|
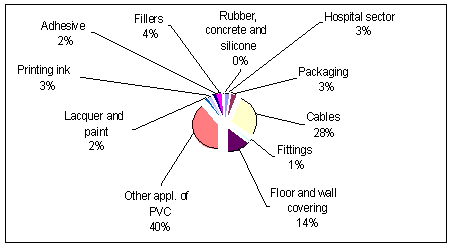
Environmental and Health Assesment of Alternatives to Phthalates and to 4.Use patterns and substitutes4.1 Use patterns of phthalates 4.1 Use patterns of phthalates4.1.1 Assessment of use of phthalate plasticisersMany polymer products need to be flexible and soft so they can take on a different shape and form depending on their application. This plastification is often conducted using plasticisers such as phthalates, adipates, trimelli-tates and citrates. The major uses of flexible PVC in Western Europe is in the product groups of film and sheet, wire and cable, floor covering, extrusions, coated fabrics and plastisols (European Council for Plasticisers and Intermediates, 2000). PVC plasticisers In the Danish Product Register, close to 180 different plasticisers are registered in a wide range of products. According to the European industry 95% of the plasticiser production is for PVC end-use. In Denmark phthalate in PVC contributes with 90% of the turnover of phthalates (Hoffmann, 1996). Plasticisers are used in a wide range of products from toys, babycare items, medical devices, wall-coverings, electrical cables, automotive parts, pack-aging, coatings and in the manufacture of clothing and footwear. Smaller quantities of plasticisers are also used in paints, rubber products, adhesives and some cosmetics. A small amount is used as denaturant in cosmetics such as "sun-tan oil". In Denmark, phthalates are used as plasticisers in various PVC-products for medical utilities, packaging, cables, fittings, floor/wall covering, but there is still a an extensive, not specified, consumption of phthalates in plasticised PVC products (Hoffmann, 1996). This "Other application of PVC" covers e.g. the use of phthalates in plastisol (coating materials) and toys. Artificial leaders are e.g. produced by coating textile with plastisol. The plastisol con-sists of a PVC-resin, solvent and a plasticiser e.g. phthalates. In Denmark, phthalates are used as plasticisers in non-PVC materials such as lacquer, paint, printing inks, adhesives, fillers and denaturants in cosmet-ics. The unspecified consumption of phthalates in non-PVC applications is small compared to the use in PVC (Hoffmann, 1996). Capacitors with di-electric fluid may contain phthalates (notably DEHP), but in Denmark most capacitors are dry and therefore without fluids. Another use of phthalates in non-PVC products is in ceramics for electronic products. It is assumed that the used amounts associated with these applications are relatively small in Denmark. The use of phthalates as plasticisers a.o. in 1992 in Denmark was assessed in the Substance Flow Analysis (Hoffmann, 1996). This analysis indicates that the Danish distribution of the applications of phthalates in 1992 can be illustrated as in Figure 4.1.
Figure 4.1 The distribution of phthalates for applications in Denmark in 1992 (Hoffmann, 1996). As mentioned earlier, the use of phthalates in PVC-toys is included in "other applications of PVC". This amount is assumed equal to the use of DEHP for flexible PVC-toys. This includes phthalates used for toys for children both less and more that 3 years old ~ 380 ton/year. The use of phthalates are now banned for the former category. It appears that the amount of phthalates within certain applications is de-creasing. This trend is illustrated in Table 4.1, where the development in the use pattern is listed. Table 4.1 Development in the use pattern of phthalates in different applications in Denmark.
b The Danish Plastics Federation (1996) c Hansen and Havelund (2000) d At the moment increasing globally and constant on the Nordic market, but decreasing a little on the Danish marked. e According to SFA (Hoffmann, 1996) consumption is decreasing but according to the Danish Plastics Federation (1996) it might be increasing. f The application "other applications" under non-PVC -products in Hoffmann (1996) is estimated to cover as a maximum 50 tons. g Inventory for the Consumption in 1994 made by FDLF for The Danish EPA. The trend shown in Table 4.1 for the non-PVC-products, is a decline in the consumption of phthalates. Concerning the PVC-products the general trend is difficult to deduce from Table 4.1. According to the suppliers the overall consumption is at the moment increasing but within the near future it is ex-pected to decline. The consumption of phthalates is slightly increasing in the EU as a whole, stagnant in northern Europe, and decreasing slowly in Denmark (Hansen and Havelund, 2000). 4.2 Selection of substitute substancesIn the following the background for the selection of the 11 substitutes for phthalates is described. In Table 4.2 a total of 18 compounds are listed that, in variable degree, all are potential substitutes for phthalates.
Within each of the 6 groups, one specific substance has been selected as marker for the group. The primary source of information is the industry and the initial information from The Danish Product Register. To get an impression of how the substitution will take place selected indus-trial organisations have been contacted. Suppliers and users of phthalates and/or have been contacted to give an estimate of how a complete substitu-tion of phthalates 5 years from here can be predicted. The same substance will not substitute phthalates in all applications. The substitution will within the different applications take place by a distribution of substitutes. Estimates of this distribution are given in the substitution matrix in Table 4.5 and Table 4.6. In Table 4.2, a short description of the selection of substitutes for phthalates for various applications is given. Table 4.2 The plasticiser substitutes and suggestions for example substances in the groups of plasticisers. Other possible substitutes are shown in italics.
The Danish Product Register has conducted a search on these CAS No.s and a general search to identify which CAS No.s are registered in Denmark as plasticisers. The result of the general search was that approx. 180 different substances are registered as plasticisers. These were screened with respect to their uses, and only a minority was found to be relevant substitutes for phthalates. Reduction of the list in Table 4.2 has been performed based on information from the industry, the result of comprehensive information on use patterns from The Danish Product Register, and assessment of the data availability regarding toxicological and ecotoxicological information necessary for the assessment. 4.2.1 Assessed substitutes for phthalates - substancesThe plasticisers assessed are those for which most information is expected to be available for the environmental and health assessment and which have a use pattern involving high PVC volume and/or expected high exposure of humans and/or the environment. The substances are listed in Table 4.3. Table 4.3 Substances used for the environmental and health assessment.
No specific substance to be used as a marker for polyester-substitutes has been identified in PR or from suppliers. The industry has emphasised that these substances may become important and the branch organisation has suggested polyadipate as an example substance. However, no information on health or environmental properties has been identified on this substance or group during the present project. 4.2.2 Assessed substitutes for flexible PVC - materialsPolyethylene (PE) and polyurethane (PU) are both materials which are identified as possible substitutes for flexible PVC in a number of products and they thereby contribute to the overall substitution of phthalates. The two materials polyurethane and polyethylene substitute the PVC polymer as such and not only the plasticiser additive. Both materials are polymers. The two alternative materials PE and PUR PU is expected to substitute plasticised PVC in waterproof cloths, shoes, boots and waders, and PUR based on the diphenylmethane-4,4'-diisocyanate (MDI) monomer is selected for the environmental and health assessment. LDPE and HDPE High density versions (HDPE) are produced in linear forms which allow the polymer chains to pack closely together. This structure results in a dense and highly crystalline material of high strength and moderate stiffness. Principal uses include bottles, pails, bottle caps, packaging, household appliances, and toys. Because of the strength and stiffness HDPE is more commonly used for industrial products compared to household products and consumers in general are more likely to be exposed to LDPE than HDPE. LDPE and HDPE are assumed comparable with regard to effects on environment and health. HDPE is used for toys of rigid materials, but the proportion of toys manu-factured from LDPE compared to HDPE is not known. It has been suggested that the two PE polymers have an equal share of toy market, and it is as-sumed that LDPE may lead to higher exposures than HDPE. Polyurethanes By itself, the polymerisation reaction produces a solid polyurethane. Polyu-rethane foams are made by forming gas bubbles in the polymerising mixture, which is achieved by using a blowing agent. MDI Because of the health risks ascribed to monomeric diisocyanates, much at-tention is paid to the physico-chemical and toxicological properties of the monomer in situations where substitution with a polyurethane is required. MDI has a particularly low vapour pressure compared to TDI (toluene diiso-cyanate), HDI (hexamethylene diisocyanate) and IPDI (isophorone diiso-cyanate ) and is under European legislation classified as harmful whereas the other mentioned diisocyanates are classified as toxic. This often makes MDI a better choice where it is technologically feasible. MDI is already used in the production of PU for waterproof clothes, shoes, boots and waders, application areas, which are suggested for substitution of flexible PVC in the substitution matrix, and is therefore selected to the health and environmental assessment. 4.3 Proposed use pattern for substitutesThe Danish Product Register (PR) has been used to establish an overview of the function of phthalates in chemicals until now. The Register mainly con-tains information about chemicals and to a lesser extent about materials. The historical main use of phthalates in Denmark, summarised in Table 4.1, forms the basis for the search for relevant alternatives in the PR. The most important function of phthalate has until now been plasticising, but besides this function phthalates had have the function of being denatur-ants in cosmetics (Hoffmann, 1996). The PR has conducted a search to identify the plasticisers used in selected types of chemical products or materials. The result is illustrated in Table 4.4 for the first 10 that are the substances selected for the assessment. The Poly-ester was not included due to lack of CAS no. Table 4.4 The registered use of the selected substances as plasticisers in the selected product groups. Data from the Danish Product Register. The polyester plasticiser (polyadipate) was not included due to lack of CAS no. Materials such as cables, profiles, floor and wall covering are not covered by the PR.
a Not found in the Product Register. Not all substances are registered as plasticisers in the selected products. This has to be seen in the light of the fact that the register only contains informa-tion about substances classified dangerous to the environment or the health. The result of the search is therefore as mentioned earlier supplemented with industrial information about the development of plasticisers not containing phthalates. 4.3.1 Substitution matrix for the 11 substances in tonsBy using the amounts found within the different applications in the sub-stance flow analyse (Hoffmann, 1996) and the proposed %-distribution of the alternatives in Table 4.5, the following "amount-substitution matrix" shown in Table 4.6 within the different applications can be established. Table 4.5 Substitution matrix for the 11 substances with anticipated share given in %. Note: It is only relevant to ad up the figures horizontal and not vertically because each row describe the substitution within one application. One column rep-resents non-comparable figures. Based on information from the industry, Table 4.6 represents the best pres-ent estimate on substitution of phthalates within different types of products. The dominating amount is for each substance marked in bold. The informa-tion is primarily based on interviews with industry sources rather than trade bodies, since only little overview information is available. This best present estimate has to be seen in the light of the situation in which all phthalates in a product are substituted by only one substitute. The actual substitution five years from now, will presumably not be exactly as illustrated in Table 4.6, but the information indicates in which areas the substances might be used extensively and in which areas the use is expected to be negligible. It should be emphasised that a large portion of the expected use is placed in "Other applications of PVC" (e.g. toys). Another scenario is that one substance substitutes the phthalates 100% within an application area (a 'worst worst case'). Table 4.6 Substitution matrix for the 11 substances (in tonnes)
4.3.2 Substitution matrix for the two materialsIn view of the general phase out policy for PVC the substitution of phtha-lates may obviously take place exchanging the PVC-material by other mate-rials that do not need to be plastified with phthalates. However, PE and PU cannot substitute flexible PVC across-the-board, but as seen in substitution matrix Table 4.7 PE and PU are possible substitutents for flexible PVC in different kinds of products:
Using the same procedure as for the 11 substances, but with the 1994-inventory from The Danish Plastics Federation of the consumption of plasti-cised PVC, a substitution matrix for the PVC-substituting materials can be established as in Table 4.8 Table 4.7 Substitution matrix for selected flexible PVC products in % of the total amount plasticised PVC (tonnes).
*: The vertical sum across different applications of PVC is not relevant to cal-culate. It is only the horizontal sum within the same application, which is relevant to calculate because it is describing the situation within one spe-cific application and has to add up to 100%. Table 4.8 Substitution matrix for alternative materials to flexible PVC. The unit is tons.
As for the 11 substances, the information from the industry in Table 4.8 rep-resents the most likely substitution of phthalates within different types of PVC-products. The dominating amount for each material is marked in bold. Again, the substitution five years from now, will presumably not be exactly as illustrated in Table 4.8, but the information indicates in which areas the materials might be used extensively and in which areas the use is expected to be negligible. The worst case scenario is when one material substitutes the plasticised PVC 100% within an application area. As seen in Table 4.8, polyethylene is most likely going to substitute 339 tons flexible PVC in toys. With an average concentration of phthalates in soft-PVC toys, similar to 34%, the 339 tons PVC represent 115 tons phthalates. Polyurethane is, as shown in Table 4.8, the mayor substitute for PVC in waterproof clothes. It is therefore of special interest to undertake an EUSES-calculation on the 208 tons flexible PVC, which may contain up to 100 tons phthalates. 4.4 Assessment of emission and exposureData compilation for substitution matrix In Table 4.5 and Table 4.7 the qualitative information is transferred to quantitative figures in percent. These figures form a possible scenario for how the complete substitution can take place. It will probably not corre-spond to the real situation in five years time, but it illustrates where the use of a substance might be extensive and where the use might be negligible. This overview is useful in connection with evaluation of results from calcu-lations in EUSES and in connection with priority of efforts of the environmental regulating authorities. The 11 substances and the 2 materials in this project are regarded as the main basis for the complete substitution for phthalates. According to discussion with the organisations listed in the Appendix, the most likely way to substitute phthalates is illustrated in the following sub-stitution matrixes Table 4.5 and Table 4.7. Substitution matrix for the 11 substances in % According to the Danish Plastics Federation, flexible PVC is not used in packaging, today. The actual consumption of phthalates for this purpose is therefore estimated to be of minor importance. The first five substances are expected to substitute phthalates directly. The next six are selected as markers for chemical groups from which substitutes are expected to be identified in the near future. Meanwhile the six markers are used to calculate a scenario for substitution of phthalate. Other substances and materials cover less important substitutions, conducted by other means than the 11 substances. Examples could be substances not covered by the 11 substances in Table 4.6, or new technology in the produc-tion of the mentioned products. The new technology could be the use of new materials without the need for plasticising with phthalates. The point of origin of Table 4.5 is the Danish consumption of phthalates in 1992 shown in Table 4.1 (Hoffmann, 1996). These data are selected because they are the result of a comprehensive survey, and the studies conducted later, confirm the amounts and the indicated development trends within the different applications. For the non-PVC products a decline in the use of phthalates has been identified (Hansen and Havelund, 2000). For the PVC-products the suppliers expect a decline in the near future. The background for the ratios in Table 4.5 is information gathered in con-nection with one of the substitution projects initiated by the Danish Envi-ronmental Protection Agency. It is the general impression among suppliers and users of phthalates that a complete substitution will be possible for both PVC and non-PVC products. Available substitutes for non-PVC products have been identified earlier (Hansen and Havelund, 2000). Table 4.9 Estimated use of the substitutes. These volumes are used for consumer expo-sure
4.4.1 Considerations regarding specific uses of phthalates/substitutesIndustrial processes The synthesis of phthalates for the Danish market is at the moment mainly conducted in Sweden. The identified substitutes are expected also to be synthesised in countries outside of Denmark. The main source to emissions and exposures in Denmark is expected to be from formulation of products containing plasticisers such as plasticised PVC, printing inks, adhesives and fillers. For paints and lacquers there is also an emission and exposure from the professional use of the products. Based on the substitution matrix focus in this investigation has been set on the use of the eleven substances with known potential application the fol-lowing process:
In general, uses of the products are regarded as diffuse and as minor sources to emission, but concerning consumer exposure of the 10 substances there are relevant scenarios that are described in Section 5.4. Human exposure is estimated mainly to take place in connection with:
To illustrate the potential human exposure from the 10 well defined sub-stances a calculation in EASE, which is based on the principles in the TDG has been conducted. EASE calculates a theoretical exposure of humans in the working environ-ment and private consumers. The input data takes point of origin in the scenarios in Table 4.10, which is assessed to represent the most extensive exposure. Table 4.10 Exposure scenarios of the 11 substances
To approach a situation five years from now the exposure calculation in EUSES is therefore conducted for both the most likely situation represented in bold figures and the 100%-scenario. 4.4.2 Worker and consumer exposureAssessment of the exposure of humans in the working environment and con-sumers has been conducted using the model Estimation and Assessment of Substances Exposure Physico-chemical Properties (EASE), which is a part of the model European Union System for the Evaluation of Substances (EUSES). Limitations and uncertainties of the EASE Workers exposure If reliable and representative measured data are available these can be used to overwrite the model results. The general-purpose model is called EASE (Estimation and Assessment of Substance Exposure) which is described in details in Section 2.2 of the TGD. EASE was specifically developed for the purpose of modelling inhalation and dermal workplace exposure across a wide range of circumstances. EASE is an analogue model, i.e. it is based on measured data which are as-signed to specific scenarios. The user can build scenarios by choosing be-tween several options for each of the following variables: physical proper-ties during processing (tendency to become airborne, potential for dermal contact), use pattern and pattern of control. Numerical ranges have been as-signed using measured data contained within the UK National Exposure Database for inhalation exposure, and experimental data and expert judge-ment for dermal exposure. The data used to assign ranges within the model are all 8-hour time weighted averages and the numbers generated by the model are only valid when the exposures being assessed can be related to such averages. The output of EASE is numerical ranges of concentrations or ppm. These are converted from ppm to kg.m-3 and can be used as input for risk charac-terisation in which the exposure estimates are compared to the results of the human effects assessment. Consumer-exposure A consumer product is one, which can be purchased from retail outlets by members of the general public and may be the substance itself, or a prepara-tion, or an article containing the substance. The EASE equations for consumer exposure can be used to estimate exter-nal exposure substances used as or in consumer products. Absorption or bioavailability is not taken into account by the equations implemented in EASE. The focus in EASE is on substances used indoors for a relatively short pe-riod of time per event (such as e.g. a carrier/solvent in a cosmetic formula-tion; a powder detergent). The equations in the model apply to both volatile substances and airborne particulates. It is assumed the substance is released as a vapour, gas, or air-borne particulates, and the room is filled immediately and homogeneously with the substance. Ventilation of the room is assumed to be absent. The equations can also be adapted to estimate exposure arising from 'rea-sonably foreseeable misuse', i.e. when products are not used according to the instructions, but as if they were other, allied products. To adapt the equations, the values for the parameters used in the equations are changed to reflect values foreseen in "reasonably foreseeable misuse". For example, the volume of product or the area of application is set to a dif-ferent value, reflecting reasonable foreseeable misuse. If a substance is released relatively slowly from a solid or liquid matrix (e.g. solvent in paint, plasticiser or monomer in a polymer, fragrance in furniture polish), the equation in EASE acts as a worst case estimation, estimating the maximum possible concentration. Dermal Dermal A: a substance contained in a medium. This dermal scenario also applies to
The assumption behind the equations in the calculations is that all of the substance on the skin is potentially available for uptake. This is the case when the medium is well mixed or only present as a thin film on the skin. The dermal equations apply for:
Dermal B: a non-volatile substance migrating from an article (e.g. dyed clothing, residual fabric conditioner, dyestuff/newsprint from paper). The assumption behind the equation is that only part of the substance will migrate from the article (e.g. dyed clothing, residual fabric conditioner, dye-stuff/newsprint from paper) and contact the skin. The migration is assumed to be slow enough to be represented by a constant migration rate multiplied by the time of contact. The exposure calculation will involve estimating the amount of substance which will migrate from the area of the article in contact with skin during the time of contact. Dyestuff amounts in fabrics and paper are usually given as weight of product per unit area (e.g. mg/m2). Oral Oral A: a substance in a product unintentionally swallowed during normal use (e.g. toothpaste). The exposure equations may also be used to estimate exposures arising from ingestion of the non-respirable fraction of inhaled airborne particulates. The equations may also be used to estimate exposures arising from ingestion of the non-respirable fraction of inhaled airborne particulates. Oral B: a substance migrating from an article into food or drink (e.g. plastic film, plastic-coated cups/plates). It is assumed that the substance in a layer of thickness of article (e.g. plastic film, plasticcoated cups/plates) in contact with the food will migrate to the food. The migration rate is assumed to be constant, and the migration rate multiplied by the contact duration is the fraction of substance that is migrated to the food. The equation can be used to give a conservative estimate of substance uptake by a defined volume of food. The value of the migration rate will be influenced by the type of food (e.g. fatty/dry/moist), the period of exposure and the temperature at which it occurs. Consumer exposure level will also be influenced by the proportion of contaminated food eaten. Use pattern scenarios The background for choosing scenarios is the most likely way of substitu-tion described by industrial actors and rendered in the substitution matrix in Table 4.5. Within the substitution matrix the application representing the largest estimated consumption of each substitute is selected as the most relevant scenario. This application is marked in bold figures in the substitu-tion matrix. The input data for the calculation are the substitution matrix and scenarios with the largest estimated consumption substitute for phthalate plasticisers. With point of origin in the substitution matrix the substances are distributed in the following most relevant application areas. Plasticisers in the "Cables"-application are expected to be:
For the production of "floor and wall covering" the following plasticisers are chosen:
Concerning the production of lacquer and paint the focus is on:
In connection with printing ink the relevant substances are estimated as:
The production of "fillers" is assumed to include:
The scenarios in EASE for the consumer exposure are based on the amounts for these uses and the exposure characteristics for the application. 4.4.3 Exposure in environmentSubstance parameters Two types of assessments were performed for each substance substituting phthalates. One scenario simulates the best educated guess for the future share that this particular substance would gain in the market based on inter-views with the industry. The second assessment simulates the hypothetical situation where only one of the alternatives (100% substitution case) sub-stitutes the entire tonnage of phthalates. In the 100% substitution case it is chosen to base the estimates on the most recent inventory of the phthalates in PVC and use the sum used in 1992 (10,735 tons). In various applications substitutes may be used in different volumes than the phthalates - if 1 kg new substance can substitute 2 kg phthalate or vice versa. Since the available information on this is very in-complete it has been decided not to try to include such information in the calculations. The physical parameters of some of the compounds are out of the advised range in which EUSES operates. In the cases where the physical parameters are out of the pre-set range (e.g. logPow > 6) or unknown as melting and boiling point sometimes are, it has been chosen to use the nearest maximum or minimum value as suggested in EU TDG or use a worst case approach. All results are presented in the report with two significant digits rounded off from the EUSES calculations with three significant digits (given in appen-dix). The assessment of emission to the environment and exposure of man and biota from environmental concentrations of phthalate alternatives are based on the procedures outlined in the EU TGD (EU Commission 1996). The actual concentrations are calculated by using the PC program EUSES (European Chemicals Bureau 1996), which is designed to provide decision support for the evaluation of the risks of substances to man and the envi-ronment based directly on the EU TGD. In the present evaluation EUSES is operated in three of the possible five modes. Parameters are entered for:
EUSES will calculate concentrations and doses for the assessment on three spatial scales: the local (point source), the regional (small and densely in-habited country) and the continental (Europe). The default regional scale has been changed to suit Danish conditions (see below). The local and conti-nental scenarios are included in the calculations, but no specific values have been entered. The EUSES program calculates environmental exposure based on 1) a physical scale where the use and emission takes place, 2) the use and emis-sion pattern of the substance and 3) on substance specific parameters. The specific parameters for each substance are provided for each exposure esti-mation. To assist the comparison between substances the physical dimen-sions of the scenario and the overall industrial use and emission pattern has been set identical for all substances. Use and emission scenarios
Danish regional scenario Table 4.11 Parameters of the EUSES model, which were adapted to Danish conditions
4.4.4 Migration potentialA key parameter in comparing various plasticisers is their potential for mi-grating out of the PVC polymer. Only few data has been identified on mi-gration potential for the substitutes. The information on migration potential will be used in the expert assessment process, but is not used in the exposure calculation model. To determine the total migration potential various reference methods are used which all are available as CEN standards (ENV 1186-1 - ENV 1186-12). Several new standards in this area are in preparation. In general the methods are divided in two categories: Migration from plastic to an oily extractant and migration from plastic to an aqueous solution. To determine the total migration potential using the two groups of methods either a double sided test (total submergence of plastic piece) or a single sided test (using a migration cell or by incorporating the plastic piece into a bag) is performed. The total migration potential is determined as the difference in weight be-fore and after extraction or as weight of the evaporation residue of the ex-tractant. The 3 commonly water based extractants are distilled water, 3% acetic acid and 10 % ethanol. Since most plasticisers are lipophilic it is most relevant to express the migration as migration from plastic to fat containing food. As fat simulator olive oil is the commonly used. The amount of plasticiser extracted is extractant dependent and usually the extraction time of olive oil and 95% ethanol is 10 days at 40 °C and for iso-octane 2 days at 20 °C. The difference in extraction time is due the extraction power of each ex-tractant and the ability of the extractant to make the plastic swell. When the plastic swells the dept of the layer in contact with the extractant increases and the amount of platisicer extracted increases. The extraction power of the extractant types depends on the plastic type that is investigated, but usually, when considering plastics that contains lipo-philic plasticisers the order extraction power is: isooctane > olive oil > etha-nol..
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||