Report on the Health Effects of Selected Pesticide Coformulants3 Results3.1 Toxicological effects and data availability3.2 Short reports 3.2.1 Manganese sulphate and manganese sulphide (Appendix 1) 3.2.2 Diammonium sulphate (Appendix 2) 3.2.3 Dimethyl ether (DME) (Appendix 3) 3.2.4 Hexamethylenetetramine (Appendix 4) 3.2.5 1-Methyl-1,2-ethanediyl dioleate (Appendix 5) 3.2.6 Isopropyl myristate (Appendix 6) 3.2.7 Sodium ligninsulphonate (Appendix 7) 3.2.8 Calciumdodecylbenzenesulphonate (CaDBS) (Appendix 8) 3.2.9 Ethylene Glycol (EG) (Appendix 9) 3.2.10 Propylene Glycol (PG) (Appendix 10) 3.2.11 2-Butoxyethanol (EGBE) (Appendix 11) 3.2.12 1-Methoxy-2-propanol (2PG1ME) (Appendix 12) 3.2.13 Diethylene glycol mono-n-butyl ether (DEGBE) (Appendix 13) 3.2.14 Dipropylene glycol monomethyl ether (DPGME) (Appendix 14) 3.2.15 Polyethylene glycol dodecyl ether (polyEGDE) (Appendix 15) 3.2.16 Cyclohexanone (Appendix 16) 3.2.17 1-Methylpyrrolidone (NMP) (Appendix 17) 3.2.18 4- Hydroxy-4-methyl-2-pentanone (HMP) (Appendix 18) 3.3 Critical effects and existing regulation In this project, 18 coformulants have been selected for a detailed hazard assessment; these hazard assessments are compiled in Appendices 1 to 18. The toxicological effects and the data availability for the 18 coformulants are compiled in Table 2 (section 3.1), short summaries are presented in section 3.2, and the critical effects and existing regulation are summarised in Table 3 (section 3.3). The selection procedure of the coformulants is described in section 2.1 and the principles for the hazard assessment is described in section 2.3. 3.1 Toxicological effects and data availabilityThe toxicological effects described in the public available literature and the data availability for the 18 coformulants are compiled in Table 2 and based on the hazard assessments. For every of the following end-points, acute toxicity, irritation, sensitisation, repeated dose toxicity, toxicity to reproduction, mutagenicity and genotoxicity, and carcinogenicity, the available animal and human data regarding oral, inhalation, and dermal exposure are summarily described. Whenever possible, effect levels and LOAEL(C)s or NOAEL(C)s are included. An overview on the data availability is included in the Table in the following way: Available data are summarised for each substance under each end-point. If no data are available for a specific end-point, the notation “No data” is listed. If data regarding a specific end-point are available for only one species and one exposure route, no specific mention on missing data for other species or other exposure routes for this specific end-point is made, i.e., no mention of e.g., human data means that no human data are available for this specific end-point. Data for oral and dermal exposures are listed in the unit mg/kg b.w. or mg/kg b.w./day, while inhalation exposure data are listed in mg/m3. The following abbreviations are used in the Table: anim: animal; app: approximately; AST: aspartate amino transferase; av: available; aq: aqueous; bw: body weight; CNS: central nervous system; conc: concentration; d: day(s); decr: decreased; derm: dermal; dev: developmental toxicity; eff: effect(s); gi: gastrointestinal; gp: guinea pig; gpmt: guinea pig maximisation test; hist: histological; hr: hour(s); hum: human; incr: increased; inh: inhalation; irr: irritation; LAS: linear alkylbenzene sulphonate; LC50: lethal concentration for 50% of the animals in the study; LD50: lethal dose for 50% of the animals in the study; LO(A)EL(C): lowest observed (adverse) effect level (concentration); mamm: mammalian; mat: maternal toxicity; min: minimal; Mn: manganese; mod: moderate; mth: month(s); neg: negative; NO(A)EL(C): no observed (adverse) effect level (concentration); pos:
anim: animal; app: approximately; AST: aspartate amino transferase; av: available; aq: aqueous; bw: body weight; CNS: central nervous system; conc: concentration; d: day(s); decr: decreased; derm: dermal; dev: developmental toxicity; eff: effect(s); gi: gastrointestinal; gp : guinea pig; gpmt: guinea pig maximisation test; hist : histological; hr: hour(s); hum: human; incr: increased; inh: inhalation; irr: irritation; LAS: linear alkylbenzene sulphonate; LC50: lethal concentration for 50% of the animals in the study; LD50: lethal dose for 50% of the animals in the study; LO(A)EL(C): lowest observed (adverse) effect level (concentration); mamm: mammalian; mat: maternal toxicity; min: minimal; Mn: manganese; mod: moderate; mth: month(s); neg: negative; NO(A)EL(C): no observed (adverse) effect level (concentration); pos: positive; rab: rabbit; resp: respiratory; sev: severe; sol : solution, wk: week(s); yr: year(s).
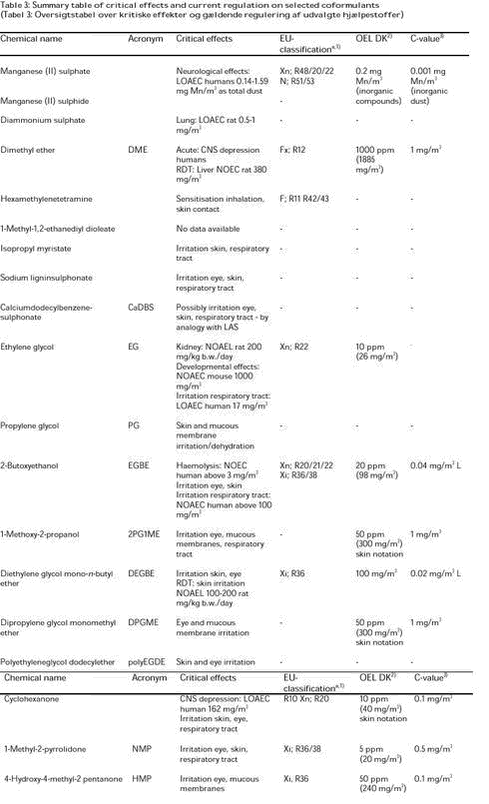
Data for oral and dermal exposures are listed in the unit mg/kg b.w. or mg/kg b.w./day, while inhalation exposure data are listed in mg/m3. 3.2 Short reportsThe hazard assessments for each of the 18 coformulants selected for this project are compiled in Appendices 1 to 18 to this report. This section contains a short summary on every substance, summarising the data found as well as the evaluation of the individual substances. 3.2.1 Manganese sulphate and manganese sulphide (Appendix 1)3.2.1.1 Toxicokinetics In humans, absorption of ingested inorganic manganese is about 3-5% and pulmonary absorption can be significant, both increasing with the solubility of the compound. 3.2.1.2 Single dose toxicity No human data were found. Oral LD50-values in rats ranged from 275 to 1082 mg Mn/kg b.w. 3.2.1.3 Irritation and sensitisation Inhalation of high concentrations of manganese dust (dioxide, tetroxide) can cause inflammation of the lung in humans and in animals. 3.2.1.4 Repeated dose toxicity Occupational exposure to manganese dusts (mainly manganese dioxide) over longer periods (years) can lead to neurological effects (manganism), characterised by weakness, muscle rigidity, tremor, apathy and speech disturbances. Symptoms can start following 1-3 months of exposure. In most cases, the symptoms are irreversible. The levels of exposure causing manganism are in the range of 0.027-0.215 mg Mn/m3 as respirable dust or 0.14-1.59 mg Mn/m3 as total dust. 3.2.1.5 Reproductive toxicity Male workers suffering from manganism following exposure to high concentrations of manganese dust have shown decreased libido and decreased sperm quality and sperm count. School children exposed to increased levels of manganese in the drinking water and food showed poorer performance in school and in neurobehavioural tests as compared to children exposed to lower levels. However, other metals may have influenced these developmental effects. Studies in mice and rats treated orally with manganese tetroxide at doses from about 1050 mg Mn/kg b.w./day indicate that the reproductive function of offspring can be delayed; the reproductive effect was worsened by diets low in iron. 3.2.1.6 Mutagenicity and genotoxicity Manganese sulphate was negative in one Ames test, but positive in another Ames test (one strain) as well as in other in vitro tests with and without metabolic activation. The substance caused an increased incidence of micronuclei and chromosomal aberrations in the bone marrow of mice treated orally, but was negative in a sex-linked recessive lethal mutation test in germ cells of fruit flies. 3.2.1.7 Carcinogenicity A small increase in pancreatic adenomas and carcinomas was seen in male rats treated for 2 years with up to 331 mg Mn kg b.w./day as manganese sulphate. In a study with manganese sulphate in mice and rats over 2 years, a significantly increased incidence of thyroid gland follicular cell hyperplasia and a marginally increased incidence of thyroid gland follicular cell adenomas were seen in mice treated orally with 731 mg Mn/kg b.w./day, while no effects were seen at 228 mg Mn/kg b.w./day, or in rats at doses up to 232 mg Mn/kg b.w./day. In another chronic oral mice study with manganese sulphate, small increases in pituitary adenomas were observed in females at 905 mg Mn/kg b.w./day, but not in males at 722 mg Mn/kg b.w./day. 3.2.1.8 Evaluation The critical effect of manganese following inhalation is neurotoxicity, occurring at concentrations of manganese in respirable dust in the range of 0.02-0.215 mg Mn/m3 and in total dust in the range of 0.14-1.59 mg Mn/m3. Manganese has also a potential to cause reproductive and developmental effects as evidenced by reproductive effects seen in workers suffering from manganism and by reproductive and development effects observed in rodents. However, most of the reproductive and developmental effects seen in rodent studies occurred after exposure via gavage or injection of the substance, administration routes which both lead to a high systemic concentration of manganese and are of minor relevance regarding exposure of workers and the general population. In most studies, no biochemical or behavioural signs of neurotoxicity were evident in pups exposed in utero to manganese. Manganese sulphate showed positive results in several in vitro and in vivo tests. Small increases in thyroid gland follicular cell adenomas, pituitary adenomas, and pancreatic adenomas and carcinomas were observed in rodents exposed orally to relatively high doses of manganese sulphate for 2 years. 3.2.2 Diammonium sulphate (Appendix 2)3.2.2.1 Toxicokinetics When hamsters inhaled 0.2 mg/m3 diammonium sulphate (particles), a substantial proportion was found in the nose; the clearance from the lungs was determined to be about 20 minutes. Oral toxicity data indicate absorption of diammonium sulphate from the gastrointestinal tract. 3.2.2.2 Single dose toxicity Changes in pulmonary function (decreased flow rates, potentiation of the bronchoconstrictor action of carbachol) occurred in healthy and asthmatic workers exposed by inhalation to 1 mg/m3 diammonium sulphate aerosols for 4 hours in combination with ozone, sulphur dioxide, or the bronchoconstrictor carbachol, while no changes were seen up to 0.5 mg/m3. For rats, the reported LC50-value is above 1200 mg/m3 following inhalation of diammonium sulphate for 8 hours. 3.2.2.3 Irritation and Sensitisation Volunteers exposed to 0.5 mg/m3 reported irritation of the upper respiratory tract and of the eyes. 3.2.2.4 Repeated dose toxicity No consistent changes in pulmonary function or in symptoms resulted from exposure of workers to 0.1 mg/m3 for 2 hours/day for 2-3 days. 3.2.2.5 Reproductive toxicity No data were found for humans or for animals. 3.2.2.6 Mutagenicity and genotoxicity Diammonium sulphate was negative in a number of in vitro tests, while no in vivo tests are available. Diammonium sulphate increased the mutagenicity of the known mutagen ethylmethanesulphonate in a chromosomal aberration study in V79 hamster cells. 3.2.2.7 Carcinogenicity In a carcinogenicity study in Syrian hamsters treated intratracheally by intubation with 5 mg benzo(a)pyrene once a week for 15 weeks, simultaneously exposure by inhalation to 0.2 mg/m3 diammonium sulphate 6 hours/day for 5 days/week did not have any effect on benzo(a)pyrene carcinogenicity. 3.2.2.8 Evaluation The critical effects of diammonium sulphate are the local effects observed in the lungs, e.g., small changes in pulmonary function (humans and animals), and transient increased alveolar fibrosis, alveolar cord length, and hypertrophy and hyperplasia of non-ciliated epithelial cells in the alveoli and bronchioles of rats and guinea pigs. These effects occurred at concentrations around 0.5-1 mg/m3 diammonium sulphate (mass median aerodynamic diameter of about 0.5 µm). 3.2.3 Dimethyl ether (DME) (Appendix 3)3.2.3.1 Toxicokinetics DME is rapidly taken up after inhalation and distributed to various organs and tissues, where steady state is reached within 30 minutes. After end of exposure, the concentration of DME in organs and tissues falls very rapidly again. The elimination is described as a two-phase process. No tissue storage is seen. 3.2.3.2 Single dose toxicity The target organ in humans after exposure to very high acute concentrations of DME is the central nervous system, covering effects from incoordination, indistinct vision, and inability to do simple tasks, to unconsciousness (exposure levels from 157000 to 382000 mg/m3). 3.2.3.3 Irritation and sensitisation No data have been found. 3.2.3.4 Repeated dose toxicity Short-term studies (2 weeks), in which rats were exposed to concentrations of DME of 96000 mg/m3, caused sedation, body weight gain suppression, haematology and organ weight changes, but no histopathological organ changes; all changes were completely reversed after cessation of exposure. At high concentrations of DME, effects on the liver (higher ALT (alanine amino transferase) and AST (aspartate amino transferase) values suggesting a possible onset of a hepatotoxic effect) and changes in white blood cell counts have been observed. The NOEC in subchronic studies (13 or 30 weeks) for haematological effects was reported to be 19000 mg/m3 for rats and 9600 mg/m3 for hamsters. In a 30-week study on rats, the NOEC for increased levels of ALT was reported to be 3800 mg/m3 and for increased levels of AST 380 mg/m3. In a lifetime study in rats, the NOEC was stated to be 38000 mg/m3 (ALT and AST were not assessed). 3.2.3.5 Toxicity to reproduction No signs of teratogenicity or embryotoxicity were observed in offspring of female rats exposed to DME at concentrations up to 76000 mg/m3 from day 6 to 15 of gestation (two studies). In one study, the female animals showed evidence of a narcotic effect from 9600 mg/m3; no maternal effects were observed in the other study. Retarded ossification of the rib bones and some of the phalangeal bones in the extremities of the foetuses, and an increase in the number of extra ribs was considered as variations reflecting developmental delay rather than a specific effect on the foetuses. 3.2.3.6 Mutagenicity and genotoxicity DME showed no signs of a mutagenic or genotoxic potential in three in vitro and two in vivo test systems. 3.2.3.7 Carcinogenicity In a lifetime study in rats (exposed to DME at concentrations up to 48000 mg/m3), there was no increased incidence of tumours in any of the tissues or organs of the animals. 3.2.3.8 Evaluation The critical target organ at acute high concentrations is the CNS resulting in a narcotic effect. Based on the limited and old data on human exposure to DME, it is not possible to estimate a NOAEC for the narcotic effects in humans. No human data are available in relation to CNS changes such as neurobehavioral disturbances. 3.2.4 Hexamethylenetetramine (Appendix 4)3.2.4.1 Toxicokinetics In humans, hexamethylenetetramine was rapidly absorbed following oral administration and distributed to various organs. Following single or repeated oral administration, approximately 82 and 88%, respectively, of the compound was recovered unchanged in the urine. From 10 to 30% of a single oral dose was hydrolysed to formaldehyde and ammonia in the gastric fluid. 3.2.4.2 Single dose toxicity A case of bladder inflammation was reported following accidental ingestion of hexamethylenetetramine-mandelate. The acute toxicity in animals is low, with reported oral LD50-values in rats from 9200 to over 20000 mg/kg b.w. 3.2.4.3 Irritation and sensitisation Hexamethylenetetramine has been reported to cause skin and eye irritation in workers. No to mild skin irritation was observed in rabbits and guinea pigs from 2% hexamethylenetetramine. No eye irritation occurred in rabbits from 0.2% hexamethylenetetramine, while a mascara containing 0.1% hexamethylenetetramine caused mild eye irritation in this species. Hexamethylenetetramine was reported to cause allergic contact dermatitis in workers in the rubber, lacquer and plastic industry and positive patch test results have been observed in patients treated with 2% hexamethylenetetramine. Cross-reaction with formaldehyde has been reported. A maximisation test in 25 adults using mascara containing 0.1% hexamethylenetetramine was negative. Hexamethylenetetramine was positive in a Guinea pig maximisation test using a 30% solution for induction and a 50% solution for challenge, while another maximisation test using a concentration of 0.2% was negative. 3.2.4.4 Repeated dose toxicity Adverse effects have been reported in less than 3.5% of patients receiving hexamethylenetetramine and its salts orally as a drug. The most frequent findings were gastrointestinal disturbances; some patients showed hypersensitivity reactions. 3.2.4.5 Reproductive toxicity No increase in the incidence of congenital abnormalities was observed in 200 newborns exposed to hexamethylenetetramine in utero during the first trimester. 3.2.4.6 Mutagenicity and genotoxicity In vitro assays with hexamethylenetetramine showed both positive and negative results. In vivo assays were negative except when hexamethylenetetramine was administered at very high doses. 3.2.4.7 Carcinogenicity No evidence of treatment-related tumours were seen in rats and mice in oral studies with doses up to about 2500 mg/kg b.w./day for up to 2 years or in dermal studies with concentrations up to 30% for up to 2 years. 3.2.4.8 Evaluation The critical effect of hexamethylenetetramine is evaluated to be sensitisation following exposure by inhalation or by skin contact; the sensitising potential of hexamethylenetetramine is possibly due to its metabolite, the known strong sensitiser formaldehyde. 3.2.5 1-Methyl-1,2-ethanediyl dioleate (Appendix 5)No toxicological studies regarding effects following exposure to 1-methyl-1,2-ethanediyl dioleate have been found. Only one reference to 1-methyl-1,2-ethanediyl dioleate has been found in which it was stated that vapour of heated 1-methyl-1,2-ethanediyl dioleate can cause irritation in humans when inhaled. This study is of limited value because of the lack of information on exposure levels and duration. In addition, the irritation might be caused by degradation products of 1-methyl-1,2-ethanediyl dioleate formed by the heating of the substance. No hazard assessment of 1-methyl-1,2-ethanediyl dioleate is possible because of lack of data. 3.2.6 Isopropyl myristate (Appendix 6)3.2.6.1 Toxicokinetics Only 0.25% of the substance was absorbed by monkeys exposed for 5 seconds to a spray containing isopropyl myristate. Dermal application of isopropyl myristate resulted in local penetration in rabbits and guinea pigs, but not in hairless mice. Subcutaneous injection to mice indicated that if absorbed, the substance will be distributed into almost all organs. In humans, isopropyl myristate has been shown to enhance the penetration rate of several other chemicals through human skin. 3.2.6.2 Single dose toxicity Isopropyl myristate has a low acute oral and dermal toxicity with LD50-values of > 13700 mg/kg b.w. for rats and > 5000 mg/kg b.w., respectively, being reported. No deaths or evidence of systemic toxicity occurred in rats exposed to an aerosol containing 16-20% isopropyl myristate for 6.5 seconds/minute for an hour; the only effect observed was lethargy. 3.2.6.3 Irritation and Sensitisation No or minimal skin irritation has been observed in humans exposed dermally to isopropyl myristate for up to 21 days. When applied in petrolatum under cover for 48 hours, 10% isopropyl myristate was the lowest non-irritating concentration. Rabbits treated dermally on the ears with 1% or more isopropyl myristate in propylene glycol twice daily for 2 weeks developed comedones. Application of isopropyl myristate to the eyes of rabbits daily for 3 days caused no or slight irritation that had vanished after 7 days. 3.2.6.4 Repeated dose toxicity Increased lung weights, but no histological changes, were observed in guinea pigs exposed for an hour three times a day, seven days a week for 4 or 13 weeks to isopropyl myristate (in an aerosol antiperspirant) in concentrations from 10 mg/m3. Monkeys exposed for 13 weeks to 0.95-6.7 mg/m3 isopropyl myristate in an aerosol antiperspirant coughed and wheezed. The lung function tests were normal, but histological examination revealed a dose-related accumulation of macrophages within the alveolar and bronchiolar walls of the lungs. No significant effects were seen in rats and hamsters exposed up to 0.16 mg/m3 isopropyl myristate aerosol (in a complex fragrance mixture) for 4 hours/day for 13 weeks. 3.2.6.5 Reproductive toxicity No gross abnormalities were seen in offspring of mice treated with 0.1ml of 1% isopropyl myristate in acetone applied to the skin once a week for 18 months. No human data have been found. 3.2.6.6 Mutagenicity and genotoxicity Isopropyl myristate was negative in an Ames with and without metabolic activation. 3.2.6.7 Carcinogenicity No significant differences were observed in the incidence of skin or internal tumours between negative control animals (mice and rabbits) and animals, which were given dermal applications of undiluted (or diluted) isopropyl myristate twice a week in lifetime (or shorter) studies. In a mice skin painting study, a 50% solution of isopropyl myristate accelerated the carcinogenic activity of 0.15% benzo(a)pyrene, a known skin carcinogen. No human data have been found. 3.2.6.8 Evaluation The critical effect of isopropyl myristate is the local effects (mainly irritation) it might cause. In animals, undiluted isopropyl myristate was moderately to severely irritating to the skin following repeated exposure and at most slightly irritating to the eyes. However, in the majority of studies with human volunteers no or minimal skin irritation has been observed following repeated dermal administration of undiluted isopropyl myristate. In one human study the highest non-irritant concentration of isopropyl myristate was 10%. The wheezing and coughing of monkeys exposed by inhalation to a formulation containing isopropyl myristate is probably a result of respiratory tract irritation. It should be noted, that in the inhalation studies, isopropyl myristate has only been tested as part of a formulation and not as the pure substance. It is a cause of concern that isopropyl myristate has the ability to enhance the dermal absorption of other chemicals since it, as an inert ingredient in pesticide formulations, might alter the absorption of the active substance or of other of the inert ingredients and thus possibly alter the toxicity of these chemicals. 3.2.7 Sodium ligninsulphonate (Appendix 7)3.2.7.1 Toxicokinetics Systemic effects following oral administration indicate that sodium ligninsulphonate is absorbed by this route. However, because of the size of the molecule and its ionisation in solution, the absorption is probably limited. 3.2.7.2 Single dose toxicity LD50-values for oral administration of sodium ligninsulphonate of 6000 mg/kg b.w. and greater than 40000 mg/kg b.w. have been reported for mice and rats, respectively, and the LC50-value for inhalatory administration to rats was reported to be greater than 480 mg/m3. 3.2.7.3 Irritation and Sensitisation Limited data on experimental animals indicate that sodium ligninsulphonate may irritate eyes, skin, and the upper respiratory tract. However, there is no information about the dose levels causing irritation, or whether the irritation was caused by the sodium ligninsulphonate powder or by the chemical in a solution. No human data have been found. 3.2.7.4 Repeated dose toxicity Guinea pigs exposed to sodium ligninsulphonate in the drinking water in doses at or above 1700 mg/kg b.w./day for up to 6 weeks developed ulcers in the upper part of the colon; at higher doses, stomach ulcers as well as weight loss, diarrhoea and deaths also occurred. Rats exposed (drinking water) to doses at about 10000 mg/kg b.w./day for 16 weeks had histological changes of the liver and kidneys, and an increased weight of the same organs as well as the spleen; sodium ligninsulphonate caused no adverse effects at a dose level up to about 2500 mg/kg b.w./per day. 3.2.7.5 Reproductive toxicity No data have been found regarding reproductive and developmental effects following exposure by inhalation, oral administration, or dermal contact. Sodium ligninsulphonate showed no estrogenic activity in a yeast screening assay. 3.2.7.6 Mutagenicity and genotoxicity No data were found. 3.2.7.7 Carcinogenicity 3.2.7.8 Evaluation Based on the available data, the critical effect of sodium ligninsulphonate is probably the irritative that it may cause to the eyes, skin, and upper respiratory tract. However, no details on the exposure were available. The hazard assessment is limited by the lack of data, as no data regarding reproductive and developmental effects, mutagenic and genotoxic potential, and effects following long-term exposure, including carcinogenicity, are available. 3.2.8 Calciumdodecylbenzenesulphonate (CaDBS) (Appendix 8)3.2.8.1 Toxicokinetics CaDBS is readily absorbed from the gastrointestinal tract and excreted equally via urine and faeces. 3.2.8.2 Single dose toxicity CaDBS is of low acute toxicity with reported LD50-values in rats and mice of about 4000 mg/kg b.w. 3.2.8.3 Irritation and sensitisation No data were available. 3.2.8.4 Repeated dose toxicity No data were available. 3.2.8.5 Reproductive toxicity No data were available. 3.2.8.6 Mutagenicity and genotoxicity No data were available. 3.2.8.7 Carcinogenicity No data were available. 3.2.8.8 Evaluation No toxicological studies regarding effects following exposure to CaDBS have been found and thus, no hazard assessment is possible. Analogy considerations with the structural analogues linear alkyl benzene sulphonates (LAS) indicate that CaDBS may be irritating to the skin, eyes and respiratory tract. 3.2.9 Ethylene Glycol (EG) (Appendix 9)3.2.9.1 Toxicokinetics EG is rapidly absorbed and distributed following inhalation (rats: 75-80%), oral (rats and mice: 90-100%), and dermal administration (rats: 30%; dermal, mice: 85-100%). EG is metabolised by oxidation via glycol aldehyde and glycolic acid to glyoxylic acid, which is converted either to carbon dioxide or to oxalic acid. Generally, metabolism begins immediately after administration of EG, and excretion of most of the parent compound and metabolites is complete 12 to 48 hours after dosing. The major excretory end products are carbon dioxide in exhaled air, and glycolate and unchanged EG in the urine. 3.2.9.2 Single dose toxicity There are numerous case reports in the literature of poisoning in humans due to accidental or intentional ingestion of EG; the minimal lethal oral dose for humans has been estimated to be about 1600 mg/kg b.w. for adults. 3.2.9.3 Irritation and sensitisation EG has not shown a particularly irritating potential to eyes or skin in humans and did not show irritating properties when applied to the skin of rabbits; prolonged dermal exposure to humans can result in skin maceration. The data on eye irritation in experimental animals are conflicting but overall, the data indicate an eye irritating potential of EG. Male volunteers complained of irritation of the throat following exposure to 17 to 49 mg/m3 and concentrations greater than about 200 mg/m3 were intolerable due to strong irritation of the upper respiratory tract. 3.2.9.4 Repeated dose toxicity Numerous studies in experimental animals have revealed that repeated oral administration of EG resulted primarily in toxic effects in the kidneys; overall, a NOAEL for renal effects in male rats, the most sensitive species, of 200 mg/kg b.w./day is considered taking into account the reliability of the various studies. 3.2.9.5 Toxicity to reproduction Dietary exposure of rats to EG at dose levels up to 1000 mg/kg b.w./day (the highest dose level in the study) for three generations produced no effects on fertility, fecundity, or reproductive performance. When EG was administered to mice in the drinking water for 14 weeks (continuous breeding study), reduced fertility and fecundity, and foetotoxic effects, including malformations were observed at about 1640 mg/kg b.w./day; the NOAEL was about 840 mg/kg b.w./day. 3.2.9.6 Mutagenicity and genotoxicity Most of the mutagenicity and genotoxicity tests available indicate that EG is not a mutagenic or genotoxic substance although some positive results have been reported. In a micronucleus assay in mice, increased numbers of micronuclei was observed following administration (oral, intraperitoneal injection) of very high doses (2800 to 13900 mg/kg b.w.) and thus, the result is not considered as being reliable. Overall, EG is considered not to be a mutagenic or genotoxic substance. 3.2.9.7 Carcinogenicity No evidence of a carcinogenic effect of EG was observed at dietary concentrations of up to approximately 2000 mg/kg b.w./day for 2 years in rats or of up to approximately 12000 mg/kg b.w./day for 2 years in mice. 3.2.9.8 Evaluation The critical effects following exposure to EG are the effects in the kidneys, which are observed in both humans and experimental animals; the developmental effects observed in experimental animals; and the irritative effects observed in humans and experimental animals following inhalation of EG. 3.2.10 Propylene Glycol (PG) (Appendix 10)3.2.10.1 Toxicokinetics PG is rapidly absorbed from the gastrointestinal tract and following dermal contact through damaged skin. It is metabolised to lactic and pyruvic acid, which enter the energy production. From 20 to 45% PG is recovered unchanged in the urine. 3.2.10.2 Single dose toxicity In humans, high concentrations of PG cause CNS depression and acidosis. Animal data show low acute oral and dermal toxicity with reported oral LD50-values being of 18000 to 33500 mg/kg b.w. and a dermal LD50-value in the rabbit of 20800 mg/kg b.w. 3.2.10.3 Irritation and sensitisation Exposure by inhalation did not result in effects in the respiratory tract of humans. In rats, nose bleeding and goblet cell enlargement were reported from inhalation exposure to aerosols at concentrations from 160 and 1000 mg/m3, respectively, for 90 days, probably related to the hygroscopic character of the substance. PG was reported to be a mild skin and eye irritant in rabbits. It has been reported to cause contact dermatitis in humans, which is considered to be primarily of irritative nature, but which occasionally may be of allergic nature. 3.2.10.4 Repeated dose toxicity No effects were seen in humans following repeated exposure by inhalation to concentrations up to 94 mg/m3 for several weeks. Rats exposed by inhalation to 2200 mg/m3 as an aerosol over 90 days showed an effect (not dose-related) on haematological parameters. 3.2.10.5 Toxicity to reproduction No developmental effects were seen in different animal species (rats, mice, hamsters) treated orally with PG at doses greater than 1000 mg/kg b.w./day. No effects on fertility were reported in mice treated orally with doses up to 10000 mg/kg b.w./day or in rats treated by inhalation with up to 354 mg/m3. 3.2.10.6 Mutagenicity and genotoxicity Only one of several in vitro assays with PG, a chromosome aberration test was positive, and all in vivo tests were negative. 3.2.10.7 Carcinogenicity No carcinogenic effect was reported in a 2-year oral study in rats or in a 120-week dermal study in mice. 3.2.10.8 Evaluation PG is considered to be of low toxicity, the critical effects being the irritative effects on the skin and the dehydrating effect on the mucous membranes. 3.2.11 2-Butoxyethanol (EGBE) (Appendix 11)3.2.11.1 Toxicokinetics EGBE is absorbed and distributed throughout the body (humans and rats) following inhalation, oral administration, and dermal contact. EGBE is metabolised to 2-butoxyacetic acid (2-BAA), the toxic metabolite. 3.2.11.2 Single dose toxicity EGBE seems of low acute toxicity in humans with haematological changes and metabolic acidosis being the primary effects after acute oral ingestion of large doses of EGBE (combined with other solvents). 3.2.11.3 Irritation and sensitisation Irritation of the nose and throat, and eyes was noted in human volunteers exposed by inhalation to EGBE at concentrations from 490-957 mg/m3 for 4-8 hours, but not in volunteers exposed to EGBE (98 mg/m3) for 2 hours. The NOAEL for irritative effects of EGBE in humans is above 100 mg/m3. 3.2.11.4 Repeated dose toxicity Haematological effects, particularly haemolysis, have been identified as the critical end-point in toxicological studies following both acute and repeated exposures to EGBE. In addition to the haemolytic effect, effects in the liver, spleen and kidney have also been observed following exposure to EGBE; the available data indicate that these effects are secondary to haemolysis. For repeated inhalation exposure, 152 mg/m3 was a LOAEC for haematological changes for rats (both sexes) and for female mice, and a NOAEC for male mice. For repeated oral exposure, a LOAEL for haematological effects of 69 and 82 mg/kg b.w./day is considered for male and females rats, respectively, and a NOAEL of 357 mg/kg b.w./day for mice. Certain species differences in sensitivity have been observed regarding the haematological effects of EGBE, with rats being particularly sensitive, mice sensitive, and guinea pigs appearing relative insensitive. Humans appear to be less sensitive than are rats to the haemolytic effects of EGBE as no or only very slight haemolytic effects were observed in the poisoning cases after acute oral ingestion of large doses. Furthermore, the only indication of haemolysis (small changes for haematocrit and mean corpuscular haemoglobin concentration MCHC) observed in workers exposed to an average airborne concentration of EGBE of 2.9 mg/m3 was in the range of normal clinical values; the NOEC for haemolytic effects in humans is therefore above 3 mg/m3. This difference in sensitivity between rats and humans is supported by in vitro studies, which have shown that erythrocytes from humans were unaffected by incubations with 2-butoxyacetic acid (2-BAA, the toxic metabolite of EGBE) at concentrations, which produced total rat erythrocyte haemolysis. 3.2.11.5 Toxicity to reproduction The reproductive and developmental toxicity of EGBE has been studied in several studies in rats, mice and rabbits following inhalation, oral administration, or dermal application (developmental toxicity only). It can be concluded from these studies that EGBE does not affect the reproductive organs of parents (both males or females), and only results in adverse reproductive and developmental effects at dose levels, which also result in parental toxicity. No malformations were observed in any of the studies. No data have been located regarding toxicity to reproduction in humans. 3.2.11.6 Mutagenicity and genotoxicity No increases in micronuclei or sister chromatid exchanges were observed in workers exposed to both EGBE and to 2-ethoxyethanol (EGEE). EGBE has been tested for its potential to induce gene mutations in in vitro systems and cytogenetic damage in both in vitro and in vivo systems. In most of the tests, EGBE has given negative results. Overall, the available data do not support a mutagenic or clastogenic potential for EGBE. 3.2.11.7 Carcinogenicity Two-year inhalation studies have shown no evidence of carcinogenic activity in male rats, equivocal evidence in female rats, and some evidence in mice. The relevance of the observed tumours to an assessment of the carcinogenicity of EGBE to humans has been questioned. As EGBE is generally negative in the genotoxicity tests and as glycol ethers generally appear unlikely to be carcinogenic, the concern for a carcinogenic potential of EGBE is low. 3.2.11.8 Evaluation The critical effects following exposure to EGBE are the irritative effects on the respiratory tract and eyes observed in humans and in experimental animals, and the haemolytic effect observed in experimental animals and probably also indicated by the sparse human data available. The NOAEC for irritative effects of EGBE in humans is above 100 mg/m3. Data indicate that humans are less sensitive to the haemolytic toxicity of EGBE than are rats and mice. For humans, the NOEC for haemolytic effects is above 3 mg/m3. For repeated inhalation exposure in experimental animals, 152 mg/m3 was a LOAEC for haematological changes for rats (both sexes) and for female mice, and a NOAEC for male mice. For repeated oral exposure, a LOAEL for haematological effects of 69 and 82 mg/kg b.w./day is considered for male and females rats, respectively, and a NOAEL of 357 mg/kg b.w./day for mice. 3.2.12 1-Methoxy-2-propanol (2PG1ME) (Appendix 12)3.2.12.1 Toxicokinetics 2PG1ME appears to be absorbed by all routes of exposure. It is primarily metabolised via O- demethylation and oxidation to carbon dioxide; a minor part being excreted via the urine in conjugated form. The toxic metabolite methoxyacetic acid is not formed by 2PG1ME, but only by its â-isomer 2-methoxy-1-propanol, 1PG2ME. 2PG1ME makes out minimum 95% commercial propylene glycol monomethyl ether (PGME) and maximum 5% is the â-isomer. 3.2.12.2 Single dose toxicity In humans, inhalation exposure to PGME vapours at concentrations from 1125 mg/m3 for 1 to 7 hours caused slight CNS-depression. An LC50-value in rats and guinea pigs of approximately 54600 mg/m3 has been reported for 4 or 10 hours exposure, respectively. CNS-depression was the major symptom reported from acute inhalation studies in experimental animals. Oral LD50-values of 5000 to 10800 mg/kg b.w. have been reported for rats, mice, rabbits and dogs. A dermal LD50-value of about 13000 mg/kg b.w. has been reported in rabbits. 3.2.12.3 Irritation and sensitisation Volunteers complained of eye and nose irritation from inhalation exposure to 938 mg/m3 for 15-30 minutes, and of throat irritation after 45 minutes. In rats, respiratory tract irritation was reported from 4 hours exposure to 37500 mg/m3. PGME was not irritating or mildly irritating to the eyes of rabbits. No information was available on skin irritation from PGME exposure in humans. Rabbits showed no or slight skin irritation following exposure to PGME. 3.2.12.4 Repeated dose toxicity No human data were available. In animals, CNS depression and effects in the liver (increased weight, hypertrophy, and occasionally slight non-fatty degeneration and granulation)were reported for rats, mice, guinea pigs, and rabbits following repeated exposure to 5450-21800 mg/m3 for up to 6 months. In a two-year inhalation study in rats, increased liver weight and incidence of eosinophilic hepatocellular foci were observed at 11250 mg/m3, and development of glomerulonephritis was significantly higher in male F344-rats at this concentration; however, this finding was related to increased levels of á2u-globulin, which is considered specific to male rats of that strain. In dogs, oral administration of PGME for 14 weeks resulted in CNS depression at doses from 920 mg/kg b.w./day. 3.2.12.5 Reproductive toxicity PGME did not affect the testes of rats following inhalation exposure at concentrations up to 2250 mg/m3 for 10 days or in mice following oral exposure to 2500 mg/kg b.w./day for 25 days. Occurrence of macrophages in the testes and epididymides of dogs treated orally with 462-2772 mg/kg b.w./day for 14 weeks was reported, but the finding is of unknown significance. Delayed ossification of the sternebrae or the skull was reported in the offspring of rats treated during gestation by inhalation of 11250 mg/m3 PGME or orally with 739 mg/kg b.w./day, respectively; maternal toxicity was seen in the inhalation at this concentration as well. In a continuous breeding study in mice given PGME in the drinking water, reduced birth weight and weights of epididymides and prostate were observed at 3300 mg/kg b.w./day; no effects were observed in the dams. No foetotoxicity was seen in mice treated orally with doses up to 1848 mg/kg b.w./day, or in rabbits following inhalation of up to 11250 mg/m3 or orally at doses up to 924 mg/kg b.w./day. 3.2.12.6 Mutagenicity and genotoxicity PGME was negative in three different in vitro tests (Ames test, unscheduled DNA synthesis, chromosomal aberration); no in vivo tests were available. 3.2.12.7 Carcinogenicity No increase in tumour incidence was seen in a 2-year inhalation study in rats and mice exposed to concentrations up to 11250 mg/m3 PGME. 3.2.12.8 Evaluation 2PG1ME is considered to be of low systemic toxicity, the critical effects being the irritative effects to the eyes, the mucous membranes and the respiratory tract, and depression of the CNS. 3.2.13 Diethylene glycol mono-n-butyl ether (DEGBE) (Appendix 13)3.2.13.1 Toxicokinetics In rats, the absorption is about 85% following oral administration and following dermal contact, about 30-50% at low dose levels (200 mg/kg b.w.) and about 3-18% at high dose levels (2000 mg/kg b.w.). The major urinary metabolite was 2-(2-butoxyethoxy)acetic acid at both exposure routes; only minor amounts (a few percent) were excreted in faeces and about 5% as carbon dioxide. No data regarding toxicokinetics following inhalation have been found. 3.2.13.2 Single dose toxicity DEGBE is of low acute toxicity following oral administration and dermal application in experimental animals with oral LD50-values ranging from 2000 to 9600 mg/kg b.w. (rats, mice, rabbits, and guinea pigs) and dermal LD50-values greater than 2000 mg/kg b.w. (rats and rabbits). An LC50-value of about 73000 mg/m3 for rats following exposure to the acetate of DEGBE indicate a low order of acute inhalation toxicity for DEGBE as well. No human data have been found. 3.2.13.3 Irritation and sensitisation A few human case reports of irritation (skin, eyes, and upper respiratory tract) and sensitisation to DEGBE have been reported. 3.2.13.4 Repeated dose toxicity Two-week inhalation studies in rats have revealed effects indicative of local lung effects at exposure levels from 100 mg/m3; these types of effects were, however, not reported in a 5-week or in a 13-week inhalation study in rats. In the 5-week study, effects on the liver were reported (changes in weight, slight paleness, and slight hepatocyte vacuolisation from 13 mg/m3). However, in the 13-week study (OECD Guideline 413), no toxicologically relevant effects were observed. Overall, a NOAEC of 95 mg/m3 is established for the various effects, both systemic as well as local effects in the lungs, observed in the studies of rats following exposure by inhalation to DEGBE. 3.2.13.5 Toxicity to reproduction No indications of reproductive and developmental effects were observed in rats in two one-generation studies at dose levels of up to 1000 mg/kg b.w./day (oral study) and 2000 mg/kg b.w./day (dermal study); in the oral study, post-natal effects (decreased weight of pups at day 14 of lactation) were observed in offspring from females administered 1000 mg/kg b.w./day and mated with untreated males whereas no post-natal effects were noted in the dermal study. 3.2.13.6 Mutagenicity and genotoxicity The data on mutagenicity and genotoxicity indicate that DEGBE is not a mutagenic or genotoxic substance neither in vitro nor in vivo. 3.2.13.7 Carcinogenicity No data on carcinogenic effects in humans or experimental animals have been found. 3.2.13.8 Evaluation The critical effects following exposure to DEGBE are the irritative effects on the skin and eyes observed in humans and in experimental animals, and the haemolytic effect observed in studies in experimental animals. 3.2.14 Dipropylene glycol monomethyl ether (DPGME) (Appendix 14)3.2.14.1 Toxicokinetics DPGME appears to be readily absorbed by all routes of exposure. Commercial DPGME consists of minimum 95% secondary alcohol isomers, which are metabolised to propylene glycol or dipropylene glycol, or conjugated to glucuronic acid. Excretion primarily occurs through urine. The reproductive toxicant methoxypropionic acid, which is a metabolic product of the primary alcohol isomers, was not found in urine in metabolism studies with DPGME. 3.2.14.2 Single dose toxicity Inhalation of DPGME (vapour and aerosol) at 3080 mg/m3 caused CNS depression in rats. Oral LD50-values in rodents and dogs were reported to range from 5000 to 7500 mg/kg b.w., and dermal LD50-values in rabbits to range from 9400 to > 19000 mg/kg b.w. 3.2.14.3 Irritation and sensitisation In humans, inhalation of 456 mg/m3 DPGME was irritating to the respiratory tract. Transient eye irritation was reported from application of a 20% aqueous solution of DPGME. Animal data indicated that DPGME is a mild eye irritant, but the substance is not a skin irritant in rabbits. In humans, no skin irritation or sensitisation resulted from a repeated patch test with DPGME. No sensitisation test in animals was available. 3.2.14.4 Repeated dose toxicity Transient CNS depression was reported in rats exposed to the maximum attainable vapour concentration of 1848 mg/m3 for 6 to 8 months. Slight granulation and non-fatty vacuolation of the liver was reported in rabbits, guinea pigs and monkeys at 1848 mg/m3 for 6 to 8 months. No effects were observed in other subchronic inhalation studies (90 days) in rats and rabbits at levels of up to 1232 mg/m3. No treatment-related effects were noted in rats following dermal application of up to 1000 mg/kg b.w./day for 28 days or in rabbits of up to 4700 mg/kg b.w./day for 90 days. 3.2.14.5 Toxicity to reproduction No effect on testes was reported in rodents exposed by inhalation at concentrations of up to 1232 mg/m3 for 90 days (rats, rabbits), or dermally with up to 1000 mg/kg b.w. (rats). Developmental toxicity studies showed no effects of DPGME in rats and rabbits at up to 1756 mg/m3. 3.2.14.6 Mutagenicity and genotoxicity DPGME was negative in three different in vitro tests (Ames test, unscheduled DNA synthesis, cytogenetic assay); no in vivo tests were available. 3.2.14.7 Carcinogenicity No data were found. 3.2.14.8 Evaluation DPGME is considered to be of low toxicity, the critical effect being irritation of the eye and the mucous membranes. 3.2.15 Polyethylene glycol dodecyl ether (polyEGDE) (Appendix 15)3.2.15.1 Toxicokinetics No data were found. 3.2.15.2 Single dose toxicity No data on systemic effect in humans from exposure to polyEGDE were available. Oral LD50-values in rats of 4150 and 8600 mg/kg b.w. were reported for polyEGDE. 3.2.15.3 Irritation and Sensitisation PolyEGDE was reported as a moderate skin irritant in humans. In rabbits, 75-500 mg/kg b.w. polyEGDE was mildly to moderately skin irritating, depending on the chain length. The substance was also moderately eye irritating in rabbits. Severe, but reversible, nasal irritation was caused by direct application of polyEGDE to nostril of rats, but this application way is considered irrelevant in normal use. No information was available on sensitising potential of polyEGDE. 3.2.15.4 Repeated dose toxicity 3.2.15.5 Reproductive and developmental effects No data were found. 3.2.15.6 Mutagenic and genotoxic effects PolyEGDE was negative in in vitro tests and in in vivo tests. 3.2.15.7 Carcinogenicity No data were found. 3.2.15.8 Evaluation On the basis on the scarce information available on polyEGDE, the critical effect from this substance is considered to be the irritation to the skin and eyes. 3.2.16 Cyclohexanone (Appendix 16)3.2.16.1 Toxicokinetics Cyclohexanone is absorbed by all routes of exposure and rapidly metabolises to cyclohexanol. Excretion occurs mainly through urine as glucuronide conjugates. 3.2.16.2 Single dose toxicity In animals, cyclohexanone is moderately toxic by inhalation (LC50-values in rats of 6200-32500 mg/m3 ), oral administration (LD50-values in rats of 1296-3460 mg/kg b.w), and dermal contact (LD50-values in rabbits of 794-3160 mg/kg b.w.). In humans, CNS symptoms and acidosis have been recorded after accidental ingestion of an unknown dose of cyclohexanone. 3.2.16.3 Irritation and sensitisation Cyclohexanone was reported to be irritating to eyes, nose and throat of humans following exposure at 306 mg/m3 for a few minutes, and to skin from 162 mg/m3. The substance is also a skin and eye irritant in animals. 3.2.16.4 Repeated dose toxicity Neurological symptoms in the central nervous system, including cognitive changes, have been reported from long-term occupational exposure to concentrations at 162-368 mg/m3 and confirmed in rat and rabbit studies after short time and prolonged exposure. Also peripheral nervous system effects were reported in humans at this level, but confirmation lacks from animal studies. 3.2.16.5 Toxicity to reproduction In a two-generation inhalation study in rats, fertility of male rats in the F1 was reported to be reduced at 5712 mg/m3, however, no details were available and thus, an evaluation of the effect of cyclohexanone on fertility is not possible on this basis. Slight developmental toxicity was reported in rats and mice following inhalation or oral administration of cyclohexanone, but at maternally toxic levels only. 3.2.16.6 Mutagenicity and genotoxicity In vitro mutagenicity tests with metabolic activation were negative, a few positive results have been reported without metabolic activation. All in vivo studies but one, of poor quality, were negative. Overall, the available data indicate that cyclohexanone is not a genotoxic or mutagenic substance. 3.2.16.7 Carcinogenicity In a chronic study in mice and rats exposed orally to cyclohexanone in the drinking water, tumours were reported in the lymphatic tissue, the liver, and in the lungs of mice, and in the adrenals and in the thyroid of rats. Thyroid tumours in rats may, in some cases, not be relevant for humans; however, the carcinogenic mechanism for cyclohexanone is not elucidated and no conclusion can be drawn on this effect. Some of the other tumour-types found are also of questionable relevance for humans, and the lack of dose-response indicate that the substance is not carcinogenic in these studies. However, no conclusive evaluation on the carcinogenic effect of cyclohexanone can be performed on basis of the available data. 3.2.16.8 Evaluation The available data indicate that CNS-depression and irritation of skin, eyes and respiratory tract are the critical effects of cyclohexanone. However, evaluation of the carcinogenic potential of cyclohexanone cannot be performed on the available data. 3.2.17 1-Methylpyrrolidone (NMP) (Appendix 17)3.2.17.1 Toxicokinetics NMP is readily absorbed following inhalation, oral ingestion and dermal contact, and distributed widely to organs and tissues with the highest concentrations occurring (rats) in the liver, small and large intestine, testes, stomach, and kidneys. NMP is rapidly metabolised and excreted in humans and experimental animals with the major route of excretion being the urine (rats: 85-88%). 3.2.17.2 Single dose toxicity No human data have been found. NMP is of low acute toxicity in the rat with reported 4-hour LC50-values being greater than 5100 mg/m3 or in the range of 3100-8800 mg/m3. The reported oral and dermal LD50-values ranged from 3600-7900 mg/kg and from 2500-10000 mg/kg, respectively, in the rat. 3.2.17.3 Irritation and sensitisation Several workers have experienced skin irritation and contact dermatitis on the hands after a few days of working with NMP; no signs of contact sensitisation have been reported. 3.2.17.4 Repeated dose toxicity Volunteers exposed to NMP at levels up to 50 mg/m3 for 8 hours did not report any discomfort to eyes or upper airways. Workers have reported severe eye irritation following exposure for a short time (30 minutes) to levels of about 3 mg/m3 (8-hour TWA), exposures around 66 mg/m3 were reported as being immediately uncomfortable (within 30 seconds) with minor eye irritation, and exposures above 200 mg/m3 were found unbearable following a few seconds of exposure. 3.2.17.5 Toxicity to reproduction No reproductive effects were noted in a two-generation study on rats (inhalation, 480 mg/m3). In a multigeneration study on rats, oral administration (500 mg/kg b.w./day for 13 months) affected reproduction and parental effects were noted. Several teratology studies have investigated the developmental toxicity of NMP in rats (most studies) and in rabbits; generally, no malformations were observed at dose levels, which did not induce maternal toxicity. Foetotoxic effects in form of a lower foetal body weight have been observed in some studies on rats at dose levels (480-620 mg/m3 (inhalation); 400-500 mg/kg b.w./day (oral administration); 750 mg/kg b.w./day (dermal administration)) that did not induce maternal toxicity. A neurobehavioral teratology study has shown an impairment of higher cognitive functions related to solving difficult tasks in rats exposed at 620 mg/m3 on gestation days 7-20, a dose level that did not induce maternal toxicity. 3.2.17.6 Mutagenicity and genotoxicity The mutagenicity and genotoxicity tests available indicate that NMP is not a mutagenic or genotoxic substance. 3.2.17.7 Carcinogenicity No carcinogenic effects were observed in rats exposed by inhalation (up to 400 mg/m3, 6 hours a day, 5 days per week for 2 years). 3.2.17.8 Evaluation Based on the available data, the critical effect in humans following exposure to airborne NMP is considered to be the irritative effects on the eyes and the respiratory tract; the critical effect in humans following dermal contact is considered to be skin irritation. Data obtained from studies on experimental animals do not indicate that other effects, including neurotoxic effects, than the irritative ones should be expected to occur following exposure to the levels of NMP eliciting these irritative effects. 3.2.18 4- Hydroxy-4-methyl-2-pentanone (HMP) (Appendix 18)3.2.18.1 Toxicokinetics HMP is apparently absorbed both by inhalation and by oral intake. No date have been found regarding metabolism and excretion, but the substance is expected to be eliminated in urine as conjugates, to enter the intermediary metabolism, or to be incorporated in the tissues. 3.2.18.2 Single dose toxicity Animal data indicate a low acute toxicity by all three routes of exposure, oral from 2520 to 4000 mg/kg b.w., a LD50-values in rats and mice reported dermal LD50-value in rabbits being reported at 13750 mg/kg b.w., while the lowest lethal inhalation exposure in rats was 4830 mg/m3 over 4 hours. 3.2.18.3 Irritation and sensitisation In humans, HMP was irritating to the eyes, nose and throat from 15 minutes exposure to 483 mg/m3. Mucous membrane irritation occurred in animals from 10143 mg/m3, and the substance is mildly irritating to rabbit eyes and skin. In humans, HMP is defatting to the skin. No data were available on the sensitisation potential of HMP. 3.2.18.4 Repeated dose toxicity Inhalation exposure of rats at 4830 mg/m3 HMP for 6 weeks resulted in slight lethargy during and after exposure, increased liver and kidney weights, and unspecified histological changes in the proximal renal tubules of male rats. The kidney toxicity of male rats is evaluated not to be relevant to humans, but a species and gender specific finding associated with accumulation of alpha-2-microglobulin. 3.2.18.5 Toxicity to reproduction No information was found. 3.2.18.6 Mutagenicity and genotoxicity HMP was negative in in vitro assays, while no in vivo assays were available. 3.2.18.7 Carcinogenicity No information was found. 3.2.18.8 Evaluation The limited available toxicological information on HMP indicates that the substance is irritating to the eyes and the mucous membranes. Other endpoints could not be evaluated because of insufficient data. 3.3 Critical effects and existing regulationTable 3 summarises the critical effects identified for each coformulant as a result of the hazard assessment as well as the existing regulations for that substance. The following abbreviations are used in the Table: -: no regulation; b.w.: body weight; C-value: quality criteria in ambient air; CNS: central nervous system; L: the C-value is based on odour, not health based; LAS: linear alkyl benzene sulphonate; LO(A)EL(C): lowest observed (adverse) effect level (concentration); Mn: manganese; NO(A)EL(C): no observed (adverse) effect level (concentration); OEL DK: occupational exposure limit in Denmark; RDT: repeated dose toxicity.
Abbreviations related to EU-classification are explained below the Table. -: no regulation; b.w.: body weight; C-value : quality criteria in ambient air; CNS: central nervous system; L: the C-value is based on odour, not health based; LAS: linear alkylbenzene sulphonate; LO(A)EL(C): lowest observed (adverse) effect level (concentration); Mn: manganese; NO(A)EL(C): no observed (adverse) effect level (concentration); OEL DK: occupational exposure limit in
a EU-classification and labelling system consists of classes of danger and risk phrases noted in abbreviated form as shown below. R-phrases can be combined in order to indicate the route of exposure, e.g. R48/20/22 “Harmful: Danger of serious damage to health by prolonged exposure by inhalation and if swallowed”.
|