|
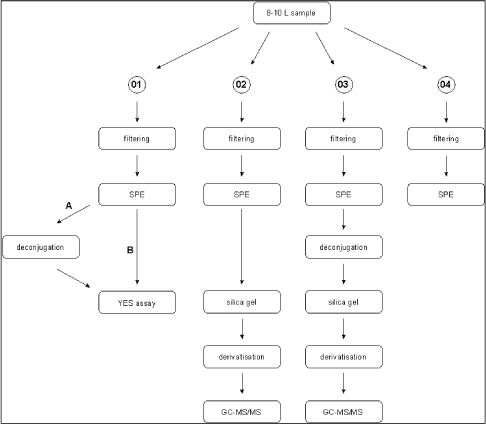
Survey of Estrogenic Activity in the Danish Aquatic Environment 3 Overview of activities3.1 Sampling methodology This chapter gives an overview of the sampling, conservation and storage procedures applied to ensure sample integrity as well as the chosen methodologies for biological testing and chemical analysis, respectively. 3.1 Sampling methodologyAll samples were taken as spot samples as the flow-proportional sampling were not considered to be feasible for the survey, see section 2.1.2. To limit the error introduced by the use of spot samples all samples were taken by “qualified spot sampling” (German Ministry of Environment 1997), a sampling procedure where each sample is mixed from equal parts of five sub-samples taken with at least 2 minutes time intervals within a maximum of 2 hours (for practical reasons typically about 30 minutes in this project). A special investigation was undertaken to document the possible error introduced by the use of spot sampling instead of the ideal flow-proportional sampling. The investigation is described further in Section 4.1. Two-litre and ten-litre glass bottles were used for sampling. The glass bottles from Schott (Blue Cap) were equipped with Teflon lined screw caps. Before use the bottles were cleaned by the usual laboratory washing procedure followed by 4 hours ignition at 430°C. Each of the five sub-samples were taken in a two-litre bottle and equal amounts, generally 2 litres, transferred to one ten-litre bottle. The samples were immediately preserved by the addition of sulphuric acid to pH 3. After the preservation the mixed sample was further transferred to 4 two-litre glass bottles. The samples were kept cool during the field operations, and after the day's work the samples were transported to the laboratory under cooled condition. For all samples the analysis was started the in the morning of the next day i.e. within a maximum of 30 hours after field sampling and preservation. In the laboratory the sample consisting of four identical sub-samples were treated as described in the following. 3.2 Analysis of steroid estrogens and estrogenic activityIn the project the samples were analysed either for their content of free estrogens or for the content of estrogens after cleavage of conjugated estrogens (i.e. total estrogen concentration). It was possible to analyse both total and free estrogens with the chemical as well as the biological method. The overall sample handling procedure is illustrated in Figure 3.1. Samples were divided intro four sub-samples which were treated differently. Sub-sample 01 was used for the biological analysis of estrogenic activity of both total and free estrogens. Sub-sample 02 was used for chemical analysis of free estrogens in the sample. In sub-sample 03, the deconjugation step enabled the determination of total estrogens with the chemical method. Sub-sample 04 was stored in the “sample-bank” for later purposes. In the following, brief descriptions of the different methods used in the determination of estrogens and estrogenic activity are given. The detailed descriptions and documentation of each method are given in the appendices to this report.
Figure 3.1 overall sample handling procedure. 3.2.1 Sample pre-treatmentAs illustrated above all samples were filtered prior to extraction on solid phase columns. GF/C glass fibre filters were used for filtration. Solid phase extraction (SPE) was made by eluting 2 litre samples through C18-cartridges (Varian, Mega Bond Elut® 1 g/6 mL) which were stored in the freezer until further analysis. After storage 5 mL of acetone was used to elute the analytes from the cartridges. The acetone extract was evaporated to dryness under a gentle stream of nitrogen. The dried acetone extracts from SPE-cartridges were treated with -glucuronidase enzyme 2 from Helix pomatia to cleave the conjugated estrogens and thus enable free and conjugated estrogens to be determined in one analysis. This procedure was applied to 03 sub-samples and half of the acetone extract used for biological analysis (sub-sample 01). To remove substances interfering with the chemical analysis, a cleanup step using silica gel was applied prior to GC-MS-MS analysis. More details on methods and testing related to the handling and pre-treatment of samples can be found in Appendix 1. 3.2.2 Biological testingThe estrogenicity of the water samples was determined by means of the YES-assay as described by Routledge and Sumpter (1996). Sub-samples from the project were analysed as follows: Initially, the samples were evaporated to dryness under N2 at 35°C after which the evaporation residue was dissolved in 300 µl ethanol. This is referred to as the undiluted sample. The sample was transferred to a vial which was stored at -20°C. 100 µL of each sample was transferred to a microtiter plate (the dilution plate) and a dilution series was produced; the dilution factor in the series was 2 and 12 diluted samples were produced. The assay plate contained one row of standard (17β-estradiol), one row of blank and six rows of samples. 10 µL of each dilution was transferred to a new microtiter plate (the assay plate). This step was carried out in a Laminar Air Flow bench. The assay plates were allowed to dry in the bench after which 200 µL yeast culture were added. Subsequently, the assay plates were incubated at 32°C for 3 days. The absorbance at 540 and 630 nm was determined and sample absorbance was converted to E2 equivalents by comparison with the E2 standard curve. The slope of a fairly large number of the sample response curves deviated from the slope of the E2 standard curve and the maximum absorbance was lower than the absorbance of the E2 standard curve (shown in Appendix 3). For these samples only the lower part of the response curve was used for the calculation of E2 equivalents (described in Appendix 3). 3.2.3 Chemical analysisSeparation and detection of the steroid estrogens were accomplished using a gas chromatographic-tandem mass spectrometry system (GC-MS/MS) consisting of a gas-chromatograph (Varian, CP-3800) and a triple quadrupole mass spectrometer (Varian, MS 1200 Quadrupole MS/MS system). Extracts of samples cleaned up with silica gel were evaporated to dryness and derivatized using a 50 µL of a mixture of N-methyl-N-(trimethylsilyl)-trifluoroacetamide (MSTFA), N-trimethylsilylimidazole (TMSI) and 1,4-dithioerythritol (DTE). After one hour of incubation at 60 °C the liquid was evaporated to dryness and re-dissolved in 200 µL heptane. Similarly, the initial sample volume of 2000 mL was reduced 10,000 times and the analytes pre-concentrated proportionally. The analytical method is described in more detail in Appendix 2. Concentrations of the steroid estrogens were calculated using a calibration curve for the ratio of the responses of the analytes and their deuterated internal standards. The calibration curve is fitted to a linear equation. 3.2.4 Data treatment and E2 response factorsThe chemical analysis provided concentrations of four individual estrogens, which were recalculated to total estrogenic activity based on equivalence factors determined in this study to be 0.29, 0.88, and 0.04 for E1, EE2 and -E2, respectively. The results in the main report are mainly presented as the calculated E2-equivalents, while the data for the individual steroid estrogens can be found in Appendix 6. It is attempted to give an unbiased expression of the data and the data sets are shown without correcting for the deviation in control samples (see section 4.3). 3.3 Quality Assurance3.3.1 Quality Control samplesTo monitor the performance of the analytical method during the project, an internal quality control scheme has been established involving blind samples and control samples that are prepared and analyzed together with every series of real samples. Results from these analyses are monitored by registration in control charts. 3.3.2 Inter-laboratory comparisonSince two different laboratories are responsible for conducting the chemical analyses in this project a small scale inter-laboratory comparison between the two laboratories has been conducted. A total of 12 identical authentic samples were analyzed by both laboratories in order to reveal any disagreement between results from the two laboratories. An inter-calibration of the YES assay was made between our laboratory and the laboratory of Prof. John Sumpter at Brunel University, UK, where this assay was developed originally. The implemented quality assurance measures are described more thoroughly in Appendix 4.
|