|
Impact categories, normalisation and weighting in LCA 5. Stratospheric ozone depletion
The concentration of the reactive oxygen compound ozone O3 is significantly higher in the stratosphere than in other parts of the atmosphere, e.g. the troposphere A number of substances, some of which occur naturally in the stratosphere, are involved in the breakdown of ozone: long-lived chlorine and bromine compounds, methane (CH4), nitrous oxide (N2O), and water vapour H2O. The reaction systems are complex. However, there is an international consensus about the issue and there is an international agreement based on the Montreal protocol concerning phase out of the relevant substances. The atmosphere receives ultraviolet radiation from the sun. Ozone molecules in the stratosphere absorb large quantities of this UV radiation, thus removing the life-threatening UV-C radiation and reducing the harmful UVB radiation. The reduction in the ozone concentration in the stratosphere will probably have a serious effect on the life on the surface of the Earth. It can cause damage to plants, animals, and humans. In the area around the South Pole (which is considered to be the most affected) effects on phytoplankton have been observed. Phytoplankton is a primary producer in every aquatic food chain and consequences can therefore be expected to be dramatic. The ozone depletion will also effect humans in the form of skin cancer, reduced immune defence etc. 5.1 Substances contributing to stratospheric ozone depletionThe substances contributing to stratospheric ozone depletion are defined as substances which:
The substances included are:
The substances are regulated in the Montreal Protocol (UNEP 1987). 5.2 Ozone Depletion PotentialIn EDIP (Hauschild & Wenzel, 1998), the potential depletion of stratospheric ozone is quantified by using ozone depletion potentials (ODP) for substances having the same effect as CFC's. CFC-11 has been chosen as reference substance in EDIP as it is in most other contexts. This is due to the fact that it is well studied and that it is one of the substances having the largest effect on ozone depletion. The ODP describes the ozone depletion potential from a substance relative to CFC-11. The modelling of the effect is complex. Data for modelling consist of e.g. the stability of the substance, its lifetime and the time horizon considered. Ozone depletion potentials have been presented by the World Meteorological Organisation (WMO) for a number of halogenated compounds (Solomon & Wuebbles 1995; Pyle et al. 1991). The actual ozone depletion potential is determined by a number of factors e.g. the content of chlorine and bromine and the chemical and photochemical stability of the substances. More recent reports containing information on production and consumption of ozone depleting substances (UNEP (1998), UNEP (2000)) also report ozone depleting potentials on the controlled substances. 5.3 Normalisation references and weighting factors for ozone depletionThe normalisation references and weighting factors for ozone depletion potential are calculated according to the formula in chapter 1, Introduction. Table 5.1 presents the normalisation references and the weighting factors for ozone depletion. Table 5.1 Normalisation reference and weighting factors for stratospheric ozone depletion (Hoffmann 2005; Busch 2005).
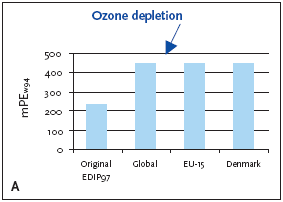
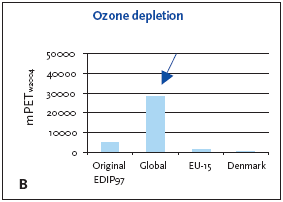
1. Industrialised countries/Developing countries 5.3.1 Recommendation of applying normalisation reference and weighting factors for ozone depletion Ozone depletion is a global impact and therefore only a global normalisation reference is relevant. 5.4 Example of applying the normalisation reference and weighting factor for ozone depletionBelow an example of applying the normalisation reference and weighting factor for a product (a refrigerator) is presented. Figure 5.1 illustrates the normalised ozone depletion potential calculated by applying the global normalisation reference. Irrespective of the geographical boundaries, the global normalisation reference combined with the global weighting factor for ozone depletion are recommended. Based on an impact potential for the product in question at 0.046 kg CFC-11-eq./year the actual normalised and weighted values are 448 mPEW94 and 28230 mPETW2004 respectively. Please note that the Danish weighting factor is infinite. Figure 5.1 Normalised (A) and weighted (B) ozone depletion potentials for production of a refrigerator at different localities.
5.5 If you would like to know moreAlbritton, D.L., & Kuijpers, L. (eds.) 1999, Synthesis of the Reports of the Scientific, Environmental Effects, and Technology and Economic Assessment Panels of the Montreal Protocol. A Decade of Assessment for Decision Makers Regarding the Protection of the Ozone Layer: 1988-1999. Nairobi: United Nations Environment Programme (UNEP). Available at http://www.unep.ch or http://www.unep.org. Busch, N.J. 2005, Calculation of weighting factors. In Stranddorf, H.K., Hoffmann, L. & Schmidt, A. Update on impact categories, normalisation and weighting in LCA. Environmental Project no. 995, Danish EPA, 2005. Hansen, J.H. 1995, Ozonlagsnedbrydende stoffer og HFC - forbrug i 1994. Miljøprojekt nr. 302. København: Miljøstyrelsen. Hauschild, M. & Wenzel, H. 1998, Stratospheric ozone depletion as a criterion in the environmental assessment of products, in Environmental assessment of products.Volume 2: Scientific background, eds. Hauschild M, Wenzel H. London: Chapman & Hall. Hoffmann, L. 2005, Stratospheric ozone depletion. In Stranddorf, H.K., Hoffmann, L. & Schmidt, A. Update on impact categories, normalisation and weighting in LCA. Environmental Project no. 995, Danish EPA, 2005. Pyle, J.A., Wuebbles, D., Solomon, S. & Zvenigorodsky, S. 1991, Ozone depletion and chlorine loading potentials. World Meteorological Organisation: Scientific assessment of stratospheric ozone: 1991 Global ozone research and monitoring project. Report no. 25. Geneva. Solomon, S. & Albritton, D.L. 1992, Time-dependent ozone depletion potentials for short- and long-term forecasts. Nature, vol. 357, pp. 33-37. Solomon, S. & Wuebbles, D. (lead authors) 1995, Ozone depletion potentials, global warming potentials and future chlorine/bromine loading in Scientific assessment of ozone depletion, 1994 eds. Albritton, D.L., Watson, R.T. & Aucamp, P.J. World Meteorological Organisation, Global Ozone Research and Monitoring Project - Report No. 37. Geneva: WMO. UNEP 1987, The 1987 Montreal Protocol on Substances that Deplete the Ozone Layer as adjusted and amended by the second Meeting of the Parties (London, 27-29 June 1990) and by the fourth Meeting of the Parties (Copenhagen, 23-25 November 1992) and further adjusted by the seventh Meeting of the Parties (Vienna, 5-7 December 1995) and further adjusted and amended by the ninth Meeting of the Parties (Montreal, 15-17 September 1997). Available at: http://www.unep.org. UNEP 1998, Data report on production and consumption of ODSs - 1986 - 1996. United Nations Environment Programme, Ozone Secretariat. UNEP (2002). Production and consumption of ozone depleting substances under the Montreal Protocol 1986-2000. UNEP Ozone Secretariat, Apreil 2002. (http://www.unep.org/ozone).
|