Toxicity testing with the collembolans Folsomia fimetaria and Folsomia candida and the results of a ringtest
2 Biology and ecotoxicology of F. fimetaria and F. candida
- 2.1 Introduction to F. fimetaria and F. candida
- 2.2 Comparison of the two species
- 2.3 Genetic variability
- 2.4 Alternative Collembolan test species
- 2.5 Differences in susceptibility of the two species
- 2.6 Variability in Reproduction Rates
2.1 Introduction to F. fimetaria and F. candida
The use of F. candida and F. fimetaria for ecotoxicological testing purposes has been covered in various publications including: (Riepert and Kula, 1996; Wiles and Krogh, 1998; Fountain and Hopkin, 2005; Scott-Fordsmand and Krogh, 2005; Environment-Canada, 2007). As F. fimetaria is not yet included in internationally approved standards and is less studied than F. candida, it is briefly introduced here. A bibliographic search in Science Citation Index (ISI Web of Knowledge/Web of Science accessed Jan 2008) revealed about 400 papers referring to F. candida and 74 papers referring to F. fimetaria. Of the F. fimetaria papers, about 35 deal with ecotoxicology and some 27 originate from the NERI Soil Fauna laboratory or authors affiliated to this laboratory.
The selection of F. fimetaria for ecotoxicological testing was done by curator, senior researcher, Henning Petersen, Mols Laboratory, Natural History Museum, Aarhus Denmark, in a project supported by the Danish Environmental Protection Agency (DK-EPA) (Petersen and Gjelstrup, 1995), however it was used even earlier in studies to test for DDT effects (Van de Bund, 1965; Scopes and Lichtenstein, 1967). Scopes and Lichtenstein even published a filter paper method on how to use F. fimetaria for general insecticide residue testing (Scopes and Lichtenstein, 1967). Adults of F. fimetaria are 0.8-1.4 mm long (Folker-Hansen et al., 1996), e.g. males 0.9 mm and females 1.3. mm, with a dry weight of 10-40 mg per individual at 20o C. Female F. candida can become 2.0-2.5 mm long (Crouau and Moia, 2006; Widarto et al., 2007), and has a dry weight of 140 mg for adults at the asymptotic maximum size. Adult F. candida males although rarely found are about 1.25 mm long. F. fimetaria reproduces only sexually, and sexual dimorphism is not detectable at low magnification before an age of 20 days after hatching. Males have a more slender body, and they are only half as big as the females (Fig. 2).
Both species are widely distributed (Fig. 1), but maps created particularly from older records cannot be fully trusted due to confusion of the two species (Hopkin, 2008a). F. fimetaria is common in a range of habitats including agricultural soil, and its preference for high organic matter hot spots seems similar to F. candida (Fjellberg, 1980). It occurs less frequently in meadows and in the soils of urban settlements (Chernova et al., 2003). The easiest way to get F. fimetaria is to collect soil samples from agricultural fields, meadows or grassland and make a heat/dry extraction of the soil. In buried lumps of organic hotspots like manure or sludge F. fimetaria can be found in huge numbers (Krogh et al., 1997), and the collection of the lumps is a good source for starting a F. fimetaria culture.
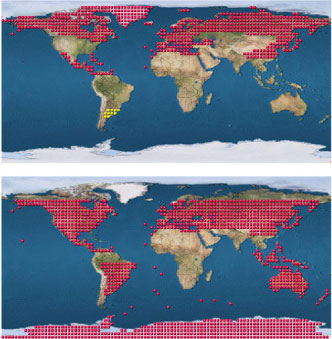
Fig. 1. Biogeographically distribution of F. fimetaria, upper, and F. candida, lower, (Bellinger et al., 1996-2008)[1]. The dotted areas indicate that the species have been found in the corresponding biogeographically region.
F. candida is a cosmopolitan species found almost all over the globe (Fig. 1) and is considered a tramp species (Hopkin, 1997). However, only few outdoor records exists for F. candida who prefers high organic matter like in compost, green-houses, flower pots or manure (Fjellberg, 1980; Chernova et al., 2003; Fjellberg, 2007a), hence the records used to generate the maps of Fig. 1 refers mainly to these domestic habitats. In line with this it is rare in Australian soils (Greenslade and Vaughan, 2003). However, it should be noted that the lack of presence of a standard test species in certain parts of the world may not at all invalidate its general use; it may well have a similar response as other collembolans under the simplified artificial conditions offered in a standard test (see section 2.5).
Discrimination of F. candida and F. fimetaria from species of the same genus is not problematic with the unique position of manubrial setae and other characteristics (Fjellberg, 1980; Potapov, 2000; Potapov and Babenko, 2000; Fjellberg, 2007b).
However, when establishing cultures from field populations, care should be taken to avoid confusion between white and eyeless relatives from the F. fimetaria group such as Folsomia lawrencei, Folsomia kerni and Folsomia litsteri. Using recent keys, e.g. Fjellberg (2007b), should prevent such mistakes. Small F. litsteri was considered to be juvenile F. candida and bigger F. litsteri to be F. lawrencei (Josef Rusek pers. comm.), but later Steve Hopkin considered F. litsteri to be a true species (Hopkin, 2008b) and this is maintained by Fjellberg (2007b).

Fig. 2. Adult F. fimetaria female and male, left, and F. candida female and male, right.
2.2 Comparison of the two species
While the size difference is very obvious for the two species behavioural differences have also been observed, but have rarely been explored scientifically (Chernova et al., 2003). The collembolan family, Sminthuridae, has long been known to display relatively complex mating behaviour (Schaller, 1952), and similarly, the podurids have a sperm transfer requiring male-female interactions as otherwise believed to be non-interactive for the arthropleone collembolans (Schliwa and Schaller, 1963). Although not yet reported for Folsomia the observations by Goloschapova et al. (2006) indicate that isotomids may have more complex mating behaviour than usually assumed.
When being disturbed F. fimetaria will respond by bending down the head and retracting the antenna downwards and inwards to the head, in contrast F. candida will start scattering and jumping. Only few studies have made direct comparisons between the basic biological properties of these two species, however aspects such as fecundity and preference responses to a range of fungi have been demonstrated to be significantly different (Larsen et al., 2008).
At 20°C, the average duration of the five juvenile instars are 3 days for F. candida (Snider, 1973) and maximum 4 days for F. fimetaria (Jensen et al., 2001). Sexual maturity is attained in the 6th instar occurring around age 15-16 days for F. candida and a few days later for F. fimetaria[2] (Snider, 1973; Holmstrup and Krogh, 1996; Widarto et al., 2007).
It is generally assumed that sexually reproducing collembolans need fertilisation for every reproductive instar (Hopkin, 1997). To substantiate this hypothesis specifically reported for only a few non-isotomid species, 24 couples of 25-28 days old, 8th instar, F. fimetaria males and females, and 24 single females were isolated and the oviposition pattern of reproduction was followed for 3 weeks at 20o C (Krogh, 2006). None of the single females produced any eggs and the couples produced averages of 10 and 30 eggs in instars 8 and 10, respectively, with a maximum clutch size of 60 eggs. The same figures for F. candida were 48 and 71 eggs with a maximum clutch of eggs of 114 (Snider, 1973). Egg development for F. fimetaria took 9.5 days, hence similar to 9-11 days observed for F. candida (Snider, 1973). The time between the 8th and 10th reproductive instars were 7 days, with 9 days between the 10th to 12th instars; 1-2 days shorter then the same instars for F. candida. The infertility of isolated females stresses that even if females are coming from a mixed male-female population, as is the case for the reproductive test, this does not enable a female to produce fertile eggs, so the uptake of spermatophores is crucial just at oviposition time shortly after shedding the cuticle.
One of the most interesting differences between the two collembolans is the intracellularly presence of Wolbachia bacteria in F. candida and the absence of it in F. fimetaria[3]. F. candida has always been reported to reproduce parthenogenetically in laboratory cultures and the presence of males in laboratory cultures has never been reported in the literature, since early studies by Goto (1960), Milne (1960), Marshall & Kevan (1962) and Green (1964). Presence of intracellular bacteria in F. candida ovaries has been known since the study by Palévody (1972), and Vandekerckhove et al. (1999) demonstrated the presence of Wolbachia in F. candida ovary cells, fat bodies and institial cells. However, the exact mechanism by which Wolbachia operates in F. candida has not yet been resolved and neither is it yet established if Wolbachia indeed is the reason for parthenogenesis in F. candida (Riparbelli et al., 2006), although it seems plausible (Koivisto and Braig, 2003). When males and females have been found in field populations the population are supposed to reproduce sexually, however as sex rarely has been determined in specimens from field samples, it has never been realized whether naturally occurring F. candida populations reproduce sexually or could have a very low rate of male production. Elin Jørgensen, environmental technician at NERI, discovered few F. candida males in our laboratory cultures in 1993. At that time it was not clear if these males actively took part in sexual reproduction and if a sexually reproducing F. candida population could emerge with these males. A second question arising from the presence of Wolbachia in F. candida was whether the rate of males would change during the life-time of female F. candida. We now know that the males, when reared with females in 10:10 proportion, do not seem to enable establishment of a sexual population with a normal ratio of males and females. Our observations indicate that only about 1 male is produced per 10,000 female offspring during the 8th and 10th reproductive instars, however for older F. candida females, it increases to one for every thousand juveniles.
2.3 Genetic variability
When investigating genetic differences, low variability was found in laboratory populations of F. candida compared to F. fimetaria (Simonsen and Christensen, 2001). Low genetic variability is considered a benefit for a standard test species because it may decrease variability of survival and reproduction between individuals as well as response to toxicants. The variability between clones has been demonstrated to convey minor differences in responses to chemicals and for some chemicals, no differences in sensitivity could be detected at all (Crommentuijn et al., 1995; Chenon et al., 2000). Genetic variability of F. fimetaria has not yet been investigated. To ensure that the species used for testing is well characterised, species cultures would have to be delivered by laboratories with a quality assurance system, such as GLP, who can certify the genetic strain and clone variability.
2.4 Alternative Collembolan test species
Several authors have suggested alternative collembolan species to be used for testing standards because F. candida has limited ecological relevance due to its absence from many natural or agricultural habitats. This has led to suggestions of such species as Paronychiurus kimi (Son et al., 2007), Sinella communis and Proisotoma minuta (Greenslade and Vaughan, 2003) as appropriate test species. Other collembolan species could be selected for testing such as e.g. Isotoma viridis (Wiles and Krogh, 1998), Isotoma anglicana, Orchesella cincta, Sinella curviseta, Orthonychiurus folsomi (Environment-Canada, 2007), and Mesaphorura macrochaeta. The result of a bibliographic search of papers referring to single collembolan species is presented in Annex 3 to give an indication of the present level of scientific knowledge. A number of prerequisites must be fulfilled in advance before using alternative species:
- an unequivocal identification
- a sound rationale for the selection of the species
- ensuring that the reproductive biology is included in the testing phase so it will be a potential target during the exposure
- life-history must be known: age at maturation, duration of egg development and instars subject to exposure
- optimal growth and reproduction conditions are provided with the test substrate and food supply
- variability is sufficiently low for precise and accurate toxicity estimation.
The choice of F. fimetaria as a test species was supported in an evaluation based on practical arguments, acceptability of tests and ecological significance (Van Gestel, 1998).
2.5 Differences in susceptibility of the two species
While Krogh (1995) reported no crucial differences between F. fimetaria and F. candida, Diao et al. (2007) found a difference which proved to be significant for mortality. Pedersen et al. (2000) found that male F. fimetaria differed from females in their copper body burden but reported no statistically significant differences between the growth and reproduction endpoints for the two species.
2.6 Variability in Reproduction Rates
Variability of F. candida reproduction is obvious from different scientific publications. Van Amelsvoort and Usher (1989) observed probably the lowest reproduction rate of F. candida fed Baker’s yeast, with the population already declining after the first clutch appeared; this was in remarkable contrast to the classical findings by Snider (1973)[4] where F. candida produced eggs throughout its lifetime. According to her findings, 10 F. candida females would on the average produce 628 juveniles on plaster-charcoal during the first two reproductive instars, instar 6 and 8; this is probably possible in soil as well. However, if the eggs of the third clutch produced by instar 10, hatched before the 4 week test duration of the F. candida test, a mean of 1342 juveniles would be produced per replicate. This would require that the duration of instars and egg development are faster than the average. For F. fimetaria, which would produce 400 juveniles during the 3 week test suggested here (section 2.2), the variability may be due to similar changes in timing and instar duration. Attempts to clarify the sources of variability was done by Axelsen et al. (1998) in a modelling exercise. They found that a precise sexual differentiation, when individuals for testing are selected from a synchronous culture of F. fimetaria, was important for variability. Crouau and Cazes (2003) demonstrated that the individual age and test duration was important for F. candida testing when performed according to ISO 11267 (ISO, 1999).
[1] Maps reproduced with permission from the authors
[2] Life history data on F. fimetaria are not yet precise enough to give accurate figures.
[3] We have made an analysis of Wolbachia in F. candida and F. fimetaria (Krogh et al in prep.)
[4] This observation led to the conclusion that yeast would affect the life history tactics by F. candida.
Version 1.0 December 2008, © Danish Environmental Protection Agency