|
| Front page | | Contents | | Previous | | Next |
The Influence of Sorption on the Degradation of Pesticides and other Chemicals in Soil
4 Degradation
The degradation of a chemical in soil can take place through either chemical or microbiological processes. Often one or more steps take place chemically (e.g. a hydrolysis) whereas the following steps are microbiological. The degradation processes that take place chemically come to an end relatively quickly whereby the microbiological processes become the more interesting subject to study. The degradation happens gradually through the formation of one or more metabolites. By a total degradation of a chemical, CO2, salts, and water are formed, and parts of the chemical are built into new molecular structures in the soil humus or in biomass. Thus, it will not be a question of all the carbon atoms added to the soil through a chemical becoming CO2 (mineralised) within a foreseeable time. This is illustrated in Figures 2 and 3. Figure 2 shows a general diagram of the degradation of the herbicide mecoprop whereas Figure 3 shows the measurements of collected 14CO2, developed from the incubation of 14C-labelled mecoprop in soil in the concentration of 5 µg g-1. At the time when the incubation has stopped, about 62% of the added substance has been converted to 14CO2, but at this time no extractable residues of mecoprop or metabolites are found. The remaining 38% of the added radioactivity is thus either built into the organic constitutents of the soil or bound very strongly to these constituents.
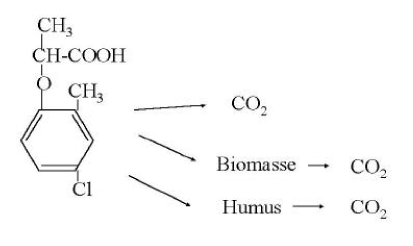
Figure 2. Diagram illustrating the degradation of the pesticide mecoprop. An inter-stage may occur in which the metabolite 2-methyl-4-chlorphenol is formed.
Figur 2. Skema der illustrerer nedbrydningen af pesticidet mechlorprop. Et mellemtrin, hvor metabolitten 2-methyl-4-chlorphenol dannes, kan forekomme.
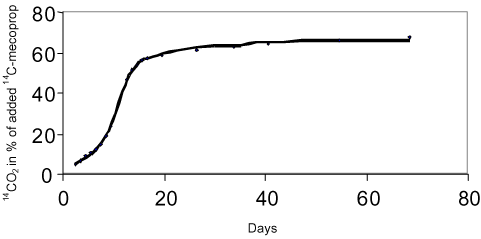
Figure 3. Mineralisation of mecoprop 5 µg g-1 in soil.
Figur 3. Mineralisering af mechlorprop 5 µg g-1 i jord.
Degradation experiments can be carried out either by measuring the disappearance over time of the added pesticide or the formation of the mineralisation product 14CO2 from 14C-labelled pesticides. The soil microorganisms manage most of the degradation processes, and the degradation can either take place metabolically – that is microorganisms making use of the substances that are being degraded for growing – or it can take place cometabolically where the substances are degraded by microorganisms without these being able to make use of the pesticide as a source of energy or nourishment. In the metabolic degradation process the degradation rate of the substance is increased as the microorganisms are growing. Figure 4 shows an example of the illustration of degradation/mineralisation experiments when the process takes place with no-growth kinetics and growth kinetics, respectively. Growth kinetics may take place for selected chemicals but will in plough layer soil typically not be seen until the concentration of the substance is relatively high (Fomsgaard, 1999).

Figure 4. Examples of illustrating degradation and mineralisation of chemicals in soil when the processes take place with and without growth, respectively.
Figur 4. Eksempler på afbildning af nedbrydning og mineralisering af kemiske stoffer i jord, når processerne foregår henholdsvis med og uden vækst.
Many factors influence the degradation of chemicals in soil. Factors such as temperature, water content, and other climatic factors, soil texture, microbiological activity, the composition of the other organic matter of the soil and the soil microbial biomass as well as the biological diversity and plant coverage. The fact that the depth of the soil influences the degradation rate of pesticides because of the very variable chemical and biological conditions has been described in many publications (Dictor et al., 1992; Mueller et al., 1992; Minton et al., 1990; Moorman & Harper, 1989; Pothuluri et al., 1990).
The effect of temperature on the degradation rate of pesticides is also well described (Helweg, 1993; Helweg, 1987; Matoba et al., 1995; Ismail & Lee, 1995; Walker et al., 1996; Jones, 1986; Eberbach, 1998). Walker et al. (1996) reviewed a large number of studies of pesticide degradation and calculated mean Q10 -values. The water content of the soil has also often been described as being of importance (Ismail & Lee, 1995; Helweg, 1993; Helweg, 1987) just as the initial concentration of the pesticide (Helweg, 1993; Helweg, 1987; Reffstrup et al., 1998; Jacobsen & Pedersen, 1992; Parker & Doxtader, 1982; Mueller et al., 1992; Alexander, 1985; Helweg, 1993; Fomsgaard & Kristensen, 1999b). Temperature, water content, and soil depth are factors taken into account as having an influence on the degradation rate of pesticides in the 9 dynamic leaching models PRZM-2, PRZM, PELMO, GLEAMS, PESTLA, VARLEACH, LEACHM, MACRO, and PLM used in the fOC US comparisons (Boesten et al., 1995). Biological activity/biomass have also often been measured just as it has been attempted to relate them to the degradation rate of pesticides, either directly or through the variation in soil depth (Anderson, 1984; Torstensson & Stenström, 1986; Monrozier et al., 1993; Dictor et al., 1992). The amount of organic matter is normally also related to the soil depth, and its influence on the degradation of xenobiotic chemicals has also often been studied (Reddy et al., 1995; Duah-Yentumi & Kuwatzuka, 1980; Greer & Shelton, 1992; Knaebel et al., 1994). The pH-value in the soil (Smelt et al., 1983; Smelt et al., 1978), the oxygen conditions in the soil (Sinclair & Lee, 1992; Pothuluri et al., 1990; Ou et al., 1988), the water content of the soil Helweg, 1987; Helweg, 1993; Konopka & Turco, 1991), and repeated sprayings with the same substance (Moorman, 1990; Rahima et al., 2000) influence the degradation rate as well. Koetler et al. (2001) described how wetting/drying cycles reduced the extractable amount of atrazine and phenanthrene. Finally, there is the influence of the factor on the degradation, which the present report deals with, viz. sorption. To a great extent, the sorption of the substance determines whether it is available to microbial degradation.
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 March 2004, © Danish Environmental Protection Agency
|