|
Biological Control of Weevils (Strophosoma melanogrammum and S. capitatum) in Greenery Plantations in Denmark 4 Biology of Strophosoma melanogrammum and S. capitatum4.1 Feeding periods of adult weevils Any control measure, chemical or biological, rests on detailed knowledge about the biology and ecology of the target insects. Occurrence, activity, feeding periods and life cycle of the pest will inevitably affect the success of treatment, and therefore a description of these elements and of the factors influencing them is prerequisite in the development of control methods and strategies. In spite of the incontrovertible status of S. melanogrammum and S. capitatum as insect pests in European forestry, knowledge of the biology of these weevil species is very sparse. Most investigations have dealt with S. capitatum as this is the more important one in European forestry whereas S. melanogrammum, which appears to be of major interest in Denmark, is only little studied. Knowledge is more or less confined to the feeding periods during which the damage on trees takes place. The activity studies all agree in showing that both Strophosoma species have two feeding periods per year: One begins in early spring and lasts for a couple of months. This feeding precedes oviposition, which takes place after about three weeks of maturation feeding on the foliage (Urban, 1999). The next feeding period occurs in late summer and autumn, when a new generation of adult beetles emerges from the soil (Grimm, 1973; Schauermann, 1973; Sedlag and Kulicke, 1979; Bejer, 1989; Palm, 1996; Thorbek and Ravn, 1999; Urban, 1999; Harding et al., 2002). Until now, it has generally been believed, although never documented, that the eggs are laid in the soil (Kleine, 1911; Balachowski, 1963; Grimm, 1973; Schauermann, 1973; Sedlag and Kulicke, 1979; Bejer, 1989; Palm 1996). Reissig (1928), however, mentioned wavy hair grass, Deschampsia flexuosa (L.) Trin. and Hylocomium mosses as oviposition sites and, based on laboratory observations, Breese (1948) suggested that eggs also can be laid above ground in cracks of the bark and below budscales. In laboratory experiments, oviposition in soil has never been observed (Breese, 1948; Urban, 1999; Frølander and Nielsen, unpubl.). Recent investigations connected to this project have demonstrated that Strophosoma eggs are deposited in the canopy of A. procera and Picea abies (Karst.) below budscales and at the base of the needles. The first instar larvae then drop to the ground for further development in the soil (Harding et al., 2002). Egg densities were highest in the top of the trees (Harding, unpubl.). These observations, however, did not specify this oviposition behaviour for both Strophosoma species as neither eggs nor larvae can be identified to species level based on morphological characters. In the literature the development time, i.e. the period from the eggs are laid until the adults emerge, varies between 3 months and 1½ year. Schauermann (1973) reports a development of 12 – 15 months, Sedlag and Kulicke (1979) 17 months; Schindler (1974), Urban (1999) and Thorbek and Ravn (1999) describe the period to last only 3-5 months. Palm (1996) reports that larvae of S. melanogrammum are occasionally found in the soil during winter, indicating that this species may have the possibility of developing from eggs to adults in either 3-5 or 15-17 months. In forest zoology handbooks and other extension service tools for management of forest pests, there is, however, a consensus of opinion that both Strophosoma species are univoltine, stating that the offspring of the beetles feeding and ovipositing in spring emerge and feed in the autumn the same year. Hence, chemical control of the weevils in spring is in general anticipated to have a significant effect not only at the time of spraying, but also on the population levels and, in consequence, on the damages in autumn. In this project the investigations have focussed on the following elements of the biology of S. melanogrammum and S. capitatum as these are of main relevance to control strategies. Further, they will altogether give the first description of the life cycle of each of the two weevil species:
4.1 Feeding periods of adult weevils4.1.1 Materials and methodsThe activity of the adult weevils was recorded from spring 2001 until autumn 2003 in a greenery stand of A. procera established in 1983 in “Storskoven”, Bidstrup, Odsherred State Forest District. The activity was investigated by 30 evenly distributed emergence traps recording adult beetles emerging from the soil to feed in the vegetation. Each emergence trap caught weevils from an area of 1/8 m² (Fig. 4.1). The traps were emptied weekly, and the weevils were counted and determined to species.
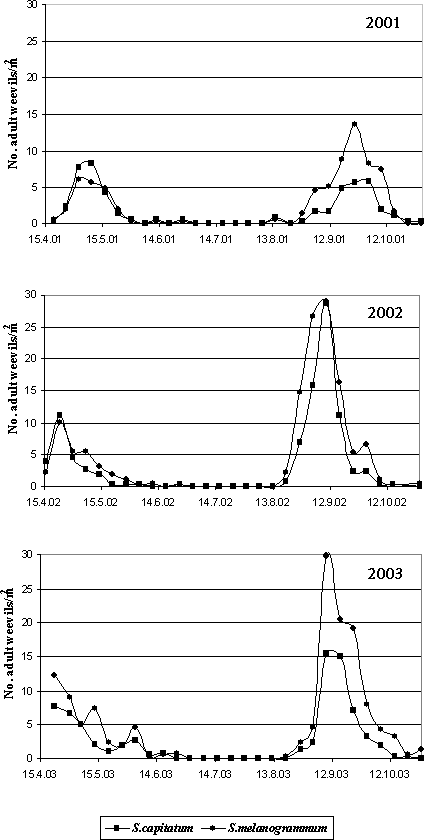
Figure. 4.1 Emergence trap for monitoring of adults of Strophosoma emerging from the soil. 4.1.2 ResultsThe monitoring of the emergence from the soil of S. melanogrammum and S. capitatum confirmed that both species have two feeding periods per year (Fig. 4.2).
Figure 4.2. Emergence of Strophosoma melanogrammum and S. capitatum from the soil in 2001-2003 given as number of weevils per m² per week as a function of date traps were collected. Numbers were recorded by weekly counting of adult weevils in 30 emergence traps in a 20-year-old greenery stand of A. procera. In spring, emergence for maturation feeding was initiated in early April and lasted for about 6-8 weeks. The emergence of the new generation was recorded from the beginning of August and proceeded for another two months, peaking late August or in September. Minor differences in the pattern of emergence occurred between years: In spring as well as in autumn 2001, emergence occurred about two weeks later than in 2002 and 2003. The two Strophosoma species were almost identical in their pattern of emergence, showing no major differences in the timing of initiation of emergence or of the peak of emergence activity. Population levels of S. melanogrammum were higher than of S. capitatum during all three years, except in spring 2001 (Table 4.1). Autumn densities were markedly higher than spring densities in both species, with the exception of S. capitatum in 2001. Table 4.1: Accumulated density (no. per m² ± SE) of Strophosoma melanogrammum and S. capitatum in the experimental greenery stand of noble fir in three successive years.
4.2 Larval drop from the canopy4.2.1 Materials and methods4.2.1.1 Larval behaviourFirst instar larvae dropping to the ground from the oviposition site in the canopy were recorded by means of funnel traps placed below the tree crowns. The traps consisted of a plastic funnel with a diameter of 21.5 cm, fitted into a 1 litre plastic bottle. The bottles were dug 2/3 into the soil to prevent it from falling over. 10 funnel traps were placed scattered in the A. procera stand (Fig. 4.3). The bottles were filled with a 2 % solution of benzoic acid. The traps were emptied by changing the bottles, which were then brought to the laboratory, where the contents were filtered through a 125µm wire mesh and preserved in 70% alcohol until Strophosoma larvae were sorted out and counted.
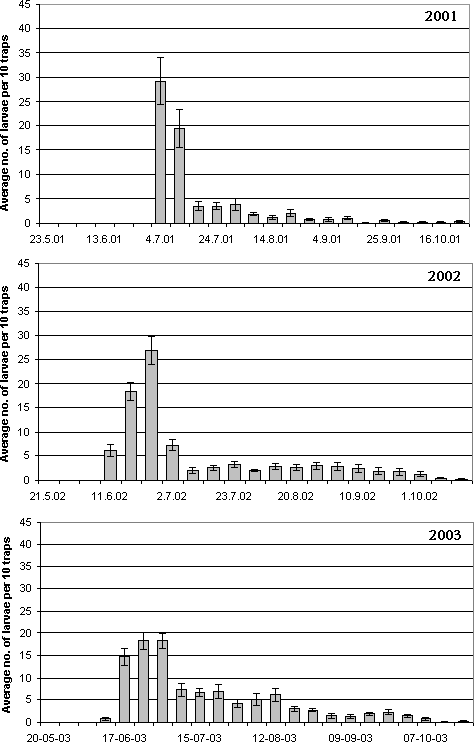
Figure 4.3. Funnel traps and sticky traps for recording of first instar larvae of Strophosoma dropping to the ground from the canopy. The activity was recorded weekly during summer until early November. In 2001 recording was, however, not initiated until early July as the observation of oviposition in the canopy was not made before that time. In 2002 recording started in early June, in 2003 in mid May. The activity of the larvae dropping from the canopy was in 2001 and 2002 also recorded by means of sticky traps. By 10 trees, a 514 cm² blue sticky trap was placed horizontally at 1.5 m's height below the crowns next to the funnel traps (Fig. 4.3). The traps were changed weekly. The sticky traps were wrapped in cling film and taken to the laboratory. Larvae adhering to the traps were counted under microscope. Both recording methods were effective; as they, however, showed a similar pattern of activity, only data from funnel traps are presented here. 4.2.1.2 Species identification of larvaeIn 2003, a fraction of the first instar larvae collected by funnel traps were identified to species by PCR analysis according to the method described in Chapter 3. At each date during the period June 17 – July 30, 10 larvae were randomly selected for analysis. However, at June 24, during the peak of activity, 46 larvae were analysed for identification. For the remaining period, bulks of 10 larvae or less were analysed and only qualitative occurrence in the sample was recorded. 4.2.2 Results4.2.2.1 Larval behaviourFirst instar larvae of Strophosoma drop to the ground during summer and early autumn (Fig. 4.4). The activity has a very distinguished peak lasting 2-3 weeks and then abruptly declining to insignificant levels. A few larvae still fall to the ground during the whole summer period, but this part of the larval population is of minor importance compared to the peak. The time of maximal activity varied only slightly between years, in 2002 peaking around June 18 – 25, in 2003 June 17 – July 1. In 2001 the peak may not have been recorded, but high levels were recorded during the first two weeks of July.
Figure 4.4. Activity of first instar Strophosoma larvae dropping from the canopy in a greenery stand of noble fir 2001-2003 . Activity is recorded as means of larvae trapped per funnel trap per week (± SE). The density of Strophosoma larvae dropping through the vegetation to seek to the soil was very high, up to several thousand larvae per m² (Table 4.2). Local densities exceeded more than 4000 larvae per m² (data not shown). The densities showed great variation between years, and showed an increase during the experimental period. Table 4.2: Accumulated density (no. per m² ± SE) of first instar Strophosoma larvae dropping from the canopy in a greenery stand of noble fir. Densities were estimated from numbers of larvae recorded in funnel traps.
4.2.2.2 Species identification of larvaePCR analysis showed that larvae of both Strophosoma species drop to the ground from the canopy. Also, both species occurred in the traps during the whole period (Table 4.3). Absence of S. capitatum in samples in October may be due to a small number of specimens analysed (6 individuals). Table 4.3. Occurrence of Strophosoma melanogrammum and S. capitatum in funnel traps recording larvae dropping to the ground from the foliage. Figures in parentheses indicate numbers of specimens identified.
4.3 Dispersal of Strophosoma adults and larvae4.3.1 Materials and methods4.3.1.1 Dispersal of adults482 adult S. melanogrammum collected in the field were marked with red nail polish in the laboratory and released on June 6, 2002, around a tree trunk of a 2½ meter high A. procera. On day 7 and 28 after the release of the weevils, the infested tree and surrounding trees were monitored for marked individuals by beating the branches over a white tray. Marked individuals captured were counted. 4.3.1.2 Dispersal of larvaeThe possibility of dispersal of the weevil population by first instar larvae when they leave the canopy was investigated by two methods: a) sticky traps placed at crown level, and b) water traps below crown level outside the stand. Trapping by sticky traps was preliminarily tested in 2002: During the peak of larval activity, three 9-meter poles were raised within the greenery stand but without physical contact with the canopy. At 5, 6, 7, and 8 m's height, respectively, a 514 cm² sticky trap was fastened vertically. After two weeks, the sticky traps were taken down and analysed for Strophosoma larvae as described in paragraph 4.2.1.1. In 2003, the 3 poles were raised at different distances from the stand trees: i) within the stand but without contact with the canopy, ii) within a glade in the stand, at least 5 m from the nearest trees, and iii) in an open area ca. 10 m from stand edges of noble fir. Sticky traps were placed at an angle of 105° to the pole to minimise turbulence. One set of traps was fit up on June 17 and taken down after one week; a second trapping period elapsed from July 1 to 15. In 2003 7 water traps were placed on the ground along the centre of a wide track within the greenery stand. These traps were circular plastic containers (diameter 15 cm, depth 9 cm) with a 2% solution of benzoic acid and detergent (soap). No traps were closer to the stand trees than 2 m. Another 3 traps were placed in the glade close to the pole with sticky traps. The total trapping area thus was ca. 0.35 m². Further, in the open area and close to the sticky trap pole, water traps were placed on a platform elevated 3 m above the vegetation: 8 circular containers (diameter 45 cm, depth 30 cm) each with a trapping area of ca. 0.16 m² and 31 plastic containers with a trapping area of 0.035 m² each. The total trapping area on the platform thus was approx. 2.37 m². All water traps were put out on July 1 and emptied twice at weekly intervals. The contents of the traps were poured into plastic cans and treated as described for funnel traps in 4.2.1.1. 4.3.2 Results4.3.2.1 Dispersal of adultsAfter seven days, 23% (109) of the released individuals were recaptured. The majority was recaptured in the tree around which the weevils were released and mainly in the top of the tree; however, marked individuals were recaptured up to 3 m away. After 28 days, 34 specimens (7%) were recaptured. The majority of weevils were again caught in the top of the tree of release, but individuals were recaptured at a distance of 7.5 meters. 4.3.2.2 Dispersal of larvaeIn 2002, only one larva was found on the sticky traps. In 2003, a total of 5 larvae were found on the sticky traps during the first period, of these 4 were found in the dense vegetation – three at 5 m and one at 7 m, and only one larva adhered to the traps in the open area. During the second period, six larvae were captured by the sticky traps, again five within the dense stand and only one in the open area. None of the water traps in the open area collected any dispersing larvae. However, in the track and glade within the stand, a total of 17 larvae were found in 2003 (nine on July 8, 2002 and eigth on July 15, 2002). The eigth larvae captured on July 15, 2002 were analysed by PCR and only S. melanogrammum was found. 4.4 Development time of S. melanogrammum and S. capitatum4.4.1 Materials and methodsStrophosoma larvae were collected from the soil of the greenery stand at irregular intervals, during late autumn (November 26, 2002), spring (April 8, 2003) and early summer (May 27 and June 25, 2003). In the field, the soil was carefully examined, and larvae and pupae were sorted out, transferred to vials with soil and taken to the laboratory, for further examination. A total of 79 larvae were collected. As investigations had demonstrated that the width of the head capsule and body length were linearly correlated in Strophosoma (data not shown) as is known from other insect larvae (Kéler, 1952), the width of the head capsule was used for classification of larval stage. The larvae were identified to species by PCR according the method described in Chapter 3. In addition, the soil was searched for the presence of pupae in August 2002 and in April and May 2003. 4.4.2 ResultsPupae were only found in the soil in August. At all other sampling dates, only larvae were found. Of the 79 larvae collected, 69 were successfully identified by PCR. This analysis showed that larvae of both Strophosoma species are present in the soil throughout the year (Fig. 4.5). In late autumn (November, 2002), medium sized larvae of S. melanogrammum were found with an average width of the head capsule of 0.71 ± 0.12 mm. In spring (April 8, 2003), the majority of larvae were still in the same stage of development, but some had developed into the next stage. In May and June 2003, the S. melanogrammum larvae had all entered their last stage of development (width of head capsule: 0.96±0.05 mm). For S. capitatum, larval sizes were very different in late autumn, ranging between 0.3 and 1.08 mm. In spring, only few larvae were found, but in early summer all larvae had developed into the last stage, having a head capsule size of 0.99±0.13 mm. There was thus a tendency that larvae of S. capitatum were a little larger than larvae of S. melanogrammum, but differences were not statistically significant. Figure. 4.5. Size of larvae, measured as width of head capsule, of Strophosoma melanogrammum (left) and S. capitatum (right) collected at different times of the year from the soil of a greenery stand of Abies procera. 4.5 DiscussionThe period of emergence observed in this investigation is consistent with previous investigations of S. capitatum (Sedlag and Kulicke, 1979) and S. melanogrammum (Thorbek, 1998; Frølander unpubl.). The feeding period is, however, longer than the emergence period, as the adult weevils spend at least 3 weeks feeding prior to oviposition (Urban, 1999). Damages from adults feeding on the foliage thus develop from mid April to early – mid June and again during autumn, August – early October. In warm autumns, extensive feeding may continue during October and even November (S. Harding, personal observation) and also in early winter active weevils can be found in the canopy if temperatures are sufficiently high (Parry, 1983; Thorbek, 1998). The higher population levels in autumn than in spring were also documented by Grimm (1973) in Germany and by Thorbek (1998) and Frølander (unpubl.) for S. melanogrammum in Denmark, but appear not to be a general pattern in Central Europe for either species (Sedlag and Kulicke, 1979). This may indicate that the life cycle may vary within the area of distribution. Although hypothesised from laboratory experiments that oviposition may not take place in the soil (Breese, 1948; Urban, 1999), the occurrence of the eggs and first instar larvae in the canopy of the greenery plantation and the falling down of the larvae through the canopy and the ground vegetation have not been documented before. This new knowledge is of great importance to the development of a control strategy as it makes it possible to target directly the microbial control against the small larvae when they drop to the ground. The application method must ensure that the larvae get in contact with the conidia of the insect pathogenic fungus. This will be possible by a treatment of the soil surface timed immediately prior to the period when the larvae pass through. In this connection, it is noteworthy that the peak of this activity is very narrow in as much as feeding and oviposition periods are very prolonged; this has the practical implication that optimal soil treatment can be timed to early June. Knowledge about dispersal is relevant in relation to the ability of the weevils to spread a pathogen within the population. As the wings of Strophosoma spp. are fused, walking is the most likely strategy of dispersal. The adults are capable of dispersing by walking up to several meters per week, but although marked individuals were recaptured 7.5 m from the point of release, dispersal over long distances is hardly possible, which was also indicated by preliminary studies by Skov (2000). Horizontal movement of the larvae in the soil has been documented by Schauermann (1973) but is unlikely to amount to more than a few meters. Wind dispersal over long distances by the small larvae dropping to the ground was near hand, as this strategy is well known in eg. Lymantriidae and Adelgidae, but our results do not lend support to this hypothesis. From our studies of dispersal it must be concluded that the ability of S. capitatum and S. melanogrammum to disperse is rather restricted compared to most other pest insects. By sampling of larvae throughout the year, it was documented that an exclusively univoltine development as suggested by Schindler (1974), Urban (1999) and Thorbek and Ravn (1999) can be totally rejected. Univoltinism would imply either absence of larvae during winter (3-5 months development) or the occurrence of last stage larvae and pupae in the spring (12 months development), and neither was found to be the case. It was found that both S. melanogrammum and S. capitatum can have a development time of 15-17 months from the eggs are laid in spring until emergence of the new generation in the autumn the following year. This sequence of development is in accordance with investigations undertaken in Germany by Schauermann (1973) who reports on the only previous detailed investigation of the development of Strophosoma spp. He found a mixture of 3rd and 4th stage larvae in the soil from September until August the following year and, being unable to discriminate between the two species, he suggested one species to hibernate in the 3rd and the other species in the 4th larval stage. This difference was not found in the present study in which larvae of both species developed from medium sized (3rd stage) to large (4th stage) larvae during early summer. The results do not enable us to definitely make out if part of the populations may be able to complete development in 3-5 months. More frequent sampling during summer and higher numbers of larvae are needed to follow in detail the exact sequence of development (cf. e.g. Axelsson et al., 1973) and any variations within populations . The practical consequence of a 2-year life cycle is that an immediate effect of control of larvae on the population levels in autumn cannot be expected. 4.5.1 Life cycleOur studies enable us to describe the general life cycle of the two Strophosoma species in Danish greenery plantations: Adults of both species emerge from their hibernation site in the soil in late April and seek to the canopy of the stand trees for maturation feeding prior to oviposition. Feeding lasts approx. 3 weeks and takes place in the treetops on the leader and upper whirls (Harding, unpubl.). The eggs, which are laid in the canopy, hatch after about 3 weeks, and 6-8 weeks after the peak of emergence the 1st stage larvae drop to the ground to complete their development in the soil. The larvae complete another 3 stages in the soil and overwinter primarily in the 3rd larval stage. The larvae enter their 4th stage in May. Pupation takes place in late July/August and the new generation emerges from late August. It cannot be excluded that part of the populations can develop in 3-5 months. Clear differences have not been found in the life cycles of the two Strophosoma species. However, S. capitatum may have a greater flexibility in its development as indicated by a high variation in the size of the larvae at a given time. It is not known whether this life cycle is general for S. melanogrammum and S. capitatum in their whole area of distribution and at different stand characteristics. 4.6 Conclusions
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||