|
Environmental Project nr. 1039, 2005 Mapping and development of alternatives to chlorinated lubricants in the metal industry (KLORPARAFRI)Contents2 Metalworking - processes, materials and lubricants
3 Demands and specifications for non-chlorinated lubricants
5 Mapping of alternative lubricants 6 Technical assessment of non-chlorinated lubricants 7 Health and environmental assessment of components in non-chlorinated lubricants
8 Health and environmental screening of non-chlorinated lubricants
ForewordThis report is a result of the project “Mapping and development of alternatives to chlorinated lubricants in the metal industry (KLORPARAFRI)”. The project is initiated by the Danish Environmental Protection Agency under the Programme for Cleaner Products. The overall objective of the project is to promote substitution of chlorinated paraffins in lubricants for metal processing. The report contains a summary of the overall activities and conclusions of the project. Following the summary, Chapter 1 describes the background as well as the objective and course of the project. Chapter 2 gives an introduction to the metal forming processes in focus in this project and to lubricant formulations and present status of lubricant technology. Chapter 3 describes the technical demands and specifications for lubricants established in the project in order to identify potentially suitable lubricants for the metal processes in focus and for technical testing in the project. Chapter 3 also describes health and environmental demands and specifications for proposed alternative lubricants established in the project in order to define the scope for health and environmental assessment of lubricants and to avoid technical testing of those with known unwanted health and environmental properties. Chapter 4 describes the strategy of the data search, while chapter 5 provides and discusses the results of the mapping of non-chlorinated lubricants. Chapter 6 describes the methods and results of the technical testing of selected lubricants. Chapter 7 describes the health and environmental assessments of selected substance groups that are typically found in non-chlorinated lubricants for heavy-duty metal working. Chapter 8 gives the results of a health and environmental screening of proposed non-chlorinated lubricants, while chapter 9 contains exposure assessments and risk characterisation at a screening level of two critical substance groups commonly occurring in non-chlorinated lubricants for heavy-duty metal working. The working group of the project comprises the following members:
Christine Skak, CETOX – Danish Toxicology Centre The project has been supervised by a steering committee. The members of the steering committee are as follows:
Kim Petersen, Danish Environmental Protection Agency
Hørsholm, October 2004 Summary and conclusionsBackground Medium-chained chloroparaffins are used in substantial amounts as extreme pressure (EP) additives in lubricants for metal working. Chloroparaffins are persistent and bio-accumulating substances. They are found widespread in the external environment and biota, including in food stuffs and human beings. The substances are toxic to aquatic organisms and may have adverse effects on human health. A health and environmental risk assessment has been performed by the EU Commission for short-chained chloroparaffins, while a risk assessment for medium-chained chloroparaffins is presently being prepared. Short-, medium- and long-chained chloroparaffins are included in the List of unwanted substances 2004 published by the Danish Environmental Agency due to their serious health and environmental properties. In the past decade, a number of projects have been carried out in the Danish metal industry aiming at finding alternatives to chlorinated lubricants for metal working. Thus, there has been a substantial reduction in the use of chlorinated lubricants by Danish large-scale users in the metal industry. Chlorinated lubricants for cutting operations as milling, screw-cutting and drilling have been completely substituted. However, for non-cutting operations, particularly demanding processes such as forming in stainless steel and titanium, chlorinated lubricants are still widely used. This is due to a lack of technically satisfying alternatives so far. At the same time, the need for lubricants to be used under very demanding production conditions is increasing due to demands for material and energy saving, increased productivity and improved quality. Objective The overall objective of this project “Mapping and development of alternatives to medium-chained chloroparaffins in lubricants in the metal industry (KLORPARAFRI)” is to promote substitution of chlorinated lubricants for metal working. The focus of the project is on alternative lubricant systems for heavy-duty metal forming, including deep drawing, punching and extrusion, in stainless steel and titanium, where the use of chlorinated lubricants still persists. The specific objectives of the project are to map existing non-chlorinated lubricant alternatives for heavy-duty metal forming, to technically test the lubricating performance of selected lubricant alternatives, to assess the health and environmental properties of alternative lubricants and, if possible, to develop promising lubricant alternatives for production. Mapping of non-chlorinated lubricant systems A mapping of existing non-chlorinated lubricant systems for heavy-duty metal forming has been performed by contact to international lubricant suppliers, including both large companies and smaller, highly specialized companies. Approximately 50 lubricant systems have been proposed as alternatives to the specified metal forming operations. Three types of lubricant systems have been mapped in this project. The dominant type is traditional oil-based wet lubricants with extreme pressure components added. Chlorinated lubricants are normally of this type. Wet lubricants are applied to the metal surface without any drying step prior to the forming operation. A second type of alternative lubricants is the so-called dry lubricants, which are also applied to the metal surface. However, they require drying prior to the forming operation. Dry lubricants are water-based emulsions and may contain polymers. The EP additives of wet and dry non-chlorinated lubricants are typically combinations of sulphur and/or phosphorous compounds. Some non-chlorinated lubricants may contain inorganic components as EP additives. While the above-mentioned wet and dry lubricant systems require a single lubricant layer on the metal surface, a third type of lubricant system has been mapped which requires two lubricant layers on the metal surface: a carrier layer and a lubricating layer. Prior to application of a lubricant, a carrier layer is applied to the metal surface by phosphating or oxalating the metal surface. The lubricant to be applied subsequently on the carrier layer can be either a wet or dry lubricant as mentioned above or it can be soaps. The mapping phase of non-chlorinated lubricants for heavy-duty metal forming has shown that substitution of chloroparaffins in metal forming lubricants requires a complete reformulation of the lubricant. It is not possible to substitute chloroparaffins in lubricants with a single component. Most often the composition of the lubricants becomes substantially more complex than the traditional chlorinated lubricant. Technical testing of non-chlorinated lubricant systems The lubricating qualities of 19 of the proposed non-chlorinated lubricants have been tested in simulating and process technical tests developed at the Technical University of Denmark (DTU). Four of the lubricants exhibited promising lubricating properties in these tests and were further tested in a full scale production test at Danfoss A/S. None of the four tested alternative lubricants exhibited sufficient lubricant performance. The production test comprised a several-step sheet forming of a work piece in stainless steel, including deep-drawing, extrusion and punching operations. Not all proposed lubricant systems were technically tested in the project. This was due to either failure to comply with technical or health and environmental criteria established in the project or that the demands of application of the lubricant could not be fulfilled in the test system. The last-mentioned includes lubricants system that require an initial phosphating or oxalating of the metal surface prior to the test. Health and environmental assessment of non-chlorinated lubricants A health and environmental assessment has been carried out for selected components that typically occur in non-chlorinated lubricants. The objective has been to obtain an assessment of the inherent health and environmental properties of non-chlorinated lubricant components in addition to an overview of the data quality and availability for the substances. As a substitution of chloroparaffins in lubricants normally implies a complete reformulation of the lubricant, it was estimated in this project that the most suitable frame of reference for comparison of the health and environmental properties of chlorinated and non-chlorinated lubricants respectively, is comparison of total lubricants. Thus, the health and environmental assessments of lubricant components has included not only components with a primary function as extreme pressure additives but also components with other functions as lubrication-enhancing additives. The result of the health and environmental assessment of non-chlorinated lubricants indicates that especially the extreme pressure additives alkyl sulphides (polysulphides) and phosphorous compounds include substances which may cause adverse health and environmental effects. The result also demonstrates that substitution of chlorinated lubricants for heavy-duty metal working implies a movement from a reasonable data platform regarding potential health and environmental effects to a substantially poorer data platform. A combined health and environmental screening for non-chlorinated lubricants has been performed for 14 of the 19 lubricants tested in the simulating and process technical tests. This has been done with the aim to establish an overview of the health and environmental properties of the lubricants at a screening level in addition to identifying and eventually sorting out lubricants with obvious unwanted health and environmental properties prior to a production test. The result of the screening, based on the sparse available data, is that non-chlorinated lubricants seem to be better than chlorinated lubricants with regard to health and environmental properties compared to chlorinated lubricants. However, some of the lubricants suggested contain components exhibiting a sensitizing potential. In addition, many of the lubricants contain substances with an environmental hazard potential at the same level as chlorinated paraffins. However, the substances are present in the lubricants at substantially lower concentrations than chloroparaffins in the chlorinated lubricants. Several of the proposed lubricants contain substances for which no or very limited data on potential health and environmental effects could be retrieved. Worst case exposure assessments and risk characterizations in the working environment have been carried out at a screening level for two polysulphides and two phosphorous compounds considered to represent the most critical substance groups in non-chlorinated lubricants for metal forming regarding health and environmental effects. The result of these assessments indicate that worst case dermal exposure or inhalation of vapours may involve a risk of adverse effects on health for some compounds in these two groups. The risk of adverse effects by dermal contact is, however, quite easily reduced by use of personal protection equipment, especially gloves. Discussion and conclusion Four of the non-chlorinated lubricants have demonstrated promising lubricating qualities in an initial simulating test and a process technical test involving deep drawing and extrusion in stainless steel. However, none of the four lubricants showed satisfactory lubricating qualities in a full scale production test comprising a several-step forming of a work piece in stainless steel, including deep-drawing, extrusion and punching. Thus, under the test conditions given it was not possible to identify non-chlorinated lubricants capable of substituting chlorinated lubricants in full scale production involving several-step sheet forming operations in stainless steel. Very often production of work pieces in the metal industry implies several-step operations, including various types of operations as in the full scale production test. However, the demands on the lubricating properties of a lubricant may vary considerably in various metal forming processes. It is known that non-chlorinated lubricant systems exist which operate with satisfying lubricating properties in individual forming operations. However, the limits for these lubricants are that they are specially designed for a certain forming operation and often also a certain customer. Thus, these lubricants can only work under a narrow set of production conditions. Not all alternative lubricant systems proposed were technically tested in the project. In some cases, this was due to failure to comply with the testing conditions available in this project. Danfoss A/S has previously developed a lubricating system - the Extreme Pressure Lubricant System (EPL) - for cold forging of stainless steel. Cold forging involves high demands on the lubricating performance of the lubricant due to large plastic deformation rates with high energy release. The EPL system is a two-layer lubricating system comprising a carrier layer and a lubricant. The new technology in the system is the application of a phosphate carrier layer on stainless steel. A ordinary soap is subsequently applied on the metal surface as the lubricant. The EPL technique has previously demonstrated very good results in production tests at Danfoss A/S for the preparation of shell pipes in stainless steel. For financial reasons, Danfoss A/S has not implemented the EPL method as a standard technique in their production. Their production is estimated to be too small to establish the necessary production equipment. A market analysis of the sale of phosphated stainless steel demonstrated surprisingly low interest. Thus, technically satisfying alternatives to chlorinated lubricants in heavy-duty metal forming may exist. However, they involve substantial costs due to large investments in production equipment. By contrast, chlorinated lubricants are multi-functional and can be successfully added to most metal forming processes. They have well-documented lubricating properties and are cheap. The health and environmental assessment of components in non-chlorinated lubricants and the screening of proposed alternative lubricants indicate that non-chlorinated lubricants seem to possess improved inherent health and environmental properties. However, they may contain substances that are potentially sensitising and substances that may have adverse environmental effects at a level corresponding to that for chloroparaffins though the substances occur in the alternative lubricants in substantially lower concentrations than chloroparaffins in chlorinated lubricants. Furthermore, substitution of chlorinated lubricants for heavy-duty metal working implies a movement from a reasonable data platform to a significantly poorer data platform. The overall conclusion of the project is that further development of non-chlorinated lubricants for heavy-duty metal forming remains in order to obtain technically satisfying alternatives while simultaneously improving the health and environmental properties. Sammenfatning og konklusionerBaggrund Mellemkædede klorparaffiner bruges i betydelige mængder som højtryksadditiver i smøremidler til metalbearbejdning. Klorparaffiner er persistente og bioakkumulerende stoffer. De forekommer vidt spredt i det eksterne miljø og økosystemer, herunder i fødevarer og mennesker. Stofferne er giftige for organismer, der lever i vand og kan medføre skadelige effekter på menneskers sundhed. EU-kommissionen har gennemført en miljø- og sundhedsmæssig risikovurdering af kortkædede klorparaffiner, mens en risikovurdering af mellemkædede klorparaffiner er under udarbejdelse. Kort-, mellem- og langkædede klorparaffiner er opført på Listen over uønskede stoffer 2004 fra Miljøstyrelsen idet stoffernes anvendelse anses for at være sundheds- og miljømæssigt betænkelig. Indenfor de seneste 10 år er der blevet gennemført en række projekter i den danske metalindustri med det formål at finde alternativer til klorerede smøremidler til metalbearbejdning. Det har ført til en væsentlig reduktion i brugen af klorerede smøremidler hos større danske virksomheder i metalindustrien. Klorerede smøremidler til brug ved skærende operationer såsom fræsning, gevindskæring og boring er gradvist blevet fuldstændig substitueret. Ved ikke-skærende operationer, især krævende processer som formgivning af rustfrit stål og titanium, bruges klorerede smøremidler dog stadig i vidt omfang. Dette skyldes, at der i dag ikke findes teknisk tilfredsstillende alternativer. Samtidig er der et voksende behov for smøremidler til brug under meget krævende produktionsforhold på grund af krav om materiale- og energibesparelser, øget produktivitet og forbedret kvalitet. Formål Det overordnede formål med projektet ”Kortlægning og udvikling af alternativer til mellemkædede klorparaffiner i smøremidler i metalindustrien (KLORPARAFRI)” er at fremme substitutionen af klorerede smøremidler til metalbearbejdning. Projektets fokus er på alternative smøresystemer til krævende spånløs metalbearbejdning herunder dybtrækning, stansning og ekstrudering i rustfrit stål og titanium, hvor brugen af klorerede smøremidler stadigvæk fastholdes. Projektets specifikke formål er at kortlægge eksisterende alternative ikke-klorerede smøremidler til krævende spånløs metalbearbejdning, at teste udvalgte alternative smøremidlers smøreevne, at vurdere alternative smøremidlers miljø- og sundhedsmæssige egenskaber samt, hvis muligt, at produktionsmodne lovende smøremiddelalternativer. Kortlægning af ikke-klorerede smøresystemer Der er sket en kortlægning af eksisterende ikke-klorerede smøresystemer til krævende spånløs metalbearbejdning ved kontakt til internationale leverandører af smøremidler, både store virksomheder og mindre, højt specialiserede firmaer. Omkring 50 smøresystemer er blevet foreslået som alternativer til specificerede spånløse metalbearbejdningsoperationer. Tre typer smøresystemer er blevet kortlagt i dette projekt. Den dominerende type er traditionelle oliebaserede våd-smøremidler tilsat højtryksadditiver. Klorerede smøremidler er normalt af denne type. Våd-smøremidler påføres metaloverfladen uden nogen form for tørring forud for bearbejdningsprocessen. En anden type alternative smøremidler er de såkaldte tør-smøremidler, som også påføres metaloverfladen, men de kræver en tørring forud for bearbejdningsprocessen. Tør-smøremidler er vandbaserede emulsioner og kan indeholde polymerer. Højtryksadditiverne i ikke-klorerede våd- og tør-smøremidler er typisk kombinationer af svovl og/eller fosforforbindelser. Nogle ikke-klorerede smøremidler kan indeholde uorganiske forbindelser som højtryksadditiver. Mens de ovennævnte våd- og tør-smøresystemer indbefatter et enkelt smørelag på metaloverfladen, er en tredje type smøresystem blevet kortlagt, som omfatter to smørelag på metaloverfladen: Et bærelag og et smørelag. Før påføringen af et smøremiddel påføres metaloverfladen et bærelag ved en fosfatering eller oxalatering af metaloverfladen. Smøremidlet, som herefter påføres på bærelaget, kan være enten et våd- eller et tør-smøremiddel, som nævnt ovenfor, eller det kan være sæber. Kortlægning af ikke-klorerede smøremidler til krævende spånløs metalbearbejdning har vist, at substitution af klorparaffiner i smøremidler til spånløs metalbearbejdning forudsætter en fuldstændig reformulering af smøremidlet. Det er ikke muligt at substituere klorparaffinen i smøremidler med et enkelt komponent. Som oftest bliver smøremidlets sammensætning væsentligt mere kompleks end de traditionelle klorerede smøremidler. Teknisk afprøvning af ikke-klorerede smøresystemer Smøreevnen af 19 af de foreslåede ikke-klorerede smøremidler er testet i simulerings- og procestekniske prøver udviklet af Danmarks Tekniske Universitet (DTU). Fire af smøremidlerne viste lovende smøreegenskaber i disse test og blev efterfølgende afprøvet i en fuld produktionstest hos Danfoss A/S. Her viste ingen af de fire smøremidler tilstrækkelige smøreegenskaber. Produktionstesten omfattede en fler-trins pladebearbejdning af et emne rustfrit stål herunder dybtræknings-, stansnings- og ekstruderingsprocesser. Ikke alle de foreslåede smøresystemer er blevet teknisk testet i dette projekt. Dette skyldes enten, at smøremidlerne ikke overholdt de tekniske eller sundheds- og miljømæssige kriterier, som blev fastsat i projektet, eller at smøremidlernes krav til påføring ikke kunne opfyldes i testsystemet. De sidstnævnte inkluderer smøresystemer, som forudsætter en indledende fosfatering eller oxalatering af metaloverfladen forud for testen. Sundheds- og miljøvurdering af ikke-klorerede smøremidler Der er gennemført en sundheds- og miljøvurdering af udvalgte komponenter, som typisk forekommer i ikke-klorerede smøremidler. Dette med henblik på at få en vurdering af de iboende sundheds- og miljømæssige egenskaber af ikke-klorerede smøremiddelkomponenter samt at få et overblik over mængden og kvaliteten af data for stofferne. Da der ved substitution af klorparaffiner i smøremidler normalt sker en fuldstændig reformulering af smøremidlet, blev det vurderet i projektet, at den bedste referenceramme til sammenligning af sundheds- og miljømæssige egenskaber af henholdsvis klorerede og ikke-klorerede smøremidler er en sammenligning af totale smøremidler. Derfor har sundheds- og miljøvurderingen af smøremiddelkomponenterne omfattet ikke bare komponenter med en primær funktion som højtryksadditiver, men også komponenter med en funktion som eksempelvis smøreforbedrende additiv. Resultatet af sundheds- og miljøvurderingen af ikke-klorerede smøremidler viser, at specielt højtryksadditiverne alkylsulphider (polysulphider) og fosforforbindelser inkluderer stoffer med uønskede sundheds- og miljøeffekter. Resultatet viser også, at substitution af klorerede smøremidler til krævende spånløs metalbearbejdning indebærer et skift fra en rimelig dataplatform vedrørende potentielle sundheds- og miljømæssige effekter til en væsentlig ringere dataplatform. En screening af de samlede sundheds- og miljøeffekter af ikke-klorerede smøremidler er gennemført for 14 af de 19 produkter, som blev testet i simulerings- og procestekniske test. Dette med henblik på at få et overblik smøremidlernes sundheds- og miljømæssige egenskaber på et screeningsniveau, samt at identificere og eventuelt frasortere smøremidler med umiddelbart uønskede sundheds- og miljømæssige egenskaber før en produktionstest. Resultatet af screeningen, baseret på sparsomme tilgængelige data, er, at ikke-klorerede smøremidler tilsyneladende er bedre smøremidler i miljø- og sundhedsmæssig henseende end klorerede smøremidler. Dog indeholder nogle af de foreslåede smøremidler potentielt allergifremkaldende komponenter. Samtidig indeholder flere af smøremidlerne stoffer, som er potentielt ligeså miljøskadelige som klorparaffiner. Dog forekommer stofferne i væsentlig lavere koncentrationer end klorparaffiner i klorerede smøremidler. Flere af de foreslåede smøremidler indeholder stoffer, hvor der ikke er fundet data, eller kun meget få data, om potentielle sundheds- og miljøeffekter. Der er gennemført worst-case eksponeringsvurderinger og risikokarakterisering i arbejdsmiljøet på et screeningsniveau for to polysulphider og to fosforforbindelser, som vurderes at repræsentere de mest kritiske stofgrupper i ikke-klorerede smøremidler med hensyn til sundheds- og miljøeffekter. Resultatet af denne vurdering indikerer, at worst-case hudeksponering eller indånding af dampe kan medføre en risiko for uønskede effekter på helbredet. Risikoen for uønskede effekter på helbredet ved hudeksponering kan dog forholdsvis let minimeres ved brug af personlige værnemidler, specielt handsker. Diskussion og konklusion Fire ikke-klorerede smøremidler har udvist lovende smøreegenskaber i en indledende simuleringstest og en procesteknisk test, som omfatter dybtrækning og stansning i rustfrit stål. Ingen af de fire smøremidler viste dog tilfredsstillende smørende egenskaber i en fuld produktionstest, som omfatter en flertrins pladebearbejdning af et emne rustfrit stål, herunder dybtrækning, ekstrudering og stansning. Det var, under de givne testforhold, således ikke muligt at identificere ikke-klorerede smøremidler, som kan erstattet klorerede smøremidler i fuld produktion ved krævende flertrins pladebearbejdning i rustfrit stål. Produktion af emner i metalindustrien omfatter meget ofte flertrins operationer, herunder forskellige typer af operationer som i den fulde produktionstest. Kravene til et smøremiddels smørende egenskaber kan dog variere en del i forskellige spånløse metalbearbejdningsprocesser. Det vides, at der findes ikke-klorerende smøresystemer, som fungerer med tilfredsstillende smøreegenskaber i individuelle bearbejdningsprocesser. Disse smøremidlers begrænsning er dog, at de er udviklet til en speciel bearbejdningsproces og ofte endog til en speciel kunde. Derfor fungerer disse smøremidler kun under begrænsede produktionsforhold. Ikke alle alternative smøresystemer, som er foreslået, er teknisk testede i projektet. Det skyldes i nogle tilfælde, at smøremidlernes krav til påføring ikke kunne opfyldes under projektets tekniske testforhold. Danfoss A/S har tidligere udviklet et smøresystem – Extreme Pressure Lubricant System (EPL) – til massiv formgivning af rustfrit stål. Massiv formgivning stiller store krav til smøremidlers smøreegenskaber på grund høje plastiske deformeringsforhold med store energifrigivelser. EPL-systemet er et to-lags smøresystem, som består af et bærelag og et smøremiddel. Den nye teknologi i systemet er påføring af et fosforbærelag på rustfrit stål. En almindelig sæbe påføres derefter på metaloverfladen som smøremiddel. EPL-teknikken har vist gode resultater i produktionsforsøg på Danfoss A/S ved fremstilling af bælgrør i rustfrit stål. Danfoss A/S har af økonomiske årsager ikke implementeret EPL metoden som standardteknik i deres produktion, idet deres produktion vurderes at være for lille til at etablere det nødvendige produktionsudstyr. En markedsundersøgelse af salg af fosfateret rustfrit stål viste en overraskende lav interesse. Der kan således være teknisk tilfredsstillende alternativer til klorerede smøremidler i krævende bearbejdningsprocesser i rustfrit stål, men de involverer store omkostninger på grund af store investeringer i produktionsudstyr. Samtidig er klorerede smøremidler multi-funktionelle og kan med stor succes anvendes i de fleste spånløse metalbearbejdningsprocesser. De har veldokumenterede smøreegenskaber og er billige. Sundheds- og miljøvurderingen af komponenter i ikke-klorerede smøremidler og screeningen af foreslåede alternative smøremidler viser, at ikke-klorerede smøremidler tilsyneladende har forbedrede iboende sundheds- og miljømæssige egenskaber. De kan dog indeholde stoffer, som er potentielt allergifremkaldende og stoffer med potentielt miljøskadelige effekter på et niveau svarende til klorerede paraffiner, dog i væsentligt lavere koncentrationer. Resultatet viser også, at substitution af klorerede smøremidler til krævende spånløs metalbearbejdning indebærer et skift fra en rimelig sundheds- og miljømæssig dataplatform til en væsentligt ringere dataplatform. Projektets overordnede konklusion er, at yderligere udvikling af ikke-klorerede smøresystemer til krævende spånløs metalbearbejdning forestår for at opnå teknisk tilfredsstillende alternativer og samtidig forbedre de sundheds- og miljømæssige egenskaber. 1 Introduction1.1 BackgroundThe fields of application of chlorinated paraffins are many. One of the main fields are as extreme pressure additives in metal working lubricants (1). The chlorinated paraffins are chemically and physically stable and have excellent lubricating properties at high pressure. These characteristics in combination with low production costs make the substances technically and economically well suited as high pressure additives in lubricants for metal working. Chlorinated paraffins are complex mixtures of chlorinated n-alkanes characterized by an average carbon chain and chlorination degree. The chain length of commercially available chlorinated paraffins varies from 10 to 30 carbon atoms. From the number of carbon atoms in the carbon chain, the chlorinated paraffins are divided into three main groups: C10-13 – short-chained chlorinated paraffins, C14-17 – medium-chained chlorinated paraffins and C18-30 – long-chained chlorinated paraffins (2). Both short- and medium-chained chlorinated paraffins are used as high pressure additives in metal working lubricants. Due to reduction initiatives for short-chained chlorinated paraffins in the EU, there has been a change within the EU towards an increased use of medium-chained chlorinated paraffins in metal working lubricants (3). Chlorinated paraffins are suspected to cause harmful effects on human health. The EU classification of short-chained chlorinated paraffins (alkanes, C10-13, chloro, CAS No. 85535-84-8) regarding health effects is carcinogenic in category 3 (Carc3) with risk phrase R40 ”Limited evidence of a carcinogenic effect” (4). This classification is based on animal test data (5). A risk assessment of medium-chained chlorinated paraffins (alkanes, C14-17, chloro, CAS No. 85535-85-9) is presently being carried out in the EU. In the latest draft for human risk assessment of medium-chained chlorinated paraffins it is proposed that the substance group should be classified as toxic to reproduction in category 3 (Rep3) with risk phrases R63 ”Possible risk of harm to the unborn child” and R64 ”May cause harm to breastfed babies” in addition to R66 “Repeated exposure may cause skin dryness or cracking”. The classification is based on animal test data (3). The chlorinated paraffins are persistent and bio-accumulative substances. This is reflected in the fact that the substances are found widespread in the external environment, including sediments, aquatic organisms and marine mammals. The substances have also been found in foodstuffs and mother's milk (2,3). In the EU, short-chained chlorinated paraffins (CAS No. 85535-84-8) are classified as dangerous for the environment (N) with the risk phrase R50/53 ”Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment” (4). Medium-chained chlorinated paraffins (CAS No. 85535-85-9) exhibit similar effects in the aquatic environment. In the draft EU risk assessment of the substance group in the external environment, an environmental classification N;R50/53 similar to that for short-chained chlorinated paraffins is proposed (3). Long-chained chlorinated paraffins are currently undergoing a health and environmental risk assessment in the EU. Chlorinated paraffins, including both short-, medium- and long-chained chlorinated paraffins, are included in the Danish Environmental Protection Agency's list of unwanted substances due to the serious health and environmental properties of the substances (6). In the past decade, a number of projects have been carried out in the Danish metal industry aiming at finding alternatives to chlorinated paraffins in metal working fluids. Thus, there has been a substantial reduction in the use of chlorinated lubricants by Danish large-scale consumers in the metal industry. Chlorinated metal working lubricants for cutting operations such as milling, screw-cutting and drilling have gradually been completely substituted. Technically satisfying non-chlorinated lubricants for these types of metal working processes are now readily available on the market. For non-cutting operations, particularly demanding processes such as forming stainless steel and titanium, chlorinated metal working lubricants are still widely used. The reason for this is that so far there have not been technically satisfactory alternatives available on the market (7,8). Thus, chlorinated metal working lubricants are still used in the Danish metal industry in demanding non-cutting operations in stainless steel etc. At the same time, metal working processes making heavy demands on the lubricants become more and more common in the industrial production due to demands for material and energy savings, increased productivity, improved quality etc. Therefore, the need for lubricants to be used under very demanding production conditions is increasing (9). 1.2 ObjectiveThe overall objective of this project is to promote the complete substitution of chlorinated paraffins used in lubricants for metal working by technically acceptable and health and environmentally improved alternatives. As the use of chlorinated lubricants continues especially in heavy-duty metal forming operations in stainless steel and in other metals, the project concentrates on these types of operations. The specific objectives of the project are:
1.3 The projectThe project has been divided into five phases.
Phase 1 In phase 1, technical criteria were set up for lubricants. This was done in order to be able to identify lubricants with potentially suitable lubricating properties in the metal forming processes in focus and secondly to identify lubricants with suitable properties regarding corrosion, stability, application and degreasing etc.. A stepwise selection of lubricants was planned with an initial identification of suitable lubricants based on supplier information, secondly an identification of promising lubricants based on results in simulating technical tests and thirdly an identification of the most suitable lubricants for testing in the production equipment at Danfoss A/S. Also in phase 1, health and environmental criteria for lubricants were set up. This was done in order to define an acceptable lubricant in a health and environmental context and to avoid production tests of lubricants with obvious unwanted health or environmental properties. Phase 2 A mapping of existing non-chlorinated lubricant systems for heavy-duty metal forming in stainless steel was performed in phase 2 by contacting international lubricant suppliers and suppliers of lubricant additives. Phase 3 In phase 3, the non-chlorinated lubricants for which supplier information showed that they possessed suitable lubricating properties in the metal processes in focus, and for which the supplier agreed to forward a test probe, were tested in simulating and process technical tests. Simultaneously, a health and environmental screening of proposed non-chlorinated lubricants was performed in order to get an overview of the health and environmental characteristics compared to chlorinated lubricants and to identify lubricants with obvious unwanted health and environmental properties prior to the production tests of selected lubricants. Phase 4 Selected lubricants exhibiting promising lubricating properties in the simulating and process technical tests were tested in production at Danfoss A/S. A health and environmental assessment of selected components typically occurring in non-chlorinated lubricants was performed. This was done in order to provide an overview of the health and environmental data platform for components in non-chlorinated lubricants and to perform assessments of inherent health and environmental properties of the components based on available data. In addition, exposure assessments at a screening level of selected components in non-chlorinated lubricants were performed. Phase 5 In phase 5, the project results were communicated. Project partners Partners in the project has been Danfoss A/S, Esti Chem A/S, the Department of Chemistry of the Technical University of Denmark and CETOX (Centre for Integrated Environment and Toxicology). Danfoss A/S is the largest industrial group in Denmark and one of the leading companies in the world within refrigeration and air conditioning, industrial controls, heating and water and motion controls. Danfoss A/S produces a long line of products by forming stainless steel. Esti Chem A/S is part of the Dow Chemical Company. Esti Chem A/S has specialised in development of synthetic ester based raw materials for formulation of industrial lubricants. Substitution of chlorinated lubricants in metal working has long been a high-priority development area for Esti Chem A/S. The Department of Chemistry of the Technical University of Denmark is working in the area of inorganic chemistry, molten salts chemistry and materials chemistry including relations between chemical and lubricating characteristics. For several years, the department has been engaged in projects dealing with the development of non-chlorinated lubricants for metal working and has expert knowledge on tribological tests. Tribology is the science of lubrication, theory and technique. CETOX is a formal cooperation between the Danish Toxicology Centre (DTC) and DHI Water and Environment. The working area of CETOX is consultancy for industry and authorities in the area of integrated human toxicology and eco-toxicology. Both DTC and DHI are independent, non-profit technological service institutes approved by the Danish Minister for Science, Technology and Development. 2 Metalworking – processes, materials and lubricants
2.1 Introduction to chapter 2The objective of chapter 2 is to give a short introduction of both the metal working processes in focus in this project and the lubricant formulation including the present status of lubricant technology for heavy-duty metal working. 2.2 Processes and materialsMetalworking processes are normally separated into two main types, chip removing (cutting) operations and chip-less (forming) operations. Chip removing operations include milling, threading and drilling. Chip-less operations are subdivided into sheet- and cold forging operations. Sheet metal forming involves operations such as punching and deep drawing. Relevant cold forging operations in this project are extrusion and backwards can extrusion. This project focuses on chip-less machining of metallic materials and especially on sheet forming operations. Thus, lubricants developed for sheet forming operations are the principal interest in this project. However, if lubricants developed for cold forging operations show properties, which might contribute positively to lubrication in sheet forming operations, they will be included in the project. Danfoss A/S, a large Danish company, participates in this project as the metal industry partner. Production tests of selected lubricants, described in chapter 5, are performed at Danfoss A/S. The metal working operations at Danfoss A/S, where chlorinated lubricants are used at present, are forming operations in stainless steel including:
The forming processes may include combinations of the mentioned operations. The three types of forming operations are briefly described below. In sheet metal forming, cutting intended for creating a controlled break in the material, is the process most often used. Punching which is a process, where holes of different shapes are made in the blank is an example of a metal forming process including cutting (see figure 2.1). Deep drawing is defined as a pull-pressure forming of a plane sheet to a hollow body, or a hollow body changing shape to a hollow body of a smaller circumference without changing the thickness of the sheet (see figure 2.2). Extrusion is a cold forging process where the body is severely deformed during great heat generation. An example of extrusion is shown in figure 2.3 where a cup is formed with reduced wall thickness. Figures 2.1, 2.2 and 2.3 illustrate the three forming operations. 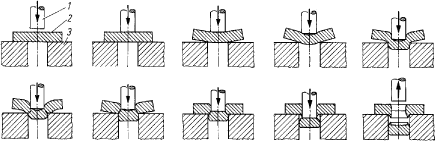
Figure 2.1 Sequence of punching: 1. Punch, 2. Sheet, 3. Support 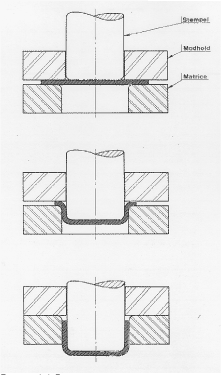
Figure 2.2 Deep drawing 
Figure 2.3 Extrusion Materials for which chlorinated paraffins are required at Danfoss A/S in the forming operations are:
Figure 2.4 and 2.5 shows work pieces produced by Danfoss A/S. In the production process of these work pieces chlorinated lubricants are required. The shown work pieces and the forming operations are representative of the use of chlorinated lubricants. To a less extent more extreme deep drawing, extrusions and punching forming operations are also conducted. 
Figure 2.4 Deep drawing in several steps with slight extrusion in final step. Performed in a multistage deep drawing including slight extrusion in the last step. Produced in a step press. 0,8 mm stainless steel (W.nr. 1.4306) 
Figure 2.5 The three work pieces are made in a step press at Danfoss A/S with up to seven draws and a final calibration (extrusion to final thickness of material). The materials are stainless steel (W.nr. 1.4301), acid resistant steel (W.nr. 1.4404), and a copper coated acid resistant steel ((W.nr. 1.4404 / Cu). The forming operations in focus in this project are divided in the following groups: A1 Deep drawing of highly alloyed steel (>4% Cr) B1 Punching of sheets of highly alloyed steel (>4% Cr) 2.3 Lubricants2.3.1 Traditional formulation of lubricants for chip-less manufacturingThe traditional lubricants for use in chip-less processing of metals are normally based on mineral oil, polar additives and so-called extreme pressure (EP) additives. EP additives in the lubricant prevents sliding metal surfaces from seizing under conditions of extreme pressure. At the high local temperatures associated with metal-to-metal contact, an EP additive combines chemically with the metal to form a surface film that prevents the welding of opposing asperities and the consequent scoring that is destructive to sliding surfaces under high loads (10). Other additives may also be present in the lubricant such as biocides, de-foamers, yellow metal inhibitors etc.. However, these additives do not contribute to the lubricating properties of the formulation. The lubricants are most often used as straight oils (oil based wet lubricants). However, water-miscible formulations (water miscible wet lubricants) and dry-film lubricants (dry lubricants), which are applied in wet form and dried up as a solid film before processing of the metal, are also seen in industrial applications. The traditional lubricants for chip-less manufacturing usually consist of the following components:
The below-mentioned parameters are particularly important in the design of the qualities of the finished lubricant.
2.4 Metal forming and lubricants – technical development2.4.1 Problems when replacing chlorinated paraffinsWhen replacing chlorinated paraffins, the following problems are particularly encountered:
One of the basic problems encountered when replacing chlorinated paraffins is thus connected to their ”multi-functionality” which cannot be found in any other EP additive. Instead of using a single EP additive, the formulator is often forced to choose a “package” of lubrication additives. Doing so, the finished lubricant becomes much more complex than a traditional chlorinated lubricant. Naturally, this causes increased expenses and subsequently problems when selling the products on a market where the use of chlorinated paraffins is not directly banned. Another problem is to document the technical properties of an alternative lubricant to the customers. There is a lack of standardised lubricating test methods where results from laboratory testing can be correlated with full-scaled production trials. 2.4.2 Present statusParticularly in Western Europe, the producers of lubricants have worked intensively to replace chlorinated paraffins in metal working lubricants. This has been a success in most lubricants for cutting processing of ordinary steel, copper, brass and aluminium and less demanding chip-less metal forming processes. However, until today, it has been problematic to find technically satisfying alternatives to chlorinated paraffins, for instance demanding chip-less processing of stainless steel and titanium. Non-chlorinated lubricant alternatives using new formulation technologies such as inorganic fillers and polymers are now on the market. However, these products are often developed for one particular process and one particular client. Thus, the supply of products is huge and an overall view of non-chlorinated lubricant alternatives is not possible. In addition, testing of new chlorine-free lubricants is costly for the metalworking companies as it implies breaking off in production, possibly repairs of tools due to breakdown of the test lubricant, and increased scrap percentage of the final work pieces. Subsequently, this leads to downtime and increased costs. Thus, there is still a substantial need for development of new non-chlorinated lubricant alternatives, especially for heavy-duty metal forming operations which are universal, reliable, economic, and possible to use in the existing production lines without major modifications of the technical equipment. 3 Demands and specifications for non-chlorinated lubricants
3.1 Introduction to chapter 3The objective of chapter 3 is to describe the technical criteria set up by project partners in this project in order to be able to identify non-chlorinated lubricants exhibiting promising lubricating properties in the metal processes in focus and to identify lubricants suitable for testing in simulating and process technical tests and in production at Danfoss A/S. In addition, the objective of chapter 3 is to describe the health and environmental criteria set up by project partners in this project in order to define an acceptable lubricant in a health and environmental context. Based on criteria set up for acceptable lubricants, those with obvious unwanted health and environmental properties could be deselected for production test. 3.2 Technical demands and specifications for non-chlorinated lubricantsA three stage technical identification and priority of proposed alternative lubricants has been performed in the project based on specified technical criteria initially set up by project partners. In the first step, an identification of lubricants was made based on technical supplier documentation. The primary focus was on non-chlorinated lubricants with promising lubricating properties in heavy-duty forming operations in stainless steel etc. as specified in chapter 2. However, attention was also paid to secondary lubricant properties such storage stability and aggressiveness to common construction materials etc, in addition to the lubricants' compatibility with application conditions in the subsequent technical tests and with conditions at Danfoss A/S. In the second step, the technical efficiency of proposed lubricants that passed step one was tested in two technical tests described further in chapter 6. Lubricants exhibiting promising lubricating properties based on the results in the technical test where selected for a full scale production test at Danfoss A/S. In the third step, lubricants selected in step 2 were tested in a full scale production test at Danfoss A/S. The test, which is described further in chapter 6, was performed without adjustment of the production. Thus, lubricant systems which exhibited promising lubricant properties in specified metal forming operations based on supplier information but did not comply with the technical test conditions for instance regarding application, were not technically tested in this project. For the selection of alternative lubricants, the following technical criteria were set up: Primary technical criteria:
Secondary technical criteria:
Demands specific for Danfoss A/S:
3.3 Health and environmental demands and specifications for non-chlorinated lubricantsA health and environmental evaluation at the screening level was carried out for proposed alternative lubricants that passed step 1 of the technical identification and priority of suitable non-chlorinated lubricants and for which sufficient compositional information had been obtained from the supplier. The results of the screening of the proposed lubricants are described in chapter 8. A health and environmental evaluation has been performed for components commonly occurring in non-chlorinated lubricants for heavy-duty metal forming. The results of the health and environmental assessment of representative lubricant components are described in chapter 7. Both the screening of proposed lubricants and the assessments of representative lubricant components involve health and environmental rating systems. The assessment methods including the rating systems are described in chapters 7 and 8. Exposure assessments have been carried out in the working environment for four substances representing two types of components that typically occur in non-chlorinated lubricants for heavy-duty metal forming. The method and results are described in chapter 9. In the project, criteria for an acceptable lubricant in a health and environmental context were defined by the project partners. In the project, there is a focus on the inherent health and environmental properties of chemical substances. The parameters, on which the health and environmental assessments of the proposed non-chlorinated lubricants and representative lubricant components are based, in addition to criteria for health and environmental acceptable lubricants, are described in sections 3.3.1 and 3.3.2, respectively. 3.3.1 Health parameters and criteria for non-chlorinated lubricantsHealth parameters The following parameters are included in the health evaluation of proposed non-chlorinated lubricants and representative lubricant components. As mentioned above, all parameters and criteria focus on inherent properties of substances and products.
The health evaluation is based on principles in the EU health classification of chemical substances and preparations (11). Health criteria Substances and products with the following effects, classifications and health scores should be avoided:
Substances and products which, based on adequate data, do not comply with the above-mentioned criteria and thus are assigned health score 1 or 2 are considered as good alternatives to chlorinated lubricants in a health context. 3.3.2 Environmental parameters and criteria for non-chlorinated lubricantsAs in the health assessment of lubricants and lubricant components, the environmental assessment focuses on the inherent properties of substances and products. The following parameters are included in the environmental evaluation.
The environmental evaluation is based on principles in the EU environmental classification of substances and preparations (11) and the global classification system (OECD 2001). Substances and products with high toxicity to aquatic organisms (EC50 < 10 mg/l) and substances that are not readily biodegradable or bioaccumulate (BCF > 500, Log Pow > 4) should be avoided. According the EU environmental classification system, these substances are classified as dangerous to the environment (N) with risk phrase R50: “Very toxic to aquatic organisms” or R51: Toxic to aquatic organism” and risk phrase R53: “May cause long-term adverse effects in the aquatic environment (N;R50/53 or N;R51/53). These classifications correspond to environmental scores 4 and 5 respectively (for a description of environmental scores, see also chapter 7). 4 Strategy of data search4.1 Introduction to chapter 4Chapter 4 describes the strategy of data search for retrieval of relevant and sufficient information from suppliers of non-chlorinated lubricants and subsequently to retrieve further health and environmental data on components in non-chlorinated lubricants. This in order to be able to make an identification of technically suitable lubricants, to perform a health and environmental screening of proposed non-chlorinated lubricants and to perform health and environmental assessments of lubricant components. 4.2 The strategy of data search4.2.1 Retrieval of information on proposed lubricant alternativesInitially, technical and compositional information on non-chlorinated lubricant alternatives was obtained from the suppliers of the proposed lubricants. If possible, the following documentation was requested from the lubricant suppliers:
4.2.2 Retrieval of further health and environmental dataThe health and environmental screening of proposed lubricants is based on information from suppliers of the lubricants, a search in Annex 1 of Directive 67/548/EEC on classification and labelling of dangerous substances in addition to a data search in well-recognized toxicological and ecotoxicological handbooks and databases. The health and environmental assessment of components typically occurring in non-chlorinated lubricants for heavy-duty metal working is based on information retrieved from suppliers of proposed alternative lubricants, on data in well-recognized handbooks, reviews and databases of toxicological and eco-toxicological relevance, on internet searches for the specified CAS Nos. and substance names, on internet searches for home pages of raw material suppliers in addition to personal communication with industry experts. The strategy of data search is listed below:
The health and environmental assessments of proposed lubricants and lubricant components is performed at a screening level. The data quality is not considered in details and rated though data is retrieved from common acknowledged sources. The methods and results of the health and environmental assessment of typical lubricant components in non-chlorinated lubricants are described and discussed in chapter 7. The health and environmental screening of proposed alternative lubricants is based on information from the lubricant suppliers. The methods and results of the health and environmental screening of proposed lubricants are described and discussed in chapter 8. 5 Mapping of alternative lubricants5.1 MethodIn order to get an overview of non-chlorinated metal forming lubricant technology available on the international market, contact has been made to a number of companies in Europe and the US. These are mainly larger companies with expertise in the field of metalworking lubricants and extreme pressure additives. However, there are also a number of smaller, highly specialized companies. Some of the companies contacted are Castrol, Henkel Surface Technologies, Master Chemical Cooperation, Klüber Lubrication, Houghton, Holifa, Rhenus Lub, Vulcan Oil & Chemical Products, Pfau Oil Products, Fuchs DEA Schmierstoffe, Uniqema, Chemetall, Quaker Chemical, Rocol Lubricants, Hangsterfers and Dover Chemical Corporation. In addition, contact has been made to a large steel supplier, Avesta Polarit. This in order to clarify whether pre-coated steel plates are being marketed. The purpose of the pre-coating of steel plates should be to diminish or remove the demand for lubrication in the subsequent manufacturing processes. The lubricant suppliers have been asked about lubricants for deep drawing, several step deep drawing, stamping, several step stamping, extruding, several step extruding and calibration as well as ironing in stainless steel, acid-proof steel, titanium, alloys and metal combinations – e.g. coatings (see also chapter 2). Not only the process type and material define the technical demands to a metal working lubricant. Also tools and application and washing conditions influence the ultimate technical suitability of a lubricant. However, mapping of alternative lubricants has mainly focused on compliance with technical demands for the core property of the lubricant, namely the lubricating ability in specified processes. Demands for application and washing etc. are considered to be secondary properties of the lubricant, for which it is most often possible to subsequently adjust. Contact to lubricant and additive suppliers has been made in two steps. In step one, a request is made to the suppliers for non-confidential sales information such as use, technical suitability and capacity, physical/chemical data, application, washing and removal as well as a material safety data sheet. Based on the information obtained, an evaluation has been made of the technical suitability of the lubricants for the manufacturing processes that are the focus of the project. In step two, contact has been made to the suppliers who, based on the forwarded information, will possess promising alternative lubricants. This step to obtain information on product composition including any data on effects on health and environment. The lubricants suggested are divided into categories with regard to suitable manufacturing processes and materials, type of lubricant based on chemical composition and application of the lubricant. For manufacturing processes and materials, the following categories are used: A Deep drawing, unspecified B Stamping, unspecified C Extruding, unspecified With regard to type of lubricant, the lubricants are categorized according to their basic content. Basic content:
Type 1 are lubricants based on various mineral oil fractions and free from water. The agents are used undiluted. Type 2 are solvent-based lubricants, free from water and used undiluted. The agents have a high content of solvents. Type 3 are emulsifiable lubricants with a mineral oil content of 20-30 %. The agents are used diluted with water. Type 4 are semi-synthetic lubricants with a mineral oil content of less than 10-15 %. The agents are used diluted with water. Type 5 are lubricants based on synthetic oils and free from mineral oil. The agents may be diluted with water prior to use. Type 6 are lubricants based on vegetable and/or animal oils and free from water. The agents are used undiluted. Type 7 are emulsions of vegetable and/or animal oils. The agents do not contain mineral oil and should usually be diluted with water. In addition, the lubricants have been categorized as dry lubricants (D) or wet lubricants (W), respectively depending on the type of application. See also chapter 2. 5.2 ResultsIn step 1 of the mapping phase, contact has been made to approximately 50 international companies marketing either metal working lubricants or lubricant additives. Of these, 16 companies have forwarded suggestions for approximately 60 alternative lubricants based on technical specifications on manufacturing processes and materials. As contact has been made to suppliers of both lubricants and EP additives, the suggestions for alternative lubricants include finished products (off-the-shelf items) as well as products requiring a certain production maturation. A technical examination of the forwarded product information assessed that 15 of the 16 suppliers should possess lubricants that have a potential for being technically suited for the processes and materials described in chapter 2. The result of step 1 of the mapping phase is stated below in table 5.1. As can be seen from the table, 53 products are estimated as potential alternatives to chlorinated lubricants in the processes specified. Seven of the initially proposed lubricants are not included in table 5.1 due to failure in complying with technical criteria established in step 1 of the technical assessment of proposed lubricants.
Table 5.1 Overview of proposed alternative lubricants for the metal working processes in focus and described in chapter 2. An * indicates that use of the lubricant in question depends on a preceding phosphating or oxalating of the metal surface. W indicates a wet lubricant, D indicates a dry lubricant. See also chapter 2. As can be seen from table 5.1, it varies how specifically the supplier has stated the processes suitable for the lubricants. Most of the lubricants suggested are traditionally formulated as wet lubricants similar to chlorinated lubricants. Six lubricants are dry lubricants which means that a drying step is needed in the manufacturing process, see also chapter 2. Two proposed lubricating systems marked with an * require a preceding phosphating or oxalating of the metal surface prior to lubrication, see also chapter 2. Wet lubricants are the dominating type of lubricants. As regards chemical composition, the majority of the lubricants suggested are based on either synthetic esters, polymers or on mineral oil. However, virtually all types of lubricants are represented, including a solvent-based lubricant – type 2. The last-mentioned was dropped in step 1 of the mapping phase due to a low flash point (< 100°C).
Table 5.2. Overview of suggested alternative lubricants that are subsequently tested in the simulating test in step 2 as described in section 5.1. In step 2 of the mapping process, contact has been made to the 15 suppliers who, based on the information forwarded, are in a position to offer lubricants that are technically suitable for demanding forming operations in high alloy steel and other metals, as specified in section 5.1. Product samples for testing and detailed composition information for the relevant products are requested at this stage. As can be seen from table 5.2, 11 suppliers have forwarded a total of 19 lubricants for testing. Of the 19 proposed lubricants, sufficient information regarding the chemical composition has been received for 15 lubricants in order to perform the health and environmental assessments of lubricants and selected lubricant components. Despite our request for non-chlorinated lubricants, one product turned out to contain polychlorinated alkanes. This product was dropped. One dry lubricant is represented among the 15 products. Lubricants based on mineral oil, synthetic esters as well as vegetable and animal oils are represented. As mentioned in section 5.1, contact has also been made to a large supplier of steel during step 1 of the mapping phase. The supplier has been contacted in order to clarify whether a preceding coating of steel plates can take place and function as a lubricant in the subsequent metal working processes or reduce the demand for subsequent lubricants. The result was that no coating of steel plates takes place prior to resale to remove or reduce the need for lubricants during steel work. No efforts are being made to develop such processes (12). 6 Technical assessment of non-chlorinated lubricants6.1 IntroductionThe objective of chapter 6 is to describe the methods and results of the testing of proposed lubricants in technical tests and in production at Danfoss A/S, in addition to a discussion of the test results. 6.2 Test methodsBelow is a description of the test methods used to evaluate the technical performance of the proposed lubricants for heavy-duty metal forming, which passed step 1of the technical selection sequence described in chapter 3. A description of the used reference lubricants is also enclosed. Simulative test: In previous projects involving the Technical University of Denmark as a co-worker, several qualified test methods to evaluate the performance of lubrication were identified. Figure 6.1 shows different simulative tests (A-H) to be used for characterization and scaling of the performance of a lubricant. The most critical partial processes are: B) ”bend under tension” and H) ”strip reduction”. Strip reduction is mainly a cold forging operation, but is part of the deep drawing process. H) ”strip reduction” is chosen as the simulative test method. 
Figure 6.1 Simulative technical test: Identified partial processes. The equipment for strip reduction is shown in figure 6.2. Strip reduction is carried out by pressing a tool with a polished surface (Ra = 0,01 mm) into a sheet material (strip) to a certain depth. The strip is pulled, while the tool is being pressed into it, and the thickness of the strip is being reduced. The cylindrical tool is not rotating during the reduction. In table 6.1, the most important test parameters are summarized. Table 6.1 Parameters for strip reduction
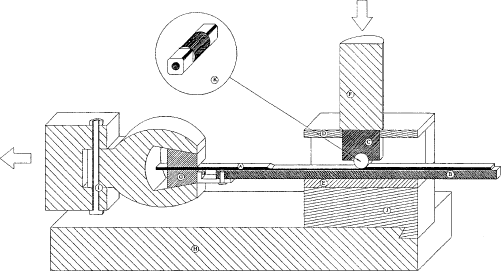
Figure 6.2 Set-up for strip reduction. (A) Strip, (B) Hardened steel rod, (C) Pressing block, (D) Distance sheet, (F) Vertical piston, (G) Horizontal piston with claw, (K) Tools When the lubricant is breaking down in strip reduction, the strip and tool will cold weld, and the soft material from the strip is welded to the hardened tool. This material causes severe scratches in the strip parallel to the pull direction. Few scratches can occur even with a good lubricant, but if the performance of the lubricant is not satisfactory under the specific test conditions, these scratches are developed due to a rise in temperature. The rise in temperature will breakdown the lubricant further, and the scratches will develop to a larger band on the strip. Figure 6.3 shows strips reduced in strip reduction. On the top of the figure a good lubricant is used and in the bottom, a bad lubricant is used. In the bottom of the figure is seen that the scratches develop into to a wide band (marked with the grey area). The band formation is defined as the position from the start of the reduction (left side in figure 6.3) to the position where the individual scratches cannot be separated under a stereo microscope. The performance in strip reduction of different lubricants are determined by comparing the results of a well known bad lubricant (pure mineral oil) and a good lubricant (chlorinated paraffin). The lubricants are scaled according to performance in the individual tests. 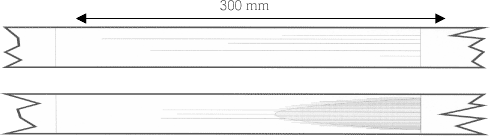
Figure 6.3 Strips reduced in strip reduction from left to right. In the top is seen some scratches in the strip. In the bottom the scratches have developed to a wide band (marked with a grey area) Process technical test: A deep drawing test is carried out with the selected lubricants using a press and tool. This test includes severe process parameters. The test, which is called backwards can extrusion, is carried out. A can is created by pressing a blank over a punch. This process is illustrated in figure 6.4. 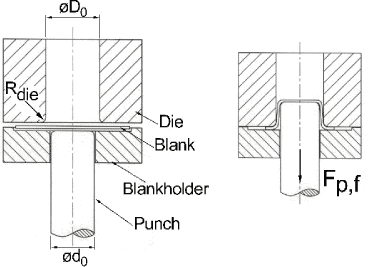
Figure 6.4 Backwards can extrusion. To the left is shown a situation before forming and to the right is shown the forming operation with the die pressing the blank over the punch. The ratio between the diameter of the punch and the blank is called the draw ratio and the larger the draw ratio the more stressing for the lubricant. The most important parameters for the tests are shown in table 6.2. Table 6.2 Parameters for backward can extrusion.
During the entire operation (forming and following separation of the tool and can) the affecting power is collected to computer. In this forming operation, lubricant breakdown between the outer of the can and the die will occur often, whereas it will occur more seldom between the inner side of the can and the punch. The collected powers are:
During normal process conditions, the can will often stay on the punch after forming, but in case of severe lubricant breakdown, the can may be dragged off the punch when the die is withdrawn. In this case, the force measurement Fd,w (withdrawal force between can and die after forming) will actually be measured as Fp,w (withdrawal force between can and punch after forming). In these cases the Fd,ej (ejection force between can and die) is the sensitive force measurement. When dry film lubricants are used, it is observed that the can is often located in the die after forming, which is probably due to a liquid viscose lubricant causing the can to be sucked on the punch. To evaluate the development of the involved forces and the development of lubricant breakdown, a number of cans (more than 3) are formed. Scaling of the performance for the tested lubricants will primarily be based on the mentioned force measurements, and secondary (if needed) on a visual inspection of the cans. Full scale production test: In this project, the lubricants showing the best performances in the simulative and the technical test are tested in full scale production at Danfoss A/S (Nordborg, Denmark). In the production test, the lubricants are tested in a multi stage tool forming a work piece as shown in figure 6.5. The work piece is produced in seven process steps, including deep drawing, extrusion and punching without any annealing of the work piece between each step. As seen to the right in figure 6.5, the middle hole is flanged, which causes a reduction of the thickness of the sheet. Danfoss A/S has identified this flanging as the most critical process in the production of the shown flange, because the rim is torn off the work piece if lubricant breakdown occurs. 
Figure 6.5 Flange of stainless steel (W. no. 1.4301) produced by Danfoss A/S using of lubricants containing chlorinated compounds. Shown in real size. During the flanging process, the thickness of the sheet is reduced approximately 50%. Scaling of the efficiency of the lubricants is based on number of produced work pieces at a given production speed before a lubricant breakdown occurs. Reference lubricants: For the simulative test is chosen a well known bad lubricant, a pure mineral oil mixture without any additives. The mixture contains 50 wt% Sunoco Sun 60N (low viscosity naphthenic mineral oil) and 50 wt% Houghton Plunger CR5 (high viscosity paraffinic mineral oil). A viscosity of 60 cSt at 40°C (8 cSt at 100°C) is obtained from this mixture. The lubricating properties of this mineral oil mixture is only due to a physical separation of tool and work piece and removal of heat from the deformation zone. For both the simulative and the process technical tests is chosen a well known good lubricant, Castrol Iloform TDN 81 containing 80% of a medium-chained chloroparaffin with a chlorination degree of 50%. In the full scale production test at Danfoss A/S, a chlorinated lubricant (Holifa HFF 13) is chosen as reference. This lubricant is usually used in the production at Danfoss A/S. Holifa HFF 13 contains a medium-chained chloroparaffin with an average degree of chlorination of 20%. Table 6.3 Reference lubricants.
6.3 Test resultsSimulative test: As mentioned in section 6.1, simulative test, the performance of a lubricant can be evaluated in the simulative test ”strip reduction” by comparing the result with the results of a bad and a good lubricant. In this test, the performance of the lubricant is evaluated from two visual criteria, namely the distance to lubricant breakdown (band formation) from the beginning of the reduction and the amount of scratches at the end of the reduction (sliding length = 300 mm). In strip reduction, a reduction of 45% (a strip is reduced 45% in thickness) is the limit for the chosen test materials and this is only obtained when applying the best lubricants. As a starting point, the lubricants are tested with 30% reduction, because this reduction is large enough to reject some lubricants to further testing. In strip reduction, each lubricant was tested three times, except lubricant no. 12, 39 and 40. The performance of these particular lubricants was so bad that a repetition was not advisable. For the simulative test, all results are presented as an average of the three measurements for each lubricant with a standard deviation incorporated. Figure 6.6 shows the distance (in mm) from the start of the reduction until band formation is visually observed in the stereo microscope for the reference lubricants (MO - mineral oil mixture, CP1 - Castrol Iloform TDN 81 and CP2 - Holifa HFF 13) and the tested lubricants. On the figure, the top limit is 300 mm (sliding length = 300 mm), which is the assigned value, if reduction has occurred without band formation. From the first three columns in figure 6.6 (the reference lubricants) it is seen that the mineral oil mixture (well-known bad lubricant) breaks down after a reduction length of 169±57 mm, and the two chlorinated lubricants (well-known good lubricants) can be reduced in the entire length (300 mm) without band formation. 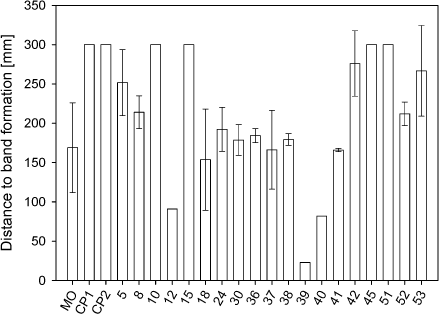
Figure 6.6 Distance to band formation of the tested lubricants at 30%'s reduction. It is seen from figure 6.6 that lubricant numbers 10, 15, 45 and 51 can be reduced in the entire length (300 mm) without band formation, whereas the remaining lubricants break down between 140 to 276 mm. Lubricants only tested once (lubricant numbers 12, 39 and 40) all break down and create band before 100 mm sliding. From appendix 1 it is seen that lubricant numbers 42 and 53 only create band in one of the three measurement, but since band formation is due to a severe lubricant breakdown, these two lubricants were not chosen for further tests in the project. Lubricant numbers 10, 15, 45 and 51 are the only lubricants, which were identified by the strip reduction test as having good enough performance to continue testing in the project. A visual evaluation of the amount of scratches after reduction is only possible for lubricants, which have not created band during the test. Figure 6.7 shows the result of the counted scratches after 300 mm's reduction for the tested lubricants. 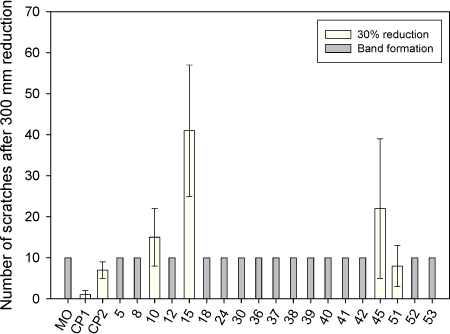
Figure 6.7 Result of the simulative test: Number of scratches after 300 mm's reduction of 30%. From figure 6.7 it is seen that after 300 mm's reduction, only few scratches were observed on strips reduced with CP1 (Castrol TDN81) compared to the strips reduced with CP2 (Holifa HFF13). The fewer scratches are probably due to the higher degree of chlorination of the CP1 chlorinated compound (see table 6.3, section 6.2). The figure clearly shows that the number of scratches in the tested lubricants, which do not create band is quite large (8 to 41 in average). Figure 6.7 also shows that the performance under the specified test conditions is good for lubricant number 10 and especially lubricant number 51 compared to the two chlorinated lubricants. 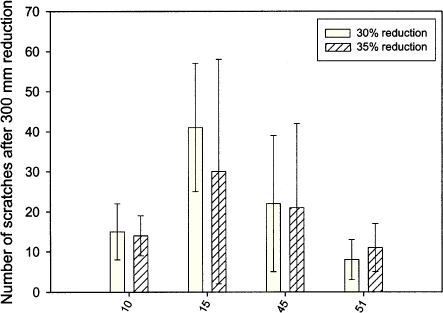
Figure 6.8 Result of the simulative test: Number of scratches after 300 mm reduction (30 and 35 %) for lubricant numbers 10, 15, 45 and 51. To further separate, and thereby scale, the performance of the four lubricants, which did not create band at 30 %'s reduction, two additional measurements were carried out in strip reduction with each of the four lubricants at 35 %'s reduction. Even at 35 %'s reduction, none of the four tested lubricants created band. The results from counting number of scratches after 300 mm's reduction are shown in figure 6.8. From the figure it is seen that the amount of scratches reduces at increased level of reduction for lubricant number 10, 15 and 45 and increases a bit for lubricant number 51. It is not expected that the amount of scratches are falling at 35% reduction compared to 30% reduction, since an increased reduction should cause more critical condition for the lubricant and thereby more locally lubricant breakdown (increased amount of scratches). The test result may be due to statistical uncertainty in the reductions. Another explanation could be that in case of very good EP additives, in the lubricants, a change (increase) in pressure and temperature may cause an increased activity of the EP additive, and consequently – an improved performance of the lubricant. A further increase of the reduction to e.g. 40 % in order to improve scaling of the performance of these four lubricants is not advisable, since other conditions may interfere with the results when approaching the technical limit (45% reduction). The performance of lubricant numbers 10, 15, 45 and 51 is far better than the other tested lubricants. The performance of these four lubricants under the chosen test conditions is quite good, not better, however, compared to the two chlorinated reference lubricants. The performance of lubricant number 10 and 51 is slightly better than lubricant number 15 and 45. Process technical test: From the experiments carried out in the simulative test it was decided that lubricant numbers 10, 15, 45 and 51 were to be tested in the process technical test ”backwards can extrusion” where a deep drawing is carried out. It was decided to test the lubricants with a draw ratio of 1.8 and 2.0. From section 6.2 other process parameters can be found. It was decided to form ten cans in the testing of each of the four lubricants. In a previous investigation, lubricant number 15 has shown quite good performance in backwards can extrusion. Therefore, it was decided not to test this lubricant with a draw ratio of 1.8. 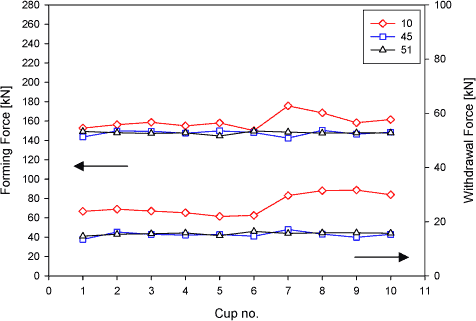
Figure 6.9 Result of the process technical test The upper graphs in figure 6.9 shows the development of the forming force for lubricant numbers 10, 45 and 51, and the lower graphs show the development of the withdrawal force at a draw ratio of 1.8 in backwards can extrusion (see section 6.2 for a description of the mentioned forces). The forming force for the three tested lubricants at this draw ratio (1.8) starts at the same level (about 150 kN). However, when more cans are drawn, the forming force for lubricant number 10 is seen to increase up to about 180 kN. at can number seven. The forming force for the other two lubricants (numbers 45 and 51) does not increase within the number of drawn cans. The withdrawal force (lower graphs in figure 6.9) shows the same tendency in force development, also here, an increase in force is observed for lubricant number 10, but not for lubricant numbers 45 and 51. The lubricant film breaks down for lubricant number 4 at the seventh drawn can. The maximum number of cans, which can be drawn at a draw ration of 1.8, is six for lubricant number 10. Lubricant numbers 45 and 51 can draw all 10 cans without any lubricant film breakdown at this draw ratio. 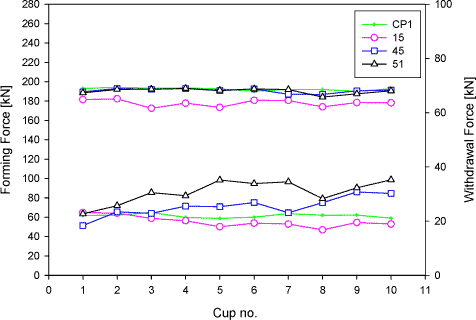
Figure 6.10 Result of technical test. Forming (upper graphs) and withdrawal force (lower graphs) in backwards can extrusion for CP1 and lubricants number 6, 18 and 19 at a draw ratio of 2.0 At the draw ratio of 2.0, lubricant numbers 15, 45 and 51 and the chlorinated reference lubricant, CP1, are tested. The results from this test are shown in figure 5.10. The forming force for CP1 and the lubricants numbers 45 and 51 are even about 190 kN and does not increase with the number of drawn cans. The forming force for lubricant number 15 is not as stable as the other lubricants, but in return, the forming force are about 10 kN (about 5%) lower than for the other lubricant. This observation is most likely due to the nature of lubricant number 15, since this is a dry film lubricant, lubricating with a very low viscous film and a efficient EP additive. The forming force for other liquid lubricants (also lubricants tested outside this project) are almost independent of the viscosity at the same level at the same test parameters. From figure 6.10 it is observed that the withdrawal force for CP1 and lubricant number 15 are at an even level (about 60 kN), whereas the withdrawal force for lubricant numbers 45 and 51 is less stable and increases slightly with the number of cans drawn, but without any sudden increase in force. The only explanation for the slight increase in withdrawal force for lubricant number 45 and 51 is that the lubricating limit for these lubricants is a draw ratio of approximately 2.0 The result of the technical test is that the performance of lubricant number 15 is equal to the chlorinated reference lubricant under the test conditions. The performance of lubricant numbers 45 and 51 is slightly worse, although not critical, but force development, which indicates that the lubrication limit is reached at a draw ratio of 2.0 for these lubricants is observed. Lubricant number 10 breaks down at a draw ratio of 1.8. Full scale production test: Lubricant number 15 has previously been tested in full scale at Danfoss A/S in another project without showing good performance. It was concluded that the bad performance was due to its nature (dry film lubricant), as previously discussed. Therefore, this type of lubricants is not suitable for operations, such as punching, where lubricants need to be pulled with the tool. Lubricant number 18 is a liquid variant of number 15 (same producer and same EP additive) and since lubricant number 15 has shown good performance in both preliminary tests, it was decided to test lubricant number 18 instead of number 15 in the full scale production test. In the full scale production, a work piece (described in section 6.2) is formed in a progress tool at a production speed of 60 work pieces per. minute. The usual production speed at Danfoss A/S is 120 work pieces per minute when using lubricants containing chlorinated compounds. The progress tool is adjusted, thus lubricant break down cause an emergency stop. Unnecessary war of the tools is hereby avoided. Under the chosen test conditions none of the four lubricants (numbers 10, 18, 45 and 51) could produce more than 40 work pieces before lubricant break down occurred. This caused an automatic emergency stop of the test in all cases. The attached pick-up to the tool prevented further automatic production with the test lubricants, but it was possible to continue manual production of more work pieces with all lubricants. This manual production (slow and piece by piece) probably prevented elevated temperature (automatic work temperature) and thereby continued building of pick-up on the tool. Due to the bad performance of the tested lubricants in the full scale production test, it was not possible to scale the lubricants individually. 6.4 ConclusionsThe strip reduction test used to evaluate the lubricating performance of proposed lubricants in this project causes severely demanding conditions due to large surface expansion, high temperature and low speed. From the results in the strip reduction test (30% reduction), it may be surprising that only four lubricants (numbers 10, 15, 45 and 51) out of 20 tested showed satisfying lubricating properties since all 20 lubricants were proposed by suppliers based on a thorough description of test methods, test conditions and test materials (both tool and sheet material). The results of the strip reduction test indicate that it is difficult to design a lubricant to be used under severe conditions even for a single forming operation. Four lubricants (numbers 10, 15, 45 and 51) continued to the process technical test where they all demonstrated more or less the same lubricating properties. In the full scale production test at Danfoss A/S, lubricants are stressed further than in the simulating and the process technical tests due to several subsequent forming operations. In this test, lubricant number 18 was tested instead of number 15. Number 18 is a liquid variant of number 15. Number 15 had been tested in the full scale production at Danfoss A/S in another project without demonstrating a good performance, probably due to the fact that number 15 is a dry lubricant. Even though three of the four lubricants tested in the full scale production test demonstrated promising results in the simulative technical and the process technical tests, neither of the lubricants could be used in the full scale production test. Thus, none of the lubricants tested in this project demonstrated satisfying lubricating performance in the full scale production test where a chlorinated lubricant is currently used. As mentioned in the introduction in chapter 1, non-chlorinated alternatives to chlorinated lubricants already exist for use in less demanding metal working operations such as drilling and milling. For metal forming operations such as sheet forming, rolling and extrusion, the demands on the lubricant are greatly influenced by the process conditions including the degree of deformation, speed, temperature and other not as easily identifiable parameters. Experience shows that under moderate or less severe metal forming conditions, some non-chlorinated lubricants developed for forming operations such as sheet forming, rolling and extrusion show adequate lubricating properties. However, the limits for these non-chlorinated lubricants are that they are specially designed for a certain forming operation and therefore only work under a narrow set of technical conditions. The lubricants are mainly developed from the knowledge of the performance of existing non-chlorinated EP additives present in commercially available lubricants and are rarely produced with new types of additives. The demands from the industry to produce faster and cheaper (material and energy savings) with better tolerances causes more severe process conditions and naturally greater demands on the lubricant. Often a production line involves more than one single metal working operation such as cutting, bending, stamping, deep drawing etc. to produce a specimen with the desired shape. The process conditions for all the single forming operations can individually be gentle and non-chlorinated substitutes may be available even though difficult materials such as stainless steel and titanium are used. However, the successive forming operations in a production line demands that the lubricant has to exhibit good lubricating performance not only in the individual forming operations but also during the entire process. Thus, the lubricant film formed on the surface of the material has to be able to sustain the entire process or rebuild in the same rate as it is degraded. The only way to obtain this high performance from a lubricant during very severe process conditions is to use a proper (very effective) EP additive. So far, chlorinated paraffins have shown superior performance in severe metal forming operations compared to other tested EP additives. Other kind of additives such as polar additives only have a minor impact on the lubricating properties though large synergy effects sometimes improve the lubricity. It is obvious from the technical tests that at the present state there are no non-chlorinated lubricant alternatives commercially available for use in severe metal forming operations. Other metal forming operations such as cold forging cause severe conditions for the lubricant due to large plastic deformation rates (with simultaneous higher energy release). At Danfoss A/S, steel tubes are produced by forming of a steel rod in a cold forging operation. The lubrication in this particular forming operation on steel is conducted by a carrier layer of phosphate applied underneath a soap. Due to a demands to form these tubes in stainless steel, this lubrication technique was tested on stainless steel. However, the phosphate layer did not attach to the surface of stainless steel. In order to solve the problem, a collaboration between Danfoss A/S and Department of Chemistry (DTU) was established with the purpose of developing an electrochemical technique capable of applying a phosphate carrier layer on stainless steel. This collaboration resulted in the development of a technique called the Extreme Pressure Lubricant System (EPL). The EPL technique has previously demonstrated very good results in the strip reduction test and the backwards can extrusion test described in section 6.2, in addition to in a full scale production test (tube forming) at Danfoss A/S. The EPL method was not tested in this project due to failure to comply with the testing equipment in this project. Danfoss A/S assess their own production to be too small to build equipment to make use of the EPL method as a standard technique. A marked inquiry by Danfoss AS/ to sell surface treated stainless steel has demonstrated surprisingly low or no interest in the metal industry. Based on test results of the EPL method, it is expected that the lubricating method is a true alternative to chlorinated lubricants, at least for some heavy-duty metal forming operations. 7 Health and environmental assessment of components in non-chlorinated lubricants
7.1 IntroductionChapter 7 describes the methods and results of the health and environmental assessments of components typically found in non-chlorinated lubricants for heavy-duty metal working. The assessments of the various substance groups are performed on the basis of available data. The strategy for the data search is described in chapter 4. The primary function of chlorinated paraffins in metal working lubricants is as extreme pressure additives. As described in chapter 2 it is not possible to substitute the chlorinated paraffins with a single component. A total reformulation of the lubricant normally takes place. It is estimated in this project that the most suitable frame of reference for comparison of the health and environmental properties of chlorinated and non-chlorinated lubricants, respectively, is a comparison of total lubricants. Thus, the health and environmental assessment of substance groups in non-chlorinated lubricants includes both compounds functioning mainly as lubricant bases, compounds functioning mainly as polar additives and compounds functioning mainly as extreme pressure additives (see also chapter 2). There is virtually no literature on the chemical composition of metal forming lubricants. The selection of substance groups for the health and environmental assessment is based on information retrieved from lubricant suppliers and raw material suppliers contacted in this project in addition to information retrieved through an internet search for home pages of raw material suppliers and by personal contact to industry experts. The chosen substance groups generally occur in non-chlorinated lubricants proposed in this project and are considered as representative of non–chlorinated lubricants. A health and environmental assessment of medium-chained chlorinated paraffins is included as a point of reference. Table 7.1 provides an overview of the substance groups occurring in non-chlorinated lubricants and for which a health and environmental assessment has been performed and described.
Table 7.1 Overview of substance groups in non-chlorinated lubricants for which a health and environmental assessment has been performed. Medium-chained chloroparaffins are included as a point of reference. Some of the substance groups assessed commonly occurring in non-chlorinated lubricants for heavy-duty metal working comprise a huge number of substances. The health and environmental assessments of the substance groups are performed at a screening level. The result of the health and environmental assessments of selected components should only be regarded as an indication of the health and environmental properties. As described in chapter 2, there is only to a certain degree a general concept of formulation of non-chlorinated lubricants. Lubricant formulation is also based on the specific knowledge of the individual supplier. Besides the substance groups, which are chosen for health and environmental assessment due to their common occurrence in lubricant alternatives, several of the non-chlorinated lubricants proposed as alternatives in this project contain specific substances. These substances only appear in the individual lubricant and cannot be discussed further due to confidentiality. However, they are included in the health and environmental screening and rating of the proposed alternative lubricants described and discussed in chapter 8. 7.2 Health and environmental assessments of substance groups in non-chlorinated lubricantsThe health and environmental assessment of substance groups in alternative lubricants focuses entirely on inherent properties. The use of lubricants for metal forming is not considered. The parameters that are included in the health and environmental assessment are described in chapter 3. Based on the assessment of the individual substance groups, each substance group is assigned a health score and an environmental score. The scores are assessed on the basis of a rating system developed by CETOX (Centre for Integrated Environment and Toxicology). The system was developed as a tool to facilitate the differentiation between chemical substances and between products with respect to health and environment. The rating system is described in further detail below in sections 7.3.1 and 7.3.2. 7.2.1 Assignment of health scoresTable 7.2 shows the rating system for the health assessment of chemical substances and products. The system was developed based on the EU classification system for chemical substances which focuses on the inherent properties of substances. The rating system for the health assessment is based on five health hazard groups. Group 1 comprises substances with no or few less severe health hazardous effects. The higher the group, the more serious the health effects are considered to be. Group 5 comprises substances that may cause the most severe health effects. Groups 4 and 5 include only substances classified according to the EU classification system while groups 1 through 3 include both substances classified according to the EU classification system and substances for which the documentation available is too weak to make a proper classification. The latter is true for group 3 – sensitizing which includes substances for which data is available that indicate sensitizing effects but not sufficient documentation for an actual EU classification. This is also the case for group 2 – sensitization which includes substances for which data is available on a few, isolated cases of allergy. Finally, substances exhibiting weak to no irritation to skin and eyes are included in group 1 – irritation. An additional group, ND, is included in the scoring system. This group comprises substances for which no data is available or for which the data available is insufficient to place the substance in a health hazard group. If a substance has more than one hazardous property, the most serious one determines the assignment of the score for that particular substance. The designations “health hazard group” (group) and “health score” (score) corresponds to each other.
7.2.2 Assignment of environmental scoresTable 7.3 shows the rating system for the environmental assessment of chemical substances and products. The system was developed based on the EU classification system for chemical substances, which focuses on the inherent properties of substances. The rating system for the environmental assessment is based on five health hazard groups. The following environmental data will typically be available: *ready biodegradability under aerobic conditions, *potential for bioaccumulation (determination of n-octanol/water partition coefficient, log KOW, or the bioconcentration factor, BCF), *toxicity towards algae, *toxicity towards crustaceans (Daphnia) and *toxicity towards fish. The properties marked with an asterisk (*) form part of the basis of the environmental hazard assessment of chemical substances according to EU Directive 67/548/EEC. The environmental assessment is based on the principles used in relation to the EU environmental hazard classification and the global classification system (OECD 2001). Using the OECD harmonized system for classification of chemical substances on the basis of their hazard towards the aquatic environment, the individual substances are given an environmental rating from 1 to 5 (groups 1 to 5), of which 1 is best. The rating is based on the biodegradability and potential for bioaccumulation of the chemical substances and their toxic effects on aquatic organisms. The environmental assessment is made for single substances, if at all possible, or for groups of substances when similarities in their chemical structure justify an analogous assessment.
Table 7.3 Environmental rating system developed by CETOX (Centre for Integrated Environment and Toxicology). 7.3 Mineral base oils7.3.1 FunctionMineral base oils enter non-chlorinated as wells as chlorinated lubricants as lubricant bases (13,14). 7.3.2 IdentificationThe base oils are high boiling fractions of crude oil extracted by distillation. Initially, the crude oil passes through a distillation at atmospheric pressure followed by a further distillation of the distillation rest in vacuum. A range of vacuum distillates are produced by this. The distillates are further treated by solvent extraction and /or hydro-fining in order to increase viscosity index, enhance the colour and to convert unwanted structures such as unsaturated hydrocarbons and aromatics to less chemically reactive species. Finally, solvent de-waxing is used to reduce the wax content of the base oils so as to prevent wax crystals forming within the normal working temperature range of the lubricant. High viscosity grades of lubricating oil base stocks are produced by solvent de-asphalting of the vacuum residue and subsequent solvent extraction and/or hydrogenation (15). All crude oils contain polycyclic aromatic hydrocarbons (PAH). Some of these are known carcinogens. The content of PAH in the mineral base oil is primarily dependent on the severity of the refining process, which the oil has passed through. Severe solvent extraction and/or hydro-treatment substantially reduces the total content of aromatics in the mineral oil including PAH (15). Mineral lubricating oils are described as paraffinic or naphthenic depending on the dominating kind of hydrocarbons. Besides hydrocarbons, the mineral lubricating oils also contain varying amounts of sulphur, nitrogen and traces of metals. In the end, the composition of the base oils depends on the origin of the crude oil and the refining process. The final base oil can be a blend of lubricant base stocks and additives can be added (15). Lubricating base oils found in non-chlorinated as wells as in chlorinated lubricants for metal forming are severely refined mineral oils. They include a large number of different CAS numbers. Table 7.4 states the CAS numbers which are included in the health and environmental assessment of mineral base oils.
Table 7.4 Chemical names and CAS Nos. of substances included in the health and environmental assessment of mineral base oils. 7.3.3 Physical/chemical dataMineral lubricating oils are complex mixtures of hydrocarbons. They are composed of varying amounts of paraffins, naphthenes and aromates. The number of carbon atoms in the single molecules varies from 15 to 30. The boiling point of the oils ranges from 300 to 600°C. Mineral base oils have low vapour pressures at room temperature and very low solubility in water (15,16). 7.3.4 Health assessmentAcute toxicity Highly refined mineral base oils have low acute toxicity by ingestion and skin contact. As an example, LD50 (oral, rat) > 5000 mg/kg bw for distillates (petroleum), hydro-treated heavy naphthenic (CAS No. 64742-52-5), and LD50 (skin, rabbit) > 2000 mg/kg bw for CAS No. 64742-52-5 (16). There are only few data regarding exposure by inhalation. These data indicate low acute toxicity also by this route. LC50 (rat, 4 hours) > 4 mg/l for residual oils (petroleum), solvent deasphalted (CAS No. 64741-95-3) (16). Irritation Based on animal tests and experience from the working environment, highly refined mineral base oils are in general not more than slightly irritating to skin and eyes (16). Repeated and prolonged exposure to mineral oils may degrease the skin and cause dermatitis and oil acne (17). Sensitization Highly refined mineral base oils exhibit a low potential for skin sensitization by standard test in guinea pigs and by experience from humans. No data is available on sensitization by respiration, but it not considered relevant considering absence of sensitizing potential by skin contact and general experience from the working environment (15,16). Repeated dose toxicity There are no data for the relevant mineral oils on toxicity by repeated oral exposure. Highly refined mineral base oils exhibit low toxicity by repeated exposure to skin. In a standard test, rabbits were exposed five days a week for four weeks of doses not exceeding 1000 mg/kg bw of five different paraffinic oils covered by CAS Nos. 64742-56-9, 101316-70-5, 101316-71-6, 101316-72-7 and 64742-62-7. Minor dermal irritation was observed following prolonged exposure but there were no treatment related effects observed by necropsy or clinical observations and no effects on body weight. NOAEL (No Observed Adverse Effect Level) for all five oils > 1000 mg/kg bw (16). In another study, rabbits were exposed to two highly refined naphthenic base oils three times a week for four weeks at doses of 200, 1000 and 2000 mg/kg bw distillates (petroleum), hydro-treated light naphthenic (CAS No. 64742-53-6) and distillates (petroleum), hydro-treated heavy naphthenic (CAS No. 64742-52-5). There were no deaths, and minimal to moderate irritation in dose groups. Erythema and flaky skin were observed in all dose groups, oedema in medium and high dose groups, and statistically significant lower overall body weight gain were observed in medium and high dose groups. NOAEL > 200 mg/kg bw (16). The dominating effect of repeated skin exposure to highly refined mineral oils in animal test is slight to moderate skin irritation (15). There exists only a few data on effects of highly refined mineral oils by repeated exposure by inhalation. These indicate low toxicity. In a study consistent with OECD Guideline 412, rats were exposes to aerosol concentrations of 50, 210 or 1000 mg/m³ of one of three different highly refined mineral oils (CAS No. 64742-70-7, 64742-54-7, 8042-47-5). The exposure period was 6 hours/day, 5 days/week for four weeks. Body weights and clinical signs were not affected by treatment. A dose dependent increase in wet lung weight and dry/wet lung weight ratio was observed. This was associated with accumulations of foamy alveolar macrophages in the lung tissue (16). In another study with rats consistent with OECD Guideline 412, a two week exposure, 6 hours per day for 10 days of 55, 507 or 1507 mg/m³, resulted in treatment-related clinical signs of central nervous system and dermal effects at >500 mg/m³ (16). Carcinogenicity All mineral base oils in the proposed non-chlorinated lubricants are highly refined mineral oils with a content of PAH measured by DMSO extraction (IP 346) less that 3 % by weight (Concawe, 1994, IP , 1993). Several skin painting studies in mice show that mineral oils with a PAH content measured by DMSO below 3 % are not carcinogenic by skin contact (15,16). There are very few data regarding the carcinogenicity of highly refined mineral oils by oral exposure and inhalation. These data indicate, that the mineral oils are not carcinogenic by these exposure routes (15). Genotoxicity Similar to carcinogenicity of mineral oils, a connection is observed between genotoxic effects of the oils and the content of PAH. Highly refined mineral base oils with a low content of PAH below 3 % (measured by DMSO-extraction) do not exhibit mutagenic effects in various standard test systems (15,16). Reproductive toxicity Very few data exist on effects of mineral base oils on reproduction and the developing foetus. In a study, rats were dermally exposed to one of three highly refined mineral oils from day 0-19 of gestation at levels up to 2000 mg/kg/day. No abnormal development was observed in the offspring (15). Health rating Mineral oils entering into proposed non-chlorinated lubricants are assigned health score 1. 7.3.5 Environmental assessmentAquatic toxicity The few data available show that mineral base oils have a low toxicity towards aquatic organisms. Available LC50 values are above 1000 mg/l. Distillates (petroleum), hydro-treated light naphthenic (CAS No. 64742-53-6) have an LC50(48h) towards Daphnia magna above 1000 mg/l (tested on the water accommodated fraction (WAF)). A WAF can be made by rapid stirring, ultra sound or other ways to ensure maximum concentration of test substance in the water fraction. The test period was 21 days with WAF replacement every 3 days. No effects were observed. The European Oil Industry Association (CONCAWE) refers in its report No. 01/54 similar results from short-term as well as long-term testing (15). Environmental fate All referred tests showed that base oils are not readily biodegradable, e.g., distillates (petroleum), hydro-treated heavy naphthenic (CAS No. 64742-52-5) with 6% degradation in 28 days (OECD 301 B) (16). Similar results were found for other base oils. Bioaccumulation All base oils contain compounds with log POW > 4 indicating that bioaccumulation may be a potential concern (16). Environmental rating Based on the sparse available data on this large group of substances, base oils are assigned environmental score 2. 7.4 Calcium sulphonates (“over-based”)7.4.1 FunctionThe function of sulphonates in metal working lubricants is primarily as extreme pressure additives. In addition, they act as metallic dispersants and corrosion inhibitors. Sulphonates may occur in the lubricant in such high amounts, that they also partly make up the lubricant base (13,14,18). 7.4.2 IdentificationSulphonates are produced by neutralization of a sulphonic acid group with a metallic base – a divalent metal oxide (MO) or a divalent metal hydroxide (MOH). By this process, a salt is formed. R-SO3H + MO or MOH → R-SO3M + H2O R represents an organic radical. The radical can be a straight-chained or branch-chained alkyl group or alkaryl group. There are two types of commercial sulphonates: petroleum sulphonates and synthetic sulphonates. Formerly, petroleum sulphonates were by-products of the sulphonic treatment of oil fractions in the manufacture of white oils. Currently, with the high demand for detergent oils, sulphonates rather than white oils have become the principal product. The structure of the organic portion of petroleum sulphonates are not completely known. Depending on the crude oil source, the structure can have varying aliphatic, naphthenic, and aromatic hydrocarbon groups. Synthetic sulphonates are metal salts of acids produced from the sulphonation of alkylated aromatics by reaction with sulphur trioxide. In many cases, synthetic sulphonates are derivates of benzene with long alkyl substitutes. The structure is illustrated below in Fig 7.1, where R and R' are aliphatic radicals with a combined number of carbon above 20. 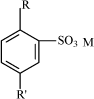
Fig. 7.1 Synthetic sulphonate. R and R' are aliphatic radicals with a combined number of carbon above 20. The molecular weight of the organic radical in commercial sulphonates is 350 or more. The radical is considered necessary for the oil solubility of the sulphonate. The metal ion in the sulphonates, which are used as extreme pressure additive in metal working lubricants, is most often calcium, however it can also be sodium or magnesium. Oil soluble sulphonates containing metal in excess of the stoichiometric amount are called over-based sulphonates. The advantage of over-based sulphonates is a greater ability to neutralize acidic bodies in addition to serving as dispersant for contaminants (18,19,20). Calcium sulphonates in the non-chlorinated lubricants for metal forming are mixtures of petroleum sulphonates and synthetic sulphonates. They are over-based. Calcium sulphonates include a large number of different CAS numbers. Comparable substances can be sulphurized detergents as long chained alkyl phenols and alkylbenzenes, which are included in the environmental assessment. Table 7.5 states the CAS numbers of substances which are included in the health and environmental assessment of the over-based calcium sulphonates.
Table 7.5 Chemical names and CAS Nos. of substances included in the health and environmental assessment of calcium sulphonates, over-based. 7.4.3 Physical/chemical dataOver-based calcium sulphonates are dark coloured viscous liquids with very high boiling points (> 500°C), high flash points (> 180°C), low vapour pressures (< 0.001 hPa at 20°C) and low solubility in water (20,21). 7.4.4 Health assessmentThere are very few data on sulphonates in common toxicological literature. The health assessment of over-based calcium sulphonates is thus based on information retrieved from suppliers and on the internet. Acute toxicity Calcium sulphonates exhibit low acute oral and dermal toxicity in animal studies. LD50 (rat, oral) > 5.000 mg/kg sulphonic acids, petroleum, calcium salts (CAS No. 61789-86-4), sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0), C14-C24 alkaryl calcium salt, over-based derivative (CAS No. 115733-09-0) (20). LD50 (rabbit, dermal) > 2000 – 5000 mg/kg (16). Limited data indicate that calcium sulphonates (sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68786-96-0)) also exhibit low acute toxicity by inhalation (16,21). Irritation Over-based calcium sulphonates (sulphonic acids, petroleum, calcium salts, over-based (CAS-Nr. 68783-96-0)) do not exhibit irritating potential towards skin and eye in standard tests with rabbits (16). There are no data on irritation to respiratory organs. Sensitization An over-based calcium sulphonate (sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0)) did not exhibit a sensitizing potential in the Guinea Pig Maximization test (16). There are no data available regarding sensitization towards respiratory organs. Repeated dose toxicity Data on effects by repeated exposure indicates low toxicity of calcium sulphonates. In a four weeks study (OECD 407), rats were orally exposed to a C20-24- alkaryl calcium sulphonate (Benzenesulphonic acid, mono-C16-24-alkyl derives, calcium salt (CAS No. 70024-69-0)) at dose levels of 100, 500 and 1000 mg/kg/day. Decreased serum cholesterol was observed at 1000 mg/kg/day. NOAEL was 500 mg/kg bw/day in this study (20). In another four weeks study (OECD 410), rats were dermally exposed to an over-based calcium sulphonate (sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0)) at dose levels of 100, 300 and 1000 mg/kg/day 6 hours/day and under occlusion. A low incidence of erythema, desquamation and scabbing was sporadically observed in treated animals. The NOAEL was estimated at 1000 mg/kg/day in this study (20). In a four weeks inhalation study with rats (OECD 412), the animals were exposed to an over-based calcium sulphonate (CAS No. 68783-96-0) in concentrations of 49.5, 156 and 260 mg/m³ respectively. Signs of toxicity including adverse effects on lungs were observed at the two highest dose levels. NOAEL was 49.5 mg/m³ in this study (20). Data from standard tests in different cell systems for CAS No. 68783-96-0 and CAS No. 70024-69-0 indicate that calcium sulphonates do not exhibit genotoxic properties (20,21). Reproduction toxicity and carcinogenicity There are no data for the assessment of the reprotoxic and carcinogenic potential of calcium sulphonates. Health rating On behalf of the available data, over-based calcium sulphonates are assigned health score 1. 7.4.5 Environmental assessmentAquatic toxicity Only few data were available on ecotoxicity and they mainly indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, higher toxicity, EC/LC50 below 1000 mg/l, has been referred for the following substances:
Environmental fate Two tests are referred and demonstrate that sulphonated petroleum is not readily biodegradable. A test for ready biodegradability (OECD 301 B) with sulphonic acids, petroleum, calcium salts, over-based (CAS No. 68783-96-0) and benzene, C9-13-alkyl deriv., distu. ,residues, sulphonated, calcium salts (CAS No 97675-24-6) showed 16% degradation in 28 days (16). The two tests refer the same result and it is not possible determine whether one or two tests have actually been made. Bioaccumulation No test data on sulphonated petroleum substances are referred. QSAR calculations of log POW show that sulphonated petroleum products contain compounds with log POW > 4 indicating that these substances are potentially bioaccumulative. Environmental rating Based on the sparse available data on this large group of substances, sulphonated petroleums and related substances are assigned environmental scores 2-4. In general, these substances are scored 2 as they are not readily biodegradable, they contain compounds with log POW > 4 and the EC/LC50 values are above 100 mg/l. Some sulphonated petroleum substances are, however, more toxic and will be scored 3 or 4. 7.5 Alkyl sulphides (polysulphides)7.5.1 FunctionAlkyl sulphides (also named polysulphides) are added to metal forming lubricants as extreme pressure additives (13,22). 7.5.2 IdentificationThe molecular structure of polysulphides are R-[S]x-R, where x 2, and R are organic radicals (23). In alkyl sulphides, the R is linear or branched alkyl groups. Alkyl sulphides entering into non-chlorinated lubricants for heavy-duty metal working are generally of the type di-tertiary alkyl polysulphides. Di-tertiary alkyl pentasulphides are dominating. This implies that the average number of sulphur atoms in the molecules is five, however the number varies between two and five (23,24). Dialkyl polysulphides are manufactured by reacting corresponding thiols with sulphur: 2 R-SH + S → R-Sx-R (22). Figure 7.2 gives an example of the chemical structure of a dialkyl polysulphide commonly entering non-chlorinated lubricants as an EP additive. 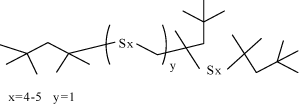
Fig. 7.2 The chemical structure of the polysulphide; sulphurized trimethyl pentane (CAS No. 68515-88-8). Table 7.6 states the CAS numbers of substances which are included in the health and environmental assessment of the substance group alkyl sulphides.
Table 7.6 Chemical names and CAS Nos. of substances included in the health and environmental assessment of alkyl sulphides. 7.5.3 Physical/chemical dataDi-tertiary alkyl polysulphides are clear, yellow liquids at room temperature. They have high boiling points (> 200°C) relatively high flash points (85°C – 150°C) and low vapour pressure (< 0.1 hPa at 20°C). They are virtually insoluble in water (16). 7.5.4 Health assessmentThere are few toxicological data available on alkyl sulphides in literature and data bases. In addition to these data, the health assessment of the alkyl sulphides is also based on information from suppliers of dialkyl polysulphides used as extreme pressure additive in metal forming lubricants. Acute toxicity Dialkyl polysulphides exhibit low acute toxicity by ingestion and skin contact. Acute oral toxicity data for di(tert-dodecyl) pentasulphide (CAS No. 31565-23-8) was: LD50 (mouse, oral) > 20000 mg/kg and LD0 (rat, dermal) > 2000 mg/kg (16). LD50 (rat, oral) > 5000 mg/kg for di-tert-butyl polysulphides (CAS No. 68937-96-2) (16). LD50 (rat, oral) was 19500 mg/kg and LD50 (rabbit, dermal) > 2000 mg/kg for di-tert-nonyl polysulphides (CAS No. 68425-16-1) (16). Toxicity by inhalation is considered less relevant for the alkyl sulphides in focus due to very low vapour pressures. However, data on acute toxicity by inhalation for three di-tertio-alkyl polysulphides indicate low to moderate toxicity by this exposure route. LC50 (rat, 4 hours aerosol exposure) > 15.5 mg/l for di-tert-nonyl polysulphides (CAS-No., 68425-16-1) (25). LC50 (rat, exposure period not stated, aerosol exposure) > 5,6 mg/l for male rats and equal to 2.17 mg/l for female rats for sulphurized 2,4,4-trimethyl-pentene (CAS No. 68515-88-8). No treatment-related gross lesions were observed in surviving rats (26). LC50 (rat, 4 hours vapour exposure) > 0.39 mg/l for sulphurized 2-methyl-1-propene (CAS No. 68511-50-2). No significant clinical signs were observed after initial post-exposure observations at any dose level. No treatment-related macroscopic or microscopic findings were observed (26). Irritation Di-tertiary alkyl polysulphides (CAS Nos. 31565-23-8, 68937-96-2, 68425-15-0, 68425-16-1 and 68515-88-8) cause in general not more that slight irritation to skin and eyes in standard tests (16). However a single compound (CAS no. 68937-96-2) is stated to be irritating to skin in a study with rabbits (27). The study is not specified in further details. There are no data on the irritating potential of ditertiary alkyl polysulphides towards respiratory organs. Sensitization There are varying data regarding the sensitizing potential of dialkyl polysulphides by skin contact. The skin sensitizing potential of di-tert-butyl polysulphides (CAS No. 68937-96-2) was tested in the Guinea pig maximization. The result of the test was ambiguous, as the material (5 % solution in mineral oil) appeared to be a sensitizer at challenge, but not at re-challenge (16). A supplier states that the same CAS No. provoked sensitization in another test with Guinea pigs. There are no further details regarding this study (27). The supplier classifies the polysulphide as sensitizing by skin contact (Xi;R43) in accordance with EU regulations on classification and labelling of dangerous substances and preparations (27). Di-tert-nonyl polysulphides, (CAS No. 68425-16-1) is also stated to be sensitizing by skin contact by a supplier. Test data are not further specified (25). Di-tert-dodecyl polysulphides (CAS No, 31565-23-8) was examined in a standard test with Guinea pigs performed in accordance with the maximization method established by Magnusson and Kligman, the OECD 406 and GLP. After challenge the substance caused an a-specific, weak skin irritation in 4 of 20 animals. It was concluded in the study, that the substance may have a weak skin sensitizing potential (16). Repeated dose toxicity B: In a 28-day study, rats were daily exposed to di-tert-dodecyl penta-sulphides, (CAS No. 31565-23-8) by gavage at dose levels of 0, 50, 250 or 1000 mg/kg bw/day. There was a post observation period of two weeks. No treatment related deaths occurred. Salivation was observed in all animals in the highest dose group only during the treatment period. No treatment-related effects were observed on the mean food consumption, the body weight gain, the haematological and blood biochemical parameters, the urine analysis, the organ weights, and the macro- and microscopic examinations. NOAEL was defined as 1000 mg/kg/day in this study (28). In another 28-day study with rats orally exposed to 1-(tert-dodecylthio) propan-2-ol (CAS No. 67124-09-8) at dose levels of 100, 300 or 1000 mg/kg/day, gross and microscopic observations at study termination showed alterations in kidneys and liver. The effects in liver are considered an adaptive response and the effects on kidney are not considered relevant to humans (26). A: Four sub-chronic dermal toxicity studies (in rats or rabbits) have been conducted with either sulphurized 2-methyl-1-propene (CAS No. 68511-50-2) or sulphurized 2,4,4-trimethyl pentene (CAS No. 68515-88-8). The predominant effect observed was dermal irritation at the site of the test material administration. The lowest reported NOAEL was 50 mg/kg bw/day for a 13-week rat dermal study with sulphurized 2-methyl 1-propene (CAS No. 68511-50-2) at dose levels of 10, 50, 100, 250, 500 or 2000 mg/kg bw/day. In this study, a decrease in red blood cell number, an increase in neutrophils and an increase in spleen size and pigments in spleen were observed at 250 mg/kg bw/day and above, in addition to a decreased body weight gain in males. At 100 mg/kg/day and above, an increased production of white blood cells in spleen and bone marrow was observed. There is no information on whether these observed effects were reversible at cessation of exposure (26). C: A 28-day inhalation study in rats has been conducted with sulphurized 2,4,4-trimethyl pentene (CAS No. 68515-88-8) at dose levels of 15, 50 or 150 mg/m³. At dose levels of 15 mg/m³ and above there was a trend toward lower body weight gain (all males and two highest doses in females); increased kidney weights (males only); globular casts and hyaline droplets in the proximal tubule cells of the kidney (all males and recovery high dose males); increased liver weight (high dose males and females and mid-dose males). At 150 mg/m³ a decrease in hemoglobin concentration was observed. The NOAEL was < 15 mg/m³. Effects on the liver are considered to be adaptive responses and the effects on rat kidneys are not considered relevant to humans (26). Thus the available data on alkyl sulphides indicate moderate toxicity by repeated exposure. Genotoxicity There are data on genotoxic potential for several dialkyl polysulphides. CAS No. 68425-15-0, 68425-16-1, 31565-23-8 and 68937-96-2 did not exhibit a genotoxic potential in a standard bacterial gene mutagenicity test (Ames test), with and without metabolic activation or standard chromosomal aberration test in human lymphocytes (16,25,28). In another study of gene mutations of sulphides and polysulphides in the mouse lymphoma assay, di-tert-nonyl polysulphides (CAS No. 68425-16-1) produced a clear, dose-dependent mutagenic response while its homologue, di-tert-dodecyl polysulphides (CAS No. 68425-15-0) was inactive. Only the non-activated portions of the test was performed. The results of the total study suggest that the mutagenic species may be the hydrosulphide ion. Thus, the relatively stable alkyl mono- and disulphides are inactive, while the more easily hydrolized sodium sulphide and di-tertiary-nonyl polysulphide are mutagenic (29). No further data is available, including tests for chromosome aberrations. The mutagenicity of dialkyl polysulphides has not been fully investigated. Together, there are discrepancy regarding mutagenicity data for di-tert-nonyl polysulphides (CAS No. 68425-16-1), while data for the remaining polysulphides indicate non-genotoxicity. Reproduction toxicity There are test data on effects on prenatal development for a single compound di-tert-dodecyl polysulphides (CAS No. 31565-23-8). In a standard study (OECD 414), rats were exposed by oral route from day 6 to day 15 of pregnancy to dose levels of 0, 50, 250 and 1000 mg/kg/day of the polysulphide. On day 20, dams were sacrificed, litter values determined and foetuses subsequently examined for visceral or skeletal anomalies. No treatment-related effects were observed on the clinical signs, mortality, abortions or total re-sorption, food consumption, body weight gain, macroscopic findings, post-implantation loss, number of live foetuses per animal, foetal body weight, external anomalies or malformations, soft tissue malformations or anomalies, skeletal malformations, anomalies and variations. The maternal and foetal NOAEL was 1000 mg/kg/day in this study (30). Carcinogenicity There are no data on the carcinogenic potential of polysulphides. Health rating Di-tert-dodecyl polysulphides and di-tert-dodecyl pentasulphides (CAS Nos. 68425-15-0 and 31565-23-8) are assigned health score 1 based on the available data. Sulphurized 2,4,4-tri-methyl pentene (CAS No. 68515-88-8) is assigned health score 2 due to acute toxicity by inhalation (LC50 for female rats was 2.17 mg/l). Di-tert-nonyl polysulphides (CAS No. 68425-16-1) is assigned health score 4 due to a skin sensitizing potential. Di-tert-butyl polysulphides (CAS No. 68937-96-2) is assigned health score 4 due to a skin irritating and sensitizing potential. Sulphurized 2-methyl 1-propene (CAS No. 68511-50-2) is assigned health score 1 –2 due to data which may indicate a health hazard by repeated exposure to skin. In conclusion, the health score of the polysulphides in focus varies from 1 to 4 based on sparse data. 7.5.5 Environmental assessmentPolysulphides are a large group of chemicals and only few environmental data are available. Aquatic toxicity Of the few available data, most indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, EC/LC50 values below 100 mg/L are not unusual either. Toxicity tests with EC/LC50 values below 100 mg/l are referred for di-tert-butyl polysulphides (CAS No. 68937-96-2) with an EC50(96h) towards Selenastrum capricornutum (now Pseudokirchneriella subcapitata) of 29-39 mg/l and di(tert-dodecyl) pentasulphide (CAS No. 31565-23-8) with an LC50(24h) towards Daphnia magna of 0.449 mg/l (16). Environmental fate Only one test concerning biodegradation is available for di(tert-dodecyl) pentasulphide (CAS No. 31565-23-8) demonstrating 0% biodegradation after 28 days (Closed Bottle test) (16). Bioaccumulation No experimental data are available. QSAR calculations with the online estimation program KowWin (100) state log POW values above 4 for pentasulphides, e.g.:
Based on calculated log POW values, it is assessed that pentasulphides bioaccumulate in aquatic organisms. Environmental rating Based on the sparse available data on this large group of substances and on additional secondary data from material safety data sheets, in which some products are classified N;R50/53 or N;R51/53, polysulphides are assigned environmental scores 3, 4 or 5. 7.6 Vegetable and animal oils7.6.1 FunctionNatural oils of animal and vegetable origin enter lubricants for metal working as lubricity improving additives (polar additives) and as lubricant bases (14). 7.6.2 IdentificationRape oil and lard oil are the most frequent used oils in metal working lubricants. Also used are, however, soybean oil, palm oil, palm kernel oil and castor oil. Pure fatty acids extracted from the natural oils may also enter metal working lubricants. In addition, different types of modified natural oils including hydrogenated, polymerized and epoxidized oils are used. 7.6.3 Physical/chemical dataThe oils are liquids or solids at room temperature (16). In general, they have high boiling points (> 200°C), high flash points (> 200°C), low vapour pressures and very low solubility in water (16,31,32). Table 7.7 states the CAS numbers of substances which are included in the health and environmental assessment of the substance group vegetable and animal oils.
Table 7.7 Chemical names and CAS Nos. of substances included in the health and environmental assessment of vegetable and animal oils. 7.6.4 Health assessmentRape oil and lard oil are assessed here in a health context as representatives of the vegetable and animal oils group. 7.6.4.1 Rape oilRape oil (CAS No. 8002-13-9) is obtained from several species of the cruciferous genus Brassica. The oil is separated either by solvent extraction or by cold or hot pressing. Cold-pressed rape oil is used for edible purposes whereas refined oil is used as a lubricant. (33) Rape oil consists primarily of triglyerides of the fatty acids linoleic, oleic and erucic acid (34). Physical/chemical data Rape oil is a yellow, oily liquid with a high boiling point (> 350°C), a high flash point (> 300°C), a low vapour pressure (< 1 mbar (20°C) and it is insoluble in water (35). Health assessment It is well-known that high-erucic acid rape oil causes necrosis in the heart muscle by repeated ingestion (36) and today high-erucic acid rapeseed oil is produced only in small quantities for industrial non-food use. Rape oil for human consumption usually contains less that 2 % erucic acid (CAS No. 112-86-7) in the EU countries (37). Due to this effect of erucic acid, cultivation of rape variants containing a low content of erucic acid now prevails. Rape oil for human consumption may not contain more than 2 % erucic acid. Industrial oils may have higher contents (37). Acute toxicity There are no animal test data for rape oil in standard toxicological literature and databases. Fatty acids of rape oil exhibits very low acute toxicity by ingestion and skin contact. For oleic acid (CAS No. 112-80-1), LD50 (rat, oral) is 64000 – 74000 mg/kg bw and LD50 (Guinea pig, dermal) > 3000 mg/kg (16). Rape-oil fatty acids (CAS No. 85711-54-2) typically contains 9 – 25 % of oleic acid. Toxicity by inhalation is considered of minor relevance due to the very low vapour pressure. Irritation There are several studies with rabbits and humans of the skin irritating potential of rape oil fatty acids (CAS No. 85711-54-2). In general, oleic acid (CAS No. 112-80-1), linoleic acid (CAS No. 60-33-3) and erucic acid (CAS No. 112-86-7) caused no or slight to moderate irritation (16). However, there is no indication that the fatty acids occurring as triglycerides in rape oil causes irritation (35). Rape oil is used as a skin conditioning agent in cosmetic (37). Fatty acids of rape oil (oleic acid CAS No. 112-80-1, linoleic acid CAS No. 60-33-3 and erucic acid CAS No. 112-86-7) caused slight irritation in rabbit eyes (16). Sensitization Oleic acid (CAS No. 112-80-1) did not provoke sensitization in a standard sensitization test with Guinea pigs. Rape-oil fatty acids (CAS No. 85711-54-2) typically contain 9 – 25 % of oleic acid (16). Repeated dose toxicity As mentioned above, it is well-documented in animal studies that rape oil with a high content of erucic acid causes necrosis in the heart muscle (36). The NOAEL value for this effect has been estimated at 95 mg/kg bw in a two-year feeding study with rats given rape oil fatty acids (CAS No. 85711-54-2) (16). In a 24-week study, rats were exposed to oleic acid (CAS No. 112-80-1) in the diet at a dose level of approximately 7500 mg/kg bw/day. The content of erucic acid was not stated. Normal growth and general good health was reported. The NOAEL was estimated at > 7500 mg/kg bw/day. Rape-oil fatty acids (CAS No. 85711-54-2) typically contain 9 – 25 % of oleic acid (16). Genotoxicity The mutagenicity of oleic acid (CAS No. 112-80-1) has been tested in several laboratory mutagenicity tests, some with and without metabolic activation, with negative result. Thus, there is no indication of genotoxic effects. Rape oil fatty acids (CAS No. 85711-54-2) typically contain 9 – 25 % oleic acid (16). Carcinogenicity The carcinogenic potential of oleic acid by oral and dermal exposure has been studied in several studies in rats and mice exposed to rape oil fatty acids. The results of these studies indicate that rape oil fatty acids including oleic acid (CAS No. 112-80-1) do not posses a carcinogenic potential (16). Reproduction toxicity In a 16-week study, rats were orally fed to 7500 mg/kg bw/day of oleic acid (CAS No. 112-80-1) in the diet. Rape-oil fatty acids typically contain 9-25 % oleic acid. There are no further details on test conditions. No adverse effects on fertility were observed in male rats, however the exposure appeared to impair the reproductive capacity in female rats by interfering with parturition and mammary gland development. Mortality in the offspring was increased (16). There was no evidence of maternal or foetal toxicity and no teratogenicity was observed in an older study with rats dermally exposed to 2000 mg/kg of a hair dye formulation containing 15 % oleic acid (CAS No. 112-80-1) every third day during pregnancy until day 19 of gestation. Rape oil fatty acids (CAS No. 85711-54-2) typically contains 9 - 25 % oleic acid (16). In an older study, rats were fed 10 or 30 % erucic acid (CAS No. 112-86-7) in the diet between 9-45 weeks for male and 9-28 weeks for female rats in a pre-mating exposure period. Rape oil fatty acids (CAS No. 85711-54-2) may contain 30 - 60 % erucic acid. Male rats fed 10 % became completely sterile after 5 months due to degeneration of testes tissue and failure of spermatogenesis. The female fertility was also affected. There was impairment of parturition, a high mortality rate in offspring and surviving pups were underweight due to deficient mammary gland development and lactation. Recovery of fertility of rats returned to the stock diet proceeded slowly and may be limited. The rats were otherwise healthy and vigorous and there were no lesions in organs other than those of reproduction. Rats fed 30 % erucic acid in the diet for 5 months suffered only a temporary retardation in growth rate and thereafter remained healthy and continued to grow at a normal rate (16). Health rating – rape oil Rape oil and the fatty acids oleic and linoleic acid occur naturally in human diets in substantial amounts. Low-erucic acid rape oil is based on animal test data for rape oil acids and general human experience assigned health score 1. High-erucic acid rape oil is assigned health score 5 due to the effects on the heart muscle and fertility by repeated exposure. 7.6.4.2 Lard oilLard oil (CAS No. 8016-28-2) is of animal origin. It is extracted by fractional crystallization and cold pressing of lard. The main constituent in lard oil is the triglyceride olein (CAS No. 122-32-7). Minor constituents are glycerides of solid fatty acids, e.g. stearin (29),(40). There are very few data on lard oil in toxicological standard literature and databases. Therefore, the health assessment of lard oil is thus based on supplier data, long term experience and data for the main ingredient olein (CAS No. 122-32-7) and tallow (CAS No. 61789-97-7) resembling the composition of lard oil to a high degree (40). Acute toxicity The acute toxicity of lard oil by ingestion is low. LD50 (rat) > 2000 mg bw/kg (41). The corresponding LD50 for tallow is > 18.000 mg/kg (16). LD50 (oral, rat) for the fatty acid of olein, oleic acid (CAS No. 112-80-1) is 64000 – 74000 mg/kg bw and LD50 (Guinea pig, dermal) > 3000 mg/kg (16). There is no further animal test data available on lard oil. Genotoxicity A standard mutagenicity test (Ames test) with tallow oil (CAS No. 61789-97-7) indicates that tallow oil does not possess genotoxic properties (16). Carcinogenicity Carcinogenicity studies in rats, mice and hamsters of minor reliability indicate that a life time high fat intake or an intake of heated fat may have a tumour promoting effect in the experimental animals (16). The studies are considered of less relevance in this context due to very high doses of exposure. Reproduction toxicity No repro-toxic effects were observed in a study with pigs which were daily fed with 8 % tallow (CAS No. 61789-97-7) in the diet from day 90 of gestation (16). Health rating – lard oil Lard oil is extracted from lard, which has been a natural part of human food for several thousand years and which may occur in the food in substantial amounts. Olein, which is the main constituent of lard, is a constituent of many fluid vegetable and animal oils which are part of the human diet (40). Olein is used as an ingredient in cosmetics and pharmaceuticals (40,42). Lard oil is based on the available data and experience assigned health score 1. 7.6.5 Environmental assessmentVegetable and animal lipids are a large group of chemicals. Environmental data are available on oils, e.g. rape oil, soybean oil, castor oil, tallow fat and their fatty acids. This group also includes hydro-treated (hydrogenated) and epoxidized oils. Aquatic toxicity The most of few available data indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, EC/LC50 values below 100 mg/l are not unusual either. Toxicity tests with EC/LC50 values below 100 mg/l are referred for the following oils:
Environmental fate Several tests concerning biodegradation were available. All tests showed that fatty acids and lipids are readily biodegradable (16). Bioaccumulation No experimental bioaccumulation data were available but IUCLID refers log POW data (21). All data show log POW higher than 4, which indicates that fatty acids and natural lipids have a potential for bioaccumulating e in aquatic organisms. Environmental rating Based on the available data, this large group of substances is assigned environmental score 1 but some epoxidized lipids can be assigned environmental score 3 or 4. 7.7 Phosphorous compounds7.7.1 FunctionOrganic phosphorous compounds enter lubricants for metal working as extreme pressure additives. The compounds may have other functions, such as corrosion inhibition and anti-wear (13,14,18,19). 7.7.2 IdentificationThe phosphorous extreme pressure additives is a broad group of substances, - including phosphate esters (mono-, di- and tri-ester compounds), salts and amines of phosphate esters, complex phosphate esters, mono- and diester compounds of phosphite, diphosphoric acid esters and trialkyl phosphines. The organic radicals (R) in the phosphorous additives can be aliphatic as well as aromatic groups. The aliphatic phosphate esters comprises C4 – C10-compounds as well as polyethylene- and polypropoxylene oxide derivates (18,43). Fig. 7.3 illustrates the chemical structure of some of the groups of phosphorous compounds entering non-chlorinated lubricants as extreme pressure additives. 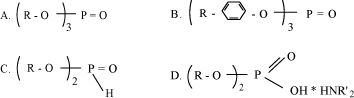
Fig. 7.3 Molecular structures for some of the groups of phosphorous compounds used as extreme pressure additives in metal forming lubricants. A. Trialkyl phosphate B. Triaryl phosphate C. Dialkyl phosphate D. Amine phosphate Another type of phosphorous compounds – the phosphorous-sulphur compounds, also enters lubricants as extreme pressure additives. Dialkyldithiophosphates – including zinc dialkyldithiophosphates, - are used as extreme pressure additives especially in motor oils, but also in lubricants for metal working (18). Fig. 7.4 illustrates the molecular structure of zinc dialkyldithiophosphates. 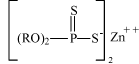
Fig. 7.4 Molecular structure of zinc di-alkyldithiophosphates Phosphate esters are produced by reacting phenols or alcohols with anhydrides or chlorides of phosphoric acid (18). Zinc dialkyldithiophosphates are produced by reacting phosphorous pentasulphide with the corresponding alcohols at 80 –150°C. The resulting dialkyldithiophosphoric acid is neutralized with zinc oxide at 25- 75°C (18). Table 7.8 states the CAS numbers of substances which are included in the health and environmental assessment of the phosphorous compounds.
Table 7.8 Chemical names and CAS Nos. of substances included in the health and environmental assessment of phosphorous compounds. 7.7.3 Physical/chemical dataThe assessed phosphorus extreme pressure additives are liquids at room temperature. The substances have boiling points varying from about 50°C to more than 200°C, flash points > 100 °C and low vapour pressures. The solubility in water is varying (16). 7.7.4 Health and environmental assessmentHealth and environmental effects of phosphate esters and other phosphorous compounds used as extreme pressure additives in metal working lubricants show a great variation. Therefore, it is not possible to consider the phosphorus additives as a uniform group. The health effects of selected CAS Nos. known to occur in metal working lubricants and representing some of the different groups of phosphorous extreme pressure additives are assessed below. 7.7.4.1 Triaryl phosphates and aryl phosphitesHealth assessment Acute toxicity A triaryl phosphate – phenol, isopropylated , phosphate 3:1 (ITAP) (CAS No. 68937-41-7) exhibits low acute toxicity by ingestion, skin contact and inhalation in animal studies. LD50 (rat, oral) > 5000 mg/kg) (16). LD50 (rabbit, dermal) > 5000 mg/kg (16). LC50 (rat/4 hours) > 6350 mg/m³) (43). Irritation ITAP does not exhibit irritating properties to skin or eye in standard tests (OECD 404 and 405) (16). Sensitization ITAP has been tested in Guinea Pigs in accordance with OECD Guideline 406. The test substance was not sensitizing in this model (16). Repeated dose toxicity A: A standard study with rats indicates moderate toxicity of ITAP by repeated dermal exposure. In accordance with OECD Guideline 410, rats were dermally exposed to ITAP (Kronitex 50) 6 hours/day, 5 days/week for four weeks at doses of 0, 100, 500 and 2000 mg bw/kg/day. There was a slight inhibition of plasma cholinesterase activity in females receiving 500 mg/kg bw/day as well as in both sexes of the 2000 mg/kg bw/day group. The erythrocyte cholinesterase activity was significantly inhibited in the males treated with 2000 mg/kg bw/day. Adrenal weights were increased in males receiving 500 and 2000 mg/kg bw/day. Microscopic examination of tissues showed a slight fatty change in the adrenal cortex of 2/5 males receiving 500 mg/kg bw/day and in 3/5 males receiving 2000 mg/kg bw/day. The NOAEL was 100 mg/kg bw/day in males and 500 mg/kg bw/day in females (16). B: In an analogous study, rats were dermally exposed to ITAP (Reolube HYD 46) at doses of 0, 40, 200 and 1000 mg/kg bw/day. A slight inhibition of the plasma cholinesterase activity was observed in the females receiving 1000 mg/kg bw/day. A decrease in absolute and relative testicular weight was observed in the males receiving 1000 mg/kg bw/day. Microscopic examination of the testes showed slight tubular atrophy in both controls and treated groups. Slightly increased absolute and relative adrenal weights were observed in the treated group but no microscopic findings were observed. There are no further details for this study, however it was concluded that dermal application over a period of four weeks at a dosage of 200 mg/kg bw/day did not produce observable adverse effects. NOAEL was 200 mg/kg/day (16). Neurotoxicity C: Several studies with hens exposed to ITAP (Reofos 50, Reofos 65 and Reofos 95) by ingestion have been performed. A number of these studies indicate delayed neurotoxic effects including signs of ataxia and axonal degeneration. The lowest observed dose level for neurotoxic effects in hens by ingestion was 90 mg/kg/day in a 13 weeks study. However other neurotoxicity studies with hens exposed to ITAP by ingestion at higher dose levels (up to 12000 mg/kg) did not result in neurotoxic effects (16). In a study with rats exposed to ITAP in a single oral dose of 2 g/kg, a decrease in serum cholinesterase in addition to significant inhibition of brain cholinesterase was observed in treated animals. However no clinical signs were observed (16). D: Hens were exposed by inhalation to ITAP in aerosol form (Reofos 50) in a single eight hour period and the hens were examined for the next 21 day period for signs of neurotoxicity. Actual does levels were 620, 2400, 2540 and 3090 mg/m³. Mild or modest ataxia was seen in 2/10 hens exposed to 2400 mg/m³ and in 4/10 hens exposed to 3090 mg/m³. Histologic examination of nervous tissues confirmed that degenerative changes were observed at these two dosage levels. No effects were observed at the 620 mg/m³ exposure level. NOAEC was 620 mg/m³ (16). A couple of epidemiological examinations of possible neurotoxic effects of ITAP in the working environment are available. The results of these studies are inconclusive (16). Genotoxicity Several studies of effects of ITAP on genes indicate, that the substance is not genotoxic (16). Reproduction toxicity and carcinogenicity There are no data on effects of ITAP on reproduction, foetal development or carcinogenicity. Health rating Some data indicate that ITAP may have neurotoxic effects by repeated exposure at high doses. However, in total the available data are incomplete to make an assessment regarding this effect. Thus, ITAP is assigned health score 1. ITAP has been selected for the health assessment of aryl phosphates and aryl phosphites. However, the toxicity of the group varies considerably implying that the health score of individual substances in the group may be between 1 – 5. Environmental assessment Aquatic toxicity Tri-p-tolyl phosphate (CAS No. 78-32-0) and triphenyl phosphite (CAS No. 101-02-0) are classified as dangerous to the environment; N;R51/53 and N;R50/53, respectively (4). Toxicity tests with EC/LC50 values are referred for the following compounds:
Environmental fate Several tests concerning biodegradation are available. The referred tests indicate both ready biodegradability and not ready biodegradability. It is not possible to find any relation between structure and biodegradability (16). Bioaccumulation All phosphate esters have log POW values above 4 and one test showed a bio-concentration factor above 1000. E.g., 2-ethylhexyl diphenyl phosphate (CAS No. 1241-94-7) had a BCF of 1241 (16). Many tests showed BCF values of 10-250. Based on the available data, phosphate esters are assessed to have a potential for bioaccumulation in aquatic organisms. Environmental rating Phosphate esters in general are assigned environmental scores 4 or 5. However, it is expected that certain phosphate esters fulfil criteria for environmental scores 3, 2 and even 1. 7.7.4.2 Trialkyl phosphatesHealth assessment Acute toxicity A trialkyl phosphate – tributyl phosphate (TBP) (CAS No. 126-73-8) is classified as harmful by ingestion (Xn;R22) (4). LD50 (rat, oral) is 1500 - 3000 mg/kg (16,46). TBP exhibits low acute toxicity by skin contact and inhalation (16). Irritation TBP is slightly irritating to skin and eyes in standard studies (OECD 404 and 405). However, other studies indicate moderate to strong skin irritation and moderate irritation to mucous membranes (16,46). Sensitization There is no evidence of a sensitizing potential of TBP (16,46). Repeated dose toxicity TBP shows moderate toxicity by repeated exposure by inhalation. A: Rats exposed to 5.1 or 13.6 mg/m³ TBP 5 hours/day, 5 days/week for 4 months showed decreased cholinesterase activity after 3 months in addition to effects on physiological and biochemical parameters, especially in the liver, in the high dose group. The effect retained to normal during a one-month post exposure period. The NOAEC was 5.1 mg/m³ (16). Several studies in rats, mice and rabbits of TBP effects by repeated oral exposure have been performed and indicate moderate toxicity (16,46). In a 13-week study rats were daily exposed to TBP by gavage at doses of 32, 100 and 325 mg/kg bw/day. Mortality, salivation and muzzle staining were observed in the 325 mg group and less severe in the 100 mg group. In addition, reduced body weight, body weight gain, reduced food intake and initial weight loss were observed in the highest dose group. Motor activity test results were not significantly different between the groups. There were no abnormal gross pathology findings and the neuropathological assessment revealed no effects of treatment (16). B: In a 2-weeks study with rats exposed to 270 or 400 mg/kg bw/day by gavage, no overt signs of toxicity were observed. However, reduction in conduction velocity of the caudal nerve was observed in high dose males. Electron microscopic examination revealed morphological changes of the nerve. The NOAEL was 270 mg/kg bw/day (16). C: In another 2-weeks study with rats exposed to TBP by gavage at dose levels of 136 or 400 mg/kg bw/day, no overt signs of toxicity were observed. However in the high dose group microscopic degenerative changes were observed in the seminiferous tubules of one of four male rats, in addition to some changes in clinical chemistry, an increase in relative and absolute liver weight and a decrease in spleen weight (female rats). The NOAEL was 136 mg/kg bw/day (16). There are no data regarding repeated exposure of TBP by skin contact. Genotoxicity In mutagenicity studies, equivocal results have been obtained by using the Ames test in the presence and absence of metabolic activation. However, all other types of mutagenicity tests indicate that TBP is non-mutagenic (16,46). Carcinogenicity In a 18-week study, TBP was administered by gavage once a day, 5 days/week to rats. Low dose animals received 200 mg/kg/day throughout the study, high dose animals received 300 mg/kg/day for the first 6 weeks and 350 mg/kg/day for the remaining 12 weeks. Histopathological examination revealed that all treated animals developed diffuse hyperplasia of the urinary bladder epithelium (47). D: In another study, groups of 50 male and female rats each obtained TBP with their diet (up to 140 and 180 mg/kg bw/day respectively) for 2 years. In this experiment hyperplasia and also neoplastic lesions dependent on the dose (papilloma and especially for the males also carcinoma) were found in the urine bladder. The male and female NOAEL was 9 and 12 mg/kg bw/day respectively, assuming that TBP is a non genotoxic oncogene (48). On the EU provisional list Existing substances for the 29th ATP Rev. 18, TBP is classified as a carcinogen in category 3 with risk phrase R40: “Limited evidence of a carcinogenic effect” (49). Reproduction toxicity E: In a single two-generation reproduction study, rats were daily exposed to TBP in food at dose levels of 200, 700 and 3000 mg/kg diet (approximately 15, 53 and 225 mg/kg bw/day). Reduction in body weight, weight gain and food consumption were observed during F0 and F1 pre-bred dosing periods in the two highest dose groups. In the 200 mg/kg dose groups, transient effects on body weight and food consumption were observed. No signs of toxicity and no treatment related mortality were observed in any dose group. There was no evidence of reproductive organ histopathology at any dose level and no effect on pre- and postnatal mortality. A NOAEL for reproductive toxicity could not be established. LOAEL (reduced pup weight) was < 15 mg/kg bw/day (16). Two studies in rats and two studies in rabbits do not indicate embryotoxic or fetotoxic effects of TBP (16). Other effects TBP is a weak plasma cholinesterase inhibitor in rats and causes toxic effects to the peripheral nervous system at high dose levels (1000 mg/kg bw) (16). TBP is readily absorbed through the skin (16,4046). Health rating Based on the available data, TBP is assigned health score 5 due to the potential carcinogenic effect. TBP has been selected for the health assessment of trialkyl phosphates. However, the toxicity of the group varies considerably implying that the health score of individual substances in the group may be between 1 – 5. Environmental assessment Environmental rating Based on available data on triaryl phosphates (section 7.7.4.1), phosphate esters in general are assigned environmental scores 4 or 5. However, it is expected that certain phosphate esters fulfil criteria for environmental scores 3, 2 and even 1. 7.7.4.3 Dialkyl phosphates, dialkyl phosphates, monoalkyl phosphates and complex phosphate estersHealth assessment Health rating - dialkyl phosphates The dialkyl phosphate – bis(2-ethylhexyl) hydrogen phosphate (CAS No. 298-07-7) -exhibits low acute toxicity by ingestion (40). The substance is harmful by skin contact (LD50, rabbit, dermal > 1250 mg/kg) and corrosive to skin and mucous membranes. The substance is assigned health score 3. Health rating - dialkyl phosphites The dialkyl phosphite – didodecyl phosphite (CAS No. 21302-09-0) - exhibits low acute toxicity by ingestion, inhalation and skin contact (43). Based on the limited data, the substance is assigned health score 2 due to a skin irritating potential. Dimethyl hydrogen phosphite (CAS-No. 868-85-9) exhibits low acute toxicity by ingestion and inhalation (43). The substance should be classified as harmful by skin contact (LD50 (rabbit, dermal) = 681 mg/kg) (43). Dimethyl phosphite is mildly to moderately irritating to skin and eyes (43). Limited evidence from animal studies indicates that the substance may have a carcinogenic potential. IARC classifies the substance as carcinogenic in category 3 (40,44). The compound is assigned health score 5 due to a carcinogenic potential. Health rating – monoalkyl phosphate The monoalkyl phosphate – 2-ethylhexyl hydrogen phosphate (CAS No. 1070-03-7) exhibits low acute toxicity by ingestion and skin contact. The substance is strongly irritating to eyes (43). A supplier classifies the mono alkylphosphate as corrosive (C;R34) (50). The substance is assigned health score 3. Health rating – complex phosphate esters There are virtually no toxicological data available on complex phosphate esters, including polypropylene and polyethylene oxide derivates. Polyethoxy oleyletherphosphate (CAS No. 39464-69-2), which is used as an extreme pressure additive for metal working lubricants, should not be classified as dangerous to the health based on the available supplier information (51,52). This corresponds to health score 1. The compound is used in cosmetics (42). Environmental assessment Environmental rating - dialkylphosphates, dialkylphosphites and monoalkylphosphates Based on available data (section 7.7.4.1), phosphate esters in general are assigned environmental scores 4 or 5. However, it is expected that certain phosphate esters fulfil criteria for environmental scores 3, 2 and even 1. 7.7.4.4 Zinc dialkyldithiophosphatesZinc alkyl thiophosphates are a large group of chemicals. The database IUCLID (16) contains 10 zinc alkyl thiophosphates that are used as lubricant additives. However, only little data is available for these substances especially concerning biodegradation and bioaccumulation. Zinc dialkyldithiophosphates are manufactured and commercially distributed in highly refined lubricant base oil (IP 346 DMSO extractables < 3%). The zinc dialkyldithiophosphates are never isolated from base oil at any time during their life cycle. Hence all testing for environmental and health effects are performed on zinc dialkyldithiophosphates in highly refined lubricant base oils. Health assessment Acute toxicity In general, commercial zinc dialkyldithiophosphates exhibit low acute toxicity. Acute oral LD50 in rats ranges from 2000 - 3500 mg/kg. Acute dermal LD50 in rabbits > 2000 mg/kg (53). Irritation Zinc dialkyldithiophosphates (CAS Nos. 4259-15-8, 28629-66-5, 68457-79-4) are moderately to strongly irritating to skin and eyes implying a risk of serious eye damage (16). Sensitization There is no indication of a sensitizing potential (53). Genotoxicity Several standard mutagenicity tests have been performed for a number of zinc dialkyldithiophosphates. Findings indicate that commercial zinc dialkyldithiophosphates have a low potential for inducing genetic toxicity (53). Repeated dose toxicity Data from several repeated-dose toxicity studies of commercial zinc dialkyldithiophosphates have been reviewed. Repeated dermal exposure of experimental animals results in moderate-to-severe dermal irritation, behavioural distress, body weight loss and emaciation, reduction in haematological parameters and adverse effects on male reproductive organs. Oral administration causes significant gastric irritation and related gastrointestinal disturbances, signs of distress but no evidence of adverse effects on male reproductive organs (53). Reproduction toxicity Data from a study on zinc bis[0,0-bis(2-ethylhexyl)] bis(dithiophosphate) (CAS No. 4295-15-8) indicates a low concern for reproduction/ developmental toxicity. Furthermore, an epidemiological study on workers exposed to oil-based zinc dialkyldithiophosphates (range C4-C8) in an additive manufacturing plant revealed no adverse effects on worker reproductive health (53). Carcinogenicity There are no data regarding the carcinogenic potential of zinc dialkyldithiophosphates. Health rating Based on available data, zinc dialkyldithiophosphates are irritating to skin. The eye irritating potential of zinc dialkyldithiophosphates ranges from moderately irritating to risk of serious damage to eyes. This classification implies that zinc dialkyldithiophosphates are assigned health score 2 – 3. Environmental assessment Aquatic toxicity Few data indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. Most of the data indicate EC/LC50 values below 100 mg/l. Toxicity tests of zinc dialkyldithiophosphates showed the following EC/LC50 values:
Environmental fate No test results for biodegradation were available. In a MSDS, the used zinc additive 2-ethylhexyl zinc dithiophosphate was referred to as not readily biodegradable according to an OECD 301 D test (5% degraded). Bioaccumulation No experimental data were available. The information on the structure of the zinc salts was considered insufficient for QSAR calculations to be made. Environmental rating Based on the sparse available data on this large group of substances and on additional secondary data from one MSDS, in which the zinc additive is classified R51/53, zinc additives are assigned environmental scores 4 or 5. 7.8 Sulphurized fatty compounds7.8.1 FunctionSulphurized fatty compounds are added to lubricants for metal working as extreme pressure additives (13,14). 7.8.2 IdentificationSulphurized fatty compounds are obtained by reaction of unsaturated compounds such as triglycerides from soy, rape seed or lard oil or synthetic esters produced from natural fatty acids, e.g. rapeseed methyl ester, with elemental sulphur at high temperatures or with hydrogen sulphide. By this, sulphur is added to double bonds forming sulphur links or ring structures. Fig. 7.5 shows an example of the chemical structure of sulphurized compounds used as extreme pressure additives in non-chlorinated lubricants. 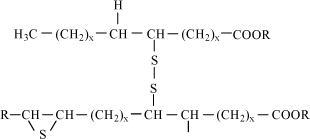
Fig. 7.5 Sulphurised fatty acid The function as extreme pressure additives depends on the degree of sulphurization. There are compounds with active and inactive sulphur on the market. The more active sulphur the compound contains the lower is the response temperature for the extreme pressure function (18,54). 7.8.3 Physical/chemical dataSulphurized fatty compounds are liquids at room temperature with high flash points (> 110°C to 200°C), negligible vapour pressures and very low solubility in water (55,56,57). 7.8.4 Health assessmentThere are virtually no health data in literature and databases on sulphurized fatty compounds. Thus, the health assessment of the group is based on sparse supplier information for a mixture of sulphurized rape seed oil and vegetable fatty acid methyl esters and a mixture of sulphurized animal oils and vegetable fatty acid methyl esters, in addition to conclusions by analogy from health data on starting materials for sulphurized fatty compounds. Acute toxicity Supplier data are available from studies in rats of acute oral toxicity. These data indicate low acute toxicity. LD50-values range from > 2000 mg/kg - > 5000 mg/kg (55,56,57). There are no data on acute dermal toxicity or toxicity by inhalation. Hydrogen sulphide may be formed by decomposition due to heating above 150° to 180°C. Hydrogen sulphide is very toxic by inhalation. Irritation Standard irritation tests (OECD Guideline 404 and 405) in rabbits of the mentioned mixtures of sulphurized vegetable or animal fatty oils and vegetable methyl esters indicate that the compounds are not irritating to skin and eyes (55,56,57). There are no data regarding inhalation. Sensitization Standard sensitization tests with Guinea pigs (OECD Guideline 406) have been performed for the compounds. The sulphurized fatty compounds do not possess a sensitizing potential based on the results of the tests (55,56,57). Repeated dose toxicity There are no further toxicological data on the sulphurized fatty compounds. Health rating There are very sparse data regarding the health effects of sulphurized fatty compounds. However, there exists considerable experience and knowledge of health effects of the starting materials, fatty vegetable and animal oils, esters synthesized from natural fatty acids and elemental sulphur. See also section 7.6.4. This knowledge indicates that health effects of sulphurized fatty compounds are limited. Sulphur dust is irritating to skin and mucous membranes (40,48). Thus based on the available supplier data and conclusion by analogy, sulphurized fatty compounds are assigned health score 1. 7.8.5 Environmental assessmentAquatic toxicity Only few data are available. According to a few material safety data sheets (MSDS), sulphurized oils and esters show EC/LC50 values above 100 mg/l. Environmental fate Very few data are available. According to a few material safety data sheets (MSDS), sulphurized esters and oils are readily biodegradable. Bioaccumulation No experimental bioconcentration factors were available. Based on the general structure of sulphurized oils, it is expected that log POW is above 4 for many compounds in this group. Environmental rating Based on these very sparse data, sulphurized natural and synthetic oils and esters are assigned environmental score 1. 7.9 Synthetic ester oils7.9.1 FunctionSynthetic ester oils, predominantly aliphatic ester oils, enter into lubricants for metal forming as lubricity improving additives (polar additives – see also section 2.2) and as lubricant bases (13,14). 7.9.2 IdentificationAs mentioned above, aliphatic ester oils are the most frequently used type of synthetic ester oils in metal forming lubricants. Thus, the health and environmental assessment of synthetic ester oils will focus on this group of esters (13,14). An ester is the condensation product of an acid, most often an organic acid, and an alcohol. Aliphatic ester oils are produced in a condensation process at 120-240°C under water cleavage (14). Aliphatic ester oils are a large and varying group of substances. They are synthesized from relatively pure and simple starting materials as natural fatty acids and alcohols (monoalcohols, glycols and polyols). There is an incredible versatility in the design of ester molecules due to the large number of commercially available acids and alcohols, from which to choose. A large number of esters can be designed with different chemical structures selected for specific desired properties. Performance properties, which can be varied in the ester design include viscosity, viscosity index, volatility, biodegradability, lubricity, hydrolytic stability, additive solubility, high temperature coking tendency and seal comparability (58). Synthetic ester oils for metal working lubricants can be divided into five groups:
Monoesters are used primarily as lubricant base oils. The function of diesters, polyglycol esters, polyol esters and complex esters in lubricants for metal working are mainly as lubricity improving additives (polar additives). However they can also enter as lubricant base oils. The polyglycol esters also have emulsifying properties which are used in water based lubricants (14,18). 7.9.3 Monoesters7.9.3.1IdentificationMonoesters are synthesized from natural or synthetic carboxylic acids and monoalcohols. Typical starting materials for monoesters in lubricants are oleic acid, palmitic acid, cocos fatty acids and methanol, isopropanol, isobutanol, 2 –ethylhexanol, n-octanol/n-decanol and isotridecanol (14). Fig. 7.6 illustrates the chemical structure of an aliphatic organic monoester. Fig. 7.6 The chemical structure of an organic monoester. R and R' are aliphatic radicals. Table 7.9 states the name and CAS number of substances which are included in the health and environmental assessment of monoesters.
Table 7.9 Chemical names and CAS Nos. of substances included in the health and environmental assessment of monoesters in metal working lubricants. 7.9.3.2 Physical/chemical dataThe monoesters are liquids at room temperature. They have high boiling points (< 200°C), high flash points (> 100°C) and low vapour pressure. The solubility in water is generally low (16,59,60). 7.9.3.3 Health assessmentAcute toxicity Monoesters of higher fatty acids (C12 or more) in general exhibit low toxicity and a number are cleared for use in the food industry (59,60). In addition, the majority of the monoester compounds included in this health assessment is used as skin conditioning agents in cosmetics (38,42). Data for acute toxicity by ingestion (LD50, rat, oral) for the specified CAS Nos. are in the range of more than 2000 mg/kg to 27000 mg/kg. Data for acute toxicity by skin contact (LD50, rabbit or rat, dermal) are in the range of 3000 mg/kg to more than 5000 mg/kg (16,44,61,62). Inhalation exposure at toxicological significant levels is not expected due to the low volatility of the mentioned monoesters. Irritation The monoesters have varying irritating properties. Methyl oleate (CAS No. 112-62-9) is moderately irritating to rabbit skin. Methyl laurate (CAS No. 111-82-0) is stated as slightly irritating to rabbit skin in an unspecified test. However the substance is stated as highly irritating to rabbit skin when tested in accordance with EU Directive 84/449/EEC, B.4. The same substance is stated as non-irritating to human skin in the Epicutan test according to Burckhardt (16). The remaining monoesters exhibit slightly irritating properties on rabbit skin (16,44). Methyl laurate (CAS No. 111-82-0) and 2-ethylhexyl laurate (CAS No. 20292-08-4) are non-irritating in rabbit eyes, while 2-ethylhexyl-2-ethyl hexanoate (CAS No. 7425-14-1) is moderately to strongly irritating in rabbit eyes (16). Sensitization Data for sensitization potential of the monoesters indicate that they do not possess this property. Methyl laurate (CAS No. 111-82-0) did not provoke an allergic response when tested in Guinea pig (test method not specified), while 2-ethylhexyl laurate (CAS No. 20292-08-4) and decyl oleate (CAS No. 3687-46-5) were non-sensitizing in the Guinea pig maximization test (16,61). Methyl oleate (CAS No. 112-62-9) was not sensitizing by skin contact in a test with volunteers, exposed to the substance in 10 % petrolatum solution (16,61). Repeated dose toxicity A 28-day oral gavage study in rats with decyl oleate (CAS No. 3687-46-5) at doses of 100, 500 and 1000 mg/kg showed no toxicity as observed with respect to clinical symptoms, biochemistry, hematology, gross lesions or tissue/organ histopathology (16). The NOAEL was 1000 mg/kg. In a corresponding 28-day gavage study of 2-ethylhexyl laurate (CAS No. 20292-08-4) in rats, the NOAEL was 1000 mg/kg (16). The findings support a low order of toxicity of monoesters by repeated exposure. Genotoxicity Genotoxicity has been studied in bacterial cells for three of the mentioned monoesters (decyl oleate (CAS No. 3687-46-5), methyl laurate (CAS No 111-82-0) and 2-ethylhexyl laurate (CAS No. 20292-08-4)). The test results were negative in all three cases. In addition, 2-ethylhexyl laurate has been tested in vivo in mice in the micronucleus assay with negative result (16). The findings indicate that the monoesters do not posses a genotoxic potential. Reproduction toxicity Fertility, litter size and survival of offspring were normal in rats fed diets containing 6,25 % (approx. 3125 mg/kg bw/day) of butyl stearate (CAS No. 123-95-5) for 10 weeks. However, growth was reduced in offspring during the pre-weaning and post-weaning periods. No gross lesions were observed among the offspring killed at the end of the 21-day post-weaning periods. These results indicate that long-chain fatty acid esters do not cause reproductive toxicity to rats (59). Assessment of developmental effects for long chain fatty acid monoesters is primarily based on data for C16-18, 2-ethylhexyl ester (CAS No. 91031-48-0). In oral gavage studies in rats administered doses of 100, 300 and 1000 mg/kg during gestation, the maternal NOAEL was 1000 mg/kg and the NOAEL for foetal effects was 1000 mg/kg (59). The findings indicate that long chain fatty acid monoesters do not posses a foetotoxic potential. Carcinogenicity There is data available from carcinogenicity studies of a single monoester, methyl oleate CAS No. 112-62-9. Studies in mice exposed to the substance by oral ingestion, dermal application or injection under the skin for a life long period indicate, that methyl oleate may have a weak tumour promoting activity (16). Health rating Monoesters used in metal working lubricants are based on the available data, assigned score 1. However, some monoesters as methyl oleate (CAS No. 112-62-9) and 2-ethylhexyl-2-ethylhexanoat (CAS No. 7425-14-1) exhibit an eye irritating potential and are thus assigned health score 2. 7.9.3.4 Environmental assessmentEnvironmental data are available for methylesters of different fatty acids. Aquatic toxicity Few data are available for this group of chemicals. The available data indicate low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. Two toxicity tests with EC/LC50 values below 100 mg/l were referred, i.e. fatty acid C6-C10 methyl esters (CAS No. 68937-83-7) with LC50(48h) towards Leuciscus idus of 95 mg/l and 88 mg/l, respectively (16). Environmental fate Several biodegradation tests are available. All tests indicate that fatty acids methyl esters are readily biodegradable (16). Bioaccumulation No experimental bioconcentration data are available but many log POW data are referred in IUCLID (16). All data showed log POW values above 4, which indicates that fatty acid methyl esters have a potential for bioaccumulating in aquatic organisms Environmental rating Based on the available data, fatty acid monoesters are assigned environmental score 1. 7.9.4 Diesters7.10.4.1 IdentificationDiesters are ester compounds of diacids and monoalcohols. Starting materials for diesters in metal working lubricants are fatty acids as adipic acid, sebacic acid and dimer acid and monoalcohols as methanol, isopropanol, isobutanol, 2-ethylhexanol and isotridecanol (14). Adipates are the most widely used diesters in lubricants (58). Fig. 7.7 illustrates an example of the chemical structure of an aliphatic organic diester. 
Fig. 7.7 The chemical structure of an organic diester. R1 and R' are aliphatic organic radicals. Table 7.10 states the name and CAS number of substances which are included in the health and environmental assessment of diesters.
Table 7.10 Chemical names and CAS Nos. of substances included in the health and environmental assessment of diesters in metal working lubricants. 7.9.4.1 Physical/chemical dataShort-chain alkyl (e.g. methyl, isopropyl and butyl) diesters are generally more water soluble and more volatile than the corresponding long-chain alkyl (C7-C13 alcohol) diesters. The diesters included in the assessment are liquids at room temperature with high boiling points (> 200°C), high flash points (> 100°C), low vapour pressures and moderate to very low solubility in water (16,59). 7.9.4.2 Health assessmentAcute toxicity The diesters in general demonstrate a low order of acute toxicity. Oral rat LD50 values range from > 2000 mg/kg to > 45.000 mg/kg (16,59). Dermal rabbit LD50 values for diisodecyl adipate (CAS No. 27178-16-1) > 5000 mg/kg and for bis(2-ethylhexyl) adipate (CAS No. 103-23-1) > 8.000 mg/kg (16). Inhalation exposure at toxicological significant levels is not expected due to the low volatility of the diesters in focus. Irritation Standard skin and eye irritation test with rabbits are available for several of the diesters in focus. The results mainly indicate slightly irritating properties. (16). Sensitization Sensitization data are available for bis(2-ethylhexyl) adipate (CAS No. 103-23-1) and diisodecyl adipate (CAS No. 27178-16-1). Bis(2-ethylhexyl) adipate was not sensitising in Guinea pig tested by the Draize test and two unspecified tests, and when tested in rabbits in a Patch test. Diisodecyl adipate was not sensitising in the Guinea pig maximization test (16). Thus there is no dating indicating a sensitising potential of diesters in metal working lubricants. Repeated dose toxicity There are a number of animal studies of repeated exposure to bis(2-ethylhexyl) adipate (CAS No. 103-23-1). In 90-day sub-chronic dietary studies, the NOAEL was approximately 300 mg/kg /day in rats and 230 mg/kg/day in mice. The LOAEL was approximately 600 mg/kg/day in rats and 460 mg/kg/day in mice. Hepatic hypertrophy and increased peroxisomal enzyme activity occurred in rats and mice (16,59). Data on repeated dose toxicity have also been reported for diisononyl adipate (CAS No. 33703-08-1) and bis(tridecyl) adipate (CAS No. 16958-92-2). In 90-day toxicity study, rats were administered diisononyl adipate in the diet at levels equivalent to 0, 50, 150 and 500 mg/kg/day. The NOAEL was 500 mg/kg/day. Feeding studies were also carried out in beagle dogs for 13 weeks at dietary concentrations of 0, 0.3, 1 and 3 % of diisononyl adipate (increased to 6 % at week 9). The NOAEL was determined to be 1 % in the diet or approximately 274 mg/kg /day. In another 13-week study, bis(tridecyl) adipate was well tolerated in rats given dermal doses of 800 and 2000 mg/kg/day (59). Overall the findings indicate a low order of toxicity by repeated exposure to the diesters in focus. Several of the adipates and sebacates have Indirect Food Additive Status for use in food wrapping materials (63). In addition, several of the adipates and sebacates are used as ingredients in cosmetic (38,42). Genotoxicity Bis(2-ethylhexyl) sebacate (CAS No. 122-62-3), bis(tridecyl) adipate (CAS No. 16958-92-2) and diisononyl adipate (CAS No. 33703-08-1) were shown to be negative in the Ames assay. Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) has also been evaluated for mutagenicity and was found to be negative in both the Ames and mouse lymphoma assay (59). Regarding chromosomal aberration, bis(tridecyl) adipate (CAS No. 16958-92-2) has been tested in the micronucleus assay, and bis(2-ethylhexyl) adipate (CAS No. 103-23-1) has been tested in the Chinese hamster ovary cell with negative results (16,59). Thus the available findings do not indicate genotoxic properties of the diesters in focus. Carcinogenicity Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) was tested for carcinogenicity by oral administration in one experiment in mice and one experiment in rats. In mice, liver adenomas and carcinomas were produced in both males and females. No treatment-related tumours were observed in rats. The International Agency for Cancer Research (IARC) evaluates that there is limited evidence in experimental animals for the carcinogenicity of bis(2-ethylhexyl) adipate. According to the IARC evaluation criteria, the substance is not classifiable as to its carcinogenicity in humans (Group 3) (64). There are no further data on carcinogenicity of the diesters in focus. Reproduction toxicity Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) has been evaluated for reproductive effects in a one-generation study. Male and female rats were administered the substance in their diets at same levels (0, 28, 170 or 1080 mg/kg/day). After 10 weeks on the diet, the animals were mated to produce one generation of offspring. Test diets were administered continuously throughout the study (18 –19 weeks of exposure). No effects were seen on male or female fertility. However, at the highest dose, there was a reduction in body weight in the dams, and reduction in offspring body weight, total litter weight and litter size. The NOAEL in this study was 170 mg/kg/day. The LOAEL was 1080 mg/kg/day (59). A developmental toxicity/teratogenicity study in rats is available for bis(2-ethylhexyl) adipate (CAS No. 103-23-1). In accordance with OECD Guideline 414, rats were exposed to the substance in the diet during the gestation period at dose levels of 28, 170 and 1080 mg/kg/day. A significant reduction in the maternal body weight gain and feed intake was observed in the highest dose group, however no maternal toxicity was observed at 28 and 170 mg/kg/day. The foetal weight, litter weight and number of external and internal abnormalities were not influenced by the treatment. However, a dose dependent fetotoxicity was observed as minor skeletal defects in the groups receiving 170 and 1080 mg/kg/day. It was concluded that bis(2-ethylhexyl) adipate caused a dose-related foetotoxic effect, however no teratogenic effect (16). A newer study support the observation of foetotoxicity of bis(2-ethylhexyl) adipate (CAS No. 103-23-1). In rats orally exposed to pre- and postnatal doses of 0, 200 , 400 or 800 mg/kg bw/day of bis(2-ethylhexyl) adipate, a prolonged gestation period was observed at a dose level of 800 mg/kg bw/day, and an increased frequency of pre- and postnatal death of pups was observed at dose levels of 400 and 800 mg/kg bw/day. Bis(2-ethylhexyl) adipate at a dose level of 800 mg/kg bw/day also decreased the pup weight and the decrease in body weight persisted until adulthood. The NOAEL was 200 mg/kg bw/day in this study (65). In 13-week dermal studies with bis(tridecyl) adipate (CAS No. 16958-92-2), no sperm morphological changes were observed in rats treated at levels of 2000 mg/kg. Increase in organ weight of the epididymides and uterus was observed at dermal exposure to 2000 mg/kg but not at 800 mg/kg (59). In a 19-week oral feeding study with bis(2-ethylhexyl) sebacate (CAS No. 122-62-3), no adverse reproductive effects were reported for this substance (59). There are no data on developmental toxicity/ teratogenicity for the remainder diesters in focus. Health rating Bis(2-ethylhexyl) adipate (CAS No. 103-23-1) is assigned health score 5 due to an indication of a foetoxic effect. Based on the available data, the remaining diesters in focus are assigned health score 1. 7.9.4.3 Environmental assessmentAquatic toxicity Few environmental test data are available on adipates. Some of these data indicate EC/LC50 values below 1 mg/kg. Toxicity tests of diesters showed the following EC/LC50 values:
Environmental fate Several biodegradation tests are available. All the tests show that adipates are readily biodegradable except one test result for diisotridecyl adipate (CAS No. 26401-35-4) (16). Bioaccumulation Only few test data were available. Test results were found for the following compounds:
On this basis, adipates are assessed to have a potential for bioaccumulating in aquatic organisms. Environmental rating Based on the available data on this large group of substances, adipates are in general assigned environmental scores 4 or 5. This assessment is based on the few available data on bioaccumulation (diethylhexyl adipate and diisononyl adipate). 7.9.5 Polyglycol esters7.9.5.1IdentificationPolyglycol esters are ester compounds of mono- or dicarboxylic acids and polyglycols. Typical starting materials for production of polyglycol esters for lubricants are co-polymers of ethylene oxide, propylene oxide and butylene oxide, eventually with glycerine, trimethylol propane (TMP) or pentaerythritol (PENTA) as starters, and carboxylic acids as oleic acid, palmitic acid, cocos fatty acids, adipic acid, sebacic acids and dimer acid (14). Fig. 7.8 shows an example of the chemical structure of an aliphatic polyglycol ester. 
Fig. 7.8 The chemical structure of an aliphatic polyglycol ester. R and R' are aliphatic radicals. Short chain aliphatic glycol esters are included in the health and environmental assessment of polyglycol esters. Table 7.11 states the name and CAS number of substances which are included in the health and environmental assessment of polyglycol esters.
Table 7.11 Chemical names and CAS Nos. of substances included in the health and environmental assessment of polyglycol esters in metal working lubricants. 7.9.5.2 Physical/chemical dataThe polyglycol esters and short chain aliphatic glycol esters in focus are liquids at room temperature with high boiling points (> 250°C), high flash points (> 200°C) and very low vapour pressures. The solubility in water varies from completely soluble to very limited solubility (16,59,66,67). 7.9.5.3 Health assessmentAcute toxicity Polyethyleneglycol monooleate (CAS No. 9004-96-0) exhibits low acute toxicity by oral exposure (LD50 rat > 2000 mg/kg) (68). Data from rat studies with shorter chain aliphatic glycol esters also indicate a low order of toxicity (LD50 values ranging from 2000 mg/kg to 34600 mg/kg) (59). Irritation Polyethyleneglycol monooleate (CAS No. 9004-96-0) exhibits mild skin and eye irritating potential in standard tests with rabbits (43). A polyoxyalkylene glycol (56 % solution in water) (CAS No. not specified) is stated to be non-irritating to rabbit skin and eyes by the supplier (67). A short chain aliphatic glycol ester, triethyleneglycol diheptanoate (CAS No. 7434-40-4) is non-irritating to rabbit skin and eyes in standard tests (16). Polyethyleneglycol monooleate (CAS No. 9004-96-0) and a number of the short chain aliphatic glycol esters in focus are used as ingredients in cosmetics (16). Sensitization There is no data available regarding the sensitizing potential of polyglycolesters or short chain aliphatic glycol esters. Repeated dose toxicity There are no data available on repeated exposure for polyglycol esters. However, sub-chronic studies have been carried out with short chain glycol esters. In a 28–day oral gavage studies in rats exposed to PEG-4 heptanoate (CAS No. 70729-68-9), the NOAEL was determined to be 1000 mg/kg. No clinical signs of toxicity were observed and no treatment-related changes in hematology or clinical chemistry were reported (59). Propylene glycol monostearate (CAS No. 1323-39-3), which was administered for 13 weeks at dietary concentrations up to 7.52% (approx. 3760 mg/kg bw/day), showed no signs of toxicity in rats (59). Similarly, in 6-month oral studies, no signs of toxicity, gross or histological pathology were observed in rats and dogs fed diets containing up to 10% propylene glycol monostearate (CAS No. 1323-39-3) (59). Doses up to 1000 mg/kg/day (approx. 5000 mg/kg bw/day) of 2,2,4-trimethyl-1,3-pentanediol heptanoate (CAS No. 71839-38-8) were well tolerated in rats that were orally (gavage) administered the test material for 28 days (59). The findings indicate a low toxicity by repeated exposure of short chain aliphatic glycol esters and polyglycol esters as the toxicity of the last-mentioned are expected to be lower than that of the short chain aliphatic glycol esters. Genotoxicity There are no data on the genotoxic potential of polyglycol esters. Four short chain aliphatic glycol esters, PEG-4 diheptanoate (CAS No. 70729-68-9), 2,2,4-trimethyl-1,3-pentanediol heptanoate (CAS No. 71839-38-8), triethyleneglycol diheptanoate (CAS No. 7434-40-4) and propyleneglycol monostearate (CAS No. 1323-39-3) have shown to be negative in the Ames assay (59). PEG-4 diheptanoate (CAS No. 70729-68-9) has been tested in the Chinese hamster ovary cell assay and did not cause chromosomal aberrations. 2,2,4-Trimethyl-1,3-pentanediol heptanoate (CAS No. 71839-38-8) has also been evaluated in the in-vitro cytogenetics test using human peripheral lymphocytes with negative result (59). Together, these findings indicate that glycol esters do not cause gene mutations or chromosomal aberrations. Carcinogenicity and reproductive toxicity There are no data on the carcinogenic, reprotoxic or teratogenic potential of polyglycol esters or shorter chain aliphatic glycol esters. Health rating CESIO (The European trade organisation for manufacturers of surfactants and their intermediates) recently concluded in a report that ethoxylated fatty acids should not be classified with respect to health hazards according to the Dangerous Substances EU Directive (69). Polyethylenglycol esters are based on the available data on polyglycol esters and short chain aliphatic glycol esters, assigned health score 1. 7.9.5.4 Environmental assessmentAquatic toxicity The few available data showed that polyglycol esters have a low toxicity towards water-living organisms. All available EC/LC50 values are above 100 mg/l, e.g. (60):
Environmental fate All referred tests showed that polyglycol esters are readily biodegradable, e.g. (60):
Bioaccumulation All assessed polyglycolesters have log POW below 3. Polyglycol esters made by long-chained acids may have log POW above 4 (60). Environmental rating Based on the sparse available data on this large group of substances, polyglycol esters are assigned environmental score 1. 7.9.6 Polyol esters and complex esters7.9.6.1 IdentificationPolyol esters are ester compounds of poly-functional alcohols and monocarboxylic acids. Starting materials for manufacture of polyol esters are neopentylglycol (NPG), trimethylolpropane (TMP) and pentaerythritol (PENTA) in addition to oleic acid, palmitic acid, cocos fatty acids and isononanoic acid (14). Complex esters are ester compounds of poly-functional alcohols and mono- and dicarboxylic acids. Starting materials for manufacture of complex esters are the same as for polyol esters in addition to dicarboxylic acids, such as adipic acid, sebacic acid and dimer acids (14). Fig. 7.9 gives an example of the chemical structure of an aliphatic polyol ester using pentaerythritol as a starting materials. 
Fig. 7.9 The chemical structure of an aliphatic polyolester using pentaerythritol as one of the starting materials. The R's are aliphatic radicals. Complex esters can be considered a subgroup of polyolesters. As both ester groups to a great extent are manufactured from the same raw materials, they are considered as one substance group in the health and environmental assessment. There are very few toxicological data on longer chain (C16 and more) fatty acid polyol esters, and consequently data on shorter chain (< C11) will be included in the assessment. Table 7.12 states the name and CAS number of substances which are included in the health and environmental assessment of polyol esters including complex esters.
Table 7.12 Chemical names and CAS Nos. of substances included in the health and environmental assessment of polyol esters and complex esters in metal working lubricants. 7.10.6.2 Physical/chemical dataPolyolesters and complex esters are large molecules with molecular weights above 400. They are liquids at room temperature with high boiling points (> 200°C), high flash points (> 200°C), very low vapour pressure and very low solubility in water (59,70). 7.9.6.2 Health assessmentAcute toxicity Oral LD50-values from studies with rats indicate a low order of toxicity (LD50 > 2000 mg/kg) for a number of polyol esters (CAS Nos. not stated) (59). Irritation A single polyol ester (CAS No. not stated) was not irritating to rabbit skin and eyes (70). A polyol ester, (PENTA mixed esters, C6,7,8,10 acids (CAS No. 68130-55-2)) applied to the skin of rats five days a week for four weeks at dose levels of 0, 125, 500 and 2000 mg/kg/day caused no visible signs of irritation. However, microscopically treated skin of rats in the two highest dose groups exhibited a dose-related increased incidence and severity of hyperplasia and hyperkeratosis of the epidermis and sebaceous gland hyperplasia. The effects were reversible (59). Sensitization There are no data available on the sensitizing potential of polyol esters or complex esters. Repeated dose toxicity Data from five 28-day oral toxicity studies in rats and one 28-day dermal toxicity study in rats are available for polyolesters designated a) TMP esters of heptanoic and octanoic acid, b) heptanoic acid, ester with 2,2,4-trimethyl-1,3-pentanediol, c) hexanedioic acid, mixed esters with C10-rich, C9-11 isoalcohols and TMP, d) fatty acid, C6-10, tetraesters with PENTA, e) hexanedioic acid mixed esters with decanoic acid, heptanoic acid, octanoic acid and PENTA. No CAS Nos. are stated. The polyol esters a) through d) were well tolerated by rats in the 28-day oral studies. The NOAEL for these studies was 1000 mg/kg/day in rats. The polyol ester a) did not produce signs of overt systemic toxicity at any dose level tested (100, 300, and 1000 mg/kg/day). There were no treatment-related changes in clinical symptoms, functional observation battery or gross post mortem findings. There were no treatment-related mortality, no adverse effects on body weight, food consumption, clinical laboratory parameters, or organ weights. However, an increased number of hyaline droplets in kidneys of the 300 and 1000 mg/kg/day in male rats was observed. Based on these findings, the NOAEL was established at 100 mg/kg/day for male rats. The observed hyaline droplet formation is considered to be of little relevance to humans (59). The polyol ester e) was applied to the skin on groups of 10 (male and female) rats for five days a week for four weeks at dose levels of 0, 125, 500 and 2000 mg/kg/day. Treated animals exhibited no signs indicative of systemic toxicity. No visible signs of irritation were observed at treatment sites. Microscopically, treated skin (500 mg/kg/day or more) exhibited a dose related increased incidence and severity of hyperplasia and hyperkeratosis of epidermis and sebaceous gland hyperplasia. These effects were reversible. None of the minor changes in hematology and serum chemistry parameters were considered biologically significant. High dose females (2000 mg/kg/day) exhibited a significant increase in relative adrenal and brain weight in the female animals. The NOAEL in this study was 500 mg/kg/day for systemic toxicity and 125 mg/kg/day for skin irritation (59). A TMP ester (C8, C10 acid) (CAS No. 11138-60-6), was evaluated for repeated dose toxicity in a 28-day dermal study. Dose levels in the study are not stated. The effects observed as a result of treatment (decrease in body weight and serum protein values) were insignificant and of little toxicological concern. No evidence of microscopic changes was observed in the histopathological evaluation. Thus, the NOAEL for the TMP ester (C8, C10 acid) was 2000 mg/kg bw (59). Together, the findings of the repeated dose toxicity studies suggest that polyol esters exhibit a low order of toxicity following repeated application. Genotoxicity Standard bacterial cell mutagenicity tests (Ames test) and chromosomal aberration tests have been carried out for a number of polyolesters (TMP esters, C7 and C8 acids (CAS No. not stated), TMP esters, C7 acids (CAS No. 71839-38-8), TMP and other alcohols mixed, C6 dioic acids (CAS No. 180788-27-6), TMP ester, C8, C10 acid (CAS No. 11138-60-6), PENTA tetraester, C5-9 acids (CAS No. 67762-53-2), PENTA mixed esters, C6,7,8,10 acids (CAS No. 68130-55-2) (59). The results are in all studies negative, indicating that polyol esters do not possess a genotoxic potential. Reproduction toxicity and carcinogenicity There are no data available on the toxicity of polyol esters towards reproduction and foetal development or the carcinogenic potential. Health rating Based on the available data, polyol esters are assigned health score 1. 7.9.6.3 Environmental assessmentAquatic toxicity The few available data indicate that polyol esters have a low toxicity towards water-living organisms. All available EC/LC50 values are above 100 mg/l. Toxicity tests of polyol esters showed the following EC/LC50 values (16,60):
Environmental fate Referred tests of ready biodegradability showed variable results, e.g. (60):
Bioaccumulation All assessed polyol esters have log POW above 4 (60). Environmental rating Based on the sparse available data on this large group of substances, polyol esters are assigned environmental scores 1 or 2. 7.10 Soaps7.10.1 FunctionThe function of soaps in lubricants for metal working is as lubricant improving additives (polar additives) and corrosion inhibitors (14). 7.10.2 IdentificationSoaps are metal salts of fatty acids. They are produced by reaction of fatty acid esters from animal or vegetable fats heated with aqueous alkali as potassium or sodium hydroxide. Glycerol is produced as a by-product. The reaction is called a saponification. An example of a saponification is stated below (71). Ex.: (C17H35COO)3C3H5 + 3 NaOH → 3C17H35COONa + C3H5(OH)3 The health assessment of soaps focuses on potassium and calcium soaps of long-chain fatty acids (C 16). Experiments have shown that the behaviour of an acid and its salt is very similar in a physical, chemical and toxicological context (72). Thus, the health assessment of the soaps is based on animal and human test both on soaps and on the pure fatty acids of the soaps in addition to experience. Table 7.13 states the name and CAS number of substances which are included in the health and environmental assessment of soaps.
Table 7.13 Chemical names and CAS Nos. of substances included in the health and environmental assessment of soaps in metal working lubricants. 7.10.3 Physical chemical dataSoaps seen in lubricants for heavy-duty metal forming are typically potassium and calcium soaps of longer-chain fatty acids (C ≥ 16). These soaps are solids with melting point above 100°C (16). Sodium soaps are so-called hard soaps, while potassium softs are soft soaps. Both types of soaps are water soluble. The so-called metallic soaps (aluminium, calcium, cobalt, lead and zinc) are not water soluble. (71,73). 7.10.4 Health assessmentAcute toxicity Test data with rats indicate a low acute toxicity of longer-chain fatty acid salts of calcium and potassium. Oral LD50-values range from > 2000 mg/kg for C18 and C18-unsaturated fatty acid salts of potassium (CAS No. 68647-90-5) to > 10.000 mg/kg for C16-18 fatty acid calcium salts (CAS No. 85251-71-4) (16). Dermal LD50-values in rats, rabbits or guinea pigs for long-chain fatty acids and their salts and a single inhalation study in rats for decanoic acid (C10) (CAS No. 334-48-5) also indicate low acute toxicity by these exposure routes (72). Irritation Tests in animals and humans show that the skin irritating potential of fatty acids and their salts decrease with increasing chain length, to the effect that medium chain lengths (C10) are irritants, whereas C12 is minimally irritant and the longer chain lengths, C14 and above, are not irritant (72). To some extent, soaps degrease the skin and make the surface of the skin alkaline. This may increase the skin permeability of substances, which are irritating (74). As with skin irritation, animal test data show that the eye irritation potential of fatty acids and their salts decrease with increasing chain lengths, to the effect that chain lengths C10 and C12 are irritant, whereas the longer chain lengths, C14 and above, are not irritant (72). There are very few reports on eye damage in humans caused by soaps. The effects have been transient (75). Soaps in commercial detergents with free alkali and high alkalinity may cause severe and irreversible eye damage (76). Sensitization Soaps are based on test data for fatty acids and their salts notoriously not sensitizing by skin contact. However soaps degrease the skin and make it more permeable for substances as perfumes and preservatives, which may provoke an allergic response (72,74). Repeated dose toxicity Available tests demonstrate a low toxicity of fatty acids and their salts by repeated exposure. This is consistent with the long history of safe use in foods for both fatty acids and glycerides (72). In a 24-week oral study, rats were fed doses of 15 % oleic acid (C18) (CAS No. 112-80-1) (approximately 7500 mg/kg bw/day). Normal growth and general good health were reported in the rats and the NOAEL was reported to be 7.500 mg/kg bw/day (16). A formulation ”bath soap and detergent” containing 10-25% sodium stearate (C18) was used to conduct a dermal toxicity study in rabbits. Formulations at a dose of 2000 mg/kg bw were applied daily for three months to the skin by syringe, five days a week. No untoward reactions were observed (72). Together the available data indicate low toxicity of fatty acids and their salts by repeated exposure. Genotoxicity Potassium salts of fatty acids, C18 and C18-unsaturated (CAS No. 68647-90-5) has been tested in a standard bacterial mutagenicity assay (the Ames test), with and without metabolic activation, with negative result (16). The fatty acids, capric acid (C10), lauric acid (C12), stearic acid (C18), oleic acid (C18) and fatty acids (C18-22) have produced negative results in the Ames test (72). There is no data available regarding the potential to cause chromosome aberration. Based on available data, there is no indications that fatty acids and their salts possess a genotoxic potential. Carcinogenicity Numerous studies of mechanisms for the role of dietary fat in tumorigenesis have been studied. In a two-year study, groups of male and female rats, initially 7 weeks old, were given sodium oleate (C18) (CAS No. 143-19-1) for 108 weeks at concentrations of 2,5 and 5,0 % in the drinking water. The conclusion of this study was that sodium oleate did not cause cancer in rats exposed to the substance through the diet (72). Reproduction toxicity 15 % oleic acid (C18) (CAS No. 112-80-1) in the diet (approximately 7.500 mg/kg bw/day) for 10 to 19 weeks did not affect the fertility of male rats but appeared to impair reproductive capacity in the female rats by interfering with parturition and mammary gland development. Increased mortality in the offspring was observed. No further information is available (16). In a three-generation study in mice, which were reared on semi-purified diets containing 8.6 % (approx. 12900 mg/kg bw/day) Cuphea oil (contained 76 % capric acid (C10)), no adverse effects on reproductive parameters or any tissue pathology were observed (72). Soap (not specified further) was examined for foetotoxic potential following percutaneous administration. Groups of rats and mice were treated with concentrations of 0.3, 3 and 30 % (corresponding to 50, 500 and 5000 mg/kg/day in mice) of a standard soap solution. The formulated solutions were applied to the skin at the rate of 0.5 ml/rat or mouse per day with rats being dosed on days 2 –15 and mice on days 2 –13 of gestation. There was no evidence of reproductive or foetotoxic effects (72). Health rating Fatty acid esters in the form of triglycerides are occurring in substantial amounts as a natural part of the human diet. Several of the fatty acids are normal degradation products of fats in the human metabolism. Their degradation pathway and fate in the organism are well-known. Potassium, calcium and sodium are all essential nutrients in the human diet. Several of the fatty acids are approved as direct and as indirect food additives. Long experience with safe use of fatty acids and their salts in foods and available test data demonstrate that soaps do not posses carcinogenic, reprotoxic or teratogenic potential (72). Long chain fatty acid soaps of potassium and calcium are assigned health score 1. 7.10.5 Environmental assessmentAquatic toxicity Most of the available data indicated low toxicity towards aquatic organisms with EC/LC50 values above 1000 mg/l. However, toxicity tests with EC/LC50 values below 100 mg/l and even below 1 mg/L were referred (soap with chain length C12-C14) with a LC50(96h) towards Oncorhynchus mykiss of 0.6 mg/L (the geometric mean of five tests) (16). Environmental fate Several biodegradation tests are available. All tests show that soaps are readily biodegradable (16). Bioaccumulation No experimental bioaccumulation data are available. QSAR calculations show log POW values from 0.3 (C7) to 4.7 (C17) (95). Environmental rating Based on the available data, soaps are assigned environmental scores 1 or 2. 7.11 Reference point – medium-chained chlorinated paraffins7.11.1 FunctionThe function of chlorinated paraffins in lubricants for metal working is mainly as extreme pressure additives. In addition, the chlorinated paraffins may constitute a substantial part of the lubricant implying that the compounds also function as a lubricant base (1). 7.11.2 IdentificationChlorinated paraffins are produced by adding chlorine gas to n-alkane fractions (straight-chained saturated hydrocarbons). The general structure of chlorinated paraffins is CxH(2x-y+2)Cly., The chain length of commercial chlorinated paraffins is normally between 10 and 30. Chlorine content varies between 40 –70 %. The chlorinated paraffins are divided into three groups: Short-chained chlorinated paraffins (C10 – C13), medium-chained chlorinated paraffins (C14 – C17) and long-chained chlorinated paraffins (C18 – C30). There may be a further classification of the chloroparaffins depending on their chlorination degree. Low chlorinated paraffins have a chlorination degree < 50 %, whereas high chlorinated paraffins have a chlorination degree > 50 % (2). There exist more than 200 different grades of commercial chloroparaffins. They are all complex mixtures of n-alkanes characterized by an average chain length and chlorination degree (2,3). Table 7.14 states the names and CAS numbers of substances which are included in the health and environmental assessment of medium-chained chlorinated paraffins.
Table 7.14 Chemical names and CAS numbers of substances included in the health and environmental assessment of medium-chained chloronated paraffins. 7.11.3 Physical/chemical dataChloroparaffins are viscous, colourless or yellow liquids. Chloroparaffins with a chain of more than 20 carbon atoms and high chlorination degree (70 %) are solids. Chloroparaffins are chemically very stable, but decompose above 300°C forming hydrogen chloride. The substance group have very low vapour pressures at room temperature, high flash points and are virtually insoluble in water (2). Due to risk reduction measures in the EU for short-chained chlorinated paraffins (SCCPs), medium-chained chlorinated paraffins (MCCPs) are the dominating type of chloroparaffins in lubricants for metal working (3). 7.11.4 Health assessmentThe health assessment of MCCPs are based on data for MCCPs (CAS No. 85535-85-9 (alkanes, C14-17, chloro)) and the similar SCCPs (CAS No. 85535-84-8 (alkanes, C10-13, chloro). Acute toxicity MCCPs have very low oral acute toxicity when ingested. LD50 (rat) > 15.000 mg/kg (16). Animal test data for SCCPs indicate low acute toxicity by skin contact and inhalation. Based on data for SCCP, which is structurally very similar to MCCP, MCCP may also have a very low acute toxicity by skin contact and inhalation (3). Irritation Studies in rats and rabbits have demonstrated slight irritation at most to the skin caused by MCCPs. However, there was some potential for cracking of the skin following repeated dermal application of liquid MCCPs, probably caused by de-fatting properties (3). Studies in rabbits indicate that MCCPs have low eye irritation potential (3). There are no data on respiratory irritation of MCCPs. As there are no reports relating to this endpoint and due to the widespread use of chloroparaffins, this suggests a lacking potential to cause such an effect. The low skin and eye irritation potential and the generally unreactive nature of the substance group lends further support to this (3). Sensitization There was no evidence of skin sensitization in standard tests in Guinea pigs. These data and the generally un-reactive nature of MCCPs indicate that they do not possess a sensitizing potential (3). Repeated exposure A number of studies in rats, mice and Guinea pigs in addition to a single study in dogs examine the effects of MCCPs by repeated oral exposure. These studies demonstrate that liver, thyroid gland and kidneys are the target organs. Only some of the observed effects are considered to be of toxicological significance to humans. However, in rats, single cell necrosis was observed in the liver at 360 mg/kg/day. In addition, in the same study minor effects were observed in kidney tissue at dose levels of 4 mg/kg bw/day and 10 mg/kg bw/day and considered of toxicological significance to humans (3). Genotoxicity Studies of genotoxic effects of MCCPs and the structurally similar SCCPs in bacterial and mammal cell systems and rats and mice indicate that MCCPs do not possess a genotoxic potential (3). Carcinogenicity There are no data on the carcinogenic potential of MCCPs. Rodent carcinogenicity studies using a SCCP (60 % chlorination) produced toxicologically significant dose-related increases in the incidence of several tumour types. Due to the underlying mechanisms of these tumours, they were considered of little or no relevance to human health. However, kidney tubular call adenomas were seen in male rats as a result of exposure to the SCCPs. The underlying mechanism for the kidney tumours has no yet been fully elucidated. The carcinogenic potential to humans cannot be entirely excluded based on the available data. However, recent mechanistic evidence strongly suggests that the mechanism of the kidney tumours are probably not of toxicological significance to humans. This is supported by the general un-reactive nature of chloroparaffins and the fact that MCCPs do not exhibit a mutagenic potential (3). Reproduction toxicity There is no data available in humans regarding effects of MCCPs on fertility and foetal development. One limited animal study is available in relation to the effects of MCCPs on fertility. The administration of a MCCP (52 % chlorination) to rats in a 2-generation reproduction study at up to 384 mg/kg/day for females and 463 mg/kg/day for males in the diet had no apparent effect on fertility (3). No adverse effects during gestation were produced in two reproductive studies with rats and rabbits orally exposed to a MCCP in doses up to 5000 and 100 mg/kg/day respectively (3). In contrast, exposure of rats (dams) to a MCCP (52 % chlorination) at approximately 400 mg/kg/day in the diet produced internal haemorrhaging and deaths in the pups. A mechanistic study has indicated interference of the MCCP to the vitamin-K-dependent neonatal blood clotting system. It is considered that either direct exposure to the MCCP via transferral to the pups in the breast milk disrupted the vitamin-K-dependent clotting system or the pups received less vitamin K in the breast milk due to treatment-related effects upon their mothers. This would appear to be a phenomenon specific for neonates as there is no indication of haematological effects in adult animals in conventional repeated-exposure studies. From the studies available, a NOAEL of 8 mg/kg/day as a maternal dose can be identified for this effect (3). In summary, based on limited animal data, MCCPs do not exhibit an effect on fertility or foetal development when administered to rats up to approximately 400 mg/kg/day in the diet. However, MCCPs may present a hazard to the neonatal offspring via the lactating mother (3). Health rating MCCPs are based on the available data proposed a classification as reproduction toxic in category 3 with risk phrase R63 ”Possible risk of the unborn child”, risk phrase R64 ”May cause harm to breastfed babies” and risk phrase R66 ”Repeated exposure may cause skin dryness or cracking” (Rep3;R63 R64 R66) (3). The substance group is assigned health score 5. 7.11.5 Environmental assessmentAquatic toxicity The medium-chained chlorinated paraffins (MCCPs) seem to have low acute toxicity towards. Data refers LC50(96h) towards fish above 5,000 mg/l. Long-term tests also showed little effect on fish (1). Studies show, however, that MCCPs are very toxic to daphnids. In a short-term toxicity test, LC50(48h) was 6-8 µg/l. Results from long-term tests with NOEC of 10 µg/l are, however, conflicting with the results from the acute test. Other short-term studies show LC50 values of approx. 1 mg/l (1). Environmental fate More tests indicate that biodegradation of MCCPs is limited. BOD5 values of <10 and 20 mg O2/g have been reported for a MCCP (41% Cl) and MCCP (49% Cl), respectively. Other experiments show a decrease in biodegradability with increasing degree of chlorination (1). Bioaccumulation A large number of studies concerning bioconcentration from water are reported in the EU risk assessment of MCCPs (CAS No. 85535-85-9). However, in a number of these studies, calculated test concentrations exceeded the solubility, and it could be suspected that not all of the MCCPs were truly dissolved. A BCF(fish) of 1087 measured for Oncorhynchus mykiss is referred as a reliable result (1). Environmental rating In the draft for EU's environmental risk assessment of MCCPs (CAS No. 85535-89-5), it is proposed that MCCPs are classified as dangerous to the environment: very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment (N;R50/53) (1). Based on this proposal, MCCPs are assigned environmental score 5. 7.12 Conclusion7.12.1 ResultsThe chosen representative substance groups of non-chlorinated lubricants for metal forming are assigned a health and an environmental score. The scores are based on a health and environmental rating system developed by CETOX (Centre for Integrated Environment and Toxicology). The system is developed on the basis of the EU classification system for chemical substances which focus on the inherent properties of substances. The higher the score, the more adverse health or environmental properties of a compound. Thus, score 5 implies the most serious health or environmental effects while score 1 implies the least adverse effects. The rating system is described in further detail in section 7.2. Table 7.14 provides an overview of the result of the health and environmental assessment of representative components in non-chlorinated lubricants.
Table 7.14 The result of the health and environmental assessment of representative substance groups in non-chlorinated lubricants for metal forming. Subgroups are assigned a health and environmental score respectively. Assignment of health or environmental score 5 implies the most serious health or environmental effects, while health or environmental score 1 implies the least adverse effects. For further details see section 7.2. 7.12.2 ConclusionIn the EU human risk assessment draft of medium-chained chloroparaffins (MCCPs) it is proposed, that MCCPs should be classified as toxic to reproduction in category 3 (Rep3) with risk phrases R63 ”Possible risk of harm to the unborn child” and R64 ”May cause harm to breastfed babies” in addition to R66 “Repeated exposure may cause skin dryness or cracking” (3). This classification implies that MCCPs are assigned health score 5. The substance groups mineral base oils, over-based calcium petroleum sulphonates, vegetable and animal oils, sulphurized fatty compounds, synthetic ester oils and soaps enter non-chlorinated lubricants for metal forming lubricants as lubricant bases, lubricity enhancing additives (polar additives) or extreme pressure additives. The health assessment of these substance groups has resulted in an assignment of health score 1 or 2 (except for synthtetic diesters). This indicates that these compounds do not possess properties which may cause serious adverse health effects. The substance groups alkyl sulphides (polysulphides) and phosphorous compounds are added to non-chlorinated lubricants primarily as extreme pressure additives, while synthetic diesters are added as lubricity improving additives and lubricant bases. The three substance groups are assigned health score 1 – 4, 1 – 5 and 1 -5, respectively. This implies that there is a considerable variety in the toxicity of individual substances in the groups. The substance groups includes substances which posses properties which may cause adverse health effects. Some of these substances have the potential to cause adverse health effects as serious as assessed for MCCPs. In the EU environmental risk assessment draft of MCCPs it is proposed that MCCPs should be classified as dangerous to the environment (N) with the risk phrase R50/53 ”Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment” (1). This implies that the substance group is assigned environmental score 5. The environmental assessment of mineral base oils, over-based calcium petroleum sulphonates, vegetable and animal oils, sulphurized fatty compounds, synthetic ester oils (except for some diesters) and soaps result in assignment of environmental score 1 or 2. This indicates that these substance groups possess properties which may cause less severe effects in the environment. Similar to the health assessment of the selected substance groups in non-chlorinated lubricants, the environmental assessment indicates that alkyl sulphides (polysulphides) and phosphorous compounds possess properties which may cause the most severe environmental effects. These two substance groups are assigned environmental score 3 – 5 and 1 – 5. Thus the eco-toxicity of especially phosphorous compounds may vary considerably. Both substance groups include substances which may cause severe environmental effects at a rating level as assessed for MCCPs. Finally, the health and environmental assessment of selected substance groups in non-chlorinated lubricants demonstrates that the health and especially the environmental data platform is poor for a number of the substance groups generally occurring in non-chlorinated lubricants. This includes alkyl sulphides, some of the phosphorous compounds, sulphurized fatty acids and some of the synthetic ester oils. Thus, substitution of chlorinated lubricants to the currently available non-chlorinated lubricant alternatives imply a movement from a reasonable health and environmental data platform to a substantially poorer data platform. 8 Health and environmental screening of non-chlorinated lubricants
8.1 IntroductionA health and environmental screening of proposed non-chlorinated lubricant alternatives has been performed in phase 3 of the project. This was done with the aim to establish an overview of the health and environmental properties of the proposed non-chlorinated lubricants at a screening level in addition to identify and eventually sort out lubricants with obvious unwanted health and environmental properties prior to full scale production test. The original objective of this project was merely to focus on the extreme pressure additives (EP additives) of non-chlorinated alternatives, as the primary function of chlorinated paraffins in metal working lubricants is as EP additives. However, this quickly showed to be difficult. Substitution of chlorinated paraffins in lubricants for metal forming implies a complete reformulation of the lubricant. Most often it is impossible to precisely point out which of the components in non-chlorinated lubricants substitutes the chloroparaffins. Together, the individual components of the non-chlorinated lubricant form a complex chemistry resulting in lubrication during the metal forming process. Thus, it was rather quickly estimated in the project that the best basis for comparison between health and environmental effects of chlorinated and non-chlorinated lubricants was obtained through an evaluation of not only the entering components but also the entire lubricant. The health and environmental screening includes lubricants which possess promising lubricating properties in the focused metal processes based on supplier information and at the same time, where satisfactory compositional information has been obtained. In total a health and environmental screening has been performed for 14 non-chlorinated lubricants. The objective of chapter 8 is to describe the methods for the health and environmental screening of the proposed non-chlorinated lubricants and to provide and discuss the results of the screening. 8.2 Method for health screening of proposed non-chlorinated lubricantsAs in the health assessment of entering components in non-chlorinated lubricants, the health and environmental screening of alternative lubricants focuses entirely on the inherent properties of the components contained in the products. This means that the use of the product is not considered in the assessment. The screening of the proposed alternative lubricants is based on information retrieved at the suppliers (see also chapter 4) in addition to a search in Annex 1 to the EU Directive 67/548/EEC on dangerous substances and toxicological and eco-toxicological data in well-recognized handbooks and databases. The quality of the data is not rated. Thus, the screening of proposed non-chlorinated lubricants should be regarded only as an indication of the health and environmental properties. Based on the health and environmental classification of the individual substances entering into a lubricant in a concentration above 0,1 %, each substance is assigned a health and an environmental score respectively. The scores are assessed on the basis of a health and environmental rating system developed by CETOX (Centre for Integrated Environment and Toxicology). The system is developed as a tool to facilitate the differentiation between chemical substances and products with respect to health and environment. Both the health and the environmental rating systems comprise five hazard groups – health or environmental groups 1 to 5. Group 5 includes substances with the most severe health or environmental effects, while group 1 includes substances with no or few less severe health or environmental effects. The designation score corresponds to hazard group. The rating systems are described further in section 7.2 of this report. No or insufficient data (Score ND) Both the health rating and the environmental rating systems include an additional group comprising substances for which no data is available or for which the data available is insufficient to place the substance in a hazard group. For components in a lubricant placed in group ND there is either no or insufficient information available for a health or environmental assessment resulting in a classification and subsequently a rating. Score ND – health Assignment of a health score of a component demands as a minimum the availability of test data from animal studies on acute oral toxicity as well as data for skin and eye irritation and sensitizing potential by skin contact. Alternatively, a health score of a component can be established based on data from long term experience with human exposure by relevant routes. Score ND – environment Assignment of an environmental score demands as a minimum data for aquatic toxicity in addition to data on biodegradability. If there are no data on bio-accumulation, this effect can be evaluated on experimental or calculated data for water-octanol distibution. Health and environmental assessment of total lubricants Based on health and environmental classifications for the individual components in a lubricant, the product classification is calculated in accordance with the EU classification principles for preparations described in EU Directive 99/45/EC on classification, packaging and labelling of dangerous chemical preparations. A product health and environmental score respectively are assigned based on the product classification. Reference point A chlorinated lubricant (approximately 50 % Cl) used by Danfoss A/S in relevant metal forming processes at the time of this project is used as the reference point in the health and environmental screening of alternative lubricants. The lubricant contains 80 % of a medium-chained chlorinated paraffin. 8.3 Results of health and environmental screening of proposed lubricantsA health and environmental screening has been performed for 16 non-chlorinated lubricants. Below are stated the results of the health and environmental screening of the proposed non-chlorinated lubricants in addition to the results of a health and environmental screening of a reference lubricant - a chlorinated lubricant. The figures representing the individual products illustrate the percentage content of components placed in the individual health and environmental hazard groups. The designation score corresponds to hazard group. The product health score and the product environmental score is stated in the text below the figures. 8.3.1 Product no. 10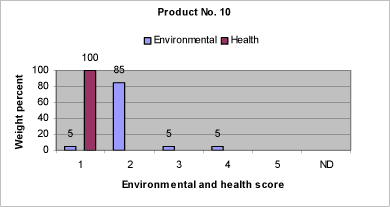
Fig 8.1: Relative content of components in product no. 10 assigned the individual environmental and health scores As can be seen from fig. 8.1, 5.0 % of the components in product no. 10 is assigned environmental score 1, 85.0 % is assigned score environmental 2, 5.0 % is assigned environmental score 3 and 5 % is assigned environmental score 4. All components in product no. 10 are assigned health score 1. The total product is assigned environmental score 3 and health score 1. 8.3.2 Product no. 12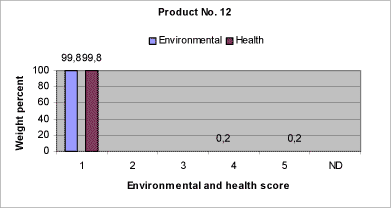
Fig.8.2: Relative content of components in product no. 12 assigned the individual environmental and health scores. As can be seen from fig. 8.2, 99.8 % of the components in product no. 12 are assigned environmental and health score 1, respectively. 0.2 % of the components are assigned environmental score 4 and health score 5. The total product is assigned environmental score 1 and health score 1. 8.3.3 Product no. 15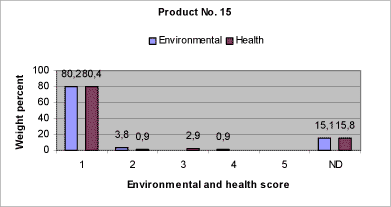
Fig 8.3: Relative content of components in product no. 15 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 15 are illustrated in fig. 8.3. As regards environmental hazard, 80.2 % of the components in product no.15 is assigned score 1, 3.8 % is assigned score 2, and 0.9 % is assigned score 4. Regarding health hazard, 80.4 % of the components in product no.15 is assigned score 1, 0.9 % is assigned score 2, and 2.9 % is assigned score 3. For 15.1 % and 15.8 % respectively of the components in product no. 15 it is not possible to assign an environmental and health score due to insufficient data. Based on available data, the total product is assigned environmental score 1 and health score 1. 8.3.4 Product no. 18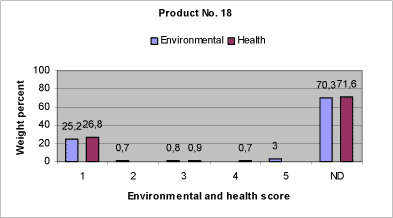
Fig. 8.4: Relative content of components in product no. 18 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 18 are illustrated in fig. 8.4. As regards environmental hazard, 25.2 % of the components in product no. 18 is assigned score 1, 0.7 % is assigned score 2, 0.8 % is assigned score 3 and 3 % is assigned score 5. Regarding health hazard, 26.8 % of the components in product no.18 is assigned score 1, 0.9 % is assigned score 3 and 0.7 % is assigned score 4. For 70.3 % and 71.6 % respectively of the components in product no. 18, it is not possible to assign an environmental and health score due to insufficient data. Based on available data, the product is assigned environmental score 4 and health score 1. 8.3.5 Product no. 24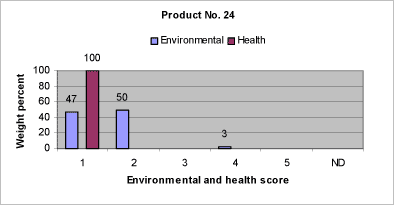
Fig 8.5: Relative content of components in product no. 24 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 24 are illustrated in fig. 8.5. As regards environmental hazard, 47.0 % of the components in product no. 24 is assigned score 1, 50.0 % is assigned score 2 and 3.0 % is assigned score 4. All components in product no. 24 are assigned health score 1. The total product is assigned environmental score 1 and health score 1. 8.3.6 Product no. 37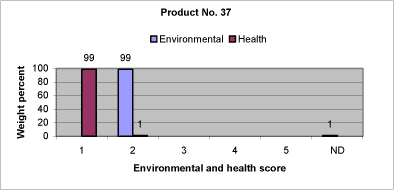
Fig. 8.6: Relative content of components in product no. 37 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 37 are illustrated in fig. 8.6. As regards, environmental hazard 99.0 % of the components in product no.37 is assigned score 2. 99.0 % of the components in product no. 37 is assigned health score 1, and 1 % health score 2. For 1 % of the components in product no. 37, it is not possible to assign an environmental score due to insufficient data. Based on available data, the total product is assigned environmental score 2 and health score 1. 8.3.7 Product no. 38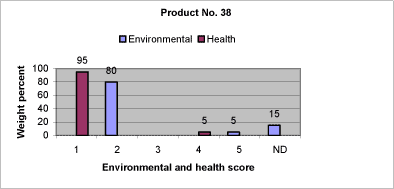
Fig. 8.7: Relative content of components in product no. 38 assigned the individual environmental and health scores. As can be seen in fig. 8.7, 80 % of the components in product no. 38 is assigned environmental score 2 and 5 % are assigned environmental score 5. 95 % of the components in product no. 38 is assigned health score1 and 5 % is assigned health score 4. For 15 % of the entering components it is not possible to establish an environmental score due to insufficient data. Based on available data, the total product is assigned environmental score 4 and health score 4. 8.3.8 Product no. 39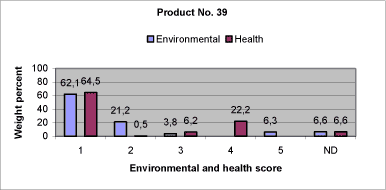
Fig. 8.8: Relative content of components in product no. 39 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 39 are illustrated in fig. 8.8. As regards environmental hazard, 62.1 % of the components in product no. 39 is assigned score 1, 21.2 % is assigned score 2, 3.8 % is assigned score 3 and 6.3 % is assigned score 5. Regarding health hazard 64.5 % of the components in product no. 39 is assigned score 1, 0.5 % is assigned score 2, 6.2 % is assigned score 3 and 22.2 % is assigned score 4. For 6.6 % of the components in product no. 39 it is not possible to establish an environmental and health score due to insufficient data. Based on available data, the total product is assigned environmental score 4 and health score 4. 8.3.9 Product no. 40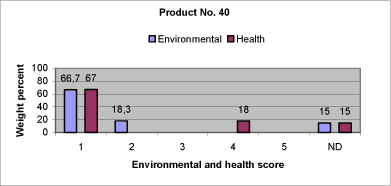
Fig. 8.9: Relative content of components in product no. 40 assigned the individual environmental and health scores. As can be seen in fig. 8.9, 66.7 % of the components contained in product no. 40 is assigned environmental score 1 and 18.3 % is assigned environmental score 2. 67.0 % of the components in product no. 40 is assigned health score 1 and 18.0 % is assigned health score 4. For 15 % of the entering components it is not possible to establish an environmental and health score due to insufficient data. Based on available data, the total product is assigned environmental score 1 and health score 4. 8.3.10 Product no. 41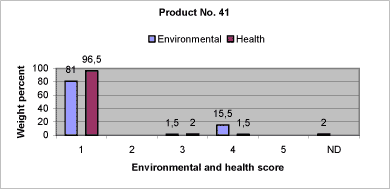
Fig. 8.10: Relative content of components in product no. 41 assigned the individual environmental and health scores. As can be seen in fig. 8.10, 81.0 % of the components in product no. 41 is assigned environmental score 1, 1.5 % is assigned score 3 and 15.5 % is assigned environmental score 4. 96.5 % of the components in product no. 41 is assigned health score 1, 2.0 % is assigned score 3 and 1.5 % is assigned health score 4. For 2 % of the entering components it is not possible to establish an environmental score due to insufficient data. Based on available data, the total product is assigned environmental score 3 and health score 1. 8.3.11 Product no. 42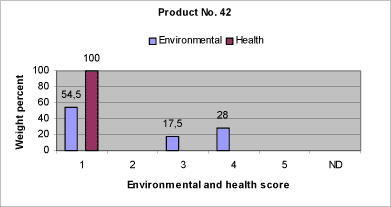
Fig 8.11: Relative content of components in product no. 42 assigned the individual environmental and health scores. As can be seen in fig. 8.11, 54.5 % of the components entering into product no. 42 is assigned environmental score 1, 17.5 % is assigned environmental score 3 and 28 % is assigned environmental score 4. All components in product no. 42 are assigned health score 1. The total product is assigned environmental score 4 and health score 1. 8.3.12 Product no. 45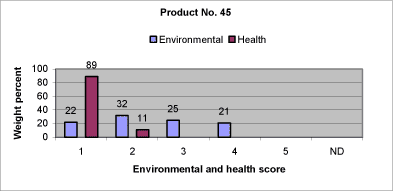
Fig. 8.12: Relative content of components in product no. 45 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 45 are illustrated in fig. 8.12. As regards environmental hazard, 22.0 % of the components in product no. 45 is assigned score 1, 32.0 % is assigned score 2, 25.0 % is assigned score 3 and 21.0 % is assigned score 4. Regarding health hazard 89.0 % of the components in product no. 45 is assigned score 1 and 11.0 % is assigned score 2. The total product is assigned environmental score 3 and health score 1. 8.3.13 Product no. 51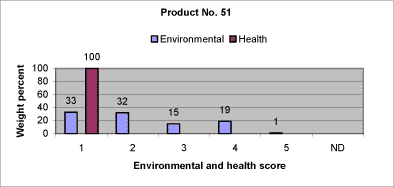
Fig 8.13: Relative content of components in product no. 51 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 51 are illustrated in fig. 8.13. As regards environmental hazard, 33.0 % of the components in product no. 51 is assigned score 1, 32.0 % is assigned score 2, 15.0 % is assigned score 3, 19.0 % is assigned score 4 and 1 % are assigned score 5. All components in product no. 51 are assigned health score 1. The total product is assigned environmental score 4 and health score 1. 8.3.14 Product no. 53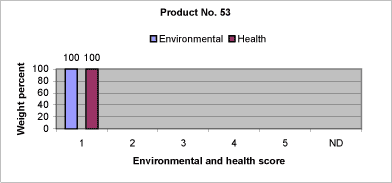
Fig 8.14: Relative content of components in product no. 53 assigned the individual environmental and health scores. The individual environmental and health scores for product no. 53 are illustrated in fig. 8.14. All components in product no. 21 are assigned environmental score 1 and health score 1 respectively. The total product is assigned environmental score 1 and health score 1. 8.3.15 Chlorinated lubricant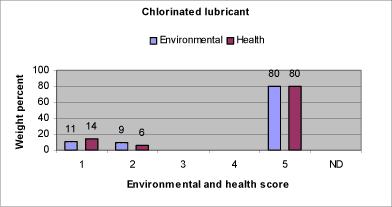
Fig. 8.15: Relative content of components in the reference product, a chlorinated lubricant, assigned the individual environmental and health scores. As can be seen from figure 8.15, 80 % of components in the reference product, a chlorinated lubricant, is assigned environmental and health score 5. This is due to the content of a medium-chain chlorinated paraffin. Medium-chain chlorinated paraffins (CAS-No. 85535-85-9) are proposed a classification as reprotoxic in category 3 with risk phrases R63: “Possible risk of harm to the unborn child“, R64: “May cause harm to breastfed babies”, R66: “Repeated exposure may cause skin dryness or cracking” and dangerous to the environment with risk phrase R50/53: “Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment”. This classification is proposed in the EU Risk Assessment on medium-chained chlorinated paraffins (CAS-No. 85535-85-9) human health draft February 2002 (3) and environment draft August 2000 (1). 11.0 % of the entering components are assigned environmental score 1 and 9 % is assigned environmental score 2. 14.0 % of entering components are assigned health score 1 and 6 % is assigned health score 2. The total product is assigned environmental score 5 and health score 5. 8.4 ConclusionsThe total product environmental scores of the proposed alternative lubricants are illustrated in fig. 8.16. The reference product – a chlorinated lubricant – is marked with an asterisk. 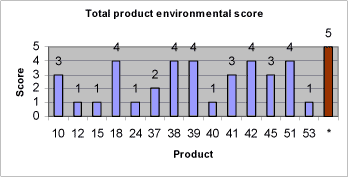
Figure 8.16 Total product environmental scores for proposed non-chlorinated lubricants in addition to the product environmental score for the reference lubricant – a chlorinated lubricant - marked with an asterisk ( * ). Based on the available data, the results of the environmental screening of the suggested alternative lubricants show that five non-chlorinated lubricants are assigned a total product environmental score 1, one lubricant is assigned a total product score 2 and eight non-chlorinated lubricants are assigned a total product environmental score 3 or 4. The total product health scores of the suggested alternative lubricants are illustrated in fig. 8.17. The reference product – a chlorinated lubricant – is marked with an asterisk. 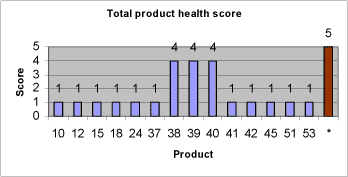
Figure 8.17 Total product health scores for proposed non-chlorinated lubricants in addition to the product health score for the reference lubricant – a chlorinated lubricant - marked with an asterisk ( * ). Eleven non-chlorinated lubricants are assigned a total product health score 1 while three non-chlorinated lubricants are assigned a total product health score 4. The reference product, a chlorinated lubricant, is assigned a total product health score 5. It can be concluded, that with reference to the health and environmental criteria established in section 3.3.1 and 3.3.2, apparently the majority of the non-chlorinated lubricants have acceptable health qualities based on available data while a substantial number of the same lubricants have environmental properties which should be avoided based on available data. Three of the proposed alternative lubricants are assigned a total product health score of 4 due to a content of component classified as sensitizing by skin contact. In the two cases, this classification is due to an organic amine and in the third case due to a polysulphide. Eight out of fourteen of the non-chlorinated lubricants are assigned a total product environmental score of 3 or 4. These environmental scores are due to a content of polysulphides, phosphorous compounds and/or calcium sulphonates. The health and environmental properties of all three substance groups are discussed further in chapter 7. The products considered as the best alternatives in a total health and environmental context based on criteria established in sections 3.3.1 and 3.3.2 are stated in table 8.1.
Table 8.1 The proposed lubricant alternatives considered as the health and environmentally best alternatives. For about half of the proposed non-chlorinated lubricants, there are no or insufficient data available for a number of entering components. This implies that there is no or insufficient data regarding potential health effects including data on acute oral toxicity, skin and eye irritation and sensitizing potential by skin contact. Regarding potential effects in the environment, this implies, that there is no or insufficient data available on aquatic toxicity and biodegradeability. Thus, assignment of a health and environmental score only implies that there is a minimum data set available concerning health and environmental effects. For a substantial part of the components in non-chlorinated lubricants there exists very limited data on health and environmental properties compared to chlorinated paraffins. See also chapter 7 on health and environmental assessment of components in non-chlorinated lubricants. It can be concluded that based on the sparse available data, non-chlorinated lubricants seem to possess improved inherent health and environmental properties compared to chlorinated lubricants. However, it should be kept in mind, that a number of the proposed non-chlorinated lubricants contain substances which are assigned environmental score 3 or 4 or even 5, though in lower concentrations than the typical concentration of chlorinated paraffins in the chlorinated lubricants. Further, substitution of chlorinated lubricants to the currently available non-chlorinated lubricant technologies implies a movement from a reasonable health and environmental data platform to a substantially poorer data platform. 9 Exposure assessment in the working environment of selected components in non-chlorinated lubricants
9.1 Introduction to chapter 9The health and environmental screening of proposed non-chlorinated lubricants and the health and environmental assessments of components typically found in non-chlorinated lubricants focus on the inherent properties of chemical substances and products. The use of the lubricants is not considered. In order to also consider the use of the lubricants and thereby the health risk involved when using the non-chlorinated lubricants in the working environment, exposure assessments have been performed for four substances. Together these substances represent two groups of components typically found in non-chlorinated lubricants for heavy-duty metal working with the main function as extreme pressure additives (EP additives). The exposure assessment and evaluation of the health risk in the working environment for the use of non-chlorinated lubricants are very much performed at a screening level and only provide an indication of the health risk involved by use of the products. The exposure assessments only consider exposure in the working environment. Exposure of the specified substances may also occur in other spheres – for instance by diffuse exposure via the environment. Exposure in other spheres than the working environment is not considered in this project. Chapter 9 describes the method and results of the exposure assessments and discuss the results. 9.2 Substances in focusOrganic phosphorous compounds and alkyl sulphides (polysulphides) are considered to be the two most critical substance groups found in non-chlorinated lubricants with respect to potential adverse health effects in the occupational environment. This evaluation is based on the health assessments performed in this project on components in non-chlorinated lubricants. Both organic phosphorous compounds and polysulphides are typically entering non-chlorinated lubricants primarily as EP additives. The inherent health and environmental properties of the substance groups are discussed in chapter 7. Exposure assessments in the working environment have been performed for two organic phosphorous compounds and two polysulphides. The substances selected for the exposure assessments are the organic phosphates; tributyl phosphate (TBP) (CAS No. 126-73-8) and phenol, isopropylated, phosphate (3:1) (ITAP) (CAS No. 68937-41-7), and the polysulphides sulphurized; 2,4,4-trimethyl-pentene (CAS No. 68515-88-8) and di(tert-dodecyl) pentasulphide (CAS No. 31565-23-8). The health assessment of the four substances is described in sections 7.5.4 and 7.7.4, respectively. The choice of these four substances were determined by the availability of physical/chemical and health data for the individual substances. The selected substances demonstrated the best data platform. 9.3 The exposure scenarioToday, chlorinated lubricants are used at Danfoss A/S for metal forming operations, such as extrusion, stamping and deep drawing in stainless steel. Exposure conditions at Danfoss A/S during these forming operations are used as scenarios in the exposure assessment. The relevant exposure routes for chemicals in the working environment are skin contact and inhalation. At Danfoss A/S, metal forming operations are performed by machines in machining rooms, which are generally fenced from the working areas. The degree of dermal exposure to the lubricant may vary depending on the type of forming operation. However, in general dermal exposure occurs during continuous surveillance and regulation and mounting of the machines, cleaning and renovation of used tools, and control of finished work pieces. This may imply skin exposure of hands and underarms, in addition to drip on shoulders and hair. The oil is heated to 70 –80°C during some of the forming operations. Thus, dermal exposure to metal forming lubricants occurs through non-dispersive use with direct and extensive handling. Hot vapours may be generated during intentional heating of the lubricant to 70 – 80°C to adjust the viscosity, but also during friction heating generated at the metal surface under the forming operations. There is no data on the temperature at the metal surface under the forming operations. However, presumably it may reach the decomposition point of the substances in focus which are informed to be > 200° C. Decomposition products of TBP may be toxic fumes of phosphor oxides (31). There is no information on decomposition products of ITAP, while decomposition products of the polysulphides may be toxic fumes of hydrogen sulphide and sulphur oxides (30). Metal forming operations do not give rise to mechanically produced mist (aerosols). Mist may be formed by condensation of hot vapour. However, the exposure to mist during metal forming is considered to be very limited. Exposure to lubricants by inhalation of vapours and mist occurs in the machining room during surveillance and regulation, shift of tools and manual emptying of boxes containing finished work pieces. 9.4 Dermal exposureA worst-case assessment of dermal exposure is calculated for the four substances in focus by use of the EUSES EASE model (77). The use pattern is non-dispersive use and direct and extensive handling. In addition, skin absorption estimates of the four substances are, if possible, calculated using three different skin absorption models. The models used are SkinPerm (78), DermWin (79) and the method described by Sartorelli (80). 9.4.1 Worst case dermal exposure calculated by EUSES EASE modelThe calculated worst case dermal exposure for metal working lubricants with direct and extensive handling using the EUSES EASE model lies in the range of 1- 5 mg/cm²/day (3). The estimated exposure area (hands and underarms) is 2700 cm² and the average human body weight is 70 kg. Thus, the calculated exposure of the total lubricant will be approximately 39 – 193 mg/kg bw/day. The calculated worst case range of dermal exposure for the four substances in focus are stated below in table 9.1.
Table 9.1 Calculated worst case dermal exposure concentrations for four non-chlorinated lubricant EP-additives estimated by the EUSES EASE model (77). 9.4.2 Dermal absorption calculated by various skin absorption modelsSkin absorption estimates of the four substances are, if possible, calculated using three different skin absorption models. The models used are SkinPerm (78), DermWin (79) and the method described by Sartorelli (80). All three models are developed based on data set for a limited number of substances. Thus, the sensitivity of the models varies depending on the physical/chemical characteristics of the chosen substances. In addition, all three models are based on skin absorption of substances in aqueous solution. This is indeed not an optimal absorption model for components in metal forming lubricants as they are most often mineral oil based. However to our knowledge, there are no skin absorption models available at present, which are developed on the basis of substances in oil-based solutions. The three skin absorption models underestimate the skin absorption of substances with very low water solubility. A scenario with an exposure time of 8 hours/day and an exposure area (hands and arms) of 2700 cm² is used in the three models. Mean average human body weight is 70 kg. The calculated total absorption in/through the skin for the four substances in focus are stated in table 9.2. The Sartorelli model is not suitable for calculation of skin absorption for substances with a Log Pow above 6. Thus a skin absorption estimate calculated by the Sartorelli model is only available for tributyl phosphate (TBP) having a Log Pow below 6 (16).
Table 9.2 Calculated total dermal skin absorption for four non-chlorinated lubricant EP-additives using three different skin absorption models. Log Pows marked with an asterisk ( * ) are estimated by EpiWin (81). A ( - ) indicates that the absorption model is not applicable for the specific substance. 9.5 Exposure by inhalationThe EUSES EASE model (77) has been used to calculate the vapour concentrations of the substances in focus in the working environment during conditions as described in the exposure scenario. An operating temperature of approximately 80° C are used in the model. Decomposition products are not considered. Below in table 9.3 are stated the vapour concentrations at a process temperature at 80°C calculated by the EASE method in addition to the saturated vapour concentrations of the four substances in focus.
Table 9.3 Vapour concentrations and saturated vapour concentrations at a process temperature on 80°C for four EP additives in non-chlorinated lubricants for metal forming. * Estimated by EPIWIN (81) and EUSES/EASE (77) 9.6 Discussion of exposure results and risk characterization9.6.1 IntroductionA risk characterization has been performed in the working environment for the four substances in focus – two phosphorous compounds (TBP, CAS No. 126-73-8 and ITAP, CAS No. 68937-41-7) and two polysulphides (sulphurized 2,4,4-trimethyl pentene, CAS No. 68515-88-8 and di(tert-dodecyl) pentasulphide, CAS No. 31565-23-8). The risk characterization has been performed by comparison of the calculated exposure levels by dermal contact and inhalation and the NOAEL, LOAEL or NOAEC for critical health effects of the four substances by repeated exposure. Critical health effects of a substance is the effects observed on health at the lowest dose/concentration levels. NOAEL is an abbreviation for the “no observed adverse effect level” while LOAEL is the “lowest observed adverse effect level”. NOAEC is the “no adverse effect concentration” 9.6.1.1 Tributyl phosphate (TBP) (CAS No. 126-73-8)Dermal exposure The worst case dermal exposure scenario for tributyl phosphate (TBP) estimated by EASE is 0,4 – 29 mg/kg bw/day (table 9.1), while the calculated skin absorption estimates for TBP using three different skin absorption models range from 0,42 – 5,01 mg/kg/day (table 9.2). It can be concluded that there is reasonable consistency between the magnitude of the skin absorption estimates in the three models. There is also a reasonable consistency between the magnitude of the skin absorption estimates and the worst case dermal exposure calculated by EASE. Thus the calculated worst case exposure seems to be a reasonable estimate of the internal exposure of TBP by skin contact. Exposure by inhalation The calculated vapour concentration of TBP at 80°C is in the range of 554 - 1110 mg/m³, while the saturated vapour concentration (SVC) of TBP at 80°C is 153 mg/m³ (table 9.3). The SVC is the theoretical maximum in a steady state environment and will rarely, if ever, be achieved in practice in an industrial situation. The actual vapour concentration of TBP is therefore likely to be lower than the SVC. Thus the worst case vapour concentration of TBP in the working air calculated by EASE is obviously an overestimate. The contribution from aerosols to the inhaled exposure is considered to be very limited and is, thus not included in the exposure assessment. Risk characterization There are no data on the health effects of TBP by repeated dermal exposure. Available data on effects by repeated oral exposure, indicate that the critical effects seem to be effects on the peripheral nerve system, effects on the seminiferous tubules, effects on reproduction (reduced pup weight) and possible carcinogenicity in the urine bladder. It should be emphasized that the importance of these results to humans are not thoroughly assessed. Health effects by repeated exposure of TBP assessed as critical are summarized below in table 9.4. The studies and results mentioned are also described in the health assessment of TBP in section 7.7.4.2 (studies A, B, C, D and E).
Table 9.4 summarizes critical health effects of tributyl phosphate (CAS No. 126-73-8) by repeated exposure. Effects on peripheral nerve system and seminiferous tubules are observed at 400 mg/kg bw/day, whereas effects on reproduction (pup weight) and carcinogenic effects are observed at lower exposure levels. LOAEL for effects on pup weight was 15 mg/kg bw/day while NOAEL for neoplastic lesions in the urine bladder was 9 mg/kg bw/day for males and 12 mg/kg bw/day for females, respectively. Comparison of these effect levels with the calculated worst case dermal exposure level for TBP (0.4-29 mg/kg bw/day) indicates that a worst-case dermal exposure scenario to lubricants containing TBP (CAS No. 126-73-8) at a common lubricant concentration levels implies a risk of adverse effects on health. In a 4-month inhalation study with rats exposed to TBP, effects on cholinesterase activity and liver were observed at 13,6 mg/m³. The vapour concentration of TBP at 80°C will never exceed the SVC at the same temperature. Thus the theoretical maximum vapour concentration of TBP will be 153 mg/m³. In reality, the vapour concentration will be considerably lower than SVC. Comparison of the theoretical maximum vapour concentration of TBP (153 mg/m³) with the effect level of 13.6 mg/m³ indicates that the vapour concentration of TBP at 80°C may reach levels during metal forming operations implying a risk of adverse effects on health. 9.6.1.2 Phenol isopropylated phosphate (3:1) (ITAP) (CAS No. 68937-41-7)Dermal exposure The worst case dermal exposure scenario for ITAP estimated by EASE is 0,4 – 29 mg/kg bw/day (table 9.1), whereas the skin absorption estimate using two skin absorption models ranges from 0 to 0,5 µg/kg/day (table 9.2). There is considerable inconsistency between the magnitudes of the calculated skin absorption estimates and between the skin absorption estimates and the worst case dermal exposure estimate. The calculated water solubility of ITAP is very low (2,58 x 10-5 mg/l). The suitability of the skin absorption models for calculation of skin absorption of non-water soluble substances is limited. The calculated skin absorptions are underestimations. The actual internal exposure of ITAP by skin contact are presumably somewhere between the worst case dermal exposure calculated by EASE and the estimated skin absorption. Exposure by inhalation As illustrated in table 9.3, the calculated vapour concentration of ITAP at 80°C is in the range of 0 – 1,88 mg/m³, while the saturated vapour concentration (SVC) of ITAP at 80°C is 0,12 mg/m³. As mentioned for TBP, the SVC is the theoretical maximum in a steady state environment and will rarely, if ever, be achieved in practice in an industrial situation. The vapour exposure of ITAP is therefore likely to be lower than the SVC. The contribution to exposure by inhalation from aerosols is considered to be very limited. Risk characterization Two 4-weeks dermal exposure studies of triisopropylated phenyl phosphate (ITAP) (CAS No. 68937-41-7) with rats indicate that the critical health effects by repeated dermal exposure are slight inhibition of plasma cholinesterase activity in both sexes and effects on adrenals in males. The lowest NOAEL for inhibition of plasma cholinesterase was 200 mg/kg/day, while the NOAEL for adrenal effects in male rats was 100 mg/kg/day. Studies in hens and epidemiological studies are inconclusive with respect to toxicity of ITAP to the peripheral nerve system. The critical health effects of ITAP are summarized below in table 9.5. The studies and results are also described in the health assessment of ITAP in section 7.7.4.1 (studies A, B, C and D).
Table 9.5 Critical health effects of triisopropylated phenyl phosphate (ITAP) (CAS No. 68937-41-7) by repeated exposure. Comparison of the lowest NOAEL from a 4-week dermal exposure study with rats (100 mg/kg bw/day) with the worst case dermal exposure estimate of 0.4-29 mg/kg bw/day for ITAP (table 9.1) indicates that the rise of adverse health effects in the working environment due to dermal exposure of lubricants containing ITAP should be considered limited. Dermal exposure from vapour concentrations of ITAP contribute to the dermal exposure. However, this contribution is considered to be negligible compared to direct dermal contact. Available inhalation data for ITAP from studies in hens indicate that inhalation of ITAP in aerosol form may cause harmful effects on the nervous system. However, the contribution from aerosols generated during metal forming operations is considered to be very limited. An estimated NOAEC for the effect was 0,12 mg/m³. This indicates that the risk of harmful effects on health caused by inhalation of ITAP vapours and aerosols is considered to be minimal at expected concentrations during metal forming. 9.6.1.3 Sulphurized 2,4,4-Trimethyl pentene (CAS No. 68515-88-8) and di-tert-dodecyl pentasulphide (CAS No. 31565-23-8)Dermal exposure As can be seen from table 9.1, the worst case dermal exposure range of the polysulphides estimated by EASE is 1,2 - 58 mg/kg bw/day, whereas the skin absorption estimates using two skin absorption models ranges from 0 to 5,0 x 10-2 mg/kg bw/day for sulphurized 2,4,4-trimethyl pentene and from 0 to 7,3 x 10-5 mg/kg bw/day for di-tert-dodecyl pentasulphide (se table 9.2). There is considerable inconsistency between magnitudes of the skin absorption estimates, and between the skin absorption estimates and the worst case dermal exposure. The calculated water solubility of the two polysulphides are very low (7,825 x 10-3 mg/L and 5,36 x 10-8 mg/L). The suitability of the skin absorption models for calculating skin absorption of non-water soluble substances is limited and underestimates the skin absorption. The actual internal exposure of the polysulphides by skin contact is presumably somewhere between the worst case dermal exposure estimate and the skin absorption estimates. Exposure by inhalation As summarized in table 9.3, the calculated vapour concentration for sulphurized 2,4,4-trimethyl pentene (CAS No. 68515-88-8) at 80°C is 298 – 596 mg/m³, while the saturated vapour concentration (SVC) at 80°C is 810 mg/m³. Thus the calculated vapour concentration of sulphurized 2,4,4-trimethyl pentene is possible in the working environment under metal forming operations. For di-tert-dodecyl pentasulphide (CAS No. 31565-23-8), the calculated vapour concentration at 80°C is 0 – 2.08 mg/m³ while the saturated vapour concentration (SVC) at 80°C is 0.05 mg/m³, as can be seen from table 8.3. The SVC is the theoretical maximum in a steady state environment and will rarely, if ever, be achieved in practice in an industrial situation. The actual vapour exposure of di-tert-dodecyl pentasulphide is therefore lower than the SVC. Risk characterization Limited health data are available for polysulphides, especially data by repeated exposure. Sensitization by skin contact seems to be one effect of some polysulphides. Systemic effects by repeated exposure includes decreased body weight gain and effects on the blood and immune system. Four sub-chronic dermal toxicity studies in rats and rabbits have been conducted with either sulphurized 2,4,4-trimethyl-pentene (CAS No. 68515-88-8) of sulphurized 2-methyl-1-propene (CAS No. 68511-50-2). The predominant effect observed was dermal irritation at the site of the test material. The lowest reported NOAEL for systemic toxicity in a 13-weeks rat study was 50 mg/kg bw/day for sulphurized 2-methyl-1-propene (CAS No. 68511-50-2). The critical health effects of polysulphides are summarized below in table 9.6. The studies and results are also described in the health assessment of polysulphides in section 7.5.4 (studies A, B and C).
Table 9.6 Critical health effects of polysulphides by repeated exposure. Comparison of the worst case dermal exposure estimates on 1,2 - 58 mg/kg bw/day for sulphurized 2,4,4-trimethyl pentene and di(tert-dodecyl) pentasulphide calculated by EASE with the estimated NOAEL on 50 mg/kg/day for systemic toxicity by dermal exposure of another polysulphide, sulphurized 2-methyl-1-propene (CAS No. 68511-50-2) indicates that repeated dermal exposure to some polysulphides may involve a risk of adverse effects on health. In addition, data indicates, that some polysulphides exhibit a sensitizing potential by repeated skin contact. Available inhalation data for sulphurized 2,4,4-trimethyl pentene from a single study in rats indicate that inhalation of the substance may cause systemic effects (reduced body weight gain) below 15 mg/m³. The maximum vapour concentration of sulphurized 2,4,4-trimethyl pentene at 80°C was calculated to be 596 mg/m³, which is well below the calculated SVC on 810 mg/m³. The maximum vapour concentration of di-tert-dodecyl pentasulphide at 80oC was 2.08 mg/m³ which is considerably above the SVC for this substance at 0.05 mg/m³. Comparison of the NOAEC < 15 mg/m³ with maximum vapour concentrations at 80oC of 596 mg/m³ for sulphurized 2,4,4-trimethyl pentene indicates that there is a risk of harmful effects on health caused by repeated inhalation of vapours of sulphurized 2,4,4-trimethyl pentene during metal forming operations. As the possible vapour concentration of di(tert-dodecyl) pentasulphide at 80°C (the SVC at 0.05 mg/m³) is considerably below 15 mg/m³, the risk of adverse health effects caused by inhalation of ditert-dodecyl pentasulphide during metal forming operations is considered very limited. 9.7 ConclusionExposure assessments and risk characterization in working environment have been performed at a screening level for two phosphates representing the substance group phosphorous compounds and two polysulphides representing the substance group polysulphides. Both substance groups typically occur in non-chlorinated lubricants as extreme pressure additives and are considered to be the most critical substance groups in non-chlorinated lubricants regarding health effects. This evaluation is based on the results of the health and environmental assessment of components in non-chlorinated lubricants described in chapter 7. Dermal exposure The results of the exposure assessments and risk characterizations indicate that worst-case dermal exposure to lubricants containing tributyl phosphate (TBP) (CAS No. 126-73-8) at common lubricant concentrations involve a risk of adverse effects on health, while the risk of adverse health effects due to dermal exposure of lubricants containing phenol isopropylated phosphate (3:1) (ITAP) (CAS No. 68937-41-7) at common lubricant concentrations should be considered limited. Worst case dermal exposure estimates of polysulphides indicates, that repeated dermal exposure to some polysulphides may involve a risk of adverse effects on health including skin sensitisation. However, the actual dermal exposure levels in the working environment are presumably lower than the worst case estimations as the exposure time and especially the exposure area are lower. Further, the dermal exposure to metal forming lubricants can easily be reduced substantially by use of personal protection equipment, in particular, gloves. Exposure by inhalation Regarding exposure by inhalation, the results of the exposure assessments indicate that the vapour concentration of TBP at 80°C may reach levels during metal forming operations implying a risk of adverse effects on health. The risk of harmful effects on health caused by inhalation of ITAP vapours is considered to be minimal at worst case estimated vapour concentrations. Based on worst case estimates of the vapour concentration at 80°C, there is a risk of harmful effects on health caused by repeated inhalation of sulphurized 2,4,4-trimethyl pentene (CAS No. 68515-88-8). The risk of adverse health effects caused by inhalation of di-tert-dodecyl pentasulphide (CAS No. 31565-23-8) at 80°C is considered to be very limited. The exposure assessments do not consider exposure from decomposition products of the four substances in focus formed due to friction heating at the metal surface during forming operations. Decomposition products of phosphorous compounds may be toxic fumes of phosphor oxides, while decomposition products of polysulphides may be toxic fumes of hydrogen sulphide and sulphur oxides. Thus decomposition products of phosphorous compounds and polysulphides may involve an additional risk of adverse health effects. In addition, only exposure in the working environment is considered in the exposure assessments. Exposure from other spheres – most probably diffuse exposure via the environment - may involve an additional exposure. Reference list
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||