Calibration of Models Describing Pesticide Fate and Transport in Lillebæk and Odder Bæk Catchment
Appendix D
1 Pesticide Characteristics used for the Pond and Stream Model
1.1 Bentazone (CAS-RN: 25057-89-0)
Basic properties:
Molecular weight: 240.3 g/mol
- pKa: 3.28 (24°C) (Danish EPA), 2.92 (Syracuse); 3.3 (Pesticide Handbook)
- LogKOW: 1.41 (pH=2), 1.21 (pH=4), -0.03 (pH=6), 2,34 (pH not stated) (Syracuse); 0.77 (pH=5); -0.45 (pH=7); -0,55 (pH=9) (Pesticide Handbook). See Figure 1.
- Boiling point:
- Melting point: 141 (Danish EPA), 138 (Syracuse), 137-139 (Verscueren)
- Water solubility: 570 mg/L (pH=7, 20°C) (Danish EPA), 490 mg/L (pH=3, 20°C) (Danish EPA), 500 mg/L (Syracuse, Verschueren).
- Vapor pressure: 1.28(10-6 mmHg (pH not stated, 20 °C) (Danish EPA), 3.45(10-6 mm Hg (pH not stated, 20 °C) (Syracuse)
- Henry's constant: 7.11(10-10 atm(m3/mol (neither temperature or pH stated) (Danish EPA), 2.2(10-9 atm(m3/mol (Syracuse, estimated from water solubililty and vapor pressure at 20°C)
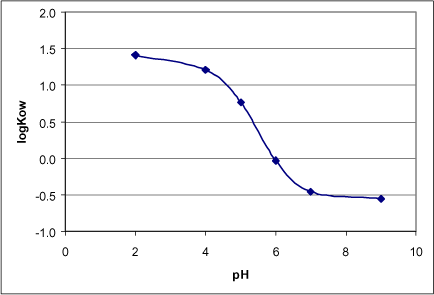
Figure 1. LogKow as a function of pH
Hydrolysis: Does not hydrolyze (HSDB, DK EPA, US EPA, Verschueren, Pesticide Handbook)
Photolysis: Is transformed by light.
Quantum yield:
Absorption spectrum:
Measured half-lives in aqueous solutions. From Danish EPA
| Half-life (h) | pH | Temp (°C) | Concentration (ppm) | Duration (h) | Light |
| 122 | 5 | 24-26 | 250 | 257 | Xenon, 850 W/m2 |
| 93 | 7 | 24-26 | 250 | 116 | Xenon, 850 W/m2 |
| 14 | 9 | 24-26 | 250 | 30.7 | Xenon, 850 W/m2 |
Biodegradation: Bentazone mineralises very slowly in water systems (Christian Helweg, 2001). The soil half-lives on primary degradation reported in the Danish EPA are between 17 days and 78 days. In sediment systems (in darkness), a very quick transformation of around 20% bentazone to an unknown metabolite was observed. After 28 days up to around 80-100% of bentazone persisted and the unknown metabolite constituted the major transformation product. No major mineralisation was observed. The results obtained in the studies of biodegradation in water-sediment systems, are not adequate to be used for determining a rate constant of the transformation of bentazone.
For the calibration of the model (only chemical analysis of the mother product was carried out in the pond experiments), a primary biodegration rate in water of bentazone 0 hr-1 is recommended, but it can be necessary to adjust the biodegradation rate, as transformation of bentazone has been observed in the water-sediment system.
Sediment-water partition coefficient (KD): The KD for the pond sediment has not been measured for bentazon. A KD-value of 0.8 L/Kg is used (average KOC=23 L/kg, a organic carbon content of 3.3% in the pond).
| Recommeded values for bentazone pKa 3.28 logKOW (at the pH of the pond) -0.5 Volatilization Henrys constant: 7.11(10-10 atm(m3/mol (undissociated part) Hydrolysis: Hydrolysis_Constant_Acid=0 Hydrolysis_Constant_Alkaline=0 Hydrolysis_Constant_Neutral=0 Photolysis: Use the photolytic half-life as a calibration parameter. Take basis in a depth averaged half-life of 28 days. Sediment-water partition coefficient (KD) Use 0.8 l/kg Retention factor in sediment 1.15 Sorption rate: 0.0363 hr-1 (g/L SS)-1 Desorption rate: 45.375 (g/L SS)-1 hr-1 Degradation rate in water column (dissolved part) 0 hr-1 Degradation rate in water column (sorped part) 0 hr-1 Degradation rate in sediment (dissolved part) 0 hr-1 Degradation rate in sediment (sorped part) 0 hr-1 |
1.2 Ioxynil (CAS-RN: 1689-83-4)
Basic properties:
Molecular weight: 370.9 g/mol
pKa: 3,96 (25°C)(Syracuse)
LogKOW: 3.51 (24°C) (Danish EPA), 6.12 (Danish EPA, Ioxynil octonoate), 3.43 (Syracuse)
Boiling point:
Melting point: 209 (Danish EPA), 212.5 (Syracuse)
Water solubility: 50 mg/L (pH=not stated, 25°C) (Danish EPA), 50 mg/L (Syracuse, Verschueren).
Vapor pressure: 5.1(10-6 mmHg (pH not stated, 25 °C) (Danish EPA), 1.38(10-7 mm Hg (pH not stated, 25 °C) (Syracuse)
Henry's constant: 5.48(10-10 atm(m3/mol (Syracuse, estimated from water solubililty and vapor pressure at 25°C)
Hydrolysis: Ioxynil octonoate is rapidly hydrolized to octanoic acid and ioxynil. Ioxynil is not expected to hydrolyze.
Photolysis: Is transformed by light.
Quantum yield (Millet et al, 1996):
| pH | Lamda range | %CH3CN | Quantuum Yield |
| 275-320 | 0.5% | (2.5 0.3)10-3 | |
| HPLC | (2.5 1.3)10-3 | ||
| 3.5 | 235-259 | 0.5 | (2.6 0.3)10-3 |
| 7.0 | 275-315 | (2.6 1.3)10-3 | |
| 7.0 | HPLC | 0.5 | (2.5 1.2)10-3 |
| 9.0 | 275-315 | 0.5 | (2.1 1.3)10-3 |
Absorption spectrum:
Biodegradation: Ioxynil mineralizes slowly in water systems (Christian Helweg, 2001). The rate of mineralization depends on the type of sediment. The rate of mineralization was measured to 0.004 d-1 (Lillebæk), 0.008 d-1 (Odderbæk) and 0.008 d-1 in a Norwegian sediment water system. Data reported in the Danish EPA indicates that no mineralization of ioxynil took place during 28 days (20°C, 10 ppm) in an aerobic sediment water suspension.
Another study reported in the Danish EPA of the transformation of radio labelled ioxynil octanoate (14C labeled in the phenylring) in a sediment/water system (concentration 50g dry sediment in 150 mL water) during 26 week shows that ioxynil octanoate is transformed/degraded with a half-life of 13.8 days (1 order rate constant = 0.05 d-1). The degradation products are ioxynil, 3-iodo-4-hydroxy-benzonitrile, 4-hydroxy-benzonitrile, CO2 and residues. After 26 week, 14CO2 accounted for 48% of the initial activity, 10.8% was found in sediment (12.6% ioxyniloctanote, 1.4% ioxynil, 1.3% 4-hydroxy-benzonitrile and 84.8 residues) and the remaining 39% was not found.
The soil half-lives on primary aerobic degradation reported in the Danish EPA are between 2.5 days and 75 days (average 7 days, std.dev. 5.5 days). The highest half-lives are observed for a soil with a very high organic matter content (34.4%).
Assuming
- a first order kinetix
- degradation takes only place for dissolved pesticide
- the maximum water holding capacity (if nothing stated) is 50%
- if notthing is stated then the water content is set to 50% of the maximum holding capacity
- KOC for ixoynil is equal to 23 L/kg
- the temperature dependency of the rate of biodegradation is assumed to follow the expression: DT50 (T) =DT50(20°C)(exp{A((T-20°C)}
where A is temperature coefficient. It is here estimated as the value giving the lowest correlation coefficient between temperature and degradation rate. A is estimated to 0.092 °C-1, corresponding to that the half-lives is 2.5 times higher for every 10°C decrease in temperature.
- The hereby calculated rate of primary degradation of dissolved pesticide at 20°C varies between 0.7 and 1.6 days-1, average 1.2 days-1, stdev. 0.3 days-1. This rate is thus between 150 and 300 times higher than the rate observed in a water phase containing suspended sediment. In case of that no data is available but for soil systems, then extrapolation from studies in sediment/water systems could be carried out by
- calculate the rate of biodegradadation in the water phase at a reference temperature
- divide this rate with 150-300 in order to get a rate of degradation in the water phasee
For the calibration of the model (only chemical analysis of the mother product was carried out in the pond experiments), a primary biodegration rate in water of ioxynil 0.004-0.008 days-1 is recommended, but it can be necessary to adjust the biodegradation rate.
Volatilization: Henrys constant: 7.11(10-10 atm(m3/mol.
Sediment-water partition coefficient (KD): The KD for the pond sediment has not been measured for ioxynil. A KD-value of 11 L/Kg is used (average KOC=335 L/kg, a organic carbon content of 3.3% in the pond).
Sorption rate:
Desorption rate:_
| Recommeded values for ioxynil pKa 3.96 logKOW (at the pH of the pond) 3.51 Volatilization Henrys constant: 5.5(10-10 atm(m3/mol (undissociated part) Hydrolysis: Hydrolysis_Constant_Acid=0 Hydrolysis_Constant_Alkaline=0 Hydrolysis_Constant_Neutral=0 Photolysis: Use the photolytic half-life as a calibration parameter. Take basis in a depth averaged half-life of 17 days. Quantuum yield: 0.0024 Sediment-water partition coefficient (KD) Use 11 l/kg Retention factor in sediment 5.75 Sorption rate: 0.0363 hr-1 (g/L SS)-1 Desorption rate: 3.3 (g/L SS)-1 hr-1 Degradation rate in water column (dissolved part) 0.0003 hr-1 Degradation rate in water column (sorped part) 0.0 hr-1 Degradation rate in sediment (dissolved part) 0.05 hr-1 Degradation rate in sediment sorped part) 0.0 hr-1 |
1.3 Pendimethalin (CAS-RN: 40487-42-1)
Basic properties:
Molecular weight: 281.3 g/mol
- pKa: 2.8 (Danish EPA). No other supporting information on that pendimethalin behaves as an acid have been found. It is quite questionable, whether the value in the Danish EPA is correct.
- LogKOW: 2.18 (Danish EPA); 5.18 (Pesticide Handbook, SRC),
- Boiling point: 330 (EPISuite)
- Melting point: 54-58°C (Pesticide Handbook, Verschueren); 56-57°C (HSDB), 56°C (SRC, EPISuite)
- Water solubility: 0.275 (25°C) (Danish EPA); 0.3 mg/L (20°C) (Pesticide Handbook, HSDB, SRC), 0.275 mg/L (25°C); (EPISuite)
- Vapor pressure: 7.1(10-6 mmHg (25°C) (Danish EPA); 1.45(10-5 mmHg (25°C) (Danish EPA); 9.38(10-6 mmHg (25°C) (Danish EPA); 3.70(10-5 mmHg (35°C) (Danish EPA); 6.0(10-5 mmHg (35°C) (Danish EPA); 2.35(10-4 mmHg (45°C) (Danish EPA); 3.0(10-5 mmHg (25°C) (Pesticide Handbook, HSDB, SRC, EPISuite)
- Henry's constant: 2.7(10-5 atm(m3/mole (25°C) (Danish EPA); 1.1(10-6 atm(m3/mole (35°C) (Danish EPA); 8.6(10-7 atm(m3/mole (HSDB, SRC)
Hydrolysis: Data in the Danish EPA indecates insignificant hydrolysis. Stable to hydrolysis (HSDB)
Photolysis: Is transformed by light. N-Propyl-3,4-dimethyl-2,6-dinitroaniline is the major degradation product (Verschueren)
Quantum yield:
Absorption spectrum:
Biodegradation: Pendimethalin mineralizes to a certain degree in water systems (Christian Helweg, 2001).
For the calibration of the model (only chemical analysis of the mother product was carried out in the pond experiments), a primary biodegration rate in water of pendimethalin 0.008 hr-1 is recommended, but it can be necessary to adjust the biodegradation rate, as transformation of bentazone has been observed in the water-sediment system.
Sediment-water partition coefficient (KD): The KD for the pond sediment was measured for pendimethalin in the pond system to be between 551 and 811 L/kg (average 702 L/kg, stdev: 140 L/kg). The average KD-value of 702 L/Kg is recommended to be used for pendimethalin sorption to the pond sediment.
| Recommeded values for pendimethalin pKa - logKOW (at the pH of the pond) Volatilization Henrys constant: 2.7(10-5 atm(m3/mole (25°C) Hydrolysis: Hydrolysis_Constant_Acid=0 Hydrolysis_Constant_Alkaline=0 Hydrolysis_Constant_Neutral=0 Photolysis: Pendimethalin degrades photolytically. Use the rate of photolysis as a calibration parameter. Start with a photolytic rate of degradation of 0.001 hr-1. Sediment-water partition coefficient (KD) 702 L/kg Retention factor in sediment 352 Sorption rate: 0.0363 hr-1 (g/L SS)-1 Desorption rate: 0.052 (g/L SS)-1 hr-1 Degradation rate in water column (dissolved part) 0.008 hr-1 Degradation rate in water column (sorped part) 0 hr-1 Degradation rate in sediment (dissolved part) 0.79 hr-1 Degradation rate in sediment (sorped part) 0 hr-1 |
1.4 Fenpropimorph (CAS-RN: 67306-03-0)
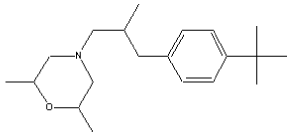
Molecular weight: 303
pKa: 6.98 (25°C) (Danish EPA). As fenpropimorph is a base, this pKa is interpreted as the base constant (gives a perfect fit to the meaured LogKow (see below).
LogKOW: 2.60 (pH=5), 4.06 (pH=7), 4.39 (pH=9), 3.60 (pH not stated), 2.44(pH not stated), 2.43(pH not stated), 2.46 (pH not stated) (all Danish EPA), 4.93 (pH not stated) (Syracuse). A KOW for the non-dissociated part respectively the dissociated part is estimted to 25376 respectively 146 using the measured logKOW, where pH has been stated.
Boiling point: > 250°C (Danish EPA), 120°C at 0.05 mmHg (Syracuse)
Melting point: <25°C (Syracuse)
Water solubility: 3.53 mg/L (pH=9-11, 20°C) (Danish EPA), 7300 (pH=4.4, 20°C) (Danish EPA). A water solubility of the non-dissociated part is thus around 3.53 mg/L and for the dissociated part around 7300 mg/L (>99% is expected to be dissociated at pH 4.4).
Vapor pressure: 1.7(10-5 mmHg (20°C, pH not stated) (Danish EPA), 3.4(10-5 (25°C, pH not stated) (Danish EPA), 2.5(10-5 (20°C, pH not stated) (Syracuse)
Henry's constant: 1.92(10-6 (atm(m3/mol) (pH=9-11, 20°C) (estimated from vapor pressure and water solubility), 9.30(10-10(atm(m3/mol) (pH=4.4, 20°C) (estimated from vapor pressure and water solubility)
Hydrolysis: Does not hydrolyze (Danish EPA)
Photolysis: Does not photolyze at pH 5 in water systems(Danish EPA), photolytic half-life in soil 30 days (Danish EPA). Biodegradation products from the soil photolysis: N-[3-(4-tert-butylphenyl)-2-methyl, 1-oxopropyl]-cis-2,6-dimethylmorpholine and N-[3-(4-tert-butylphenyl)-2-methylpropyl]-cis-2,6-dimethyl, 3-oxo-morpholine (Danish EPA).
Quantum yield:
Absorption spectrum:
KOC: 3 values of soil partition coefficient KD is reported in Danish EPA: 34.47 L/kg (pH=7.3, %OC=0.58) 73.73 L/kg (pH=7.2, %OC=2.66) and 22.62 L/kg (pH=7.0, %OC=0.51). At a pH between 7.0 and 7.3 around 34-50% of fenpropimorph is expected to be dissociated. Unfortunately, no data is reported at lower and higher pH.
An average KOC of 4384 L/kg (std.dev. 1586 L/kg) is calculated from the three data points.
The dissociated part of fenpropimorph is positively charged and is thus expected to sorp much stronger to the soil particles than the non-dissociated part. The highest KOC would thus be exptected to occur at the lowest pH. This is however not the case, as the highest KOC is obtained at pH=7.3, and the lowest KOC at pH=7.2. The KOC values, which can be derived from the Danish EPA is thus considered to be very uncertain, and care should be taken when using these data to other soil/sediment systems.
Sediment-water partition coefficient (KD):
The slurry pH of the pond sediment is 7.6, which is pretty close to the pH, at which the KOC was derived. At a pH of 7.6 around 20% is expected to be dissociated. For this system it is suggested to use the average KOC of 4384 L/kg with the awareness that is determined with a high degree of uncertainty. This give a KD of 145 L/kg (%OC=3.3%).
Sorption rate:
Desorption rate:
Biodegradation:
Metabolite 1: after derivation reaction cis-4[3-[4-(1-methoxy-carbonyl-1-methyl-ethyl)phenyl]-2-methyl-propyl]-2,6-dimethylmorpholine could be synthesized and metabolite 2: 2,6-dimethylmorpholine (Danish EPA)
BF 421-1: N-[3-(4-(2-hydroxy, 1,1-dimethyl)ethylphenyl)-2-methylpropyl]-cis-2,6-dimethyl-morpholine and BF 421-2: 2-methyl, 2-[4-(2-methyl, 3-N-cis-2,6-dimethyl-morpholine)propyl]phenyl-propionic acid. Both metabolites are found in the Rhine system and in the pond system.
RO 15-4422: [4-Aza, 6-methyl, 7-(4-tert-butylphenyl)]-2-heptanol and RO 15-4423: [3-Aza, 5-methyl, 6-(4-tert-butylphenyl)]-1-carboxyl. (Danish EPA)
Four laboratory studies of aerobic biodegradation of fenpropimorph are reported in the Danish EPA. Two of the studies are carried out with 14C-labelled fenpropimorph and it is thus probably the rate of mineralization being measured (it is not completely clear from the data reported in the database). A half-life of 20 respectively 36 days was observed in these two studies. It is not clearly stated in the two other studies, whether the measurements were carried out with 14C-labellede pesticide or if the primary degradation is measured. The reported half-lives for these two studies are 5 days respectively 13 days.
The field half-lives specified in the Danish EPA is between 9 and 181days. It is probably the half-life for the primary degradation. The observed half-lives are correlated with the temperature (correlation coefficient –0.7), time of experiment (0.5), humic content/pH (0.3/0.3), and weekly with concentration (-0.2) (see Table).
Introducing a temperature dependency of the half-life (T is the temperature i °C):
DT50 (T) =DT50(20°C)(exp{A((T-20°C)}
where A is temperature coefficient. It is here estimated as the value giving the lowest correlation coefficient with temperature and observed half-lives. A is estimated to 0.099 °C-1, corresponding to that the half-lives is 2.69 times higher for every 10°C decrease in temperature.
Correcting for the temperature effects assuming that the half-life is doubled for every 10°C decrease in temperature gives a variation in the half-lives at 20°C between 11 and 73 days. The effect of humic content (the half-lives increases with increases organic matter content due to higher sorption) and water content (the dissolved fraction increases with increasing water content causing a higher degradation rate and thus a lower half-life) is expected. The effects of pH on the field half-life is not expected, as fenpropimorph is expected to sorp stronger with decreasing pH causing a higher half-life. The effects of time of experiment on the half-life could be explained by a lag-phase due to adaption and that the degradation does not follow a complete first order degradation path-way.
The rate of biodegradation in the water phase –assuming all biodegradation takes place in the water phase – and using a KOC of 4384 L/kg – gives a biodegradation rate in water phase between 10 and 23 days-1 for the laboratory studies (a single outlier of 173 days-1) is omitted and between 7.7 and 33 days-1 for the field studies. Merging the field data and laboratory data and applying a conversion factor between 150-300 for converting soil degradation data to water column degradation data gives a degradation rate in the water column between 0.03 and 0.22 days-1, average 0.06 days-1.
| Observed DT50 | Correlation coefficients | Range in DT50 (d) |
|||||
| Concentration | Humic content | pH | Temperature | Time of experiment | Water content | ||
| -0,17 | 0,32 | 0,33 | -0,70 | 0,49 | -0,16 | 9-181 | |
| DT50 temperature corrected | -0,57 | 0,66 | 0,37 | 0,00 | 0,42 | -0,50 | 11-73 |
1.4.1 Conclusions
Volatilization: Use a Henrys constant for the non-dissociated part of 1.92(10-6 (atm(m3/mol).
Biodegradation rate: Take basis in a value of 0.06 days-1, but if required adjust the rate (can vary between 0.03 and 0.22 days-1).
Rate of photolysis:
Quantuum yield is set to 0
Rate of hydrolysis:
The three rate coefficients are set to 0.
Sediment water partition coefficient: 145 L/kg
Desorption rate:
Sorption rate:
| Recommeded values for fenpropimorph pKb 6.98 (fenpropimorph is a base) logKOW (at the pH of the pond) 1.48 Volatilization Henrys constant: 1.92(10-6 atm(m3/mol (undissociated part) Hydrolysis: Hydrolysis_Constant_Acid=0 Hydrolysis_Constant_Alkaline=0 Hydrolysis_Constant_Neutral=0 Photolysis: Use the photolytic half-life as a calibration parameter. Take basis in a rate of photolysis of 0 hr-1. A relatively high photolytic half-life is expected. Sediment-water partition coefficient (KD) Use 145 L/kg Retention factor in sediment 73.3 Sorption rate: 0.0363 hr-1 (g/L SS)-1 Desorption rate: 0.25 (g/L SS)-1 hr-1 Degradation rate in water column (dissolved part) 0.0025 hr-1 (at 20°C); temperature correction factor: 0.099 (°C)-1 Degradation rate in water column (sorped part) 0.0 hr-1 Degradation rate in sediment (dissolved part) 0.59 hr-1 (at 20°C); temperature correction factor: 0.099 (°C)-1 Degradation rate in sediment sorped part) 0.0 hr-1 |
1.5 Deltamethrin (CAS-RN: 52918-63-5)
Molecular weight: 505,205
pKa:
LogKOW: 4.6 (Pesticide Handbook), 6.2 (HSDB, Syracuse)
Boiling point:
Melting point: 99 (HSDB), 101-105°C (HSDB), 98-101°C (Verschueren)
Water solubility: 0.002 mg/L (HSDB), <0.1 mg/L (Veschueren), <0.0002 mg/L (25°C) (Pesticide Handbook)
Vapor pressure: 9.3(10-11 mmHg (25°C) (EPIWIN), 1.5(10-8 mmHg (25°C) (Syracuse, HSDB), 2(10-8 mbar (25°C) (Verschueren).
Henry's constant: 4.99(10-6 atm(m3/mole (Syracuse, estimated water solubility and vapor pressure), 3.231(10-6 atm(m3/mole (EPIWIN, estimated water solubility and vapor pressure), 3.09(10-7 atm(m3/mole, 5(10-6 atm m3/mole (estimated) (HSDB)
Hydrolysis: Does hydrolyze under alkaline conditions, but very slowly under neutral and acidic conditions. T1/2 (pH=9)=2.5 days (Pesticide Manual) – corresponds to kOH = 0.32 L/(mol(s). EPIWIN predictions: total Kb (pH>8)=6.124(10-3 L/mol s. Below pH=8 hydrolysis is expected to be indsignificant.
Photolysis: A cistrans isomerization, splitting of the ester bond, and loss of bromine occurs under UV and sunlight. The photodegradadtion half-life of deltamethrin in distilled and natural water solutions exposed to sunlight was found to range from 1 to less than 5 days (HSDB).
Quantum yield:
Absorption spectrum:
Biodegradation: Half-lives in soil: 4.9 – 6.9 weeks in sandy clay loam soil(HSDB).
Volatilization:
Sediment-water partition coefficient (KD): KOC = 46,000 – 1,630,000 L/kg (Pesticide Manual, HSDB). KOC=108,000 L/kg (EPIWIN estimated). Can only be set with a high degree of uncertainty due to the large variation in the KOC. A value of 46,000 L/kg used. This gives a KD of 1518 L/kg.
Sorption rate:
Desorption rate:
| Recommeded values for Deltamethrin pKa - logKOW (at the pH of the pond) 6.2 Volatilization Henrys constant: 5(10-6 atm(m3/mole (25°C) Hydrolysis: Hydrolysis_Constant_Acid=0 Hydrolysis_Constant_Alkaline=0.32 L/(mol(s) Hydrolysis_Constant_Neutral=0 Photolysis: Does degradade in sunlight. Use the rate of photolysis as a calibration parameter. Is expected to vary between 0.0008-0.004 hr-1. Start with a value of 0.001 hr-1. Sediment-water partition coefficient (KD) 1518 L/kg. Very uncertain Retention factor in sediment 760. Very uncertain. Can eventually be used as a calibration parameter. Sorption rate: 0.0363 hr-1 (g/L SS)-1 Desorption rate: 0.048 hr-1 (g/L SS)-1 Degradation rate in water column (dissolved part) Very difficult to estimate due to lack of precise data. A degradation rate in water column around 0.003 hr-1 is suggested to be used. Degradation rate in water column (sorped part) 0 hr-1 Degradation rate in sediment (dissolved part) Very uncertain. A value equal to 0.45 hr-1 is suggested (150 times the rate in the water column). Degradation rate in sediment (sorped part) 0 hr-1 |
1.6 Permethrin (CAS-RN: 52645-53-1)
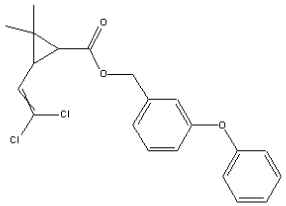
Molecular weight: 391,29
pKa:
LogKOW: 6.1 (Pesticide Handbook), 6.5 (Syracuse, HSDB)
Boiling point: 200 °C (0.1 mmHg) (Pesticide Handbook, HSDB); >290 °C at 760 mmHg; 220 °C at 0.05 mmHg
Melting point: 34-35 (Pesticide Handbook, HSDB); cis-isomeres 63-65°C. (pesticide Handbook), 34 °C (Syracuse)
Water solubility: 0.2 mg/L (Pesticide Handbook), 0.006 mg/L (20°C) (Syracuse); 0.006 mg/L (20°C) (HSDB)
Vapor pressure: 9.75(10-9 mmHg (20°C) (Spectrum Laboratories), 2.18(10-8 mmHg (25°C) (HSDB); 0.0025 Pa (Cis)/0.0015 Pa (Trans) (HSDB)
Henry's constant: 2.51(10-8 atm m3/mole (20°C) (Spectrum Laboratories). 1.87(10-6 atm m3/mole (25°C) (Syracuse, estimated from water solubility and vapor pressure)
Hydrolysis: More stable in acid than in alkaline media (Pesticide Handbook). Hydrolyzes to 3-(2,2-dichlorovinyl)-2,2-dimethylcycloprppanecarboxylic acid and 3-phenoxbenzylalcohol. Estimated half-life at pH=9: 20 days (Spectrum Laboratories) – corresponds to kOH = 0.0336 L/(mol(s).
Photolysis: Some photochemical degradation observed in laboratory studies. Pholysis half-lives of 27.1 and 19.6 hours were determined for cis- and trans-isomers in 800 mL pond water exposed to sunlight.
Quantum yield:
Absorption spectrum:
Biodegradation: DT50 (soil, pH 4.2-7.7, o.m. 1.3-51.3) < 38 days. Field dissipaiton rate between 6 and up to 106 days reported in the ARS. An aerobic half-life of 30 days is recommended by ARS.
For the calibration of the model
Volatilization:
Sediment-water partition coefficient (KD): KOC : 19340-60870 L/kg avg: 39300 L/kg. (ARS). KOC 63100 L/kg (Spectrum Laboratories). The average value 39300 is used, giving a KD for the pond sediment : 1297 L/kg,
Sorption rate:
Desorption rate:
| Recommeded values for Permethrin pKa - logKOW (at the pH of the pond) 6.5 Volatilization Henrys constant: 1.9(10-6 atm(m3/mole (25°C) Hydrolysis: Hydrolysis_Constant_Acid=0 Hydrolysis_Constant_Alkaline=0.0336 L/(mol(s) Hydrolysis_Constant_Neutral=0 Photolysis: Does degradade in sunlight. Use the rate of photolysis as a calibration parameter. Is expected to vary between 0.0008-0.004 hr-1. Start with a value of 0.001 hr-1. Sediment-water partition coefficient (KD) 1297 L/kg. Very uncertain Retention factor in sediment 649. Very uncertain. Can eventually be used as a calibration parameter. Sorption rate: 0.0363 hr-1 (g/L SS)-1 Desorption rate: 0.028 hr-1 (g/L SS)-1 Degradation rate in water column (dissolved part) Very difficult to estimate due to lack of precise data. A degradation rate in water column around 0.08 hr-1 is suggested to be used. Degradation rate in water column (sorped part) 0 hr-1 Degradation rate in sediment (dissolved part) Very uncertain. A value equal to 11.4 hr-1 is suggested (150 times the rate in the water column). Degradation rate in sediment (sorped part) 0 hr-1 |
1.7 Esfenvalerate (CAS-RN: 66230-04-4)
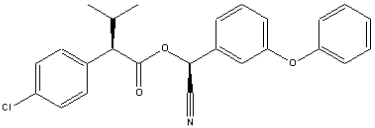
Molecular weight: 419.9 g/mole
pKa: -
LogKOW: 6.22 (Danish EPA), 6.22 (25°C) (Pesticide Handbook); 6.20 (EPISuite), 6.77 (HSDB)
Boiling point: 157-167°C (Pesticide Handbook); 300°C at 37mmHg; 151-167°C (HSDB)
Melting point: 59.0-60.2°C (Pesticide Handbook, HSDB): 59.5 °C (EPISuite),
Water solubility: 0.002 mg/L (25°C) (Pesticide Handbook); 0.002 mg/L (25°C); 0.024 mg/L (25°C) (EPISuite); <0.1 mg/L (20°C)(HSDB)
Vapor pressure: 2.6(10-7 mmHg (20°C) (Danish EPA); 1.5(10-9 mmHg (25°C) (Pesticide Handbook); 1.5(10-9 mmHg (25°C); 2.8(10-7 mmHg (25°C) (HSDB); 5(10-7 mmHg (25°C) (HSDB)
Henry's constant:
Hydrolysis: Kb for pH>8: 5.49(10-3 L/(mol(s) (EPISuite). Estimated halflives of 16.3 days, 1.63 days and 3.9 hours at pH 7, 8 and 9 respectively, at 25°C (HSDB). Hydrolysis halflife (derived from data in Danish EPA): 39.2 days (pH=7, 24-26°C).
67.2 days (pH=9) (Danish EPA); 217.1 (pH=5) (Danish EPA); 129.2 (pH=5) (Danish EPA), 64.4 (pH=9) (Danish EPA).
Photolysis: The data given in the Danish EPA on photolytic degradation:
| Photolytic half-life | Conditions |
| 8.7 hours | On petri dishes precoated with a silica gel treated with 14C-esfenvalerate (0.56 microg/cm2) 25°C Artificial UV-light > 290 nm. |
| 3.3 hours | 10 ml solution with esfenvalerate were transferred to the test tube. Artificial UV-light > 290 nm. pH = 3.9 25°C |
| 5.7 hours | 10 ml solution with esfenvalerate were transferred to the test tube. Artificial UV-light > 290 nm. 25°C pH=7.2 |
| 2.5 hours | 10 ml solution with esfenvalerate were transferred to the test tube. Artificial UV-light > 290 nm. 25°C pH=9.9 |
| 19.4 hours | Artificial UV-light > 290 nm. A thin-layer plate was precoated with a silica gel treated with 14C-esfenvalerate (0.56 microg/cm2) 25°C |
| 6.8 hours | Soil (OM=3.4%; clay 18.5%; Sand: 68.5%; Silt 13.0% ) was coated on a glass plate with a thickness of ca. 500 micrometer and irradiated. Esfenvalerate in diethyl ether was evenly applied to the soil thin layer plates at a rate of approximately 0.56 microg/cm2. Artificial UV-light > 290 nm pH = 5.9 25°C |
| 2.8 hours | Soil (OM=13.2%; clay 8%; Sand: 61.5%; Silt 30.5%) was coated on a glass plate with a thickness of ca. 500 micrometer and irradiated. Esfenvalerate in diethyl ether was evenly applied to the soil thin layer plates at a rate of approximately 0.56 microg/cm2. Artificial UV-light > 290 nm pH = 4.4 25°C |
| 9.4 hours | Soil (OM=0.5%; clay 11%; Sand: 79.1%; Silt 9.9%) was coated on a glass plate with a thickness of ca. 500 micrometer and irradiated. Esfenvalerate in diethyl ether was evenly applied to the soil thin layer plates at a rate of approximately 0.56 microg/cm2. Artificial UV-light > 290 nm pH = 7.1 25°C |
| 26.4 hours (1.1 days | Soil (OM=0.5%; clay 11%; Sand: 79.1%; Silt 9.9%) was sieved and air dried and coated on thin layer plates. Esfenvalerate was then applied to the soil plates and these were exposed to outdoor conditions for 30 days. Esfenvalerate in an acetone solution was applied on the plates at a rate equivalent to 0.3 - 0.4 ng/cm2. The sunlight intensity was 520, 1640 and 130 micro W/cm2 at the begining, middle and end of the day. pH = 5.3 |
Relatively stable to heat and light (Pesticide Handbook). "Photolytic degradation may contribute to esfenvalerate's degradation on soil surfaces and water exposed to sunlight". Dominant photodegradation reaction are hdyration of the cyano group and ether cleavage in the alcohol moiety. (HSDB).
Quantum yield:
Absorption spectrum:
Biodegradation:
For the calibration of the model
Volatilization:
Sediment-water partition coefficient (KD): The following Freundlich coefficients for the soil water partitioning of esfenvalerate were found in the Danish EPA:
KD
| Kd | n | Conc (ppm). | Soil |
| 4.41 | 1.13 | 0.1; 1.0; 10.0 | OM: 0.4%; Clay: 7.0%; Sand: 84.6%; Silt: 8.4%; CEC: 9.0; Temperature: 25°C; pH=Not determined |
| 6.36 | 0.96 | 0.1; 1.0; 10.0 | OM: 1.1%; Clay: 7.2%; Sand: 81.6%; Silt: 11.2%; CEC: 7.5; Temperature: 25°C; pH=7.3 |
| 71.32 | 1.02 | 0.1; 1.0; 10.0 | OM: 2.0%; Clay: 27.5%; Sand: 19.6%; Silt: 62.8%; CEC: 20.2; Temperature: 25°C; pH=5.3 |
| 104.83 | 1.18 | 0.1; 1.0; 10.0 | OM: 1.5%; Clay: 32.4%; Sand: 52.0%; Silt: 15.6%; CEC: 5.2; Temperature: 25°C; pH=6.4 |
The KOC for esfenvalerate is estimated by estimating the average KD for each type of soil for the concentrations (0.1, 1.0, 10.0 ppm). KOC is found to vary between 1038 and 12755 L/kg, with an average value of 5547 L/kg.
Sorption rate:
Desorption rate:
| Recommeded values for Esfenvalerate pKa - logKOW (at the pH of the pond) 6.22 Volatilization Henrys constant: 7.7(10-5 atm(m3/mole (25°C) (estimated from water solubility, vapor pressure) Hydrolysis: Hydrolysis_Constant_Acid= Hydrolysis_Constant_Alkaline=0.0336 L/(mol(s) Hydrolysis_Constant_Neutral=0 Photolysis: Does degradade in sunlight. Use the rate of photolysis as a calibration parameter. Is expected to vary between 0.0008-0.004 hr-1. Start with a value of 0.001 hr-1. Sediment-water partition coefficient (KD) 1297 L/kg. Very uncertain Retention factor in sediment 649. Very uncertain. Can eventually be used as a calibration parameter. Sorption rate: 0.0363 hr-1 (g/L SS)-1 Desorption rate: 0.028 hr-1 (g/L SS)-1 Degradation rate in water column (dissolved part) Very difficult to estimate due to lack of precise data. A degradation rate in water column around 0.003 hr-1 is suggested to be used. Degradation rate in water column (sorped part) 0 hr-1 Degradation rate in sediment (dissolved part) Very uncertain. A value equal to 0.45 hr-1 is suggested (150 times the rate in the water column). Degradation rate in sediment (sorped part) 0 hr-1 |
1.8 Glyphosate (CAS-RN: 1071-83-6)

Molecular weight: 169,07
pKa:
LogKOW:
Boiling point:
Melting point:
Water solubility:
Vapor pressure:
Henry's constant:
Hydrolysis:
Photolysis:
Quantum yield:
Absorption spectrum:
Biodegradation:
Fenpropimorph acid major degradation product.
For the calibration of the model
Volatilization:
Sediment-water partition coefficient (KD):
Sorption rate:
Desorption rate:
Version 1.0 November 2004, © Danish Environmental Protection Agency