Survey and health assessment of mercury in compact fluorescent lamps and straight fluorescent lamps
3 Release of mercury from broken lamps
- 3.1 Mercury compounds and quantities
- 3.2 Release from broken lamps
- 3.3 Impact from flooring and evaporation of mercury after accidents
- 3.4 Description of accident
- 3.5 Release of mercury to the outer environment
- 3.6 Risk of breakage
- 3.7 Discussion and summary
This chapter describes information found in various literature regarding release of mercury when compact/straight fluorescent lamps break.
Quantities of mercury released when a lamp breaks depend on the quantity of mercury in the lamp, the form of the mercury (i.e. the mercury compound, unbound/bound to the material in the lamp and casing in some cases) and other factors such as room temperature (Aucott et al., 2003). The form of mercury also depends on age and use pattern of the lamp (Aucott et al., 2003). These factors are described in more detail below.
3.1 Mercury compounds and quantities
Aucott et al. (2003) states based on information from industry that a compact fluorescent lamp generally contains less than 0.02 mg of vapour-phase mercury at room temperature (temperature of lamp when not in use). In addition, around 0.1 mg of mercury is bound as solid mercury compounds such as HgO.
At an operating temperature of around 40° C the share of vapour-phase mercury increases, but according to industry quantities do not exceed 0.05 mg. According to another report industry states that only 0.5 % of mercury is in vapour-phase when the lamp is on and 0.3 % is in vapour-phase when the lamp is off. This corresponds to 0.025 mg and 0.015 mg of mercury respectively for a lamp with a total content of 5 mg of mercury (NEMA, 2000).
Jang et al. (2005) states that for new straight fluorescent lamps around 0.17 % of metallic mercury is in vapour-phase while for used straight fluorescent lamps this amounts to 0.04 %. This corresponds to 0.009 mg and 0.002 mg of mercury respectively for a straight fluorescent lamp with a total content of 5 mg of mercury.
One manufacturer states that mercury is only in vapour-phase when the lamp is on. When off, it is bound to, for instance, the phosphor coating on the inner side of the glass (Manufacturer C). This, however, is not in line with information from industry stated above from Aucott et al. (2003). Another manufacturer states that mercury vapour is not released from the added amalgam at room temperature (Manufacturer G). Manufacturer D states that when the lamp is on the major part of mercury is in metallic form regardless of the compound added.
According to Truesdale et al. (1992) mercury is probably also found in metal form in used compact/straight fluorescent lamps, since the inactive atmosphere in the lamp should prevent significant oxidation of the mercury. Due to repeated evaporation and condensation during use it seems probable that the mercury is more evenly distributed at the end of the lifetime of the lamp than at the start (Truesdale et al., 1992; Aucott et al., 2003). This more evenly distributed mercury is expected to have a larger evaporation due to larger surface (Aucott et al., 2003). Along with the ageing of the lamp during use an increasing amount of the metallic mercury is converted into mercury compound (mainly HgO) and is mainly bound to the phosphor coating on the inner side. Thereby, it is no longer accessible for release as vapour (Manufacturer D; Truesdale, 1992; Aucott et al., 2003; NEMA, 2000; Raposo et al., 2003; Snijkers-Hendrickx, 2007). According to NEMA (2000) an average of 1.5 mg of mercury was bound to the glass per used lamp in 1994 – at that time the average content of mercury per lamp was at 22.8 mg. In other words, 6.5 % of the total quantity of mercury was bound to the glass.
Bound mercury can be released again if the glass/phosphor coating is heated to a minimum of 400° C in a given period of time (Manufacturer D; Raposo et al., 2003). This factor is exploited, for instance, in recycling operations. Experience from recycling has shown that most mercury in used straight fluorescent lamps can be removed from the lamps together with the phosphor coating (Truesdale et al., 1992). It is also possible that some of the mercury forms amalgam with the electrode material (Truesdale, 1992; Jang et al., 2005).
3.2 Release from broken lamps
In an USEPA report an estimate of mercury emissions to the air from breakage of used lamps is given at around 6.8 % of total mercury contents. This estimate is based, among others, on 12 measurements of mercury concentrations in the phosphor coating on the inner glass surface. Concentrations were between 0.0868 % and 1.02 %. USEPA assumes in its calculations that 100 % of metallic mercury is in vapour-phase and that quantities of mercury in vapour-phase correspond to 0.2 % of total quantities of mercury at the end of the lifetime of the lamp. There is no statement of the period of time over which these 6.8 % of total mercury content is expected to evaporate, but the project deals with emissions of mercury in waste treatment of compact/straight fluorescent lamps (USEPA, 1998).
As described above there are large variations in quantities of mercury in vapour-phase in a lamp (between 0.04 and 0.5 % or 0.002 – 0.05 mg Hg). Basically, this is expected to depend on the temperature (whether the lamp is on or off) and on the inner volume of the lamp. Saturated mercury vapour has a concentration of 20 mg Hg/m³ at 25 °C. This may result in an amount of around 0.001 mg Hg in a ball with a radius of 5 cm. Naturally, the concentration of mercury vapour will increase along with the temperature.
Chandrasekhar (2007) has made a calculation model for concentrations of mercury in a room when mercury is released from a compact fluorescent lamp that breaks. The calculation model takes account of the background concentration of mercury in the surrounding air and of air exchange/ventilation in the room. Model calculations show that with open windows and extra ventilation from a fan it is possible to reach levels below recommended limit values in less than 20 minutes after a lamp breaks.
A fan is used in the model, partly to allow for the assumption that the mercury concentration is evenly distributed in the entire room (otherwise the heavy mercury vapours will concentrate along the floor), and partly to ensure faster ventilation. The calculation assumed that all mercury in a lamp evaporates momentarily at the time t=0, i.e. the initial concentration in a room of 32.6 m³ will be 0.150 mg Hg/m³, if it is assumed that the broken lamp contains 5 mg mercury.
These concentrations are far above the recommended limit values and higher than concentrations measured by Aucott et al. (2003) in practical tests – as described below. But it is well in line with the fact that up to 0.140 mg/m³ were measured in the air in connection with mercury spills in a home (Baughman, 2006).
Aucott et al. (2003) has made measurements of rates of release of mercury vapour from used straight fluorescent lamps. Two straight fluorescent lamps with an assumed average content of 4.55 mg of mercury[4] were broken in a plastic drum with a volume of 0.121 m³. Following concentrations were measured at different temperatures:
- 0.651 mg/m³ at 5° C after two minutes
- 1.152 mg/m³ at 15° C after one minute
- 1.440 mg/m³ at 30° C after one minute[5].
If it is assumed that the concentration is the same in the entire drum this means that around 0.9 %, 1.5 % and 1.9 % of the mercury content evaporates during the first one to two minutes. It is also stated that between 4 and 7 % (depending on temperature) of the total mercury content is released during the first minutes. Aucott et al. (2003) further states that between 17 and 40 % (depending on temperature) of the mercury content will be released during a period of two weeks and that one third of this is released during the first 8 hours after breakage of the fluorescent lamp.
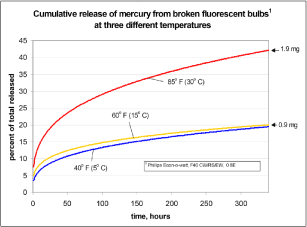
Figure 3-1 Cumulative amount of released mercury from one broken fluorescent tube Aucott et al, 2003).
Figure 3-1 shows a graph of cumulative release of mercury at tests with one broken fluorescent lamp. In these tests only metallic mercury was measured and therefore it is not known whether mercury is also released in other forms. Aucott et al. (2003) describes a formula for the rate of release of mercury per time unit, but this formula (as illustrated with the graph above) only applies to the average content of mercury of 4.55 mg of mercury per straight fluorescent lamp.
Stahler et al. (2008) has also made measurements of release of mercury from compact fluorescent lamps in a room of around 12.7 m² and with a floor-to-ceiling height of around 3 m², corresponding to a volume of around 39 m³. The tests were made with different lamps, flooring, ventilation scenarios and cleaning scenarios. Many different tests were made, but one lamp was broken at a time after which a new test (new lamp with new flooring material etc.) was analysed. The concentration of mercury was measured at a height of around 30 cm (corresponding to the inhalation height of a child) and at a height of around 1.5 m (corresponding to the inhalation height of an adult). To simulate worst case new compact fluorescent lamps were broken with a hammer, and measurements showed a tendency to higher concentrations at 30 cm height than at 1.5 m height in tests without vacuuming. This reflects that mercury vapours are very heavy and concentrate near the floor. The tests showed that when one compact fluorescent lamp broke mercury concentration in the air of the room often exceeded 0.0003 mg Hg/m³ for a period of time (corresponding to the USEPA reference long-term concentration without hazardous effects (RfC)). Short fluctuations with concentrations above 0.05 mg/m³ (upper measurable limit) were also registered. In comparison, Danish occupational threshold limit values for mercury vapours are at 0.025 mg Hg/m³ during a workday (AT, 2007).
It was seen, however, that a short period of ventilation of the room (open window) in most cases reduced the mercury concentration significantly, both in 30 cm’s height and in 1.5 m’s height. For all tests (a total of six) the concentration in 30 cm’s height decreased to below 0.0003 mg Hg/m³ within 9½ minutes after breakage of the lamp. Concentrations increased again, however, when the room was no longer ventilated, especially for some types of lamps as well as during and after vacuuming. Measurements showed that there are large differences between different types of compact fluorescent lamps, and between the period of time before mercury concentrations decrease to below 0.0003 mg/m³.
One test was also conducted with a cracked lamp instead of a broken lamp as well as one test where lamps were warm further to use. The results from these tests were similar to results from previous studies (Stahler et al., 2008).
The main conclusion of the Stahler et al. (2008) study was that the release of mercury vapour is much more variable for scenarios with compact fluorescent lamps from different manufacturers than between different accident and cleaning scenarios with compact fluorescent lamps from the same manufacturer. In other words, release of mercury depends more on type of lamp, i.e. especially quantities of mercury in the lamp and it may also depend on the mercury compound found in the lamp. In the study six different brands of compact fluorescent lamps with different effect were used so results from the study are assumed to represent a general picture if a compact fluorescent lamp should break in a home.
Stahler et al. (2008) is concerned about postponing cleaning after an accident with a broken compact fluorescent lamp. Three tests were made with exactly the same type of lamp, but with cleaning after 1 minute and 46 minutes after the accident. It was seen that even if the initial mercury concentration was the same in both 30 cm’s and 1.5 meter’s height, the delay meant that the mercury spread in the room and resulted in higher average concentrations (at both 30 cm’s and 1.5 meter’s height) – even if a window was opened immediately after the accident in all three cases. Due to the high concentrations measured just after the accident (up to 0.05 mg Hg/m³) Stahler et al. (2008) recommends to wait – not too long, but some 5-15 minutes – before cleaning.
Literature describes that compact fluorescent lamps are available that are covered by a silicone film, which will reduce the risk of release of mercury during breaks. [6] As mentioned above consumers may be better protected against mercury exposure through use of dosage techniques with encapsulated mercury compared with liquid mercury or if an additional outer casing is used for compact fluorescent lamps and only this outer casing breaks.
3.3 Impact from flooring and evaporation of mercury after accidents
Stahler et al. (2008) has also studied the impact of flooring and vacuuming on mercury concentrations in the air after accidents, i.e. when compact/straight fluorescent lamps break in a home.
It was common to all types of flooring (long and short-pile carpets and laminate wooden floor) that even if they looked clean all types of flooring still released mercury even after cleaning and vacuuming. In the tests significantly higher concentrations of mercury were measured after cleaning if the floors were impacted physically (e.g. walking, vacuuming or washing) than if the floors were left untouched. Measurements of mercury concentrations in the room (unventilated) were continued until the concentration was below 0.0003 mg Hg/m³. In most cases it took up to four days before the concentration decreased to below 0.0003 mg Hg/m³ for wooden floors, but for two of ten measurements for wooden floors it took more than 20 days before the concentration dropped to below 0.0003 mg Hg/m³. For carpet flooring it took generally longer before the concentration decreased to below 0.0003 mg Hg/m³ – 6, 15, > 27, 34, 52 and > 59 days respectively[7] for a total of six measurements. In all cases the carpets were impacted physically (simulated vacuuming) several times after cleaning. Results showed that the concentration close to the floor may reach as high as 0.029 mg Hg/m³ several weeks after removal of the lamp (measured after vacuuming/impacting of the flooring material).
The tests showed that particularly carpets seemed to contain more ”mercury residues” after cleaning compared with wooden floors. The wooden floor used was a laminate wooden floor. It may well be that an old wooden floor with, for instance, broad planks with large spacing would be just as hard to clean as a carpet, since droplets of mercury may compile in the spaces and be difficult to collect.
The surveys in Stahler et al. (2008) also showed that it is not expedient in the first cleaning stage to use a vacuum-cleaner for cleaning broken compact fluorescent lamps/straight fluorescent lamps. The vacuum-cleaner will disperse the mercury and become contaminated with mercury to an extent that it is difficult to clean. However, by removing the dust bag and cleaning the mouthpiece and hoses thoroughly, for instance with wet tissues, the concentration of mercury in the vacuum-cleaner can be reduced.
3.4 Description of accident
One of the interviewed manufacturers describes that the older the lamp, the more mercury will be bound to the glass and the phosphor coating on the inner side of the glass. Normally, this is seen by a greying of the inner side of the glass and the inner phosphor coat. The area near the electrodes will gradually turn black. In addition, in some cases it is possible to see fine dispersed mercury drops when a lamp breaks. Due to the breakage of the lamp some of the phosphor coating may be loosened from the glass surface. For the consumer it is therefore relevant to remove all visible glass, powder and mercury droplets, if any, after an accident with a broken lamp.
3.5 Release of mercury to the outer environment
Using the study by Aucott et al. (2003) to indicate how much mercury will evaporate to the outer environment when a broken fluorescent lamp is discarded in the waste bin – before incineration of the waste – the following will be the result: As mentioned between 17 and 40 % of the mercury will be released in the course of a two-weeks period. Amounts depend on the temperature but also a number of other factors such as air volume surrounding the mercury. Evaporation will presumably be lower if the mercury is packed airtight, for instance.
USEPA estimates that around 11 % of the mercury in a fluorescent lamp will be released to the air or water when the lamp is landfilled as waste. Evaporation of mercury to the outer environment is not dealt with further in this report since it is not the purpose of the study. However, it is evident that ventilation in connection with breakage of a fluorescent lamp will also contribute to mercury in the surrounding air.
3.6 Risk of breakage
It is difficult to indicate the frequency of breakage of fluorescent lamps. It has only been possible to find trade figures from the UK showing that less than 1 % of lamps break (Defra, 2009). This has not been studied in detail in this project since focus is on health impacts from broken fluorescent lamps in private homes.
3.7 Discussion and summary
The below tables sum up the most significant figures from the different studies. The first table shows values for content of mercury in vapour form and amounts bound to the glass before a lamp breaks. The second table shows concentrations of mercury measured at different times after breakage of a fluorescent lamp as well as relevant references. The values originate mostly from tests and, primarily, maximum values are stated.
Table 3-1 Values for contents of Hg in vapour form and bound to the glass – before breakage
| Compact fluorescent lamps | Straight fluorescent lamps | |
|---|---|---|
| Mercury in vapour form within the lamp | Max. 0.05 mg Hg (Aucott et al., 2003) Max. 0.025 mg Hg (0.5 %) in warm lamp and max. 0.015 mg Hg (0.3 %) in cold lamp (for lamp with total of 5 mg Hg) (NEMA, 2000) |
New lamps contain around 0.17 % Hg in vapour form. Used lamps contain around 0.04 % Hg in vapour form. (Jang et al., 2005) |
| Mercury bound to the glass | 6.5 % of total quantity of mercury is bound to the glass in used lamps (NEMA, 2000) | |
Table 3-2 Values for concentrations of mercury in accidents with broken compact fluorescent lamps/straight fluorescent lamps
| Compact fluorescent lamps | Straight fluorescent lamps | |
|---|---|---|
| Maximum concentration/ ”peak” values | Theoretical calculation without ventilation: 0.150 mg Hg/m³ (Chandrasekhar, 2007) |
|
| Measurements during accident in a private home: 0.140 mg Hg/m³ (Baughmann, 2006) Measurements during test (room 39 m³): 0.05 - > 0.1 mg Hg/m³ (Stahler et al., 2008) |
||
| Concentration one minute after accident | Test where two lamps are broken in a drum: 1.152 mg Hg/m³ at 15 °C 1.440 mg Hg/m³ at 30 °C (Aucott et al., 2003) |
Measurements during test (2 lamps with 4.55 mg Hg): 1.440 mg Hg/m³ (at 30 °C) corresponding to 1.9 % has evaporated (Aucott et al., 2003) |
| Concentration a few minutes after accident | Test where two lamps are broken in a drum: 0.651 mg Hg/m³ at 5 °C after two minutes (Aucott et al., 2003) |
Measurements during test (2 lamps with 4.55 mg Hg): 4 – 7 % Hg has evaporated depending on temperature (Aucott et al., 2003) |
| Concentration 8 hours after accident | Measurements during test (2 lamps with 4.55 mg Hg): 6 – 13 % Hg has evaporated depending on temperature (Aucott et al., 2003) |
|
| Concentration two weeks after accident | Measurements during test (2 lamps with 4.55 mg Hg): 17 – 40 % Hg has evaporated depending on temperature (Aucott et al., 2003) |
|
| Concentration > 59 days after accident after cleaning | Measurements during test (room 39 m³): > 0.0003 mg Hg/m³. Measurements were made until the value 0.0003 mg Hg/m³ was no longer exceeded. It took between < 4 days to > 59 days depending on flooring type. (Stahler et al., 2008) |
|
Information shows that around 0.5 % of total quantities of mercury (maybe up to 1 % or a maximum of 0.05 mg Hg) will evaporate immediately from a broken compact fluorescent lamp. When it is warm (30 °C) up to around 2 % may have evaporated after one minute and up to 7 % after a few minutes. After 8 hours up to 13 % of total quantities of mercury contained in the fluorescent lamp may have evaporated. It is not stipulated precisely, but it can be read from the graph (Figure 3-1) that around 10 % will have evaporated within around 30 minutes – the maximum time presumed necessary for cleaning after an accident. Therefore it is assumed that 10 % of the total amount of mercury evaporates in 30 minutes in the exposure calculations.
Thus, in practice a maximum of 0.5 % of the total amount of mercury will presumably evaporate momentarily after which in the following minutes up to 7 % of the total amount of mercury contained in the lamp will evaporate and up to 10 % after 30 minutes. In the worst-case calculations it is therefore assumed that 10 % of the total amount of mercury will evaporate immediately when the lamp breaks.
A number of measured values show that maximum concentrations can easily exceed the occupational threshold limit value – even with a very high factor. These maximum values, however, are only present for a brief period of time. Both calculation models and tests show that the concentration of mercury decreases very quickly to low levels far below relevant limit values through continuous ventilation of the room.
Tests also show that concentrations of mercury are higher 30 cm above floor height than 1.5 m above floor height. In addition to the height above floor level the concentration of mercury in the room after breakage of a lamp depends on the quantities of mercury in the lamp and, possibly, the form in which mercury is present in the lamp.
In one study a certain concern is expressed for postponing cleaning after breakage of a compact fluorescent lamp, since three different tests with the same broken lamp showed that a long waiting time (46 minutes) compared with a short period of time (1 minute) before cleaning up resulted in higher average concentrations (both in 30 cm’s and in 1.5 metre’s height) – despite the fact that a window was opened immediately after the accident in all three cases. Due to the high concentrations measured immediately after the breakage (up to 0.05 mg Hg/m³) it is recommended in the study to put off cleaning for a few minutes – some 5-15 minutes, but no longer than that.
Furthermore, tests have shown that in a room cleaned after an accident and where visible residues of the fluorescent lamp and mercury have been removed, concentrations of mercury can exceed the USEPA RfC value (reference concentration without harmful effects) for short periods of time during and after vacuuming: tests show that when floors containing mercury residues are impacted physically, concentrations at floor height may increase substantially.
Particularly carpets seem to contain more ”mercury residues” after cleaning compared with a smooth wooden laminate floor. Old wooden floors with large spaces may, however, be just as difficult to clean as a carpet.
A vacuum cleaner can easily be contaminated with mercury if it is used to vacuum glass residues from the fluorescent lamp and thereby release mercury vapours to the indoor climate for a long period of time.
One of the interviewed manufacturers stated that during an accident it is relevant to remove all visible glass, powder and even droplets of pure mercury. Both glass and powder contain mercury.
Several references state that the broken lamp should be removed using materials that are subsequently discarded, i.e. use cardboard pieces to pick up pieces of broken glas and adhesive tape to collect other physical residues. In this way it is avoided that cleaning utensils such as brooms and vacuum cleaners are contaminated with mercury so that they release mercury vapours in the weeks and months after the accident. Pieces from the broken lamp and various collection tools should be placed in a closed container, e.g. a canning jar, to avoid spreading of mercury vapours. After removal of residues the contaminated area should be cleaned.
[4] The manufacturer stated a content in straight fluorescent lamps between 4.4 and 4.7 mg Hg. Therefore, an average content of mercury of 4.55 mg has been assumed.
[5] These concentrations are far below the concentration for saturated mercury vapours (20 mg Hg/m³ at 25 °C). This means that the drum used for measuring mercury concentrations has been large enough to measure maximum quantity of mercury that can evaporate.
[6] http://www.defra.gov.uk/environment/business/products/roadmaps/lightlamps.htm; http://www.megaman.cc/global/greenroom/silicone_protection.php; http://www.clear-lite.net/docs/_sub_products_1.html).
[7] For two of the tests the concentration of 300 ng Hg/m³ was not reached before the test ceased after 27 and 59 days, respectively.
Version 1.0 August 2010, © Danish Environmental Protection Agency