|
| Front page | | Contents | | Previous | | Next |
The Influence of Sorption on the Degradation of Pesticides and other Chemicals in Soil
3 Sorption
Even though it till now has not been possible to give a complete description of the binding of xenobiotic chemicals to soil, a vast number of studies of this subject have been carried out just as a large number of processes have been described because the binding of the substances in the soil is of great importance to their fate. The sorption of chemicals to soil can vary from being completely reversible to being completely irreversible. For certain combinations of substance and soil, the desorption rate can be considerably slower than the sorption rate. The sorption depends on the molecular structure of the chemical and the soil properties, such as the amount and the type of organic matter, the distribution of particle size, and the cation composition. The sorption may be purely physical or chemical by nature. Gevao et al. (2000) distinguished between ionic bonding, hydrogen bonding, van der Waals forces, ligand exchange, complexes formed by charge transfer, hydrophobic partitioning, covalent bonding, and sequestration. Figure 1 shows an outline from Gevao et al. (2000) of different types of pesticides.
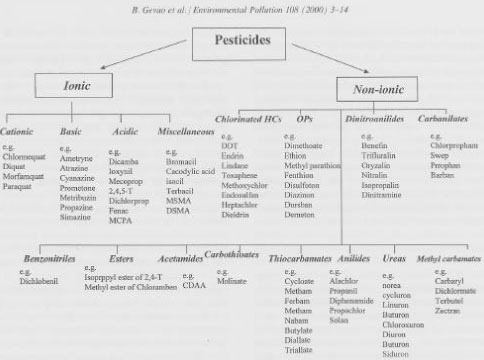
Figure 1. Outline of pesticides classified according to their molecular structure (from Gevao et al., 2000). The figure is reproduced with the kind permission from Elsevier.
Figur 1. Oversigt over pesticider inddelt efter molekylestruktur (fra Gevao et al., 2000). Figuren er gengivet med tilladelse fra Elsevier.
Ionic bonding occurs when the chemicals occur in cationic form in the soil or can be protonated, and the substances are bound to hydroxyl groups in humus, either phenolic or carboxyl groups. Cationic substances are e.g. diquat and paraquat while e.g. the triazines, which are alkaline, can form ionic bonds if both the chemical and the hydroxyl groups on humus have been ionised by the present pH-value in the soil. Hydrogen bonds can be formed between functional groups in humus that contains oxygen or OH and similar groups in the pesticide molecules. Hydrogen bonds are supposed to be of great importance to the sorption of non-ionic pesticides. If the pH-value in the soil is below the pKa-value of acidic and anionic pesticides such as phenoxy acids, phenoxyacetic esters, and dicamba, these are brought on a non-ionic form by which they also can form part of hydrogen bonds. The triazines can also form part of hydrogen bonds with the parts of the molecule that are not ionised. E.g. hydrogen bonds can be formed between carbonyl groups in humic acids and secondary amine groups in the triazines. Glyphosate is often mentioned as a substance that does not bind to the soil organic matter. However, Piccolo and Celano (1994) showed that glyphosate [N-(phosphonomethyl)glycine] via its phosphono group formed hydrogen bonds with O-atoms in soluble humic acids. Van der Waals forces are weak dipolar attractions that occur frequently where non-ionic or non-polar chemicals come into contact with humic acid molecules. Van der Waals forces are thought to be the dominant type of binding for picloram and 2,4-D (Khan, 1973). In hydrophobic partitioning the soil organic matter is regarded as an organic phase that is immiscible with water whether the organic matter is solid or dissolved. When referring to hydrophobic partitioning, the part of the chemical that is found on the hydrophobic phase is regarded as being dissolved in this phase. Hydrophobic partitioning is an important element in the binding of the "old" chlorinated compounds such as DDT, but it also occurs as one of the binding processes for trazine and urea herbicides. Covalent bonds are chemically well-defined bonds, which most frequently are irreversible and which by definition result in the originally well-defined structure of the pesticide, metabolite or other chemicals no longer existing. The chemicals that most frequently form covalent bonds are substances that have functional groups similar to the functional groups in humus (Bollag & Myers, 1992; Bollag et al., 1992; Senesi, 1992). E.g. substances that have phenolic groups will often form covalent bonds with humus. The covalent bonds are often formed by oxidative coupling and catalysed chemically, photochemically or enzymatically (Bollag & Myers, 1992; Dec & Bollag, 1997). In some cases, the microbial activity is the cause of the formation of the covalent bonds while some groups of substances can form covalent bonds without the participation of microorganisms (Parris, 1980).
The sorption is often described by a simple equilibrium reaction between the concentration of substance sorbed to the solid matrix Cs (mg/kg) and the concentration in the aqueous phase Cw (mg/l). Several empirical expressions for describing this relation exist; the most common is the Freundlich-isotherm or a simple linear isotherm:
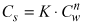
where K is called the Freundlich constant and n is a constant describing the "non-linearity" of the isotherm. If n = 1, the isotherm will be linear.
If it is assumed that the sorption isotherm is linear for the individual substance to a given soil, a sorption coefficient can be calculated:
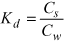
As most of the pesticides and other foreign chemicals that are found in soil are bound to the organic matter of the soil, a distribution coefficient between organic carbon and water KOC is often calculated on the basis of the sorption coefficient:
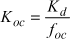
or
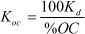
where fOC is the fraction of organic carbon. This relation is expected to be valid for soils with more than 0.1% organic carbon.
By far the majority of the published studies on measurements of sorption have used the OECD method (OECD, 2000) in a more or less adapted form. This is a batch method by which a given volume of water with a known concentration of the substance is added to a known amount of air-dried soil. This mixture is shaken until equilibrium after which the concentration in the aqueous phase is measured. Based on the difference between the initial – and the equilibrium concentrations in the aqueous phase, the sorbed amount is measured. In recent times, however, a number of attempts at applying techniques for measuring the sorption have been carried out by means of which a natural soil/water ratio is retained (Weber & Young, 1997; Rochette & Koskinen, 1997). In addition, studies have been published in which more complex models have shown to describe the sorption better than simple one-compartment models (Pignatello & Xing, 1996; Ma & Selim, 1994; Streck, 1995; de Jonge et al., 2000).
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 March 2004, © Danish Environmental Protection Agency
|