Report on the Health Effects of Selected Pesticide Coformulants2 Methodology2.1 Prioritisation of substances for the project2.2 Literature search 2.3 Principles for the hazard assessment 2.1 Prioritisation of substances for the projectThe Danish EPA established the following criteria, prioritised in the following succession, for the selection of substances to be included in this project:
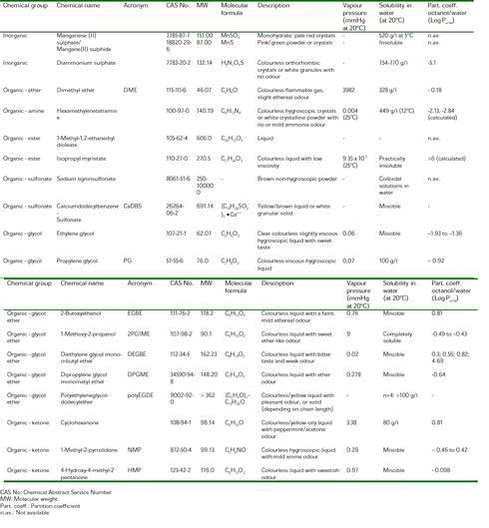
The result was the selection of the 18 substances listed in Table 1. The Table compiles identity and physico-chemical properties of the substances, and groups the coformulants according to chemical classes. 2.2 Literature searchFor the hazard assessments of the coformulants selected in this report, data have been collected from national and international criteria documents and monographs, from original scientific literature, and from the International Uniform Chemical Information Database (IUCLID) on High Production Volume Chemicals reported by European Industry in the frame of the EU Existing Chemicals risk Assessment programme. The standard references consulted in the literature search for the selected coformulants are given below: ACGIH (1991). TLV‘s Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices for 1991-1992. Cincinnati, OH. Arbete och Hälsa. Nordiska Expertgruppen för Gränsvärdesdokumentation. Arbetarskyddsverket. Alarie Y (1981). Dose-response analysis in animal studies: prediction of human responses. Environ Health Perspect 42 , 9-13. Amoore JE and Hautala E (1983). Odor as an aid to chemical safety: Odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J Appl Toxicol , 272-290. At (1996). Grænseværdier for stoffer og materialer. Arbejdstilsynets At-anvisning Nr. 3.1.0.2, December 1996.10 ATSDR. Toxicological Profiles. U.S. Department of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry. Berlin A, Draper M, Krug E, Roi R and van der Venne MTh. The Toxicology of Chemicals. 1. Carcinogenicity. Summary Reviews of the Scientific Evidence, Luxembourg, Commission of the European Communities. BUA. Beatergremium für umweltrelevante Alstoffe (BUA), Geschellschaft Deutscher Chemiker. Chemfinder. Http://www.chemfinder.com HSDB. Hazardous Substances Data Base. IARC. IARC Monographs on the Evaluation of the Carcinogenic Risk to Humans, Lyon. IRIS. Integrated Risk Information System. Database quest. US-EPA. IUCLID (2000). International Uniform Chemical Information Database. European Commission, ECB, JRC, Ispra. Merck Index (1996). 12th. Ed., Rahway, New Jersey, Merck & Co., Inc. Miljøministeriets bekendtgørelse af listen over farlige stoffer. (Statutory Order from the Ministry of the Environment on the List of Dangerous Chemical Substances).11 MM (1988). Bekendtgørelse om vandkvalitet og tilsyn med vandforsyningsanlæg. Miljøministeriets bekendtgørelse nr. 515 af 29. august 1988. (Statutory Order from the Ministry of the Environment no 515 of 29 August 1988 on water quality and control of water supply facilities).12 MST (1996). B-værdier. Orientering fra Miljøstyrelsen nr 15, 1996.13 MST (1990). Begrænsning af luftforurening fra virksomheder. Vejledning fra Miljøstyrelsen nr 6 1990. RTECS. Registry of Toxic Effects of Chemical Substances database. Ruth JH (1986). Odor thresholds and irritation levels of several chemical substances: a review. Am Ind Hyg Assoc J 47 , A142-A151. Sullivan FM, Watkins WJ and van der Venne MTh (1993). The Toxicology of Chemicals. 2. Reproductive Toxicity. Vol. 1, Summary Reviews of the Scientific Evidence, Luxembourg, Commission of the European Communities. Toxline plus 1999-2000/01. WHO (1998). Guidelines for drinking-water quality. Second edition. World Health Organization, Geneva. WHO (1987). Air Quality Guidelines for Europe. WHO Regional Publications, European Series No. 23, Copenhagen. WHO. Environmental Health Criteria. World Health Organisation, International Programme on Chemical Safety, Geneva. 2.3 Principles for the hazard assessmentThe scientific basis for the hazard assessment (hazard identification and hazard characterisation) of chemical substances as e.g., the coformulants consists of data elucidating the toxicological effects in humans and in experimental animals. Ideally, a complete database including information on toxicokinetics, acute toxicity, irritation, sensitisation, repeated dose toxicity, mutagenicity and genotoxicity, carcinogenicity, and toxicity to reproduction should be available for the hazard assessment of a coformulant. Direct information about health effects in humans may be obtained from well-planned and documented epidemiological studies. Some types of effects as e.g., neurotoxicological effects, which are assessed by examining intellectual and psychological end-points can only be revealed from human studies as no adequate experimental models are currently available. In addition to epidemiological studies, information on effects in humans may be obtained from case reports (e.g., poisonings), clinical examinations, studies on volunteers, and experiences from the working environment. For most chemical substances including coformulants, however, adequate human data are not available for a hazard assessment. Therefore, toxicological studies in experimental animals play an important role in hazard assessments. In the hazard assessment, the quality and relevance of the available studies on experimental animals are evaluated. Studies performed according to international guidelines as e.g., the OECD guidelines and the EU guidelines on studies of chemical substances in experimental animals (Annex V to Council Directive 67/548/EEC) are preferred because of their high scientific standard and comparability of the results. However, such studies are generally not available for most of the chemical substances in use. Exposure to a chemical substance can result in a broad spectrum of effects varying from mild effects as e.g., irritation to fatal poisonings. The type and severity of the effects observed is most often correlated with the exposure concentration. According to WHO14, the NOAEL is the “greatest concentration or amount of a substance, found by experiment or observation, which causes no detectable adverse alteration of morphology, functional capacity, growth, development or life span of the target organism under defined conditions of exposure. Alterations of morphology, functional capacity, growth, development or life span of the target may be detected which are judged not to be adverse”. Similarly, the LOAEL is the “lowest concentration or amount of a substance, found by experiment or observation, which causes an adverse alteration of morphology, functional capacity, growth, development or life span of the target organism distinguishable from normal (control organisms of the same species and strain under the same defined conditions of exposure”. When all the relevant toxicological data have been evaluated, the hazard(s) considered most important, “the critical effect(s)”, is identified, i.e., the effect(s), which is judged to be most crucial following exposure to the substance in question. ______________________________________________________________
|