|
Evaluation of Health Hazards by exposure to Triazines and Degradation Products 1 General description
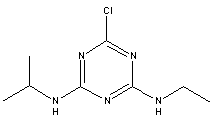
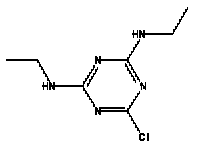
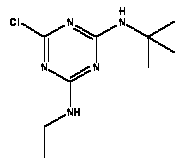
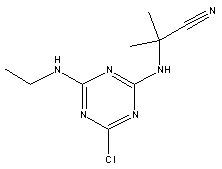
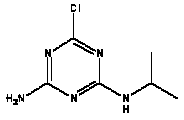
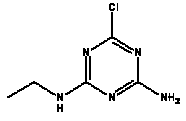
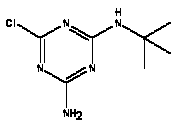
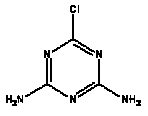
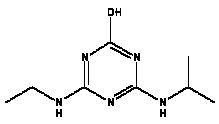
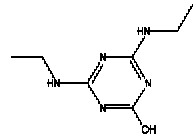
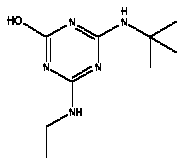
Atrazine, simazine, terbutylazine and cyanazine are triazines, which have been used (atrazine, cyanazine) or still are in use (simazine, terbutylazine) in agriculture as herbicides in Denmark (MST 2003b). Desethyl atrazine (DEA), desisopropyl atrazine (DIA), desethyl terbutylazine, desethyldesisopropyl atrazine (DACT), hydroxyatrazine, hydroxysimazine, and hydroxyterbutylazine are some of the degradation products of these triazines (IARC 1999a,b, Jørgensen 2002, WHO 1996a,b, WHO 1998a,b). This document does not necessarily include all studies that have been performed with the triazines and their degradation products. The included studies are viewed as representative for the toxicology of these chemical substances. In this and in subsequent chapters, no data were found for a triazine or its degradation products if the chemical is not mentioned. 1.1 IdentityName: Molecular formula: Structural formula: a) b) c) d) e) f) g) h) i) j) k) Molecular weight: CAS-no.:
a) 1912-24-9
Synonyms: b) 6-Chloro-N,N'-diethyl-1,3,5-triazine-2,4-diamine; 2-Chloro-4,6-bis(ethylamino)-s-triazine; 2-Chloro-4,6-bis(ethylamino)-1,3,5-triazine; 1-Chloro-3,5-bis(ethylamino)-2,4,6-triazine c) 6-Chloro-N-(1,1-dimethylethyl)-N'-ethyl-1,3,5-triazine-2,4-diamine; 2-tert-Butylamino-4-chloro-6-ethylamino-1,3,5-triazine d) 2-Chloro-4-ethylamino-6-(cyano-1-methyl)-(ethylamino)-1,3,5-triazine; 2-[[4-Chloro-6-ethylamino-1,3,5-triazine-2-yl]amino]-2-methylpropanenitrile e) 6-Chloro-N-(1-methylethyl)-1,3,5-triazine-2,4-diamine; 2-Amino-4-isopropylamino-6-chloro-1,3,5-triazine f) 6-Chloro-N-ethyl-1,3,5-triazine-2,4-diamine; 2-Amino-4-chloro-6-ethylamino-1,3,5-triazine g) 6-Chloro-N-(1,1-dimethylethyl)-1,3,5-triazine-2,4-diamine; 2-Amino-4-tert-butylamino-6-chloro-1,3,5-triazine h) 6-Chloro-1,3,5-triazine-2,4-diamine; 2-Chloro-4,6-diamino-1,3,5-triazine; diaminochlorotriazine i) 4-ethylamino-6-[(1-methylethyl)amino]-1,3,5-triazin-2 (1H)-one; 2-hydroxy-4-ethylamino-6-isopopylamino-s-triazine 1.2 Physical / chemical propertiesDescription: Purity: Melting point: Boiling point: Density: Vapour pressure: Flash point: Flammable limits: Autoignition temp.: Solubility: Considerably more soluble (110-183.000 mg/l) in organic solvents (acetone, chloroform, dichloromethan, diethyl ether, dimethyl sulfoxide, ethanol, ether, ethyl acetate, n-hexane, methanol, n-octanol, toluene) than in water b) Water: 5 mg/l (at °C) c) Water: 8.5 mg/l (at 20°C) d) Water: 171 mg/l (at 25°C) Considerably more soluble (15.000-210.000 mg/l) in organic solvents (benzene, chloroform, ethanol, hexane) than in water logPoctanol/water: pKa-value: Stability: a,b,d) Relatively resistant to decomposition by ultraviolet radiation Incompatibilities: No data were found Odour threshold, air: Odour threshold, water: Taste threshold, water: References: 1.3 Production and useAtrazine, simazine, terbutylazine and cyanazine are produced by the reaction of 2,4,6-trichloro-1,3,5-triazine with appropriate intermediates (US-EPA 2002). For instance, atrazine is produced by a reaction between 2,4,6-trichloro-1,3,5-triazine and isopropyl amine under basic conditions followed by a reaction with monoethyl amine and dilute caustic (IARC 1999a). Atrazine, simazine, terbutylazine and cyanazine are used in agriculture, in forestry and/or in nurseries as herbicides. Atrazine has also been used as a soil sterilant for airfields, parking lots and industrial sites and as an algaecide in swimming pools. In the European Union, where a limit of 0.1 μg/l has been set for all pesticide residues in drinking- and groundwater, the use of atrazine-containing herbicides has been limited mainly to agricultural uses on corn and on sorghum. (IARC 1999a,b, WHO 1996a,b, WHO 1998a,b). In Denmark, atrazine has not been sold since 1994 (Brüsch 2002). Today, both atrazine and cyanazine are forbidden to sell or use in Denmark. Simazine is allowed for use in Denmark in forestry and nurseries on a limited amount of plants, bushes and trees. Terbutylazine is allowed for use in Denmark in forestry and in corn for fodder. (MST 2003b). But simazine-containing products will be withdrawn in EU in 2004-5 and products containing terbutylazine are being reviewed. 1.4 Environmental occurrenceThere are no known natural sources of atrazine. Virtually the entire production volume is released to the environment, primarily soils, mainly as a result of agricultural and other weed-control practices. (ATSDR 2001). 1.4.1 AirAtrazine has been detected in the atmosphere both nearby and distant from areas where it has been applied as a pesticide and has been detected up to 300 km from the closest application area. The concentration of atrazine in air will vary with application season and measured concentrations have ranged from just above the detection limit (about 0.03 ng/m3) to more typical concentrations of 0.2-0.3 μg/m3 (measured in regions in and around Paris, France). Once in the air, atrazine will exist in both the particulate and vapour phases, but with a tendency to exist more in the particulate phase than in the vapour phase. Atrazine has been found in the vapour phase in the atmosphere in association with fog and rainwater and is commonly found in rainwater in the seasons following agricultural applications; a concentration of up to 1 g/l has been found in Ohio, USA. (ATSDR 2001, IARC 1999a). Simazine has been detected at low concentrations in ambient air and rainwater (IARC 1999b). 1.4.2 WaterAtrazine is found in surface and groundwater and in drinking water wells as a result of its application to crop fields as a herbicide as well as from its disposal. Table 1 contains data from 1993 to 2001on triazine concentrations measured in Danish ground water and in Danish ground water abstraction wells. The triazines and their degradation products are found both in ground water rich in and deficient in oxygen and also in ground water containing high concentrations of nitrate. (Jørgensen 2002). Results from an investigation of 628 small water supply plants in 4 different counties (København, Sønderjylland, Storstrøm, Viborg) in Denmark also included analyses of selected triazines, Table 1. Small water supply plants are defined as wells or borings providing water for 1-9 households. The data are based on double water samples from all 628 plants. Analysis was not performed for desethyldesisopropyl atrazine (DACT) and for the hydroxy triazines, which were found in the national ground water monitoring and in the ground water abstraction wells. (Brüsch et al. 2004). Atrazine, cyanazine, simazine and terbutylazine have also been detected in surface- and/or groundwater at concentrations above 0.1 g/l in other European countries (WHO 1996a,b, WHO 1998a,b). The concentration of atrazine varies during the season in waters receiving runoff from agricultural lands with the highest concentrations generally being found during the 1½ to 2 months after application. Typically, atrazine is found more frequently and usually at lower concentrations in groundwater than in surface water. Simazine and its degradation products are detected less frequently and at lower concentrations than atrazine. (IARC 1999a,b). Table 1. Triazine herbicides or degradation products in Danish water.
1.4.3 SoilThe triazines are commonly found in agricultural soils following their application to crop fields as herbicides. They can also be detected in soils that have been impacted by runoff or by atmospheric deposition. Following application to crop soils, most atrazine is found at the highest concentrations in the upper layers of soil, as a result of sorption. Atrazine has been shown to be relatively mobile in soils and it can leach through the soil column and contaminate groundwater. Transport through soil occurs via macro pores, along roots and through earthworm burrows. The rate of transport is dependent on many soil factors including the soil type, the amount of water that is applied to the soil, the presence of crop residues, and the types of fertilisers used. Atrazine has been reported to move more rapidly through soils than its breakdown products. (ATSDR 2001). An investigation of the dry soil concentration of triazines and their degradations products in a limited number of point sources (gardening, machine pools, feedstuff businesses, and nurseries) in Denmark has shown the following results (AVJ 2001):
1.4.4 FoodstuffsSee chapter 5 for maximum residue limits (MRLs) in foods. No residues (< 6 μg/kg) of atrazine were found in 1339 samples of fruit and vegetables in Denmark in 2002. Out of 1339 fruit and vegetable samples, simazine was found in only one (a lemon from Spain) at a concentration of 9 μg/kg. Analysis was not performed for cyanazine, terbutylazine, and any of the degradation products. (Andersen et al. 2003). No residues (< 50 μg/kg) of atrazine, simazine or cyanazine were found in more than 75000 food samples in USA. Uptake of atrazine in corn and sorghum is relatively low and the metabolism is rapid. (IARC 1999a,b). However, hydroxy metabolites of atrazine have been found in plants grown in soil treated with atrazine (WHO 1996a). No residues (< 500 μg/kg) of terbutylazine were found in almost 7000 vegetable and fruit samples in USA (WHO 1998b). 1.5 Environmental fateTriazines can be degraded by biological or chemical (e.g., photolysis) processes via N-dealkylation and hydrolysis of the chloro substituent. Atrazine is degraded slowly in most environmental compartments, whether by biological or chemical processes. Chemical (abiotic) degradation of atrazine occurs by hydrolysis to hydroxyatrazine; no direct photolytic degradation has been detected. Biodegradation of atrazine occurs primarily by dealkylation resulting in the formation of desethyl atrazine (DEA), desisopropyl atrazine (DIA) and desethyldesisopropyl atrazine (DACT). Hydroxyatrazine can also be formed by biodegradation. Biodegradation may lead to a complete degradation of atrazine, but this is not always observed. In some cases, atrazine residues become incorporated into unextractable residues, which are considered to be less bioavailable than the free parent or metabolite compounds. (ATSDR 2001). Terbutylazine is degraded to e.g. hydroxyterbutylazine, desethyl terbutylazine, desisopropyl atrazine (DIA), and desethyldesisopropyl atrazine (DACT). (Brüsch 2002, WHO 1998b). 1.5.1 AirIn the atmosphere, atrazine will exist in both the particulate and vapour phases. Particulate phase atrazine will be removed from the atmosphere by wet and dry deposition. Vapour phase atrazine can be degraded in the atmosphere by reaction with photochemically produced hydroxyl radicals; a half-life of 14 hours has been estimated. Atrazine has not been observed to undergo direct photolytic degradation in the atmosphere. (ATSDR 2001). Terbutylazine in air is degraded by reactions with hydroxyl radicals with a half-life of about 1.5 days (WHO 1998b). 1.5.2 WaterAtrazine is stable to abiotic hydrolysis at pH 5, 7 and 9. The photolysis in water is very slow with an estimated half-life of 805 days. In controlled aerobic water-sediment systems atrazine disappeared from the water phase with a half-life of 28 - 134 days, while the degradation half-life was found to be 45 - 253 days for the whole system. (EC 1996a). Simazine is stable to abiotic hydrolysis at pH 5, 7 and 9. The photolysis in water is described with contrasting half-lives from 0.7 to 382 days. In controlled aerobic water-sediment systems the dissipation half-life for the water phase was 12 - > 77 days, while the degradation half-life was 26 - 201 days for the whole system. (EC 1996b). The abiotic hydrolysis of terbutylazine occurs only slowly with a half-life of 73 days (pH 5), 205 days (pH 7) and 194 days (pH 9). The photolysis in water also seems to be slow with reported half-lives of > 40 to 70 days and 172 days in the presence of photosensitisers. Terbutylazine shall be classified not ready biodegradable. In two controlled aerobic water-sediment systems terbutylazine disappeared from the water phase with half-lives of 6 and 7 days, while the degradation half-lives were 33 and 80 days for the whole system, respectively. High microbial activity enhanced the degradation. (MST 2003a). 1.5.3 SoilUnder aerobic conditions at 20°C in the laboratory the half-life of atrazine was in the range of 41 – 146 days with an average of 81 days. The mineralisation was very slow with a reported CO2 production of 6 % in 300 days. An anaerobic half-life of 160 days has been reported. Field studies carried out in Switzerland, Austria, France and USA showed half-lives in the range of 5 - 60 days with an average of 29 days. Regarding ground water contamination atrazine and the three metabolites desethyl atrazine (DEA), desisopropyl atrazine (DIA) and hydroxyatrazine are in risk to possess an unacceptable leaching. (EC 1996a). The aerobic half-life of simazine at 20°C in the laboratory was in the range of 20 – 270 days with an average of 53 days. The anaerobic degradation rate was comparable to the aerobic rate. The mineralisation accounted for 19 % in 120 days. Field studies conducted in Switzerland, UK, Germany, Italy, Netherlands and Sweden showed half-lives in the range of 14 - 146 days, average rates were 64 and 130 days at spring and fall application, respectively. Regarding ground water contamination the highest risk comes from the metabolite desisopropyl atrazine (DIA). (EC 1996b). Degradation of terbutylazine under aerobic conditions at 20°C in laboratory studies occurred with half-lives in the range of 61 – 169 days with a median value of 79 days (n=10). High microbial activity enhanced the degradation. Ten metabolites with the triazine structure intact have been identified. The mineralisation was very slow with an average CO2 production of about 5 % in half a year. Under anaerobic conditions the degradation was somewhat slower and no mineralisation was observed. Field studies carried out in Germany and Switzerland showed half-lives of terbutylazine in the range of 10 - 36 days with a median of 22 days (n=7). Regarding ground water contamination the most important metabolites from terbutylazine were hydroxyterbutylazine and desethyl terbutylazine, the former having the highest leaching potential. The leaching potential of terbutylazine is less than the potential of the two metabolites mentioned. Hydroxyterbutylazine can be produced both abiotic and biotic, while desethyl terbutylazine is only produced under biotic conditions. Therefore, risk to groundwater from hydroxyterbutylazine is highest when terbutylazine is applied under conditions not favourable for the microbial processes. The lowest risk to ground water pollution in Denmark was found to be from soils with a high amount of organic matter and a high biological activity as exemplified by cornfields receiving manure every year. (MST 2003a). The half-life is commonly 14-98 days for cyanazine (WHO 1998a). 1.5.4 BioaccumulationAtrazine has a slight to moderate tendency to bioconcentrate in microorganisms, algae, aquatic invertebrates, worms, snails, or fish (ATSDR 2001). 1.6 Human exposureOccupational exposure to triazines may occur through dermal contact or inhalation during the manufacture, formulation or application of the herbicides. (IARC 1999a,b). The general population may be exposed to the triazines and their degradation products through their widespread occurrence in the environment especially in drinking water. No significant exposure is expected to occur via foodstuffs.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||