Screening for health effects from chemical substances in textile colorants
3 Health assessment
- 3.1 Assessment method
- 3.2 Assessment scenarios
- 3.3 Assessment of individual substances
- 3.3.1 1-Butanol
- 3.3.2 Caprolactam
- 3.3.3 1,4-Dichlorobenzene
- 3.3.4 Diethylene glycol
- 3.3.5 Diisopropylene glycol
- 3.3.6 1,4-Dioxane
- 3.3.7 Glycerine
- 3.3.8 Hexamethylentetramine (methenamine)
- 3.3.9 Isobutane
- 3.3.10 3-Isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate
- 3.3.11 N-Methyl-2-pyrrolidone
- 3.3.12 2-Phenoxyethanol
- 3.3.13 Propylene glycol
- 3.3.14 Antimony
- 3.3.15 Copper
3.1 Assessment method
For the chemical substances detected by the analyses, an evaluation of which substances appeared immediate to be the most interesting was performed. The selection is performed in agreement with the Danish Environmental Protection Agency. Data on the individual substances then are retrieved from available sources in order to perform a health hazard evaluation based on known information from previous prepared Danish or foreign monographs, etc. The obtained data for toxicity are then compared with the concentrations estimated in the used scenarios described below.
The methods used are approximately the same as recommended in connection with risk assessment of chemical substances in the European Union (EU) and described in the Technical Guidance Document, TGD (EC 2003). In the TGD the potential risk to the consumer is estimated as the ratio between the concentration where no adverse effect is expected (No Observed Adverse Effect level, NOAEL) and the predicted exposure concentration, i.e. NOAEL / the estimated uptake in the exposed consumers. NOAEL is based on mammalian data: typically rats, mice and rabbits. Therefore, it is necessary to introduce a safety factor (SF) to cover potential differences when extrapolating from these mammals to humans.
The safety factor is interpreted as a margin of safety applied to a NOAEL to produce a value below which exposures are presumed to be without significant health risk. The safety factor is traditionally composed of a factor 10 for extrapolation between species (animal to human, interspecies variation), a factor 10 to protect the most sensitive individuals of the population such as e.g. children (intraspecies variation). A third factor is applied depending on the data and may vary. For instance 10 is used if LOAEL (Lowest Observed Adverse Effect Level) is used instead of NOAEL or using subchronic data instead of chronic data. The total safety factor is a result from multiplication of the three factors.
In the EU risk assessment, the risk of health effect is expressed by NOAEL dicided with the exposure, i.e. by expressing the margin of safety (MOS) to reflect whether the distance from the level where no adverse effect is expected to the estimated exposure level is sufficient. Typically MOS is preferred to be above 100.
The classification authorised in Denmark (Miljøministeriet 2002), which is an implementation of the European Union classification (28th amendment to EU directive 67/548/EEC), is used in the evaluation. The amendments performed in the 29th amendment and adopted in Directive 2004/73/EC (EC 2004) and not yet implemented in Denmark are included, however, as the implementation may be expected within the near future.
For the evaluation of the individual substances is used the threshold limit values derived and evaluated by the authorities or recognised institutions. The limit values thus contain an evaluation of the data used including a safety factor that qualified persons have evaluated as acceptable.
Of relevant limit values included in the health evaluation if available are:
| ADI: | Acceptable Daily Intake. A value calculated from NOAEL by an official authority as an acceptable daily intake (mg/kg body weight/day. ADI is usually based on chemical substances in food. |
| C-value: | Contribution value: The C-value is defined as the total maximal allowed contribution to the air pollution from an enterprise to the environment outside the production site. The C-value usually is derived from NOAEL levels and includes safety factors (Miljøstyrelsen 2002). |
| RfC: | Reference concentration. RfC is an inhalation reference concentration based on the assumption that a threshold limit value for certain toxic effects exists. The value is based on NOAEC from inhalation studies of subchronic or chronic character and includes safety factors. The value is given in mg/m3. |
| RfD: | Reference dosis. RfD is an oral reference dosis based on the assumption that
a threshold limit for certain toxic effects exists. The value is based on NOAEL
from subchronic or chronic studies using oral administration and includes safety
factors. The value is given in mg/kg body weight/day.
. |
| TLV: | Threshold Limit Value in force for the working environment is set by the Danish Working Environment Authority (Arbejdstilsynet, AT 2002). The threshold limit values of the Danish Working Environment Authority is valid where the chemical substances are used in the production. The threshold limit values are based on 8-hour time-weighted average (a working day). It is important to note that the threshold limit value does not include the consumer at home. |
| TDI: | Tolerable Daily Intake. Almost identical to ADI but usually based on chemical pollutants. |
3.2 Assessment scenarios
Scenarios for the assessment of textile colorants are based on the products used by the consumer for decoration or dyeing of textiles at home.
The exposure of the consumer therefore varies according to the end use. Nevertheless it is chosen to use as starting point the exposure scenarios where the exposure is presumed to be the highest.
The highest primary exposure takes place when using the product, i.e. when the consumer decorates or dyes the textile. The exposure route will be by inhalation of volatile substances that evaporate during use and dermal exposure by contact to the product or the non-dried textile.
A further exposure may occur by contact next to the skin of the coloured textile (e.g. clothes and bed linen). The direct exposure from the coloured textile will be lower and to a higher degree of another character such as e.g. inhalation of evaporated substances or substances adsorbed to detached fibres as dust. The contact exposure is in the current evaluation assumed to be included in the used scenarios.
The oral exposure of the consumer may occur if for instance a child puts fingers with colours or the product into the mouth or if the coloured/dyed textile is mouthed.
Scenarios are presented for:
- Exposure via inhalation
- Dermal exposure
- Oral exposure
3.2.1 Exposure via inhalation
Exposure to the substance via inhalation of volatile substances may be direct exposure to evaporated substances from the use of the colorant product, either directly from the "source" or after its application to the textile. Further inhalation may occur of dust from fibres that contain the chemical substance or dust to which the chemical substance is absorbed
The exposure period theoretically may extend from the use of the colour product until it is completely dry. However, it is considered likely that the consumer leaves the spot where the dyeing has taken place following the use and therefore not necessarily is so highly exposed during the actual drying period.
The exposure via inhalation is expressed as the concentration of the chemical substance in the air in the breathing zone and is given as an average concentration over a reference period, e.g. 8 hours for the working environment. For the consumer of textile colorants the exposure duration may be from a few minutes to a few hours at the home. In the assessment is used an exposure duration of 30 minutes (half an hour).
For the estimation of exposure via inhalation you have to know the inhalation rate, the size of the breathing zone or the room, and the release rate of the substance to the room or the concentration in the breathing zone or the room.
The inhalation rates for a child and adult by sedentary activities are 0.4 and 0.5 m3/hour, respectively, by exposure for a short period (TGD, EC 2003). By prolonged exposure which is assumed only to apply to adults is used an inhalation rate of 15.2 m3/day (standard in TGD, EC 2003). The exposure by dyeing is assumed rarely to comprise a whole day. As young humans are considered the most sensitive group in this context an inhalation rate of 0.4 m3/hour is used in the assessment.
The concentration in the breathing zone is based on an estimate of the concentration in 1 m3 of air right around the consumer during the use of the colorant product to colour/dye the textile. This concentration will be somewhat diluted relative to the consumer sitting with the head close to the product when working intensely with decorating colours. On the other hand the exposure is assessed as a repeated exposure which is assumed to compensate for the dilution.
The concentration in closed rooms is assumed to be higher than by outdoor use of the textile colorants. For the calculation of the concentration in the room is used an equation for volatile substances and airborne particles where it is assumed that the substance is released instantaneously to the breathing zone or the entire room and distributed homogeneously. It is assumed that the consumer is in a room where the ventilation is too small to contribute further to the dilution already used (immediate breathing zone of 1 m3).
For the calculation of the theoretical maximum concentration of the substance in the air is used the Law on ideal gasses in an adapted form (EC 2003):
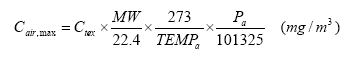
Where
| Cair, max | Maximum concentration obtainable in air | mg/m3 |
| Ctex | Concentration of the substance in product | mg/kg |
| MW | Molecular weight | g/mol |
| 22.4 | The volume that 1 mol of a substance occupies in gaseous form at 0°C and 1 atm | l |
| 273 | Temperature 0°C in °K | °K |
| TEMPa | Actual temperature in °K | °K |
| Pa | Vapour pressure of the substance in Pascal | Pa |
| 101325 | Standard normal atmospheric pressure | Pa |
The same equation is used in the EASE model designed for the working environment (EC 2003).
The concentration in inhaled air then can be calculated according to the equation:
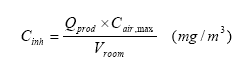
| Cinh | Concentration in inhaled air | mg/m3 | |
| Qprod | Amount of product used | kg | 0.005 kg |
| Cair, max | Maximum concentration obtainable in the room | mg/m3 | |
| Vroom | Volume of the room / breathing zone | m3 | Used: 1 m3 |
The amount of inhaled substance is then (EC 2003):
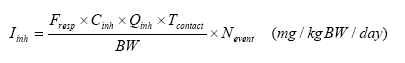
| Iinh | Amount of substance inhaled | mg | mg/kg bw/day |
| Fresp | Inhalable or respirable fraction of the substance | (e.g. 1 (i.e. 100%)) | |
| Cinh | Concentration in air | mg/m3 | |
| Qinh | Inhalation rate | m3/hour | 0.4 m3/hour |
| Tcontact | Duration of exposure | hours | 0.5 |
| Nevent | Number of events | day-1 | 1 |
| BW | Body weight | kg | Used: 20 kg |
The consumers in this case are assumed to be children 7 to 10 years old. According to realistic worst case the body weight is set to the 5 percentile body weight (AUH 1995) of children 7 to 8 years of age which is approx. 20 kg (AUH 1995, US-EPA 2002).
As starting point is used a scenario where the highest exposure is expected. The consumer is a child of 20 kg body weight decorating/dyeing a piece of textile for 30 minutes. The respiration rate is 0.4 m3/hour. The exposure is directly to the breathing zone set to 1 m3.
In professional dyeing mills, a large number of dyes are used and the consumption varies from 2 to more than 80 g/kg of textile with an average of 20 g/kg according to the required depth of colour (Laursen et al. 1997). The TGD indicates that commercial dye factories use 10 kg dye/ton of textiles, i.e. 1% (ECB 2003). The Swedish Chemicals Inspectorate indicates that for both dyeing and printing 500 to 30000 ppm dyes or pigments, i.e. up to 3%, may be used (KemI 1997). OECD presents data indicating that the consumption of dyestuff in the textile finishing industry varies between 11 and 88 g/kg textile (OECD 2004).
Assuming that the coloured textile weights approx. 100-200 gram, that decorations may occupy a smaller area than total dyeing of the textile but using more colorant/area, and that home dyeing consumers probably use a little more colorant than the professionals the used amount is set to 5 g.
For the organic substances is assumed that the maximum obtainable concentration is reached in the breathing of 1 m3 by the use of 5 g of the colour product.
3.2.2 Dermal exposure
Before uptake via the skin the chemical compound has to migrate from the colour in or on the textile to the skin or directly from skin contact with the colorant from the product (pen, powder etc.). When the substance has reached the skin the substance may be absorbed percutaneous to the blood stream and then distributed throughout the body.
The uptake after contact may be from "free" chemical substances released from the colour in/on the textile or from degradation products. The degradation of the substance may take place in the textile or via bacteria or enzymes on the skin or in the gastro-intestinal-tract after absorption.
The exposure can be expressed shortly by the equation (EC 2003) which is modified to the used exposure scenario:
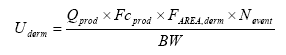
Where:
| Uderm | Potential uptake of the chemical substance | μg/kg bw/day |
| Qprod | Amount of product | g |
| Fcprod | Weight fraction of chemical substance | μg/g |
| Farea, derm | Fraction of exposed skin | |
| Nevent | Number of exposure events | per day |
| BW | Body weight | kg |
For the evaluation of dermal exposure is used a scenario with skin contact during the use of the colorant and the coloured textile. The exposed skin area such as hands is assumed to be the palm of the hand for 30 minutes (half an hour) which is assumed to be the period used to decorate or dyeing the textile. The palm of the hands varies as humans grow but assuming that the ratio between hands and the body surface is reasonably constant the exposed surface may be estimated. Both hands constitute approx. 5% of the total body surface (US-EPA 1997). It is assumed that maximum 1/5 of the hands are exposed thus 1% of the body may directly get into contact with the colorant during the use.
Skin contact with the textile after the application is assumed less relevant for solvents where the main part is expected to evaporate. Besides in the assessment is used a repeated exposure and in total the assessment is assumed to involve both exposure routes.
As in the scenario on inhalation the exposure is assumed to be 5 gram of colorant and a body weight of 20 kg.
Absorption
After exposure to the skin the chemical compound has to pass the skin before actual absorption is taking place. Only a few data of percutaneous absorption of the studied compounds have been found. The dermal absorption is therefore estimated.
Depending on the exposure and/or the compound's lipophilicity the dermal penetration is assumed to be insignificant for very lipophilic compounds with a log Kow more than 5 (OECD 1993).
Dermal penetration is considered very small for compounds with a log Kow less than -1 (i.e. very hydrophilic) and for compounds with a molecular weight above 700 (Vermeire et al. 1993). According to a Dutch model the dermal absorption is estimated to 10% for compounds with a molecular weight above 500 g/mol and a log Kow <-1 or >4 (De Heer 1999). The latter values are also included in the TGD (EC 2003).
In standard assessments or when no informations are available a typical dermal absorption of 100% is used (EC 2003). This has been performed with all organic compounds. If information on absorption was available the information has been used in refining of the estimates. It has been performed by multiplying the dermal exposure (Uderm) with the absorption factor (Fabs):
Aderm = Uderm x Fabs
3.2.3 Oral exposure
Oral exposure may take place by chewing or sucking on fingers that have been in contact with colorant or the dyed textile (children). By oral exposure the absorption takes place after intake by uptake over the epithelium in the mouth cavity or the gastro-intestinal-tract.
The oral intake can be estimated by the equation (OECD 1993, EC 2003):
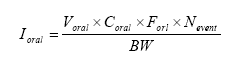
Where
| Ioral | Intake of the compound | μg/kg bw/day |
| Vprod | Weight of product mouthed | kg |
| Coral | Concentration of substance in the product | mg/kg |
| Nevent | Number of events per day | assumed 1 time/day |
| BW | Body weight | kg |
| Foral | Fraction absorbed (bioavailable part) |
As starting point is chosen that the consumer in the current case a child sucks/chews on textile corresponding to 1010 cm = 100 cm2 textile. In the TGD (EC 2003) is assumed use of 0.5 g/m2 by total dyeing. In the remaining scenarios are assumed a use of 5 g of colorant for decorating that will be dispersed unevenly.
It is therefore assumed that maximum 1/10 of the coloured material might be mouthed. This may be set high but it also includes other sources such as contaminants on hands or felt-pens, which are then mouthed, getting into contact with food or in other ways bring the substance in indirect contact with the mouth.
This is assumed in total to be included in the selected parameters that is then a total oral exposure to textile with 0.5 g colorant.
The body weight is set to 20 kg as in the other scenarios.
3.3 Assessment of individual substances
3.3.1 1-Butanol
Identification:
| Name | 1-Butanol |
| CAS no. | 71-36-3 |
| EINECS no. | 200-751-6 |
| Molecular formula | C4H10O |
| Molecular structure | |
| Molecular weight | 74.12 g/mol |
| Synonyms | Butan-1-ol |
| n-Butanol | |
| Butylalcohol | |
| 1-Hydroxybutane | |
| Propylcarbinol |
The melting point is –89.8°C. The boiling point is 117.7°C. The water solubility is 70000 mg/l at 25°C. The vapour pressure is 640 Pa at 20°C and 910 Pa at 25°C (6.7 mmHg, Boublik et al. 1984). The octanol/water partition coefficient is measured to log Kow 0.88 (Hansch et al. 1995).
Classification
1-Butanol is adopted on the List of dangerous substances (Miljøministeriet 2002) and classified:
| R10 | Flammable. |
| Xn;R22 | Harmful if swallowed. |
| Xi;R37/38-41 | Irritating to respiratory system and skin. Risk of serious damage to eyes. |
| R67 | Vapours may cause drowsiness and dizziness. |
Use
1-Butanol is used among others as additive in the paint and lacquers industry, especially as solvent.
Effects on health
Some data have been found on acute toxicity. Of those can be mentioned:
| Acute oral, rat | LD50 | 790 mg/kg | IUCLID 2000 |
| Acute oral, mouse | LD50 | 2680 mg/kg | IUCLID 2000 |
| Acute dermal, rabbit | LD50 | 3400 mg/kg | IUCLID 2000 |
| Acute inhalation, rat | LC50, 4 h | >24000 mg/m3 | IUCLID 2000 |
| Acute inhalation, mouse | RD50, 1 min | 11696 ppm | IUCLID 2000 |
Data on acute exposure to 1-butanol show a low toxicity. On the other hand several data on irritation exist. In a study on humans was observed that vapours at a concentration of 50 ppm (150 mg/m3) resulted in eye irritation in most test persons while 25 ppm (75 mg/m3) caused irritation of the nose and throat in the test persons (IPCS 1987). Irritation of skin and respiratory system is observed in several tests on test animals (IUCLID 2000).
In a 92 days inhalation study, rats were continuously exposed to 0.03 and 7.02 ppm. Based on changes in blood parameters at the high dose NOAEL was set to 0.03 ppm corresponding to 0.09 mg/m3 (IPCS 1987).
Of studies with prolonged exposure duration is found a 13 weeks rat study where the rats daily were administered 1-butanol directly into the stomach by gavage with the doses 0, 30, 125 and 500 mg/kg bw/day. Based on observed effects on the central nervous system such as ataxia and hypoactivity at 500 mg/kg bw/day the NOAEL was set to 125 mg/kg bw/day (IRIS 2004).
Threshold limit values
The threshold limit value for the working environment is 50 ppm corresponding to 150 mg/m3 with notation LH. L indicates that the threshold limit value is a ceiling value that at no time must be exceeded. H means that the substance may penetrate the skin (AT 2002).
The C-value is 0.2 mg/m3 (Miljøstyrelsen 2002).
The oral RfD value is 0.1 mg/kg bw/day. The value is based on a subchronic rat study where a NOAEL of 125 mg/kg bw/day was set and with the application of a safety factor of 1000: 10 for interspecies, 10 for intraspecies differences and 10 for subchronic to chronic extrapolation (IRIS 2004).
Absorption
Descriptions indicating readily absorption by oral intake and via inhalation have been found. No values were found for dermal absorption. Therefore 100% absorption is assumed.
Assessment
Exposure by inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Calculation example (only performed for the first substance, for further information cf. the section on methods):
Cair, max =690 (74.12/22.4) (273/298) (910/101325) = 18.785 mg/m3
C inhalation = 18.785 0.005 (kg/kg) / 1 (m3) 1000 = 93.9 μg/m3
Uptake = 93.9 1 (100% abs.) 0.4 (resp. rate) 0.5 (h) / 20 (kg) = 0.939 μg/kg bw/day.
Table 4. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 1 | 150 | 4.084 | 20.418 | 0.204 |
| 4 | 650 | 17.696 | 88.479 | 0885 |
| 5 | 690 | 18.785 | 93.924 | 0.939 |
| 7 | 77 | 2.096 | 10.481 | 0.105 |
| 9 | 190 | 5.173 | 25.863 | 0.259 |
| 12 | 620 | 16.879 | 84.396 | 0.844 |
Uptake through skin (dermal uptake) is estimated assuming that both palms are exposed to 5 g of the product 1 time per day. The skin area on both hands is approx. 5% of the total body skin area (US EPA 1997). It is assumed that maximum 1/5 of the hands is exposed thus 1% is used in the calculations:
Dermal uptake = 690 0.005 0.01 (FAREA, derm) 1 (F abs) 1 (d-1) / 20 (kg) 1000 = 1.725 μg/kg bw/day
Oral uptake assuming an exposure to 0.5 g product
Oral uptake = 690 0.0005 (kg prod) 1 (F orl) 1 (d-1) / 20 (kg) 1000 = 17.25 μg/kg bw/day
Table 5. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 150 | 0.375 | 3.75 | 4.3 |
| 4 | 650 | 1.63 | 16.25 | 18.8 |
| 5 | 690 | 1.73 | 17.25 | 19.9 |
| 7 | 77 | 0.19 | 1.93 | 2.2 |
| 9 | 190 | 0.48 | 4.75 | 5.4 |
| 12 | 620 | 1.55 | 15.50 | 17.9 |
Based on observed effects on the central nervous system such as ataxia and hypoactivity with a NOAEL of 125 mg/kg bw/day the margin of safety (MOS) for total exposure is: 125 / 0.0199 = 6280.
The distance to the RfD value is a factor of: 0.1/0.0199 = 5.
Conclusion
The content of the products of 1-butanol is assessed not to pose a health risk to the consumer of the studied textile colorants.
However, the possibility of irritation of nose and throat mucous membranes can not be excluded during the use of the products. The estimated maximum concentrations in air are approx. 1/3 of the concentration where irritation is reported (75 mg/m3).
3.3.2 Caprolactam
Identification:
| Name | Caprolactam |
| CAS no. | 105-60-2 |
| EINECS no. | 203-313-2 |
| Molecular formula | C6H11NO |
| Molecular structure | 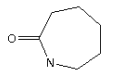 |
| Molecular weight | 113.16 g/mol |
| Synonyms | epsilon-caprolactam (EINECS name) |
| 2-Oxohexamethylenimin (AT name and C-value name) | |
| 2-Azacycloheptanone | |
| Hexahydro-2H-azepin-2-one (CA) |
The melting point is 69.2°C. The boiling point is 270°C. The water solubility is 772000 mg/l at 25°C (Yalkowsky and Dannenfelser 1992). The vapour pressure is 0.21 Pa at 25°C (0.0016 mmHg). The octanol/water partition coefficient is measured to log Kow 0.12 (IUCLID 2000).
Classification
Caprolactam is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
| Xn;R20/22 | Harmful by inhalation and if swallowed. |
| Xi;R36/37/38 | Irritating to eyes, respiratory system and skin. |
Use
Caprolactam has several uses within the chemical industry in the production of other chemical compounds, polymers and in the production of paints and lacquers. The use as solvent in polymers may explain the presence.
Effects on health
Some data on acute toxicity have been available. Of these can be mentioned:
| Acute oral, rat | LD50 | 1210 mg/kg | HSDB 2004 |
| Acute oral, mouse | LD50 | 930 mg/kg | HSDB 2004 |
| Acute dermal, rat | LD50 | >2000 mg/kg | IUCLID 2000 |
| Acute inhalation, rat | LC50, 2 h | 300 mg/m3 | HSDB 2004 |
| Acute inhalation, mouse | LC50, 2 h | 450 mg/m3 | HSDB 2004 |
Of studies using prolonged exposure duration some references have been found. Only two studies on exposure via inhalation and dietary toxicity with the lowest levels are mentioned.
In a 13 weeks inhalation study rats were exposed to 0, 23, 66 and 244 mg/m3, 6 hours/day, and 5 days/week. A NOEL was set to 70 mg/m3 for the upper respiratory tract and a NOEL of 243 mg/m3 for the lower respiratory tract, systemic and neurotoxic effect (HSDB 2004).
In an inhalation study rats were exposed via inhalation to 0.06, 0.6 and 6 mg/m3 caprolactam in the breathing air for 24 hours/day over 82 days. At the highest concentration was observed reduced body weight gain and changes in clinical-chemical parameters. Similar observations but to a lesser extent were also observed at 0.6 mg/m3. NOAEL was set to 0.06 mg/m3 (IUCLID 2000).
In a 90 days study with oral administration, rats were dosed caprolactam at the dosages 0.05, 0.1, 0.25, 0.5 and 1% in the diet (41.7, 83.3, 208.3 and 833.3 mg/kg) for 90 days. Based on degeneration of the kidneys a NOAEL was set to 41.7 mg/kg bw/day (IUCLID 2000).
In a 3-generation reproduction study, rats were dosed with 0, 1000, 5000 or 10000 ppm in the diet. The body weight in offspring and parental animals was reduced at 5000 and 1000 ppm including certain effects on the kidneys. NOAEL was set to 1000 ppm in the diet corresponding to 50 mg/kg bw/day (Serota et al. 1984 in IRIS 2004).
Threshold limit values
The threshold limit value for the working environment is 2 ppm corresponding to 10 mg/m3 (AT 2002).
The C-value is 0.01 mg/m3 (Miljøstyrelsen 2002).
The oral RfD value is 0.5 mg/kg bw/day.
The value is based on a 3-generation rat study. The NOAEL of 50 mg/kg bw/day was divided with a safety factor of 100 (10 for interspecies and 10 for intraspecies variability).
Absorption
Indications on readily absorption of the substance are found but no values are available (IUCLID 2004). Therefore, 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 6. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 7 | 1100 | 0.0106 | 0.053 | 0.00053 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 7. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 7 | 1100 | 2.75 | 27.50 | 30.25 |
For the assessment is used the 3-generation reproduction study because the threshold for the most sensitive reproductive effect, reduced body weight of offspring, was clearly identified. In this study NOAEL for kidney effects in rats, another critical effect in the most sensitive species, was also 50 mg/kg bw/day.
Based on NOAEL of 50 mg/kg bw/day the margin of safety (MOS) for total exposure is: 50 / 0.03025 = 1650.
The distance to the RfD value is 0.5/0.03025 = 17.
Conclusion
The product's content of caprolactam is assessed not to pose a health risk to the consumer of the studied textile colorant.
3.3.3 1,4-Dichlorobenzene
Identification:
| Name | 1,4-Dichlorobenzene |
| CAS no. | 106-46-7 |
| EINECS no. | 203-400-5 |
| Molecular formula | C6H4Cl2 |
| Molecular structure | 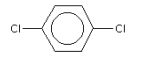 |
| Molecular weight | 147.0 g/mol |
| Synonym | p-Dichlorobenzene |
The melting point is 52.7°C. The boiling point is 174°C. The water solubility is 81.3 mg/l at 25°C (Yalkowsky and Dannenfelser 1992). The vapour pressure is 232 Pa at 25°C (1.74 mmHg). The octanol/water partition coefficient is measured to log Kow 3.44 (Hansch et al. 1995).
Classification
1,4-Dichlorobenzene is classified in the List of dangerous substances (Miljøministeriet 2002) with Xi;R39 and N;R50/53. By the addition of Carc.cat.3 in the 29th ATP (Directive 2004/73/EC, EC 2004) that will be implemented by the next revision of the Statutory Order on dangerous substances, the new classification is:
| Xi;R36 | Irritating to eyes. |
| Carc.Cat.3;R40 | 29th ATP addition: Limitd evidence of a carcinogenic effect. |
| N;R50/53 | Very toxic to aquatic organisms, may cause long-trem advrese effect in aquatic enviroment. |
Use
1,4-Dichlorobenzene is most known as air freshener in lavatories/rest rooms. The substance is used as carrier in textile dyes and in the production of colorants and pigments via the tranformation to 1,4-dichloro-2-nitrobenzene (ECB 2004a).
Effects on health
Some data on acute toxicity have been recovered. Of these are mentioned:
| Acute oral, rat | LD50 | 500 mg/kg | ECB 2004a |
| Acute oral, rat | LD50 | >2000 mg/kg | ECB 2004a |
| Acute oral, mouse | LD50 | 3000 mg/kg | ECB 2004a |
| Acute dermal, rat | LD50 | >2000 mg/kg | ECB 2004a |
| Acute inhalation, rat | LC50, 4 h | 5070 mg/m3 | ECB 2004a |
The acute toxicity thus appears low regardless of exposure route (ECB 2004a).
Irritation after inhalation that resulted in a 50% decrease of the respiration rate in mice (RD50) was 270 ppm for males and 245 ppm for females (Wilson 1990, ECB 2004a). The concentrations correspond to 1620 and 1470 mg/m3, respectively.
Of studies with prolonged exposure duration was found a 2-generation reproduction study where rats were exposed to 1,4-dichlorobenzene vapour at the concentrations of 0, 50, 150, or 450 ppm (0, 301, 902, 2705 mg/m3) for 10 weeks, 6 hours/day, 7 days/week, then the rats were mated. Next generation was exposed in the same way. Based on significant increase in liver weights of P1, parental males, NOAEC was set to 50 ppm corresponding to 300 mg/m3 (IRIS 2004).
In a two-generation oral study conducted on rats the substance was administered by gavage at 0, 30, 90, 270 mg/kg bw/day, 7 days/week. At 270 mg/kg bw/day was observed serious damages to kidneys. At 90 mg/kg/day, statistically significant (p < 0.05) reversible reduced mean body weight in foetuses was observed. Thus, the NOAEL for these developmental effects is set at 30 mg/kg/day (ECB 2004).
In a 1-year oral toxicity study on Beagle dogs were administered gelatine capsules with the substance at the doses of 0, 10, 50 and 150 mg/kg bw/day. In the liver was observed absolute and relative increase of the liver weight. NOAEL was set to 10 mg/kg bw/day (Naylor 1996 in ECB 2004a).
The carcinogenic potential of 1,4-dichlorobenzene has been demonstrated in mice which are of very high sensitivity towards hepatotoxic chemicals but the mechanism by which these hepatic tumours form, has not been clearly identified. A threshold mechanism for carcinogenicity of 1,4-dichlorobenzene is proposed in view of the liver tumours from the highest doses tested (oral and inhalation route in two species of mice). For carcinogenicity, a NOAEC of 75 ppm following inhalation exposure was obtained in mice (6 hours/day, 5 days/week for 13 weeks), equivalent to 13 ppm or 80 mg /m3, for continuous exposure. A NOAEL of 300 mg/kg/day was identified in mice following oral administration (ECB 2004).
With regard to mutagenicity, even if 1,4-dichlorobenzene has been investigated in a large number of in vitro and in vivo tests, data do not provide a coherent view of the genotoxicity of 1,4-dichlorobenzene. The so-called standard tests for genotoxicity do not suggest that 1,4-dichlorobenzene has any such potential; the evidence pointing in this direction comes from non-standard tests. The overall weight of evidence from the most reliable studies indicates that it does not have any significant genotoxic potential. According to the EU criteria for classification and labelling of dangerous substances, 1,4 dichlorobenzene is not considered as a genotoxic agent (ECB 2004).
Threshold limit values
The threshold limit value for the working environment is 10 ppm corresponding to 60 mg/m3 with notation K. K means that the substance is adopted on the list of substances considered to be carcinogenic (AT 2002).
The inhalation reference concentration value (RfC) is by US-EPA set to 0.8 mg/m3. The value is based on NOAEL of 300 mg/m3 recalculated from an exposure of 6 hours/day to 24 hours and applying a safety factor of 100 (IRIS 2004).
The TDI value is 107 μg/kg bw/day according to WHO (1993, 2004). The value is derived from a LOAEL of 150 mg/kg bw/day for kidney damages in a 2-year rat study with oral administration. A correction for 5 days/week and a safety factor of 1000 was applied (10 for interspecies, 10 for intraspecies variability and 10 for the use of LOAEL.
Absorption
The absorption of the substance after inhalation takes place readily. In rats were found that after inhalation they excrete 73% via urine and 2.5% via faeces and after oral administration 87% are excreted via urine and 2% via faeces. The dermal absorption is the lowest with 41% excreted via urine and 0.1% via faeces. The remaining amounts are assumed excreted via the exhaled air. The values vary to a large extent though depending on animal species and whether the exposure is repeated. Therefore 100% absorption is assumed in this assessment.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 8. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 1 | 9.9 | 0.136 | 0.681 | 0.0068 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 9. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 9.9 | 0.025 | 0.25 | 0.28 |
General systemic repeated-dose toxicity, carcinogenicity and the developmental toxicity are the critical end points for humans. As 1,4-dichlorobenzene is considered to be a non-genotoxic carcinogen, a threshold approach is appropriate.
The lowest and relevant NOAEL of 10 mg/kg/day was found in a chronic oral study on dogs with the critical effects on liver (significant increase of liver weight).
Based on NOAEL of 10 mg/kg bw/day the margin of safety (MOS) for total exposure is: 10 / 0.00028 = 35710.
The distance to the TDI value is a factor of 107/0.28 = 382.
Conclusion
The product's content of 1,4-dichlorobenzene is assessed not to pose an immediate health risk to the consumer of the studied textile colorant.
It should be noted that 1,4-dichlorobenzene is classified carcinogenic (category 3; R40 Limited evidence of carcinogenic effect). The assessment performed in the EU risk assessment report indicates a threshold to carcinogenic effects. Thus at the determined levels the substance may not be of significance but the manufacturer and the consumer perhaps should consider alternatives.
3.3.4 Diethylene glycol
Identification:
| Name | Diethylene glycol |
| CAS no. | 111-46-6 |
| EINECS no. | 203-872-2 |
| Molecular formula | C4H10O3 |
| Molecular structure | |
| Molecular weight | 106.12 g/mol |
| Synonyms | 2,2'-Oxydiethanol |
| 2,2' –Oxybisethanol | |
| 2,2' –Dihydroxyethylether | |
| 3-Oxapentane-1,5-diol |
The melting point is -8°C. The boiling point is 245°C. The water solubility is high (miscible with water at 25°C). The vapour pressure is 1.04 Pa at 25°C (Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow 1 (IUCLID 2000).
Classification
Diethylene glycol is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
| Xn;R22 | Harmful if swallowed |
Use
Among several uses the substance is used also as solvent in colorants and adhesives.
Effects on health
Some data on acute toxicity has been available. Of these can be mentioned:
| Acute oral, rat | LD50 | 15600 mg/kg | Clayton and Clayton 1982 |
| Acute oral, mouse | LD50 | 13000 mg/kg | Clayton and Clayton 1982 |
| Acute dermal, rabbit | LD50 | 11890 mg/kg | IUCLID 2000 |
| Acute inhalation, rat | LCLO, 4 h | 4500 mg/m3 | IUCLID 2000 |
| Acute inhalation, mouse | LCLO, 2 h | 130 mg/m3 | IUCLID 2000 |
Studies with acute exposure indicate a low acute toxicity (IUCLID 2000).
Of studies with prolonged exposure duration some are available but most are of older date or only available as insufficient references in RTECS.
For exposure via inhalation a study is found where rats were exposed 5 days/week for 6 months. LOAEL was 0.02 - 0.03 mg/l corresponding to 20 - 30 mg/m3 (IUCLID 2000).
In a 90 days oral study, rats were administered daily the substance in the drinking water at the doses 0, 200, 700, and 8000 mg/kg/day. Based on effects on the kidney a NOAEL was set to 200 mg/kg bw/day (Freundt and Weis 1989).
In a 225 days oral study, rats were administered daily the substance in the diet at the doses 0, 0.085, 0.17, 0.4 or 2% corresponding to 0, 64, 128, 300 or 1500 mg/kg bw). Based on functional disorders of the kidneys a NOAEL was set to 64 mg/kg bw/day (IUCLID 2000).
References on several poisonings of humans in 1937 with more than 100 deaths from the use of the substance as solvent in a sulphanilamide "elixir" (pharmaceutical preparation). The mixture contained 72% diethylene glycol. Pathological examination showed effects on kidneys and to a lesser extent on liver. The lowest lethal dosis (LDLO) was set to 1 ml/kg or approx. 1000 mg/kg bw/day (IUCLID 2000).
Threshold limit values
The threshold limit for the working environment is 2.5 ppm corresponding to 11 mg/m3 (AT 2002).
Absorption
No values on absorption were available. Therefore, 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 10. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 1 | 53000 | 1.725 | 8.627 | 0.086 |
| 5 | 6200 | 0.202 | 1.009 | 0.010 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 11. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 53000 | 132.5 | 1325 | 1457 |
| 5 | 6200 | 15.5 | 155 | 170 |
The critical effect is functional disorders of the kidneys. The lowest available NOAEL for this effects is therefore used in the assessment.
Based on a NOAEL of 64 mg/kg bw/day the margin of safety (MOS) for total exposure is: 64 / 1.475 = 43.
Conclusion
The products' content of diethylene glycol are assessed not to pose a health risk to the consumer of the studied textile colorants.
3.3.5 Diisopropylene glycol
Identification:
| Name | Diisopropylene glycol |
| CAS no. | 110-98-5 |
| EINECS no. | 203-821-4 |
| Molecular formula | C6H14O3 |
| Molecular structure | 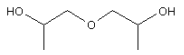 |
| Molecular weight | 134.18 g/mol |
| Synonyms | 1,1'-oxydipropan-2-ol (EINECS name) |
| 1,1'-oxydi-2-propanol (CA name) | |
| Dipropylene glycol |
The melting point is 6°C. The boiling point is 230°C. The water solubility is high (miscible with water at 25°C). The vapour pressure is 4.3 Pa at 25°C (0.032 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is estimated to log Kow -0,64 (EPI).
Classification
Dipropylene glycol is not classified in the List of dangerous substances (Miljøministeriet 2002).
Use
The substance is used as solvent and plasticiser in resins and printing inks (HSDB 2004).
Effects on health
Only few data on acute toxicity were available. Of these are mentioned:
| Acute oral, rat | LD50 | 14800 mg/kg | HSDB 2004 |
| Acute inhalation, rat | LC50, 8 h | >6000 mg/m3 * | IUCLID 2000 |
| Acute dermal, rabbit | LD50 | >20600 mg/kg | IUCLID 2000 |
*: Saturated air, aerosol. None out of 6 animals died.
Dipropylene glycol is observed to be slightly irritating to skin (IUCLID 2000).
Very few useful data is found available on acute and chronic exposure of test animals (IUCLID 2000). The data available are often insufficiently described.
Of tests a study was found where rats were exposed orally via drinking water containing 5% dipropylene glycol (approx. 3100 mg/kg bw/day) for 77 days without any effects were observed. At 10% few animals died. In these degeneration of the kidney epithelium and liver parenchyma were observed (IUCLID 2000).
In a teratogenicity study, rats were exposed orally via gavage at the doses 800, 2000, and 5000 mg/kg/day in days 6 to 15 of the gestation period. Toxic effects and deaths were observed in the maternal animals at 2000 and 5000 mg/kg bw/day (mortality rate 4% and 9%, respectively). A NOAEL was set for maternal toxicity to 800 mg/kg bw/day. No differences between exposed foetuses and control animals were observed (NTP 1992, ref. in IUCLID 2000).
Sufficient data to establish the dosis-response relationship for dipropylene glycol were not available. Data to identify the critical effect are not available though doses close to lethal dose have effects on the central nervous system and the kidney epithelium (Lundberg 1993).
Threshold limit values
No threshold limit values for the working environment were found for Denmark or other countries.
Absorption
No values for absorption were available and, therefore, 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 12. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 1 | 2300 | 0.536 | 2.68 | 0.027 |
| 5 | 5000 | 1.164 | 5.82 | 0.058 |
| 6 | 3100 | 0.722 | 3.61 | 0.048 |
| 7 | 5100 | 1.188 | 5.94 | 0.036 |
| 9 | 390 | 0.091 | 0.45 | 0.059 |
| 12 | 4900 | 1.141 | 5.71 | 0.057 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 13. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake, μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 2300 | 5.7 | 57.5 | 63.3 |
| 5 | 5000 | 12.5 | 125.0 | 137.6 |
| 6 | 3100 | 7.8 | 77.5 | 85.3 |
| 7 | 5100 | 12.7 | 127.5 | 140.3 |
| 9 | 390 | 0.98 | 9.8 | 10.7 |
| 12 | 4900 | 12.3 | 122.5 | 134.8 |
No NOEL values after repeated exposure studies were available. Based on the apparent low toxicity of the substance NOEL may be expected to be around the gram/kg bw/day level. The assessment is therefore based on the analogous glycol: diethylene glycol, which appears to have a similar acute toxicity and long-term effects on the kidneys. The assessment is thus based on the same NOAEL of 64 mg/kg bw/day. The margin of safety (MOS) for total exposure is then: 64 / 0.140 = 456.
Conclusion
The products' content of diisopropylene glycol is assessed not to pose a health risk to the consumer of the studied textile colorants.
3.3.6 1,4-Dioxane
Identification:
| Name | 1,4-Dioxane |
| CAS no. | 123-91-1 |
| EINECS no. | 204-661-8 |
| Molecular formula | C4H8O2 |
| Molecular structure | 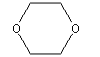 |
| Molecular weight | 88.11 g/mol |
| Synonyms | 1,4-dioxacyclohexane |
| Diethylene ether | |
| Glycolethylene ether |
The melting point is 11.8°C. The boiling point is 101.5°C. The water solubility is high (miscible with water at 25°C, Riddick et al. 1986). The vapour pressure is 5080 Pa at 25°C (38.1 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow -0.27 (Hansch et al. 1995).
Classification
1,4-Dioxane is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
| F;R11-19 | Highly flammable. May form explosive peroxides. |
| Xi;R36/37 | Irritating to eyes and respiratory system |
| Carc3;R40 | Limited evidence of carcinogenic effects |
| R66 | Repeated exposure may cause skin dryness or cracking. |
Use
1,4-Dioxane has many uses but in this context it is used as solvent in colorants and adhesives (ECB 2002).
Effects on health
Some data on acute toxicity are available. Of these are mentioned:
| Acute oral, rat | LD50 | 5170 mg/kg | ECB 2002 |
| Acute oral, mouse | LD50 | 5850 mg/kg | ECB 2002 |
| Acute dermal, rabbit | LD50 | 7855 mg/kg | RTECS 1995 |
| Acute inhalation, rat | LC50, 2 h | 46000 mg/m3 | ECB 2002 |
| Acute inhalation, mouse | LC50, 2 h | 37000 mg/m3 | ECB 2002 |
Following acute oral administration to different mammals were observed narcotic effects, coma, irritation of gastro-intestinal mucous membranes and damages to liver and kidneys (ECB 2002).
By dermal exposure similar effects are observed, i.e. the substance can be absorbed via the skin (ECB 2002).
In a 2-year rat toxicity study the rats were exposed to a concentration in the breathing air of 400 mg 1,4-dioxane vapour/m3 for 7 hours/day, 5 days/week for 104 weeks. Based on 100% absorption, 240 ml inhaled air, a body weight of 400 g and 7 hours exposure/day an exposure of 108 mg/kg bw/day was calculated. No clinical effects, effects on body weight or mortality were observed. NOAEL for toxic effects was set thus to 400 mg/m3 (Torkelson et al. 1974).
Of studies with prolonged exposure duration, several are mentioned in the EU risk assessment report (ECB 2002). Common to the studies on oral exposure via the drinking water of rats and mice for 2 to 13 weeks are a description of serious effects on liver, kidney and nose (liver and nasal tumours and organ damage) with a LOAEL of 16 mg/kg bw/day and NOAEL of 10 mg/kg bw/day.
Despite the fact that the substance is a carcinogen in two species (rats and mice), with some indication for a third species (guinea pigs), the substance is a low potent carcinogen and the available data indicate a non-genotoxic mechanism. For both liver and nasal tumours, cytotoxic effects and organ damage are considered to be involved, which are subject to non-linear kinetics, implicating a threshold (ECB 2002).
In studies on the carcinogenicity were observed that 1,4-dioxane showed inadequate evidence for the carcinogenicity in humans but sufficient evidence for the carcinogenicity in experimental animals. The conclusion was that dioxane was placed in class 2B, i.e. possibly carcinogenic to humans (IARC 1999).
Threshold limit values
The threshold limit value for the working environment is 30 ppm corresponding to 36 mg/m3 with notations HK. L indicates that the substance may penetrate the skin. K means that the substance is adopted on the list on substances that is considered carcinogenic (AT 2002).
The C-value is 0.01 – 0.1 mg/m3 (Miljøstyrelsen 2002).
The TDI value is 16 μg/kg bw/day according to WHO (2004). The value is based on a NOAEL of 16 mg/kg bw/day for hepatocellular tumours observed in a long-term study where rats were exposed via the drinking water. A safety factor of 1000 is used (100 for inter and intraspecies differences and 10 for non-genotoxic carcinogenicity (WHO 2004).
Absorption
Radioactive labelled 1,4-dioxane is absorbed readily and almost completely after oral administration and exposure via inhalation in rats. Dermal absorption is less, especially since the substance is volatile. In the EU risk assessment is used 100% absorption for oral intake or exposure via inhalation and 50% by dermal exposure (ECB 2002).
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 14. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 1 | 11 | 0.0020 | 0.010 | 0.000098 |
| 9 | 4.7 | 0.0008 | 0.004 | 0.000042 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 15. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake, μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 11 | 0.028 | 0.275 | 0.303 |
| 9 | 4.7 | 0.012 | 0.118 | 0.129 |
The starting point for the risk assessment are the exposure estimates and the overall NOAEL for oral repeated exposure of 10 mg/kg bw/day from the 2-year drinking water study in rats. As 1,4-dioxane is considered to be a non-genotoxic carcinogen, a threshold approach is appropriate.
Based on a NOAEL of 10 mg/kg bw/day the margin of safety (MOS) for total exposure is: 10 / 0.00030 = 33000.
Conclusion
The products' content of 1,4-dioxane is assessed not to pose an immediate health risk to the consumer of the examined textile colorants.
Based on all data it can be concluded that 1,4-dioxane is irritating to the eye and the respiratory tract, but not to the skin. However, being a fat solvent, 1,4-dioxane can cause eczema upon prolonged or repeated contact.
It should also be noted that 1,4-dioxane is classified carcinogenic (category 3;R40 Limited evidence of carcinogenic effect). The assessment performed in the EU risk assessment report indicates a threshold to carcinogenic effects. The report also states that 1,4-dioxane has tumour promoter but not initiator properties. Thus at the determined levels the substance may not be of significance but the manufacturer and the consumer perhaps should consider alternatives.
3.3.7 Glycerine
Identification:
| Name | Glycerine |
| CAS no. | 56-81-5 |
| EINECS no. | 200-289-5 |
| Molecular formula | C3H8O3 |
| Molecular structure | 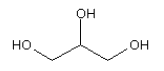 |
| Molecular weight | 92.09 g/mol |
| Synonyms | Glycerol |
| 1,2,3-Propanetriol | |
| 1,2,3-Trihydroxypropane |
The melting point is 18.2°C. The boiling point is 290°C. The water solubility is high (miscible with water at 25°C). The vapour pressure is 0.022 Pa at 25°C (1.68x10-4 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow –1.76 (Hansch et al. 1995).
Classification
Glycerine is not classified in the List of dangerous substances (Miljøministeriet 2002).
Use
Glycerine is used as solvent, thickener and softener in a series of products, among others printing inks. Glycerine may also be used as humectant i.e. retains the water and thus increase the drying period and provide time for curing of the colorant.
Effects on health
Few data on acute toxicity are available. Of these are mentioned:
| Acute oral, rat | LD50 | 12600 mg/kg | IUCLID 2000 |
| Acute oral, mouse | LD50 | 4090 mg/kg | IUCLID 2000 |
| Acute dermal, rat | LD50, >0.3 h | >21900 mg/kg | IUCLID 2000 |
Based on the few available data glycerine is not acute toxic.
Of studies with prolonged exposure duration a 13 weeks rat study is found where rats were exposed via inhalation for 6 hours/day, 5 days/week at the doses 0, 0.033, 0.167, 0.662 mg/l air. Based on damages to the nose epithelium a NOAEL was set to 0.167 mg/l air corresponding to 167 mg/m3 (Renne et al. 1992).
Of oral studies a study was found where rats were exposed to diet containing concentrations of 0, 1, 3, 6, 10, 15, 20, 30, 40, 50 or 60% (corresponding to 0, 1000, 3000, 6000, 10 000, 15 000, 20 000, 30 000, 40 000, 50 000, or 60 000 mg/kg bw/day) for 20 weeks. A reduced body weight gain at doses above 40% glycerine in the diet and tissue pathological changes in liver cells at doses above 10% were observed. NOEL was set to 5% glycerine in the diet corresponding to 5000 mg/kg bw/day (Guerrant et al. 1947).
In a 2-year study where rats were administered glycerine in the diet at concentrations of 0, 5, 10 or 20% (corresponding to 0, 2500, 5000 or 10 000 mg/kg bw/day) no significant changes in body weight or histopathological changes were observed. NOEL was thus 10 000 mg/kg bw/day (JECFA 2001).
Threshold limit values
No threshold limit value for the working environment is available for Denmark (AT 2002).
For glycerine mist a threshold limit value for 8 hours time weighted average (TLV – TWA) of 10 mg/m3 was found (ACGIH 2002).
The ADI value is not specified because the Joint FAO/WHO Expert Committee on Food Additives found that due to the low toxicity it was not necessary (JECFA 2001).
Absorption
Following oral administration glycerine is readily absorbed from the gastro-intestinal tract. The main part is distributed to the body fat and only 7-14% is excreted unchanged in the urine (HSDB 2004). Therefore, 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 16. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 5 | 11000 | 0.009 | 0.045 | 0.00045 |
| 6 | 27000 | 0.022 | 0.110 | 0.00110 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 17. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake, μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 5 | 11000 | 27.5 | 275 | 302.5 |
| 6 | 27000 | 67.5 | 675 | 742.5 |
Toxic effects appear to take place at high doses and thus the lowest available NOAEL is used in the assessment.
By a comparison to NOAEL 5000 mg/kg bw/day the margin of safety (MOS) for total uptake is: 5000/0.7425 = >6730.
Conclusion
The products' content of glycerine is assessed not to pose a health risk to the consumer of the examined textile colorants.
3.3.8 Hexamethylentetramine (methenamine)
Identification:
| Name | Hexamethylentetramine (= methenamine) |
| CAS no. | 100-97-0 |
| EINECS no. | 202-905-8 |
| Molecular formula | C6H12N4 |
| Molecular structure | 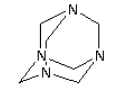 |
| Molecular weight | 140.19 g/mol |
| Synonyms | Methenamine (EINECS name) |
| 1,3,5,7-Tetraazatricyclo-3.1.1.13,7-decane (IUPAC name) |
The melting point is >250°C. The boiling point is unknown. The water solubility is 667000 mg/l at 25°C (Merck Index). The vapour pressure is 0.05 Pa at 25°C (0.004 mmHg). The octanol/water partition coefficient is estimated to log Kow –4.15 (EU RAR draft 2005, ECB 2005).
Classification
Methenamine is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
| F;R11 | Highly flammable. |
| R42/43 | May cause sensitization by inhalation and skin contact. |
Use
Methenamine is added as curative agent in resins used as binder in different products among other textile colorants. Methenamine is also used as preservative in cosmetics.
Effects on health
Methenamine is in the process of EU risk assessment but the report is not finalised (ECB 2005). Germany is rapporteur country.
Only a few data on acute toxicity have been available. Of these are mentioned:
| Acute oral, rat | LD50 | >10000 mg/kg | Della Porta 1966, ECB 2005 |
| Acute dermal, rat | LD50 | >2000 mg/kg | ECB 2005 |
Based on the few data methenamine is not acute toxic by oral or dermal exposure.
Of studies with prolonged exposure duration several studies are available using oral administration of the substance via the diet or drinking water. Almost all studies, which last from 2 to 104 weeks, use 1 dose for the duration (limit tests). Apparently the NOAEL is about 5000 mg/kg bw/day and the highest NOAEL for systemic effects is 2500 mg/kg bw/day from a 60 weeks study on mice with exposure via drinking water (Della Porta 1968).
The developmental toxicity of methenamine was studied in dogs. Methenamine was given at dietary levels of 600 or 1250 ppm (corresponding to doses of 15 and 31 mg/kg bw/day) at days 4 to 54 after mating. Growth retardation and higher mortality was observed in the high dosage group. The NOAEL was set to 15 mg/kg bw/day (ECB 2005).
In humans where methenamine has been used in the treatment of urinary tract inflammation is observed that a dose of 2-4 g/day over 3 to 4 weeks does not affect the patient while 8 g/day resulted in side-effects such as bladder irritation and blood in the urine. Based on a body weight of 70 kg this corresponds to a LOAEL of 57 mg/kg bw/day and NOAEL 27 mg/kg bw/day. This value is used in risk characterisation in the EU risk assessment report (ECB 2005).
In a dermal study where 1.3 mg/kg bw/day was applied to the skin of rabbits 5 days/week for 6 weeks no visible effects were observed (ECB 2005).
The substance is shown to be skin sensitising to humans. Allergic symptoms such as asthma are observed by exposure though with simultaneously exposure to other irritating and sensitising substances (ECB 2005).
Because no data on effects by exposure via inhalation a recalculation has been performed based on the NOAEL value for oral administration of 57 mg/kg bw/day. The absorption in both exposure routes is assumed to be 100% and the respiration rate 20 m3/dag. NOAEC for inhalation then becomes: 57/20 = approx. 3 mg/m3.
Threshold limit values
No threshold limit values for the working environment is available (AT 2002).
The ADI value is set to 0.15 mg/kg bw by WHO (1974).
According to the Statutory Order on cosmetics the maximum allowed concentration of methenamine in cosmetic products is 0.15%.
Absorption
Methenamine is readily absorbed after oral intake and is distributed to the whole body. The substance may penetrate the placenta and has been detected in mother's milk. No values are available for absorption through the skin or after inhalation (ECB 2005). Therefore, 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 18. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 4 | 49 | 0.00014 | 0.00069 | 0.0000069 |
| 5 | 800 | 0.0023 | 0.01132 | 0.0001132 |
| 7 | 11 | 0.000031 | 0.00016 | 0.0000016 |
| 9 | 22 | 0.000062 | 0.00031 | 0.0000031 |
| 10 | 570 | 0.0016 | 0.00806 | 0.0000806 |
| 12 | 860 | 0.0024 | 0.01217 | 0.0001217 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 19. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 4 | 49 | 0.123 | 1.23 | 1.35 |
| 5 | 800 | 2.000 | 20.0 | 22.00 |
| 7 | 11 | 0.028 | 0.28 | 0.30 |
| 9 | 22 | 0.055 | 0.55 | 0.61 |
| 10 | 570 | 1.425 | 14.3 | 15.68 |
| 12 | 860 | 2.150 | 21.5 | 23.65 |
As human data exist and are considered more reliable than the study on dogs the human NOAEL of 27 mg/kg bw/day are used in the assessment.
Based on a NOAEL of 27 mg/kg bw/day The margin of safety (MOS) for total uptake is: 27 / 0.024 = 1125.
The ADI value of 0.15 mg/kg bw/day is not exceeded. The distance is at least 6.
The maximum concentration of methenamine allowed in cosmetic products according to the Statutory Order on cosmetics of 0.15% (1500 mg/kg) is not exceeded.
Conclusion
The products' content of methenamine is assessed not to pose a health risk to the consumers of the examined textile colorants.
However, it should be noted that methenamine has demonstrated skin sensitising properties in humans and may cause sensitisation by inhalation.
3.3.9 Isobutane
Identification:
| Name | Isobutane |
| CAS no. | 75-28-5 |
| EINECS no. | 200-857-2 |
| Molecular formula | C4H10 |
| Molecular structure | 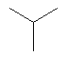 |
| Molecular weight | 58.12 g/mol |
| Synonyms | 2-Methyl-propane |
| 1,1-Dimethylethane | |
| Trimethylmethane |
The melting point is –138.3°C. The boiling point is –11.7°C. The water solubility is 48.8 mg/l at 25°C (Riddick et al. 1986). The vapour pressure is 348000 Pa at 25°C (2610 mmHg). The octanol/water partition coefficient is measured to log Kow 2.76 (Hansch et al. 1995).
Classification
Isobutane is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
| Fx;R12 | Extremely flammable |
| if >= 0.1% 1,3-butadien (CAS no. 106-99-0, EINECS no. 203-450-8, then also: | |
| Carc.cat.1;R45 | May cause cancer |
| Mut.cat.2;R46 | May cause heritable genetic damage |
NB: 1,3 butadiene was not detected in the analysis.
Use
Isobutane is a gas detected in the 2 "pop-up" textile colorants where the substance is used as blowing agent of the colour when heated.
Effects on health
Few data on acute toxicity are available. Sine the substance is a gas the data only comes from studies with exposure via inhalation. Of these are mentioned:
| Acute inhalation, rat | LC50, 15 min. | 570000 ppm, 1375000 mg/m3 | IUCLID 2000 |
| Acute inhalation, rat | LC50, 4 h | 658000 mg/m3 | IUCLID 2000 |
Acute exposure primary takes place via inhalation since the substance is very volatile. The mortalities recorded during the inhalation studies happened during and not after the studies. The test animals that survived the studies appeared normal within 10 minutes. The effects are caused by stimulation of the central nervous system (IUCLID 2000).
Of studies with prolonged exposure duration a 90 days rat study was available where the rats were exposed via inhalation for 6 hours/day, 5 days/week to 0, 1000 or 5000 ppm with a gas mixture comprising 50% butane and 50% pentane. At the highest dose no effects on kidneys or body weight were observed, and no effects were evident from haematological or biochemical parameters or from histopathology. Of clinical observations were noted hunched posture, lethargy and intermittent tremor (IUCLID 2000).
Threshold limit values
A threshold limit value for the working environment is not available for Denmark but matching values from other countries are found. For instance for Germany was found a MAK(Maksimaler Arbeitsplatz Konzentration) value of 2350 mg/m3, for USA a TLV (TWA) value of 1780 mg/m3 and for United Kingdom an OES (Occupational Exposure Standard) value of 1430 mg/m3 (IUCLID 2000).
Absorption
No values on absorption are available and, therefore, 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 20. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 4 | 3500 | 28572 | 142865 | 1428 |
| 5 | 2000 | 16327 | 81637 | 816 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 21. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake, μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 4 | 49 | 8.75 | 87.5 | 1525 |
| 5 | 800 | 5.0 | 50.0 | 871 |
Isobutane is a gas. The estimates also indicate that the primary exposure takes place via inhalation with a maximum exposure in the breathing zone of 143 mg/m3. The estimated concentration is approx. 1/10 of the lowest available value for the working environment of 1430 mg/m3. This distance seems to be small when addressing exposure at home and not at work.
An attempt to recalculate to oral exposure based on the lowest found threshold limit value for the working environment resulted in: 1430 10/20 m3 5/7 days = 510 (mg/m3/day) / 70 kg = 7.3 mg/kg bw/day. The margin of safety is then 7.3/1.5 = 4.8, which is considered low. On the other hand, the value of the used safety factor is unknown
Conclusion
The products' content of isobutane though is assessed not to pose an immediate health risk to the consumer of the examined textile colorants as the exposure is presumed to be of a short duration. However, aeration or forced ventilation should be recommended when using the products.
1,3-Butadiene was not detected in the analysis and thus the classifications as carcinogenic and mutagenic is not warranted for the analysed products.
3.3.10 3-Isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate
Identification:
| Name | 3-Isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate |
| CAS no. | 4098-71-9 |
| EINECS no. | 223-861-6 |
| Molecular formula | C12H18N2O2 |
| Molecular structure | 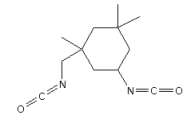 |
| Molecular weight | 222.29 g/mol |
| Synonyms | 5-isocyanato-1-(isocyanatomethyl)-1,3,3-trimethyl-cyclohexane |
| Isophorone diamine diisocyanate | |
| Isophoronediisocyanate | |
| IPDI |
The melting point is -60°C. The boiling point is 158°C. The water solubility is estimated to 3 mg/l at 25°C (EPI). The vapour pressure is 0.04 Pa at 20°C (0.0003 mmHg, Lewis 1997, HSDB). The octanol/water partition coefficient is estimated to log Kow 4.75 (EPI).
Classification
3-Isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate (IPDI) is adopted in the List of dangerous substances (Miljøministeriet 2002) and classified:
| T;R23 | Toxic by inhalation. |
| Xi;R36/37/38 | Irritating to eyes, respiratory system and skin |
| R42/43 | May cause sensitization by inhalation and skin contact |
| N;R51/53 | Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment |
The classification is depending on the concentration in the product (cf. table 3).
Use
IPDI yields polyurethanes with high stability, resistance to light discoloration and chemical resistance (HSDB). IPDI is used in colorants as hardener, binding agent and adhesive.
Effects on health
Some data on acute toxicity have been recovered. Of these are mentioned:
| Acute oral, rat | LD50 | 1270 mg/kg | IUCLID 2000 |
| Acute oral, mouse | LD50 | >2500 mg/kg | IUCLID 2000 |
| Acute dermal, rat | LD50 | 1060 mg/kg | HSDB |
| Acute inhalation, rat | LC50, 4 h | 123 mg/m3 | IUCLID 2000 |
The acute oral toxicity thus appears low but IPDI is toxic by skin absorption.
IPDI is a severe irritant, irritating to eyes, skin and the respiratory system.
Irritation after inhalation for 1 hour that resulted in a 50% decrease of the respiration rate in mice (RD50) was 1.2 ppm corresponding to 0.01 mg/m3 (IUCLID 2000).
Of studies with prolonged exposure duration was found a repeated dose toxicity study where rats were exposed to IPDI via inhalation at the concentrations of 0, 0.25, 0.64 or 1.37 mg/m3 for 4 hours/day, 5 days/week over 28 days. At the highest dose group was observed reduced weight gain and slightly oedematous lungs. At the other dose groups no symptoms and no pathological findings were observed. Thus, NOAEL was set to 0.64 mg/m3. In a similar study using 0, 0.525, 0.84, 3.57 and 33 mg/m3 a NOAEL 0.525 mg/m3 was found, but without reported effects except mortality at the 2 highest concentrations: 1 and 4 out of 20 animals/group, respectively (IUCLID).
IPDI is shown to provoke allergic dermatitis in human. One hour exposure to IPDI caused eczema in 3 of 4 workers of which only 1 had pervious contact to the substance. The others had previous contact to toluene diisocyanate and methyldiisocyanate, suggesting cross-sensitisation (ACGIH 1991 in HSDB)
Several studies on test animals and humans have confirmed the sensitisation potential of IPDI. One of the studies indicates a threshold to be present (cf. below).
The allergic contact hypersensitivity sensitising potential of IPDI was studied in female mice. The mice were sensitised with 0.1, 0.3, and 1.0% IPDI and challenged with 3.0% IPDI. Doses of IPDI were selected from assays for primary irritancy. Mice received 20 microliters by direct dermal application for 5 days to sites prepared by shaving, dermabrading, and in some cases, with intradermal injection of Freund's complete adjuvant (FCA). The rest period was 7 days. Measurement of contact hypersensitivity response in mice was by radioisotopic assay. A statistically significant hypersensitivity response was elicited in mice using a sensitising concentration of 1.0% and a challenge concentration of 3.0%, with or without pretreatment with Freund's complete adjuvant (NTP 1990).
Very little direct information on the health effects associated with exposure to IPDI has been available, except for numerous studies on exposure via inhalation causing irritation of the respiratory tract and decreases in pulmonary function, and dermal exposure causing skin sensitisation (allergy, eczema).
The isocyanates: methylenediphenyl diisocyanate (several CAS nos.) and 1,3-diisocyanatomethylbenzene (toluenediisocyanate) (several CAS nos.) are included on the List of undesirable substances (Miljøstyrelsen 2004b).
Threshold limit values
The threshold limit value for the working environment is 0.005 ppm corresponding to 0.045 mg/m3 with skin notation H. H means that the substance may penetrate the skin (AT 2002).
Absorption
No information was available on absorption. Therefore 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 22. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 7 | 270 | 0.0010 | 0.0048 | 0.000048 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 23. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 7 | 270 | 0.675 | 6.75 | 7.43 |
Because no NOAEL value on repeated exposures by oral administration was available a transformation of the inhalation data is performed:
NOAEL (in mg/kg/d) = NOAEL inh (mg/m3) x 1/bw (kg) x inhalation rate (m3/d).
Using rat body weight 0.3 kg and inhalation rate, rat = 0.0144 m3/h then:
NOAEL = 0.0525 x 1/0.3 x 0.0144 x 24/4 (h) x 7/5 (d) =0.021 mg/kg/day.
Based on the estimated NOAEL of 0.021 mg/kg bw/day the margin of safety (MOS) for total exposure is: 0.021 / 0.00743 = 2.8.
Conclusion
The product's content of IPDI is assessed not to pose an immediate health risk to the consumer of the studied textile colorant.
However, the MOS is low indicating a potential health concern and prolonged exposure should be avoided with products containing this substance. It should be noted also that the substance may cause sensitisation by skin contact and may cause problems by inhalation. Heating of the colorants should be performed under ventilation.
3.3.11 N-Methyl-2-pyrrolidone
Identification:
| Name | N-Methyl-2-pyrrolidone |
| CAS no. | 872-50-4 |
| EINECS no. | 212-828-1 |
| Molecular formula | C5H9NO |
| Molecular structure | 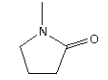 |
| Molecular weight | 99.13 g/mol |
| Synonyms | N-Methylpyrrolidinone |
| 1-Methyl-2-pyrrolidone |
The melting point is -24°C. The boiling point is 202°C. The water solubility is high at 25°C (miscible with water). The vapour pressure is 25.3 Pa at 25°C (Riddick et al. 1986). The octanol/water partition coefficient is measured to log Kow –0.46 (IUCLID 2000).
Classification
N-Methyl-2-pyrrolidone is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
| Xi;R36/38 | Irritating to eyes and skin. |
Use
The substance has several uses within the chemical industry among others in paints, dyes and lacquers. The substance is used as solvent and in the production of pigment and printing inks (IUCLID 2000, CICAD 2001).
Effects on health
N-Methyl-2-pyrrolidone has a low acute toxicity.
Some data on acute toxicity have been found. Of those are mentioned:
| Acute oral, rat | LD50 | 3084 mg/kg | IUCLID 2000 |
| Acute oral, mouse | LD50 | 4050 mg/kg | IUCLID 2000 |
| Acute dermal, rat | LD50 | 7000 mg/kg | IUCLID 2000 |
| Acute inhalation, rat | LC50, 4 h | 5100 mg/m3 | IUCLID 2000 |
Uptake of acute toxic doses by oral intake, dermal exposure or exposure via inhalation causes functional disorders and affects the central nervous system. Irritations of the respiratory system by exposure via inhalation and of the gastro-intestinal tract after oral administration have been observed (IUCLID 2000, CICAD 2001).
N-Methyl-2-pyrrolidone has a potential for skin irritation and eye irritation in rabbits. The effects are dependent of the concentration (IUCLID 2000).
In a 13 week study with exposure via inhalation, rats were exposed to 0, 500, 1000 and 3000 mg/m3 for 6 hours/day, 5 days/week. Nasal irritation and crust formations on nasal edges were observed at 1000 mg/m3. At 3000 mg/m3, decreased body weight and decreased relative weight of testis were observed. The NOAEL was then set to 500 mg/m3 (CICAD 2001).
Of studies with prolonged duration of oral exposure, a 28-day study was found where rats were orally administered (via gavage) the doses 0, 257, 514, 1028 and 2060 mg/kg bw/day. A dose-dependent increase in relative liver and kidney weights was observed at 1028 mg/kg bw/day. Thus, the NOAEL was 514 mg/kg bw/day (CICAD 2001).
In a 90-day study, rats were administered the substance in the diet at the doses 0, 3000, 7500, and 18 000 mg/kg diet/day corresponding to 0, 169, 433 and 1057 mg/kg bw/day in males and 0, 217, 565 and 1344 mg/kg bw/day in females. A dose-related decrease in body weight and effects on the central nervous system was observed at 433 and 565 mg/kg bw/day in males and females, respectively. The NOAEL was 169 mg/kg bw/day in males and 217 mg/kg bw/day in females (CICAD 2001).
Threshold limit values
The threshold limit value for the working environment is 5 ppm corresponding to 20 mg/m3 (AT 2002).
The C-value is 0.5 mg/m3 (Miljøstyrelsen 2002).
The TDI value is 0.6 mg/kg bw/day. The value is derived from the 90-day oral study with a NOAEL of 169 mg/kg bw/day divided with a safety factor of 300 (10 for interspecies and 10 for intraspecies variation and 3 for adjusting from a 90 day subchronic to chronic) (CICAD 2001).
Absorption
N-Methyl-2-pyrrolidone is absorbed easily after inhalation, oral or dermal exposure and is distributed to the whole body. Approximately 80% of the administered dosis are excreted via the urine within 24 hours (Åkesson and Paulsson 1997). Therefore, absorption of 100% is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 24. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 7 | 740 | 0.749 | 3.745 | 0.037 |
The uptake via skin (dermal uptake) is estimated assuming that 1% of the skin area is exposed to 5 g of the product, 1 time per day.
Oral uptake assumes an exposure to 0.5 g product.
Table 25. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 7 | 740 | 1.85 | 18.5 | 20.4 |
As systemic effects appear to be the most sensitive endpoint, the lowest NOAEL available is used in the assessment.
Based on NOAEL of 169 mg/kg bw/day the margin of safety (MOS) to total uptake is: 169/0.0204 = 8284.
The estimated uptake does not exceed the TDI value of 600 μg/kg bw/day. The distance is approx. a factor 30.
Conclusion
The product's content of N-methyl-2-pyrrolidone is assessed not to pose an immediate health risk to the consumer of the examined textile colorant.
The substance is classified as irritating to eyes and skin. Data from IUCLID indicate that the effects are depending on the exposure concentration and at higher levels than detected.
3.3.12 2-Phenoxyethanol
Identification
| Name | 2-Phenoxyethanol |
| CAS no. | 122-99-6 |
| EINECS no. | 204-589-7 |
| Molecular formula | C8 H10 O2 |
| Molecular structure |  |
| Molecular weight | 138.17 g/mol |
The melting point is 14°C. The boiling point is 245°C (Budavari 1996). The vapour pressure is 0.93 Pa at 25°C (0.007 mmHg, Dow 1990) or 4 Pa at 20°C (IUCLID 2000). The water solubility is 26700 mg/l at 20°C (Yalkowsky and Dannenfelser 1992). The partition coefficient log Kow is measured to 1.16 (Hansch et al. 1995).
Use
2-Phenoxyethanol is used in many industrial products as solvent and/or as preservative.
Classification
2-Phenoxyethanol is adopted on the List of dangerous substances and classified (Miljøministeriet 2002):
| Xn;R22 | Harmful. Harmful if swallowed |
| Xi;R36 | Irritant: Irritating to eyes |
Effects on health
Some data on acute toxicity have been found. Of those are mentioned:
| Acute oral, rat | LD50 | 1260 mg/kg | IUCLID 2000 |
| Acute oral, rat | LD50 | 2740 mg/kg | IUCLID 2000 |
| Acute inhalation, rat | LC50 (8 h) | >saturated atmosphere | IUCLID 2000 |
| Acute dermal, rat | LD50 | 14422 mg/kg bw | IUCLID 2000 |
| Acute dermal, rabbit | LD50 | 3660 mg/kg bw | IUCLID 2000 |
The substance was not irritating to skin in tests on humans in 48 hours closed patch tests and 24 hours tests 3 times/week for 3 weeks. The substance is found irritating to eyes in rabbits (IUCLID 2000). The substance is not sensitising in maximisation tests on guinea pigs and in patch tests on humans (IUCLID 2000).
2-Phenoxyethanol is studied in a repeated dose toxicity test for 13 weeks on rats using oral administration of 2-phenoxyethanol in the diet at the concentrations 0, 50, 100, 200 and 500 mg/kg bw. At the highest concentration was observed a significant decrease in body weight gain and an alteration in blood parameters. Thus, NOAEL is set to 200 mg/kg bw/day (IUCLID 2000).
Threshold limit values
The threshold limit value for working environment (TLV) is 20 ppm corresponding to 110 mg/m3 with skin notation (H), i.e. the substance may penetrate the skin (DF 2001).
The C-value is 0.1 mg/m3 (Miljøstyrelsen 2002).
Absorption
Because no value on absorption is available the absorption is set to 100%.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 26. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 4 | 460 | 0.024 | 0.12 | 0.0012 |
| 10 | 4900 | 0.254 | 1.27 | 0.013 |
The uptake via skin (dermal uptake) is estimated assuming that 1% of the skin area is exposed to 5 g of the product, 1 time per day.
Oral uptake assumes an exposure to 0.5 g product.
Table 27. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 4 | 460 | 1.15 | 11.5 | 12.7 |
| 10 | 4900 | 12.3 | 122.5 | 134.8 |
The lowest NOAEL from a long-term repeated dose toxicity test is used in the assessment.
Based on NOAEL of 200 mg/kg bw/day the margin of safety (MOS) to total uptake is at least: 200/0.135 = 1480.
Conclusion
The products' content of 2-phenoxyethanol is assessed not to pose an immediate health risk to the consumer of the examined textile colorants.
The substance is classified irritating to eyes but if the colorant gets in contact with the eyes the irritation may also be caused by other components.
3.3.13 Propylene glycol
Identification:
| Name | Propylene glycol |
| CAS no. | 57-55-6 |
| EINECS no. | 200-338-0 |
| Molecular formula | C3H8O2 |
| Molecular structure |  |
| Molecular weight | 76.10 g/mol |
| Synonyms | 1,2-Propanediol |
| Propan-1,2-diol (EINECS name) | |
| Methylethylene glycol |
The melting point is -60°C. The boiling point is 187.6°C. The water solubility is high (miscible with water at 25°C). The vapour pressure is 17 Pa at 25°C (0.129 mmHg, Daubert and Danner 1989). The octanol/water partition coefficient is measured to log Kow –0.92 (Hansch et al. 1995).
Classification
Propylene glycol is not classified in the List of dangerous substances (Miljøministeriet 2002).
Use
The substance is contained in several products within several industries among other the paint and lacquers industry and the textile manufacturing industry. The substance is mostly used as solvent (IUCLID 2000).
Effects on health
Some data on acute toxicity have been found. Of these are mentioned:
| Acute oral, rat | LD50 | 20300 mg/kg | IUCLID 2000 |
| Acute oral, mouse | LD50 | 23900 mg/kg | Ruddick 1972 |
| Acute dermal, rabbit | LD50 | 20800 mg/kg | IUCLID 2000 |
Data from studies using single dose exposures indicate that propylene glycol has a low toxicity by acute exposure.
Of studies with prolonged exposure duration a 90 days inhalation study has been found where rats were exposed for 6 hours/day, 5 days/week to 0, 0.16, 1 and 2.2 mg/l air. In the second week, nose bleeding was observed as a result of dehydration effect from propylene glycol on the nose mucous membranes. At the highest doses reduced body weight and diet intake were observed. NOAEL was set to 1 mg/l corresponding to 1000 mg/m3 (IUCLID 2000).
For oral intake a 2-year study was found where rats were administered daily to 0, 6250, 12500 and 50000 ppm in the diet. Because no damaging effects were observed NOAEL was set to 50000 ppm in the diet corresponding to 2500 mg/kg bw/day (IUCLID 2000).
A further 2-year oral study is available where rats were administered propylene glycol in the diet at the concentration 0, 310, 630, 1300 or 2500 mg/kg bw/day for 2 years. No adverse effects on weight gain of body or organs, haematological or clinic-chemical parameters were observed. NOAEL was 1300 mg/kg bw/day (Gaunt et al. 1972).
Threshold limit values
Threshold limit values for the working environment is not available for Denmark (AT 2002) but for the United Kingdom was found an OES value of 474 mg/m3 and for Germany an AIHA Workplace Exposure Limit Guide value of 10 mg/m3 (IUCLID 2000).
The C-value is 1 mg/m3 (Miljøstyrelsen 2002).
An ADI value of 25 mg/kg bw/day is proposed for humans based on a 2-year oral rat study with a NOAEL of 2500 mg/kg bw/day and a safety factor of 100 (10 for interspecies and 10 for intraspecies differences) (Gaunt et al. 1972).
Absorption
Propylene glycol administered orally is absorbed rapidly (Hanzlik et al. 1939, Yu et al. 1985, JECFA 2001). No values have been available, therefore, 100% absorption is assumed.
Assessment
Uptake via inhalation was based on a child of 20 kg using the textile colour product for 30 minutes/day. The consumption is set to 5 g/event, 1 time/day.
Table 28. Uptake via inhalation
| Prod.no. | Content in prod. mg/kg |
C air, max mg/m3 |
C inh μg/m3 |
Uptake via inhalation μg/kg bw/day |
| 1 | 370 | 0.193 | 0.97 | 0.010 |
| 4 | 2300 | 1.201 | 6.01 | 0.060 |
| 5 | 36 | 0.019 | 0.094 | 0.0009 |
| 7 | 3300 | 1.723 | 8.62 | 0.086 |
| 10 | 7100 | 3.707 | 18.54 | 0.185 |
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day.
Oral uptake is assuming an exposure to 0.5 g product.
Table 29. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 370 | 0.93 | 9.25 | 10.2 |
| 4 | 2300 | 5.75 | 57.5 | 63.3 |
| 5 | 36 | 0.09 | 0.90 | 0.99 |
| 7 | 3300 | 8.25 | 82.5 | 90.8 |
| 10 | 7100 | 17.75 | 177.5 | 195.4 |
Propylene glycol is apparently not very toxic except for the dehydrating effect on mucous membranes. For the assessment the lowest available NOAEL of 1300 mg/kg bw/day from the 2-year rat study is used.
Based on NOAEL 1300 mg/kg bw/day the margin of safety (MOS) for total uptake is at least 1300/0.1954 = 6650.
The estimated uptake does not exceed the ADI value of 25 mg/kg bw/day. The distance is more than a factor of 128.
Conclusion
The products' content of propylenglycol is assessed not to pose an immediate health risk to the consumer of the examined textile colorants.
3.3.14 Antimony
Identification
| Name | Antimony | |
| CAS no. | 7440-36-0 | |
| EINECS no. | 231-146-5 | |
| Molecular formula | Sb | |
| Atomic weight | 121.75 g/mol | |
| Synonym | Stibium (Sb) |
The melting point of antimony is 630°C. The boiling point is 1635°C. (Budavari 1989). The vapour pressure is 1 mmHg at 885°C (ATSDR 1992).
Classification
Antimony compounds are classified under several index numbers.
Antimony trioxide (CAS no.: 1309-64-4, EINECS no.: 215-175-0) is classified:
Carc3;R40; Harmful. Limited evidence of carcinogenic effects.
Antimony compounds, other than antimony chlorides, oxides and sulphides, are classified:
| Xn;R20/22 | Harmful by inhalation and if swallowed. |
| N;R51/53 | Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
Use
Antimony is typically present in textile colorants for two reasons. Firstly antimony is used as component in certain colorants or pigments and secondly antimony is used as synergist to flame retardants. Since antimony trioxide is used as white pigment in colours and as synergist to flame retardants in textiles the substance detected is probably antimony trioxide. However, as the analysis was performed as an element analysis it is unknown. Other antimony compounds such as antimony trisulphide and pentasulphide may alsobe detected in textile colorants (Lindell 2000).
Effects on health
Antimony trioxide is in the process of EU risk assessment with Sweden as rapporteur country.
Some data on acute toxicity have been available. Most data are derived from studies using antimony compounds such as antimony trioxide, antimony trichloride, etc. Of these can be mentioned:
| Acute oral, rat | LD50 | 100 mg Sb/kg | Sax 1989 |
| Acute oral, mouse | LD50 | 550 mg Sb/kg | Sax 1989 |
Antimony and its compounds have been reported to cause dermatitis, keratitis, conjunctivitis and nasal septal ulceration by contact, fumes or dust (Budavari 1989).
In Sloof et al. (1992) the results from several subacute studies using different antimony compounds are presented in tabular form. Many of the tests are performed as limit tests i.e. using only one concentration. Since effects were observed in most studies at the used concentrations they can not be used to establish a NOAEL. Besides, the results clearly indicate that the observed values on toxicity depend on which antimony compound that is used in the study. For instance the water solubility and lipid solubility of the used antimony compound will be decisive for bioavailability/absorption and thus the test result.
The currently best results and evaluations are therefore found in the EU draft on a risk assessment of antimony trioxide (ECB 2004b). Because the detected antimony probably are a result of the use of antimony trioxide in colorants this is considered acceptable for this assessment.
For exposure via inhalation the EU risk assessment draft uses a 1-year rat study where rats were exposed to antimony trioxide at the doses 0, 1.9 and 5 mg Sb2O3/m3 for 6 hours/day, 5 days/week over 52 weeks corresponding to 1.6 and 4.2 mg Sb/m3. Based on numerous harmful effects to the lungs a LOAEC was set to 1.9 mg antimony trioxide/m3 corresponding to 1.6 mg Sb/m3 (EU draft 2004, ECB 2004b).
For oral exposure the EU draft on antimony trioxide (ECB 2004b) uses a 90 days rat study where the rats were administered diet containing 0, 1000, 5000 or 20000 ppm antimony trioxide. Based on effects on the liver a NOAEL was set to 5000 ppm in the diet corresponding to 421 mg Sb2O3/kg bw/day for male rats and 494 mg Sb2O3/kg bw/day for female rats (Hext et al. 1999). Recalculated to antimony on the basis of molecular weight this corresponds to the lowest NOAEL being 421(2121.75)/291.52 = 351 mg Sb/kg bw/day.
In the EU risk assessment scenarios for consumers are included. From exposure to antimony trioxide the most essential effects are mutagenic and carcinogenic effects. It is assessed that a potential possibility of health problems exists. To the risk assessment of effects to the reproduction and development is used a NOAEL from inhalation of 6.3 mg/m3 recalculated to oral exposure assuming that the rat weighs 250 g and has an inhalation rate of 0.0144 m3/h to: 6.3 mg/m3 0.0144 m3/h 6 h/day / 0.25 kg = 2.2 mg/kg bw/day (ECB 2004b). Recalculated to antimony this corresponds to 1.8 mg Sb/kg bw/day.
The study that WHO (1996) has used to derive ADI is a long-term study where rats for the duration of their lives were exposed to antimony in the drinking water at 5 ml Sb/l corresponding to 0.43 mg/kg bw/day. Based on 15% reduction of lifetime and changed blood chemistry a LOAEL was set to 0.43 mg Sb/kg bw/day (Schröder et al. 1970).
However, WHO has later revised the antimony studies due to newer studies and severe criticism. WHO now bases its TDI value on a 90-day rat study where the rats were administered potassium antimony tartrate in the drinking water. Based on decreased body weight gain and reduced food intake a NOAEL was set to 6 mg Sb/kg bw/day (WHO 2004, 2004b).
Antimony may migrate out of the colorant in or on the textile even at low temperatures to liquids such as sweat, saliva and synthetic blood (Hansen et al. 2002).
Threshold limit values
The threshold limit for air in the working environment is 0.5 mg Sb/m3 (AT 2002).
The C-value for antimony compounds is 0.001 mg Sb/m3 (Miljøstyrelsen 2002).
US-EPA derives an inhalation RfC of 0.2 μg/m3 for antimony trioxide (IRIS 2002).
Of oral limit values is found that ATSDR (1992) has calculated an oral RfD to 0.4 μg Sb/kg bw/day based on a LOAEL 0.35 mg Sb/kg/day from a chronic rat study (IRIS 2002).
An acceptable daily intake (ADI) is derived by WHO to 8.610-4 mg Sb/kg bw/day (WHO 1996). The value is based on a LOAEL of 0.43 mg Sb/kg bw/day and a safety factor of 500 (10 for interspecies, 10 for intraspecies differences and 5 for LOAEL to NOAEL), i.e. ADI = 430/500 = 0.86 μg Sb/kg bw/day.
Based on the same study as WHO 1996 (LOAEL 0.43 mg Sb/kg bw/day) but using a safety factor of 1000 (used factor 10 for LOAEL to NOAEL) A TDI value can be set to 0.43 μg Sb/kg bw/day. This value is found in several references.
The revised TDI value 6 μg Sb/kg bw/day is given in WHO (2004). This TDI value is based on a NOAEL of 6 mg/kg bw/day from a 90-day rat study. A safety factor of 1000 was applied (100 for inter and intraspecies differences and 10 for subchronic to chronic) (WHO 2004c).
Absorption
The bioavailability by inhalation is not available and therefore set to 100%.
The bioavailability by dermal uptake is unknown but for other metals it is assumed to be about 1-5% (ECB 2004b-draft antimony trioxide). The value is in this report set to 10%.
The bioavailability by oral intake is estimated to 10%. The value is based on uptake from gastro-intestinal tract has been found to 2-7% (Miljøstyrelsen 2002).
Assessment
Exposure via inhalation is based on that antimony as metalloid has a very low vapour pressure. The release from the use of textile colorant products is therefore assumed to be very low and insignificant in relation to other sources of antimony exposure in the indoor climate (dust from clothes of polyester containing antimony, the releases from electronic equipment, plastic, etc. where antimony is used as flame retardant, Laursen et al. 2003).
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day. The absorption is set to 10%.
Oral uptake is assuming an exposure to 0.5 g product and absorption of 10%.
Table 30. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake, μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 78 | 0.020 | 0.195 | 0.215 |
| 2 | 64 | 0.016 | 0.160 | 0.176 |
| 3 | 84 | 0.021 | 0.210 | 0.231 |
Based on a LOAEL of 0.43 mg Sb/kg bw/day the margin of safety (MOS) for total uptake is at least 0.43/0.000231 = 1861.
Using a NOAEL of 6 mg Sb/kg bw/day the margin of safety (MOS) for total uptake is at least 6/0,00023 = 26000.
The estimated uptake does not exceed the ADI value of 0.86 μg/kg bw/day and not at all the revised TDI value of 6 μg/kg bw/day (WHO 2004).
However, it should be noted that other sources to antimony exposure also exist from the environment and in food. This means that during the exposure period (the use period of the textile colorant product) exceeding of an acceptable daily intake of antimony may occur. As antimony relatively fast is excreted and not accumulated in the body exceeding of short duration are considered acceptable.
Conclusion
The products' content of antimony is assessed not to pose a health risk to the consumer of the examined textile colorants.
However, it should be noted that antimony trioxide is classified carcinogenic (category 3; R40 Limited evidence of carcinogenic effect). The assessment to be performed in the EU risk assessment programme is not yet finalised. The determined levels the substance may not be of significance but the manufacturer and the consumer perhaps should consider alternatives until this is clarified.
3.3.15 Copper
Identification
| Name | Copper | |
| CAS no. | 7440-50-8 | |
| EINECS no. | 231-159-6 | |
| Molecular formula | Cu | |
| Atomic weight | 63.55 g/mol |
The melting point is 1083°C. The boiling point is approx. 2590°C (Budavari 1989).
Copper may occur at two valences: copper(I) and copper(II). The two forms may each be included in several different chemical compounds.
Classification
Copper compounds are classified differently depending on the specific compound. Most copper compounds are classified Harmful if swallowed as copper may cause liver damages (Larsen et al. 2000).
Certain compounds such as copper(I)chloride and copper sulphate are classified Dangerous to the environment and Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. Other compounds are classified Irritants but not with risk of sensitisation (Miljøministeriet 2002).
Use
The detection is probably caused by the application of copper compounds in different colour pigments.
Effects on health
Copper belongs to the essential metals i.e. a certain minimum uptake is necessary not to retrieve deficiency diseases. It is estimated that copper is necessary at a level of normally 1 to 5 mg Cu/adult person/day corresponding to 20 to 80 μg/kg bw/day (WHO 1996b). The need of copper is usually regulated by the organism so accurately that the body's content of copper in adults is constantly about 100 to 150 mg (Scheinberg 1983).
WHO states that the daily need of copper is covered by food and drinking water at an exposure to 1 to 5 mg/day. All other intakes (via inhalation and the skin) are considered insignificant compared to the oral exposure route. For instance inhalation is assessed to contribute with an uptake of 0.3 to 2.0 μg/day from dust and smoke (IPCS 1998).
Few data on acute toxicity have been available. Of these are mentioned:
| Acute oral, mouse | LD50 | 0.7 mg/kg | IUCLID 2000 |
| Acute oral, human | TDLO | 0.12 mg/kg | IUCLID 2000 |
| Acute inhalation, human | TCLO | 1 mg/m3 | IUCLID 2000 |
Several data exist on organic and inorganic copper compounds. Because it is unknown, which copper compounds that may be contained in the products, these data are not included in this context.
A study of 13 weeks exposure of rats and mice showed no harmful effects besides dose related decrease in body weight gain following administration of 138 mg Cu/kg bw/day for rats and 1000 mg Cu/kg bw/day for mice. NOAEL was 17 mg Cu/kg bw/day for rats and 44 and 126 mg Cu/kg bw/day for male and female mice, respectively. The effects included inflammation of liver and degeneration of kidney epithelium (Hébert et al. 1993).
In a reproduction study on rats the effect level for effects on testicles, semen quality, etc. was studied. NOAEL was set to 8000 mg copper sulphate/kg diet corresponding to 140 mg Cu/kg bw/day for males and 134 mg Cu/kg bw/day for females (Hébert et al. 1993).
Because copper is essential a "window" exists between the necessary intake and the limit of toxicity: the acceptable range of oral intake (AROI). The lower limit for AROI is set to 20 μg Cu/kg bw/day for adults and 50 μg Cu/kg bw/day for children. The upper limit for AROI is not set but lies apparently around 2-3 mg/day based on studies of the gastro-intestinal tract following intake of copper contaminated drinking water (IPCS 1998, WHO 2004c).
Effects following inhalation of copper is not well studied. A study was available with exposure of rabbits to copper chloride for 6 hours/day, 5 days/week over 6 weeks. Based on effects to the respiratory and immune system a NOAEC was set to 0.6 mg Cu/m3.
Effects following dermal exposure is insufficiently studied but effects have been reported such as contact dermatitis in patch tests using copper (Baars et al. 2001).
Threshold limit values
The threshold limit value for the working environment is 10 mg Cu/m3 based on copper as powder and dust (AT 2002).
The C-value for copper as inorganic dust is 0.01 mg Cu/m3 (Miljøstyrelsen 2002).
In the available data on acceptable daily intake/concentration, no distinction is made on which copper compound it concerns. The different forms are therefore considered as one in the following text.
Copper is mainly harmful if swallowed. The acceptable daily intake (TDI) is set to 0.14 mg/kg bw/day based on estimated maximum daily intake in the Dutch population (Vermeire et al. 1991, Baars et al. 2001).
The tolerable concentration in air by inhalation (TCA) is calculated to 110-3 mg/m3. The value is based on a subacute rabbit study where NOAEC was recalculated to continuous exposure (0.6 6/24 hours 5/7 days) = 0.1 mg Cu/m3. Applying a safety factor of 100 (for inter and intraspecies differences) the value is calculated to 0.1/100 = 110-3 mg/m3 = 1 μg Cu/m3 (Baars et al. 2001).
WHO has suggested a preliminary TDI value of 0.5 mg/kg bw/day based on a study on dogs where a NOAEL of 5 mg/kg bw/day was found. Applying a safety factor of 10, which is chosen as copper is an essential metal (WHO 1996, 1998).
WHO has suggested a preliminary drinking water criteria value of 2 mg Cu/l (WHO 1996, 1998). The value is later set as established to 2 mg/l since several studies have demonstrated the level to be acceptable (WHO 2004c).
Absorption
Merian (1991) states that approx. 50% is absorbed in the gastro-intestinal tract. Apparently the absorption is inverse proportional to the concentration in the diet in a study from 1989 on 11 young men where an absorption was observed varying from 67% at 0.38 mg/day to 12% at 7.53 mg/day (WHO 2004c). The bioavailability by inhalation is determined to 50% and the bioavailability by oral intake is estimated to 50% (Baars et al. 2001).
Assessment
Exposure via inhalation is based on copper as a metal having a very low vapour pressure. The release from the use of textile colorants is therefore assumed very low and insignificant in relation to other sources of copper exposure in the indoor environment such as dust, food etc.
Dermal uptake is estimated assuming that 1% of the skin area is exposed to 5 g of the product 1 time/day. The absorption is set to 50%.
Oral uptake is assuming an exposure to 0.5 g product and absorption of 50%.
Table 31. Dermal and oral uptake, and total uptake
| Prod.no. | Content in prod. mg/kg |
Dermal uptake μg/kg bw/day |
Oral uptake μg/kg bw/day |
Total uptake (inhalation + dermal + oral) μg/kg bw/day |
| 1 | 2400 | 0.600 | 30.0 | 30.60 |
| 2 | 170 | 0.043 | 2.125 | 2.17 |
| 3 | 38 | 0.010 | 0.475 | 0.48 |
| 12 | 54 | 0.014 | 0.675 | 0.69 |
Based on a NOAEL of 17 mg/kg bw/day the margin of safety (MOS) for total uptake is at least 17/0.0306 = 556.
The estimated uptake does exceed the Dutch TDI value of 0.14 mg/kg bw/day. The distance is at least a factor 4.6.
Using the WHO guideline value for drinking water of 2 mg/l it can be recalculated assuming an intake of 2 l/day and a weight of 70 kg and an absorption of 50% to: 2 2/70 0.5= 0.12 mg/kg bw/day. This value is close to the Dutch value.
The WHO temporary TDI of 0.5 mg/kg bw/day is not exceeded. The distance is at least a factor of 16.
However, it should be noted that other sources to copper exposure also exist from the environment and in food. This means that during the exposure period (the use period of the textile colorant product) exceeding of an acceptable daily intake of copper may occur. As copper contained in the body apparently is relatively constant indicating that copper relatively fast is excreted and not accumulated in the body an exceeding of short duration are considered acceptable.
Conclusion
The products' content of copper is assessed not to pose a health risk to the consumer of the examined textile colorants.
Version 1.0 July 2005, © Danish Environmental Protection Agency