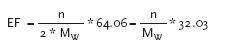|
Impact categories, normalisation and weighting in LCA 7. Acidification
Acidification is regarded as a regional effect. Acidification is caused by releases of protons in the terrestrial or aquatic ecosystems. The acidifying substances are only contributing to acidification if the anion is leached out from the system. Organic acids vil predominantly be mineralised and does therefore not leach to the system. Accordingly, they are not regarded as contributors to acidification. In certain areas, acidification leads to increased mobility of heavy metals and aluminium. In the terrestrial ecosystem the effects are seen in softwood forests (e.g. spruce) but also in hardwood forests (e.g. beech) as inefficient growth and as a final consequence forest dieback. In Europe, these effects are mainly seen in Scandinavia and in the middle/eastern part of Europe. In the aquatic ecosystem the effects are seen as (clear) acid lakes without any wildlife. Clean lakes are mainly seen in Scandinavia. Buildings, constructions, sculptures and other objects are also damaged by e.g. acid rain. 7.1 Substances contributing to the impact categorySubstances are considered to have an acidification effect if they result in (Hauschild & Wenzel, 1998):
The substances normally considered as contributors to acidification are:
The technical report (Hoffmann 2005) summarises the presently available data on emissions of acidifying substances to the Danish and the European environment. The primary contributors are:
7.2 Acidification Potential (AP)In EDIP (Hauschild & Wenzel, 1998), the potential acidification potential is quantified by using acidification potentials (AP) for substances having the same effect as SO2 in reflection of acidification. Acidification potentials are expressed as SO2-equivalents (SO2-eq), i.e. the potentials are expressed relative to the potential of SO2. 7.2.1 Definition of Acidification Potential SO2 is used as a basis for determination of the acidification potential or the equivalence factor. The method of establishing effect factors for acidifying substances is based on stoichiometric considerations and it is internationally accepted. The equivalency factors are determined as (Hauschild & Wenzel, 1998):
where The acidification potential (AP) can be estimated as SO2-equivalents:
where 7.3 Normalisation references and weighting factorsThe normalisation references and the weighting factors for acidification potential are calculated according to the formula presented in Chapter 1, Introduction. Table 7.1 presents the normalisation references and the weighting factors for acidification. Table 7.1 Normalisation references and weighting factors for acidification (Hoffmann 2001; Busch 2001).
1 Weighting factors have not been established worldwide; the European weighting factors are recommended for impact potentials located outside Europe or at unknown locality 7.3.1 Recommendation for application of normalisation references and weighting factors for acidification For acidification as a regional effect the EU-15 normalisation reference is recommended for impact potentials located in Denmark as well as Europe or the global normalisation reference if the locality is outside Europe or unknown. 7.4 Example of applying normalisation reference and weighting for acidificationBelow an example of applying the normalisation reference and weighting factor for a product (a refrigerator) is presented. Figure 7.1 illustrates the normalised and weighted acidification potential for the product. Figure 7.1 Normalised (A) and weighted (B) acidification potentials for production of a refrigerator at different localities. 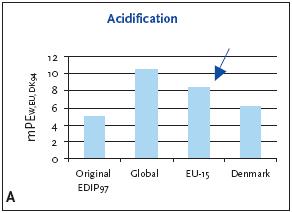 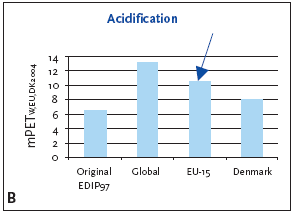
For impact potentials located in Denmark the EU-15 normalisation reference combined with the EU-15 weighting factor for acidification are recommended. Based on an impact potential for the product in question at 0.62 kg SO2-eq./year the actual normalised and weighted values are 8.3 mPEEU94 and 11 mPETEU2004 respectively. As can be deduced from Table 7.1 and seen in Figure 7.1, the normalised result can vary with about 25%, depending on which normalisation references are chosen. In the case of the refrigerator, acidification is more important on the European and global level. This can be explained by the fact that the contribution from the average person is higher in Denmark than in the other regions, but the political weight on the issue is about the same. Please note that a global weighting factor has not been calculated. 7.5 If you would like to know moreBusch, N.J. 2005, Calculation of weighting factors. In Stranddorf, H.K., Hoffmann, L. & Schmidt, A. Update on impact categories, normalisation and weighting in LCA. Environmental Project no. 995, Danish EPA, 2005. EEA 1998a, Europe's Environment:The Second Assessment. Eurostat, European Commission, European Environment Agency. Office for Official Publications of the European Commission, Luxembourg. EEA 1998b,. Europe's Environment: statistical compendium for the Second Assessment. Eurostat, European Commission, European Environment Agency. Office for Official Publications of the European Commission, Luxembourg. Hauschild, M. & Wenzel, H. 1998, Acidification as a criterion in the environmental assessment of products in Environmental assessment of products.Volume 2 Scientific background eds. Hauschild, M. & Wenzel, H. London: Chapman & Hall. Hoffmann, L. 2005. Acidification. In Stranddorf, H.K., Hoffmann, L. & Schmidt, A. Update on impact categories, normalisation and weighting in LCA. Environmental Project no. 995, Danish EPA, 2005. Koch, D. 1998, Air emissions - Annual topic update 1997. Topic Report no.4. European Environment Agency. Ritter, M. 1997, CORINAIR 94 - Summary Report - European Emission Inventory for Air Pollutants. Copenhagen: European Environment Agency. UN-ECE 1979, Convention on Long-range Transboundary Air Pollution. United Nations, Economic Commission for Europe. Available: http://www.unece.org.
|