloys being in contact with the skin forms nickel chlorides and partly in the risk assessment from the Danish EPA from 2005 (Andersen et al., 2005a), which claims that corrosion of metal in sweat primarily is dependent on the content of chloride and oxygen.
The values for oral and dermal absorption are in this way associated with some uncertainty because it is not known for sure exactly which metal compounds that will be formed when the jewelleries are in contact with sweat (and saliva). The optimum would be to conduct studies that demonstrate precisely which metal compounds that are formed and which oral and dermal absorption these can expect to have. However, it has not been possible within the scope of this project to perform these kind of comprehensive studies.
7.1.4 Highest value chosen in duplicate determinations
In situations where the duplicate determinations differed significantly the highest value has been used. In Table 4-1 the results from the migration analysis for all samples are presented. In this table it is also illustrated which samples showed significant difference in the duplicate determinations. A significant difference is defined as a difference of approximately 50% or more.
7.1.5 Migration is presumed constant over time
During the risk assessment it is presumed that the migration is constant over time. It should be mentioned here that a piece of jewellery cannot be expected to release the same amount of metal per hour during several years. The migration will decrease with time. Based on the analysis performed in this project it is, however, not possible to determine how fast the migration will decrease and therefore it is assumed that in a period of days/weeks the migration will stay relatively constant.
7.2 Dermal exposure
The potential dermal intake (the exposure) by wearing a piece of jewellery can be expressed through the following equation (European Commission, 2003):
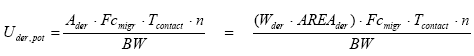
Where:
| Uder,pot | Potential absorption of the chemical substance | µg/kg bw/day |
| Ader | Total amount of substance which the skin is potentially exposed to | G |
| Wder | The weight of the product on the skin | g/cm2 |
| AREAder | Area of contact between the product and the skin | cm2 |
| Fcmigr | Fraction of substance which migrates | µg/g per hour |
| Tcontact | The durance of exposure per occurrence | hours |
| N | The number of occurrences per day | per day |
| BW | Body weight | Kg |
For use in these exposure scenarios the formula can be rewritten to:
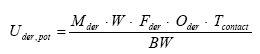
Where:
| Uder, pot | Potential absorption of the chemical substance | µg/kg bw/day |
| Mder | Fraction of chemical substance which migrates | µg/g/4 hours |
| W | Weight of jewellery | g |
| Fder | Part of the jewellery (W) having contact to the skin | % |
| Tcontact | Duration of the exposure | hours |
| BW | Body weight | Kg |
| Odermal | Dermal absorption | % |
Mder represents the amount (µg) of chemical substance (here metal) which migrates per gram of jewellery during a period of 4 hours, i.e. the result of the migration analysis.
W represents the real weight of the part/s of the jewellery which can be assumed to have a problematic content of heavy metal, i.e. textile strings etc. are deducted. W is defined by looking at the metal content of the different jewellery parts of the jewellery (from the XRF-screening) and based on this information perform an individual assessment of which metal parts of the jewellery that can be assumed to have a problematic content, i.e. the weight of the jewellery parts which resemble the analysed part. In other words, it is here determined (by comparing the results from the XRF screening) which metal parts of the jewellery that resemble the part on which a migration analysis was made - and thus can be expected to have the same content of metal (and migration).
In cases where a migration test is performed on two different jewellery parts (with a different result) belonging to the same piece of jewellery, separate calculations are made where W is set to the weight of each of the two jewellery parts. Finally, the two results (Uder) are added to give a total dermal absorption by wearing the specific jewellery.
Fder represents the part of the weight (W) of the jewellery which has contact to the skin. Fder is determined based on a visual assessment of each jewellery. However, in most cases it is assumed that 50% of the jewellery has contact to the skin.
Tcontact represents the time period of which the jewellery has contact with the skin. Two scenarios are taken into consideration (16 and 24 hours respectively) as it is assumed that some types of jewelleries (as for instance rings, piercings etc.) are worn day and night while other types of jewelleries (as for instance large necklaces and earrings etc.) are most probably removed during the night.
BW represents body weight in kilograms. Based on TGD, it is here assumed that children weigh 20 kg while adults weigh 60 kg. The value of 60 kg is chosen as it is assumed that it is primarily women who wear jewelleries. According to TGD values for adults of both 60 and 70 kg can be used.
Odermal represents the dermal absorption (in percentage) of the specific metal. Theses values are different from metal to metal (see Table 7-1) and are described during the health assessment of the different metals.
When Uder, pot is calculated the value is compared with the “Margin to TDI” value which is described in Table 7-2. As earlier described, the “Margin to TDI” value shall be regarded as the tolerable daily dose of metal which people can tolerate, deducted the exposure which the Danes are already exposed to through food, beverages and air. If the Uder, pot value exceeds this ”Margin to TDI” value it means that the total exposure (from food, beverages, air and jewelleries) is higher than the tolerable daily intake. In these cases, there might be a potential health risk by wearing the jewelleries. Here it must be mentioned that the “Margin to TDI” value is multiplied by the oral absorption rate (belonging to the specific metal) before it is compared with the dermal exposure as dermal exposure shall be regarded as an absorption of the metal and not an intake which TDI values are normally based on. At an intake (ingestion) some of the metal is excreted through for instance the faeces and is thus not absorbed in the body, while the amount “coming in” through the skin shall be regarded as absorbed in the body (i.e. in plasma, blood, organs etc.).
Example of a calculation of absorption through dermal exposure
Below the exposure- and risk calculation for cadmium exposure from jewellery no. 62 is described. The calculations are made by extrapolating the result from the migration test of the jewellery part (belonging to the specific jewellery) to apply to all the metal parts of the jewellery which can be expected to have the same content of metal as the jewellery part examined (i.e. W). The exposure scenario is 16 hours and valid for children (20 kg). The migration analysis showed that 3 µg cadmium migrated per gram of jewellery during 4 hours. The part of the whole jewellery which is assumed to have a problematic content of metal (W) - i.e. the weight of jewellery parts resembling the analysed part - weighed 40 gram of which 50% is assumed to have skin contact while the dermal absorption rate is 0.6%.
Therefore, the following formula is valid:
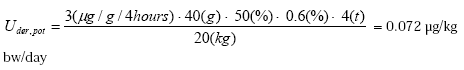
Here it shall be noted that the migration analysis is made during a period of 4 hours and therefore the calculations are multiplied by 4 to illustrate an exposure scenario of 16 hours (and 6 for exposure scenarios related to 24 hours).
The TDI value for cadmium is set to 0.5 µg/kg bw/day. If the amount of cadmium which an average child (4-6 yours) is exposed to daily through air, food and beverages (0.39 µg/kg bw/day) is deducted, a “Margin to TDI “ value of 0.11 µg/kg bw/day is achieved (see Table 7-2). This “Margin to TDI” value is multiplied by the oral absorption rate for cadmium (8.9%) in order to “imitate” a TDI value for absorbed metal. I.e. the value with which the dermal exposure shall be compared is (0.089x0.11) 0.00979 µg/kg bw/day.
Therefore the following applies:
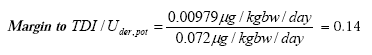
In this situation the dermal exposure exceeds the “Margin to TDI” value and therefore the result is below 1, i.e. there might be a potential health risk for a child who wears this jewellery for 16 hours.
Here it shall be emphasized that a TDI value is an expression of what a person may get on a daily basis through an entire lifetime without experiencing health related effects. Thus, for a shorter period the TDI value can be exceeded without this causing any effects, if the person in a corresponding period later in life takes in an equivalent lesser amount. Thus, to wear the above-mentioned jewellery for 16 hours during a single day will not result in effects harmful to health unless an amount equal to the TDI value is taken in every day for the rest of the life.
7.2.1 Results – dermal exposure
Background exposure - average
In the tables below the results of dermal exposure of the jewelleries for 16 and 24 hours respectively at children and adults are presented – at an average background exposure of the four metals.
Table 7-3: Results for dermal exposure at children for 16 and 24 hours respectively – at an average background exposure
| No. | Product category | Margin to TDI value / Uder, children – at an average background exposure | |||||||
| Cd | Pb | Ni | Cu | ||||||
| 16 h | 24 h | 16 h | 24 h | 16 h | 24 h | 16 h | 24 h | ||
| 169.1 | Silver coated | 117 | 78 | 21 | 14 | 1103 | 735 | ||
| 164.1 | Silver coated | 2799 | 1866 | 26224 | 17482 | ||||
| 152.2 | Clearly non-precious metal | 34027 | 22685 | ||||||
| 138.1 | Silver alloy | 0.3 | 0.2 | 2.4 | 1.6 | 5454 | 3636 | ||
| 136.2 | Silver coated | 0.3 | 0.2 | 3.3 | 2.2 | 2460 | 1640 | ||
| 130.1 | Silver-like | 431 | 287 | ||||||
| 125.1 | Silver coated | 4.3 | 2.9 | 4.6 | 3.1 | 20.9 | 14.0 | 20815 | 13876 |
| 107.1 | Gold coated | 65 | 43 | ||||||
| 101.3 | Gold coated | 1241 | 827 | ||||||
| 101.1 | Gold coated | 14.3 | 9.6 | 5002 | 3334 | ||||
| 99.2 | Gold coated | 1138 | 759 | ||||||
| 99.1 | Gold coated | 4713 | 3142 | ||||||
| 95.3 | Gold coated | 43.7 | 29.1 | 20144 | 13430 | ||||
| 91.1 | Clearly non-precious metal | 4.9 | 3.3 | 162 | 108 | ||||
| 91.3 | Clearly non-precious metal | 3.9 | 2.6 | 46 | 31 | ||||
| 88.1 | Silver-like | 0.2 | 0.12 | 401 | 267 | 5308 | 3539 | ||
| 70.3 | Gold coated | 5.7 | 3.8 | 55397 | 36931 | ||||
| 70.1 | Gold coated | 4.0 | 2.6 | 9979 | 6653 | ||||
| 68.1 | Silver coated | 0.3 | 0.2 | 6.2 | 4.1 | 5915 | 3944 | ||
| 62.1 | Silver-like | 0.1 | 0.1 | 7.0 | 4.7 | 2480 | 1653 | ||
| 60.1 | Golden-like | 507 | 338 | ||||||
| 56.1 | Silver-like | 25.8 | 17.2 | 4793 | 3195 | ||||
| 38.1 | Gold coated | 0.2 | 0.2 | 8.1 | 5.4 | 18303 | 12202 | ||
| 26.2 | Clearly non-precious metal | 2116 | 1411 | ||||||
| 6.1 | Gold coated | 0.1 | 0.1 | 18.3 | 12.2 | 36470 | 24313 | ||
NB: Dark grey fields indicate that the TDI value is exceeded while pale grey fields indicate that the TDI value is close to being exceeded.
The results show that for nearly all the jewelleries from where cadmium migrated in an amount above the detection limit, there is a potential health risk for children (who already are exposed to an average background exposure) by wearing the jewelleries for 16 and 24 hours respectively a day. For two of the jewelleries the total tolerable daily dose is exceeded by a factor of 10. I.e. children wearing these jewelleries get 10 times the tolerable dose of cadmium a day. However, here it shall again be mentioned that the daily tolerable dose (TDI) is an expression of what a person may daily get through an entire lifetime without this causing health related effects. Thus, for a shorter period, the TDI value can be exceeded without this causing any effects if in a corresponding period later in life an equally lesser amount is taken in.
Only one piece of jewellery was close to the TDI value of lead while another piece of jewellery was close to the TDI value of nickel. The results indicate that there is no health risk associated with the content of copper in the jewelleries.
Table 7-4: Results for dermal exposure at adults for 16 and 24 hours respectively – at an average background exposure.
| No. | Product category | Margin to TDI value / Uder, adults - at an average background exposure | |||||||
| Cd | Pb | Ni | Cu | ||||||
| 16 h | 24 h | 16 h | 24 h | 16 h | 24 h | 16 h | 24 h | ||
| 169.1 | Silver coated | 397 | 265 | 9.2 | 6.1 | 11718 | 7812 | ||
| 164.1 | Silver coated | 9487 | 6325 | 278536 | 185691 | ||||
| 152.2 | Clearly non-precious metal | 361426 | 240951 | ||||||
| 138.1 | Silver alloy | 2.9 | 1.9 | 8.2 | 5.5 | 57933 | 38622 | ||
| 136.2 | Silver coated | 2.7 | 1.8 | 11.1 | 7.4 | 26129 | 17419 | ||
| 130.1 | Silver-like | 4574 | 3049 | ||||||
| 125.1 | Silver coated | 39.0 | 26.0 | 15.7 | 10.4 | 9.2 | 6.1 | 221085 | 147390 |
| 107.1 | Gold coated | 686 | 457 | ||||||
| 101.3 | Gold coated | 13184 | 8789 | ||||||
| 101.1 | Gold coated | 48.6 | 32.4 | 53126 | 35418 | ||||
| 99.2 | Gold coated | 12091 | 8061 | ||||||
| 99.1 | Gold coated | 50055 | 33370 | ||||||
| 95.3 | Gold coated | 148.0 | 98.7 | 213967 | 142644 | ||||
| 91.1 | Clearly non-precious metal | 2.2 | 1.4 | 1722 | 1148 | ||||
| 91.3 | Clearly non-precious metal | 1.7 | 1.1 | 486 | 324 | ||||
| 88.1 | Silver-like | 1.7 | 1.1 | 1359 | 906 | 56381 | 37587 | ||
| 70.3 | Gold coated | 19.4 | 12.9 | 588408 | 392272 | ||||
| 70.1 | Gold coated | 35.6 | 23.7 | 105994 | 70663 | ||||
| 68.1 | Silver coated | 3.1 | 2.0 | 20.9 | 14.0 | 62830 | 41887 | ||
| 62.1 | Silver-like | 1.2 | 0.8 | 23.7 | 15.8 | 26343 | 17562 | ||
| 60.1 | Golden-like | 5386 | 3591 | ||||||
| 56.1 | Silver-like | 87.5 | 58.4 | 50907 | 33938 | ||||
| 38.1 | Gold coated | 2.0 | 1.3 | 27.3 | 18.2 | 194406 | 129604 | ||
| 26.2 | Clearly non-precious metal | 22475 | 14983 | ||||||
| 6.1 | Gold coated | 1.1 | 0.8 | 62.2 | 41.4 | 387366 | 258244 | ||
For adults being exposed to an average background exposure the TDI value related to cadmium is exceeded for two of the examined jewelleries, when they are worn over a period of 24 hours. Five other jewelleries are close to the TDI value.
None of the jewelleries gave problems related to lead and copper. Three other jewellery parts were close to the TDI value for nickel.
Background exposure – 95 percentiles
In the tables below the results of dermal exposure of the jewelleries for 16 and 24 hours respectively at children and adults are presented – at a background exposure corresponding to the 95 percentile. Copper is not included as no background exposure corresponding to the 95 percentile for copper exposure in Denmark was found.
Table 7-5: Results of dermal exposure at children for 16 and 24 hours respectively – at a background exposure corresponding to the 95 percentile.
| No. | Product category | Margin to TDI value / Uder, children - at 95 percentile background exposure | |||||
| Cd | Pb | Ni | |||||
| 16 h | 24 h | 16 h | 24 h | 16 h | 24 h | ||
| 169.1 | Silver coated | 91 | 61 | 17 | 11.3 | ||
| 164.1 | Silver coated | 2179 | 1453 | ||||
| 152.2 | Clearly non-precious metal | ||||||
| 138.1 | Silver alloy | 1.89 | 1.26 | ||||
| 136.2 | Silver coated | 2.55 | 1.70 | ||||
| 130.1 | Silver-like | ||||||
| 125.1 | Silver coated | 3.60 | 2.40 | 16.8 | 11.2 | ||
| 107.1 | Gold coated | ||||||
| 101.3 | Gold coated | ||||||
| 101.1 | Gold coated | 11.16 | 7.44 | ||||
| 99.2 | Gold coated | ||||||
| 99.1 | Gold coated | ||||||
| 95.3 | Gold coated | 34.00 | 22.67 | ||||
| 91.1 | Clearly non-precious metal | 4.0 | 2.6 | ||||
| 91.3 | Clearly non-precious metal | 3.2 | 2.1 | ||||
| 88.1 | Silver-like | 312 | 208 | ||||
| 70.3 | Gold coated | 4.46 | 2.97 | ||||
| 70.1 | Gold coated | ||||||
| 68.1 | Silver coated | 4.81 | 3.21 | ||||
| 62.1 | Silver-like | 5.45 | 3.63 | ||||
| 60.1 | Golden-like | ||||||
| 56.1 | Silver-like | 20.11 | 13.41 | ||||
| 38.1 | Gold coated | 6.28 | 4.18 | ||||
| 26.2 | Clearly non-precious metal | ||||||
| 6.1 | Gold coated | 14.28 | 9.52 | ||||
NB: A red field indicates that the TDI value is already exceeded without including the contribution from jewelleries.
No calculations are performed for cadmium as the TDI value for cadmium is already exceeded for the 5% of Danish children being exposed to the maximum background exposure of cadmium.
Three jewellery parts are close to the TDI value related to lead.
| No. | Product category | Margin to TDI value / Uder, adults - at 95 percentile background exposure | |||||
| Cd | Pb | Ni | |||||
| 16 h | 24 h | 16 h | 24 h | 16 h | 24 h | ||
| 169.1 | Silver coated | 344 | 229 | 4 | 2.6 | ||
| 164.1 | Silver coated | 8205 | 5470 | ||||
| 152.2 | Clearly non-precious metal | ||||||
| 138.1 | Silver alloy | 1.91 | 1.27 | 7.13 | 4.75 | ||
| 136.2 | Silver coated | 1.80 | 1.20 | 9.59 | 6.39 | ||
| 130.1 | Silver-like | ||||||
| 125.1 | Silver coated | 26.02 | 17.35 | 13.54 | 9.03 | 3.9 | 2.6 |
| 107.1 | Gold coated | ||||||
| 101.3 | Gold coated | ||||||
| 101.1 | Gold coated | 42.01 | 28.00 | ||||
| 99.2 | Gold coated | ||||||
| 99.1 | Gold coated | ||||||
| 95.3 | Gold coated | 128 | 85.33 | ||||
| 91.1 | Clearly non-precious metal | 0.9 | 0.6 | ||||
| 91.3 | Clearly non-precious metal | 0.7 | 0.5 | ||||
| 88.1 | Silver-like | 1.12 | 0.75 | 1175 | 784 | ||
| 70.3 | Gold coated | 16.8 | 11.2 | ||||
| 70.1 | Gold coated | 23.70 | 15.80 | ||||
| 68.1 | Silver coated | 2.04 | 1.36 | 18.1 | 12.1 | ||
| 62.1 | Silver-like | 0.82 | 0.54 | 20.5 | 13.7 | ||
| 60.1 | Golden-like | ||||||
| 56.1 | Silver-like | 75.7 | 50.5 | ||||
| 38.1 | Gold coated | 1.33 | 0.88 | 23.6 | 15.8 | ||
| 26.2 | Clearly non-precious metal | ||||||
| 6.1 | Gold coated | 0.75 | 0.50 | 53.8 | 35,8 | ||
Table 7-6: Results of dermal exposure at adults for 16 and 24 hours respectively – at background exposure corresponding to the 95 percentile.
The results show that for the 5% adults being exposed to the maximum background exposure of cadmium (i.e. the value belonging to the 95 percentile or higher) the TDI value is exceeded for four of the jewelleries when they are worn for a period of 24 hours, while only two of them exceed the TDI value when they are worn for only 16 hours.
None of the jewelleries gives problems related to lead while two jewellery parts exceed the TDI value related to nickel when they are worn for 16 and 24 hours respectively.
Added results for jewelleries which had jewellery parts with different migration results
For 5 jewelleries a migration analysis has been made on two different jewellery parts belonging to the same jewellery. To get a correct picture of the potential skin absorption on these jewelleries it is necessary to add the results for the two different jewellery parts. Below the added results are presented. These calculations are only relevant to carry out for dermal exposure as it is not expected that humans will have two different charms in the mouth at the same time. As an explanation of the table it can be mentioned that among the jewelleries showing a migration there was a jewellery from where nickel migrated from two different jewellery parts (jewellery no.91). There was no case of a jewellery showing migration of cadmium from two different parts on the same jewellery and therefore there are no results for cadmium.
Table 7-7: Added results for the 4 jewelleries which each had two jewellery parts with different migration of metals. Dermal exposure time: 24 hours. Background exposure: the 95 percentile.
| Jewellery no. | Margin to TDI value/ Uder, 24 h – at 95 percentile background exposure | |||||
| Pb | Ni | Cu | ||||
| Children | Adults | Children | Adults | Children | Adults | |
| 101 | 663.0 | 7041.8 | ||||
| 99 | 611.3 | 6492.5 | ||||
| 91 | 1.2 | 0.3* | 23.8 | 252.9 | ||
| 70 | 5637.2 | 59876.7 | ||||
* At exposure of 16 hours the value is 0.4.
It is seen that jewellery no. 91 exceeds the total TDI when adults wear it for 24 hours (and 16 hours). This is valid for the 5% of adults in Denmark being exposed to maximum background exposure of nickel.
As the results from this project show that there might potentially be a hazardous health risk by wearing just one piece of jewellery at a time it shall of course be emphasised that if several different jewelleries are worn at the same time the exposure will increase and the potential health risk of course increase as well.
7.3 Oral exposure
When people suck jewelleries (as for instance a pendant) the metals, which migrate from the jewellery, will be ingested directly. In these cases, the total amount of migrated metal (measured in the migration analysis) will be ingested (though not necessarily absorbed in the body).
The TDI value, which is used during the risk assessment, is based on studies that indicate an effect at a certain amount of ingested metal. Thus, during the risk assessment in this project the amount of ingested metal is compared directly to the TDI value (i.e. an oral absorption rate is not multiplied, as in the calculations related to dermal exposure).
It is assumed that as a worst case jewellery is sucked for a period of maximal two hours per day. Calculations regarding oral ingestion are based on the equation for migration of substances from a product to food/beverages, which is then ingested (European Commission, 2003). However, sucking jewellery is not fully comparable with the situation of which the above mentioned equation is based, thus the equation is adjusted.
Thus, oral ingestion can be calculated based on the following equation (European Commission, 2003):
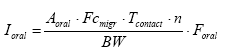
Where:
| Ioral | Amount of substance ingested | µg/kg bw/day |
| Aoral | Total amount of product being sucked | G |
| Fcmigr | Fraction of substance, which migrates per unit of time | µg/g/hour |
| Tcontact | Duration of exposure per incident | Hours |
| N | Number of incidents per day | per day |
| BW | Body weight | Kg |
| Foral | Fraction, which is absorbed (bioavailable part) |
By oral exposure of the jewelleries the equation can be adjusted to:
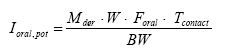
Where:
| Ioral, pot | Potential uptake of chemical substance | µg/kg bw/day |
| Mder | Fraction of chemical substance, which migrates | µg/g/4 hours |
| W | Weight of jewellery | G |
| Foral | Part of jewellery (w), which is assumed to be sucked | % |
| Tcontact | Duration of exposure | Hours |
| BW | Body weight | Kg |
Mder, W, and BW represent the same as described in the section regarding dermal exposure calculation.
Foral represents the part of the weight of the jewellery (W) (which has a problematic content of heavy metal) that can be assumed to be sucked. Foral is determined by visually judging how large a part of each jewellery that realistically can be assumed to be put into the mouth. Thus, for jewelleries such as rings, bracelets and piercings Foral is 0 since these jewelleries are not assumed to be put into the mouth.
Tcontact represents the duration of the exposure which here is assumed to be 2 hours.
When Ioral, pot is calculated, the value is compared to the value representing “Margin to TDI” which is described in Table 7-2. The “Margin to TDI” value must, as described earlier, be regarded as the amount of metal which people can tolerate to take in on a daily basis, subtracted the amount of metal which the Danes already take in via food, beverages and air – in other words, the “Margin to TDI” value must be thought of as the extra amount of metal which people can tolerate on a daily basis without experiencing health related effects. If the Ioral, pot value exceeds this “Margin to TDI” value there is a potential health related risk by sucking the jewelleries.
Example of a calculation on uptake via oral exposure
Below is described how the exposure calculation related to lead is performed for jewellery no. 62. The calculation is performed by extrapolating the result derived from the migration test of the jewellery part (belonging to that specific jewellery) to account for the metal part of the jewellery which can be expected to be put into the mouth.
The duration of the exposure (Tcontact) is 2 hours and the “target” of interest is children (20 kg). The migration analysis showed that 39 µg lead migrated per gram of jewellery during a period of 4 hours. The part of the jewellery which is assumed to have a problematic content of metal (W) – i.e. the weight of the part of the jewellery, which resembles the analysed part – weighs 40 gram. The part of W, which is assumed to be put into the mouth (Foral) is 0.50 (since the pendant is of such a size that it is judged that only half of it can be put into the mouth) – thus the weight of the part of the jewellery that can be put into the mouth is 20 gram.
Thus, the following equation is valid:
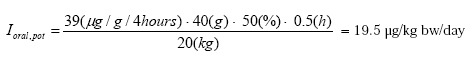
It should be noted that the migration analysis is performed during a period of 4 hours. In order to illustrate an exposure scenario of 2 hours a factor of 0.5 is therefore multiplied in the calculations.
The TDI value for lead is set at 1.8 µg/kg bw/day. If the amount of lead, which an average child (4-6 years) is exposed to on a daily basis via air, food and beverages (0.49 µg/kg bw/day) is subtracted, a “Margin to TDI” value of 1.31 µg/kg bw/day arises (see Table 7-2).
Thus, the result is as follows:
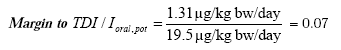
In this case the result is below 1, which indicates that there is a potential health related risk related to lead for children who suck this particular jewellery for a period of 2 hours per day. This is only valid for children that are exposed to an average background concentration of lead.
It should be pointed out that a TDI value represents the amount of substance that a person can tolerate to take in per day during an entire lifetime, without the occurrence of health related effects. Thus, it is possible for a short period of time to exceed the TDI value without this causing any effects, provided that in a similar time period later in life the person takes in an equivalent lesser amount. Thus, sucking the above mentioned jewellery for a period of 2 hours per day will not result in health related effects unless the person every day for the rest of his/her life takes in an amount corresponding to the tolerable daily dosage (the TDI value).
7.3.1 Results – oral exposure
Background exposure - average
The table below presents the results related to oral exposure of the jewelleries during a period of 2 hours per day in adults and children respectively – who already are exposed to an average background exposure of the four metals.
Table 7-8: Results related to oral exposure during a period of 2 hours in adults and children respectively – calculated in scenarios representing an average background exposure.
| No | Product category | Margin to TDI value / Ioral, pot – at average background exposure | |||||||
| Cd | Pb | Ni | Cu | ||||||
| Child | Adult | Child | Adult | Child | Adult | Child | Adult | ||
| 164.1 | Silver coated | ||||||||
| 152.2 | Clearly non-precious metal | ||||||||
| 138.1 | Silver alloy | 0.09 | 0.77 | 0.01 | 0.04 | 87 | 921 | ||
| 136.2 | Silver coated | 0.88 | 7.94 | 0.06 | 0.21 | 153 | 1629 | ||
| 130.1 | Silver-like | ||||||||
| 125.1 | Silver coated | ||||||||
| 107.1 | Gold coated | ||||||||
| 101.3 | Gold coated | 19 | 205 | ||||||
| 101.1 | Gold coated | 0.12 | 0.41 | 97 | 1035 | ||||
| 99.2 | Gold coated | ||||||||
| 99.1 | Gold coated | ||||||||
| 95.3 | Gold coated | 0.21 | 0.71 | 314 | 3335 | ||||
| 91.1 | Clearly non-precious metal | 1.9 | 0.8 | 3.61 | 38 | ||||
| 91.3 | Clearly non-precious metal | 5.3 | 2.3 | 3.57 | 38 | ||||
| 88.1 | Silver-like | ||||||||
| 70.3 | Gold coated | 0.47 | 1.58 | 863 | 9170 | ||||
| 70.1 | Gold coated | 1.07 | 9.59 | 156 | 1652 | ||||
| 68.1 | Silver coated | ||||||||
| 62.1 | Silver-like | 0.07 | 0.66 | 0.07 | 0.23 | 77 | 821 | ||
| 60.1 | Golden-like | ||||||||
| 56.1 | Silver-like | 0.10 | 0.34 | 60 | 635 | ||||
| 38.1 | Gold coated | 0.06 | 0.54 | 0.04 | 0.13 | 285 | 3030 | ||
| 26.2 | Clearly non-precious metal | 412 | 4378 | ||||||
| 6.1 | Gold coated | 0.07 | 0.61 | 0.09 | 0.30 | 1137 | 12074 | ||
The results show that when sucking the jewelleries for a period of 2 hours potential health related risks related to cadmium, nickel and lead arise. It is most pronounced for lead and cadmium since almost all of the jewelleries (which showed migration of the specific metal) exceeded the TDI value related to lead and cadmium. This is valid for children and adults exposed to an average background exposure.
Background exposure – 95-percentiles
The table below presents the results related to oral exposure of the jewelleries during a period of 2 hours per day in children and adults respectively exposed to a background exposure corresponding to 95-percentiles.
Table 7-9: Results related to oral exposure during a period of 2 hours in children and adults respectively exposed to a background exposure corresponding to 95-percentiles.
| No. | Product category | Margin to TDI value / Ioral, pot – at 95-percentiles background exposure | |||||
| Cd | Pb | Ni | |||||
| Child | Adult | Child | Adult | Child | Adult | ||
| 164.1 | Silver coated | ||||||
| 152.2 | Clearly non-precious metal | ||||||
| 138.1 | Silver alloy | 0.51 | 0.01 | 0.03 | |||
| 136.2 | Silver coated | 5.30 | 0.05 | 0.18 | |||
| 130.1 | Silver-like | ||||||
| 125.1 | Silver coated | ||||||
| 107.1 | Gold coated | ||||||
| 101.3 | Gold coated | ||||||
| 101.1 | Gold coated | 0.09 | 0.35 | ||||
| 99.2 | Gold coated | ||||||
| 99.1 | Gold coated | ||||||
| 95.3 | Gold coated | 0.16 | 0.61 | ||||
| 91.1 | Clearly non-precious metal | 1.5 | 0.4 | ||||
| 91.3 | Clearly non-precious metal | 4.2 | 1.0 | ||||
| 88.1 | Silver-like | ||||||
| 70.3 | Gold coated | 0.36 | 1.36 | ||||
| 70.1 | Gold coated | 6.39 | |||||
| 68.1 | Silver coated | ||||||
| 62.1 | Silver-like | 0.44 | 0.05 | 0.20 | |||
| 60.1 | Golden-like | ||||||
| 56.1 | Silver-like | 0.08 | 0.29 | ||||
| 38.1 | Gold coated | 0.36 | 0.03 | 0.11 | |||
| 26.2 | Clearly non-precious metal | ||||||
| 6.1 | Gold coated | 0.41 | 0.07 | 0.26 | |||
NB: a red box indicates that the TDI value is exceeded without taken into account the contribution from sucking jewelleries.
As described in section 7.2.1 5% of the Danish children are already exposed to an amount of cadmium which exceeds the tolerable daily dosage. Thus, no calculations are performed for cadmium.
However, almost all of the jewelleries (which showed migration of lead) exceed the TDI value for lead in children as well as adults. Finally, one jewellery exceeds the TDI value related to nickel in situations where adults suck the jewellery for a period of 2 hours.
Table 7-10 shows the content of lead in those jewelleries, where the results from the migration analysis indicated a potential health related problem by sucking them for a 2-hour period. The table also shows the time period of which a person can suck the jewelleries before the total TDI value is exceeded.
Table 7-10: Oral exposure calculations for lead in relation to content of lead in the specific jewelleries. Calculated for persons that are exposed to an average background exposure.
| Jewellery part no. | Content of Pb (%) | Pb - Children (TDI /Ioral, pot) | Minutes of which a person can suck the jewellery before the TDI values is exceeded | Pb - Adults (TDI/ Ioral, pot) | Minutes of which person can suck the jewellery before the TDI values is exceeded |
| 95.3 | 69.59 | 0.21 | 25 | 0.71 | 21312 |
| 70.3 | 40.95 | 0.47 | 947 | 1.58 | 32975 |
| 136.2 | 33.49 | 0.06 | 30 | 0.21 | 306 |
| 56.1 | 26.14 | 0.10 | 12 | 0.34 | 313 |
| 62.1 | 15.62 | 0.07 | 16 | 0.23 | 53 |
| 38.1 | 9.21 | 0.04 | 5 | 0.13 | 562 |
| 6.1 | 7.64 | 0.09 | 11 | 0.30 | 1540 |
| 101.1 | 2.58 | 0.12 | 24 | 0.41 | 591 |
| 138.1 | 1.77 | 0.01 | 1 | 0.04 | 49 |
| 152.2 | 0.16 | - | - | ||
| 164.1 | 0.11 | - | - | ||
| 91.3 | 0.03 | - | - | ||
| 60.1 | 0.03 | - | - | ||
| 91.1 | 0.02 | - | - | ||
| 99.2 | 0.00 | - | - | ||
| 99.1 | 0.00 | - | - | ||
| 88.1 | 0.00 | - | - | ||
| 26.2 | 0.00 | - | - |
NB: The content of Pb is determined by an XRF-screening and therefore only gives an approximate estimate of the content of lead (see section 3.4.1 for description of the uncertainty). A line indicates that no migration occurred, thus it was not possible to calculate a TDI/Ioral, pot value.
The results show that the lowest content of lead, which causes potential health related problems by sucking the jewelleries for a period of 2 hours, is a lead content of 1.77%, i.e. a great deal above the claim of a maximum content of 0.01% lead stated by the new Statutory order on ban on import and sale of products containing lead. At the same time it is seen that none of the jewelleries containing a maximum amount of 100 ppm lead cause potential health related problems.
The table above likewise shows the number of minutes of which an adult/child can suck the jewelleries before the tolerable daily dosage is exceeded. For jewellery no. 138.1 it can be seen that children as a maximum can suck the jewellery for 1 minute before the tolerable daily dosage is exceeded. It is here interesting to note that this jewellery proved to have the lowest content of lead (out of the jewelleries that showed migration of lead). Of this can be concluded that in general it must be recommended not to suck jewelleries.
7.4 Total health risk by using jewelleries
Based on the findings of this project it cannot be excluded that by wearing or sucking some of the metal jewelleries examined in this project potential health risks related to especially cadmium and nickel arise. This is valid for people exposed to the background exposure found in Denmark. For lead there are primarily problems when sucking the jewelleries. None of the jewelleries examined caused problems related to copper.
The health related risks associated with the three metals are effects on the kidneys (cadmium), increased mortality risk on foetuses (nickel) and reduced IQ at children (lead).
If the jewelleries comply with the legal demand related to content of lead, the results in this project indicate that no health related risks arise by wearing or sucking the jewelleries.
A number of reservations are decisive in terms of health related problems arising. Among others, the jewellery must be worn/sucked every day over an extended period of time, since a short-term exceeding of the tolerable daily dosage not necessarily results in health related effects, unless during the remaining life time the person takes in an amount equal to the tolerable daily dosage. Furthermore it is assumed in the calculations, that the migration of metals from the jewelleries is constant. The migration will in time decrease.
Additionally, the findings of this project show that no correlations seem to exist between the appearance of the jewellery (i.e. gold coated, silver-like etc.) and the content/migration of heavy metals. Based on the findings of this project there is thus no reason for avoiding for instance silver-like or gold coated jewelleries. Though, it should be mentioned that jeweller jewelleries (genuine silver and gold), that is jewelleries submitted to control by the Precious Metal Assay and Inspection, generally are not expected to contain heavy metals at a problematic level.
Finally it is in the project concluded that there exist textile necklaces on the Danish market, which contain benzidine in amounts exceeding the allowable.
8 References
Andersen, L.K., Larsen, H.S., Larsen, P.B. Tyle, H. 2005a. “RISK ASSESSMENT – Nickel Cas-No: 7440-02-0.” Draft, November 2005. Danish Environmental Protection Agency. http://ecb.jrc.it/DOCUMENTS/Existing-Chemicals/RISK_ASSESSMENT/DRAFT/R311_0601_hh.pdf
Andersen, L.K., Larsen, H.S., Larsen, P.B. Tyle, H. 2005b. “RISK ASSESSMENT – Nickel Sulphate Cas-No: 7786-81-4.” Draft, November 2005. Danish Environmental Protection Agency.
Andersen, L.K., Larsen, H.S., Larsen, P.B., Tyle, H. 2006. ”Nickel and nickel compounds. Background Document in support of individual RISK ASSESSMENT REPORTS of nickel compounds – prepared in relation to Council Regulation (EEC) 793/93. 2006. Danish Environmental Protection Agency.
Andersen, NL et al. ”The Danes’ dietary habits 2000-2002”. Main results (In press). In Danish. Søborg: Danish Institute for Food and Veterinary Research.
American Biogenics Corporation. 1988. “Ninety day gavage study in albino rats using nickel”. Final report submitted to U.S. Environmental Protection Agency, Office of Solid Waste. Submitted by Research Triangle Institute and American Biogenics Corporation.
ATSDR (1999). ”Toxicological profile for cadmium”. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 1999.
ATSDR (2004). “Toxicological profile for copper”. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 2004.
ATSDR (2005). ”Draft – Toxicological profile for lead”. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 2005.
ATSDR (2005a). “Toxicological profile for nickel”. U.S. Department of Health and Human Services. Public Health Service. Agency for Toxic Substances and Disease Registry. 2005.
Baars, A.J., Theelen, R.M.C, Janssen, P.J.C.M, Hesse, J.M., van Apeldoorn, M.E., Meijerink, M.C.M, Verdam, L. and Zeilmaker, M.J. 2001. ”Re-evaluation of human-toxicological maximum permissible risk levels”. Research for man and environment RIVM report 711701 025.
Berg, KK., MD, Hennepin County, Minnesota Office of the Medical Examiner; HF Hull, MD, Minnesota Dept of Health; EW Zabel, PhD, Minnesota Childhood Lead Poisoning Prevention Program. PK Staley, MPA, MJ Brown, ScD, DM Homa, PhD, Div of Emergency and Environmental Health Svcs, National Center for Environmental Health, CDC. “Death of a Child After Ingestion of a Metallic Charm”. Minnesota, 2006.
Briefing from the Danish Environmental Protection Agency no. 8, 2004. ”Listen over uønskede stoffer 2004”. Danish Environmental Protection Agency 2004.
Buckler, H.M., Smith, W.D., Rees, W.D. 1986. “Self poisoning with oral cadmium chloride”. Br Med J. 292:1559-1560.
Chemfinder. Chemfinder.com. Found on: http://chemfinder.cambridgesoft.com/.
Cross, H., Wheatley, A. Sadhra, S. 2006 ”Copper, copper sulphate pentahydrate, copper(I)oxide, copper(II)oxide, dicopper chloride trihydroxide. Voluntary risk assessment. Chapter 4.1.2. Human Health – effects. 2006. Draft.
Environmental Project no. 635, 2001. ”Rapport om vejledende liste til selvklassificering af farlige stoffer”. Environmental Project no. 635, 2001, Danish Environmental Protection Agency.
Environmental Project no. 557, 2000. ”Massestrømsanalyse for cadmium”. Danish Environmental Protection Agency.
European Commission, 2003. TGD. “Technical Guidance Document on Risk Assessment in support of Commission Directive 93/67/EEC on Risk Assessment for new notified substances. Commission Regulation (EC) No 1488/94 on Risk Assessment for existing substances. Directive 98/8/EC of the European Parliament and of the Council concerning the placing of biocidal products on the market”. Part I. European Commission, Joint Research Centre, Institute for Health and Consumer Protection, European Chemicals Bureau, 2003.
EU risk assessment – cadmium oxide. Part II - Human health. 2007. Institute for Health and Consumer Protection. European Chemicals Bureau.
Fromberg, A., Larsen, E.H., Hartkopp, H., Larsen, J.C., Granby, K., Jørgensen, K., Rasmussen, P.H., Cederberg, T., Christensen, T. 2005. “Chemical contaminants. Food monitoring, 1998-2003”. Part 1. Ministry of Family and Consumer Affaris. Danish Veterinary and Food Administration.
HSDB. Cadmium chloride. Hazardous Substances Data Bank. Cas no. 10108-64-2.
IARC (1997). “Chromium, Nickel and Welding”. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 49. International Agency for Research on Cancer. World Health Organization.
IARC (1997a). ”Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry”. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 58. International Agency for Research on Cancer. 1997 World Health Organization.
IARC (2006). ”Inorganic and organic lead compounds”. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 87. International Agency for Research on Cancer. 2006 World Health Organization.
IRIS (1996). Nickel, soluble salts (CASRN Various). U.S. Environmental Protection Agency. Integrated Risk Information System.
IUCLID, 2000. Dataset on lead (Cas no. 1317-36-8). European Commission – European Chemicals Bureau. 2000.
IUCLID, 2000a. Dataset on lead monoxide (Cas no. 1317-36-8). European Commission – European Chemicals Bureau. 2000.
IUCLID, 2000b. Dataset on copper (Cas no. 7440-50-8). European Commission – European Chemicals Bureau. 2000.
IOM, 2001. ”Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium and Zinc. Institute of Medicine. National Academy Press, Washington, D.C. 2001. (Abstract).
Kemp, K., Ellermann, T., Brandt, J., Christensen, J., Ketzel, M. “The Danish Air Quality Monitoring Programme”. Annual Summary for 2006. NERI Technical Report No. 623, 2007. National Environmental Research Institute. University of Aarhus, Denmark.
Larsen, P. B., and Tyle, H. 2008 – Draft. “Nickel; Nickel Carbonate; Nickel Chloride; Nickel Dinitrate; Nickel Sulphate”. Cas No: 7440-02-0; 3333-67-3; 7718-54-9; 13138-45-9; 7786-81-4”. European Union Risk Assessment Report. Final draft version, April 2008. Danish Environmental Protection Agency in collaboration with NiPERA and VITO.
Lassen, C., Christensen, C.L., Skårup, S. 2004. ”Massestrømsanalyse for bly 2000 – revideret udgave”. Environmental project no. 917. Danish Environmental Protection Agency. ISBN no. 87-7614-231-0.
Laurson, S.E., Hansen, J., Drøjdahl, A., Hansen, C.O., Pommer, K., Pedersen, E. and Bernth, N. 2003. “Kortlægning af kemiske stoffer i tekstilmetervarer”. Survey of chemical substances in consumer products, no. 23 2003. The Danish Ministry of Environment, Danish Environmental Protection Agency.
”Lov om kontrol med arbejder af ædle metaller m.v.” LAW no. 308 of 17.05.1995.
Nogawa K, Honda R, Kido T et al., 1989. A dose-response analysis of cadmium in the general environment with special reference to total cadmium intake limit. Environmental Research 48:7-16.
Stat. Ord. 1199 of 23.12.1992. Bekendtgørelse om forbud mod salg, import og fremstilling af cadmiumholdige produkter. The Danish Ministry of Environment.
Stat. Ord. 1012 of 13.11.2000. Bekendtgørelse om forbud mod import og salg af produkter, der indeholder bly. The Danish Ministry of Environment. (historic).
Stat. Ord. no. 24 of 14.01.2000. Bekendtgørelse om forbud mod import og salg af visse nikkelholdige produkter. The Danish Ministry of Environment.
Stat. Ord. 627 of 01.07.2003. Bekendtgørelse om forbud mod import, salg og eksport af kviksølv og kviksølvholdige produkter. The Danish Ministry of Environment.
Stat. Ord. 755 of 15.08.2003. Bekendtgørelse om forbud mod import, salg og anvendelse af visse azofarvestoffer. The Danish Ministry of Environment.
Stat. Ord. no. 923 of 28.09.2005. Bekendtgørelse om listen over farlige stoffer. The Danish Ministry of Environment.
Stat. Ord. no. 789 of 12.08.2005. Bekendtgørelse om ændring af bekendtgørelse om forbud mod import og salg af visse nikkelholdige produkter. The Danish Ministry of Environment.
Stat. Ord. no. 1082 of 13.09.2007. Bekendtgørelse om forbud mod import og salg af produkter, der indeholder bly. The Danish Ministry of Environment. (valid).
Strandesen, M., Birkved, M., Holm, P.E. and Hauschild, M.Z. 2007. “Fate and distribution modelling of metals in life cycle impact assessment.” Ecological Modelling 203. 327-338. 2007.
Turnland, R.J., Keyes, W.R., Peiffer, G.L., Scott, K.C. 1998. ”Copper absorption, excretion and retention by young men consuming low dietary copper determined by using the stable isotope 65Cu1,2. The American Journal of Clinical Nutrition. 1998. 67;1219-25.
Vermeire, T.G., van Apeldoorn, M.E., de Fouw, J.C., Janssen, P.J.C.M. 1991. ”Voorstel voor de humaan-toxicologische onderbouwing van C-(toetsings)waarden”. Rapportrn. 725201005. Rijksinstituut voor Volksgezondheid en Milieuhygiene. Bilthoven.
Wester, et. al. (1992). Abstract of Wester R.C., Maibach H.I, Sedik, L., Melendres J., DiZio S. and Wade, M. ”In vitro percutaneous absorption of cadmium from water and soil into human skin”. Fundam Appl. Toxicol. 1992. Jul. (19(1): 1-5.).
WHO (1991). Environmental Health and Safety Guide no. 62. ”Nickel, nickel carbonyl, and some nickel compounds”. IPCS INCHEM Home. World Health Organization. 1991.
WHO (1995). Safety evaluation of certain food additives and contaminants. WHO Food Additives Series: 44. IPCS INCHEM Home. World Health Organization. 1995.
WHO (1998). Environmental Health Criteria 200. ”Copper”. IPCS INCHEM Home. World Health Organization. 1998.
WHO (2000). “Safety evaluation of certain food additives and contaminants” WHO Food additives series: 44. Lead. 2000 World Health organisation.
WHO (2003). “Lead in Drinking-water”. Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organisation. 2003.
WHO (2004). “Cadmium in Drinking-water”. Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organisation. 2004.
WHO (2004a). “Copper in Drinking-water”. Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organisation. 2004.
WHO (2007). “Nickel in Drinking-water”. Background document for development of WHO Guidelines for Drinking-water Quality. World Health Organisation. 2007.
9 List of Appendixes
Appendix A: Extracts from the database of jewelleries
Appendix B: Note on ”Heavy metal in precious metals jewelleries”
Appendix C: DST categorization of jewelleries
Appendix D: Amount of jewelleries on the market in Denmark
Appendix E: Banned azo-dyes
Appendix F: Aromatic amines
Appendix G: The requirements of the Toys Statutory Order on maximum release of heavy metals
Appendix H: Samples where duplicate determinations varied
Appendix I: Results from screening of textile necklaces
Appendix J: Results from the migration analysis calculated in relation to areal jewellery
Appendix K: Selected jewellery parts for migration test
Appendix L: Correlation between migration and content of metal
Footnotes
[1] Source: (http://www.ldaint.org/-information.htm#Info)
[2] http://www.enhis.org/object_document/o4736n27387.html
[3] http://www.netdoktor.dk/sunderaad/fakta/pigevaekstTabel.htm
[4] http://da.wikipedia.org/wiki/Organisk_syre
[5] http://en.wikipedia.org/wiki/Phenylbutazone
[6] Information from IOM (2001) also confirms that the tolerable daily intake of copper is 10 mg per day.
[7] http://glwww.mst.dk/udgiv/publikationer/2000/87-7944-304-4/html/kap13.htm
[8] Statutory order on ban on import and sale of certain nickel-containing products. Stat. Ord. No. 24 of 14.01.2001.
[9] Statutory order on change of statutory order on ban on import and sale of certain nickel-containing products. Stat. Ord. No. 789 of 12.08.2005.
Appendix A: Extracts from the database of jewelleries
1 Overview of the content in the database
This appendix contains a description of the content of the completed database of the purchased jewelleries.
1.1 Database
For the in total 170 purchased pieces of jewellery a database in Microsoft Access 2000 has been developed for the Danish Environmental Protection Agency. The purpose of the database is to create an overview of the different parameters which characterise the purchased jewelleries, including the analysis results from the XRF screening and the migration analyses as well. Furthermore, it is possible to sort in the information in the database as well as extract information criss-cross in the entered data.
In the table below the registered information in the database of all purchased metal jewelleries is presented.
Table 1-1: Information registered for each piece of metal jewellery in the database
| Information of type | Remark |
| Jewellery no. | |
| Product type | The database operates with the following product types: Necklace, bracelet, earring, ring, ankle chain, piercing, other. |
| Product description | Here the immediate appearance of the product is described, e.g. gold-coated necklace with angel charm. |
| Bar code | Registered if available. |
| Article or order number | Registered if available. |
| Trademark | Here a possible jewellery trademark is noted, as for instance “Pilgrim”. |
| Name of shop | |
| Address of shop | |
| Type of shop | The database operates with the following types of shops: Jewellery shop, internet shop, shop with owners of another ethnic background, department store, clothes shop, 10 kroner’s’ shop, supermarket, other type of shop. |
| Time of purchase | Stated as d/m/y. |
| Name of picture file | A picture of each piece of jewellery is linked to the database. |
| Price (DKK) | |
| Weight (g) | The weight for the whole piece of jewellery, i.e. including chain, but exclusive price ticket etc. |
| Price per gram | The weight of the piece of jewellery divided by the price. |
| CBR no. | CBR numbers are the identification number of the companies. In Denmark, the number is eight-digit and is registered in this database if immediately available. |
| Information from the shop about the content of precious metal | Here possible information is registered which shop assistants/shop owners etc. could immediately find in relation to the content of precious metal in the piece of jewellery. |
| Comments | Here information regarding where to locate further information related to suppliers, producers and country of origin, is registered (i.e. typically telephone number to supplier, head office etc.). |
| Name and address of importer | |
| Name and address of producer | |
| Country of origin | Here the database operates with the following options: The shop does not know, England, the Netherlands, India, China, South Korea, Thailand, Checked but could not be stated, the East. |
| Screening data | Here a possible content (in percent) of the following metals is registered (for maximum three different parts of each piece of jewellery): Au, Ag, Cu, Sb, Pb, Se, Cr, Cd, Hg, Sn, Al, Mo, Nb, Zr, Bi, W, Zn, Ni, Co, Fe, Mn, V, Ti, In, Pt, Pd. Furthermore, it is registered whether the jewellery parts contain As and Ba. |
| Description of jewellery part | For instance ”charm (angel)” or ”chain”. |
| Appearance of jewellery part | Here the database operates with the following product categories: genuine gold, genuine silver, clearly non-precious metal, gold coated, silver coated, silver alloy, golden-like, silver-like. |
| Estimated fraction of jewellery part being in contact with the skin | Estimate based on the appearance of the individual jewellery part. |
In the following the registered information for a few of the above-mentioned points is described in details.
1.1.1 Bar code
The bar code is registered for all products having an imprinted bar code. Stating a bar code (EAN bar code) is optional. The bar code is a tool for registration of goods in connection with stock control and sales. The bar code is stated both through a number (the EAN bar code) and the actual bar code. The two first digits state in which country the good is registered, but not necessarily where it is produced. 57 is the code for Denmark. The next five digits represents a marketing number for producer, importer or retail. Hereafter five digits follow being the internal part number related to the distributor and the last digit is a control digit. The price of the good is not stated in the EAN number or the bar code (Mærkningsguiden, 1997).
2 Results of the screening
This chapter shows the content (in percentage) of the different metals in the examined jewelleries.
2.1 Content of Pb in all jewellery parts
| Jewellery part ID | Pb | Pb Error | Product type | Appearance of jewellery part | Description jewellery part |
| 95.3 | 69.59 | 0.59 | Necklace | Gold coated | catch |
| 151.1 | 49.18 | 0.40 | Bracelet | Clearly non-precious metal | clover charm from behind |
| 94.2 | 47.97 | 0.36 | Necklace | Silver-like | catch |
| 70.3 | 40.95 | 0.39 | Necklace | Gold coated | catch |
| 136.2 | 33.49 | 0.21 | Other | Silver coated | disc |
| 79.2 | 33.24 | 0.29 | Necklace | Gold coated | catch |
| 159.3 | 31.22 | 0.29 | Necklace | Silver-like | catch |
| 85.1 | 30.06 | 0.16 | Necklace | Gold coated | charm backside |
| 90.2 | 28.87 | 0.20 | Necklace | Gold coated | catch |
| 19.3 | 26.90 | 0.33 | Bracelet | Silver coated | small ball |
| 56.1 | 26.14 | 0.18 | Necklace | Silver-like | charm backside |
| 82.2 | 25.82 | 0.26 | Necklace | Gold coated | catch |
| 151.3 | 25.79 | 0.30 | Bracelet | Silver coated | catch |
| 20.1 | 25.66 | 0.19 | Earring | Gold coated | golden side |
| 85.2 | 24.89 | 0.28 | Necklace | Gold coated | catch |
| 134.1 | 24.81 | 0.21 | Necklace | Silver alloy | butterfly |
| 81.3 | 23.28 | 0.22 | Necklace | Gold coated | catch |
| 80.1 | 22.35 | 0.19 | Necklace | Gold coated | heart inner side |
| 19.2 | 22.30 | 0.20 | Bracelet | Silver-like | catch |
| 70.1 | 21.42 | 0.16 | Necklace | Gold coated | charm |
| 41.1 | 20.82 | 0.15 | Necklace | Gold coated | piece of jewellery |
| 147.2 | 20.23 | 0.21 | Bracelet | Silver-like | catch |
| 71.2 | 19.47 | 0.20 | Necklace | Silver coated | catch |
| 43.1 | 19.25 | 0.18 | Necklace | Gold coated | piece of jewellery |
| 100.1 | 18.16 | 0.19 | Earring | Silver coated | backside of charm |
| 153.2 | 18.10 | 0.20 | Earring | Silver-like | connecting link |
| 8.2 | 16.68 | 0.21 | Necklace | Gold coated | catch |
| 57.1 | 16.56 | 0.19 | Earring | Silver-like | piece of jewellery backside |
| 27.1 | 16.31 | 0.16 | Necklace | Gold coated | backside of charm |
| 62.1 | 15.62 | 0.16 | Necklace | Silver-like | charm |
| 96.1 | 15.14 | 0.15 | Earring | Silver-like | backside of needle |
| 74.3 | 15.08 | 0.22 | Necklace | Silver-like | catch |
| 14.1 | 14.77 | 0.15 | Bracelet | Clearly non-precious metal | metallic side |
| 19.1 | 14.74 | 0.13 | Bracelet | Silver coated | disc |
| 104.3 | 14.48 | 0.14 | Bracelet | Silver-like | pins |
| 90.1 | 14.47 | 0.14 | Necklace | Gold coated | big heart backside |
| 68.1 | 14.35 | 0.14 | Ring | Silver coated | ring |
| 136.1 | 14.19 | 0.13 | Other | Silver coated | charm |
| 96.2 | 14.01 | 0.20 | Earring | Silver-like | side |
| 142.1 | 13.07 | 0.19 | Bracelet | Silver-like | catch pin |
| 32.1 | 12.01 | 0.14 | Necklace | Silver-like | side 2 without stone |
| 169.3 | 12.00 | 0.18 | Ankle chain | Silver-like | catch |
| 10.1 | 11.47 | 0.14 | Ring | Clearly non-precious metal | ring |
| 29.1 | 11.07 | 0.11 | Ring | Clearly non-precious metal | ring disc |
| 46.1 | 10.89 | 0.12 | Bracelet | Gold coated | flower |
| 2.1 | 10.88 | 0.12 | Earring | Gold coated | flower |
| 12.2 | 10.74 | 0.12 | Ring | Clearly non-precious metal | death's head |
| 12.1 | 9.95 | 0.12 | Ring | Clearly non-precious metal | ring/other |
| 124.1 | 9.89 | 0.17 | Necklace | Silver-like | catch |
| 38.1 | 9.21 | 0.10 | Necklace | Gold coated | piece of jewellery without stone |
| 42.1 | 9.10 | 0.11 | Necklace | Silver-like | leaf |
| 54.1 | 9.06 | 0.11 | Necklace | Silver-like | charm |
| 78.2 | 8.96 | 0.20 | Necklace | Silver-like | catch |
| 8.1 | 8.92 | 0.12 | Necklace | Gold coated | flower |
| 40.1 | 8.92 | 0.11 | Necklace | Silver-like | piece of jewellery |
| 15.1 | 8.70 | 0.10 | Ring | Silver-like | ring |
| 139.2 | 8.33 | 0.13 | Necklace | Clearly non-precious metal | catch |
| 6.1 | 7.64 | 0.11 | Necklace | Gold coated | angle |
| 156.1 | 7.50 | 0.10 | Earring | Silver-like | backside and needle |
| 63.1 | 6.73 | 0.12 | Necklace | Gold coated | charm without stone |
| 103.1 | 5.41 | 0.08 | Earring | Silver-like | bright side |
| 31.3 | 4.16 | 0.08 | Necklace | Silver-like | cartridge |
| 76.2 | 4.07 | 0.11 | Necklace | Silver-like | catch |
| 107.1 | 3.86 | 0.09 | Bracelet | Gold coated | chain with balls |
| 78.1 | 3.65 | 0.08 | Necklace | Silver-like | heart leaf side |
| 101.3 | 3.65 | 0.08 | Necklace | Gold coated | cone |
| 82.1 | 3.25 | 0.07 | Necklace | Gold coated | charm backside |
| 81.1 | 3.23 | 0.07 | Necklace | Gold coated | charm backside |
| 113.1 | 3.02 | 0.09 | Bracelet | Golden-like | chain |
| 3.2 | 2.98 | 0.09 | Necklace | Gold coated | small charm |
| 101.1 | 2.58 | 0.07 | Necklace | Gold coated | backside of heart |
| 4.1 | 2.56 | 0.07 | Ring | Silver-like | ring |
| 76.1 | 2.31 | 0.07 | Necklace | Silver-like | butterfly |
| 105.1 | 2.30 | 0.08 | Ring | Silver-like | metallic side |
| 77.1 | 2.24 | 0.07 | Necklace | Silver-like | heart leaf side |
| 138.3 | 2.13 | 0.07 | Necklace | Silver coated | catch |
| 138.1 | 1.77 | 0.05 | Necklace | Silver alloy | charm from behind |
| 82.3 | 1.39 | 0.05 | Necklace | Gold coated | connecting piece |
| 150.1 | 1.25 | 0.05 | Bracelet | Silver-like | charm |
| 130.1 | 1.21 | 0.08 | Earring | Silver-like | needles |
| 125.1 | 1.20 | 0.06 | Ring | Silver coated | ring |
| 159.1 | 0.88 | 0.04 | Necklace | Silver-like | heart backside |
| 63.2 | 0.59 | 0.04 | Necklace | Gold coated | heart |
| 116.2 | 0.56 | 0.04 | Bracelet | Silver coated | backside of flower and chain |
| 95.1 | 0.54 | 0.03 | Necklace | Gold coated | charm |
| 46.2 | 0.52 | 0.06 | Bracelet | Gold coated | bracelet |
| 77.2 | 0.52 | 0.05 | Necklace | Silver-like | catch |
| 84.2 | 0.51 | 0.05 | Earring | Gold coated | star |
| 31.2 | 0.47 | 0.03 | Necklace | Silver-like | balls 2 pcs. |
| 129.1 | 0.45 | 0.05 | Earring | Gold coated | needles |
| 39.1 | 0.41 | 0.03 | Ring | Genuine silver | ring |
| 143.1 | 0.40 | 0.03 | Bracelet | Silver coated | bracelet |
| 169.1 | 0.36 | 0.02 | Ankle chain | Silver coated | heart |
| 3.1 | 0.33 | 0.02 | Necklace | Gold coated | large charm |
| 147.3 | 0.32 | 0.03 | Bracelet | Silver-like | eye |
| 152.2 | 0.16 | 0.02 | Ring | Clearly non-precious metal | matt ring |
| 57.2 | 0.13 | 0.02 | Earring | Silver-like | catch |
| 102.1 | 0.13 | 0.02 | Bracelet | Silver-like | metallic side |
| 152.1 | 0.13 | 0.02 | Ring | Silver-like | bright ring |
| 159.2 | 0.13 | 0.02 | Necklace | Silver-like | chain |
| 161.1 | 0.13 | 0.02 | Bracelet | Silver-like | bracelet |
| 164.1 | 0.11 | 0.01 | Piercing | Silver coated | heart backside |
| 70.2 | 0.10 | 0.03 | Necklace | Gold coated | cu-coloured chain |
| 30.1 | 0.09 | 0.03 | Earring | Gold coated | ear studs |
| 53.3 | 0.09 | 0.02 | Ankle chain | Gold coated | chain |
| 106.1 | 0.09 | 0.01 | Earring | Genuine silver | discs |
| 145.1 | 0.09 | 0.02 | Earring | Gold coated | rings |
| 153.3 | 0.09 | 0.01 | Earring | Silver-like | chains |
| 76.3 | 0.07 | 0.01 | Necklace | Silver-like | chain |
| 85.3 | 0.07 | 0.02 | Necklace | Gold coated | chain |
| 128.3 | 0.07 | 0.02 | Piercing | Silver-like | charm backside |
| 162.1 | 0.07 | 0.02 | Ring | Gold coated | ring |
| 6.2 | 0.06 | 0.01 | Necklace | Gold coated | chain |
| 33.1 | 0.06 | 0.01 | Necklace | Gold coated | 4 edged piece of jewellery |
| 56.2 | 0.06 | 0.02 | Necklace | Silver-like | chain |
| 123.1 | 0.06 | 0.01 | Ring | Gold coated | ring |
| 165.2 | 0.06 | 0.01 | Necklace | Genuine silver | chain |
| 35.1 | 0.05 | 0.01 | Necklace | Clearly non-precious metal | holder and connecting piece |
| 153.1 | 0.05 | 0.02 | Earring | Silver-like | needles |
| 21.1 | 0.04 | 0.01 | Earring | Clearly non-precious metal | metallic part |
| 34.2 | 0.04 | 0.01 | Necklace | Clearly non-precious metal | chain Cu-coloured |
| 98.2 | 0.04 | 0.01 | Bracelet | Silver-like | Twisting strings |
| 132.1 | 0.04 | 0.01 | Bracelet | Golden-like | Bracelet |
| 151.2 | 0.04 | 0.02 | Bracelet | Silver coated | chain |
| 53.1 | 0.03 | 0.01 | Ankle chain | Gold coated | round disc |
| 53.2 | 0.03 | 0.01 | Ankle chain | Clearly non-precious metal | ball |
| 60.1 | 0.03 | 0.01 | Bracelet | Golden-like | bracelet |
| 80.2 | 0.03 | 0.01 | Necklace | Gold coated | chain |
| 98.1 | 0.03 | 0.01 | Bracelet | Silver-like | bracelet |
| 118.1 | 0.03 | 0.01 | Bracelet | Gold coated | bracelet |
| 124.2 | 0.03 | 0.01 | Necklace | Silver-like | chain |
| 134.2 | 0.03 | 0.01 | Necklace | Silver coated | chain |
| 135.1 | 0.03 | 0.01 | Earring | Clearly non-precious metal | chain |
| 148.1 | 0.03 | 0.00 | Ring | Silver coated | ring |
| 91.3 | 0.03 | 0.00 | Necklace | Clearly non-precious metal | chain |
| 1.1 | 0.02 | 0.01 | Bracelet | Silver-like | bracelet |
| 32.2 | 0.02 | 0.01 | Necklace | Silver-like | chain |
| 59.1 | 0.02 | 0.01 | Bracelet | Silver-like | bracelet |
| 67.3 | 0.02 | 0.01 | Necklace | Silver-like | chain |
| 91.1 | 0.02 | 0.01 | Necklace | Clearly non-precious metal | large ball |
| 146.1 | 0.02 | 0.01 | Bracelet | Golden-like | bracelet |
| 147.1 | 0.02 | 0.01 | Bracelet | Silver-like | bracelet |
| 170.1 | 0.02 | 0.01 | Bracelet | Golden-like | golden pattern disc |
| 119.1 | 0.01 | 0.00 | Ring | Silver coated | |
| 3.3 | 0.00 | 0.02 | Necklace | Gold coated | part of the string |
| 5.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | ring |
| 7.1 | 0.00 | 0.02 | Ankle chain | Silver-like | disc circle with hole |
| 7.2 | 0.00 | 0.02 | Ankle chain | Silver-like | small disc circle |
| 7.3 | 0.00 | 0.02 | Ankle chain | Silver-like | chain |
| 9.1 | 0.00 | 0.02 | Necklace | Golden-like | heart |
| 11.1 | 0.00 | 0.04 | Ring | Gold coated | ring |
| 13.1 | 0.00 | 0.02 | Earring | Silver-like | charm |
| 13.2 | 0.00 | 0.02 | Earring | Silver coated | chain |
| 16.1 | 0.00 | 0.02 | Other | Silver-like | chain |
| 16.2 | 0.00 | 0.02 | Other | Silver-like | lock |
| 17.1 | 0.00 | 0.03 | Ring | Gold coated | side with green colour |
| 17.2 | 0.00 | 0.03 | Ring | Gold coated | without green colour |
| 18.1 | 0.00 | 0.02 | Necklace | Silver-like | charm |
| 18.2 | 0.00 | 0.03 | Necklace | Silver-like | chain |
| 22.1 | 0.00 | 0.04 | Ring | Silver alloy | finger rings |
| 23.1 | 0.00 | 0.02 | Bracelet | Gold coated | bracelet |
| 24.1 | 0.00 | 0.02 | Ring | Gold coated | ring side with name |
| 25.1 | 0.00 | 0.02 | Necklace | Clearly non-precious metal | charm |
| 25.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 26.1 | 0.00 | 0.02 | Other | Clearly non-precious metal | chain |
| 26.2 | 0.00 | 0.02 | Other | Clearly non-precious metal | catch |
| 28.1 | 0.00 | 0.02 | Necklace | Gold coated | charm |
| 28.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 31.1 | 0.00 | 0.02 | Necklace | Silver-like | cylinder with hole |
| 33.2 | 0.00 | 0.02 | Necklace | Golden-like | leaf |
| 33.3 | 0.00 | 0.03 | Necklace | Golden-like | chain |
| 34.1 | 0.00 | 0.02 | Necklace | Clearly non-precious metal | piece of jewellery copper side |
| 36.1 | 0.00 | 0.02 | Ring | Silver-like | finger part |
| 37.1 | 0.00 | 0.02 | Necklace | Silver coated | piece of jewellery without stone |
| 37.2 | 0.00 | 0.02 | Necklace | Silver coated | chain |
| 38.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 40.2 | 0.00 | 0.02 | Necklace | Silver-like | balls 2 pcs. |
| 41.2 | 0.00 | 0.02 | Necklace | Golden-like | chain |
| 42.2 | 0.00 | 0.02 | Necklace | Silver-like | chain |
| 43.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 44.1 | 0.00 | 0.04 | Earring | Gold coated | the sticks |
| 45.1 | 0.00 | 0.03 | Bracelet | Golden-like | chain |
| 46.3 | 0.00 | 0.02 | Bracelet | Gold coated | catch |
| 47.1 | 0.00 | 0.02 | Bracelet | Silver coated | bracelet |
| 48.1 | 0.00 | 0.02 | Necklace | Gold coated | heart |
| 48.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 49.1 | 0.00 | 0.02 | Bracelet | Golden-like | bracelet |
| 50.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | ring |
| 51.1 | 0.00 | 0.02 | Earring | Silver coated | piece of jewellery bright side |
| 52.1 | 0.00 | 0.02 | Bracelet | Gold coated | bracelet |
| 54.2 | 0.00 | 0.02 | Necklace | Silver-like | chain |
| 55.1 | 0.00 | 0.02 | Bracelet | Golden-like | bracelet |
| 58.1 | 0.00 | 0.02 | Ring | Gold coated | the finger ring |
| 61.1 | 0.00 | 0.02 | Necklace | Golden-like | charm without stone |
| 61.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 62.2 | 0.00 | 0.02 | Necklace | Silver-like | chain |
| 63.3 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 64.1 | 0.00 | 0.02 | Earring | Gold coated | ball |
| 64.2 | 0.00 | 0.02 | Earring | Golden-like | needle |
| 65.1 | 0.00 | 0.02 | Ring | Silver-like | ring |
| 66.1 | 0.00 | 0.02 | Bracelet | Silver coated | bracelet |
| 66.2 | 0.00 | 0.02 | Bracelet | Silver coated | catch |
| 66.3 | 0.00 | 0.02 | Bracelet | Silver-like | end piece |
| 67.1 | 0.00 | 0.02 | Necklace | Silver-like | ball |
| 67.2 | 0.00 | 0.02 | Necklace | Silver-like | catch |
| 69.1 | 0.00 | 0.02 | Bracelet | Silver coated | bracelet |
| 69.2 | 0.00 | 0.02 | Bracelet | Silver coated | butterfly backside |
| 71.1 | 0.00 | 0.02 | Necklace | Silver-like | heart |
| 71.3 | 0.00 | 0.02 | Necklace | Silver coated | chain |
| 72.1 | 0.00 | 0.04 | Ring | Gold coated | the finger ring |
| 73.1 | 0.00 | 0.02 | Bracelet | Gold coated | gold coloured side |
| 74.1 | 0.00 | 0.02 | Necklace | Silver-like | heart |
| 74.2 | 0.00 | 0.02 | Necklace | Silver-like | chain |
| 75.1 | 0.00 | 0.02 | Bracelet | Golden-like | bracelet |
| 77.3 | 0.00 | 0.03 | Necklace | Silver-like | chain |
| 78.3 | 0.00 | 0.02 | Necklace | Silver-like | chain |
| 79.1 | 0.00 | 0.03 | Necklace | Golden-like | flower |
| 79.3 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 81.2 | 0.00 | 0.03 | Necklace | Golden-like | neck bar |
| 83.1 | 0.00 | 0.02 | Necklace | Silver-like | heart |
| 83.2 | 0.00 | 0.02 | Necklace | Silver coated | catch |
| 83.3 | 0.00 | 0.02 | Necklace | Silver coated | chain |
| 84.1 | 0.00 | 0.04 | Earring | Golden-like | the sticks |
| 86.1 | 0.00 | 0.02 | Necklace | Silver coated | charm |
| 86.2 | 0.00 | 0.02 | Necklace | Silver coated | chain |
| 86.3 | 0.00 | 0.03 | Necklace | Silver coated | catch |
| 87.1 | 0.00 | 0.02 | Bracelet | Golden-like | flower backside |
| 87.2 | 0.00 | 0.02 | Bracelet | Golden-like | catch |
| 87.3 | 0.00 | 0.02 | Bracelet | Gold coated | chain |
| 88.1 | 0.00 | 0.02 | Ring | Silver-like | ring without stone |
| 89.1 | 0.00 | 0.02 | Earring | Golden-like | piece of jewellery |
| 89.2 | 0.00 | 0.03 | Earring | Golden-like | 2 needles |
| 91.2 | 0.00 | 0.02 | Necklace | Clearly non-precious metal | small ball |
| 92.1 | 0.00 | 0.02 | Other | Silver-like | side without stone |
| 93.1 | 0.00 | 0.02 | Bracelet | Golden-like | the side of bracelet |
| 94.1 | 0.00 | 0.02 | Necklace | Silver-like | heart |
| 94.3 | 0.00 | 0.03 | Necklace | Silver-like | small ball |
| 95.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 97.1 | 0.00 | 0.02 | Bracelet | Gold coated | bracelet |
| 99.1 | 0.00 | 0.04 | Earring | Gold coated | twisted rings |
| 99.2 | 0.00 | 0.08 | Earring | Gold coated | non-twisted part |
| 100.2 | 0.00 | 0.08 | Earring | Silver coated | needles |
| 101.2 | 0.00 | 0.02 | Necklace | Gold coated | chain |
| 103.2 | 0.00 | 0.02 | Earring | Silver-like | chains |
| 104.1 | 0.00 | 0.02 | Bracelet | Silver-like | chain without leather |
| 104.2 | 0.00 | 0.02 | Bracelet | Silver-like | heart |
| 106.2 | 0.00 | 0.03 | Earring | Genuine silver | thread |
| 108.1 | 0.00 | 0.03 | Bracelet | Silver coated | side with stone |
| 108.2 | 0.00 | 0.02 | Bracelet | Silver coated | metallic side |
| 109.1 | 0.00 | 0.02 | Earring | Gold coated | star golden side |
| 109.2 | 0.00 | 0.04 | Earring | Golden-like | thread |
| 110.1 | 0.00 | 0.04 | Earring | Gold coated | rings |
| 110.2 | 0.00 | 0.03 | Earring | Gold coated | holders with balls |
| 111.1 | 0.00 | 0.02 | Bracelet | Gold coated | golden edge |
| 112.1 | 0.00 | 0.04 | Earring | Gold coated | thread thick |
| 112.2 | 0.00 | 0.08 | Earring | Gold coated | thin thread catch |
| 113.2 | 0.00 | 0.03 | Bracelet | Gold coated | golden balls |
| 114.1 | 0.00 | 0.02 | Earring | Genuine silver | side and needle |
| 115.1 | 0.00 | 0.04 | Ring | Gold coated | the finger ring |
| 116.1 | 0.00 | 0.04 | Bracelet | Silver alloy | catch |
| 116.3 | 0.00 | 0.05 | Bracelet | Silver alloy | chain |
| 117.1 | 0.00 | 0.02 | Bracelet | Silver coated | catch |
| 117.2 | 0.00 | 0.02 | Bracelet | Silver-like | discs etc. |
| 117.3 | 0.00 | 0.02 | Bracelet | Silver coated | chain with ball |
| 120.1 | 0.00 | 0.02 | Earring | Gold coated | large disc |
| 120.2 | 0.00 | 0.03 | Earring | Gold coated | small disc |
| 120.3 | 0.00 | 0.03 | Earring | Gold coated | thread |
| 121.1 | 0.00 | 0.02 | Earring | Gold coated | large flower |
| 121.2 | 0.00 | 0.02 | Earring | Gold coated | small flowers |
| 121.3 | 0.00 | 0.08 | Earring | Gold coated | thread |
| 122.1 | 0.00 | 0.04 | Bracelet | Gold coated | royal crown |
| 126.1 | 0.00 | 0.02 | Earring | Genuine silver | hearts |
| 126.2 | 0.00 | 0.03 | Earring | Genuine silver | needles |
| 127.1 | 0.00 | 0.03 | Other | Gold coated | finger ring |
| 128.1 | 0.00 | 0.02 | Piercing | Silver-like | navel stick |
| 128.2 | 0.00 | 0.02 | Piercing | Silver-like | large ball without stone |
| 131.1 | 0.00 | 0.02 | Bracelet | Silver-like | bracelet without stone |
| 133.1 | 0.00 | 0.02 | Ring | Genuine silver | finger ring |
| 134.3 | 0.00 | 0.03 | Necklace | Silver alloy | catch |
| 135.2 | 0.00 | 0.04 | Earring | Clearly non-precious metal | needles |
| 137.1 | 0.00 | 0.02 | Necklace | Silver coated | flower metal |
| 137.2 | 0.00 | 0.02 | Necklace | Silver alloy | chain |
| 138.2 | 0.00 | 0.07 | Necklace | Clearly non-precious metal | chain |
| 139.1 | 0.00 | 0.02 | Necklace | Clearly non-precious metal | chain |
| 140.1 | 0.00 | 0.02 | Earring | Gold coated | silver coloured area |
| 140.2 | 0.00 | 0.06 | Earring | Gold coated | catch |
| 140.3 | 0.00 | 0.05 | Earring | Gold coated | golden area |
| 141.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | ring |
| 142.2 | 0.00 | 0.02 | Bracelet | Silver-like | star |
| 142.3 | 0.00 | 0.02 | Bracelet | Silver coated | chain |
| 144.1 | 0.00 | 0.02 | Earring | Golden-like | piece of jewellery |
| 144.2 | 0.00 | 0.04 | Earring | Silver coated | needles with balls |
| 149.1 | 0.00 | 0.02 | Bracelet | Silver-like | bracelet |
| 149.2 | 0.00 | 0.02 | Bracelet | Silver-like | edge protector |
| 149.3 | 0.00 | 0.02 | Bracelet | Silver-like | backside of bracelet |
| 154.1 | 0.00 | 0.02 | Bracelet | Clearly non-precious metal | bracelet |
| 155.1 | 0.00 | 0.02 | Earring | Gold coated | ring |
| 155.2 | 0.00 | 0.06 | Earring | Golden-like | needles |
| 155.3 | 0.00 | 0.02 | Earring | Golden-like | catch |
| 157.1 | 0.00 | 0.02 | Ankle chain | Silver coated | catch |
| 157.2 | 0.00 | 0.02 | Ankle chain | Silver coated | disc |
| 158.1 | 0.00 | 0.02 | Ankle chain | Silver-like | feet |
| 158.2 | 0.00 | 0.02 | Ankle chain | Silver-like | chain |
| 158.3 | 0.00 | 0.04 | Ankle chain | Silver-like | catch |
| 160.1 | 0.00 | 0.02 | Bracelet | Silver-like | metallic side |
| 163.1 | 0.00 | 0.03 | Piercing | Genuine silver | ring with ball |
| 164.2 | 0.00 | 0.02 | Piercing | Silver-like | ball |
| 165.1 | 0.00 | 0.03 | Necklace | Genuine silver | charm from behind |
| 165.3 | 0.00 | 0.03 | Necklace | Genuine silver | catch |
| 166.1 | 0.00 | 0.10 | Earring | Genuine gold | rings |
| 166.2 | 0.00 | 0.11 | Earring | Genuine gold | catches |
| 167.1 | 0.00 | 0.02 | Earring | Gold coated | butterflies |
| 167.2 | 0.00 | 0.17 | Earring | Genuine gold | thread |
| 168.1 | 0.00 | 0.03 | Ankle chain | Gold coated | heart |
| 168.2 | 0.00 | 0.02 | Ankle chain | Gold coated | chain |
| 168.3 | 0.00 | 0.04 | Ankle chain | Gold coated | catch |
| 169.2 | 0.00 | 0.02 | Ankle chain | Silver-like | chain |
| 170.2 | 0.00 | 0.02 | Bracelet | Golden-like | the side of bracelet |
2.2 Content of Cd in all jewellery parts
| Jewellery part ID | Cd | CD Error | Product type | Appearance of jewellery part | Description jewellery part |
| 34.1 | 29.15 | 0.22 | Necklace | Clearly non-precious metal | piece of jewellery copper side |
| 120.1 | 7.65 | 0.13 | Earring | Gold coated | large disc |
| 120.2 | 6.42 | 0.11 | Earring | Gold coated | small disc |
| 106.1 | 5.51 | 0.11 | Earring | Genuine silver | discs |
| 88.1 | 4.08 | 0.10 | Ring | Silver-like | ring without stone |
| 14.1 | 3.42 | 0.08 | Bracelet | Clearly non-precious metal | metallic side |
| 134.1 | 3.23 | 0.11 | Necklace | Silver alloy | butterfly |
| 19.1 | 1.87 | 0.05 | Bracelet | Silver coated | disc |
| 70.1 | 1.45 | 0.05 | Necklace | Gold coated | charm |
| 81.1 | 0.98 | 0.04 | Necklace | Gold coated | charm backside |
| 82.1 | 0.78 | 0.03 | Necklace | Gold coated | charm backside |
| 99.2 | 0.64 | 0.15 | Earring | Gold coated | non twisted part |
| 82.3 | 0.53 | 0.05 | Necklace | Gold coated | connecting piece |
| 99.1 | 0.49 | 0.06 | Earring | Gold coated | twisted rings |
| 6.1 | 0.46 | 0.03 | Necklace | Gold coated | angel |
| 116.2 | 0.43 | 0.05 | Bracelet | Silver coated | backside of flower and chain |
| 38.1 | 0.32 | 0.03 | Necklace | Gold coated | piece of jewellery without stone |
| 67.1 | 0.32 | 0.04 | Necklace | Silver-like | ball |
| 120.3 | 0.32 | 0.11 | Earring | Gold coated | thread |
| 54.1 | 0.30 | 0.03 | Necklace | Silver-like | charm |
| 96.2 | 0.27 | 0.05 | Earring | Silver-like | the side |
| 36.1 | 0.26 | 0.03 | Ring | Silver-like | finger part |
| 28.1 | 0.25 | 0.06 | Necklace | Gold coated | charm |
| 68.1 | 0.24 | 0.03 | Ring | Silver coated | ring |
| 62.1 | 0.23 | 0.03 | Necklace | Silver-like | charm |
| 64.1 | 0.22 | 0.03 | Earring | Gold coated | ball |
| 83.1 | 0.22 | 0.03 | Necklace | Silver-like | heart |
| 151.3 | 0.22 | 0.06 | Bracelet | Silver coated | catch |
| 100.1 | 0.20 | 0.04 | Earring | Silver coated | backside of charm |
| 104.2 | 0.19 | 0.04 | Bracelet | Silver-like | heart |
| 107.1 | 0.17 | 0.04 | Bracelet | Gold coated | chain with balls |
| 142.2 | 0.16 | 0.04 | Bracelet | Silver-like | star |
| 159.3 | 0.16 | 0.05 | Necklace | Silver-like | catch |
| 74.1 | 0.15 | 0.03 | Necklace | Silver-like | heart |
| 96.1 | 0.15 | 0.03 | Earring | Silver-like | backside and needle |
| 128.2 | 0.13 | 0.05 | Piercing | Silver-like | large ball without stone |
| 161.1 | 0.13 | 0.03 | Bracelet | Silver-like | bracelet |
| 142.3 | 0.12 | 0.04 | Bracelet | Silver coated | chain |
| 41.1 | 0.10 | 0.02 | Necklace | Gold coated | piece of jewellery |
| 26.2 | 0.08 | 0.03 | Other | Clearly non-precious metal | lock |
| 79.2 | 0.08 | 0.04 | Necklace | Gold coated | catch |
| 103.1 | 0.08 | 0.02 | Earring | Silver-like | bright side |
| 46.1 | 0.07 | 0.02 | Bracelet | Gold coated | flower |
| 6.2 | 0.06 | 0.02 | Necklace | Gold coated | the chain |
| 147.1 | 0.06 | 0.03 | Bracelet | Silver-like | bracelet |
| 2.1 | 0.05 | 0.02 | Earring | Gold coated | flower |
| 46.3 | 0.05 | 0.02 | Bracelet | Gold coated | catch |
| 77.1 | 0.05 | 0.03 | Necklace | Silver-like | heart leaf side |
| 91.3 | 0.03 | 0.00 | Necklace | Clearly non-precious metal | chain |
| 148.1 | 0.00 | 0.00 | Ring | Silver coated | the finger ring |
| 119.1 | 0.00 | 0.00 | Ring | Silver coated | |
| 1.1 | 0.00 | 0.04 | Bracelet | Silver-like | bracelet |
| 3.1 | 0.00 | 0.04 | Necklace | Gold coated | large charm |
| 3.2 | 0.00 | 0.06 | Necklace | Gold coated | small charm |
| 3.3 | 0.00 | 0.05 | Necklace | Gold coated | part on the string |
| 4.1 | 0.00 | 0.04 | Ring | Silver-like | ring |
| 5.1 | 0.00 | 0.03 | Ring | Clearly non-precious metal | ring |
| 7.1 | 0.00 | 0.06 | Ankle chain | Silver-like | disc circle with a hole |
| 7.2 | 0.00 | 0.05 | Ankle chain | Silver-like | small disc circle |
| 7.3 | 0.00 | 0.04 | Ankle chain | Silver-like | chain |
| 8.1 | 0.00 | 0.05 | Necklace | Gold coated | flower |
| 8.2 | 0.00 | 0.08 | Necklace | Gold coated | catch |
| 9.1 | 0.00 | 0.04 | Necklace | Golden-like | heart |
| 10.1 | 0.00 | 0.05 | Ring | Clearly non-precious metal | ring |
| 11.1 | 0.00 | 0.06 | Ring | Gold coated | ring |
| 12.1 | 0.00 | 0.05 | Ring | Clearly non-precious metal | ring/other |
| 12.2 | 0.00 | 0.05 | Ring | Clearly non-precious metal | death’s head |
| 13.1 | 0.00 | 0.04 | Earring | Silver-like | charm |
| 13.2 | 0.00 | 0.07 | Earring | Silver coated | chain |
| 15.1 | 0.00 | 0.04 | Ring | Silver-like | ring |
| 16.1 | 0.00 | 0.04 | Other | Silver-like | chain |
| 16.2 | 0.00 | 0.02 | Other | Silver-like | lock |
| 17.1 | 0.00 | 0.05 | Ring | Gold coated | side with green colour |
| 17.2 | 0.00 | 0.05 | Ring | Gold coated | without green colour |
| 18.1 | 0.00 | 0.04 | Necklace | Silver-like | charm |
| 18.2 | 0.00 | 0.10 | Necklace | Silver-like | chain |
| 19.2 | 0.00 | 0.06 | Bracelet | Silver-like | catch |
| 19.3 | 0.00 | 0.14 | Bracelet | Silver coated | small ball |
| 20.1 | 0.00 | 0.06 | Earring | Gold coated | golden side |
| 21.1 | 0.00 | 0.03 | Earring | Clearly non-precious metal | metallic part |
| 22.1 | 0.00 | 0.18 | Ring | Silver alloy | finger rings |
| 23.1 | 0.00 | 0.05 | Bracelet | Gold coated | bracelet |
| 24.1 | 0.00 | 0.06 | Ring | Gold coated | ring side with name |
| 25.1 | 0.00 | 0.04 | Necklace | Clearly non-precious metal | charm |
| 25.2 | 0.00 | 0.04 | Necklace | Gold coated | chain |
| 26.1 | 0.00 | 0.04 | Other | Clearly non-precious metal | chain |
| 27.1 | 0.00 | 0.05 | Necklace | Gold coated | backside of charm |
| 28.2 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 29.1 | 0.00 | 0.06 | Ring | Clearly non-precious metal | ring disc |
| 30.1 | 0.00 | 0.07 | Earring | Gold coated | ear sticks |
| 31.1 | 0.00 | 0.06 | Necklace | Silver-like | cylinder with a hole |
| 31.2 | 0.00 | 0.06 | Necklace | Silver-like | balls 2 pcs. |
| 31.3 | 0.00 | 0.04 | Necklace | Silver-like | cartridge |
| 32.1 | 0.00 | 0.05 | Necklace | Silver-like | side 2 without stone |
| 32.2 | 0.00 | 0.06 | Necklace | Silver-like | chain |
| 33.1 | 0.00 | 0.06 | Necklace | Gold coated | 4 edged piece of jewellery |
| 33.2 | 0.00 | 0.02 | Necklace | Golden-like | Leaf |
| 33.3 | 0.00 | 0.06 | Necklace | Golden-like | chain |
| 34.2 | 0.00 | 0.05 | Necklace | Clearly non-precious metal | chain Cu-coloured |
| 35.1 | 0.00 | 0.06 | Necklace | Clearly non-precious metal | holder and connecting link |
| 37.1 | 0.00 | 0.06 | Necklace | Silver coated | piece of jewellery without stone |
| 37.2 | 0.00 | 0.06 | Necklace | Silver coated | chain |
| 38.2 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 39.1 | 0.00 | 0.16 | Ring | Genuine silver | ring |
| 40.1 | 0.00 | 0.04 | Necklace | Silver-like | piece of jewellery |
| 40.2 | 0.00 | 0.02 | Necklace | Silver-like | balls 2 pcs. |
| 41.2 | 0.00 | 0.05 | Necklace | Golden-like | chain |
| 42.1 | 0.00 | 0.04 | Necklace | Silver-like | leaf |
| 42.2 | 0.00 | 0.04 | Necklace | Silver-like | chain |
| 43.1 | 0.00 | 0.06 | Necklace | Gold coated | piece of jewellery |
| 43.2 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 44.1 | 0.00 | 0.18 | Earring | Gold coated | the sticks |
| 45.1 | 0.00 | 0.05 | Bracelet | Golden-like | chain |
| 46.2 | 0.00 | 0.11 | Bracelet | Gold coated | bracelet |
| 47.1 | 0.00 | 0.04 | Bracelet | Silver coated | bracelet |
| 48.1 | 0.00 | 0.04 | Necklace | Gold coated | heart |
| 48.2 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 49.1 | 0.00 | 0.02 | Bracelet | Golden-like | bracelet |
| 50.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | ring |
| 51.1 | 0.00 | 0.05 | Earring | Silver coated | piece of jewellery bright side |
| 52.1 | 0.00 | 0.04 | Bracelet | Gold coated | bracelet |
| 53.1 | 0.00 | 0.04 | Ankle chain | Gold coated | round disc |
| 53.2 | 0.00 | 0.07 | Ankle chain | Clearly non-precious metal | ball |
| 53.3 | 0.00 | 0.07 | Ankle chain | Gold coated | chain |
| 54.2 | 0.00 | 0.05 | Necklace | Silver-like | chain |
| 55.1 | 0.00 | 0.04 | Bracelet | Golden-like | bracelet |
| 56.1 | 0.00 | 0.05 | Necklace | Silver-like | charm backside |
| 56.2 | 0.00 | 0.09 | Necklace | Silver-like | chain |
| 57.1 | 0.00 | 0.07 | Earring | Silver-like | piece of jewellery backside |
| 57.2 | 0.00 | 0.05 | Earring | Silver-like | catch |
| 58.1 | 0.00 | 0.06 | Ring | Gold coated | the finger ring |
| 59.1 | 0.00 | 0.05 | Bracelet | Silver-like | bracelet |
| 60.1 | 0.00 | 0.04 | Bracelet | Golden-like | bracelet |
| 61.1 | 0.00 | 0.04 | Necklace | Golden-like | charm without stone |
| 61.2 | 0.00 | 0.04 | Necklace | Gold coated | chain |
| 62.2 | 0.00 | 0.06 | Necklace | Silver-like | chain |
| 63.1 | 0.00 | 0.06 | Necklace | Gold coated | charm without stone |
| 63.2 | 0.00 | 0.05 | Necklace | Gold coated | heart |
| 63.3 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 64.2 | 0.00 | 0.13 | Earring | Golden-like | needle |
| 65.1 | 0.00 | 0.04 | Ring | Silver-like | ring |
| 66.1 | 0.00 | 0.04 | Bracelet | Silver coated | bracelet |
| 66.2 | 0.00 | 0.07 | Bracelet | Silver coated | catch |
| 66.3 | 0.00 | 0.05 | Bracelet | Silver-like | connecting piece |
| 67.2 | 0.00 | 0.09 | Necklace | Silver-like | catch |
| 67.3 | 0.00 | 0.05 | Necklace | Silver-like | chain |
| 69.1 | 0.00 | 0.09 | Bracelet | Silver coated | bracelet |
| 69.2 | 0.00 | 0.04 | Bracelet | Silver coated | butterfly backside |
| 70.2 | 0.00 | 0.09 | Necklace | Gold coated | Cu-coloured chain |
| 70.3 | 0.00 | 0.13 | Necklace | Gold coated | catch |
| 71.1 | 0.00 | 0.14 | Necklace | Silver-like | heart |
| 71.2 | 0.00 | 0.07 | Necklace | Silver coated | catch |
| 71.3 | 0.00 | 0.05 | Necklace | Silver coated | chain |
| 72.1 | 0.00 | 0.06 | Ring | Gold coated | the finger ring |
| 73.1 | 0.00 | 0.06 | Bracelet | Gold coated | golden coloured side |
| 74.2 | 0.00 | 0.05 | Necklace | Silver-like | chain |
| 74.3 | 0.00 | 0.09 | Necklace | Silver-like | catch |
| 75.1 | 0.00 | 0.04 | Bracelet | Golden-like | bracelet |
| 76.1 | 0.00 | 0.05 | Necklace | Silver-like | butterfly |
| 76.2 | 0.00 | 0.07 | Necklace | Silver-like | catch |
| 76.3 | 0.00 | 0.05 | Necklace | Silver-like | chain |
| 77.2 | 0.00 | 0.12 | Necklace | Silver-like | catch |
| 77.3 | 0.00 | 0.08 | Necklace | Silver-like | chain |
| 78.1 | 0.00 | 0.05 | Necklace | Silver-like | heart leaf side |
| 78.2 | 0.00 | 0.11 | Necklace | Silver-like | catch |
| 78.3 | 0.00 | 0.05 | Necklace | Silver-like | chain |
| 79.1 | 0.00 | 0.11 | Necklace | Golden-like | flower |
| 79.3 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 80.1 | 0.00 | 0.06 | Necklace | Gold coated | heart inner side |
| 80.2 | 0.00 | 0.04 | Necklace | Gold coated | chain |
| 81.2 | 0.00 | 0.08 | Necklace | Golden-like | neck pin |
| 81.3 | 0.00 | 0.07 | Necklace | Gold coated | catch |
| 82.2 | 0.00 | 0.09 | Necklace | Gold coated | catch |
| 83.2 | 0.00 | 0.08 | Necklace | Silver coated | catch |
| 83.3 | 0.00 | 0.07 | Necklace | Silver coated | chain |
| 84.1 | 0.00 | 0.10 | Earring | Golden-like | sticks |
| 84.2 | 0.00 | 0.08 | Earring | Gold coated | star |
| 85.1 | 0.00 | 0.04 | Necklace | Gold coated | charm backside |
| 85.2 | 0.00 | 0.09 | Necklace | Gold coated | catch |
| 85.3 | 0.00 | 0.06 | Necklace | Gold coated | chain |
| 86.1 | 0.00 | 0.05 | Necklace | Silver coated | charm |
| 86.2 | 0.00 | 0.08 | Necklace | Silver coated | chain |
| 86.3 | 0.00 | 0.10 | Necklace | Silver coated | catch |
| 87.1 | 0.00 | 0.05 | Bracelet | Golden-like | flower backside |
| 87.2 | 0.00 | 0.08 | Bracelet | Golden-like | catch |
| 87.3 | 0.00 | 0.05 | Bracelet | Gold coated | chain |
| 89.1 | 0.00 | 0.04 | Earring | Golden-like | piece of jewellery |
| 89.2 | 0.00 | 0.14 | Earring | Golden-like | 2 needles |
| 90.1 | 0.00 | 0.05 | Necklace | Gold coated | large heart backside |
| 90.2 | 0.00 | 0.05 | Necklace | Gold coated | catch |
| 91.1 | 0.00 | 0.05 | Necklace | Clearly non-precious metal | large ball |
| 91.2 | 0.00 | 0.05 | Necklace | Clearly non-precious metal | small ball |
| 92.1 | 0.00 | 0.05 | Other | Silver-like | side without stone |
| 93.1 | 0.00 | 0.07 | Bracelet | Golden-like | the side of bracelet |
| 94.1 | 0.00 | 0.04 | Necklace | Silver-like | heart |
| 94.2 | 0.00 | 0.09 | Necklace | Silver-like | catch |
| 94.3 | 0.00 | 0.14 | Necklace | Silver-like | small ball |
| 95.1 | 0.00 | 0.04 | Necklace | Gold coated | charm |
| 95.2 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 95.3 | 0.00 | 0.15 | Necklace | Gold coated | catch |
| 97.1 | 0.00 | 0.05 | Bracelet | Gold coated | bracelet |
| 98.1 | 0.00 | 0.07 | Bracelet | Silver-like | bracelet |
| 98.2 | 0.00 | 0.06 | Bracelet | Silver-like | twisted strings |
| 100.2 | 0.00 | 0.31 | Earring | Silver coated | needles |
| 101.1 | 0.00 | 0.04 | Necklace | Gold coated | backside of heart |
| 101.2 | 0.00 | 0.05 | Necklace | Gold coated | chain |
| 101.3 | 0.00 | 0.04 | Necklace | Gold coated | cone |
| 102.1 | 0.00 | 0.06 | Bracelet | Silver-like | metallic side |
| 103.2 | 0.00 | 0.09 | Earring | Silver-like | chains |
| 104.1 | 0.00 | 0.05 | Bracelet | Silver-like | chain without leather |
| 104.3 | 0.00 | 0.05 | Bracelet | Silver-like | pins |
| 105.1 | 0.00 | 0.06 | Ring | Silver-like | metallic side |
| 106.2 | 0.00 | 0.25 | Earring | Genuine silver | thread |
| 108.1 | 0.00 | 0.14 | Bracelet | Silver coated | side with stone |
| 108.2 | 0.00 | 0.06 | Bracelet | Silver coated | metallic side |
| 109.1 | 0.00 | 0.03 | Earring | Gold coated | star golden side |
| 109.2 | 0.00 | 0.14 | Earring | Golden-like | thread |
| 110.1 | 0.00 | 0.18 | Earring | Gold coated | rings |
| 110.2 | 0.00 | 0.18 | Earring | Gold coated | holder with balls |
| 111.1 | 0.00 | 0.05 | Bracelet | Gold coated | golden edge |
| 112.1 | 0.00 | 0.20 | Earring | Gold coated | thread thick |
| 112.2 | 0.00 | 0.39 | Earring | Gold coated | thin thread catch |
| 113.1 | 0.00 | 0.06 | Bracelet | Golden-like | chain |
| 113.2 | 0.00 | 0.04 | Bracelet | Gold coated | golden ball |
| 114.1 | 0.00 | 0.18 | Earring | Genuine silver | side and needle |
| 115.1 | 0.00 | 0.06 | Ring | Gold coated | the finger ring |
| 116.1 | 0.00 | 0.18 | Bracelet | Silver alloy | catch |
| 116.3 | 0.00 | 0.24 | Bracelet | Silver alloy | chain |
| 117.1 | 0.00 | 0.09 | Bracelet | Silver coated | catch |
| 117.2 | 0.00 | 0.05 | Bracelet | Silver-like | discs etc. |
| 117.3 | 0.00 | 0.09 | Bracelet | Silver coated | chain with ball |
| 118.1 | 0.00 | 0.04 | Bracelet | Gold coated | bracelet |
| 121.1 | 0.00 | 0.12 | Earring | Gold coated | large flower |
| 121.2 | 0.00 | 0.14 | Earring | Gold coated | small flowers |
| 121.3 | 0.00 | 0.15 | Earring | Gold coated | thread |
| 122.1 | 0.00 | 0.20 | Bracelet | Gold coated | royal crown |
| 123.1 | 0.00 | 0.06 | Ring | Gold coated | finger ring |
| 124.1 | 0.00 | 0.08 | Necklace | Silver-like | catch |
| 124.2 | 0.00 | 0.05 | Necklace | Silver-like | chain |
| 125.1 | 0.00 | 0.06 | Ring | Silver coated | finger ring |
| 126.1 | 0.00 | 0.13 | Earring | Genuine silver | hearts |
| 126.2 | 0.00 | 0.21 | Earring | Genuine silver | needles |
| 127.1 | 0.00 | 0.18 | Other | Gold coated | finger ring |
| 128.1 | 0.00 | 0.06 | Piercing | Silver-like | navel stick |
| 128.3 | 0.00 | 0.07 | Piercing | Silver-like | charm backside |
| 129.1 | 0.00 | 0.11 | Earring | Gold coated | needles |
| 130.1 | 0.00 | 0.10 | Earring | Silver-like | needles |
| 131.1 | 0.00 | 0.06 | Bracelet | Silver-like | Bracelet without stone |
| 132.1 | 0.00 | 0.04 | Bracelet | Golden-like | Bracelet |
| 133.1 | 0.00 | 0.20 | Ring | Genuine silver | finger ring |
| 134.2 | 0.00 | 0.07 | Necklace | Silver coated | chain |
| 134.3 | 0.00 | 0.13 | Necklace | Silver alloy | catch |
| 135.1 | 0.00 | 0.06 | Earring | Clearly non-precious metal | chain |
| 135.2 | 0.00 | 0.14 | Earring | Clearly non-precious metal | needles |
| 136.1 | 0.00 | 0.06 | Other | Silver coated | charm |
| 136.2 | 0.00 | 0.07 | Other | Silver coated | disc |
| 137.1 | 0.00 | 0.05 | Necklace | Silver coated | flower metal |
| 137.2 | 0.00 | 0.10 | Necklace | Silver alloy | chain |
| 138.1 | 0.00 | 0.09 | Necklace | Silver alloy | charm from behind |
| 138.2 | 0.00 | 0.28 | Necklace | Clearly non-precious metal | chain |
| 138.3 | 0.00 | 0.10 | Necklace | Silver coated | catch |
| 139.1 | 0.00 | 0.05 | Necklace | Clearly non-precious metal | chain |
| 139.2 | 0.00 | 0.06 | Necklace | Clearly non-precious metal | catch |
| 140.1 | 0.00 | 0.05 | Earring | Gold coated | silver-coloured area |
| 140.2 | 0.00 | 0.19 | Earring | Gold coated | catch |
| 140.3 | 0.00 | 0.13 | Earring | Gold coated | golden area |
| 141.1 | 0.00 | 0.03 | Ring | Clearly non-precious metal | ring |
| 142.1 | 0.00 | 0.08 | Bracelet | Silver-like | catch pin |
| 143.1 | 0.00 | 0.06 | Bracelet | Silver coated | bracelet |
| 144.1 | 0.00 | 0.06 | Earring | Golden-like | piece of jewellery |
| 144.2 | 0.00 | 0.25 | Earring | Silver coated | needles with balls |
| 145.1 | 0.00 | 0.07 | Earring | Gold coated | rings |
| 146.1 | 0.00 | 0.04 | Bracelet | Golden-like | bracelet |
| 147.2 | 0.00 | 0.07 | Bracelet | Silver-like | catch |
| 147.3 | 0.00 | 0.07 | Bracelet | Silver-like | eye |
| 149.1 | 0.00 | 0.04 | Bracelet | Silver-like | bracelet |
| 149.2 | 0.00 | 0.04 | Bracelet | Silver-like | edge protector |
| 149.3 | 0.00 | 0.04 | Bracelet | Silver-like | backside of bracelet |
| 150.1 | 0.00 | 0.05 | Bracelet | Silver-like | charm |
| 151.1 | 0.00 | 0.14 | Bracelet | Clearly non-precious metal | clover charm from behind |
| 151.2 | 0.00 | 0.10 | Bracelet | Silver coated | chain |
| 152.1 | 0.00 | 0.05 | Ring | Silver-like | bright ring |
| 152.2 | 0.00 | 0.06 | Ring | Clearly non-precious metal | matt ring |
| 153.1 | 0.00 | 0.11 | Earring | Silver-like | needles |
| 153.2 | 0.00 | 0.07 | Earring | Silver-like | connecting link |
| 153.3 | 0.00 | 0.05 | Earring | Silver-like | chains |
| 154.1 | 0.00 | 0.03 | Bracelet | Clearly non-precious metal | bracelet |
| 155.1 | 0.00 | 0.04 | Earring | Gold coated | ring |
| 155.2 | 0.00 | 0.24 | Earring | Golden-like | needles |
| 155.3 | 0.00 | 0.06 | Earring | Golden-like | catch |
| 156.1 | 0.00 | 0.04 | Earring | Silver-like | backside and needle |
| 157.1 | 0.00 | 0.08 | Ankle chain | Silver coated | catch |
| 157.2 | 0.00 | 0.06 | Ankle chain | Silver coated | disc |
| 158.1 | 0.00 | 0.05 | Ankle chain | Silver-like | feet |
| 158.2 | 0.00 | 0.08 | Ankle chain | Silver-like | chain |
| 158.3 | 0.00 | 0.17 | Ankle chain | Silver-like | catch |
| 159.1 | 0.00 | 0.05 | Necklace | Silver-like | heart backside |
| 159.2 | 0.00 | 0.07 | Necklace | Silver-like | chain |
| 160.1 | 0.00 | 0.05 | Bracelet | Silver-like | metallic side |
| 162.1 | 0.00 | 0.07 | Ring | Gold coated | finger ring |
| 163.1 | 0.00 | 0.24 | Piercing | Genuine silver | ring with ball |
| 164.1 | 0.00 | 0.05 | Piercing | Silver coated | heart backside |
| 164.2 | 0.00 | 0.09 | Piercing | Silver-like | ball |
| 165.1 | 0.00 | 0.21 | Necklace | Genuine silver | charm from behind |
| 165.2 | 0.00 | 0.13 | Necklace | Genuine silver | chain |
| 165.3 | 0.00 | 0.24 | Necklace | Genuine silver | catch |
| 166.1 | 0.00 | 0.25 | Earring | Genuine gold | rings |
| 166.2 | 0.00 | 0.33 | Earring | Genuine gold | catches |
| 167.1 | 0.00 | 0.05 | Earring | Gold coated | butterflies |
| 167.2 | 0.00 | 0.32 | Earring | Genuine gold | threads |
| 168.1 | 0.00 | 0.17 | Ankle chain | Gold coated | heart |
| 168.2 | 0.00 | 0.14 | Ankle chain | Gold coated | chain |
| 168.3 | 0.00 | 0.23 | Ankle chain | Gold coated | catch |
| 169.1 | 0.00 | 0.04 | Ankle chain | Silver coated | heart |
| 169.2 | 0.00 | 0.04 | Ankle chain | Silver-like | chain |
| 169.3 | 0.00 | 0.07 | Ankle chain | Silver-like | catch |
| 170.1 | 0.00 | 0.09 | Bracelet | Golden-like | golden pattern coate |
| 170.2 | 0.00 | 0.15 | Bracelet | Golden-like | the side of bracelet |
2.3 Content of Hg in all jewellery parts
| Jewellery part ID | Hg | Hg Error | Product type | Appearance of jewellery part | Description jewellery part |
| 28.1 | 2.33 | 0.08 | Necklace | Gold coated | charm |
| 94.3 | 0.95 | 0.08 | Necklace | Silver-like | small ball |
| 117.3 | 0.86 | 0.06 | Bracelet | Silver coated | chain with ball |
| 33.2 | 0.62 | 0.01 | Necklace | Golden-like | leaf |
| 1.1 | 0.00 | 0.02 | Bracelet | Silver-like | bracelet |
| 2.1 | 0.00 | 0.03 | Earring | Gold coated | flower |
| 3.1 | 0.00 | 0.04 | Necklace | Gold coated | large charm |
| 3.2 | 0.00 | 0.03 | Necklace | Gold coated | small charm |
| 3.3 | 0.00 | 0.04 | Necklace | Gold coated | part on the string |
| 4.1 | 0.00 | 0.02 | Ring | Silver-like | Ring |
| 5.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | Ring |
| 6.1 | 0.00 | 0.03 | Necklace | Gold coated | Angle |
| 6.2 | 0.00 | 0.03 | Necklace | Gold coated | Chain |
| 7.1 | 0.00 | 0.02 | Ankle chain | Silver-like | Circle with hoal |
| 7.2 | 0.00 | 0.02 | Ankle chain | Silver-like | Small circle |
| 7.3 | 0.00 | 0.02 | Ankle chain | Silver-like | Chain |
| 8.1 | 0.00 | 0.03 | Necklace | Gold coated | flower |
| 8.2 | 0.00 | 0.05 | Necklace | Gold coated | Catch |
| 9.1 | 0.00 | 0.05 | Necklace | Golden-like | Heart |
| 10.1 | 0.00 | 0.04 | Ring | Clearly non-precious metal | Ring |
| 11.1 | 0.00 | 0.12 | Ring | Gold coated | Ring |
| 12.1 | 0.00 | 0.03 | Ring | Clearly non-precious metal | Ring/Other |
| 12.2 | 0.00 | 0.03 | Ring | Clearly non-precious metal | Death’s head |
| 13.1 | 0.00 | 0.02 | Earring | Silver-like | Charm |
| 13.2 | 0.00 | 0.02 | Earring | Silver coated | Chain |
| 14.1 | 0.00 | 0.04 | Bracelet | Clearly non-precious metal | metallic side |
| 15.1 | 0.00 | 0.02 | Ring | Silver-like | Ring |
| 16.1 | 0.00 | 0.02 | Other | Silver-like | Chain |
| 16.2 | 0.00 | 0.02 | Other | Silver-like | Catch |
| 17.1 | 0.00 | 0.06 | Ring | Gold coated | Side with green colour |
| 17.2 | 0.00 | 0.07 | Ring | Gold coated | without green colour |
| 18.1 | 0.00 | 0.02 | Necklace | Silver-like | Charm |
| 18.2 | 0.00 | 0.04 | Necklace | Silver-like | Chain |
| 19.1 | 0.00 | 0.03 | Bracelet | Silver coated | Disc |
| 19.2 | 0.00 | 0.05 | Bracelet | Silver-like | Catch |
| 19.3 | 0.00 | 0.09 | Bracelet | Silver coated | small ball |
| 20.1 | 0.00 | 0.06 | Earring | Gold coated | golden side |
| 21.1 | 0.00 | 0.02 | Earring | Clearly non-precious metal | metallic del |
| 22.1 | 0.00 | 0.04 | Ring | Silver alloy | finger rings |
| 23.1 | 0.00 | 0.17 | Bracelet | Gold coated | Bracelet |
| 24.1 | 0.00 | 0.12 | Ring | Gold coated | ringside with name |
| 25.1 | 0.00 | 0.07 | Necklace | Clearly non-precious metal | Charm |
| 25.2 | 0.00 | 0.05 | Necklace | Gold coated | Chain |
| 26.1 | 0.00 | 0.02 | Other | Clearly non-precious metal | Chain |
| 26.2 | 0.00 | 0.05 | Other | Clearly non-precious metal | Catch |
| 27.1 | 0.00 | 0.07 | Necklace | Gold coated | backside of charm |
| 28.2 | 0.00 | 0.07 | Necklace | Gold coated | Chain |
| 29.1 | 0.00 | 0.03 | Ring | Clearly non-precious metal | Ring disc |
| 30.1 | 0.00 | 0.11 | Earring | Gold coated | Sticks |
| 31.1 | 0.00 | 0.02 | Necklace | Silver-like | cylinder with hoal |
| 31.2 | 0.00 | 0.02 | Necklace | Silver-like | balls 2 pcs. |
| 31.3 | 0.00 | 0.07 | Necklace | Silver-like | Cartridge |
| 32.1 | 0.00 | 0.03 | Necklace | Silver-like | side 2 without stone |
| 32.2 | 0.00 | 0.02 | Necklace | Silver-like | Chain |
| 33.1 | 0.00 | 0.28 | Necklace | Gold coated | 4 edged piece of jewellery |
| 33.3 | 0.00 | 0.04 | Necklace | Golden-like | Chain |
| 34.1 | 0.00 | 0.03 | Necklace | Clearly non-precious metal | piece of jewellery Cu-side |
| 34.2 | 0.00 | 0.02 | Necklace | Clearly non-precious metal | chain Cu-coloured |
| 35.1 | 0.00 | 0.06 | Necklace | Clearly non-precious metal | holder and connecting link |
| 36.1 | 0.00 | 0.02 | Ring | Silver-like | Finger part |
| 37.1 | 0.00 | 0.06 | Necklace | Silver coated | piece of jewellery without stone |
| 37.2 | 0.00 | 0.03 | Necklace | Silver coated | Chain |
| 38.1 | 0.00 | 0.04 | Necklace | Gold coated | piece of jewellery without stone |
| 38.2 | 0.00 | 0.05 | Necklace | Gold coated | Chain |
| 39.1 | 0.00 | 0.04 | Ring | Genuine silver | Ring |
| 40.1 | 0.00 | 0.03 | Necklace | Silver-like | piece of jewellery |
| 40.2 | 0.00 | 0.02 | Necklace | Silver-like | balls 2 pcs. |
| 41.1 | 0.00 | 0.03 | Necklace | Gold coated | piece of jewellery |
| 41.2 | 0.00 | 0.02 | Necklace | Golden-like | Chain |
| 42.1 | 0.00 | 0.03 | Necklace | Silver-like | Leaf |
| 42.2 | 0.00 | 0.02 | Necklace | Silver-like | Chain |
| 43.1 | 0.00 | 0.07 | Necklace | Gold coated | piece of jewellery |
| 43.2 | 0.00 | 0.07 | Necklace | Gold coated | Chain |
| 44.1 | 0.00 | 0.20 | Earring | Gold coated | Sticks |
| 45.1 | 0.00 | 0.11 | Bracelet | Golden-like | Chain |
| 46.1 | 0.00 | 0.09 | Bracelet | Gold coated | Flower |
| 46.2 | 0.00 | 0.19 | Bracelet | Gold coated | Bracelet |
| 46.3 | 0.00 | 0.09 | Bracelet | Gold coated | Catch |
| 47.1 | 0.00 | 0.03 | Bracelet | Silver coated | Bracelet |
| 48.1 | 0.00 | 0.04 | Necklace | Gold coated | Heart |
| 48.2 | 0.00 | 0.09 | Necklace | Gold coated | Chain |
| 49.1 | 0.00 | 0.02 | Bracelet | Golden-like | Bracelet |
| 50.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | Ring |
| 51.1 | 0.00 | 0.02 | Earring | Silver coated | piece of jewellery bright side |
| 52.1 | 0.00 | 0.03 | Bracelet | Gold coated | Bracelet |
| 53.1 | 0.00 | 0.04 | Ankle chain | Gold coated | Round disc |
| 53.2 | 0.00 | 0.08 | Ankle chain | Clearly non-precious metal | Ball |
| 53.3 | 0.00 | 0.07 | Ankle chain | Gold coated | Chain |
| 54.1 | 0.00 | 0.03 | Necklace | Silver-like | Charm |
| 54.2 | 0.00 | 0.04 | Necklace | Silver-like | Chain |
| 55.1 | 0.00 | 0.08 | Bracelet | Golden-like | Bracelet |
| 56.1 | 0.00 | 0.04 | Necklace | Silver-like | Charm backside |
| 56.2 | 0.00 | 0.03 | Necklace | Silver-like | Chain |
| 57.1 | 0.00 | 0.05 | Earring | Silver-like | piece of jewellery backside |
| 57.2 | 0.00 | 0.02 | Earring | Silver-like | Catch |
| 58.1 | 0.00 | 0.06 | Ring | Gold coated | finger ring |
| 59.1 | 0.00 | 0.07 | Bracelet | Silver-like | Bracelet |
| 60.1 | 0.00 | 0.09 | Bracelet | Golden-like | Bracelet |
| 61.1 | 0.00 | 0.02 | Necklace | Golden-like | Charm without stone |
| 61.2 | 0.00 | 0.03 | Necklace | Gold coated | Chain |
| 62.1 | 0.00 | 0.04 | Necklace | Silver-like | Charm |
| 62.2 | 0.00 | 0.02 | Necklace | Silver-like | Chain |
| 63.1 | 0.00 | 0.04 | Necklace | Gold coated | charm without stone |
| 63.2 | 0.00 | 0.02 | Necklace | Gold coated | Heart |
| 63.3 | 0.00 | 0.03 | Necklace | Gold coated | Chain |
| 64.1 | 0.00 | 0.03 | Earring | Gold coated | Ball |
| 64.2 | 0.00 | 0.09 | Earring | Golden-like | Needle |
| 65.1 | 0.00 | 0.02 | Ring | Silver-like | Ring |
| 66.1 | 0.00 | 0.04 | Bracelet | Silver coated | Bracelet |
| 66.2 | 0.00 | 0.07 | Bracelet | Silver coated | Catch |
| 66.3 | 0.00 | 0.02 | Bracelet | Silver-like | Piece at the end |
| 67.1 | 0.00 | 0.02 | Necklace | Silver-like | Ball |
| 67.2 | 0.00 | 0.04 | Necklace | Silver-like | Catch |
| 67.3 | 0.00 | 0.03 | Necklace | Silver-like | Chain |
| 68.1 | 0.00 | 0.04 | Ring | Silver coated | Ring |
| 69.1 | 0.00 | 0.03 | Bracelet | Silver coated | Bracelet |
| 69.2 | 0.00 | 0.02 | Bracelet | Silver coated | butterfly backside |
| 70.1 | 0.00 | 0.04 | Necklace | Gold coated | Charm |
| 70.2 | 0.00 | 0.10 | Necklace | Gold coated | Cu-coloured chain |
| 70.3 | 0.00 | 0.11 | Necklace | Gold coated | Catch |
| 71.1 | 0.00 | 0.02 | Necklace | Silver-like | Heart |
| 71.2 | 0.00 | 0.05 | Necklace | Silver coated | Catch |
| 71.3 | 0.00 | 0.02 | Necklace | Silver coated | Chain |
| 72.1 | 0.00 | 0.23 | Ring | Gold coated | finger ring |
| 73.1 | 0.00 | 0.06 | Bracelet | Gold coated | Gold-coloured side |
| 74.1 | 0.00 | 0.02 | Necklace | Silver-like | Heart |
| 74.2 | 0.00 | 0.02 | Necklace | Silver-like | Chain |
| 74.3 | 0.00 | 0.05 | Necklace | Silver-like | Catch |
| 75.1 | 0.00 | 0.05 | Bracelet | Golden-like | Bracelet |
| 76.1 | 0.00 | 0.03 | Necklace | Silver-like | butterfly |
| 76.2 | 0.00 | 0.03 | Necklace | Silver-like | Catch |
| 76.3 | 0.00 | 0.02 | Necklace | Silver-like | Chain |
| 77.1 | 0.00 | 0.06 | Necklace | Silver-like | Heart leaf side |
| 77.2 | 0.00 | 0.04 | Necklace | Silver-like | Catch |
| 77.3 | 0.00 | 0.03 | Necklace | Silver-like | Chain |
| 78.1 | 0.00 | 0.07 | Necklace | Silver-like | Heart leaf side |
| 78.2 | 0.00 | 0.05 | Necklace | Silver-like | Catch |
| 78.3 | 0.00 | 0.02 | Necklace | Silver-like | Chain |
| 79.1 | 0.00 | 0.12 | Necklace | Golden-like | flower |
| 79.2 | 0.00 | 0.09 | Necklace | Gold coated | Catch |
| 79.3 | 0.00 | 0.07 | Necklace | Gold coated | Chain |
| 80.1 | 0.00 | 0.05 | Necklace | Gold coated | Heart inner side |
| 80.2 | 0.00 | 0.02 | Necklace | Gold coated | Chain |
| 81.1 | 0.00 | 0.05 | Necklace | Gold coated | Charm backside |
| 81.2 | 0.00 | 0.10 | Necklace | Golden-like | Neck stick |
| 81.3 | 0.00 | 0.08 | Necklace | Gold coated | Catch |
| 82.1 | 0.00 | 0.04 | Necklace | Gold coated | charm backside |
| 82.2 | 0.00 | 0.09 | Necklace | Gold coated | Catch |
| 82.3 | 0.00 | 0.08 | Necklace | Gold coated | Spacer |
| 83.1 | 0.00 | 0.02 | Necklace | Silver-like | Heart |
| 83.2 | 0.00 | 0.04 | Necklace | Silver coated | Catch |
| 83.3 | 0.00 | 0.03 | Necklace | Silver coated | Chain |
| 84.1 | 0.00 | 0.10 | Earring | Golden-like | Sticks |
| 84.2 | 0.00 | 0.08 | Earring | Gold coated | Star |
| 85.1 | 0.00 | 0.05 | Necklace | Gold coated | Charm backside |
| 85.2 | 0.00 | 0.10 | Necklace | Gold coated | Catch |
| 85.3 | 0.00 | 0.08 | Necklace | Gold coated | Chain |
| 86.1 | 0.00 | 0.02 | Necklace | Silver coated | Charm |
| 86.2 | 0.00 | 0.03 | Necklace | Silver coated | Chain |
| 86.3 | 0.00 | 0.04 | Necklace | Silver coated | Catch |
| 87.1 | 0.00 | 0.08 | Bracelet | Golden-like | flower backside |
| 87.2 | 0.00 | 0.09 | Bracelet | Golden-like | Catch |
| 87.3 | 0.00 | 0.09 | Bracelet | Gold coated | Chain |
| 88.1 | 0.00 | 0.03 | Ring | Silver-like | ring without stone |
| 89.1 | 0.00 | 0.02 | Earring | Golden-like | piece of jewellery |
| 89.2 | 0.00 | 0.04 | Earring | Golden-like | 2 needles |
| 90.1 | 0.00 | 0.03 | Necklace | Gold coated | large heart backside |
| 90.2 | 0.00 | 0.05 | Necklace | Gold coated | Catch |
| 91.1 | 0.00 | 0.07 | Necklace | Clearly non-precious metal | Large ball |
| 91.2 | 0.00 | 0.06 | Necklace | Clearly non-precious metal | small ball |
| 91.3 | 0.00 | 0.00 | Necklace | Clearly non-precious metal | chain |
| 92.1 | 0.00 | 0.02 | Other | Silver-like | side without stone |
| 93.1 | 0.00 | 0.07 | Bracelet | Golden-like | side of bracelet |
| 94.1 | 0.00 | 0.02 | Necklace | Silver-like | Heart |
| 94.2 | 0.00 | 0.08 | Necklace | Silver-like | Catch |
| 95.1 | 0.00 | 0.04 | Necklace | Gold coated | Charm |
| 95.2 | 0.00 | 0.07 | Necklace | Gold coated | Chain |
| 95.3 | 0.00 | 0.14 | Necklace | Gold coated | Catch |
| 96.1 | 0.00 | 0.04 | Earring | Silver-like | backside of needle |
| 96.2 | 0.00 | 0.05 | Earring | Silver-like | Side |
| 97.1 | 0.00 | 0.03 | Bracelet | Gold coated | Bracelet |
| 98.1 | 0.00 | 0.03 | Bracelet | Silver-like | Bracelet |
| 98.2 | 0.00 | 0.03 | Bracelet | Silver-like | Twisted strings |
| 99.1 | 0.00 | 0.20 | Earring | Gold coated | twisted rings |
| 99.2 | 0.00 | 0.43 | Earring | Gold coated | Non-twisted part |
| 100.1 | 0.00 | 0.04 | Earring | Silver coated | backside of charm |
| 100.2 | 0.00 | 0.14 | Earring | Silver coated | Needles |
| 101.1 | 0.00 | 0.03 | Necklace | Gold coated | backside of heart |
| 101.2 | 0.00 | 0.04 | Necklace | Gold coated | Chain |
| 101.3 | 0.00 | 0.03 | Necklace | Gold coated | Cornet |
| 102.1 | 0.00 | 0.02 | Bracelet | Silver-like | metallic side |
| 103.1 | 0.00 | 0.02 | Earring | Silver-like | Bright side |
| 103.2 | 0.00 | 0.05 | Earring | Silver-like | Chains |
| 104.1 | 0.00 | 0.02 | Bracelet | Silver-like | chain without leather |
| 104.2 | 0.00 | 0.02 | Bracelet | Silver-like | Heart |
| 104.3 | 0.00 | 0.03 | Bracelet | Silver-like | Sticks |
| 105.1 | 0.00 | 0.03 | Ring | Silver-like | metallic side |
| 106.1 | 0.00 | 0.02 | Earring | Genuine silver | Discs |
| 106.2 | 0.00 | 0.04 | Earring | Genuine silver | Thread |
| 107.1 | 0.00 | 0.07 | Bracelet | Gold coated | chain with balls |
| 108.1 | 0.00 | 0.05 | Bracelet | Silver coated | side with stone |
| 108.2 | 0.00 | 0.03 | Bracelet | Silver coated | metallic side |
| 109.1 | 0.00 | 0.02 | Earring | Gold coated | star golden side |
| 109.2 | 0.00 | 0.13 | Earring | Golden-like | Thread |
| 110.1 | 0.00 | 0.16 | Earring | Gold coated | Rings |
| 110.2 | 0.00 | 0.12 | Earring | Gold coated | Holder with balls |
| 111.1 | 0.00 | 0.05 | Bracelet | Gold coated | Golden edge |
| 112.1 | 0.00 | 0.19 | Earring | Gold coated | Thick thread |
| 112.2 | 0.00 | 0.38 | Earring | Gold coated | Thin thread catch |
| 113.1 | 0.00 | 0.11 | Bracelet | Golden-like | Chain |
| 113.2 | 0.00 | 0.20 | Bracelet | Gold coated | Golden balls |
| 114.1 | 0.00 | 0.03 | Earring | Genuine silver | side and needle |
| 115.1 | 0.00 | 0.21 | Ring | Gold coated | finger ring |
| 116.1 | 0.00 | 0.07 | Bracelet | Silver alloy | Catch |
| 116.2 | 0.00 | 0.03 | Bracelet | Silver coated | backside of flower and chain |
| 116.3 | 0.00 | 0.08 | Bracelet | Silver alloy | chain |
| 117.1 | 0.00 | 0.06 | Bracelet | Silver coated | catch |
| 117.2 | 0.00 | 0.02 | Bracelet | Silver-like | discs etc. |
| 118.1 | 0.00 | 0.04 | Bracelet | Gold coated | Bracelet |
| 119.1 | 0.00 | 0.00 | Ring | Silver coated | |
| 120.1 | 0.00 | 0.06 | Earring | Gold coated | large disc |
| 120.2 | 0.00 | 0.11 | Earring | Gold coated | small disc |
| 120.3 | 0.00 | 0.14 | Earring | Gold coated | Thread |
| 121.1 | 0.00 | 0.06 | Earring | Gold coated | large flower |
| 121.2 | 0.00 | 0.08 | Earring | Gold coated | Small flowers |
| 121.3 | 0.00 | 0.47 | Earring | Gold coated | Thread |
| 122.1 | 0.00 | 0.17 | Bracelet | Gold coated | royal crown |
| 123.1 | 0.00 | 0.06 | Ring | Gold coated | finger ring |
| 124.1 | 0.00 | 0.04 | Necklace | Silver-like | Catch |
| 124.2 | 0.00 | 0.02 | Necklace | Silver-like | Chain |
| 125.1 | 0.00 | 0.03 | Ring | Silver coated | Finger ring |
| 126.1 | 0.00 | 0.02 | Earring | Genuine silver | Hearts |
| 126.2 | 0.00 | 0.03 | Earring | Genuine silver | needles |
| 127.1 | 0.00 | 0.06 | Other | Gold coated | finger ring |
| 128.1 | 0.00 | 0.02 | Piercing | Silver-like | Navel stick |
| 128.2 | 0.00 | 0.02 | Piercing | Silver-like | large ball without stone |
| 128.3 | 0.00 | 0.02 | Piercing | Silver-like | charm backside |
| 129.1 | 0.00 | 0.06 | Earring | Gold coated | Needles |
| 130.1 | 0.00 | 0.04 | Earring | Silver-like | Needles |
| 131.1 | 0.00 | 0.06 | Bracelet | Silver-like | Bracelet without stone |
| 132.1 | 0.00 | 0.09 | Bracelet | Golden-like | Bracelet |
| 133.1 | 0.00 | 0.03 | Ring | Genuine silver | finger ring |
| 134.1 | 0.00 | 0.05 | Necklace | Silver alloy | Butterfly |
| 134.2 | 0.00 | 0.06 | Necklace | Silver coated | Chain |
| 134.3 | 0.00 | 0.10 | Necklace | Silver alloy | Catch |
| 135.1 | 0.00 | 0.07 | Earring | Clearly non-precious metal | Chain |
| 135.2 | 0.00 | 0.20 | Earring | Clearly non-precious metal | needles |
| 136.1 | 0.00 | 0.03 | Other | Silver coated | charm |
| 136.2 | 0.00 | 0.04 | Other | Silver coated | Disc |
| 137.1 | 0.00 | 0.02 | Necklace | Silver coated | flower metal |
| 137.2 | 0.00 | 0.03 | Necklace | Silver alloy | Chain |
| 138.1 | 0.00 | 0.02 | Necklace | Silver alloy | charm from behind |
| 138.2 | 0.00 | 0.09 | Necklace | Clearly non-precious metal | Chain |
| 138.3 | 0.00 | 0.03 | Necklace | Silver coated | Catch |
| 139.1 | 0.00 | 0.02 | Necklace | Clearly non-precious metal | Chain |
| 139.2 | 0.00 | 0.03 | Necklace | Clearly non-precious metal | Catch |
| 140.1 | 0.00 | 0.07 | Earring | Gold coated | Silver-coloured area |
| 140.2 | 0.00 | 0.29 | Earring | Gold coated | Catch |
| 140.3 | 0.00 | 0.21 | Earring | Gold coated | Golden area |
| 141.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | Ring |
| 142.1 | 0.00 | 0.05 | Bracelet | Silver-like | Catch stick |
| 142.2 | 0.00 | 0.03 | Bracelet | Silver-like | Star |
| 142.3 | 0.00 | 0.03 | Bracelet | Silver coated | Chain |
| 143.1 | 0.00 | 0.02 | Bracelet | Silver coated | Bracelet |
| 144.1 | 0.00 | 0.07 | Earring | Golden-like | piece of jewellery |
| 144.2 | 0.00 | 0.17 | Earring | Silver coated | needles with balls |
| 145.1 | 0.00 | 0.09 | Earring | Gold coated | Rings |
| 146.1 | 0.00 | 0.08 | Bracelet | Golden-like | Bracelet |
| 147.1 | 0.00 | 0.05 | Bracelet | Silver-like | Bracelet |
| 147.2 | 0.00 | 0.05 | Bracelet | Silver-like | Catch |
| 147.3 | 0.00 | 0.04 | Bracelet | Silver-like | Eye |
| 148.1 | 0.00 | 0.00 | Ring | Silver coated | Finger ring |
| 149.1 | 0.00 | 0.02 | Bracelet | Silver-like | Bracelet |
| 149.2 | 0.00 | 0.02 | Bracelet | Silver-like | Edge protector |
| 149.3 | 0.00 | 0.02 | Bracelet | Silver-like | backside of bracelet |
| 150.1 | 0.00 | 0.07 | Bracelet | Silver-like | charm |
| 151.1 | 0.00 | 0.07 | Bracelet | Clearly non-precious metal | clover charm from behind |
| 151.2 | 0.00 | 0.04 | Bracelet | Silver coated | chain |
| 151.3 | 0.00 | 0.06 | Bracelet | Silver coated | catch |
| 152.1 | 0.00 | 0.02 | Ring | Silver-like | bright ring |
| 152.2 | 0.00 | 0.02 | Ring | Clearly non-precious metal | Matt ring |
| 153.1 | 0.00 | 0.14 | Earring | Silver-like | Needles |
| 153.2 | 0.00 | 0.05 | Earring | Silver-like | Connection |
| 153.3 | 0.00 | 0.03 | Earring | Silver-like | Chains |
| 154.1 | 0.00 | 0.02 | Bracelet | Clearly non-precious metal | Bracelet |
| 155.1 | 0.00 | 0.02 | Earring | Gold coated | Ring |
| 155.2 | 0.00 | 0.11 | Earring | Golden-like | Needles |
| 155.3 | 0.00 | 0.03 | Earring | Golden-like | Catch |
| 156.1 | 0.00 | 0.03 | Earring | Silver-like | backside and needle |
| 157.1 | 0.00 | 0.04 | Ankle chain | Silver coated | Catch |
| 157.2 | 0.00 | 0.05 | Ankle chain | Silver coated | Disc |
| 158.1 | 0.00 | 0.04 | Ankle chain | Silver-like | Feet |
| 158.2 | 0.00 | 0.07 | Ankle chain | Silver-like | Chain |
| 158.3 | 0.00 | 0.09 | Ankle chain | Silver-like | Catch |
| 159.1 | 0.00 | 0.02 | Necklace | Silver-like | Heart backside |
| 159.2 | 0.00 | 0.04 | Necklace | Silver-like | Chain |
| 159.3 | 0.00 | 0.07 | Necklace | Silver-like | Catch |
| 160.1 | 0.00 | 0.02 | Bracelet | Silver-like | metallic side |
| 161.1 | 0.00 | 0.07 | Bracelet | Silver-like | Bracelet |
| 162.1 | 0.00 | 0.10 | Ring | Gold coated | finger ring |
| 163.1 | 0.00 | 0.03 | Piercing | Genuine silver | ring with ball |
| 164.1 | 0.00 | 0.02 | Piercing | Silver coated | Heart backside |
| 164.2 | 0.00 | 0.03 | Piercing | Silver-like | Ball |
| 165.1 | 0.00 | 0.03 | Necklace | Genuine silver | charm from behind |
| 165.2 | 0.00 | 0.02 | Necklace | Genuine silver | Chain |
| 165.3 | 0.00 | 0.04 | Necklace | Genuine silver | Catch |
| 166.1 | 0.00 | 0.52 | Earring | Genuine gold | Rings |
| 166.2 | 0.00 | 0.55 | Earring | Genuine gold | Catch |
| 167.1 | 0.00 | 0.11 | Earring | Gold coated | butterflies |
| 167.2 | 0.00 | 0.86 | Earring | Genuine gold | Threads |
| 168.1 | 0.00 | 0.13 | Ankle chain | Gold coated | Heart |
| 168.2 | 0.00 | 0.08 | Ankle chain | Gold coated | Chain |
| 168.3 | 0.00 | 0.15 | Ankle chain | Gold coated | Catch |
| 169.1 | 0.00 | 0.03 | Ankle chain | Silver coated | Heart |
| 169.2 | 0.00 | 0.02 | Ankle chain | Silver-like | Chain |
| 169.3 | 0.00 | 0.04 | Ankle chain | Silver-like | Catch |
| 170.1 | 0.00 | 0.10 | Bracelet | Golden-like | Golden disc |
| 170.2 | 0.00 | 0.12 | Bracelet | Golden-like | side of bracelet |
2.4 Content of Ni in all jewellery parts
| Jewellery part ID | Ni | Ni Error | Product type | Appearance of jewellery part | Description jewellery part |
| 148.1 | 95.08 | 0.11 | Ring | Silver coated | Finger ring |
| 119.1 | 93.16 | 0.11 | Ring | Silver coated | Finger ring |
| 92.1 | 85.97 | 0.21 | Other | Silver-like | Side without stone |
| 145.1 | 74.06 | 0.27 | Earring | Gold coated | Rings |
| 91.3 | 70.14 | 0.09 | Necklace | Clearly non-precious metal | Chain |
| 10.1 | 56.50 | 0.22 | Ring | Clearly non-precious metal | Ring |
| 139.1 | 55.31 | 0.22 | Necklace | Clearly non-precious metal | Chain |
| 169.1 | 52.79 | 0.18 | Ankle chain | Silver coated | Heart |
| 158.3 | 49.75 | 0.41 | Ankle chain | Silver-like | Catch |
| 156.1 | 49.66 | 0.19 | Earring | Silver-like | backside and needle |
| 158.1 | 43.12 | 0.18 | Ankle chain | Silver-like | Feet |
| 139.2 | 41.66 | 0.22 | Necklace | Clearly non-precious metal | Catch |
| 158.2 | 38.64 | 0.24 | Ankle chain | Silver-like | chain |
| 73.1 | 38.10 | 0.20 | Bracelet | Gold coated | Gold-coloured side |
| 111.1 | 32.92 | 0.18 | Bracelet | Gold coated | Golden edge |
| 31.2 | 29.98 | 0.28 | Necklace | Silver-like | balls 2 pcs. |
| 90.2 | 28.69 | 0.23 | Necklace | Gold coated | Catch |
| 18.2 | 28.25 | 0.26 | Necklace | Silver-like | Chain |
| 4.1 | 27.04 | 0.17 | Ring | Silver-like | Ring |
| 147.2 | 24.43 | 0.22 | Bracelet | Silver-like | Catch |
| 169.2 | 24.31 | 0.19 | Ankle chain | Silver-like | Chain |
| 15.1 | 23.90 | 0.15 | Ring | Silver-like | Ring |
| 128.2 | 22.14 | 0.17 | Piercing | Silver-like | large ball without stone |
| 31.1 | 21.43 | 0.36 | Necklace | Silver-like | cylinder with hoal |
| 18.1 | 20.92 | 0.13 | Necklace | Silver-like | Charm |
| 149.2 | 19.50 | 0.13 | Bracelet | Silver-like | Edge protector |
| 149.1 | 19.20 | 0.14 | Bracelet | Silver-like | Bracelet |
| 36.1 | 18.51 | 0.15 | Ring | Silver-like | Finger part |
| 128.3 | 16.17 | 0.17 | Piercing | Silver-like | charm backside |
| 97.1 | 14.72 | 0.13 | Bracelet | Gold coated | Bracelet |
| 31.3 | 14.38 | 0.10 | Necklace | Silver-like | Cartridge |
| 160.1 | 13.77 | 0.17 | Bracelet | Silver-like | metallic side |
| 101.2 | 13.47 | 0.12 | Necklace | Gold coated | Chain |
| 121.3 | 13.43 | 0.24 | Earring | Gold coated | Thread |
| 169.3 | 13.27 | 0.17 | Ankle chain | Silver-like | Catch |
| 164.2 | 12.95 | 0.37 | Piercing | Silver-like | Ball |
| 91.2 | 11.63 | 0.10 | Necklace | Clearly non-precious metal | small ball |
| 149.3 | 11.01 | 0.10 | Bracelet | Silver-like | backside of bracelet |
| 101.1 | 10.91 | 0.11 | Necklace | Gold coated | backside of heart |
| 93.1 | 10.25 | 0.13 | Bracelet | Golden-like | side of Bracelet |
| 138.2 | 9.15 | 0.65 | Necklace | Clearly non-precious metal | chain |
| 101.3 | 8.87 | 0.10 | Necklace | Gold coated | Cornet |
| 154.1 | 7.74 | 0.13 | Bracelet | Clearly non-precious metal | Bracelet |
| 140.2 | 7.62 | 0.22 | Earring | Gold coated | Catch |
| 142.3 | 7.28 | 0.10 | Bracelet | Silver coated | Chain |
| 128.1 | 6.83 | 0.23 | Piercing | Silver-like | Navel stick |
| 6.2 | 6.49 | 0.12 | Necklace | Gold coated | Chain |
| 12.1 | 5.93 | 0.09 | Ring | Clearly non-precious metal | Ring/Other |
| 63.1 | 5.03 | 0.10 | Necklace | Gold coated | charm without stone |
| 123.1 | 4.90 | 0.09 | Ring | Gold coated | finger ring |
| 12.2 | 4.38 | 0.08 | Ring | Clearly non-precious metal | Death’s head |
| 142.1 | 4.32 | 0.11 | Bracelet | Silver-like | Catch stick |
| 96.1 | 4.28 | 0.08 | Earring | Silver-like | backside and needle |
| 63.3 | 4.01 | 0.07 | Necklace | Gold coated | chain |
| 155.2 | 3.92 | 0.21 | Earring | Golden-like | needles |
| 140.1 | 3.77 | 0.06 | Earring | Gold coated | Silver-coloured area |
| 7.1 | 3.74 | 0.09 | Ankle chain | Silver-like | Disc circle with hoal |
| 141.1 | 3.60 | 0.12 | Ring | Clearly non-precious metal | Ring |
| 109.2 | 3.56 | 0.11 | Earring | Golden-like | thread |
| 140.3 | 3.44 | 0.11 | Earring | Gold coated | Golden area |
| 91.1 | 3.38 | 0.05 | Necklace | Clearly non-precious metal | large ball |
| 63.2 | 3.06 | 0.06 | Necklace | Gold coated | Heart |
| 155.1 | 2.85 | 0.06 | Earring | Gold coated | Ring |
| 155.3 | 2.66 | 0.11 | Earring | Golden-like | Catch |
| 142.2 | 2.33 | 0.06 | Bracelet | Silver-like | Star |
| 7.2 | 2.16 | 0.06 | Ankle chain | Silver-like | Small disc circle |
| 90.1 | 1.83 | 0.05 | Necklace | Gold coated | large heart backside |
| 7.3 | 1.64 | 0.05 | Ankle chain | Silver-like | Chain |
| 32.1 | 1.58 | 0.05 | Necklace | Silver-like | side 2 without stone |
| 164.1 | 1.40 | 0.07 | Piercing | Silver coated | heart backside |
| 6.1 | 1.11 | 0.04 | Necklace | Gold coated | Angel |
| 28.1 | 1.02 | 0.04 | Necklace | Gold coated | Charm |
| 78.1 | 0.89 | 0.03 | Necklace | Silver-like | heart leaf side |
| 150.1 | 0.66 | 0.03 | Bracelet | Silver-like | Charm |
| 27.1 | 0.41 | 0.03 | Necklace | Gold coated | backside of charm |
| 53.3 | 0.32 | 0.04 | Ankle chain | Gold coated | Chain |
| 53.1 | 0.31 | 0.03 | Ankle chain | Gold coated | Round disc |
| 74.2 | 0.31 | 0.03 | Necklace | Silver-like | Chain |
| 77.1 | 0.31 | 0.02 | Necklace | Silver-like | heart leaf side |
| 83.1 | 0.30 | 0.02 | Necklace | Silver-like | Heart |
| 152.1 | 0.30 | 0.03 | Ring | Silver-like | Bright ring |
| 88.1 | 0.29 | 0.03 | Ring | Silver-like | ring without stone |
| 5.1 | 0.28 | 0.04 | Ring | Clearly non-precious metal | Ring |
| 11.1 | 0.27 | 0.03 | Ring | Gold coated | Ring |
| 24.1 | 0.27 | 0.02 | Ring | Gold coated | ringside with name |
| 76.1 | 0.27 | 0.03 | Necklace | Silver-like | butterfly |
| 13.2 | 0.26 | 0.04 | Earring | Silver coated | Chain |
| 71.1 | 0.26 | 0.02 | Necklace | Silver-like | Heart |
| 105.1 | 0.26 | 0.03 | Ring | Silver-like | metallic side |
| 77.2 | 0.25 | 0.05 | Necklace | Silver-like | Catch |
| 19.3 | 0.24 | 0.05 | Bracelet | Silver coated | Small ball |
| 44.1 | 0.24 | 0.05 | Earring | Gold coated | the sticks |
| 94.3 | 0.22 | 0.04 | Necklace | Silver-like | Small ball |
| 96.2 | 0.22 | 0.04 | Earring | Silver-like | the side |
| 112.2 | 0.22 | 0.10 | Earring | Gold coated | thin thread catch |
| 113.1 | 0.22 | 0.02 | Bracelet | Golden-like | Chain |
| 69.1 | 0.21 | 0.03 | Bracelet | Silver coated | Bracelet |
| 74.1 | 0.21 | 0.02 | Necklace | Silver-like | Heart |
| 19.1 | 0.20 | 0.03 | Bracelet | Silver coated | Disc |
| 57.1 | 0.20 | 0.03 | Earring | Silver-like | piece of jewellery backside |
| 109.1 | 0.20 | 0.02 | Earring | Gold coated | star golden side |
| 134.2 | 0.20 | 0.03 | Necklace | Silver coated | Chain |
| 137.2 | 0.20 | 0.04 | Necklace | Silver alloy | Chain |
| 46.1 | 0.19 | 0.02 | Bracelet | Gold coated | Flower |
| 79.3 | 0.19 | 0.03 | Necklace | Gold coated | Chain |
| 81.3 | 0.19 | 0.03 | Necklace | Gold coated | Catch |
| 82.1 | 0.19 | 0.02 | Necklace | Gold coated | charm backside |
| 85.2 | 0.19 | 0.04 | Necklace | Gold coated | Catch |
| 130.1 | 0.19 | 0.06 | Earring | Silver-like | Needles |
| 143.1 | 0.19 | 0.03 | Bracelet | Silver coated | Bracelet |
| 22.1 | 0.18 | 0.05 | Ring | Silver alloy | finger rings |
| 70.2 | 0.18 | 0.03 | Necklace | Gold coated | Cu-coloured chain |
| 108.2 | 0.18 | 0.02 | Bracelet | Silver coated | metallic side |
| 113.2 | 0.18 | 0.02 | Bracelet | Gold coated | golden balls |
| 157.2 | 0.18 | 0.02 | Ankle chain | Silver coated | Disc |
| 21.1 | 0.17 | 0.02 | Earring | Clearly non-precious metal | metallic part |
| 84.2 | 0.17 | 0.03 | Earring | Gold coated | Star |
| 86.2 | 0.17 | 0.03 | Necklace | Silver coated | Chain |
| 103.1 | 0.17 | 0.02 | Earring | Silver-like | bright side |
| 117.1 | 0.17 | 0.03 | Bracelet | Silver coated | Catch |
| 117.2 | 0.17 | 0.02 | Bracelet | Silver-like | discs etc. |
| 117.3 | 0.17 | 0.04 | Bracelet | Silver coated | chain with ball |
| 161.1 | 0.17 | 0.02 | Bracelet | Silver-like | bracelet |
| 2.1 | 0.16 | 0.02 | Earring | Gold coated | Flower |
| 8.1 | 0.16 | 0.02 | Necklace | Gold coated | Flower |
| 19.2 | 0.16 | 0.03 | Bracelet | Silver-like | Catch |
| 26.1 | 0.16 | 0.03 | Other | Clearly non-precious metal | Chain |
| 54.1 | 0.16 | 0.02 | Necklace | Silver-like | Charm |
| 69.2 | 0.16 | 0.02 | Bracelet | Silver coated | butterfly backside |
| 84.1 | 0.16 | 0.03 | Earring | Golden-like | the sticks |
| 94.1 | 0.16 | 0.03 | Necklace | Silver-like | Heart |
| 100.2 | 0.16 | 0.07 | Earring | Silver coated | Needles |
| 3.2 | 0.15 | 0.03 | Necklace | Gold coated | small charm |
| 8.2 | 0.15 | 0.03 | Necklace | Gold coated | Catch |
| 16.1 | 0.15 | 0.02 | Other | Silver-like | Chain |
| 83.3 | 0.15 | 0.03 | Necklace | Silver coated | Chain |
| 86.1 | 0.15 | 0.02 | Necklace | Silver coated | Charm |
| 1.1 | 0.14 | 0.02 | Bracelet | Silver-like | Bracelet |
| 3.3 | 0.14 | 0.02 | Necklace | Gold coated | part on the string |
| 86.3 | 0.14 | 0.03 | Necklace | Silver coated | Catch |
| 100.1 | 0.14 | 0.03 | Earring | Silver coated | backside of charm |
| 112.1 | 0.14 | 0.06 | Earring | Gold coated | thread thick |
| 134.3 | 0.14 | 0.04 | Necklace | Silver alloy | Catch |
| 66.2 | 0.13 | 0.02 | Bracelet | Silver coated | Catch |
| 76.2 | 0.13 | 0.03 | Necklace | Silver-like | Catch |
| 82.2 | 0.13 | 0.04 | Necklace | Gold coated | Catch |
| 136.1 | 0.13 | 0.02 | Other | Silver coated | Charm |
| 159.1 | 0.13 | 0.02 | Necklace | Silver-like | heart backside |
| 13.1 | 0.12 | 0.02 | Earring | Silver-like | Charm |
| 28.2 | 0.12 | 0.03 | Necklace | Gold coated | Chain |
| 48.2 | 0.12 | 0.02 | Necklace | Gold coated | Chain |
| 53.2 | 0.12 | 0.03 | Ankle chain | Clearly non-precious metal | Ball |
| 65.1 | 0.12 | 0.02 | Ring | Silver-like | ring |
| 66.3 | 0.12 | 0.02 | Bracelet | Silver-like | end piece |
| 67.1 | 0.12 | 0.02 | Necklace | Silver-like | Ball |
| 71.3 | 0.12 | 0.03 | Necklace | Silver coated | Chain |
| 80.1 | 0.12 | 0.03 | Necklace | Gold coated | heart inner side |
| 40.1 | 0.11 | 0.02 | Necklace | Silver-like | piece of jewellery |
| 54.2 | 0.11 | 0.02 | Necklace | Silver-like | Chain |
| 57.2 | 0.11 | 0.02 | Earring | Silver-like | Catch |
| 70.3 | 0.11 | 0.04 | Necklace | Gold coated | Catch |
| 98.1 | 0.11 | 0.03 | Bracelet | Silver-like | Bracelet |
| 115.1 | 0.11 | 0.02 | Ring | Gold coated | the finger ring |
| 116.2 | 0.11 | 0.04 | Bracelet | Silver coated | backside of flower and chain |
| 146.1 | 0.11 | 0.01 | Bracelet | Golden-like | Bracelet |
| 32.2 | 0.10 | 0.04 | Necklace | Silver-like | Chain |
| 56.2 | 0.10 | 0.03 | Necklace | Silver-like | Chain |
| 64.1 | 0.10 | 0.02 | Earring | Gold coated | Ball |
| 72.1 | 0.10 | 0.02 | Ring | Gold coated | the finger ring |
| 82.3 | 0.10 | 0.02 | Necklace | Gold coated | connecting piece |
| 85.1 | 0.10 | 0.02 | Necklace | Gold coated | charm backside |
| 87.2 | 0.10 | 0.03 | Bracelet | Golden-like | Catch |
| 87.3 | 0.10 | 0.03 | Bracelet | Gold coated | Chain |
| 99.1 | 0.10 | 0.03 | Earring | Gold coated | twisted rings |
| 104.1 | 0.10 | 0.03 | Bracelet | Silver-like | chain without leather |
| 104.3 | 0.10 | 0.02 | Bracelet | Silver-like | Pins |
| 152.2 | 0.10 | 0.02 | Ring | Clearly non-precious metal | matt ring |
| 3.1 | 0.09 | 0.02 | Necklace | Gold coated | large charm |
| 9.1 | 0.09 | 0.01 | Necklace | Golden-like | Heart |
| 23.1 | 0.09 | 0.02 | Bracelet | Gold coated | Bracelet |
| 38.2 | 0.09 | 0.03 | Necklace | Gold coated | Chain |
| 43.2 | 0.09 | 0.03 | Necklace | Gold coated | Chain |
| 45.1 | 0.09 | 0.01 | Bracelet | Golden-like | Chain |
| 47.1 | 0.09 | 0.02 | Bracelet | Silver coated | bracelet |
| 78.2 | 0.09 | 0.04 | Necklace | Silver-like | Catch |
| 79.2 | 0.09 | 0.03 | Necklace | Gold coated | Catch |
| 81.2 | 0.09 | 0.02 | Necklace | Golden-like | neck pin |
| 103.2 | 0.09 | 0.03 | Earring | Silver-like | Chains |
| 107.1 | 0.09 | 0.02 | Bracelet | Gold coated | chain with balls |
| 108.1 | 0.09 | 0.02 | Bracelet | Silver coated | side with stone |
| 120.2 | 0.09 | 0.03 | Earring | Gold coated | small disc |
| 124.2 | 0.09 | 0.02 | Necklace | Silver-like | Chain |
| 135.1 | 0.09 | 0.04 | Earring | Clearly non-precious metal | Chain |
| 17.1 | 0.08 | 0.02 | Ring | Gold coated | side with green colour |
| 37.2 | 0.08 | 0.02 | Necklace | Silver coated | Chain |
| 43.1 | 0.08 | 0.03 | Necklace | Gold coated | piece of jewellery |
| 51.1 | 0.08 | 0.03 | Earring | Silver coated | piece of jewellery bright side |
| 67.2 | 0.08 | 0.02 | Necklace | Silver-like | Catch |
| 68.1 | 0.08 | 0.02 | Ring | Silver coated | Ring |
| 76.3 | 0.08 | 0.03 | Necklace | Silver-like | Chain |
| 83.2 | 0.08 | 0.03 | Necklace | Silver coated | Catch |
| 95.2 | 0.08 | 0.03 | Necklace | Gold coated | Chain |
| 124.1 | 0.08 | 0.03 | Necklace | Silver-like | Catch |
| 137.1 | 0.08 | 0.02 | Necklace | Silver coated | flower metal |
| 159.3 | 0.08 | 0.04 | Necklace | Silver-like | Catch |
| 26.2 | 0.07 | 0.02 | Other | Clearly non-precious metal | Catch |
| 52.1 | 0.07 | 0.02 | Bracelet | Gold coated | Bracelet |
| 61.2 | 0.07 | 0.03 | Necklace | Gold coated | Chain |
| 64.2 | 0.07 | 0.03 | Earring | Golden-like | needle |
| 71.2 | 0.07 | 0.03 | Necklace | Silver coated | Catch |
| 157.1 | 0.07 | 0.03 | Ankle chain | Silver coated | Catch |
| 16.2 | 0.06 | 0.01 | Other | Silver-like | Catch |
| 35.1 | 0.06 | 0.02 | Necklace | Clearly non-precious metal | holder and connecting link |
| 38.1 | 0.06 | 0.02 | Necklace | Gold coated | piece of jewellery without stone |
| 40.2 | 0.06 | 0.01 | Necklace | Silver-like | balls 2 pcs. |
| 75.1 | 0.06 | 0.02 | Bracelet | Golden-like | Bracelet |
| 81.1 | 0.06 | 0.02 | Necklace | Gold coated | charm backside |
| 136.2 | 0.06 | 0.03 | Other | Silver coated | Disc |
| 147.1 | 0.06 | 0.02 | Bracelet | Silver-like | Bracelet |
| 147.3 | 0.06 | 0.02 | Bracelet | Silver-like | Eye |
| 159.2 | 0.06 | 0.02 | Necklace | Silver-like | Chain |
| 170.2 | 0.06 | 0.02 | Bracelet | Golden-like | the side of bracelet |
| 14.1 | 0.05 | 0.02 | Bracelet | Clearly non-precious metal | metallic side |
| 34.1 | 0.05 | 0.02 | Necklace | Clearly non-precious metal | piece of jewellery copper side |
| 59.1 | 0.05 | 0.01 | Bracelet | Silver-like | Bracelet |
| 67.3 | 0.05 | 0.02 | Necklace | Silver-like | Chain |
| 87.1 | 0.05 | 0.01 | Bracelet | Golden-like | flower backside |
| 132.1 | 0.05 | 0.01 | Bracelet | Golden-like | Bracelet |
| 162.1 | 0.05 | 0.02 | Ring | Gold coated | finger ring |
| 46.3 | 0.04 | 0.02 | Bracelet | Gold coated | Catch |
| 55.1 | 0.04 | 0.01 | Bracelet | Golden-like | Bracelet |
| 104.2 | 0.04 | 0.02 | Bracelet | Silver-like | Heart |
| 79.1 | 0.03 | 0.01 | Necklace | Golden-like | Flower |
| 17.2 | 0.00 | 0.04 | Ring | Gold coated | without green colour |
| 20.1 | 0.00 | 0.05 | Earring | Gold coated | golden side |
| 25.1 | 0.00 | 0.04 | Necklace | Clearly non-precious metal | Charm |
| 25.2 | 0.00 | 0.05 | Necklace | Gold coated | Chain |
| 29.1 | 0.00 | 0.06 | Ring | Clearly non-precious metal | ring coate |
| 30.1 | 0.00 | 0.03 | Earring | Gold coated | ear sticks |
| 33.1 | 0.00 | 0.02 | Necklace | Gold coated | 4 edged piece of jewellery |
| 33.2 | 0.00 | 0.02 | Necklace | Golden-like | Leaf |
| 33.3 | 0.00 | 0.08 | Necklace | Golden-like | Chain |
| 34.2 | 0.00 | 0.07 | Necklace | Clearly non-precious metal | chain Cu-coloured |
| 37.1 | 0.00 | 0.03 | Necklace | Silver coated | piece of jewellery without stone |
| 39.1 | 0.00 | 0.10 | Ring | Genuine silver | Ring |
| 41.1 | 0.00 | 0.04 | Necklace | Gold coated | piece of jewellery |
| 41.2 | 0.00 | 0.05 | Necklace | Golden-like | Chain |
| 42.1 | 0.00 | 0.04 | Necklace | Silver-like | Leaf |
| 42.2 | 0.00 | 0.06 | Necklace | Silver-like | chain |
| 46.2 | 0.00 | 0.06 | Bracelet | Gold coated | Bracelet |
| 48.1 | 0.00 | 0.04 | Necklace | Gold coated | Heart |
| 49.1 | 0.00 | 0.02 | Bracelet | Golden-like | Bracelet |
| 50.1 | 0.00 | 0.02 | Ring | Clearly non-precious metal | Ring |
| 56.1 | 0.00 | 0.04 | Necklace | Silver-like | charm backside |
| 58.1 | 0.00 | 0.06 | Ring | Gold coated | the finger ring |
| 60.1 | 0.00 | 0.02 | Bracelet | Golden-like | Bracelet |
| 61.1 | 0.00 | 0.03 | Necklace | Golden-like | Charm without stone |
| 62.1 | 0.00 | 0.05 | Necklace | Silver-like | Charm |
| 62.2 | 0.00 | 0.04 | Necklace | Silver-like | Chain |
| 66.1 | 0.00 | 0.03 | Bracelet | Silver coated | bracelet |
| 70.1 | 0.00 | 0.04 | Necklace | Gold coated | Charm |
| 74.3 | 0.00 | 0.06 | Necklace | Silver-like | Catch |
| 77.3 | 0.00 | 0.06 | Necklace | Silver-like | Chain |
| 78.3 | 0.00 | 0.07 | Necklace | Silver-like | Chain |
| 80.2 | 0.00 | 0.05 | Necklace | Gold coated | Chain |
| 85.3 | 0.00 | 0.05 | Necklace | Gold coated | Chain |
| 89.1 | 0.00 | 0.05 | Earring | Golden-like | piece of jewellery |
| 89.2 | 0.00 | 0.26 | Earring | Golden-like | 2 needles |
| 94.2 | 0.00 | 0.09 | Necklace | Silver-like | Catch |
| 95.1 | 0.00 | 0.03 | Necklace | Gold coated | Charm |
| 95.3 | 0.00 | 0.12 | Necklace | Gold coated | Catch |
| 98.2 | 0.00 | 0.05 | Bracelet | Silver-like | Twisted strings |
| 99.2 | 0.00 | 0.14 | Earring | Gold coated | non twisted part |
| 102.1 | 0.00 | 0.04 | Bracelet | Silver-like | metallic side |
| 106.1 | 0.00 | 0.08 | Earring | Genuine silver | Discs |
| 106.2 | 0.00 | 0.12 | Earring | Genuine silver | Thread |
| 110.1 | 0.00 | 0.11 | Earring | Gold coated | Rings |
| 110.2 | 0.00 | 0.11 | Earring | Gold coated | Holder with balls |
| 114.1 | 0.00 | 0.09 | Earring | Genuine silver | side and needle |
| 116.1 | 0.00 | 0.08 | Bracelet | Silver alloy | Catch |
| 116.3 | 0.00 | 0.12 | Bracelet | Silver alloy | Chain |
| 118.1 | 0.00 | 0.05 | Bracelet | Gold coated | Bracelet |
| 120.1 | 0.00 | 0.07 | Earring | Gold coated | large disc |
| 120.3 | 0.00 | 0.12 | Earring | Gold coated | Thread |
| 121.1 | 0.00 | 0.07 | Earring | Gold coated | large flower |
| 121.2 | 0.00 | 0.07 | Earring | Gold coated | small flowers |
| 122.1 | 0.00 | 0.12 | Bracelet | Gold coated | royal crown |
| 125.1 | 0.00 | 0.05 | Ring | Silver coated | finger ring |
| 126.1 | 0.00 | 0.06 | Earring | Genuine silver | Hearts |
| 126.2 | 0.00 | 0.13 | Earring | Genuine silver | Needles |
| 127.1 | 0.00 | 0.11 | Other | Gold coated | finger ring |
| 129.1 | 0.00 | 0.13 | Earring | Gold coated | Needles |
| 131.1 | 0.00 | 0.04 | Bracelet | Silver-like | bracelet without stone |
| 133.1 | 0.00 | 0.10 | Ring | Genuine silver | finger ring |
| 134.1 | 0.00 | 0.09 | Necklace | Silver alloy | Butterfly |
| 135.2 | 0.00 | 0.05 | Earring | Clearly non-precious metal | Needles |
| 138.1 | 0.00 | 0.07 | Necklace | Silver alloy | charm from behind |
| 138.3 | 0.00 | 0.10 | Necklace | Silver coated | Catch |
| 144.1 | 0.00 | 0.03 | Earring | Golden-like | piece of jewellery |
| 144.2 | 0.00 | 0.12 | Earring | Silver coated | needles with balls |
| 151.1 | 0.00 | 0.09 | Bracelet | Clearly non-precious metal | clover charm from behind |
| 151.2 | 0.00 | 0.16 | Bracelet | Silver coated | Chain |
| 151.3 | 0.00 | 0.11 | Bracelet | Silver coated | Catch |
| 153.1 | 0.00 | 0.04 | Earring | Silver-like | Needles |
| 153.2 | 0.00 | 0.06 | Earring | Silver-like | connecting link |
| 153.3 | 0.00 | 0.03 | Earring | Silver-like | Chains |
| 163.1 | 0.00 | 0.14 | Piercing | Genuine silver | ring with ball |
| 165.1 | 0.00 | 0.10 | Necklace | Genuine silver | charm from behind |
| 165.2 | 0.00 | 0.08 | Necklace | Genuine silver | Chain |
| 165.3 | 0.00 | 0.15 | Necklace | Genuine silver | catch |
| 166.1 | 0.00 | 0.16 | Earring | Genuine gold | Rings |
| 166.2 | 0.00 | 0.18 | Earring | Genuine gold | Catches |
| 167.1 | 0.00 | 0.03 | Earring | Gold coated | Butterflies |
| 167.2 | 0.00 | 0.14 | Earring | Genuine gold | Threads |
| 168.1 | 0.00 | 0.09 | Ankle chain | Gold coated | Heart |
| 168.2 | 0.00 | 0.09 | Ankle chain | Gold coated | Chain |
| 168.3 | 0.00 | 0.14 | Ankle chain | Gold coated | Catch |
| 170.1 | 0.00 | 0.03 | Bracelet | Golden-like | golden pattern coate |
2.5 Content of Se and Cr in all jewellery parts
| Jewellery part ID | Sb | Jewellery part ID | Cr |
| 95.3 | 7.26 | 138.2 | 17.43 |
| 19.3 | 4.09 | 164.2 | 16.56 |
| 29.1 | 3.46 | 141.1 | 15.58 |
| 159.3 | 3.39 | 128.1 | 14.37 |
| 151.3 | 3.30 | 5.1 | 12.05 |
| 28.1 | 3.24 | 89.2 | 11.13 |
| 79.2 | 3.14 | 164.1 | 9.13 |
| 94.2 | 3.07 | 4.1 | 2.19 |
| 153.2 | 2.83 | 155.2 | 1.72 |
| 81.3 | 2.78 | 18.1 | 0.68 |
| 71.2 | 2.69 | 92.1 | 0.65 |
| 70.3 | 2.59 | 96.1 | 0.60 |
| 82.2 | 2.47 | 18.2 | 0.31 |
| 147.2 | 2.46 | 10.1 | 0.08 |
| 85.2 | 2.22 | 15.1 | 0.07 |
| 19.2 | 2.08 | 34.2 | 0.07 |
| 4.1 | 2.06 | 91.3 | 0.06 |
| 169.3 | 1.97 | 79.3 | 0.05 |
| 74.3 | 1.92 | 169.2 | 0.05 |
| 8.2 | 1.88 | 119.1 | 0.05 |
| 124.1 | 1.85 | 148.1 | 0.04 |
| 139.2 | 1.84 | 26.1 | 0.04 |
| 90.2 | 1.69 | 95.3 | 0.00 |
| 78.2 | 1.56 | 19.3 | 0.00 |
| 76.2 | 1.55 | 29.1 | 0.00 |
| 83.1 | 1.54 | 159.3 | 0.00 |
| 138.3 | 1.54 | 151.3 | 0.00 |
| 151.1 | 1.53 | 28.1 | 0.00 |
| 15.1 | 1.43 | 79.2 | 0.00 |
| 32.1 | 1.39 | 94.2 | 0.00 |
| 43.1 | 1.36 | 153.2 | 0.00 |
| 12.2 | 1.32 | 81.3 | 0.00 |
| 2.1 | 1.31 | 71.2 | 0.00 |
| 40.1 | 1.28 | 70.3 | 0.00 |
| 64.1 | 1.26 | 82.2 | 0.00 |
| 12.1 | 1.05 | 147.2 | 0.00 |
| 10.1 | 1.03 | 85.2 | 0.00 |
| 41.1 | 1.00 | 19.2 | 0.00 |
| 164.1 | 1.00 | 169.3 | 0.00 |
| 85.1 | 0.98 | 74.3 | 0.00 |
| 136.1 | 0.98 | 8.2 | 0.00 |
| 8.1 | 0.97 | 124.1 | 0.00 |
| 67.1 | 0.95 | 139.2 | 0.00 |
| 20.1 | 0.93 | 90.2 | 0.00 |
| 104.2 | 0.91 | 78.2 | 0.00 |
| 142.1 | 0.88 | 76.2 | 0.00 |
| 42.1 | 0.86 | 83.1 | 0.00 |
| 77.2 | 0.83 | 138.3 | 0.00 |
| 62.1 | 0.81 | 151.1 | 0.00 |
| 74.1 | 0.81 | 32.1 | 0.00 |
| 100.1 | 0.80 | 43.1 | 0.00 |
| 156.1 | 0.78 | 12.2 | 0.00 |
| 56.1 | 0.77 | 2.1 | 0.00 |
| 134.1 | 0.77 | 40.1 | 0.00 |
| 57.1 | 0.75 | 64.1 | 0.00 |
| 88.1 | 0.75 | 12.1 | 0.00 |
| 107.1 | 0.71 | 41.1 | 0.00 |
| 138.1 | 0.70 | 85.1 | 0.00 |
| 17.2 | 0.63 | 136.1 | 0.00 |
| 104.3 | 0.63 | 8.1 | 0.00 |
| 105.1 | 0.63 | 67.1 | 0.00 |
| 6.1 | 0.59 | 20.1 | 0.00 |
| 68.1 | 0.58 | 104.2 | 0.00 |
| 90.1 | 0.57 | 142.1 | 0.00 |
| 34.1 | 0.53 | 42.1 | 0.00 |
| 142.2 | 0.51 | 77.2 | 0.00 |
| 169.1 | 0.51 | 62.1 | 0.00 |
| 69.2 | 0.50 | 74.1 | 0.00 |
| 103.1 | 0.48 | 100.1 | 0.00 |
| 128.2 | 0.46 | 156.1 | 0.00 |
| 159.1 | 0.45 | 56.1 | 0.00 |
| 3.2 | 0.43 | 134.1 | 0.00 |
| 38.1 | 0.43 | 57.1 | 0.00 |
| 17.1 | 0.42 | 88.1 | 0.00 |
| 27.1 | 0.42 | 107.1 | 0.00 |
| 46.1 | 0.40 | 138.1 | 0.00 |
| 19.1 | 0.39 | 17.2 | 0.00 |
| 94.3 | 0.37 | 104.3 | 0.00 |
| 96.2 | 0.36 | 105.1 | 0.00 |
| 101.1 | 0.34 | 6.1 | 0.00 |
| 3.1 | 0.32 | 68.1 | 0.00 |
| 84.2 | 0.32 | 90.1 | 0.00 |
| 65.1 | 0.30 | 34.1 | 0.00 |
| 101.3 | 0.30 | 142.2 | 0.00 |
| 136.2 | 0.30 | 169.1 | 0.00 |
| 21.1 | 0.29 | 69.2 | 0.00 |
| 63.1 | 0.27 | 103.1 | 0.00 |
| 70.1 | 0.26 | 128.2 | 0.00 |
| 117.3 | 0.26 | 159.1 | 0.00 |
| 170.1 | 0.26 | 3.2 | 0.00 |
| 96.1 | 0.25 | 38.1 | 0.00 |
| 82.1 | 0.24 | 17.1 | 0.00 |
| 125.1 | 0.24 | 27.1 | 0.00 |
| 54.1 | 0.22 | 46.1 | 0.00 |
| 83.3 | 0.21 | 19.1 | 0.00 |
| 109.1 | 0.21 | 94.3 | 0.00 |
| 37.1 | 0.20 | 96.2 | 0.00 |
| 63.2 | 0.20 | 101.1 | 0.00 |
| 143.1 | 0.18 | 3.1 | 0.00 |
| 81.1 | 0.17 | 84.2 | 0.00 |
| 78.1 | 0.15 | 65.1 | 0.00 |
| 45.1 | 0.14 | 101.3 | 0.00 |
| 95.1 | 0.14 | 136.2 | 0.00 |
| 91.3 | 0.01 | 21.1 | 0.00 |
| 119.1 | 0.00 | 63.1 | 0.00 |
| 148.1 | 0.00 | 70.1 | 0.00 |
| 1.1 | 0.00 | 117.3 | 0.00 |
| 3.3 | 0.00 | 170.1 | 0.00 |
| 5.1 | 0.00 | 82.1 | 0.00 |
| 6.2 | 0.00 | 125.1 | 0.00 |
| 7.1 | 0.00 | 54.1 | 0.00 |
| 7.2 | 0.00 | 83.3 | 0.00 |
| 7.3 | 0.00 | 109.1 | 0.00 |
| 9.1 | 0.00 | 37.1 | 0.00 |
| 11.1 | 0.00 | 63.2 | 0.00 |
| 13.1 | 0.00 | 143.1 | 0.00 |
| 13.2 | 0.00 | 81.1 | 0.00 |
| 14.1 | 0.00 | 78.1 | 0.00 |
| 16.1 | 0.00 | 45.1 | 0.00 |
| 16.2 | 0.00 | 95.1 | 0.00 |
| 18.1 | 0.00 | 1.1 | 0.00 |
| 18.2 | 0.00 | 3.3 | 0.00 |
| 22.1 | 0.00 | 6.2 | 0.00 |
| 23.1 | 0.00 | 7.1 | 0.00 |
| 24.1 | 0.00 | 7.2 | 0.00 |
| 25.1 | 0.00 | 7.3 | 0.00 |
| 25.2 | 0.00 | 9.1 | 0.00 |
| 26.1 | 0.00 | 11.1 | 0.00 |
| 26.2 | 0.00 | 13.1 | 0.00 |
| 28.2 | 0.00 | 13.2 | 0.00 |
| 30.1 | 0.00 | 14.1 | 0.00 |
| 31.1 | 0.00 | 16.1 | 0.00 |
| 31.2 | 0.00 | 16.2 | 0.00 |
| 31.3 | 0.00 | 22.1 | 0.00 |
| 32.2 | 0.00 | 23.1 | 0.00 |
| 33.1 | 0.00 | 24.1 | 0.00 |
| 33.2 | 0.00 | 25.1 | 0.00 |
| 33.3 | 0.00 | 25.2 | 0.00 |
| 34.2 | 0.00 | 26.2 | 0.00 |
| 35.1 | 0.00 | 28.2 | 0.00 |
| 36.1 | 0.00 | 30.1 | 0.00 |
| 37.2 | 0.00 | 31.1 | 0.00 |
| 38.2 | 0.00 | 31.2 | 0.00 |
| 39.1 | 0.00 | 31.3 | 0.00 |
| 40.2 | 0.00 | 32.2 | 0.00 |
| 41.2 | 0.00 | 33.1 | 0.00 |
| 42.2 | 0.00 | 33.2 | 0.00 |
| 43.2 | 0.00 | 33.3 | 0.00 |
| 44.1 | 0.00 | 35.1 | 0.00 |
| 46.2 | 0.00 | 36.1 | 0.00 |
| 46.3 | 0.00 | 37.2 | 0.00 |
| 47.1 | 0.00 | 38.2 | 0.00 |
| 48.1 | 0.00 | 39.1 | 0.00 |
| 48.2 | 0.00 | 40.2 | 0.00 |
| 49.1 | 0.00 | 41.2 | 0.00 |
| 50.1 | 0.00 | 42.2 | 0.00 |
| 51.1 | 0.00 | 43.2 | 0.00 |
| 52.1 | 0.00 | 44.1 | 0.00 |
| 53.1 | 0.00 | 46.2 | 0.00 |
| 53.2 | 0.00 | 46.3 | 0.00 |
| 53.3 | 0.00 | 47.1 | 0.00 |
| 54.2 | 0.00 | 48.1 | 0.00 |
| 55.1 | 0.00 | 48.2 | 0.00 |
| 56.2 | 0.00 | 49.1 | 0.00 |
| 57.2 | 0.00 | 50.1 | 0.00 |
| 58.1 | 0.00 | 51.1 | 0.00 |
| 59.1 | 0.00 | 52.1 | 0.00 |
| 60.1 | 0.00 | 53.1 | 0.00 |
| 61.1 | 0.00 | 53.2 | 0.00 |
| 61.2 | 0.00 | 53.3 | 0.00 |
| 62.2 | 0.00 | 54.2 | 0.00 |
| 63.3 | 0.00 | 55.1 | 0.00 |
| 64.2 | 0.00 | 56.2 | 0.00 |
| 66.1 | 0.00 | 57.2 | 0.00 |
| 66.2 | 0.00 | 58.1 | 0.00 |
| 66.3 | 0.00 | 59.1 | 0.00 |
| 67.2 | 0.00 | 60.1 | 0.00 |
| 67.3 | 0.00 | 61.1 | 0.00 |
| 69.1 | 0.00 | 61.2 | 0.00 |
| 70.2 | 0.00 | 62.2 | 0.00 |
| 71.1 | 0.00 | 63.3 | 0.00 |
| 71.3 | 0.00 | 64.2 | 0.00 |
| 72.1 | 0.00 | 66.1 | 0.00 |
| 73.1 | 0.00 | 66.2 | 0.00 |
| 74.2 | 0.00 | 66.3 | 0.00 |
| 75.1 | 0.00 | 67.2 | 0.00 |
| 76.1 | 0.00 | 67.3 | 0.00 |
| 76.3 | 0.00 | 69.1 | 0.00 |
| 77.1 | 0.00 | 70.2 | 0.00 |
| 77.3 | 0.00 | 71.1 | 0.00 |
| 78.3 | 0.00 | 71.3 | 0.00 |
| 79.1 | 0.00 | 72.1 | 0.00 |
| 79.3 | 0.00 | 73.1 | 0.00 |
| 80.1 | 0.00 | 74.2 | 0.00 |
| 80.2 | 0.00 | 75.1 | 0.00 |
| 81.2 | 0.00 | 76.1 | 0.00 |
| 82.3 | 0.00 | 76.3 | 0.00 |
| 83.2 | 0.00 | 77.1 | 0.00 |
| 84.1 | 0.00 | 77.3 | 0.00 |
| 85.3 | 0.00 | 78.3 | 0.00 |
| 86.1 | 0.00 | 79.1 | 0.00 |
| 86.2 | 0.00 | 80.1 | 0.00 |
| 86.3 | 0.00 | 80.2 | 0.00 |
| 87.1 | 0.00 | 81.2 | 0.00 |
| 87.2 | 0.00 | 82.3 | 0.00 |
| 87.3 | 0.00 | 83.2 | 0.00 |
| 89.1 | 0.00 | 84.1 | 0.00 |
| 89.2 | 0.00 | 85.3 | 0.00 |
| 91.1 | 0.00 | 86.1 | 0.00 |
| 91.2 | 0.00 | 86.2 | 0.00 |
| 92.1 | 0.00 | 86.3 | 0.00 |
| 93.1 | 0.00 | 87.1 | 0.00 |
| 94.1 | 0.00 | 87.2 | 0.00 |
| 95.2 | 0.00 | 87.3 | 0.00 |
| 97.1 | 0.00 | 89.1 | 0.00 |
| 98.1 | 0.00 | 91.1 | 0.00 |
| 98.2 | 0.00 | 91.2 | 0.00 |
| 99.1 | 0.00 | 93.1 | 0.00 |
| 99.2 | 0.00 | 94.1 | 0.00 |
| 100.2 | 0.00 | 95.2 | 0.00 |
| 101.2 | 0.00 | 97.1 | 0.00 |
| 102.1 | 0.00 | 98.1 | 0.00 |
| 103.2 | 0.00 | 98.2 | 0.00 |
| 104.1 | 0.00 | 99.1 | 0.00 |
| 106.1 | 0.00 | 99.2 | 0.00 |
| 106.2 | 0.00 | 100.2 | 0.00 |
| 108.1 | 0.00 | 101.2 | 0.00 |
| 108.2 | 0.00 | 102.1 | 0.00 |
| 109.2 | 0.00 | 103.2 | 0.00 |
| 110.1 | 0.00 | 104.1 | 0.00 |
| 110.2 | 0.00 | 106.1 | 0.00 |
| 111.1 | 0.00 | 106.2 | 0.00 |
| 112.1 | 0.00 | 108.1 | 0.00 |
| 112.2 | 0.00 | 108.2 | 0.00 |
| 113.1 | 0.00 | 109.2 | 0.00 |
| 113.2 | 0.00 | 110.1 | 0.00 |
| 114.1 | 0.00 | 110.2 | 0.00 |
| 115.1 | 0.00 | 111.1 | 0.00 |
| 116.1 | 0.00 | 112.1 | 0.00 |
| 116.2 | 0.00 | 112.2 | 0.00 |
| 116.3 | 0.00 | 113.1 | 0.00 |
| 117.1 | 0.00 | 113.2 | 0.00 |
| 117.2 | 0.00 | 114.1 | 0.00 |
| 118.1 | 0.00 | 115.1 | 0.00 |
| 120.1 | 0.00 | 116.1 | 0.00 |
| 120.2 | 0.00 | 116.2 | 0.00 |
| 120.3 | 0.00 | 116.3 | 0.00 |
| 121.1 | 0.00 | 117.1 | 0.00 |
| 121.2 | 0.00 | 117.2 | 0.00 |
| 121.3 | 0.00 | 118.1 | 0.00 |
| 122.1 | 0.00 | 120.1 | 0.00 |
| 123.1 | 0.00 | 120.2 | 0.00 |
| 124.2 | 0.00 | 120.3 | 0.00 |
| 126.1 | 0.00 | 121.1 | 0.00 |
| 126.2 | 0.00 | 121.2 | 0.00 |
| 127.1 | 0.00 | 121.3 | 0.00 |
| 128.1 | 0.00 | 122.1 | 0.00 |
| 128.3 | 0.00 | 123.1 | 0.00 |
| 129.1 | 0.00 | 124.2 | 0.00 |
| 130.1 | 0.00 | 126.1 | 0.00 |
| 131.1 | 0.00 | 126.2 | 0.00 |
| 132.1 | 0.00 | 127.1 | 0.00 |
| 133.1 | 0.00 | 128.3 | 0.00 |
| 134.2 | 0.00 | 129.1 | 0.00 |
| 134.3 | 0.00 | 130.1 | 0.00 |
| 135.1 | 0.00 | 131.1 | 0.00 |
| 135.2 | 0.00 | 132.1 | 0.00 |
| 137.1 | 0.00 | 133.1 | 0.00 |
| 137.2 | 0.00 | 134.2 | 0.00 |
| 138.2 | 0.00 | 134.3 | 0.00 |
| 139.1 | 0.00 | 135.1 | 0.00 |
| 140.1 | 0.00 | 135.2 | 0.00 |
| 140.2 | 0.00 | 137.1 | 0.00 |
| 140.3 | 0.00 | 137.2 | 0.00 |
| 141.1 | 0.00 | 139.1 | 0.00 |
| 142.3 | 0.00 | 140.1 | 0.00 |
| 144.1 | 0.00 | 140.2 | 0.00 |
| 144.2 | 0.00 | 140.3 | 0.00 |
| 145.1 | 0.00 | 142.3 | 0.00 |
| 146.1 | 0.00 | 144.1 | 0.00 |
| 147.1 | 0.00 | 144.2 | 0.00 |
| 147.3 | 0.00 | 145.1 | 0.00 |
| 149.1 | 0.00 | 146.1 | 0.00 |
| 149.2 | 0.00 | 147.1 | 0.00 |
| 149.3 | 0.00 | 147.3 | 0.00 |
| 150.1 | 0.00 | 149.1 | 0.00 |
| 151.2 | 0.00 | 149.2 | 0.00 |
| 152.1 | 0.00 | 149.3 | 0.00 |
| 152.2 | 0.00 | 150.1 | 0.00 |
| 153.1 | 0.00 | 151.2 | 0.00 |
| 153.3 | 0.00 | 152.1 | 0.00 |
| 154.1 | 0.00 | 152.2 | 0.00 |
| 155.1 | 0.00 | 153.1 | 0.00 |
| 155.2 | 0.00 | 153.3 | 0.00 |
| 155.3 | 0.00 | 154.1 | 0.00 |
| 157.1 | 0.00 | 155.1 | 0.00 |
| 157.2 | 0.00 | 155.3 | 0.00 |
| 158.1 | 0.00 | 157.1 | 0.00 |
| 158.2 | 0.00 | 157.2 | 0.00 |
| 158.3 | 0.00 | 158.1 | 0.00 |
| 159.2 | 0.00 | 158.2 | 0.00 |
| 160.1 | 0.00 | 158.3 | 0.00 |
| 161.1 | 0.00 | 159.2 | 0.00 |
| 162.1 | 0.00 | 160.1 | 0.00 |
| 163.1 | 0.00 | 161.1 | 0.00 |
| 164.2 | 0.00 | 162.1 | 0.00 |
| 165.1 | 0.00 | 163.1 | 0.00 |
| 165.2 | 0.00 | 165.1 | 0.00 |
| 165.3 | 0.00 | 165.2 | 0.00 |
| 166.1 | 0.00 | 165.3 | 0.00 |
| 166.2 | 0.00 | 166.1 | 0.00 |
| 167.1 | 0.00 | 166.2 | 0.00 |
| 167.2 | 0.00 | 167.1 | 0.00 |
| 168.1 | 0.00 | 167.2 | 0.00 |
| 168.2 | 0.00 | 168.1 | 0.00 |
| 168.3 | 0.00 | 168.2 | 0.00 |
| 169.2 | 0.00 | 168.3 | 0.00 |
| 170.2 | 0.00 | 170.2 | 0.00 |
2.6 Content of 11 metals in all jewelleries
Appendix B: Note on ”Content of undesirable heavy metal in precious metals jewelleries”
In the project ”Survey and health assessment of chemical substances in jewelleries” it is suggested that the purchases of the jewelleries are focused towards the cheaper qualities of metal jewelleries with and without a content of precious metal. The argument of not including the more expensive “precious metals jewelleries” in the survey is that these are subject to control by the Precious Metal Assay and Inspection and are not expected to contain heavy metals at a problematic level.
The present note has the purpose to present the information which the Danish assay office already has regarding the content of undesirable heavy metals (primarily lead and cadmium) in precious metal jewelleries. Thus the note functions as a presentation to the decision whether precious metal jewelleries shall be included or excluded in the project.
What are precious metal jewelleries?
In the project the definition precious metal jewelleries covers genuine gold and silver jewelleries as well as jewelleries of platinum and palladium. According to the law these genuine jewelleries must have a “Fineness mark” telling what the content of precious metal in the good is, calculated in thousandth parts (per thousand), as well as a signature stamp informing about the person/company who has produced or imported the good. Foreign goods may be stamped with a so-called convention stamp which means that no Danish signature stamp is required. Furthermore, small goods of gold (below 1 gram) and silver (below 3 gram) are excluded from the requirements on stamping but they are not excluded from the requirements regarding control.
According to the legislation a genuine gold jewellery must contain at least 333 per thousand gold while a genuine silver jewellery must contain at least 800 per thousand silver. For jewelleries of platinum the content must be at least 850 per thousand platinum while jewelleries of palladium must contain at least 500 per thousand palladium. Goods with lower fineness are not allowed to be marketed as goods of precious metal. Furthermore applies that the fineness of precious metal in all parts must correspond to the stamped.
Besides gold, the gold alloys, for use in jewelleries, consist typically of silver, copper, zinc, nickel and/or palladium while besides silver, the silver alloys consists typically of copper. The alloy metals applied in platinum and palladium can be copper, silver, chromium, cobalt and tungsten. An alloy means a metallic material which does not consist of a only one type of metal but on purpose is added (by melting) one or several elements to reach certain material properties, e.g. more strength or hardness. A coating means a layer of a material upon another material, e.g. gold on a brass ring.
The Danish assay office’s test of precious metal jewelleries
FORCE Technology’s independent certification organ FORCE-Dantest CERT is selected as Precious metal control in Denmark and controls that the provisions of the law are fulfilled. This is done through unannounced visits at the companies which prepare and deal with goods of precious metals. According to the legislation the place of sale is responsible for the content of precious metal in the goods.
On average about 3000 samples are made yearly. The samples are tested whether the content of precious metal is in accordance with the stamped. The tests are primarily for the content of gold, silver, platinum and palladium. Among the tested goods are metals for all kinds of precious metal jewelleries produced in or imported to Denmark.
As the Danish assay office primarily analyzes for the content of precious metals we have as a supplement chosen to carry out a small survey among Danish precious metal wholesalers as well as companies who buy used precious metal for refining. This survey had the purpose to clarify to which extent these companies experience a problem with the content of undesirable heavy metals (primarily lead and cadmium) in their products.
Survey at precious metal companies
The Danish assay office holds a list of companies who act in the Danish precious metal trade. From this list the following 14 companies were contacted by phone:
- Two companies working with regaining of used precious metals.
- Four companies who are suppliers of raw materials and semi-products to the precious metal industry in Denmark.
- Three companies who make their own meltings for production of alloys in precious metals.
- Five companies who deal with goods produced by others, often purchased abroad.
The companies who through melting produced their own alloys could inform that silver alloys consist of Ag and Cu while a gold alloy typically consisted of Au, Ag, Cu, Zn, Ni or Pd plus perhaps Pt, Ir or Rh. Thus they could inform that they did not have a content of undesirable heavy metals in their products. A single company informed that a content of Pb and Cd was undesirable in their production as the material properties became undesirable due to among other things formations of cracks.
Suppliers of raw materials and semi-products informed that their alloys consisted of the same elements as informed above even though there were a few factory secrets of which there was no knowledge in Denmark. One of the raw material suppliers told that from this company, materials for hard-soldering containing Cd were sold but this was legal under the given circumstances. All in all the assessment was that suppliers of raw materials and semi-products for the Danish precious metal industry did not experience a problem with content of undesirable heavy metals in their products.
Companies who purchased used metal, including precious metals, could not inform which elements besides the already mentioned that could appear in material originating from precious metal jewelleries and their production.
Importers of finished goods from abroad did only relate themselves to the content of Ni or more correctly to emission of Ni which the legal requirement deals with. They had no clear knowledge of whether their materials contained other kinds of elements.
Thus the survey targeting the companies indicates that companies who themselves produce goods for the precious metal industry in Denmark do not generally experience problems with the content of undesirable heavy materials in precious metal jewelleries. The remaining companies (importers and companies working with regaining) could not right away comment whether their products contained undesirable heavy metals.
Finally it can be mentioned that in general the companies had not heard about this kind of problems before, but they had heard that there might be problems related to the content of for instance Cd in cheaper jewelleries imported from the East.
Preliminary screening of 94 metal bijouterie jewelleries
At present we have carried out XRF screenings on 94 purchased metal bijouterie jewelleries. This first screening indicate that there is a proportionally large content of Pb in about 1/3 of all analyzed jewellery parts as well as a content of Cd and in some cases even Hg in a small selection of the jewelleries. Thus this preliminary result confirms the hypothesis that it is in the cheaper metal bijouterie the problems with a content of undesirable heavy metals occur.
Conclusion
The survey indicated that the Danish companies in the precious metal trade do not experience a problem with a content of undesirable heavy metals in products related to precious metal jewelleries. However, based on the survey, it cannot be excluded that heavy metals like Pb and Cd can be found in raw materials and finished goods as it is not possible to analyze for these metals in the Danish value chain for precious metal, with exception of a few special cases.
However, our assessment is that possible problems with precious metal jewelleries will be of a substantial smaller scope than the problems with the cheaper jewelleries and therefore we maintain our proposal of not including precious metal jewelleries in the survey. However, FORCE Technology is open for making analyses of selected jewelleries, for instance small pieces of a gold and silver chain as a purchase of such products does not influence the budget unnecessarily and at the same time the same analyses as for the cheaper jewelleries can be made. However, it must be stressed that such a screening will only cover a very small part of the total market of precious metal jewelleries.
Appendix C: DST categorization of jewelleries
Jewelleries on the market in Denmark can statistically be divided into a number of categories. Eurostat and Statistics Denmark use the following codes:
- Jewelleries of precious metals, with a sub-division in seven categories (7113xxxx, 7114xxxx and 7115xxxx). The seven categories, which are not described in details here, include products of gold, silver, platinum, precious stones, semiprecious stones and pearls. These types of jewelleries are in general placed under rules in the professional jewellery industry and the content of metal is controlled by the Danish assay office.
- Bijouterie (custom jewellery), which also can be divided into a number of sub-categories:
- Bijouterie goods (except sleevelinks and collar studs) of base metals which might be coated with precious metals and which contain parts of glass (7117 1910). Text on goods: ”Bijouterie goods of base metals with parts of glass with exception of sleevelinks and collar studs”
- Bijouterie (except sleevelinks and collar studs) of base metals which might be coated with precious metals, without parts of glass (7117 1991). Text on goods:”Bijouterie goods of base metals, without parts of glass, gold-coated, silver-coated or platinum-coated”
- Bijouterie of artificial metals which are not coated with precious metal (7117 1999). Text on goods: ”Bijouterie goods of base metals, without parts of glass, not gold-coated, silver-coated or platinum-coated”
- Bijouterie of other materials than base metals (e.g. leather, plastic, wood, horn etc.) (7117 9000). Text on goods: ”Bijouterie goods, except base metals”
- Sleevelinks and collar studs of base metals, with possible coating of precious metal (7117 1100). Text on goods: ”Bijouterie goods of base metals, also silver-coated, gold-coated or platinum-coated, sleevelinks and collar studs”
Appendix D: Amount of jewelleries on the market in Denmark
| Import and export of jewelleries from all countries (extracts from Statistics Denmark) | 2005 | 2005 | 2005 | ||
| Product codes | Import | Export | Imp - Exp | ||
| 71131100 | Jeweller silver, also gilted or platinum-coated or coated with other precious metals | 0 | 0 | 0 | kg |
| 71131900 | Jeweller precious metals, except silver, also silver-coated, gilted or coated with | 0 | 0 | 0 | kg |
| 71141100 | Silverware, also gilted, platinum-coated or coated with other precious metals | 0 | 0 | 0 | kg |
| 71141900 | Gold/silverware of precious metal, not silver, also silver-coated, gilted, platinum-coated, coated with | 0 | 0 | 0 | kg |
| 71142000 | Gold and silverware including parts of precious metal duble on base metals | 11892 | 3118 | 8774 | kg (?) |
| 71159010 | Precious metal items, except catalysts, jeweller-, gold and silverware including parts | 4051 | 131780 | -127729 | kg |
| 71159090 | Precious metal duble items, except catalysts, jeweller-, gold and silverware including parts | 5969 | 2410 | 3559 | kg |
| 71171100 | Bijouterie products of base metals, also silver-coated, gold-coated or platinum-coated, sleevelinks and studs | 1802 | 106 | 1696 | kg |
| 71171991 | Bijouterie products of base metals, without parts of glass, gilted, silver-coated or platinum-coated | 383520 | 27385 | 356135 | kg |
| 71179000 | Bijouterie products, except base metals | 282229 | 265429 | 16800 | kg |
| 71171910 | Bijouterie products of base metals with parts of glass, except sleevelinks and studs | 137603 | 15143 | 122460 | kg |
| 71171999 | Bijouterie products of base metals, without parts of glass, not gilted, not silver-coated or platinum-coated | 242734 | 53112 | 189622 | kg |
| Total of all jewelleries | 1069800 | 498483 | 571317 | kg | |
| Total - Jewelleries | 21912 | 137308 | -115396 | kg | |
| Total – Bijouterie products with precious metals | 385322 | 27491 | 357831 | kg | |
| Total - Bijouterie products without precious metals | 380337 | 68255 | 312082 | kg | |
| Total - Non-metal bijouterie products | 282229 | 265429 | 16800 | kg | |
| The industry’s sale of jewelleries in Denmark (extracts from Statistics Denmark) | |||
| Product codes | 2005 | ||
| 7113110000 | Jeweller silver, also gilted or platinum-coated or coated with other precious metals | 1235.743 | kg |
| 7113190000 | Jeweller precious metals, except silver, also silver-coated, gilted or coated with | 338.974 | kg |
| 7114110000 | Silverware, also gilted, platinum-coated or coated with other precious metals | 414.18 | kg |
| 7114190000 | Gold/silverware of precious metal, not silver, also silver-coated, gilted, platinum-coated, coated with | 0 | gram |
| 7114200000 | Gold and silverware including parts of precious metal duble on base metals | 0 | not accessible |
| 7115901000 | Precious metal items, except catalysts, jeweller-, gold and silverware including parts | 0 | kg |
| 7115909000 | Precious metal duble items, except catalysts, jeweller-, gold and silverware including parts | 0 | kg |
| 7117110000 | Bijouterie products of base metals, also silver-coated, gold-coated or platinum-coated, sleevelinks and studs | 0 | kg |
| 7117199100 | Bijouterie products of base metals, without parts of glass, gilted, silver-coated or platinum-coated | 0 | kg |
| 7117900000 | Bijouterie products, except base metals | 19439 | kg |
| 7117191000 | Bijouterie products of base metals with parts of glass, except sleevelinks and studs | 0 | kg |
| 7117199900 | Bijouterie products of base metals, without parts of glass, not gilted, not silver-coated or platinum-coated | 0 | kg |
| Total sale of jewelleries | 21427.9 | kg | |
| Total – own jeweller products in Denmark | 1988.897 | kg | |
| Total - own bijouterie products with precious metals | 0 | kg | |
| Total – own non-metal bijouterie | 19439 | kg | |
| Total - own bijouterie products without precious metals | 0 | kg |
| IMPORT | TOTAL | TOTAL | TOTAL | TOTAL | ||
| Jeweller | Bijouterie – precious metal | Bijouterie - non metal | Bijouterie - without precious metal | |||
| 009 | Greece | 0 | 0 | 4 | 0 | kg |
| 010 | Portugal | 0 | 0 | 84 | 0 | kg |
| 011 | Spain | 2 | 97 | 41 | 164 | kg |
| 400 | USA (1992-) | 931 | 1209 | 2142 | 1224 | kg |
| 700 | Indonesia | 79 | 59 | 3344 | 955 | kg |
| 701 | Malaysia | 0 | 0 | 137 | 8 | kg |
| 706 | Singapore | 2 | 3 | 73 | 25 | kg |
| 720 | China | 11761 | 338813 | 143626 | 152516 | kg |
| 724 | North Korea | 0 | 0 | 0 | 0 | kg |
| 728 | South Korea | 545 | 11209 | 2375 | 5944 | kg |
| 732 | Japan | 815 | 16 | 90 | 18 | kg |
| 736 | Taiwan | 0 | 5148 | 4310 | 3180 | kg |
| 740 | Hong Kong | 2289 | 7120 | 20384 | 18725 | kg |
NB: This represents the 13 countries from where the main part of the imported jewelleries comes.
| Import from China in 2005 | |||||
| Product codes | Import | Export | Imp - Exp | ||
| 71131100 | Jeweller silver, also gilted or platinum-coated or coated with other precious metals | 0 | 0 | 0 | kg |
| 71131900 | Jeweller precious metals, except silver, also silver-coated, gilted or coated with | 0 | 0 | 0 | kg |
| 71141100 | Silverware, also gilted, platinum-coated or coated with other precious metals | 0 | 0 | 0 | kg |
| 71141900 | Gold/silverware of precious metal, not silver, also silver-coated, gilted, platinum-coated, coated with | 0 | 0 | 0 | kg |
| 71142000 | Gold and silverware including parts of precious metal duble on base metals | 10122 | 0 | 10122 | kg |
| 71159010 | Precious metal items, except catalysts, jeweller-, gold and silverware including parts | 1325 | 0 | 1325 | kg |
| 71159090 | Precious metal duble items, except catalysts, jeweller-, gold and silverware including parts | 314 | 0 | 314 | kg |
| 71171100 | Bijouterie products of base metals, also silver-coated, gold-coated or platinum-coated, sleevelinks and studs | 414 | 0 | 414 | kg |
| 71171991 | Bijouterie products of base metals, without parts of glass, gilted, silver-coated or platinum-coated | 338399 | 0 | 338399 | kg |
| 71179000 | Bijouterie products, except base metals | 143626 | 6724 | 136902 | kg |
| 71171910 | Bijouterie products of base metals with parts of glass, except sleevelinks and studs | 10524 | 5 | 10519 | kg |
| 71171999 | Bijouterie products of base metals, without parts of glass, not gilted, not silver-coated or platinum-coated | 141992 | 315 | 141677 | kg |
| Total jewelleries | 646716 | 7044 | 639672 | kg | |
| Total - Jewelleries | 11761 | 0 | 11761 | kg | |
| Total - Bijouterie with precious metals | 338813 | 0 | 338813 | kg | |
| Total - Bijouteri without precious metaller | 152516 | 320 | 152196 | kg | |
| Total - Non-metal bijouterie | 143626 | 6724 | 136902 | kg | |
Appendix E: Banned azo-dyes
According to Stat. Ord. 755 of 15.08.2003 import and sale of textile and leather goods which can get into direct contact with the skin or oral cavity for a longer period at humans is banned if they are dyed with the following azo-dyes:
| CAS-number | Index number | EF number | Substance | Banned from | |
| 1 | Not allocated Constituent 1: CAS-n0.: 118685-33-9 C 39 H 23 ClCrN 7 O 12 S.2Na Constituent 2: C 46 H 30 CrN 10 O 20 S 2 .3Na |
611-070-00-2 | 405-665-4 | Mixture of: dinatrium (6-(4-anisidino)-3-sulfonato-2-(3,5-dinitro-2-oxidophenylazo)-1 -naphtholato)(1-(5-chloro-2-oxidophenylazo)-2-naphtholato)chromate(1-); trinatriumbis(6-(4-anisidino)-3-sulfonato-2-(3,5-dinitro-2-oxidophenylazo)-1 -naphtholato)chromate(1-) |
30 June 2004 |
Appendix F: Aromatic amines regulated acc. to Stat. Ord. 755 of 15.08.2003
The table below shows an overview over the aromatic amines which according to Stat. Ord. 755 of 15.08.2003 are banned in concentrations above 30 ppm in finished goods including dyed parts.
| CAS number | Index number | EF number | Substance | |
| 1 | 92-67-1 | 612-072-00-6 | 202-177-1 | biphenyl-4-ylamine 4-aminobiphenyl xenylamine |
| 2 | 92-87-5 | 612-042-00-2 | 202-199-1 | benzidine |
| 3 | 95-69-2 | 202-441-6 | 4-chloro-o-toluidine | |
| 4 | 91-59-8 | 612-022-00-3 | 202-080-4 | 2-naphthylamine |
| 5 | 97-56-3 | 611-006-00-3 | 202-591-2 | o-aminoazotoluene 4-amino-2',3-dimethylazobenzene 4-o-tolylazo-o-toluidine |
| 6 | 99-55-8 | 202-765-8 | 5-nitro-o-toluidine | |
| 7 | 106-47-8 | 612-137-00-9 | 203-401-0 | 4-chloroaniline |
| 8 | 615-05-4 | 210-406-1 | 4-methoxy-m-phenylenediamine | |
| 9 | 101-77-9 | 612-051-00-1 | 202-974-4 | 4,4'-methylenedianiline 4,4'-diaminodiphenylmethane |
| 10 | 91-94-1 | 612-068-00-4 | 202-109-0 | 3,3'-dichlorobenzidine 3,3'-dichlorobiphenyl-4,4'-diamine |
| 11 | 119-90-4 | 612-036-00-X | 204-355-4 | 3,3'-dimethoxybenzidine o-dianisidine |
| 12 | 119-93-7 | 612-041-00-7 | 204-358-0 | 3,3'-dimethylbenzidine 4,4'-bi-o-toluidine |
| 13 | 838-88-0 | 612-085-00-7 | 212-658-8 | 4,4'-methylenedi-o-toluidine |
| 14 | 120-71-8 | 204-419-1 | 6-methoxy-m-toluidine p-cresidine |
|
| 15 | 101-14-4 | 612-078-00-9 | 202-918-9 | 4,4'-methylene-bis-(2-chloro-aniline) 2,2'-dichloro-4,4'-methylene-dianiline |
| 16 | 101-80-4 | 202-977-0 | 4,4'-oxydianiline | |
| 17 | 139-65-1 | 205-370-9 | 4,4'-thiodianiline | |
| 18 | 95-53-4 | 612-091-00-X | 202-429-0 | o-toluidine 2-aminotoluene |
| 19 | 95-80-7 | 612-099-00-3 | 202-453-1 | 4-methyl-m-phenylenediamine |
| 20 | 137-17-7 | 205-282-0 | 2,4,5-trimethylaniline | |
| 21 | 90-04-0 | 612-035-00-4 | 201-963-1 | o-anisidine 2-methoxyaniline |
| 22 | 60-09-3 | 611-008-00-4 | 200-453-6 | 4-amino azobenzene’ |
Appendix G: The requirements of the Toys Statutory Order on maximum release of heavy metals
According to the Toys Statutory Order applies that the bioavailability per day must not exceed the following limits due to the use of toys:
| Maximum permitted release from toys per day |
| 0,2 micro g antimony |
| 0,1 micro g arsenic |
| 25,0 micro g barium |
| 0,6 micro g cadmium |
| 0,3 micro g chromium |
| 0,7 micro g lead |
| 0,5 micro g mercury |
| 5,0 micro g selenium |
or other values which can be specified for these or other substances through community decisions, which are prepared on a scientific basis. Bioavailability means release of soluble substances which have significant toxicological importance.
Appendix H: Samples where double tests varied
In the table below the results from the migrations analyses of the 25 jewellery parts are presented. Values with < ahead indicate samples where migration did not take place in a concentration which exceeded the detection limit. In cases where two values are presented (e.g. 93/540), the two values represent results belonging to the two double tests. The reason why both are presented is that the value varied with more than 50%. Other values represent an average of the two double tests.
| Sample no. | Description | As | Ba | Cd | Cr | Cu | Hg | Ni | Pb | Sb | Se |
| Unit: µg/g | |||||||||||
| 169.1 | Pendant | <2.5 | <1.0 | <0.25 | <0.25 | 0.2 / 11 | <1.3 | 0.5 / 23 | 17 | <2.5 | <3 |
| 164.1 | Heart backside | <2.5 | <1.0 | <0.25 | <0.25 | 2.6 | <1.3 | <0.5 | 2 / 4 | <2.5 | <3 |
| 152.2 | Matt ring | <2.5 | <1.0 | <0.25 | <0.25 | 0.46 | <1.3 | <0.5 | <2.5 | <2.5 | <3 |
| 138.1 | Charm from behind | <2.5 | <1.0 | 2.4 | <0.25 | 0.57 | <1.3 | <0.5 | 150 / 210 | <2.5 | <3 |
| 136.2 | Plate | <2.5 | <1.0 | 1.2 | <0.25 | 0.81 | <1.3 | <0.5 | 100 | <2.5 | <3 |
| 130.1* | Needles | <250 | <100 | <25 | <25 | 25 / 1300 | <130 | <50 | <250 | <250 | <300 |
| 125.1 | Finger ring | <2.5 | <1.0 | 0.86 | <0.25 | 0.73 | <1.3 | 0.5 / 29 | 93 / 540 | <2.5 | <3 |
| 107.1 | Chain with balls | <2.5 | <1.0 | <0.25 | <0.25 | 53 | <1.3 | <0.5 | <2.5 | <2.5 | <3 |
| 101.3 | Cone | <2.5 | <1.0 | <0.25 | <0.25 | 16 / 34 | <1.3 | =0.5 | <2.5 | <2.5 | <3 |
| 101.1 | Backside of heart | <2.5 | <1.0 | <0.25 | <0.25 | 1.5 | <1.3 | <0.5 | 73 | <2.5 | <3 |
| 99.2* | Non-twisted part | <10 | <5.0 | =1 | <1 | 150 / 220 | <6 | <2 | <10 | <10 | <12 |
| 99.1 | Twisted rings | <2.5 | <1.0 | <0.25 | <0.25 | 160 | <1.3 | <0.5 | <2.5 | <2.5 | <3 |
| 95.3 | Catch | <2.5 | <1.0 | <0.25 | <0.25 | 3.3 | <1.3 | <0.5 | 250 | <2.5 | <3 |
| 91.1 | Large ball | <2.5 | <1.0 | <0.25 | <0.25 | 26 / 160 | <1.3 | 90 / 210 | <2.5 | <2.5 | <3 |
| 91.3 | Chain | <2.5 | <1.0 | <0.25 | <0.25 | 16 / 28 | <1.3 | 13 | <2.5 | <2.5 | <3 |
| 88.1 | Ring without stone | <2.5 | <1.0 | 0.6 / 16 | <0.25 | 2.3 | <1.3 | <0.5 | 2 / 5 | <2.5 | <3 |
| 70.3 | Catch | <2.5 | <1.0 | <0.25 | <0.25 | 1.5 | <1.3 | <0.5 | 140 | <2.5 | <3 |
| 70.1 | Charm | <2.5 | <1.0 | 0.31 | <0.25 | 0.50 | <1.3 | <0.5 | <2.5 | <2.5 | <3 |
| 68.1 | Ring | <2.5 | <1.0 | 3.8 | <0.25 | 0.89 | <1.3 | <0.5 | 140 | <2.5 | <3 |
| 62.1 | Charm | <2.5 | <1.0 | 3.0 | <0.25 | 0.67 | <1.3 | <0.5 | 39 | <2.5 | <3 |
| 60.1 | Bracelet | <2.5 | <1.0 | <0.25 | <0.25 | 3.4 | <1.3 | <0.5 | <2.5 | <2.5 | <3 |
| 56.1 | Charm backside | <2.5 | <1.0 | <0.25 | <0.25 | 2.1 | <1.3 | <0.5 | 23 / 64 | <2.5 | <3 |
| 38.1 | Jewellery without stone | <2.5 | <1.0 | 12 | <0.25 | 0.59 | <1.3 | <0.5 | 220 | <2.5 | <3 |
| 26.2 | Catch | <2.5 | <1.0 | <0.25 | <0.25 | 0.44 | <1.3 | <0.5 | <2.5 | <2.5 | <3 |
| 6.1 | Angle | <2.5 | <1.0 | 21 | <0.25 | 0.30 | <1.3 | <0.5 | 6 / 160 | <2.5 | <3 |
Samples with adjusted detection limit due to reduced sample size.
Appendix I: Results from screening of textile necklaces
In the table below the results from the screening of the 62 textile necklaces are presented:
| Textile jewellery no. | Colour in the water (1 = yes; 0 = no) |
Shade of colour | Degree of colouring (1 = weak; 2 = medium; 3 = strong). Visual assessment |
| T-1 | 1 | brown | 3 |
| T-2 | 1 | yellow | 1 |
| T-3 | 0 | - | - |
| T-4 | 1 | yellow | 3 |
| T-5 | 1 | dark green | 3 |
| T-6 | 1 | black | 3 |
| T-7 | 1 | turquoise | 3 |
| T-8 | 0 | - | - |
| T-9 | 1 | yellow | 1 |
| T-10 | 1 | red | 3 |
| T-11 | 1 | turquoise | 3 |
| T-12 | 1 | blue | 3 |
| T-13 | 1 | red | 2 |
| T-14 | 1 | yellow | 1 |
| T-15 | 0 | - | - |
| T-16 | 1 | brown | 1 |
| T-17 | 1 | yellow | 1 |
| T-18 | 1 | yellow | 1 |
| T-19 | 1 | yellow | 1 |
| T-20 | 1 | yellow | 1 |
| T-21 | 1 | purple | 2 |
| T-22 | 1 | yellow | 1 |
| T-23 | 1 | yellow | 2 |
| T-24 | 1 | blue | 2 |
| T-25 | 1 | orange | 3 |
| T-26 | 1 | red | 3 |
| T-27 | 1 | blue | 3 |
| T-28 | 1 | black | 3 |
| T-29 | 0 | - | - |
| T-30 | 0 | - | - |
| T-31 | 1 | red | 1 |
| T-32 | 1 | black | 3 |
| T-33 | 1 | yellow | 2 |
| T-34 | 1 | yellow | 1 |
| T-35 | 1 | black | 2 |
| T-36 | 1 | yellow | 1 |
| T-37 | 1 | blue | 1 |
| T-38 | 1 | turquoise | 3 |
| T-39 | 1 | red | 3 |
| T-40 | 1 | red | 2 |
| T-41 | 1 | yellow | 2 |
| T-42 | 1 | yellow | 2 |
| T-43 | 1 | brown | 3 |
| T-44 | 1 | black | 3 |
| T-45 | 1 | green | 2 |
| T-46 | 1 | blue | 3 |
| T-47 | 1 | blue | 3 |
| T-48 | 1 | brown, blurred | 2 |
| T-49 | 1 | red | 3 |
| T-50 | 0 | - | - |
| T-51 | 1 | red/brown | 2 |
| T-52 | 1 | brown | 3 |
| T-53 | 1 | brown | 3 |
| T-54 | 1 | black | 3 |
| T-55 | 1 | yellow | 2 |
| T-56 | 1 | green | 1 |
| T-57 | 1 | blue | 3 |
| T-58 | 1 | green | 3 |
| T-59 | 1 | red | 3 |
| T-60 | 1 | green | 1 |
| T-61 | 0 | - | - |
| T-62 | 1 | green | 3 |
Appendix J: Results from the migration analysis calculated in relation to areal jewellery
The table only shows results for the samples lying above the detection limit (i.e. where real metal release was measured).
Table: Results of migration analysis (artificial sweat) of Pb, Ni, Cd and Cu from the 25 jewellery parts. The values are calculated as release of metal per areal jewellery part during a period of 4 hours.
| Jewelly part no. | Product category | Description of jewellery part | Cd | Cu | Ni | Pb | ||
| Unit: µg/cm² | ||||||||
| 169.1 | Silver coated | Pendant | 0.2/8.1 | 0.2/17 | 15 | |||
| 164.1 | Silver coated | Heart backside | 3.3 | 3/7 | ||||
| 152.2 | Clearly non-precious metal | Matt ring | 0.14 | |||||
| 138.1 | Silver alloy | Charm from behind | 3.0 | 0.73 | 190/280 | |||
| 136.2 | Silver alloy | Plate | 1.3 | 0.84 | 100 | |||
| 130.1* | Silver-like | Needles | 0.1/100 | |||||
| 125.1 | Silver coated | Finger ring | 0.34 | 0.29 | 0.02/11 | 60/260 | ||
| 107.1 | Gold coated | Chain with balls | 11 | |||||
| 101.3 | Gold coated | Cone | 19/38 | =0.6 | ||||
| 101.1 | Gold coated | Backside of heart | 2.9 | 140 | ||||
| 99.2* | Gold coated | Non-twisted part | =0.4 | 56 | ||||
| 99.1 | Gold coated | Twisted rings | 46 | |||||
| 95.3 | Gold coated | Catch | 2.8 | 190 | ||||
| 91.1 | Clearly non-precious metal | Large ball | 7/45 | 25/56 | ||||
| 91.3 | Clearly non-precious metal | Chain | 8/12 | 6.3 | ||||
| 88.1 | Golden-like | Ring without stone | 0.6/15 | 2.1 | 2/5 | |||
| 70.3 | Gold coated | Catch | 1.0 | 100 | ||||
| 70.1 | Gold coated | Charm | 1.1 | 1.7 | ||||
| 68.1 | Silver coated | Ring | 3.0 | 0.70 | 110 | |||
| 62.1 | Silver-like | Charm | 4.2 | 0.91 | 53 | |||
| 60.1 | Golden-like | Bracelet | 1.2 | |||||
| 56.1 | Silver-like | Charm backside | 5.6 | 6/18 | ||||
| 38.1 | Gold coated | Jewellery without stone | 12 | 5.7 | 210 | |||
| 26.2 | Clearly non-precious metal | Catch | 1.0 | |||||
| 6.1 | Gold coated | Angle | 18 | 0.25 | 5/130 | |||
Appendix K: Selected jewellery parts for migration test
In the two tables below is shown how the selected jewellery parts fulfil the criteria for selection (content of heavy metal, coating, product type as well as type of jewellery part). It shall be emphasized that due to the limited number of purchased jewelleries it was not possible to carry out a 100% equal distribution.
Table: The 10 jewellery parts selected among jewellery parts containing above 75 ppm Cd.
| Jewellery part no. | 88,1 | 70,1 | 99,2 | 6,1 | 99,1 | 68,1 | 62,1 | 91,3 | 26,2 | 107,1 | Distribution |
| 0.01% < Cd < 0.2% | 1 | 1 | 1 | 3 | |||||||
| 0.2% < Cd < 0.3% | 1 | 1 | 2 | ||||||||
| 0.3% < Cd < 0.5% | 1 | 1 | 2 | ||||||||
| 0.5% < Cd < 1.45% | 1 | 1 | |||||||||
| 1.45% < Cd < 4% | 0 | ||||||||||
| 4% < Cd < 30% | 1 | 1 | |||||||||
| Gold | 1 | 1 | 1 | 1 | 1 | 5 | |||||
| Silver | 1 | 1 | 1 | 3 | |||||||
| Non-precious | 1 | 1 | 2 | ||||||||
| Gold coated | 1 | 1 | 1 | 1 | 1 | 5 | |||||
| Silver coated | 1 | 1 | |||||||||
| Silver alloy | 0 | ||||||||||
| Golden-like | - | ||||||||||
| Silver-like | 1 | 1 | 1 | 3 | |||||||
| Non-precious | 1 | 1 | 2 | ||||||||
| Oters | 1 | 1 | |||||||||
| Ankle chain | - | ||||||||||
| Bracelet | 1 | 1 | |||||||||
| Necklace | 1 | 1 | 1 | 1 | 1 | 5 | |||||
| Piercing | 0 | ||||||||||
| Ring | 1 | 1 | 2 | ||||||||
| Earring | 1 | 1 | 2 | ||||||||
| Charm | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 | |||
| Catch | 1 | 1 | 2 | ||||||||
| Chain | 1 | 1 |
NB: A dash indicates that there was no jewellery part of that specific kind among the jewellery parts containing above 75 ppm Cd..
Table: The 15 jewellery parts selected among jewellery parts containing above 100 ppm Pb.
During the selection rings are defined as charms while needles/ear plugs on ear rings are defined as catches. For bracelets applies that the bracelet itself is defined as a charm.
Appendix L: Correlation between migration and content of metal
The tables below shows the correlation between migration and content of metal.
Table: Correlation between content (according to the XRF-screening) and migration of metal. A line indicates that the XRF-screening did not show a content of metal. However, this does not guarantee with 100% certainty that the metal does not exist in a small amount in the jewellery. The reason for this being that the uncertainty associated with the XRF-screening is relatively large when it is attempted to locate small amounts.
| Cd | ||
| Content (%) |
Migration (µg/g) | |
| 88.1 | 4.08 | 0.6/16 |
| 70.1 | 1.45 | 0.31 |
| 6.1 | 0.46 | 21 |
| 38.1 | 0.32 | 12 |
| 68.1 | 0.24 | 3.8 |
| 62.1 | 0.23 | 3 |
| 138.1 | - | 2.4 |
| 136.2 | - | 1.2 |
| 125.1 | - | 0.86 |
| 169.1 | - | - |
| 164.1 | - | - |
| 152.2 | - | - |
| 130.1* | - | - |
| 107.1 | 0.17 | - |
| 101.3 | - | - |
| 101.1 | - | - |
| 99.2* | 0.64 | - |
| 99.1 | 0.49 | - |
| 95.3 | 0.16 | - |
| 91.1 | - | - |
| 91.3 | 0.03 | - |
| 70.3 | - | - |
| 60.1 | - | - |
| 56.1 | - | - |
| 26.2 | 0.08 | - |
NB: * Indicates that the detection limit is reduced due to reduced sample size.
| Ni | ||
| Content (%) |
Migration (µg/g) | |
| 91.3 | 70.14 | 13 |
| 91.1 | 3.38 | 90/210 |
| 169.1 | 52.79 | 0.5/23 |
| 125.1 | - | 0.5/29 |
| 164.1 | 1.4 | - |
| 152.2 | 0.1 | - |
| 138.1 | - | - |
| 136.2 | 0.06 | - |
| 130.1* | 0.19 | - |
| 107.1 | 0.09 | - |
| 101.3 | 8.87 | - |
| 101.1 | 10.91 | - |
| 99.2* | - | - |
| 99.1 | 0.1 | - |
| 95.3 | - | - |
| 88.1 | 0.29 | - |
| 70.3 | 0.11 | - |
| 70.1 | - | - |
| 68.1 | 0.08 | - |
| 62.1 | - | - |
| 60.1 | - | - |
| 56.1 | - | - |
| 38.1 | 0.06 | - |
| 26.2 | 0.07 | - |
| 6.1 | 1.11 | - |
| Cu | ||
| Content (%) |
Migration (µg/g) | |
| 152.2 | 94.55 | 0.46 |
| 88.1 | 93.21 | 2.3 |
| 125.1 | 91.3 | 0.73 |
| 107.1 | 88.59 | 53 |
| 6.1 | 87.6 | 0.30 |
| 101.3 | 82.52 | 16/34 |
| 62.1 | 82.26 | 0.67 |
| 38.1 | 81.4 | 0.59 |
| 101.1 | 81.22 | 1.5 |
| 56.1 | 71.8 | 2.1 |
| 68.1 | 70.49 | 0.89 |
| 26.2 | 65.72 | 0.44 |
| 91.1 | 64.77 | 26/160 |
| 169.1 | 19.56 | 0.2/11 |
| 60.1 | 62.18 | 3.4 |
| 70.1 | 61.37 | 0.50 |
| 70.3 | 48.63 | 1.5 |
| 136.2 | 42.05 | 0.81 |
| 164.1 | 40.17 | 2.6 |
| 99.2* | 37.87 | 150/220 |
| 130.1* | 35.53 | 25/1300 |
| 99.1 | 34.75 | 160 |
| 95.3 | 18.88 | 3.3 |
| 138.1 | 4.55 | 0.57 |
| 91.3 | 3.02 | 16/28 |
| Pb | ||
| Indhold (%) |
Migration (µg/g) | |
| 95.3 | 69.59 | 250 |
| 70.3 | 40.95 | 140 |
| 136.2 | 33.49 | 100 |
| 56.1 | 26.14 | 23/64 |
| 62.1 | 15.62 | 39 |
| 68.1 | 14.35 | 140 |
| 169.1 | 0.36 | 17 |
| 38.1 | 9.21 | 220 |
| 6.1 | 7.64 | 6/160 |
| 101.1 | 2.58 | 73 |
| 138.1 | 1.77 | 150/210 |
| 125.1 | 1.2 | 93/540 |
| 164.1 | 0.11 | 2/4 |
| 88.1 | - | 2/5 |
| 152.2 | 0.16 | - |
| 130.1* | 1.21 | - |
| 107.1 | 3.86 | - |
| 101.3 | 3.65 | - |
| 99.2* | - | - |
| 99.1 | - | - |
| 91.1 | 0.02 | - |
| 91.3 | 0.03 | - |
| 70.1 | 21.42 | - |
| 60.1 | 0.03 | - |
| 26.2 | - | - |
Version 1.0 October 2008, © Danish Environmental Protection Agency