|
Biological Control of Weevils (Strophosoma melanogrammum and S. capitatum) in Greenery Plantations in Denmark 6 Effects of fungi on non-target invertebrates and spatio-temporal distribution6.1 Non-target effects In general it is not possible to reduce a pest population using fungi without also affecting other invertebrates in the environment (Vestergaard et al., 2003). It is important to distinguish between the physiological host range and the ecological host range. Usually, tests to evaluate the physiological host range of biological control agents are conducted in the laboratory, and this host range therefore gives information on the potential non-target effects. The ecological host range is studied by sampling insects in the field after application and this gives information on the realistic non-target effects. Usually, the ecological host range is much narrower than the physiological host range (eg. Hajek et al., 1996) and in many aspects it better reflects the impact of biological control agents on non-target invertebrates in the ecosystem. The spatial and temporal distribution of an applied fungus will assist in an evaluation of non-target effects over time. This chapter aims to elucidate some of the environmental effects of spraying hyphomycete fungi in a greenery plantation, in particular M. anisopliae (BIPESCO 5). 6.1 Non-target effectsIn order to evaluate the effects on non-target invertebrates the most abundant insect and mite species were collected from a M. anisopliae treated A. procera plantation and incubated in the laboratory in order to determine the infection level of M. anisopliae. Furthermore, selected isolates were characterised by means of molecular methods in order to examine whether the isolates originating from the non-target organisms were identical with the applied isolate. 6.1.1 Materials and methods6.1.1.1 Field site:A 0.3 ha Abies procera plantation situated in Zealand, Denmark was chosen as field site. The plantation was established in 1983 and has for the last couples of years suffered from damages caused by Strophosoma spp. 6.1.1.2 Application of Metarhizium anisopliae:In year 2000, M. anisopliae (BIPESCO 5) was applied twice in two plots each of ~150 m² in a concentration of 1x1014 conidia per ha during summer (June and July) in order to control larvae of Strophosoma spp. In year 2001, M. anisopliae was also applied twice in a concentration of 4-10x1013 conidia per ha but this time the application took place during spring (April and May) in order to control adult Strophosoma spp. emerging after their hibernation in the soil. 6.1.1.3 Collection of non-target:Non-targets living above the soil surface were collected either carefully by hand picking or by sweep net from control plots and from treated plots 7, 14, 75 and 277 days after application. Specimens were incubated individually in 25 ml plastic cups at 20°C. Water and for most species also food were provided during incubation. Mortality was recorded at regular time intervals for up to eight weeks and dead individuals were examined for symptoms of insect pathogenic fungi. All insect pathogenic fungi were determined to species level. From M. anisopliae infected individuals representative in vitro isolates were obtained. 6.1.1.4 Characterisation of collected isolates:For selected M. anisopliae in vitro isolates, DNA was isolated and the ITS and IGS regions were amplified in 0.2 ml PCR tubes with a total volume of 25 µl in a Perkin Elmer GeneAmp PCR system. The amplified region was cut by restriction enzymes. Profiles were monitored by electrophoresis in 1.5% agarose gels and detected by staining with ethidium bromide. Sizes of amplification products were measured from a photograph of the gel (for further detail on the method used se Nielsen et al., 2001). 6.1.2 ResultsFor non-targets sampled 7 days after application of M. anisopliae high prevalences were found (above 50%) in two insect orders and in ticks (Table 6.1) but declined to 10-20% 14 days after application (except for target weevils and ticks). However, M. anisopliae infected ladybirds were found 277 days after application. Table 6.1: Percentage of targets (weevils) and non-targets infected with Metarhizium anisopliae sampled from the greenery plantation at different time after application with BIPESCO 5.
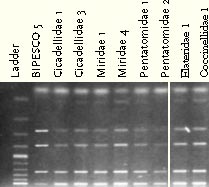
The ITS and IGS fragment pattern confirmed that the genotype isolated from infected non-targets was the same as the isolate applied (Fig. 6.1).
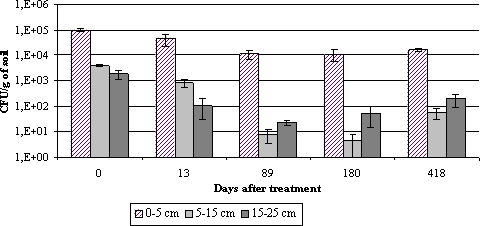
Figure 6.1: IGS fragment pattern of Metarhizium anisopliae isolates originating from non-targets collected after spraying with BIPESCO 5 6.2 Spatio-temporal distribution6.2.1 Materials and methods6.2.1.1 Soil samples:All soil samples were taken with a cylindrical soil sampler with an inner diameter of 3 cm. Samples were taken in three depths (0-5; 5-15 and 15-25 cm) both before and after application of M. anisopliae in an A. procera plantation in spring 2001. Three soil samples per treatment were taken 0, 13, 89, 180 and 418 days after application of entomopathogens. Soil samples were stored at 5°C and constant dark until analysis. 6.2.1.2 Isolation of entomopathogens from Metarhizium anisopliae treated soil:The density of M. anisopliae conidia was determined by plating soil suspensions onto Metarhizium selective medium medium (SDA with 0.5 ml l-1 dodine, 50.0 ppm Chloramphenicol, 50.0 ppm Streptomycin sulphate) using the following protocol: 1 g of soil was whirl mixed for 30 sec. in tests tubes added 9 ml of 0.05% Triton X-100. 100 l suspension or 100 l of a diluted suspension was distributed with a Drigalsky spatula onto a petri-dish with the selective medium. Plates were incubated at 23°C for 3-5 days and colony-forming units (cfu) of M. anisopliae were counted. Three replicates per sample were prepared. 6.2.2 ResultsBefore spraying with M. anisopliae the fungus did not occur naturally at detectable levels in the plantation soil. After spraying with M. anisopliae the fungus was solely found in the plots where the fungus was applied. M. anisopliae could be detected in the soil for at least 418 days after the application. The highest concentration measured was 1x105 cfu per g of dry soil in 0-5 cm's depth just after spraying of M. anisopliae. From 89 days after first spraying until 418 days after the first spraying the concentration of M. anisopliae in the upper layer remained around 1x104 cfu per g of dry soil. In the deeper layer of soil the concentration of M. anisopliae remained lower than in the upper layer throughout the season and except from just after the first spraying the concentration never exceeded 1x103 cfu per g of dry soil.
Figure 6.2: Density of Metarhizium anisopliae (cfu's per g dry soil) in soil collected from the experimental field site after treatment with M. anisopliae (BIPESCO 5) in spring 2001. 6.3 DiscussionM. anisopliae has a wide natural host range and the fungus has been reported from many insect orders (extensive review by Vestergaard et al., 2003). In studies on the physiological host range of specific isolates, transmissions between insect orders are commonly reported (James and Lighthardt, 1994; Danfa et al., 1999; Vestergaard and Eilenberg, 1999; Vestergaard et al., 2003). The natural infection of fungi in Carabids seems to be low: It was found to be around 2% in Danish lucerne and cabbage fields (Steenberg et al., 1995; Vestergaard and Eilenberg, 2000). Data from the greeneries support these data. Upon application in a lucerne field, M. anisopliae infected several Staphylinid and Carabid genera (Vestergaard and Eilenberg, 2000). It is, however, questionable if there are any population effects on non-targets. In Finland, where M. anisopliae was sprayed onto an oilseed rape field, no difference in the number of five dominant carabid species compared to the control was found (Zeng et al., 2001). It is also important to keep in mind that most of the non-targets infected by M. anisopliae have a shorter lifespan than the target Strophosoma spp. Therefore the populations of non-targets will recover much faster than the target. Also, some non-target effects can prove useful. Our finding that M. anisopliae had a significant effect on ticks, Ixodes ricinus, can actually be the first step towards the use of the fungus against this pest in Europe. In North America, a commercial product based on M. anisopliae was in summer 2003 launched for control of tick species occurring there (E. Butts, pers.comm.). It is noteworthy that M. anisopliae was not found in the soil in the control plots in the plantation. In a Danish study in Abies plantations of antagonists to the needle miner, Epinotia fraternata, Münster-Swendsen (1989) reported the occurrence of Paecilomyces farinosus in the hibernating larvae. In our study this fungus was also found naturally occurring in the stand, while M. anisopliae may occur, but at levels below the detection limit. This is opposite to the findings in agricultural fields. Here, Vestergaard and Eilenberg (2000) reported natural levels up to 300 cfu per g soil. In Finland, Vänninen et al. (2000) reported natural levels even up to 9000 cfu of M anisopliae per g soil, also in agricultural soils. It seems thus that M. anisopliae is not a significant fungus in natural greenery systems, but this needs further verification. In our studies we found non-target effects even 277 days after application and the fungus was present in the soil 418 days after application. In agricultural soils in Denmark (Vestergaard and Eilenberg, 2000) and Finland (Vänninen et al., 2000) significantly higher levels of M. anisopliae were found in treated plots compared to untreated 4 and 3 years after application, respectively. In a study from Australia it was found that three years is the maximum period for M. anisopliae to provide control in the soil after application (Milner et al., 2003). Importantly, the authors concluded that rainfall and soil type had negligible effects on the persistence of the fungus. In Tasmania, results from grassland indicate that applied M. anisopliae persists for more than seven years (Rath et al., 1997). It is still debatable, whether a long persistence of an applied fungus is simply due to 1) a long survival of single conidia, 2) a saprophytic growth and sporulation, or 3) a multiplication in hosts. Rath et al. (1997) hypothesized the long persistence in their studies to be due to a multiplication in the host. Whether or not a long persistence should be regarded as an advantage or not depends on the type of control wanted. In case of a wish to obtain long-term protection, a long persistence of the biocontrol agent should be regarded as a positive feature. 6.4 Conclusions
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||