|
Biological Control of Weevils (Strophosoma melanogrammum and S. capitatum) in Greenery Plantations in Denmark 5 Effects of hyphomycete fungi against Strophosoma spp. in greenery plantations5.1 Soil application of Metarhizium anisopliae against larvae Based on the results obtained in bioassays (chapter 2) and information on the life cycle of Strophosoma spp. (chapter 4), M. anisopliae and B. bassiana were chosen for test under field conditions in a greenery plantation. Four experiments were carried out to investigate field effects of treatment of adults and of larvae in the soil and to study the possibility of single tree treatment targeted against adults seeking to the treetops:
All experiments were performed in an A. procera plantation situated at ”Storskoven”, Bidstrup, Zealand, Denmark. The plantation was established in 1983 and has for the last years suffered from damages caused by Strophosoma spp. 5.1 Soil application of Metarhizium anisopliae against larvae5.1.1 Materials and methodsOne part of the plantation was divided into four plots each covering approximately 125 m². Untreated border zones of 4-5 m separated the plots. Two plots were treated twice with M. anisopliae in 2000 and exactly the same plots were treated again twice in the following year. In both years two plots remained untreated and served as control plots. M. anisopliae (BIPESCO 5) was applied to the two plots on July 7 and July 19, 2000 by spraying 200 ml per m² of a M. anisopliae suspension adjusted to 5.0 x 107 conidia per ml 0.05 % Triton X-100. M. anisopliae (BIPESCO 5) was applied to the same plots on June 26 and July 10, 2001 at the same concentrations as in 2000. In both years the final concentration sprayed on each date was thus 1 x 1014 conidia per ha. The conidia of M. anisopliae were produced by Prophyta (Germany). Two control plots were treated with 0.05 % Triton X-100 only. The effect of the treatment on the population levels of adult weevils was assessed by emergence traps placed on the ground. Adult weevils were sampled from 15 evenly distributed emergence traps in each plot once a week from mid July 2000 until beginning of November 2003. Each emergence trap caught weevils from 1/8 m². All weevils were counted and determined to species level. The effect of date, plot, and treatment on the number of weevils emerging from the traps was analysed by multiple regressions. The GLM procedure in SAS v. 8.02 (SAS Institute, 1999) was used for the analysis, density of weevils were log transformed [loge (weevils per trap + 1)] and each species was analysed separately. Date, plot and treatments were used as class variables. The full model including all parameters and possible interactions was reduced stepwise by excluding the least significant parameter for each step but always by excluding interactions before main factors (Jensen and Skovgaard, 1995). 5.1.2 Results5.1.2.1 Population level in 2000In autumn, the adult weevils emerged from the soil from the beginning of August until mid November with a maximum of 21 weevils per m² per week in mid September. In autumn 2000 the accumulated number of S. melanogrammum and S. capitatum caught in emergence traps in the treated plots was not significantly different from the number caught in the control plots (data not shown). 5.1.2.2 Population level in 2001The numbers of Strophosoma caught in spring 2001 are shown in figure 5.1. Adults emerged from the soil from the beginning of April until mid June, reaching a maximum of 12 S. melanogrammum and 5 S. capitatum per m² per week in the control plots in the beginning of May. In treated plots the maximum of S. melanogrammum was also reached in the beginning of May by 8 S. melanogrammum per m² per week. S. capitatum reached its maximum two weeks later by 7 individuals per m² per week. The differences in numbers between weevils caught in emergence traps in the treated plots and control plots were, however, only slightly significant for S. melanogrammum (F 1,648 = 3.94; P = 0.0476) and not significant for S. capitatum (F 1,647 = 2.49; P = 0.1151). In autumn, the adult weevils emerged from the soil from mid August until the beginning of November, reaching a maximum of 25 S. melanogrammum and 7 S. capitatum per m² per week in the control plots in the end of September. In treated plots the maximum of weevils was also reached in the end of September by 10 S. melanogrammum and 4 S. capitatum. The accumulated numbers of Strophosoma spp. caught in autumn 2001 in emergence traps were 131 per m² in control plots and 63 per m² in treated plots (Fig. 5.2). For S. melanogrammum the accumulated density of weevils in M. anisopliae treated plots was thus reduced by 60% compared to the density in the control plots. For S. capitatum the accumulated density of weevils in M. anisopliae treated plots was reduced by 38% compared to the density in the control plots. The accumulated numbers of both S. melanogrammum and S. capitatum caught in emergence cages in the treated plots were significantly lower than the numbers caught in the control plots (S. melanogrammum: F 1,588 = 41.31; P < 0.0001; S. capitatum: F 1,588 = 5.28; P = 0.0220). 5.1.2.3 Population level in 2002In spring 2002, adults emerged from the soil from the beginning of April until mid June, reaching a maximum of 24 S. melanogrammum and 7 S. capitatum per m² per week in the control plots in mid April. In treated plots the maximum of weevils was also reached in mid April by 18 S. melanogrammum and 9 S. capitatum. For S. melanogrammum the accumulated numbers of weevils in M. anisopliae treated plots was reduced significantly by 10% compared to the density in the control plots (Fig. 5.2) (F 1,648 = 3.94; P = 0.0476). For S. capitatum the accumulated numbers of weevils in M. anisopliae treated plots was not reduced compared to control plots (F 1,647 = 2.49; P = 0.1151). In autumn the adult weevils emerged from the soil from mid August until the beginning of November, reaching a maximum of 49 S. melanogrammum and 11 S. capitatum per m² per week in the control plots in the end of September. In treated plots the maximum number of S. melanogrammum was reached one week earlier by 21 weevils per m² per week. The maximum number of S. capitatum in treated plots was reached in mid September by 14 weevils per m² per week. The accumulated number of S. melanogrammum caught in autumn 2002 in emergence traps was 149 per m² in control plots and 84 per m² in treated plots (Fig. 5.2), which again was significantly different (F 1,589 = 18.14; P < 0.0001). For S. capitatum the accumulated number of weevils caught was 29 per m² in control plots and 35 per m² in treated plots (F 1,588 = 0.13; P = 0.7163). 5.1.2.4 Population level in 2003No significant differences were seen between M. anisopliae treated and control plots neither in spring nor in autumn (Spring: S. melanogrammum: F1,589 = 0.01; P = 0.9486; S. capitatum: F1,589 = 0.03; P = 0.8567. Autumn: S. melanogrammum: F1,589 = 0.82; P = 0.3656; S. capitatum: F1,588 = 0.03; P = 0.1541), which means that no long-term effect was obtained (Fig. 5.2). Figure 5.1: Density (no. per m²) in 2001 and 2002 of adult S. melanogrammum (left column) and S. capitatum (right column) on the experimental field site treated with either M. anisopliae BIPESCO 5 (Treated) or 0.05% Triton x-100 (control) in summer 2000 (July 7 and July 19) and summer 2001 (June 26 and July 10) after soil application against larvae. The density was measured by weekly counting of number of weevils caught in 30 emergence traps per treatment.
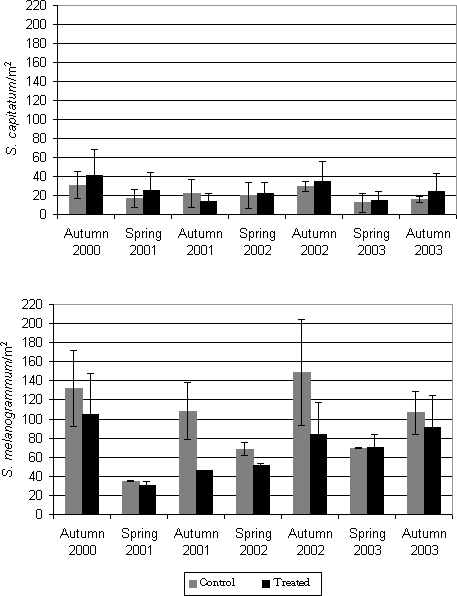
Figure 5.2: Density (accumulated numbers) in 2000, 2001, 2002 and 2003 (no. per m²) of adult S. melanogrammum (bottom) and S. capitatum (upper) on the experimental field site treated with either M. anisopliae BIPESCO 5 (Treated) or 0.05% Triton x-100 (control) in summer 2000 (July 7 and July 19, 2000) and summer 2001 (June 26 and July 10) against larvae. The density was measured by weekly counting of number of weevils caught in 30 emergence traps per treatment. 5.2 Soil application of Metarhizium anisopliae against ovipositing females5.2.1 Materials and methodsThe same A. procera plantation as used for soil application of M. anisopliae (BIPESCO 5) in summer 2000 and 2001 against larvae was chosen as field site for this experiment. Another part of the plantation was thus divided into four new plots each covering around 125 m². Untreated border zones of 4-5 m separated the plots. Two plots were treated with M. anisopliae and two plots served as control and were sprayed with 0.06% Triton-X 100 only. M. anisopliae (BIPESCO 5) was applied to two plots on April 25 and May 9, 2001 by spraying 200 ml per m² of a M. anisopliae suspension adjusted to 2.3 x 107 and 5.0 x 107 conidia per ml 0.05 % Triton X-100, respectively. The final concentration sprayed on each date was thus 4.6 x 1013 and 1 x 1014 conidia per ha. The conidia of M. anisopliae were produced by Prophyta (Germany). The two control plots were treated with 0.05 % Triton X-100 only. The effect of treatment on the population levels was determined as described in paragraph 5.1.1. In order to estimate the prevalence of the applied fungus as well as the prevalence of natural infections, the weevils were sampled from traps once a week from the beginning of April 2001 until mid June 2001. The weevils were brought to the laboratory where they were counted and determined to species. Live weevils were incubated individually in plastic cups (30 ml) at 20°C and a light regime of 16:8 L:D. Each cup contained 3% water agar in the bottom to keep humidity high and a shoot of A. procera was provided as food. The mortality was recorded weekly for fours weeks, and dead weevils were removed and placed in humid chambers and fungal infections were diagnosed based on morphological characters (Samson et al., 1988; Humber, 1997). 5.2.2 Results5.2.2.1 Prevalence study in spring 2001Prevalence data of fungal infections in adult S. melanogrammum and S. capitatum collected in spring 2001 are shown in Fig. 5.3. For S. melanogrammum the highest prevalence of fungal infection (90.2 %) was found on May 9, a result of the first release of the fungus, and the highest prevalence of fungal infection for S. capitatum was obtained on May 16 (78.1%). In the untreated control plots, 82 individuals of S. melanogrammum were sampled and incubated, but only one was infected by M. anisopliae while no infected individuals of S. capitatum were found. The infected individual was captured from one of the emergence traps on the borderline to a treated plot. Besides the applied fungus, natural infections with B. bassiana and P. farinosus and V. lecanii were observed but only at very low prevalences (below 8%).
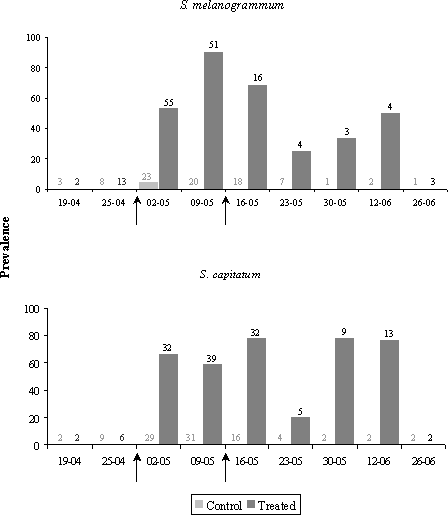
Figure 5.3: Prevalence of M. anisopliae in adult Strophosoma melanogrammum (upper graph) and Strophosoma capitatum (lower graph) populations collected from emergence traps in 2001 after treatment with Metarhizium anisopliae BIPESCO 5 (treated) or with 0.05 % Triton X-100 (control) against ovipositing females. Arrows mark application time (April 25 and May 9, 2001). 5.2.2.2 Population level in 2001In spring adults emerged from the soil from the beginning of April until mid June (Fig 5.4). The numbers of both S. melanogrammum and S. capitatum caught from emergence traps in 2001 is shown in figure 5.4. The number of both S. melanogrammum and S. capitatum caught from emergence traps in spring was not significantly different between the M. anisopliae treated plots and the Triton-X treated plots (S. melanogrammum: F1,529 = 3.48; P = 0.0625; S. capitatum: F1,529 = 3.55; P = 0.0601). In autumn, the adult weevils emerged from the soil from mid August until the beginning of November. The number of S. melanogrammum caught in emergence traps in the M. anisopliae treated plots was again higher but not significantly, than the number caught in the control plots (F1,707 = 0.88; P = 0.3490), whereas accumulated density of S. capitatum was reduced by approximately 50% in the M. anisopliae treated plots compared to the number caught in the control plots (F1,707 = 4.70; P = 0.0304). 5.2.2.3 Population level in 2002In spring 2002, adults emerged from the soil from the beginning of April until mid June, reaching a maximum of 10 S. melanogrammum and 11 S. capitatum per m² per week in the control plots in mid April. In treated plots, the maximum number of weevils was also reached in mid April by 12 S. melanogrammum and 5 S. capitatum. The density of S. melanogrammum in the M. anisopliae treated plots was higher than in the plot treated with Triton-X only but not significantly (F1,648 = 3.43, P = 0.0643). For S. capitatum the accumulated density of weevils in M. anisopliae treated plots was slightly lower compared to control plots (Fig 5.5) but again the difference was not significant (F1,648 = 2.52, P = 0.1129). In autumn the adult weevils emerged from the soil from mid August until the beginning of November, reaching a maximum of 29 S. melanogrammum and 28 S. capitatum per m² per week in the control plots in mid September. In treated plots the maximum of weevils was also reached in mid September by 26 S. melanogrammum and 17 S. capitatum. In autumn the density of both S. melanogrammum and S. capitatum were significantly lower in the M. anisopliae treated plots compared to the control plots (S. melanogrammum: F1,589 = 6.59, P = 0.0105; S. capitatum: F1,589 = 6.45, P = 0.0114). The accumulated number of S. melanogrammum caught in autumn 2002 in emergence traps was 102 per m² in control plots and 77 per m² in treated plots (Fig 5.5). For S. capitatum the accumulated number of weevils caught was 69 per m² in control plots and 46 per m² in treated plots (Fig. 5.5). Thus S. melanogrammum was reduced by 25% and S. capitatum by 33%. 5.2.2.4 Population level in 2003In 2003 no significant differences were seen between M. anisopliae treated and control plots neither in spring nor in autumn (Spring: S. melanogrammum: F1,589 = 0.01; P = 0.9486; S. capitatum: F1,589 = 0.03; P = 0.8567. Autumn: S. melanogrammum: F1,589 = 0.82; P = 0.3656; S. capitatum: F1,588 = 0.03; P = 0.1541, which means that no long-term effect of treatment was obtained (Fig. 5.5). Figure 5.4: Density (no. per m²) in 2001 and 2002 of adult S. melanogrammum (left column) and S. capitatum (right column) on the experimental field site treated with either M. anisopliae BIPESCO 5 (Treated) or 0.05% Triton x-100 (control) in spring 2001 (April 25 and May 9) after soil application against ovipositing females. The density was measured by weekly counting of number of weevils caught in 30 emergence traps per treatment.
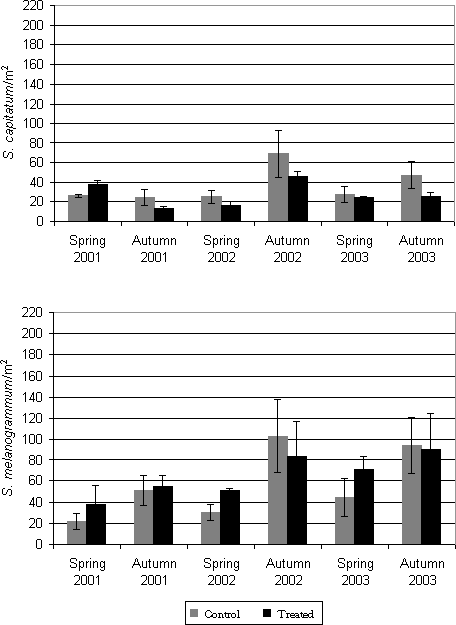
Figure 5.5: Density (accumulated numbers) in 2001, 2002 and 2003 (no. per m²) of adult S. melanogrammum and S. capitatum on the experimental field site treated with either M. anisopliae BIPESCO 5 (Treated) or 0.05% Triton x-100 (control) in spring 2001 (April 25 and May 9) against ovipositing females. The density was measured by weekly counting of number of weevils caught in 30 emergence traps per treatment 5.3 Soil application of B. bassiana and of M. anisopliae against adults emerging from the soil in spring5.3.1 Materials and methodsThe A. procera plantation used for soil application of M. anisopliae was also used for soil application of M. anisopliae (BIPESCO 5) and B. bassiana (KVL 98-20, KVL 02-04 and KVL 02-14). All the B. bassiana isolates originated from natural infections in Strophosoma collected in Denmark. Another part of the plantation was divided into five new plots each covering 9 m². Untreated border zones of 4-5 m separated the plots. Four plots were treated with one of the isolates and the last plot served as control plot. The treatments were applied to the plots on April 23, 2002 and May 7, 2002 by spraying 200 ml per m² of the fungal suspension adjusted to 5.0 x 107 conidia per ml 0.05 % Triton X-100. The final concentration sprayed on each date was thus 1 x 1014 conidia per ha. The control plot was treated with 0.05 % Triton X-100, only. Adult weevils were sampled from 9 evenly distributed emergence traps in each plot once a week from the beginning of April until June 2002. The weevils were counted and determined to species level. The prevalence of the applied fungus as well as the prevalence of natural infections was determined as described in paragraph 5.2.1. 5.3.2 ResultsPrevalence data for adult S. melanogrammum and S. capitatum collected in spring 2002 from the field experiment are shown in Table 5.1 (data for the entire season is pooled). For S. melanogrammum the highest prevalence (76 %) was found in plots treated with BIPESCO 5. The highest prevalence for S. capitatum was also obtained in the plot treated with BIPESCO 5 (80%). Table 5.1: Prevalence in adult S. melanogrammum and S. capitatum populations collected from emergence traps in 2002 after treatment with M.anisopliae (BIPESCO 5), B. bassiana (KVL 98-20, KVL 02-04 and KVL 02-14) or with 0.05 % Triton X-100 (control) on April 23 and May 7, 2002.
5.4 Application of tree trunks with lanes of Beauveria bassianaIn a preliminary experiment high infections (80 %) were initiated in Strophosoma spp. populations by treating the tree trunks with lanes covered with sporulating cultures of B. bassiana (Boverol®). The fungus showed good survival for several months. 5.5 DiscussionPopulations of Strophosoma spp. were reduced by soil application of M. anisopliae both when applied against larvae and against adults emerging from the soil to feed in the canopy. Reductions were significant. In both experiments the main reduction of the populations was monitored in autumn the year following application, most likely due to the two-year life cycle of the weevils (chapter 4). 5.5.1 Control of larvaeNo other studies have ever been reported on biocontrol of Strophosoma spp. with insect pathogenic fungi, while some experience has been obtained with other curculionid larvae. Moorhouse et al. (1993a) used M. anisopliae to control larvae of the vine weevil Otiorhynchus sulcatus Fabr. and by a prophylactic treatment in pots in greenhouses they managed to reduce the population by 78 %. Greenhouses have, however, a very controlled environment. Outdoor, Moorhouse et al. (1993b) managed to reduce O. sulcatus larvae by 43-62 %, but they noted a high variation in larval control (from zero to 96 %). Quintela et al. (1998) were less successful to control larvae of Diaprepes abbreviatus L. in soil using unformulated conidia of M. anisopliae and B. bassiana. Only minimal larval mortality was found (10.2%) and only when using B. bassiana. Even when using formulated conidia of M. anisopliae, Verkleij et al. (1992) did not obtain significant mortality in Sitona lineatus L. In comparison with for example greenhouse trials our stand is a highly diverse environment and the obtained control of Strophosoma spp. larvae in the present investigation must be regarded as successful. When M. anisopliae was applied in summer against the larvae, application had no effect on adult population levels in the autumn the same year. In contrast, a significant reduction of population levels was monitored the following autumn. Further, the effect on S. melanogrammum was greater than on S. capitatum. We can hypothesize several explanations for these delayed effects and the differences between the two species: 1) first instar larvae falling from the foliage to the ground are more easily hit by the fungus than bigger larvae developing in the soil, 2) small larvae are more susceptible to the fungus than bigger larvae, 3) the fungus can survive in the soil and infect bigger larvae over a prolonged period of time, 4) despite the apparent similar biology of the two species small differences in larval behaviour in the soil can prove significant for the success of microbial control. Due to the lack of the knowledge about the process of the first instar larvae falling from the foliage to the ground at the initiation of the field experiments, the treatment of larvae was targeted against the larvae developing in the soil. Hence, it was timed to take place mid summer. On the basis of our new knowledge, a treatment in early June would be a more optimal timing for targeting the small larvae droping to the ground. Although the population reduction obtained in the experiment was more than 50%, it is most likely that an optimisation of timing would reduce the populations even more. More extensive trials evaluating the timing of application are needed. 5.5.2 Control of adult weevilsReports on microbial control of adult curculionids are only rarely found in the literature. However, Nankinga and Moore (2000) used B. bassiana to control the adult banana weevil, Cosmopolites sordidus (Germar) and managed to reduce the population significantly, but due to the extremely different environment for banana production in contrast to greenery production in Denmark their experience is hardly comparable with ours. The effect of treatment of adult Strophosoma spp. was less than treatment of larvae, but still significant. Again, the main effect on the population levels was monitored 1½ year after treatment. However, S. capitatum responded to treatment already the same year. The explanation to this difference from S. melanogrammum is not obvious: A higher degree of flexibility in the development time from egg to adult for S. capitatum or differences in the siting or mobility of the larvae in the soil at different times of the year and hence the ability of the fungal conidia to infect one-year old larvae are possible reasons. High prevalences of M. anisopliae were recorded in both adult weevil species. The data from the bioassays indicated that such infected adults are expected die within a couple of weeks (see chapter 2). Thus, egg densities are likely to be reduced and the population levels after 15-17 months diminished accordingly. Further, there may have been an effect of the fungus on the first instar larvae passing through upper soil layer two months later. 5.5.3 Prospects for development of a microbial control strategyIn accordance with the very nature of biological control in which the control agent requires a certain period of time to act on target, no immediate lethal effect on the pest population will occur as is normally obtained by chemical pesticides. Therefore the strategy of microbial control will necessarily differ from the strategy of a traditional chemical control. Microbial control of Strophosoma spp. in greenery plantations will primarily aim at preventing damages by a general reduction of population levels. The control can be specifically targeted either against the larvae or the adults. Application against adults will significantly reduce the pest insect population levels the following year. However, some damages are potentially reduced already within some weeks after treatment, as the fungus-infected adults die within a short period of time. Also, the feeding activity may be reduced by infection, but so far it remains unexplored how fungal infection influences feeding activity of Strophosoma spp. When treating the larvae, efficiency of control appear to be higher; however, when using this strategy no effects on damage levels can be expected until approx 1½ years after treatment. Assessment of the effect of treatments on damage levels was outside the scope of this project but needs further examination. Irrespective of the target stage of Strophosoma, the overall strategy of microbial control will be prophylaxis. This implies that the fungus should be applied in the stands prior to years when clipping of greenery will take place. During the entire rotation period of the stands several applications will probably be necessary. More detailed knowledge about the behaviour and persistence of M. anisopliae in forest soils and greenery plantations is needed to fully evaluate this strategy. The number of prophylactic treatments could be reduced and the strategy thus refined by development of an early warning system enabling the growers to predict population development. It is well known that population levels vary significantly between years, but knowledge about the biotic and abiotic factors determining population densities of S. melanogrammum and S. capitatum is however not yet sufficiently available. Due to environmental and non-target reasons (see chapter 6 and Vestergaard et al., 2003), `fungal bands' placed on tree trunks should also be considered in the future control of adult weevils. The preliminary experiment showed high prevalences of the applied fungus in recaptured weevils and this method of application would also match the long activity period of the adult weevils. Furthermore, fungal bands can be removed when no longer needed. Good control has been obtained with fungal bands in China against adults of the Asian long horned beetle, Anaplophora glabripennis Motschulsky (Higuchi et al., 1997; Dubois et al., 2004). The application method needs further development for practical use. Finally, evaluation of formulated products of fungal inoculum is essential for future control of Strophosoma spp. 5.6 Conclusions
|