|
| Front page | | Contents | | Previous | | Next |
Background Report on Pre-validation of an OECD Springtail Test Guideline
OECD GUIDELINES FOR THE TESTING OF CHEMICALS
PROPOSAL FOR A NEW GUIDELINE
Annex 1
Annex 2
Annex 3
Annex 4
Collembola Reproduction Test (Folsomia fimetaria and Folsomia candida)
INTRODUCTION
1. This Test Guideline is designed for assessing the effects of chemicals on the reproductive output of the collembolans, Folsomia fimetaria and Folsomia candida in soil. It is principally based on a method
developed by the National Environmental Research Institute, Denmark [1] and the [2]. When more information is available it should be considered including more Collembola species in a test-programme.
2. Soil-dwelling Collembola are ecologically relevant species for ecotoxicological testing. Compared to for example earthworms the collembolans represent organisms with an exoskeleton, indicating a
different route (or at least different rate) of exposure.
3. Population densities of Collembola commonly reach 105 m-2 In soil and leaf litter layers in many terrestrial ecosystems [3,4]. Because of their small size however, adults typically measure 0.5 - 5 mm their
contribution to total soil animal biomass and respiration is low, estimated at being between 1 and 5% [5]. Their most important role may therefore be as potential regulators of processes through microbivory
and microfauna predation. Springtails are prey animals for a wide variety of endogeic and epigeic invertebrates, such as mites, centipedes, spiders and carabidae and rove beetles. Collembola contribute to
decomposition processes in soil and on acidic stands they may be the most important soil invertebrates as earthworms and diplopods are absent.
F. fimetaria has a worldwide distribution and is common in several soil types ranging from sandy to loamy soils and from mull to mor soils. It has been recorded in agricultural soils all over Europe [6]. It has
an omnivorous feeding habit, including fungal hyphae, bacteria, protozoa and detritus in its food. It interacts through grazing with infections of plant pathogenic fungi [7] and may influence mychorrhiza, as is
known to be the case for F. candida. It is a sexually reproducing species.
F. candida is worldwide distributed. Although it is not common in most natural soils, it often occurs in very high numbers in humus rich sites. It is a blind, unpigmented collembolan, which reproduces
parthenogenetically. It has a well-developed furca (jumping organ) and an active running movement and jumps readily if disturbed. It's a parthenogenetic species.
PRINCIPLE OF THE TEST
4. Synchronous adult Collembola are exposed to a range of concentrations of the test substance mixed into an artificial soil (or alternative soil). The test scenario can be divided into two steps:
4.a A range-finding test, in case no sufficient information is available, in which mortality is the main endpoint assessed after two weeks exposure
4.b A definitive reproduction test in which the total number of juveniles produced by parent animal and the survival of parent animals are assessed. The duration of this definitive test is three
(F. fimetaria) /four (F. candida) weeks.
The toxic effect of the test substance on the reproductive output of the animals is expressed as ECx (e.g. EC10, EC50) by using a sigmoid-regression model to estimate the concentration that would cause x
% reduction in reproductive output.
INFORMATION ON THE TEST SUBSTANCE
5. The following information relating to the test substance should be available to assist in the design of appropriate test procedures:
- water solubility
- the log Kow,
- the soil water partition coefficient
- the vapour pressure of the test substance should preferably be known.
- Additional information on the fate of the test substance in soil, such as the rates of photolysis and hydrolysis is desirable.
6. This Guideline can be used for water soluble or insoluble substances. However, the mode of application of the test substance will differ accordingly. The Guideline is not applicable to volatile substances,
i.e. substances for which the Henry's constant or the air/water partition coefficient is greater than one, or substances for which the vapour pressure exceeds 0.0133 Pa at 25°C.
VALIDITY OF THE TEST
7. The following criteria should be satisfied in the controls for a test result to be considered valid:
7.a Adult mortality should not exceed 20% at the end of the range-finding test and after the first three/four weeks of the reproduction test.
7.b F. candida: assuming that 10 adults per vessel were used in setting up the test, an average of at least 100 juveniles per vessel should have been produced at the end of the four-week
test.
7.c F. fimetaria: assuming that 20 adults (10 females and 10 males) per vessel were used in setting up the test, an average of at least 200 juveniles per vessel should have been produced at
the end of the three-week test.
7.d The coefficient of variation of juveniles should be less than 25% at the end of the reproduction test.
Where a test fails to meet the above validity criteria the test should be terminated unless a justification for proceeding with the test can be provided. The justification should be included in the test report.
REFERENCE SUBSTANCE
8. A reference substance must be tested at its EC50 concentration for the chosen test soil type either at regular intervals or possibly included in each test to verify that the response of the test organisms has
not changed significantly over time and that the test system is responding at is normal level. A suitable reference substance is -TO BE DECIDED-, which has been shown to affect survival and reproduction
of Collembola [8, 9].
DESCRIPTION OF THE TEST
Equipment
9. Containers capable of holding 30 gram of soil are suitable test vessels. The material should either be glass or inert plastic (non-toxic). The vessels should have a cross-sectional area allowing the soil depth
to be between 3-4 cm. The vessels should have transparent lids (e.g. glass or polyethylene) that are designed to reduce water evaporation whilst allowing gas exchange between the soil and the atmosphere.
The container should be at least partly transparent to allow light transmission.
10. Normal laboratory equipment is required, specifically the following:
- drying cabinet;
- stereomicroscope;
- pH-meter and photometer;
- suitable accurate balances;
- adequate equipment for temperature control;
- adequate equipment for humidity control (not essential if exposure vessels have lids);
- incubator or small room with air-conditioner;
- tweezers, hooks or loops.
Preparation of the artificial soil
11. The artificial soil is prepared according to OECD guideline 207 [10] and consist of the below components. It is highly preferable to use, in addition to the OECD soil, a natural standard soil such as Lufa
Speyer. With regard to the artificial soil its recommended composition is as follows (based on dry weights, dried to a constant weight at 105°C):
- 5 or 10% sphagnum peat, air-dried and finely ground (a particle size of 2 ± 1 mm is acceptable); it is recommended to check that a soil prepared with a fresh batch of peat is suitable for
containing the Collembola before it is used in a test. It is recommended to measure the C/N ratio, pH and CEC of the peat.
- 20% kaolin clay (kaolinite content preferably above 30%);
- approximately 0.3 to 1.0% calcium carbonate (CaCO3, pulverized, analytical grade) to obtain a pH of 6.0 ± 0.5; the amount of calcium carbonate to be added may depend principally on
the quality/nature of the peat;
- approximately 69% air-dried industrial sand (depending on the amount of CaCO3 needed), predominantly fine sand with more than 50% of the particles between 50 and 200 microns.
It is advisable to demonstrate the suitability of the test soil for use in the test and for achieving the test validity criteria before using the soil in a definitive test
11. The dry constituents of the soil are mixed thoroughly (e.g. in a large-scale laboratory mixer). This should be done at least one week before starting the test. The mixed soil should be stored for two weeks
in order to equilibrate/stabilise the acidity. For the determination of pH a mixture of soil and 1 M potassium chloride (KCl) or 0.01 M calcium chloride (CaCl2) solution in a 1:5 ratio is used (according to
Annex 3). If the soil is more acidic than the required range, it can be adjusted by addition of an appropriate amount of CaCO3. If the soil is too alkaline it can be adjusted by the addition of more of the
mixture comprising, but excluding the CaCO3.
12. The maximum water holding capacity (WHC) of the artificial soil is determined in accordance with procedures described in Annex 2. At the start of the test, the pre-moistened soil is divided into portions
corresponding with the number of test concentrations (and reference substance where appropriate) and controls used for the test. The moisture content is adjusted to about 50% of the maximum WHC
(corresponding to 50 ± 10% moisture dry mass) by the addition of the test substance solution and/or distilled or de-ionised water. However, moisture content should be optimized to attain a loose porous
texture to allow animals to enter into the pores. The weight of the containers is determined at the beginning, in the middle and at the end of the test.
Selection and preparation of test animals
13. The recommended test species are Folsomia fimetaria and Folsomia candida. These two species are some of the most accessible species of Collembola, with specimens sizes of up to 2.5 mm in
length. These collembolans are culturable and commercially available.
13.a Preparation of culturing substrate
The culturing substrate is plaster of Paris (calcium sulphate) with activated charcoal. This provides a moist substrate, the function of the charcoal being to absorb waste gases and excreta [11,
12]. Different forms of charcoal may be used to facilitate observations of the Collembola. For example, powdered charcoal is used for F. candida and F. fimetaria (producing a black/grey
plaster of Paris).
Substrate constituents:
20 ml of activated charcoal
200 ml of distilled water
200 ml of plaster of Paris
or
50 g of activated pulverized charcoal
260-300 ml of distilled water
400 g plaster of Paris.
13.c Breeding
Collembolans are held in Petri dishes (90 mm x 13 mm) with the bottom covered by a 0.5 cm layer of plaster of Paris/charcoal substrate. They are cultured at 20 ± 1°C at a light:dark cycle of
12:12 hours.
Every time the Petri dishes are 4-8 weeks old, it is necessary to transfer the animals to Petri dishes with newly prepared plaster of Paris/charcoal substrate, and they will start producing eggs
again.
Cultures are kept in an incubator at a temperature of 20±0.5°C, under a 16:8 hours light regime (<1000 Lux). Containers are kept moist at all times. Any dead individuals should be removed
from the containers immediately, as should any stale food. Stock cultures of both species should be periodically moved (e.g. every 2-3 months) to fresh plaster of Paris.
13.d Food source
Granulated dried baker's yeast is used as the principal food supply for F. candida. Fresh food is provided either once or twice a week, to avoid spoilage by fungal growth. It is placed on
filter paper discs, which are removed together with the food when necessary. The mass of baker's yeast added should be tailored to the size of collembolan population, but as a general rule
10-30 mg is sufficient.
14. The animals used in the tests are adult individuals. Synchronisation of the breeding culture is necessary, especially so for the F. fimetaria.
14.a Synchronising and culturing F. fimetaria.
1) Prepare Petri dishes with a 0.5 cm layer of plaster of Paris/charcoal substrate.
2) For egg laying transfer 150-300 adult F. fimetaria from a 4-8 weeks old substrate to the containers and feed with 15 mg baker's yeast.
3) Keep the culture at 20±1°C (means should be 20°C) and a light:dark cycle 12:12 hours (<1000 Lux).
4) After 9 days the eggs are carefully collected with a needle and spatula and moved to an “egg-paper” (small pieces of filter paper dipped in plaster of Paris/charcoal
substrate) which is placed in a fresh Petri dish with plaster of Paris/charcoal substrate. It is important that the egg-paper and substrate are humid, or else the eggs will
dehydrate.
5) Eggs and hatched juveniles are cultured in same manner as the adults.
6) After three days most of the eggs on the egg-paper have hatched, and the juveniles tend to stay under the egg-paper.
7) To have evenly aged juveniles the egg-paper with unhatched eggs is removed from the Petri dish with a tweezer. The juveniles stay in the dish. Age of the juveniles are 0-3
days.
8) Label the container with date of hatching and provide baker's yeast.
14.b. Synchronising and culturing F. candida.
1) Prepare new breeding containers with a 1 cm layer of plaster of Paris.
2) Transfer several hundred adult Collembola from stock cultures into each container, and supply with baker's yeast.
3) Keep the culture at 20±1°C (means should be 20°C) and a light:dark cycle 12:12 hours (<1000 Lux).
4) Remove adults after 24 to 48 hours.
5) Eggs and hatched juveniles are cultured in same manner as the adults.
6) Observe daily and record date of hatching. Provide food immediately after hatching.
7) After three days most of the eggs on the egg-paper have hatched, and the juveniles tend to stay under the egg-paper.
8) To have evenly aged juveniles the egg-paper with unhatched eggs is removed from the Petri dish with a tweezer. The juveniles stay in the dish. Age of the juveniles are 0-3
days.
9) Label the container with date of hatching and provide baker's yeast.
15. Other Collembolan species may also be suitable, e.g. I. viridis or O. folsomi. If other species of Collembola are used, they must be clearly identified and the rationale for the selection of the species
should be reported.
Preparation of test concentrations
16. Two methods of application of the test substance can be used: mixing the test substance into the soil or application to the soil surface. The selection of the appropriate method depends on the purpose of
the test. In general, mixing of the test substance into the soil is recommended. However application procedures that are consistent with normal agricultural practice may be required (e.g. spraying of liquid
formulation or use of special pesticide formulations such as granules or seed dressings).
Mixing the test substance into the soil
Test substance soluble in water
17. A solution of the test substance is prepared in deionised water in a quantity sufficient for all replicates of one test concentration. Each solution of test substance is mixed thoroughly with one batch of
pre-moistened soil before being introduced into the test vessel.
Test substance insoluble in water
18. For chemicals insoluble in water but soluble in organic solvents, the test substance can be dissolved in the smallest possible volume of a suitable vehicle (e.g. acetone). Only volatile solvents should be
used. When such vehicles are used, all test concentrations and an additional control should contain the same minimum amount of the vehicle. The vehicle is sprayed on or mixed with a small amount, for
example 2.5 g, of fine quartz sand. The vehicle is eliminated by evaporation under a fume hood for at least one hour. This mixture of quartz sand and test substance is added to the pre-moistened soil and
thoroughly mixed after adding an appropriate amount of de-ionised water to obtain the moisture required. The final mixture is introduced into the test vessels.
Test substance insoluble in water and organic solvents
19. For substances that are poorly soluble in water and organic solvents, the equivalent of 2.5 g of finely ground quartz sand per test vessel is mixed with the quantity of test substance to obtain the desired
test concentration. This mixture of quartz sand and test substance is added to the pre-moistened soil and thoroughly mixed after adding an appropriate amount of de-ionised water to obtain the required
moisture content. The final mixture is divided between the test vessels. The procedure is repeated for each test concentration and an appropriate control is also prepared.
Application of the test substance to the soil surface
20. The soil is treated after the Collembolans are added. The test containers are first filled with the moistened soil substrate and the weighed. The test substance is applied. It should not be added to the soil
within half an hour of introducing the Collembola so as to avoid any direct exposure to the test substance by skin contact. When the test substance is a pesticide it may be appropriate to apply it to the soil
surface by spraying. The test substance should be applied to the surface of the soil as evenly as possible using a suitable laboratory-scale spraying device to simulate spray application in the field. Before
application the cover of the test container should be removed and replaced by a liner, which protects the side walls of the container from spray. The liner can be made from a test container with the base
removed. The application should take place at a temperature within ± 2°C of variation and for aqueous solutions, emulsions or dispersions at a water application rate of between 600 and 800 µl/m². The rate
should be verified using an appropriate calibration technique. Special formulations like granules or seed dressings could be applied in a manner consistent with agricultural use.
Test containers should be left uncovered for a period of one hour to allow any volatile solvent associated with the application of the test substance to evaporate.
PERFORMANCE OF THE TESTS
Test groups and controls
21. For each test concentration, an amount of test soil corresponding to 25 g dry weight is placed into the test vessel. Controls, without the test substance, are also prepared. Food is added to each vessel.
Ten (F. candida) or twenty (F. fimetaria) collembolans are randomly allocated to each test vessel. The individuals are carefully transferred into each test vessel and placed on the surface of the soil using.
For efficient transfer of the animals a low-suction air flow devise can be used. The number of replicates for test concentrations and for controls depends on the test design used. The test vessels are
positioned randomly in the test incubator and these positions are re-randomised weekly.
22. If a vehicle is used for application of the test substance, one control series containing quartz sand sprayed or mixed with solvent should be run in addition to the test series. The solvent or dispersant
concentration should be the same as that used in the test vessels containing the test substance. A control series containing additional quartz sand (2.5 g per vessel) should be run for substances requiring
administration.
Test conditions
23. The test temperature is 20 ± 2°C. To discourage collembolans from escaping from the soil, the test is carried out under controlled light-dark cycles (preferably 16 hours light and 8 hours dark) with
illumination of 400 to 800 lux in the area of the test vessels.
24. In order to check the soil humidity, the vessels are weighed at the beginning of the test and thereafter once a week. Weight loss is replenished by the addition of an appropriate amount of deionised
water. It should be noted that loss of water can be reduced by maintaining a high air-humidity (> 80%) in the test incubator.
25. The moisture content and the pH, should be measured at the beginning and the end of both the range-finding test and the definitive test. Measurements should be made in control and treated (all
concentrations) soil samples prepared and maintained in the same way as the test cultures. Food should be added at the top of the soil samples at the start of the test and after 2 weeks. The amount of food
added should be the same as that added to the test cultures.
Feeding
26. A suitable source is granulated dried Bakers's yeast, commercially available for household use.
27. At the beginning of the test and after each 14-day interval, add about 15 mg yeast to each container.
Design for the range-finding test
28. When necessary, a range-finding test is conducted with, for example, five test substance concentrations of 0.1, 1.0, 10, 100, and 1000 mg/kg (dry weight of soil). Two replicates for each treatment and
control are sufficient.
29. The duration of the range-finding test is two weeks. At the end of the test, mortality of the Collembola is assessed. A Collembola is recorded as dead if not present in the extraction. Additional
information to mortality may also be useful in deciding on the range of concentrations to be used in the definitive test.
30. The LC50 can be determined using Probit analysis. In order for an accurate determination of the LC50 performing the test it should be ensured that the number of replicates and test concentrations
matches the power requirements for the test.
Design for the definitive reproduction test
31. For determination of the ECx (e.g. EC10, EC50), an adequate number of concentrations to cause at least four statistically significantly different mean responses at these concentrations is recommended..
At least four replicates for each test concentration and four control replicates are recommended. The spacing factor should ensure that the majority of test concentrations are on the slope of the ECx curve. It
should be considered that the power requirements of the test should be maintained.
30. Number of test individuals and duration (three test designs).
30 a. For the F. fimetaria test twenty adults per test vessel should be used. Food is added to the test vessels at the beginning of the test and then after 14 days up to and including Day 21.
On Day 21 the soil samples should be extracted and counted.
30 b. For the F. candida test ten adults per test vessel should be used. Food is added to the test vessels at the beginning of the test and then after 14 days up to and including Day 28. On
Day 28 the soil samples should be extracted and counted.
Limit test
31. If no effects are observed at the highest concentration in the range-finding test (i.e. 1000 mg/kg), the reproduction test could be performed as a limit test, using a test concentration of 1000 mg/kg. A limit
test will provide the opportunity to demonstrate that the NOEC for reproduction is greater than the limit concentration whilst minimising the number of Collembola used in the test. Eight replicates should be
used for both the treated soil and the control.
Power of the test
32. For all test designs it is advised that the Type I error is set at a 5% level and the type II error is set at maximum 20%. This should be ensured for each test.
Counting animals
33. Two methods of extraction can be performed.
33.a. First method: A controlled temperature gradient extractor based on principles by MacFayden can be used [1]. The heat coming from a heathing element at the top of the extraction box
(regulated though a thermistor placed ion the surface of the soil sample). The temperature in the cooled liquid surrounding the collecting vessel is regulated through a thermistor situated at the
surface of the collection box (placed below the soil core). The thermistors are connected to a programmable controlling unit which raises the temperature according to a pre-programmed
schedule. Animals are collected in the cooled collecting box (2°C) with the bottom layer of plaster of Paris/charcoal. Extraction is started at 25°C and the temperature is increased
automatically every 12 h by 5°C. After 12 h at 40°C the extraction is finished.
33.b. Second method: After the experimental incubation period the number of juvenile Collembola present is assessed by floatation. This involves emptying the tube of soil into a 250 ml
vessel and adding approx. 200 ml of distilled water. The soil is gently agitated with a fine paintbrush to allow Collembola to float to the water surface. A small amount, approx. 0.5 ml, of
black Kentmere photographic dye may be added to the water to aid counting by increasing the contrast between the water and the white Collembola. The dye is not toxic to the Collembola.
Counting: Counts of numbers may be carried out by eye or under a light microscope using a grid placed over the floatation vessel or by photographing the surface of each vessel and later counting
Collembola on the enlarged prints or projected slides. Count may also be performed using digital image processing techniques [13].
Summary and timetable for the test
34. The steps of the test can be summarised as follows:
| Purpose |
Range-finding test |
Definitive Reproduction test - 1 |
Definitive Reproduction test - 2 |
| Species |
F. fimetaria/F. candida |
F. candida |
F. fimetaria |
| Aim |
Mortality |
Reproduction |
Reproduction |
| Time (day) |
|
|
|
-9-12
(F. candida)
-13-26
(F. fimetaria) |
Preparation of synchronous culture |
Preparation of synchronous culture |
Preparation of synchronous culture |
| Day 5 or earlier |
- Prepare artificial soil (mixing of dry constituents) |
- Prepare artificial soil (mixing of dry constituents) |
- Prepare artificial soil (mixing of dry constituents) |
| Day 3 |
- Check pH of artificial soil
- Measure max WHC of soil |
- Check pH of artificial soil
- Measure max WHC of soil |
- Check pH of artificial soil
- Measure max WHC of soil |
| Day 2 to 1 |
- Sort Collembola for testing |
Sort Collembola for testing |
- Sort Collembola for testing |
| Day 1 |
- Pre-moisten artificial soil and distribute into batches
- Prepare stock solutions
- Apply test substance if solvent required |
- Pre-moisten artificial soil and distribute into batches
- Prepare stock solutions
- Apply test substance if solvent required |
- Pre-moisten artificial soil and distribute into batches
- Prepare stock solutions
- Apply test substance if solvent required |
| Day 0 |
- Prepare stock solutions
- Apply test substance
- Weigh test substrate into test vessels
- Mix in food
- Introduce Collembola
- Measure soil pH and moisture content |
- Prepare stock solutions
- Apply test substance
- Weigh test substrate into test vessels
- Mix in food
- Introduce Collembola
- Measure soil pH and moisture content |
- Prepare stock solutions
- Apply test substance
- Weigh test substrate into test vessels
- Mix in food
- Introduce Collembola
- Measure soil pH and moisture content |
| Day 7 |
- Check soil moisture content |
- Check soil moisture content |
- Check soil moisture content |
| Day 14 |
- Determine adult mortality
- Estimate number of juveniles
- Measure soil pH and moisture content |
- Check soil moisture content
- Feed |
- Check soil moisture content
-Feed |
| Day 21 |
|
- Check soil moisture content |
-Extract all individuals
- Check soil moisture content
-Measure pH |
| Day 28 |
|
- Extract all individuals
- Check soil moisture content
-Measure pH |
|
DATA AND REPORTING
Treatment of results
35. An overview is in the following but more detailed statistical guidance for analysing test results is given in the "OECD draft statistical report 2003".
36. In the range finding test, the main endpoint is mortality. Probit analysis [14] should normally be applied to determine the LC50. However, in cases where this method of analysis is unsuitable (e.g., if less
than three concentrations with partial kills are available), alternative methods can be used. These methods could include moving averages [14], the trimmed Spearman-Karber method [15] or simple
interpolation (e.g., geometrical mean of LC0 and LC100, as computed by the square root of LC0 multiplied by LC100).
ECx estimation (preferred method)
37. To compute any ECx value, the per-treatment means data are used for regression analysis (linear or non-linear), after an appropriate dose-response function has been selected [16]. Among suitable
functions are the normal sigmoid, logistic or Weibull functions, containing two to four parameters, some of which can also model hormetic responses. If a dose-response function was fitted by linear
regression analysis a significant r² (coefficient of determination) and/or slope should be found with the regression analysis before estimating the ECx by inserting a value corresponding to x% of the control
mean into the equation found by regression analysis. 95%-confidence limits are calculated according to [17].
38. Alternatively, one may express the treatment results as percentages of the control result or as percent inhibitions relative to control. In these cases, the normal (logistic, Weibull) sigmoid curve can often
be easily fitted to the results using the probit regression procedure [17]. In these cases the weighting function has to be adjusted for metric responses as given by [18]. However, if hormesis has been
observed, regression analysis should be replaced by a four-parameter logistic or Weibull function, fitted by a non-linear regression procedure [19]. If a suitable dose-response function cannot be fitted to the
data, one may use alternative methods to estimate the ECx, and its confidence limits, such as Moving Averages after Thompson [14] and the Trimmed Spearman-Karber procedure [15].
NOEC estimation (alternative method)
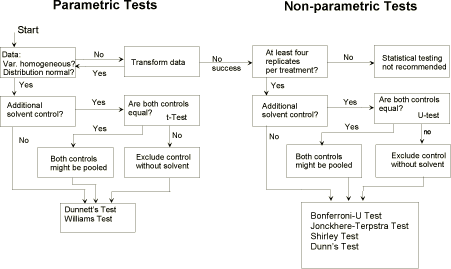
39. If an analysis of variance has been performed, the standard deviation, s, and the degrees of freedom, df, may be replaced by the pooled variance estimate obtained from the ANOVA and by its degrees
of freedom, respectively – provided variance does not depend on the concentration. In this case, use the single variances of control and treatments. Those values are usually calculated by commercial
statistical software using the per-vessel results as replicates. If pooling data for the negative and solvent controls appears reasonable rather than testing against one of those, they should be tested to see that
they are not significantly different (for the appropriate test consider Annex 4). Further statistical analysis and inferences depend on whether the replicate values are normally distributed and are homogeneous
with regard to their variance.
41. NOEC estimation: Kolmogoroff-Smirnov's [20] and Bartlett's-test procedures [21] are used respectively for testing for normality and homogeneity of variance homogeneity. With normally distributed
and homogeneous data, multiple t-tests such as Dunnett’s test [22, 23] or William’s test (a = 0.05, one-sided) [24, 25] should be performed. It should be noted that, in the case of unequal replication, the
tabulated t-values must be corrected as suggested by Dunnett and Williams. Sometimes, because of large variation the responses do not increase/decrease regularly. In this case Dunnett’s test does not lead
to reasonable NOEC/LOEC values and analysis by Williams-test is to be preferred. Alternatively, Otherwise, a multiple U-test, e.g. the Bonferroni-U-test according to Holm [26], could be used.
42. If a limit test has been performed and the prerequisites of parametric test procedures (normality, homogeneity) are fulfilled, the pair-wise Student t-test can be used or otherwise the
Mann-Whitney-U-test procedure [20].
40. The test report should include the following information:
40.a. Test substance:
- physical nature and, where relevant physical-chemical properties (e.g. water solubility, vapour pressure);
- chemical identification of the test substance according to IUPAC nomenclature, CAS-number, batch, lot, structural formula and purity;
- expiry date of sample.
40.b. Test species:
- test animals used: species, scientific name, source of organisms and breeding conditions.
40.c. Test conditions:
- ingredients and preparation details of the artificial soil; (minimum: C/N ratio, pH, WHC, CEC)
- method of application of the test substance (data verifying homogeneity of application);
- description of the test conditions, including temperature, moisture content, pH, etc.;
- full description of the experimental design and procedures.
40.d. Test results:
- Actual concentration of the tested compound in the test media (at minimum for control exposures)
- mortality of adult Collembola after two weeks and the number of juveniles at the end of the range-finding test;
- mortality of adult Collembola after three weeks exposure and the full record of juveniles at the end of the definitive test;
- any observed physical or pathological symptoms and behavioural changes in the test organisms;
- the LC50, the NOEC and/or ECx (e.g. EC50, EC10) for reproduction if some of them are applicable with confidence intervals, and a graph of the fitted model used for its
calculation, the slope of the dose-response curve and its standard error;
- all information and observations helpful for the interpretation of the results.
- Power of the actual test.
Deviations from procedures described in this guideline and any unusual occurrences during the test.
LITERATURE
(1) Wiles JA and Krogh PH (1998) Testing with the collembolans I. viridis, F. candida and F. fimetaria. In Handbook of soil invertebrate toxicity tests (ed. H. Løkke and C. A. M. Van Gestel), pp.
131-156. John Wiley & Sons, Ltd., Chichester.
(2) ISO (1999) Soil Quality - Effects of soil pollutants on Collembola (Folsomia candida):method for determination of effects on reproduction. No. 11267. International Organisation for Standardisation,
Geneve.
(3) Burges A and Raw F (Eds) (1967) Soil Biology. Academic Press. London
(4) Petersen H and Luxton M (1982) A comparative analysis of soil fauna popyulations and their role in decomposition processes. Oikos 39: 287-388
(5) Petersen H (1994) A review of collembolan ecology in ecosystem context. Acta Zoologica Fennnica 195: 111-118
(6) Hopkin SP (1997). Biology of the Springtails (Insecta : Collembola). Oxford University Press. 330pp (ISBN 0-19-854084-1).
(7) Ulber B (1983) Einfluss von Onychirurus fimatus Gisin (Collembola, Onychiuridae) und Folsomia fimetaria L. (Collembola, Isotomidae) auf Pythium ultimum trow. einen Erreger des Wurzelbrandes der
Zuruckrübe. In New trends in soil Biology (Lebrun Ph, André HM, De Medts A, Grégoire-Wibo, Wauthy G (Eds), Proceedings of the VI international colloquium on soil zoology, Louvain-la-neuve
(Belgium), 30 August-2 September 1982, I. Dieu-Brichart, Ottignies-Louvain.la.neuve, pp. 261-268.
(8) Scott-Fordsmand JJ and Krogh PH (2004). Background report on prevalidation of an OECD springtail test guideline. MST in press.
(9) Becker-van Slooten K, Campiche S, Feisthauer N, Stephenson G, Tarrasellas and Scroggings R (2004) Research in support of quality assurance requirements for new soil toxicity tests with Collembola.
Poster presented at SETAC 2004, Prague, Czeck.
(10) OECD (Organisation for Economic Cooperation and Development) (1984). Earthworm, Acute Toxicity Tests, Guideline No. 207. OECD, Paris.
(11) Edwards (1955) Simple techniques for raraing Collembola, Symphyla and other small soil inhabiting arthropods. In Soil Zoology (Kevan DKMcE, Ed). Butterworths, London, pp. 412-416.
(12) Goto HE (1960) Simple techniques for the rearing of Collembola and a not on the use of a fungistatic substance in the cultures. Entomologists' Monthly Magazine 96:138-140
(13) Krogh PH, Johansen K and Holmstrup M (1998) Automatic counting of collembolans for laboratory experiments. Appl. Soil Ecol. 7, 201-205.
(14) Finney DJ (1978) Statistical Method in Biological Assay. - Charles Griffin & Company Ltd, London.
(15) Hamilton MA, Russo RC and Thurston RV (1977) Trimmed Spearman-Karber Method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 11(7), 714-719;
Correction Environ. Sci. Technol. 12(1998), 417.
(16) OECD (2003) Draft Guidance Document for on the Statistical Analysis of Ecotoxicity Data. Environment Directorate, ORGANISATION FOR ECONOMIC CO-OPERATION AND
DEVELOPMENT, Paris 2003.
(17) Finney DJ (1971) Probit Analysis (3rd ed.), pp. 19-76. Cambridge Univ. Press.
(18) Christensen ER (1984) Dose-response functions in aquatic toxicity testing and the Weibull model. Water Research 18, 213-221.
(19) Van Ewijk PH and Hoekstra JA (1993) Calculation of the EC50 and its confidence interval when sub-toxic stimulus is present. Ecotox, Environ. Safety. 25, 25-32.
(20) Sokal, R.R. and F.J. Rohlf. (1981) Biometry. The principles and practice of statistics in
biological research. 2nd edition. W.H. Freeman and Company. New York.
(21) Miller, R.G., Jr. (1986). Beyond ANOVA, basics of applied statistics. John Wiley & Sons. New York.
(22) Dunnett, C.W. (1955). A multiple comparison procedure for comparing several treatments with a control. Amer. Statist. Ass. J. 50, 1096-1121.
(23) Dunnett, C.W. (1964). New tables for multiple comparisons with a control. Biometrics 20, 482-491.
(24) Williams, D.A. (1971). A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics 27, 103-117.
(25) Williams DA. (1972). The comparison of several dose levels with a zero dose control. Biometrics 28, 519-531.
(26) Holm, S. (1979). A simple sequentially rejective multiple test procedure. Scand. J. Statist. 6, 65-70.
ANNEX 1
The following definitions are applicable to this Guideline
LC50 (Median lethal concentration) is the concentration of a test substance that is statistically likely to kill 50% of exposed test organisms within a given time period. In this test the LC50 is expressed as a
mass of test substance per dry mass of the test soil or as a mass of test substance per unit area of soil.
LOEC (Lowest Observed Effect Concentration) is the lowest test substance concentration that has a statistically significant effect (p < 0.05) In this test the LOEC is expressed as a mass of test substance
per dry mass of the test soil or as a mass of test substance per unit area of soil. All test concentrations above the LOEC should normally show an effect that is statistically different from the control. Any
deviations from the above must be justified in the test report.
NOEC (No Observed Effect Concentration) is the highest test substance concentration immediately below the LOEC at which no effect is observed. In this test, the concentration corresponding to the
NOEC, has no statistically significant effect (p < 0.05) within a given exposure period when compared with the control.
ECx (Effect concentration for x% effect) is the concentration that causes an x% of an effect on test organisms within a given exposure period when compared with a control. For example, an EC50 is a
concentration estimated to cause an effect on a test end point in 50% of an exposed population over a defined exposure period. In this test the effect concentrations are expressed as a mass of test substance
per dry mass of the test soil or as a mass of the test substance per unit area of the soil.
Reproduction rate: Mean number of juvenile Collembola produced per a number of adults over the test period.
ANNEX 2
DETERMINATION OF THE MAXIMUM WATER HOLDING CAPACITY OF THE SOIL
The following method for determining the maximum water holding capacity of the soil has been found to be appropriate. It is described in Annex C of the ISO DIS 11268-2 (Soil Quality - Effects of
pollutants on earthworms (Eisenia fetida). Part 2: Determination of effects on reproduction (3)).
Collect a defined quantity (e.g. 5 g) of the test soil substrate using a suitable sampling device (auger tube etc.). Cover the bottom of the tube with a piece of filter paper fill with water and then place it on a
rack in a water bath. The tube should be gradually submerged until the water level is above to the top of the soil. It should then be left in the water for about three hours. Since not all water absorbed by the
soil capillaries can be retained, the soil sample should be allowed to drain for a period of two hours by placing the tube onto a bed of very wet finely ground quartz sand contained within a covered vessel (to
prevent drying). The sample should then be weighed, dried to constant mass at 105°C . The water holding capacity (WHC) can then be calculated as follows:
WHC (in % of dry mass) = 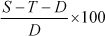
Where:
S = water-saturated substrate + mass of tube + mass of filter paper
T = tare (mass of tube + mass of filter paper)
D = dry mass of substrate
ANNEX 3
DETERMINATION OF SOIL pH
The following method for determining the pH of a soil is based on the description given in ISO DIS 10390: Soil Quality – Determination of pH (15).
A defined quantity of soil is dried at room temperature for at least 12 h. A suspension of the soil (containing at least 5 grams of soil) is then made up in five times its volume of either a 1 M solution of
analytical grade potassium chloride (KCl) or a 0.01 M solution of analytical grade calcium chloride (CaCl2). The suspension is then shaken thoroughly for five minutes and then left to settle for at least 2
hours but not for longer than 24 hours. The pH of the liquid phase is then measured using a pH-meter that has been calibrated before each measurement using an appropriate series of buffer solutions (e.g.
pH 4.0 and 7.0).
ANNEX 4
OVERVIEW OF THE STATISTICAL ASSESSMENT OF DATA (NOEC DETERMINATION)
| Front page | | Contents | | Previous | | Next | | Top |
Version 1.0 March 2005, © Danish Environmental Protection Agency
|
![]()
