Inclusion of HBCDD, DEHP, BBP, DBP and additive use of TBBPA in annex IV of the Commission's recast proposal of the RoHS Directive
1 Introduction
- 1.1 Supply chains for substances in EEE
- 1.2 Socioeconomic impact elements
- 1.3 RoHS vs. authorisation or restriction under REACH
In this report, the main socio-economic impacts of inclusion of the substances in question under the RoHS Directive are described separately for each substance. The description is mainly qualitative or semi-quantitative.
For each substance the description commences with a characterisation of the substance, its possible application in EEE and an estimate of the total quantities used. Thereafter follows a description of the availability of alternative substances and materials. Further, the socio-economic impacts are described with focus on cost of substitution, potential impacts on the waste management system and administrative costs. Finally the potential effects on human health and the environment of the substance and its alternatives are summarised based on existing reviews.
The supply chain and the socioeconomic impact elements are quite similar for all five substances and are described for all substances in common in the following sections.
1.1 Supply chains for substances in EEE
The generalised supply chain of the substances used in EEE is shown in Figure 1.1. The substances are used in different plastics and the actual manufacturers, formulators and converters may be different for the different substances. The manufacturers and formulators (and to some degree plastic converters) are more or less the same for the three phthalates DEHP, DBP and BBP and these actors are different from the manufacturers and formulators (and to some degree plastic converters) involved in the use of the flame retardants HBCDD and TBBPA.
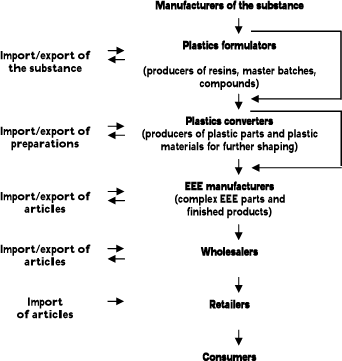
Figure 1.1
Supply chain of the substances in electrical and electronic equipment
1.2 Socioeconomic impact elements
An overview of the types of socioeconomic impacts it could have to include the substances under the RoHS Directive is given in Table 1.1. The table includes the main direct impact on each type of stakeholders including distributional effects. A distributional effect is for example that manufacturers of alternatives have benefits whereas the manufacturers of the substance have decreased sale. The socioeconomic net costs in this example concern the increased costs of the manufactured alternative if e.g. the resource consumption for manufacturing the alternative is higher than the consumption for manufacturing the substance.
Within the limits of this study, only selected impacts have been assessed further. Focus is on the estimation of the main additional socioeconomic costs to the EU, whereas the distributional effects are only described briefly.
All substitution costs are expected to ultimately be furthered to the end customers in the form of increased prices of the EEE. The substitution costs are estimated at manufacturing level, i.e. the increased costs of manufacturing the EEE parts containing the substances. The costs elements consist mainly of costs of raw materials, research and development (R&D) and investment in new tools and techniques.
In order to set the estimated costs in perspective it may be relevant to look up some of the estimates in the Commission’s Impact Assessment for the recast of the RoHS Directive. According to the Impact Assessment yearly administrative costs (in particular verification of compliance) make up approximately 67% of total costs, while the share of technical costs amounts to 33% (expected to drop to 12% in the future). The most important administrative cost is compliance verification, which is an ongoing expense. There are few data and many uncertainties about actual cost impact of the RoHS Directive, but the Commission estimated the total costs to be in the range of 165 to 23,000 million €/year, corresponding to 0.042 to 5% of the total turnover of EU companies affected by RoHS. Total turnover in EU companies are approximately 400.000 million €/year.
Administrative compliance costs of implementing RoHS are in the current study addressed qualitatively and semi-quantitatively. Most manufacturers and importers of components and EEE comprising the substances are expected to have established procedures and the necessary capacity for RoHS compliance documentation. The main extra costs are estimated to be related to compliance control; both by the manufacturers (compliance control of components from sub-contractors), importers and the authorities.
Estimation of benefits of reduced health and environment impacts by substituting the substances is still very immature and incomplete, and a quantitative assessment of these benefits has been beyond the limits of this study.
The first step in the assessment of possible benefits of reduced health and environment impacts is a comparison of the inherent properties of the substances in order to evaluate whether alternatives can be expected to be more environment-friendly and ensure at least the same level of protection for consumers. For the comparison between the substances and alternatives, data on key effects have been summarised on the basis of existing reviews. The key effects considered are carcinogenicity, mutagenicity and toxicity to reproduction (CMR properties), as well as persistence, bioaccumulation, and toxicity (PBT properties).
Basically, the assessment compares:
- the benefits to human health and the environment of substitution expressed in terms of differences in key environmental and health effects of the alternatives compared to the effects of the substances
with:
- The net costs to the society expressed in terms of increased costs at the production level and increased costs of RoHS compliance.
Table 1.1
Main Impact elements of including each of the substances under the RoHS Directive
| Stakeholders | Impact elements | Cost elements | Benefit elements |
|---|---|---|---|
| Manufacturers of the substance | Impact on producers of the substance | Decreased sale of the substance | |
| Impact on producers of alternatives | Costs of increasing the capacity for producing the alternatives | Income from increased sale of alternatives | |
| Polymer converters (including formulators and some EEE manufacturers) | Impacts on polymer converters | One-time costs of adjusting polymer formulation and adapting/changing the process line | |
| Increased costs of polymers, flame retardants or plasticisers | |||
| Impacts on working environment | Reduced costs of health effects from exposure to the substance and associated risk reduction efforts | ||
| EEE manufacturers | Impacts on EEE manufacturers | Increased costs of flame retarded plastic parts | |
| Administrative compliance costs of implementing RoHS | |||
| Impacts on working environment | Reduced costs of health effects from exposure to the substance and associated risk reduction efforts | ||
| Consumers | Health impacts from exposure to the substance | Reduced costs of health effects from exposure to the substance | |
| Impacts on the price of EEE | Increased costs of EEE | ||
| Society | Impacts on public environmental enforcement | Costs for additional chemical analyses for compliance control | |
| Impacts on the environment | Reduced costs of environmental and health effects from exposure to the substance |
1.3 RoHS vs. authorisation or restriction under REACH
HBCDD, DEHP, DBP and BBP are (November 2009) included in the draft list of substances recommended by ECHA for inclusion in the list of substances subject to authorisation in Annex XIV of REACH.
Authorisation for the placing on the market and use should be granted only if the risks arising from their use are adequately controlled, where this is possible, or the use can be justified for socio-economic reasons and no suitable alternatives are available, which are economically and technically viable. In case the Commission assess that the use of the substances in EEE do not meet these criteria authorisation should not be granted. The authorisation procedures only concern placing on the market or use of substances and do not address the import of articles containing the substances. In the case authorization is not granted for the application of the substances in EEE, European manufacturers will not be allowed to use the substances for manufacturing of EEE, whereas imported articles will not be affected.
If ECHA considers that the risk from the substances in articles (e.g. EEE) is not adequately controlled, the Agency shall prepare a dossier in relation to introduction of further restrictions and inclusion of the substances in Annex XVII of REACH. The restrictions specified in the Annex XVII concern placing on the market, manufacturing and uses of the substance on its own, in a mixture or in an article. The restriction would consequently also apply to imported EEE. By the restriction procedure a consumer safety can be achieved similar to the safety that can be achieved by inclusion of the substances in the list of prohibited substances in the RoHS Directive. The time perspective for a possible restriction in REACH of the use of the substances in EEE is unknown as no experience exist, but the procedure would probably take significantly more time than inclusion of the substances in Annex IV of the recast RoHS Directive.
The administrative costs for compliance control for manufacturers, importers and authorities would probably be the same if the substances are restricted via the restriction procedure under REACH or they are included in the list of prohibited substances in the RoHS Directive. If the use of the substances is restricted using the authorization procedure under REACH the total administrative costs for compliance control may be lower as the compliance control addresses the companies marketing or using the substances and not the final articles.
Version 1.0 March 2010, © Danish Environmental Protection Agency