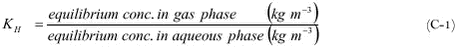Dry deposition and spray drift of pesticides to nearby water bodies
Appendix C. Derivation of the basic equation for the surface resistance of waterReferencesIn this appendix the surface resistance rc for water bodies will be derived. The exchange of gases between the atmosphere and streams, lakes and seas has traditionally been described in a way that is more familiar to engineers than to atmospheric scientists. In stead of describing the exchange with the resistances as atmospheric scientists do they describe the exchange with mass transfer coefficients. For that reason we will describe the engineering approach of exchange here first and then we will see how the derived mass transfer coefficients can be translated into resistances. The physics and chemistry of the processes involved are of course the same for both approaches, only the way they are described is different.
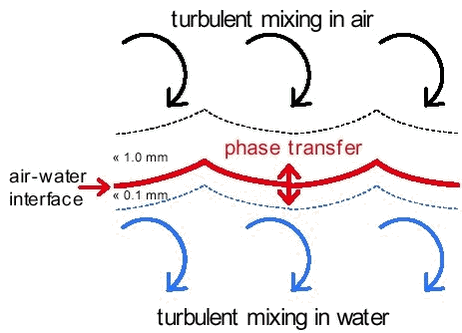
Figure C-1. Model for air-water exchange. On both sides of the air-water interface there is a stagnant or intermittently mixed boundary layer (about 1 mm thick in the air and about 0.1 mm thick in the water). The remaining parts of the air and the water are assumed to be well mixed by turbulence. Phase transfer takes place at the air-water interface. Fig.C-1. visualises the processes that play a role in the exchange of gases between the atmosphere and water ( Schwarzenbach, 1993): The mass transfer from the atmosphere to the water and vice versa depends in principle on the speed for each subsequent step. There are different models available to describe this situation (Schwarzenbach et al., 1993). The main difference between the results of these models is the fact that the transport velocity through the stagnant or intermittent layer increase with the diffusivity in model a) and with the square root of the diffusivity in model b). It should be noted that many of the parameters that are important in such models like the thickness of the stagnant or intermittent layer cannot be measured, nor can the rate at which the surface is renewed in the surface renewal model. In practice this means that both models include fitting parameters that when appropriately adjusted yield exchange rate estimates that are in agreement with experimental values. It should also be noted that the basic equations for exchange are the same in the stagnant two-film model and the surface-renewal model (Schwartz, 1992; Schwarzenbach et al., 1993). It is only the parameterization of the exchange rate that is different. In the following the basic equations are derived. In the basic equations concentrations are either expressed in the gas phase or in the aqueous phase. In case of equilibrium gas phase and aqueous phase concentrations are related through Henry’s law:
It should be noted here, that there are numerous definitions of Henry’s law coefficients, having different units and even different senses. In the model it is assumed that that the bulk of the gaseous and aqueous phases is homogeneously mixed. In the following equations are given for the fluxes in each step. The flux (Fg in kg m-2 s-1) from the bulk gas phase through the stagnant or intermittent layer in the atmosphere to the interface is described by:
where: This equation states that the flux depends on a speed, also called mass transfer coefficient kg and the difference in concentration between the bulk gas phase and the interface. Hence it will depend on the concentration differences in which way (to or from the water) the transport will go. The flux (Fi in kg m-2 s-1) across the interface is described by:
where: This flux depends on the concentration differences between both sides as well as on the molecular speed of the molecule and how big the change is that a collision will result in a transport across the interface. KH is just used to “translate” an aqueous phase concentration in a gas phase concentration, because otherwise concentrations cannot be compared. The flux (Fw in kg m-2 s-1) from the interface through the stagnant or intermittent layer in the water to the bulk aqueous phase is described by:
where: ß = factor by which the aqueous phase mass transfer enhances due to removal of the material by chemical reaction. If there is no enhancement ß is 1. If there is enhancement ß will be larger than 1. This equation indicates that the flux depends on a speed, also called mass transfer coefficient kw and the difference in concentration between the interface and the bulk water phase. Hence it will depend on the concentration differences in which way (to or from the air-water interface) the transport will go. After a very short time a steady state situation will be reached, where the fluxes Fg, Fi and Fw are equal to each other. The concentrations cg,i and cw,i at the interface are generally not known. For that reason it is useful to express the fluxes in the bulk concentrations cg and cw. This can e.g. be done by finding cw,i that still is a function of cg,i from equations (C-3) and (C-4). Then substituting the expression for cw,,i in equation (C-4) and then finding cg,i from equations (C-4) and (C-2). In this way the following equation is found for the flux:
where Kg is the overall gas phase mass transfer coefficient (m s-1), which is defined by:
Equation C-5 is in principle the same as equation (5) in the main report where cg,surface = KHcw. It should be noted that the flux can be expressed as an overall aqueous phase mass transfer coefficient as well:
where Kw is defined by:
Equations (C-7) and (C-8) are aqueous phase equivalents of (C-5) and (C-6). The terms in (C-6) or (C-8) add like electrical resistances in series, illustrating that the mass transfer is governing by three subsequent transfer processes. If the mass accommodation coefficient is greater than of the order of 1× 10-4, i.e. that the more than 1 out of 10000 gas molecules are absorbed when they hit the water surface the transport through the interface is so fast that it does not limit the overall transfer (Schwartz, 1992). The mass accommodation coefficient has only been determined for a limited number of more common atmospheric gases that have a low molecular weight (Seinfeld and Pandis, 1998) and not at all for pesticides. So it cannot be excluded that for some pesticides the transport through the interface is so slow that it limits the overall mass transfer. In the following, we will assume that the mass transfer coefficient of pesticides is so high that the second term in (C-6) and (C-4) can be neglected. If this is not correct, the mass transfer will occur at a lower rate than predicted with the simplified forms of (C-6) and (C-4). In the following the effect of chemical reactions is also neglected and it is hence assumed that ß = 1. In reality, however, reactions can be important for some pesticides, especially those that e.g. dissociate very fast in water. If there is no reaction and no resistance at the interface (i.e. the mass accomodation coefficient is large enough) (C-6) becomes:
There is in that case a critical Henry’s law coefficient KH,crit for which the gas and aqueous phase mass transfer coefficients are equal:
If KH >> KH,crit the overall mass transfer is controlled by transfer in the aqueous phase and for KH << KH,crit the overall mass transfer is controlled by the transfer gas phase. The approach in this section is the engineering approach. The mass transfer coefficient in the gas phase kg covers in fact the transport from a certain reference height in a part of the turbulent atmosphere, then through the stagnant or intermittently mixed boundary layer to the interface/surface. In the engineering approach the concentration in the bulk air phase is the same everywhere. Atmospheric scientists have a different approach and allow a vertical concentration gradient in the bulk air that is caused by dry deposition. Meteorologists use the following equation for the flux:
where (see section 2.4 of the main report): Comparing (C-11) for the situation where rc is zero with (C-2) describing the same situation leads to the following equation:
Comparing (C-11) with (C-5) with the Kg value from (C-9) leads to the equation for the surface resistance:
This relation shows that rc increases with KH and decreases with the mass transfer coefficient in the aqueous phase, which is a measure of how fast the compound is transported away from the interface into the bulk water. The molecules that have been transported away from the surface can then again be replaced by “fresh” molecules that reach the interface from the gas phase. It is equation C-13 that we will use in the main report to calculate rc. It should be noted here that the model not only describes dry deposition to water surfaces, but also emission from water surfaces, e.g. emission that can occur due to spills of chemicals. ReferencesSchwartz, S.E. (1992). Factors governing dry deposition of gases to surface water. In: Schwartz, S.E. and Slinn, W.G.N. Precipitation scavenging and atmosphere-surface exchange. Hemisphere Publishing Corporation, Washington D.C., USA, 789-801. Schwarzenbach, R.P., Gschwend, P.M., Imboden, D.M. (1993). Environmental organic chemistry. John Wiley, New York, USA. Seinfeld, J.H., Pandis, S.N. (1998) Atmospheric chemistry and physics. John Wiley, New York, USA. |