Assessment of DHA in self-tanning creams applied in spray booths
7 Annex 1 - Analysis report
Exposure to dihydroxyacetone (DHA) during self-tanning with aerosols
Summary and conclusions
Dihydroxyacetone (DHA) is used for artificial tanning. The Danish Environmental Protection Agency initiated an investigation into the health risks associated with DHA tanning by spray application. The National Environmental Research Institute in Denmark performed a study of inhalation exposure during three different spray scenarios, measuring the concentration of DHA in aerosol droplets < 12 µm in diameter. Further, we studied the exposure to DHA in rooms adjacent to the tanning booths. We developed methods for sampling and analysis of DHA in self-tan lotion aerosols. We used a series of impingers containing a derivatisation agent. The derivatisation product was analysed using LC-MS-MS.
The highest exposure occurred in a closed booth with concentrations of 115 and 238 µg/l air as an average during 16 and 14 seconds of exposure inside the booth. In an open booth working with an electrospray system, the concentration of DHA amounted to 3.3 and 17 µg/l air at an average of 35 seconds of exposure. During manual application using a turbine principle the average concentration was 0.8 µg/l air during 210 seconds of treatment.
The measurements are minimum concentrations as there may be some DHA that passed the sampling equipment without reaction with the derivatisation agent.
During one spray in the closed booth we measured size distribution of droplets between 0.5 and 20 µm in diameter. There was a big proportion of particles less than 10 µm during the application. The apparatus for measuring size distribution was not available during the measurements in open booths and with manual spray.
Introduction
The project aims to:
- determine the amount of DHA in small droplets that a person will potentially inhale during professional treatment with self-tanning lotion.
- measure the amount of DHA in small droplets in rooms adjacent to booths used for treatment with self-tanning lotion.
Dihydroxyacetone (DHA) CAS No: 96-26-4
Dihydroxyacetone or 1,3-dihydroxy-2-propanone is a white, hygroscopic, crystalline powder with a melting point of 75 oC. Formula: C3H6O3 Molecular weight 90.08. Vapour pressure is unknown. Water solubility: easily soluble (Merck 1983 p 463)
Materials and methods
Treatment places and methods
At the start of this project, the Danish EPA and Danish Toxicology Centre had already established contact to two providers of self-tanning treatments that had offered to participate in a study to elucidate the health risks of spray treatment.
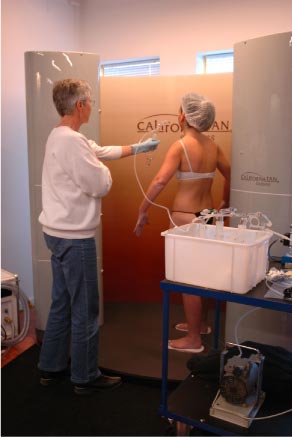
The different treatment methods are reviewed below.
- Open booth for spraying with aerosol according to the electrospray principle. The customer stands on two metal plates that work as grounders. Self-tanning lotion is sprayed from two vertical rows of nozzles in fine droplets. The droplets are subjected to a voltage of 40,000 Volts and are then sucked onto the customer's skin. Spraying is done in 2 sessions of 2, 2½ or 3 seconds. In this study, spraying was done 2 times 3 seconds. 15 ml of lotion was used per treatment. The customer is instructed to close her eyes and hold her breath during treatment. The skin did not seem wet after treatment. Samples were collected during the period when the test person was in the booth.
Open booth for auto spray treatment uses the electrospray principle. Equipment for collection of droplets < 12 µm consists of an inlet held up to the nose/mouth, four purifying flasks with derivatisation reagents in a box with crushed ice, and a pump for sucking air through the purifying flasks.
- Manual turbine spray
The spray used worked according to an HVLP (High Volume Low Pressure) turbine principle that gives a smaller lotion surplus than the turbine spray used in some places. Self-tanning lotion is applied to the entire body whilst the customer turns around. The entire treatment lasted less than three minutes. 25 ml lotion was used for the treatment. The customer is instructed to close her eyes during treatment. A small filter, placed in both nostrils, filters inhalation air. Samples were collected during the period the person was treated standing in the booth.
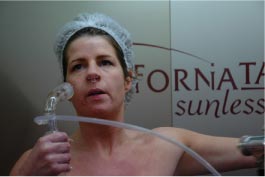
During manual spraying in booths with extractor fans, breathing is through a nose filter. Eyes are kept closed during treatment.
- Closed booth
The booth consists of two compartments. The customer hangs her kimono in the outer compartment and activates the spray programme. The customer then steps into the inner compartment, where self-tanning lotion is sprayed through three rows of nozzles. The spray programme lasts six seconds. The customer then steps into the outer compartment and closes the door behind her. The moment the treatment ends, there is a thick aerosol mist in the inner compartment. The sooner the door is closed behind the customer, the sooner the aerosol is shut off. Approx. 60 ml lotion is used per treatment. Samples were collected from the beginning of the treatment until the customer stepped out into the outer compartment. The skin was wet after treatment. Excess lotion was rubbed into the skin until it was dry. After a treatment, the inner compartment is cleaned with an automatic shower system that removes excess lotion from the air and walls in the booth.

Closed booth for auto spray. The outer compartment where the customer hangs her kimono is separated from the spraying booth by a door.
DHA in adjacent rooms.
The concentration of DHA was measured in the area outside the spraying booth at both locations. Air was collected for 30 minutes.
Collection method
We wished to collect inhalable droplets smaller than approx. 10 µm. For this purpose, an inlet was developed to remove larger drops (figure 4). The separation of the two size classes, larger than and smaller than 10 micrometres, builds on a turn/change in the airstream in the inlet through which the smallest size class follows the airstream while the larger particles separate and are collected (not analysed). The principle behind the separation of the two sizes is the same as in an impactor for collection of particles/droplets of different sizes.
The separation of the two size classes is determined by, for example, the flow at the entrance and the distance to the surface across the airstream. The aerodynamic diameter (dae) where the particles are separated is calculated on the basis of the equation (Mercer and Stafford 1969):
![]()
Here D is the diameter of the entrance hole and v is the flow at the entrance hole.
The separation was calculated at 12 µm for the actual flows in the tests. The goal was to separate at 10 µm, but this was not achievable in this study.
Figure text:
Hose for collection of small droplets
Inflow of droplets
Large drops run down here. To be cleaned subsequently – NOT to be analysed.
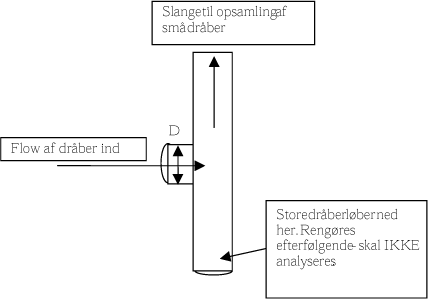
Figure 4. Outline illustrating the principle for the inlet to collect droplets.
We applied a method for collection of DHA described by Spaulding et al. 1999. These authors measured various carbonyls in outdoor air. They are the first to have been able to measure hydroxycarbonyls, such as hydroxyacetone. They collected air with low concentrations of carbonyls in periods of three hours. During collection, the air passes through four purifying flasks placed in series (Figures 1 and 5). The purifying flasks contain 150 ml derivatisation reagent that reacts with carbonyls which thus form a bond with the liquid (see analysis method). We modified the method for short-term collection of samples with a high content of DHA by increasing the concentration of derivatisation reagent in the purifying flasks from 25 mg/l to 250 mg/l. The air is sucked by means of a pump through the inlet described above and on through the purifying flasks. A needle placed in the connection tube between the purifying flasks and the pump regulates the flow rate. The flow rate aimed at was 500 ml/min. The exact flow rate was measured before each sample was taken as the mean value of five measurements measured with a Gilibrator Bubble Flow meter.
Figure text:
Sample inlet (from booth)
Flow measured at 150 ml H2O
Flow control (needle)
To pump
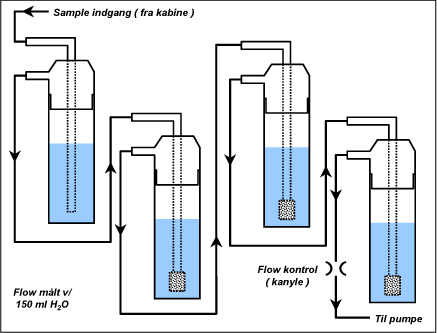
Figure 5. Outline illustrating the principle for collection setup. In the front purifying flask, the airflow passes through an open tube to catch drops. In the other three flasks, the tube ends in a frit to give the best possible contact between air and derivatisation reagent.
In order to minimise evaporation of DHA during sample collection, the derivatisation liquid was kept cold during transport, and the purifying flasks were placed in a box with crushed ice during collection. The intake to the purifying flasks has a glass inlet that is held in front of the mouth and nose during self-tanning treatment. After collection, the samples were poured into Duran bottles. At the manufacturer, this was done on a trolley in the room next to the booths. At another manufacturer it was done behind a closed door in a room next to the salon itself. First, the purifying flasks were rinsed with approx. 25 ml derivatisation reagent which was poured into the sample, and then with two-times 150 ml Millipore water, which was subsequently discarded. Before collection of air from adjacent rooms, the equipment was rinsed with more water.
Analysis method
No published method for analysis of DHA in air has been found. The method applied was modelled on Spaulding 1999. This method was developed to analyse a number of carbonyls in outdoor air, including hydroxyacetone, but not dihydroxyacetone. The carbonyl group in DHA reacts with o-(2,3,4,5,6-pentafluorobenzyl)-hydroxylamine (PFBHA), cf. figure 6. This reaction begins already during sample collection and continues at room temperature for 24 hours in darkness. In the original method, the carbonyls were analysed with GC-MS after further derivatisation with bis(trimethylsilyl)-trifluoroacetamide (BSTFA). However, preliminary lab tests showed poor recovery rates, probably because the dihydroxy compound is not sufficiently derivatised with BSTFA. Instead, a method was developed for analysis of the PFBHA derivative by means of LC-MS-MS.
When the samples were received in the laboratory, they were spiked with 13C acetone which was also derivatised. Calibration standards in concentrations ranging from 0.100 to 500 µg/ml were produced by mixing DHA standard with PFBHA solution and derivatising during the same period as the samples.
After 24 hours, the samples were pH adjusted with 1 ml 18N H2SO4 and extracted three times with 10 ml dichloromethane. The solvent was evaporated to dryness and the samples were again dissolved in 1 ml water.
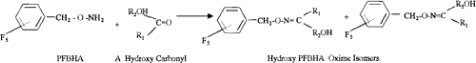

Figure 6. Reaction schemes for derivatisation of hydroxycarbonyls with PFBHA and for further derivatisation of the oxim formed with BSTFA. (Spaulding et al. 1999). The last step did not work for dihydroxyacetone.
The concentrated sample was analysed using reversed phase HPLC on an Agilent 1100 HPLC with a Thermo Hypersil column C18 250x2.1 mm. Eluent A was 1 per cent methanol, 99 per cent 5 mmol ammonium acetate and 0.01 per cent formic acid. Eluent B was 90 per cent methanol and 10 per cent 5 mmol ammonium acetate. The chromatography took place over a 45-minute period with a linear gradient. Figure 7 shows a chromatogram. Subsequently, the components were detected using a double mass spectrometry (MS-MS) on a Sciex API 2000 mass spectrometer. The substances were ionised with electrospray ionisation (ESI), which forms positive molecular ions [M+H]+. The ionised molecule is isolated in the first MS (quadrupole) and fragments when exposed to electrical energy and a collision gas (nitrogen). A characteristic fragment is isolated in the second MS (quadrupole) whereby only the component meeting these requirements as well as the retention time requirement (same retention time for standard and sample) is positively detected in the sample.
DHA derivatised with PFBHA forms a positive ion at m/z 286 and 2 specific product ions at m/z 268 and 181 after fragmentation.
The DHA content in the samples is calculated using linear regression against calibration standards.
For each sampling session, the concentration was measure in all four purifying flasks.
Distribution of droplet sizes
In connection with the sampling session, the distribution of droplet sizes was measured using an Aerodynamic Particle Sizer Spectrometer (from TSI). The instrument is on loan from the National Institute of Occupational Health (Keld Alstrup Jensen).
The Aerodynamic Particle Sizer (APS) measures the aerodynamic diameter of particles in 51 size classes from 0.5 and up to 20 micrometres. The time resolution in the tests was 1 second. The distribution of drop sizes was measured in four periods of 250 seconds each. In the room next to the booth, measurements were taken before the first treatment, during both treatments with people in the booth, and after all three spraying sessions in the booth. In the booth itself, the distribution of droplet sizes was measured during a spraying session without a person in the booth.
Figure 7. Ion chromatogram of a concentrated sample
Results and discussion
Concentration measurements
Table 1 shows the results of a measurement of DHA in drops < 12 µm in diameter in the treatment booths. The concentration is calculated on the basis of the total amount of DHA in all four purifying flasks. There was also DHA in the last bottle in the series, which seems to indicate that DHA has not had enough time to react quantitatively with the derivatisation reagent. At one sampling location, the purifying flasks were rebottled in the room next to the booths. A field blind sample, which had had the lid screwed off during rebottling, showed that there was a passive uptake of DHA in the derivatisation reagent when it was in contact with air with DHA. The samples from spray collection at this location were thus adjusted for blind value.
Table 1. Concentration of DHA in droplets < 12 µm in the air around the mouth/nose during treatment with self-tanning spray
| Treatment method | Spraying time sec. |
Collection time sec. | Flow rate ml/min | DHA in sample µg/l air |
Particle separation µm | |
| a | auto spray open booth | 2 x 3 | 35 | 538 | 3.3 | 12 |
| a | auto spray open booth | 2 x 3 | 35 | 514 | 17 | 12 |
| b | manual turbine spray | 167 | 210 | 529 | 0.8 | 12 |
| c | auto spray closed booth | 6 | 14 | 542 | 238 | 12 |
| c | auto spray closed booth | 6 | 16 | 551 | 115 | 12 |
The difference between the results of two spraying sessions in open and closed booths respectively may be because the test persons were of different heights and they may have held the inlet slightly differently in relation to the nozzles.
The low DHA content in the air during manual treatment may be because there are extractor fans in the booth and the face was only sprayed for a small part of the total treatment time. After treatment, it was possible to see that the nose filter was coloured by the bronzer in the lotion. This colour indicates immediately where the customer has been treated. There is some reaction time for DHA's reaction with the skin.
Table 2 shows the results of a measurement of DHA in drops < 12 µm in diameter in rooms adjacent to the spraying booths. At both locations, measurements were taken after three treatments, which is worst case in relation to the clinics' normal treatment frequency.
Table 2. Concentration of DHA in rooms adjacent to spray booths after three treatments with self-tanning spray
| Collection time (min) | Flow rate ml/min | DHA in sample µg/l air |
Particle separation µm | ||
| 1 | Method a and b | 30.5 | 516 | 0.29 | 12 |
| 2 | Method c | 30.0 | 553 | 0.50 | 12 |
Distribution of drop sizes
Figure 8 shows the distribution of drop sizes (measured as mass) as a five-second average after spraying in a closed booth. Each curve represents five seconds of collection. The column to the right shows the relation between the colour of the curve and the measurement time. There is a large peak of drops in the inhalable area for particles (< 10 µm). After about one minute, the level is back down to the background level for particles.
Figure text:
Y-akse: Particle mass (dM/dlogdp)
X-akse: Droplet size (micrometer)
Figure 8. Size distribution for particles (measured as mass) as a five-second average during spray session in a closed booth. The drops are differentiated within the area 0.5 to 20 µm. The table to the right shows the time for each average measurement.
Figures 9-13 show the total concentration (mg/m³) of drops < 20 µm measured in periods of 250 seconds at various times during and after treatment. The density of the liquid has been set at 1.
The background concentration is lowest before the first spray treatment. It increases a little during treatment, but is still low. During the treatment proper, the mass increases significantly, while it remains at a constantly low level after the end of the last treatment.
Figurtekster 9-13 (gentages for alle figure – testnr. ændres):
Test 1. Total conc. (mg/m³)
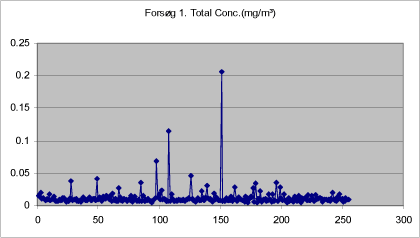
Figure 9. Total mass of drops < 20 µm measured in adjacent room before first treatment with self-tanning lotion in closed booth.
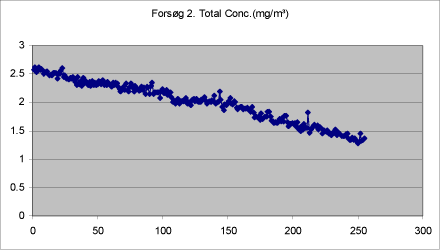
Figure 10. Total mass of drops < 20 µm measured in adjacent room during first treatment with self-tanning lotion in closed booth.
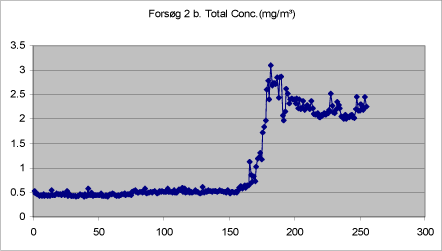
Figure 11. Total mass of drops < 20 µm measured in adjacent room during second treatment with self-tanning lotion in closed booth.
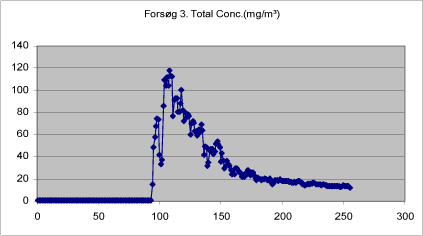
Figure 12. Total mass of drops < 20 µm measured in closed booth during treatment with self-tanning lotion.
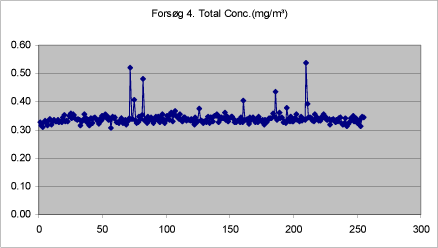
Figure 13. Total mass of drops < 20 µm measured in adjacent room after three treatments with self-tanning lotion.
Conclusion
Methods have been developed for collection and analysis of dihydroxyacetone in self-tanning lotion. Measurements have been taken during three types of treatment. The highest exposure is found during treatment in closed booths. The measurements should be regarded as minimum concentrations, as unreacted DHA may have passed the last purifying flask. During treatment in closed booths, a large proportion of drops in the inhalable area < 10 µm in diameter was detected. The instrument for measurement of distribution of drop sizes was not available during measurements in open booths and for manual turbine spray.
References
Mercer, T.T. and Stafford, R.G.: "Impaction from round jets", Ann. Occup. Hyg., vol. 12, 41-48 (1969)
Safety (MSDS) data for 1,3-dihydroxyacetone. Link: http://ptcl.chem.ox.ac.uk/MSDS/DI/1,3-dihydroxyacetone.html
Spaulding, R.S., Frazey, P., Rao, X. and Charles, M.J.: Measurement of hydroxy carbonyls and other carbonyls in ambient air using pentafluorobenzyl alcohol as a chemical ionization reagent. Analytical Chemistry 1999, 71, pp. 3420-3427.
Windholz et al. 1983: The Merck Index, 10th edition. USA
Version 1.0 September 2006, © Danish Environmental Protection Agency