Development of an analysis method for quantification of colophonium components in cosmetic products
Appendix B: A method for quantification of resin acids in cosmetics
Chemicals
Cis-5,8,11,14,17-eicosapentaenoic acid (EPA, CAS no. 10417-94-4, >98.5%), and abietic acid (CAS no. 514-10-3, technical quality, 75%) were both purchased from Fluka.
Pimaric acid (CAS no. 510-39-4) and dehydroabietic acid (CAS no. 1740-19-8) were both obtained from Helix Biotech and 7-oxo-dehydroabietic (CAS no. 18684-55-4) was synthesized according to Ayer, and Migaj, (1989).
Standard solutions for HPLC were made in acetonitrile:MilliQ water 9:1 with 0.2% formic acid. They were stored in refrigerator and were stable for at least 2 weeks.
Colophonium samples were dissolved in 100% acetonitrile to approximately 1.5 mg/ml.
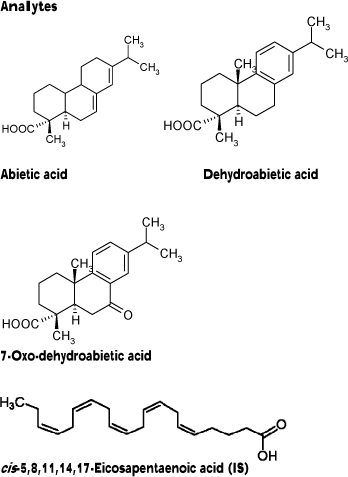
Samples
The method has been tested on Wax strips, foundation, rouge and mascara
Procedure
Extraction:
1 to 2 g samples (1 g for wax strips and mascara) were ultrasonicated in 20-100 ml (depending on sample type) of acetonitrile during 30 min. The wax strips were cut into pieces. The papers were separated to expose the wax to solvent. An aliquot of 500 ?l EPA, from a 928 micro?gram/ml solution, was added as internal standard (IS) prior to extraction.
For all cosmetic samples except wax strips: Each extract was transferred to plastic tubes and centrifuged during 15 min at 4000 rpm. The supernatant was transferred to a flask, while the pellet was redissolved in acetonitrile, ultrasonicated and centrifuged using the same procedure. The supernatants were pooled in the flask and evaporated in a rotavapor to a volume of approximately 5 ml. MilliQ water was then added to a final compostion of 7:3 acetonitrile:water and a total volume of 10 ml. Addition of water made the solution opaque. It was therefore filtrated through a syringe filter (0.45 micro?meter polypropylene membrane).
Note: Check that the syringe filter is resistant to acetonitrile.
For wax strips: After ultrasonic extraction, the sample was evaporated, using rotavapor, to about 5 ml. MilliQ water was added to a final composition of acetonitrile:water 7:3 and a total volume of 10 ml. The sample was transferred to plastic tubes. The flask was washed with a small volume of acetonitrile, which was also transferred to the tubes. The sample was then centrifuged at 4000 rpm during 15 min. The supernatant was loaded directly on the conditioned SPE, without syringe filtration.
Solid phase extraction (SPE):
MAX columns (6cc, 500mg) from Waters were used. Conditioning was performed by percolating 1) 3 ml of methanol and 2) 3 ml of MilliQ water through the SPE column. The sample was loaded and the column was then washed with 1) 3 ml of 50 mM NaOAc in MilliQ water, 2) 4 ml of methanol and finally 1 ml of 2% formic acid in methanol. The sample was then eluted with 2 ml of 2% formic acid in methanol. An additional volume of 1 ml 2% formic acid in methanol was gathered in case of breakthrough. 3 fractions were thus kept: 1) the last wash fraction (1ml), 2) the elution fraction (2 ml) and 3) an additional fraction (1ml). Only the elution fraction is to be analyzed.
Note 1: The composition of acetonitrile:water 7:3 is important for at good recovery from the SPE clean up.
Note 2: The formic acid solution has to be freshly made (daily). It is critical for good recoveries to collect exact volumes of the elution fractions.
For Colophonium samples: Internal standard, 200 ?microliter (186 ?microgram) was added to 2 ml of 1.5 mg/ml colophonium solutions. The colophonium samples were analyzed directly by HPLC after syringe filtration (without SPE clean-up).
HPLC:
HPLC system equipped with an injection loop of 20 ?l, a diode array detector (DAD) and a column heater 25ºC. The HPLC column was a RP-12 (4.6mm i.d., length 150mm, particle size 3 micrometer). The stationary phase contains both C12-chains and urea functions. Methanol:water 8:2 with 0.05% formic acid was used as mobile phase at a flow rate of 1 ml/min (yielding a pressure of approx 240 bar). An isocratic elution was performed. Four different wavelengths were used 210, 220, 240 and 250 nm. The different absorption ratios were used for identification of the compounds.
Note: Isocratic elution has to be performed to avoid increasing baseline, which would lead to higher LODs. The amount of formic acid is critical for both retention and noise level.
Chromatograms of a standard solution (200 ng/?l) at the different wavelengths are shown on next page.
The different absorption ratios used for identification (CV below 10%):
| 7-OxoDeA: | 220nm/210nm ratio 0.53 240nm/210nm ratio 0.26 250nm/210nm ratio 0.45 |
| DeA: | 220nm/210nm ratio 0.55 |
| EPA: | 220nm/210nm 0.40 |
| AbA | 240nm/250nm 1.36 |
It is recommended to control the specific absorption ratios on pure standards as these can vary depending on the specific detector.
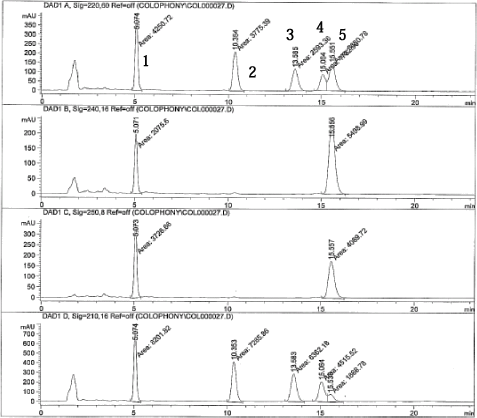
Peak 1 = 7-oxo-dehydroabietic acid
Peak 2 = Dehydroabietic acid
Peak 3 = EPA, internal standard
Peak 4 = Pimaric acid
Peak 5 = Abietic acid
The method was developed by Ulrika Nilsson and Nagmeh Berglund, Dept. of Analytical Chemistry, Stockholm University, Sweden
Reference
Ayer, W. A. and Migaj, B. S. Acids from blue-stain diseased lodgepole pine. Can J Bot. 1989: 67: 1426-1428
Version 1.0 Marts 2009, © Danish Environmental Protection Agency