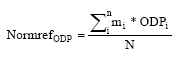|
Update on Impact Categories, Normalisation and Weighting in LCA 5 Stratospheric ozone depletion
Leif Hoffmann, dk-TEKNIK ENERGY & ENVIRONMENT 5.1 SummaryThis chapter summarises the presently available data on worldwide consumption of substances contributing to the stratospheric ozone depletion. Stratospheric ozone depletion is a global effect. The substances or emissions included are:
Consumption data are generally available worldwide as the substances are regulated according to the Montreal Protocol. The normalisation reference for stratospheric ozone depletion has been calculated to: 0.103 kg CFC-11-eq./capita/year CFC-11, CFC-12 and CFC-113 accounts for two thirds of the effect potential contributing to the normalisation reference for stratospheric ozone depletion. The normalisation reference is relatively certain as almost complete data are easy available due to the regulation of consumption according to the Montreal Protocol. 5.2 Description of the impact categoryStratospheric ozone depletion is considered as a global effect. Stratospheric ozone acts as a filter protecting the earth from incoming ultraviolet(UV)-radiation. The decomposition of ozone is enhanced by the stratospheric input of anthropogenic halogenated compounds (e.g. CFCs, HCFCs, halons etc.). The halogenated organic compounds are relatively stable and therefore a considerable amount reaches the stratosphere before they are decomposed. In the stratosphere they are decomposed to chlorine and bromine under the influence of UV-radiation. Nitrogen oxides, hydrogen oxides, methane, chlorine and bromine act as catalysing agents in the decomposition of ozone. The presence of the stratospheric ozone layer is fundamental for life on earth, as ozone absorb UV-radiation. The natural UV-radiation will make life as known today impossible. Decomposition of the stratospheric ozone layer will cause increased incoming UV-radiation (especially UV-B-radiation) leading to impacts on humans such as increased levels of e.g. skin cancer, cataracts and decreased immune defence. The increased UV-radiation has also an impact at natural organisms and ecosystems e.g. plankton in the South Pole region, where the decomposition of the ozone layer is already significant. The stratospheric ozone layer occurs at an altitude from 10 - 40 km, with maximum concentration from 15 - 25 km. The maximum generation of stratospheric ozone (O3) occurs in the top of the stratosphere at the altitude of 40 km as a result of a reaction of molecular oxygen (O2) and atomic oxygen (O). The decomposition of ozone is enhances by the stratospheric input of halogenated compounds (e.g. CFCs, HCFCs, halons etc.). See also Albritton et al. (1998) and Hauschild and Wenzel (1998) for further description of the impact category. 5.3 Substances contributing to the impact categoryThe substances contributing to stratospheric ozone depletion are defined (Solomon & Albritton 1992) as substances which:
The substances or emissions normally considered as contributors to stratospheric ozone depletion are:
The ozone depletion potentials of the relevant substances are calculated relative to the potential of CFC-11, and therefore the ozone depletion potentials are given as CFC-11 equivalents. The regulation of the production and consumption of ozone depleting substances by the Montreal Protocol (UNEP 1987) include the following substances mentioned in groups corresponding with the year for the inclusion in the protocol:
The status for ratification, accession, acceptance and approval of the different annexes varies and therefore the availability of data varies as the countries that have not ratified the annexes do not necessarily give information on consumption of the specific substances. In other words, the information on annex A substances are assumed to be more certain than information on annex B and C substances. A complete list of substances included in annex A (I and II) and annex B (I) is presented in Appendix B.2 "Calculation of normalisation references". Ozone depletion potentials (ODP) have been presented by the World Meteorological Organisation (WMO) for a number of halogenated compounds (Solomon & Wuebbles 1995; Pyle et al. 1991). A report containing information on production and consumption of ozone depleting substances (UNEP 1998) also contains ozone depleting potentials on the controlled substances. The equivalence factors differ slightly from the factors used in the EDIP methodology. The UNEP-report also mentions that the ozone depleting potentials "are estimates based on existing knowledge and will be reviewed and revised periodically". The equivalency factors are compared in Appendix C "Ozone depleting substances". The most recent ODP factors from UNEP (2002) have been used in the present calculation of normalisation reference for stratospheric ozone depletion. The values are given in Table 5-1, where they also are compared to recent values from Monztka, Frazer et al. (2002). Table 5-1
1) Where a range is given, the highest value is used in the calculations The potential depletion of stratospheric ozone as an impact from a given process can be estimated by summarising the ODPs:
where ODPi is the equivalency factor for the substance i mi is the emission of the substance i 5.4 MethodologyThe calculation of the normalisation reference for ozone depletion has been carried out according to the methodology described in Hauschild and Wenzel (1998). The normalisation factor is calculated as:
where NormrefODP is the normalisation reference for the ozone depletion mi is emitted quantity of substance i ODPi is the equivalence factor for substance i N is the number of capita in the considered area (the World) Included in the normalisation reference are the following substances:
These substances are included due to ready availability of data on emission or consumption of these substances. 5.5 Normalisation referenceOzone depletion is a global impact and therefore only a global normalisation reference is relevant. For comparison, the Danish consumption of ozone depleting substances is presented in appendix D "Consumption of ozone depleting substances". The nomalisation reference has been calculated by using information collected by UNEP (UNEP, 2002). Although the UNEP data are aggregated in classes of substances, they can be assumed to represent
the most precise and up-to-date information on ozone depleting substances. The consumption of ozone depleting substances has been used as an approximation for the emission as emission data does not
exist due to the character of the emissions. The emissions are in most cases diffuse and therefore not measured. The reason for using the above-mentioned approximation is that the relevant substances are
chemically very stable (an important quality in the use of the substances) and therefore the consumed amounts are expected to emit sooner or later. The actual delay will vary from substance to substance
depending on the actual use. The UNEP data are summarised in Table 5-2: Table 5-2
The normalisation reference for ozone depletion potential can thus be calculated as: 0.103 kg CFC-11-eq./capita/year The similar value for 1990 can be calculated to 0.226 kg CFC-11-eq./capita/year. This means that the normalisation reference is reduced by about 55%. The decrease can be explained by a significant reduction of the consumption of ozone depleting substances and an increase of the world population. At the same time the estimated ODP's for the CFC-12 and CFC-13 have been increased, adding to a higher normalisation reference. For 1994, the consumption of CFC-11, CFC-12 and CFC-113 constitutes approximately two thirds of the total consumption expressed as CFC-11 equivalents. With HCFCs as the exception, the contribution in absolute figures has decreased for all relevant compounds although most markedly for the CFCs. The reason for these findings are obviously that CFCs to a large extent have been replaced by HCFCs as refrigerants. The normalisation reference is about 50% of that calculated in the original EDIP97. The difference is of course related to the actual decrease in thee total consumption of ozone depleting substances, but it is interesting to note that there also is a large difference when examining the calculations based on the information that was available at the time of the calculations. Here, it can be seen that the calculation made in EDIP97 underestimated the normalisation reference with about 20-25%. The reason for this is probably that the data collection was not fully implemented in all relevant countries. Another difference is that some of the ODP's for specific substances have been re-evaluated, which in the total picture also causes an increase in the total ODP. The development in the assessment of ODP's for different substances is illustrated in Appendix C. 5.5.1 Alternative calculationsAppendix B.1. shows an alternative calculation of the normalisation references for 1990 and 1994. The major difference from the selected database is that in this alternative calculation, a linear decrease in the consumption of ozone depleting substances from 1990-1999 has been assumed, based on the fgures in Schiemel et al, 1996. Obviously, this causes a large overestimation –about 50% - of the ozone depleting potential in the reference year 1994. Appendix B.2. shows another alternative calculation of the normalisation reference. Here, the information collected by WMO/UNEP (Schiemel et al, 1996) and UNEP (1998) is used. A comparison between Appendix B.2. and the updated figures in Table 5-2 shows that the figures for consumption of CFC's in 1994 were underestimated in the early report. The comparison, however, also shows that the consumption of CFC's in 1990 was overestimated by about 7% in 1998, compared to the 2002 report. The two alternative calculations thus demonstrate the scientific and regulatory development in the area of stratospheric ozone depletion. The figure chosen as the normalisation reference can be assumed to represent the best possible knowledge, although there stille are some limitations in the data quality as discussed below. 5.5.2 Data qualityThe general quality of the data used in the calculations can be considered as good. There are, however, some methodological problems that must be remembered. Emissions of CFCs, HCFCs, HFCs, and halogenated carbons (CCl4 and CH3CCl3) are not measured regularly. The potential emissions are estimated by using the assumption that all the consumed amount of the actual substances will be emitted sooner or later due to their chemical stability. This methodology is problematic as the lifetime of the substances in the products differs depending on the purpose of the actual product. Substances used as degreasing agents are supposed to emit immediately during the use phase and substances used as cooling agents are supposed to emit after the use phase, i.e. when the goods are disposed off. However, the last assumption is only valid for some countries as collection of goods containing cooling agents has been established in some countries (e.g. Denmark) in order to recycle the cooling agent or to ensure proper destruction of the cooling agent. Another inherent uncertainty in calculation of the normalisation reference is that the ODP of specific substances is based on models or semi-empirical knowledge. This causes frequent changes of the ODP's, caused by the development of better models and collection of empirical data. The development is illustrated by the ODP's used for different calculations by different organisations, see Table 5-1 and Appendix C for examples. 5.6 Recommendations for future updateThe future updating of the normalisation reference for ozone depletion is recommended to be based on the same methodology and data source as used in the present update. The statistical information is compiled by UNEP and this information is also expected to be available in the future i.e. yearly updates of data on production and consumption of ozone depleting substances. 5.7 ReferencesAlbritton, D.L., & Kuijpers, L. (eds.) 1999, Synthesis of the Reports of the Scientific, Environmental Effects, and Technology and Economic Assessment Panels of the Montreal Protocol. A Decade of Assessment for Decision Makers Regarding the Protection of the Ozone Layer: 1988-1999. Nairobi: United Nations Environment Programme (UNEP). (Available at http://www.unep.ch or http://www.unep.org). Hansen, J.H. 1995, Ozonlagsnedbrydende stoffer og HFC - forbrug i 1994. Miljøprojekt nr. 302. København: Miljøstyrelsen. Hauschild, M. & Wenzel, H. 1998, Stratospheric ozone depletion as a criterion in the environmental assessment of products, in Environmental assessment of products. Volume 2: Scientific background, eds. Hauschild M, Wenzel H. London: Chapman & Hall. Pyle, J.A., Wuebbles, D., Solomon, S. & Zvenigorodsky, S. 1991, Ozone depletion and chlorine loading potentials. World Meteorological Organisation: Scientific assessment of stratospheric ozone: 1991 Global ozone research and monitoring project. Report no. 25. Geneva. Schimel, D., Alves, D., Enting, M., Heimann, M., Joos, F., Raynaud, D., Wigley, T., Prather, M., Derwent, R., Ehhalt, D., Fraser, P., Sanhueza, E., Zhou, X., Jonas, P., Charlson, R., Rodhe, H., Sadasivan, S., Shine, K.P., Fouquart, Y., Ramaswamy, V., Solomon, S., Srinivasan, J., Albritton, D., Isaksen, I., Lal, M. & Wuebbles, D. (eds.) 1996, Radiative forcing of climate change. Chapter 2 in Houghton, J.T., Meira Filho, L.G., Callander, B.A., Harris, N., Kattenberg, A. & Maskell, K. (eds.) Climate change 1995 - The science of climate change. Cambridge: Cambridge University Press. Solomon, S. & Albritton, D.L. 1992, Time-dependent ozone depletion potentials for short- and long-term forecasts. Nature, vol. 357, pp. 33-37. Solomon, S. & Wuebbles, D. (lead authors) 1995, Ozone depletion potentials, global warming potentials and future chlorine/bromine loading in Scientific assessment of ozone depletion, 1994 eds. Albritton, D.L., Watson, R.T. & Aucamp, P.J. World Meteorological Organisation, Global Ozone Research and Monitoring Project - Report No. 37. Geneva: WMO. UNEP 1987, The 1987 Montreal Protocol on Substances that Deplete the Ozone Layer as adjusted and amended by the second Meeting of the Parties (London, 27-29 June 1990) and by the fourth Meeting of the Parties (Copenhagen, 23-25 November 1992) and further adjusted by the seventh Meeting of the Parties (Vienna, 5-7 December 1995) and further adjusted and amended by the ninth Meeting of the Parties (Montreal, 15-17 September 1997). Available at: http://www.unep.org. UNEP 1998, Data report on production and consumption of ODSs - 1986 - 1996. United Nations Environment Programme, Ozone Secretariat. UNEP 1999, Status of Ratification/Accession/Acceptance/Approval of the agreements on the protection of the stratospheric ozone layer. The Vienna Convention for the Protection of the Ozone Layer (1985); The Montreal Protocol on Substances that Deplete the Ozone Layer (1987); The London Amendment to the Montreal Protocol (1990); The Copenhagen Amendment to the Montreal Protocol (1992); and the Montreal Amendment to the Montreal Protocol (1997). February 8 1999. UNEP: The Ozone Secretariat. (Available at: http://www.unep.ch). UNEP (2002). Production and consumption of ozone depleting substances under the Montreal Protocol 1986-2000. UNEP Ozone Secretariat, Apreil 2002. (http://www.unep.org/ozone). Appendix A: Data sourcesThe production and consumption of substances contributing to stratospheric ozone depletion are well registered due to the Montreal Protocol. According to the protocol the parties that have ratified the protocol has to report the consumption of ozone depleting substances every year. The data on the total production and consumption may improve in the future as more countries can be expected to ratify the protocol. The quality of the data from the countries that have ratified the protocol may also be expected to improve because more knowledge and experience is obtained. Databases (paper/electronic) UNEP (2002). Production and consumption of ozone depleting substances under the Montreal Protocol 1986-2000. UNEP Ozone Secretariat, Apreil 2002. (http://www.unep.org/ozone) Organisations The following institutions are relevant in relation to obtain data on the world production and consumption of ozone depleting substances:
Appendix B.1: Alternative calculation of normalisation references
1 Schimel et al. (1996) except consumption data for halons and methyl bromide which originates in UNEP (1998). A detailed description of the data originating from UNEP (1998) is given in Appendix B.2. The consumption is reported in ODPs and the consumption of halons and methyl bromide (in ton) has been calculated for the purpose of calculating the normalisation reference for ozone depletion as in Hauschild & Wenzel (1998). The consumption of halons (as a group) is 33,300 ton CFC-11-eq./year and is assumed to be halon-1301. Based on this assumption and that the equivalency factor for halon 1301 was set at 10 ton CFC-11-eq./ton halon 1301, the amount of consumed halons can be calculated to 3,300 ton/year. The contribution to the total ozone depleting potential in 1994 is based on the total amount of halons and the revised ODP for halon 1301. Recently, the equivalency factor has been suggesteed to be increased to 12 ton CFC-11-eq./ton halon 1301 (Montzka, Frazer et al. (2002)), and this factor has been used in the update calculations. The consumption of methyl bromide is 37,400 ton CFC-11-eq./year. Based on the equivalence factor used in 1994 (1.3 ton CFC-11-eq./ton methyl bromide), the amount of methyl bromide can be calculated to 62,300 ton/year. The new equivalence factor suggested by Montzka, Frazer et al. (2002) (i.e. 0.38 ton CFC-11-eq./ton methyl bromide) is used in the update calculations. Appendix B.2: Calculation of normalisation references, based on 1998 informationNormalisation references calculated on basis of statistical information collected by UNEP/Ozone Secretariat from the countries that have ratified/.accessed/accepted/approved the Montreal Protocol with the London and Copenhagen Amendments (UNEP 1998). The consumption data cover some countries that have ratified/accessed/accepted/approved the Montreal Protocol but not the London Amendment (regulation of annex B substances: I: "other" fully halogenated CFCs, II: carbon tetrachloride and III: methyl chloroform) and Copenhagen Amendment (regulation of annex C substances: I hydrochlorofluorocarbons, II: hydrobromofluorocarbons and annex E substance: methyl bromide). The consequence is that data are not complete for all substances. The production and consumption are reported in ODPs according to equivalency factors presented in the same publication; the equivalency factors are presented in appendix C.
For some of the countries the consumption has not been required nor reported for the years of interest i.e. 1990 and 1994. A rough extrapolation has been made from the consumption before and after the year of interest; a linear correlation has been assumed. Appendix C: Ozone depleting potentialsThe table below presents ozone depleting potentials for the most common ozone depleting substances. The calculation of ODP is based on an emission scenario, a calculation model and an atmospheric scenario. Further details on different approaches can be found in Hauschild and Wenzel (1998).
0. Solomon and Wuebbles (1995), Pyle et al. (1991) and Solomon and Albritton (1992) cited from Hauschild and Wenzel (1998). 1. UNEP (1998). 2. Montzka, Frazer et al. (2002) 3. UNEP (2002) Appendix D.1: Consumption of ozone depleting substancesInformation on the worldwide consumption of CFC, HCFC, HFC and halons are compared in the table below.
The table contains consumption and emission survey data from AFEAS (Alternative Fluorocarbons Environmental Acceptability Study). AFEAS is an organisation representing producers of CFC, HCFC and HFC. They cover less than 35% of the worldwide CFC-production and 90% - 100% of HCFC/HFC as they primarily cover European, American (North and South) and Japanese producers whereas producers from Eastern Europe and other Asiatic countries are outside AFEAS. The conclusion is that the world consumption of ozone depleting substances reported by AFEAS is below the actual consumption and therefore not useable for the present project. Appendix D.2: Consumption of ozone depleting substancesConsumption of greenhouse gasses/ozone depleting substances and emission of greenhouse gasses in Denmark in 1994.
1 Hansen (1995). Footnotes[5] The Montreal Protocol is ratified/accessed/accepted/approved by 168 countries at February 8, 1999 (UNEP 1999). [6] The London Amendment is ratified/accessed/accepted/approved by 127 countries at February 8, 1999 (UNEP 1999). [7] The Copenhagen Amendment is ratified/accessed/accepted/approved by 86 countries at February 8, 1999 (UNEP 1999).
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||