|
Assessment of Mercury Releases from the Russian Federation 1 Introduction
1.1 Previous Study on Mercury in the EnvironmentThe Committee on Ecology of the State Duma of Russia and the Government of the Russian Federation issued the Order for the State Committee on Environment Protection in 1998 to develop a National Report "On mercury pollution of the environment of the Russian Federation and its impact on population health". Unfortunately, this Order was not executed. Nevertheless, in 1999 Scientific Research Institute on Problems of Resource Saving and Wastes Management under the Ministry of Economy if RF and Goscomecologia of RF together with the specialists from other institutions developed the report titled "Analysis of mercury pollution of the environment of the Russian Federation". The main purposes of the report were to determine the main sources of mercury pollution and make recommendations for potential development of the National Mercury Releases Investigation and Pollution Abatement Program. In this report, the main sources of Hg pollution in Russian Federation were acknowledged to be production and consumption wastes. It was not possible to make comprehensive and accurate assessment of the technogenic mercury releases from each of the sources, due to the lack of reported data concerning consumption and application of mercury and Hg-containing compounds. Additionally it was determined that the distinctive features and conditions in Russia, which should be taken into account during elaboration of the National Mercury Releases Investigation and Pollution Abatement Program, such as:
In other words, the bulleted information above might be posed as: 1. undefined user – producer relationships, 2. undefined recycling incentives, 3. insufficient data due to poor monitoring, and 4. insufficient knowledge regarding technological alternatives for treating mercury under varying circumstances. Moreover the regulatory basis was reviewed and systematized to some extent. This regulatory basis for mercury pollution management was elaborated in 1970-80-ies. The existing regulations usually cover general issues related to mercury contamination and Hg and Hg-waste management and do not include specific requirements. It is the author's opinion, that one of the key causes of mercury pollution in Russia is a lack of the Hg-containing wastes management system, i.e. collection, storage, transportation and neutralization. Collection, storage and transportation of Hg-containing wastes are acknowledged to be a bottleneck in the existing system of Hg-containing wastes utilization and neutralization. Lack of the agreed documents and existence of contradictory requirements of various agencies and local authorities hamper the process of effective collection and delivery of Hg-containing wastes to the disposal site. The authors of the document concluded that the problem of mercury pollution in the Russian Federation is strongly depends on the implementation on the "Wastes" Federal target Program. The following activities were recommended for the Program' implementation targeted on Hg wastes management: 1. Forecasting on mercury consumption till 2010 and determination of the feedstock for the secondary mercury; 2. Elaboration and implementation of the Hg consumption and recycling control system (through environmental authorities); 3. Preparation of legal basis regarding Hg consumption and recycling (or regarding total losses); 4. The national inventory and certification of Hg-containing wastes covering all enterprises, which use mercury and process Hg-containing consumption wastes; 5. Design and manufacture of the reusable containers for collection, storage and transportation of Hg-containing wastes; 6. Elaboration of new utilization technologies for particular types of Hg-containing wastes. 1.2 Regulation of Mercury ReleasesThe content of mercury in different media is regulated by maximum allowed concentrations. The concentrations were fixed in the regulatory documents developed and adopted by the executive authorities of the Russian Federation and/or the USSR (see tables 1.1 – 1.6). Table 1.1 Main regulatory documents on environment and population protection from potentially dangerous pollutants including mercury and its compounds
Table 1.2 MAC of mercury and its compounds in the atmospheric air of the inhabited localities*
* Hygienic norms ÃÍ 2.1.6.695.98. Maximum allowable concentrations (MAC) in the atmospheric air of the inhabited localities. – Moscow: The Ministry of Health of RF, 1998. ** all regulated substances are related to the hazard class 1. *** MAC for Hg compounds are presented in conversion to Hg. Table 1.3 MAC of mercury and its compounds in the indoor occupational air*
* Hygienic norms 2.1.6.686-98. Maximum allowable concentrations (MAC) in the occupational air. – Moscow: The Ministry of Health of RF, 1998. Mercury. Regulations and methodological guidelines. Reference Book. Ò. 1. – Saint-Petersburg, 2001. ** All regulated substances are related to the hazard class 1. *** MAC for Hg compounds are presented in corvension to Hg (influence of inorganic compounds requires special protection of eyes and skin). Table 1.4 MAC of mercury and its compounds in potable water sources and cultural and recreational water bodies*
* Hygienic norms ÃÍ 2.1.5.690-98. Maximum allowable concentrations (MAC) of chemical substances in potable water sources and cultural and recreational water bodies. – Moscow: The Ministry of Health of RF, 1998. ** All regulated substances are related to the hazard class 1. *** Releases of inorganic mercury (Hg2+) and mercuric chloride into water bodies used for fishery are prohibited. Table 1.5 MAC of gross concentration of mercury in soil and allowable content related to the hazard levels *
* Hygienic assessment of soil quality in the inhabited localities. Methodological Guidelines. – Moscow: the Ministry of Health of RF, 1999. ** Translocative indicator of hazard indicates ability of Hg to transit to agricultural plants and to accumulate in concentrations exceeding the established MAC. Migratory water hazard factor describes the ability of Hg to migrate from soil to groundwater and surface water and to concentrate in the amounts exceeding the established MAC. Migratory air hazard factor indicates the ability of Hg to migrate from soil to the atmospheric air reaching the concentrations exceeding MAC established for the atmospheric air. A general sanitary hazard factor indicates impact of Hg on the self-purifying capacity of soil and its biological activity. Russian sanitary-hygienic legislation specifies, that this hazard factor having the lowest threshold value is selected as a limitative hazard factor and considered as the MAC of the particulate chemical substance in soil. As regards mercury – this factor is translocative and equals to 2.1 mg/kg. Table 1.6 Maximum allowable residues (MAR) of mercury in foodstuff
Regulation on collection, package, transportation and utilization of mercury-containing wastes was given in the Instruction of the Ministry of Non-ferrous Metallurgy of the USSR, adopted in October 27, 1966. Many statements of the Instruction are outdated. Therefore regional rules on mercury-containing wastes management were developed in almost each region where a company dealing with collection and treatment of mercury-containing wastes (mercury lamps first of all) is located. 1.3 Methodology of the AssessmentThe present assessment of mercury releases in the Russian Federation has been undertaken by using a life-cycle approach. For each intentional application of mercury and each field of activity by which mercury is mobilized as impurity the flow of mercury from its purchase/extraction to final release or disposal have been assessed. The methodology is based on the principle of mass balance: al mercury brought into circulation (technosphere) will sooner or later be released to the environment of end up in waste products. For each area of intentional application the use of mercury in production processes and in products is assessed on the basis of information obtained from enterprises or - in the case specific information could not be obtained - from previous studies. Releases are estimated on the basis of direct information from the enterprises in combination with the official statistics on releases of mercury to air, water and waste obtained from Goscomstat (see Annex 1). For the major uses of mercury, the enterprises have been visited in the frameworks of the present assessment. For areas in which mercury is mobilized as impurity the total mobilization is estimated by multiplying raw material consumption (e.g. amount of coal used) with the concentration of mercury in the raw materials. In general terms it can be expressed as:
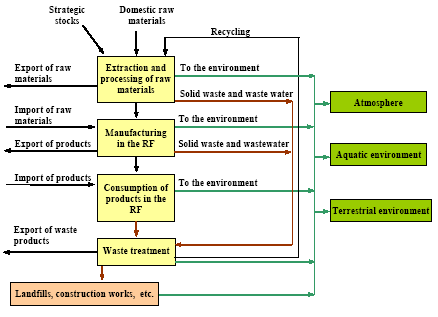
where the total amount of mobilized mercury (mobilization) is calculated by adding up the mercury content of all raw materials. The mercury content of each raw material is calculated by multiplying the consumption of the raw material (consumptionx) with the mercury concentration of this material (concentrationx). The releases from the processes (e.g. combustion of coal) to the different media are estimated by multiplying the total mobilized amount (mobilization) with media specific distribution factors (distribution) using the following equation: ReleaseAir = Mobilisation * DistributionAir where distributionAir is the distribution factor expressing the share of the total mobilised mercury that is released to air by the process. The distribution factors are either estimated on the basis of specific studies of the processes in the Russian Federation, or they are estimated on the basis of distribution factors obtained from other countries using similar technology. Almost all data used for the assessment are subject to a certain degree of uncertainty. To the extent possible the values are represented by "best estimate" and a range indicating the uncertainty. In most cases it is not possible to estimate the uncertainty using standard statistical methods, but the uncertainty estimate is rather based on expert judgements. The ranges used in the assessment represent the range within which the authors estimate that the right value will be with a probability of 90%. It means that for 10% of the estimates the true value may actually be beyond the indicated range. The probability distribution is not necessarily a symmetric distribution around the mean e.g. can very uncertain estimates rather be assumed to be lognormal distributed (the probability that the true value is twice the "best estimate" equal the probability that it is half the "best estimate"). Possible specific mercury consumption for the defence industry is not included in the assessment. The object of the present assessment is to study the flow of mercury through the technosphere (see Figure 1.1). Mercury in the environment as well as environmental and health issues are briefly discussed in a few chapters, but these issues have not been addressed uniformly through the report. Figure 1.1 Schematic illustration of the overall flow of mercury through the technosphere
1.4 Mercury ChemistryMercury has been used since ancient times. The first record of mercury made by Aristotle dates back to 350 year B.C. Mercury was also found in writings of Aristotle's teacher, a founder of mineralogy; Tirtamos from Eres, called Pheophrast. The chemical symbol of mercury "Hg" comes from Latin "hydrargyrum". It is believed that "hydrargyrum" (Greek words "hýdor" – water and "árgyros" – silver, i.e. "liquid silver" or "silver water") was first mentioned by Pliniy the Senior (I c. A. D.). Mercury was named by alchemists after the Roman god Mercury who was the god of commerce, profit and enrichment, custodian of roads, travelers and seafarers, patron of arts and crafts, connoisseur of magic and astrology, guide of souls in the other world. The god Mercury was equated to the Greek god Hermes. Origination of Russian name of mercury "“ðòóòü" is not known. The priority of adoption of mercury as an independent metal belongs to the famous Agricole. The solid mercury was first obtained in 1759 in Petersburg by M.P. Braun and M.V. Lomonosov who managed to freeze the mercury in the mixture of snow and a concentrated nitric acid. The following information has largely been extracted from the Global Mercury Assessment prepared by UNEP Chemicals (UNEP 2002), the Chemical Encyclopedia (Malaya Sovetskaya Encyclopedia, V. 2., pp. 1990. - 671), the reference book "the Properties of the Elements" (M. Metallurgia. 1997, p. 432) and the Geological Compendium on Mercury (V.P. Fedorchuk, E.F. Mintser, 1990, p. 215). Mercury is a chemical element of the Group II in Mendeleyev periodical table; its atomic number is 80, the atomic weight - 200.59. Seven stable and more than 20 radioactive isotopes of mercury are known. In normal conditions mercury is a heavy liquid metal. Textbooks specify such mercury as "elementary" or "metallic" (Hg(0) or Hg0). Mercury is relatively rarely found in nature in liquid (metallic) form, but rather within organic and inorganic compounds, as monovalent and more often divalent Hg(I) and Hg(II) (or Hg2+) respectively. The elementary mercury contained in vapourous state in the atmosphere, may transform into inorganic mercury compounds, thus providing possibility for precipitation of the technogenic mercury emitted to the atmosphere. Mercury is rather rarely found in nature; its average content in the earth crust and main rocks is estimated within 3–9 * 10-6 % (by mass). The mass of mercury accumulated in the upper layer of the earth crust of 1 km is estimated as 100,000,000,000 t (hundred billion tons), of which its natural deposits contain only 0.02%. The remaining part of mercury is extremely dispersed basically in rocks. Just this dispersed mercury creates a natural geochemical background receiving mercury contamination caused by human activity. By today more than 80 mercury minerals and dozens of mercury-containing minerals have been detected in nature. The basic ore mineral of industrial value is a cinnabar (vermilion, HgS). Globally there are about 5000 mercury deposits, ore basins and ore-bearing sites which have their own names, about 500 of these have been developed in different times. Recently it has been detected, that mercury is considerably concentrated not only in its native deposits, but in non-mercury ore deposits as well, such as pyrite, polymetallic, copper, gold and other, as well as in gas and gas-oil fields. Industrial production of metallic mercury usually applies two Hg extraction technologies: distillate oxidizing roasting (basically cinnabar ores) under the temperature above 540°C with extraction of mercury from the gas phase and (more seldom) a combined method including preliminary concentration of ores and further heating of the obtained concentrates. Elemental mercury is a shiny, silver-white metal that is a liquid at room temperature and is traditionally used in thermometers and some electrical switches. The specific gravity of metallic mercury at 20°C is 13.5 g/cm3; the melting temperature is –39°C, the boiling temperature is +357°C. Frozen mercury (at –39°C) becomes solid and easy melt able. Having high ionization potential and high positive oxidability, mercury is a relatively resistant chemical element. This conditions its ability to recover to the metal of various compounds and explains its existence in nature in its native state. Even under normal conditions, the elemental mercury has an increased saturated vapour pressure and vapourizes rather rapidly – the faster the higher temperature is. This results in a mercury atmosphere hazardous for living organisms. Impact of volt arc, electric spark and X-rays on mercury vapours creates the effects of luminescence, fluorescence and phosphorescence. The ultraviolet radiation is produced in vacuum tube between mercury electrodes under electric discharges. This is used for mercury lamps designing. Dilution of metals in the elemental mercury provides formation of amalgams – metallic systems, which include mercury as one of the components. They do not differ from the common alloys, although in case of a surplus Hg they are semi-fluid mixtures. Amalgamation is applied only for mercury wetted metals. The compounds obtained in amalgamation are easily degradable below their melting temperature and release the excess mercury, which is widely applied at gold and silver extraction from ores. Among inorganic mercuric compounds the most practically important are mercuric sulfide (HgS), mercuric oxides (HgO and Hg2O), iodic mercury (HgI), calomel (Hg2Cl2), and mercuric chloride (HgCl2). These mercury compounds are also called mercury salts. The mercuric sulfide HgS is the most widespread Hg compound, which is known in three modifications: red (similar to cinnabar), black (black mercuric sulfide or methacineabarite) and â-cinnabar (not found in nature). The iodic mercury exists in two modifications – red and yellow. The mercuric chloride is colorless rhombic crystals. Under prolonged heating up to the temperatures close to the boiling temperature, the elemental mercury is combined with the atmospheric oxygen and creates red mercuric oxide (II) - HgO, with Hg ozidization degree equal to +2. Yellow mercuric oxide (HgO) is obtained by adding alkaline to water solution of mercury salt (II). There is black mercurous oxide (Hg2O) – unstable compound with Hg oxidization degree equal to +1. In all mercury compounds (I) its atoms are connected and create divalent groups –Hg2– or –Hg–Hg–. Such connection is kept in mercuric salts solutions (I). There are some other inorganic mercury compounds such as fulminate of mercury Hg(ONC)2, mercuric nitrate Hg(NO3)2, mercuric sulfate HgSO4, mercuric sulfite HgSO3 etc. Impact of ammonia on the mercury salts creates many complex compounds. Some mercury salts (such as HgCl2) are sufficiently volatile to exist as an atmospheric gas. However, the water solubility and chemical reactivity of these inorganic (ionic) mercury gases lead to much more rapid deposition from the atmosphere than for elemental mercury. This results in significantly shorter atmospheric lifetimes for these ionic (e.g. divalent) mercury gases than for the elemental mercury vapours. There are many Hg-containing organic compounds, in which the atoms of metal are connected with the atoms of carbon. Chemical bond of carbon and mercury is very stable. It is not destroyed neither with water nor weak acids nor bases. The most known are two types of Hg-containing organic compounds: R–Hg–R` and R–HgX, where R and R` - organic radicals, Õ – acid residual. The first group includes apolar compounds which are almost insoluble in water and very volatile. The second group includes mercury compounds which are easily soluble in water and lipids and highly resistant in water, for instance methyl mercury ion (CH3–Hg+). The well-known are such mercury organic compounds as dimethylmercury, phenylmercury, ethylmercury and methylmercury, however, by far the most common organic mercury compound in the environment is methylmercury. The most toxic organic mercury compounds representing hazard for living organisms are alkylmercury compounds with a short chain in which mercury is combined with the atom of carbon from methyl, ethyl and propyl groups (methyl mercury first of all). Like the inorganic mercury compounds, both methylmercury and phenylmercury exist as "salts" (for example, methylmercuric chloride or phenylmercuric acetate). When pure, most forms of methylmercury and phenylmercury are white crystalline solids. Eexceptionally toxic dimethylmercury, however, is a colorless liquid. Methylmercury can be formed in the environment (especially in water bodies) by microbial metabolism (biotic processes) and by chemical processes that do not involve living organisms (abiotic processes). It is generally believed that its formation in nature is predominantly due to biotic processes. Significant direct anthropogenic (or human-generated) sources of methylmercury are currently not known, although some historic sources exist. Indirectly, however, anthropogenic releases contribute to the methylmercury levels found in nature because of the transformation from other mercury compounds released to the environment. Examples of direct release of organic mercury compounds are the Minamata methylmercury-poisoning event that occurred in the 1950's where organic mercury by-products of industrial-scale acetaldehyde production were discharged in the local bay, which caused accumulation of methyl mercury in marine food and mass poisoning and death of Japanese fishermen who at these products; and the Iraqi poisoning events where wheat treated with a seed dressing containing organic mercury compounds were used for bread. The recent research has shown that methylmercury can be released directly from municipal waste landfills and sewage treatment plants, but the general significance of this source is still uncertain. Being a chemical element, mercury cannot be broken down or degraded into harmless substances. Mercury may change between different states and species in its cycle, but its simplest form is elemental mercury, which itself is harmful to humans and the environment. Once mercury has been liberated from either ores or from fossil fuel and mineral deposits hidden in the earth's crust and released into the biosphere, it can be highly mobile, cycling between the earth's surface and the atmosphere. The earth's surface soils, water bodies and bottom sediments are thought to be the primary biospheric sinks for mercury. 1.5 Conceptual Model for Mercury CyclingConceptual models provide a clear written and graphical description, based on the current state of knowledge, of:
These models provide critical information about key monitoring targets (i.e., which sources, media, and receptors to monitor), as well as the temporal and spatial monitoring needs of a given compound. They also provide information on relationships between media to give a complete multimedia perspective of the behaviour of substances in the environment. The conceptual model for mercury is presented in Figure 1.2. As this figure depicts, mercury is emitted to the atmosphere from natural and anthropogenic sources, where it can cycle globally, constantly moving among various environmental compartments through a complex combination of transport and transformation, ultimately resulting in human and wildlife exposure. The most significant environmental releases of mercury are air emissions. In many countries, the largest emission sources of mercury are coal-fired electric utilities, coal-fired industrial boilers, and various types of incinerators. There are also natural sources of air emissions, e.g., volcanoes. Mercury is released in other ways as well, including discharges from industrial sources to water bodies. Though releases of mercury into water in most countries are believed to be small in comparison to atmospheric emissions, they can have significant local effects. For example, mercury discharges to surface waters from abandoned gold and mercury mines often lead to high level of methylmercury in fish. Figure 1.2 Conceptual model for mercury behaviour Click here to see the figure.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||