Health effects assessment of exposure to particles from wood smoke
2 Wood smoke, characterisation
Wood smoke consists of, besides the major combustion products carbon dioxide and water, a complex mixture of compounds, including particulate matter, inorganic gases (e.g. carbon monoxide, nitrogen oxides, sulphur dioxides), volatile organic hydrocarbons (VOC), and polycyclic aromatic compounds (PAC). Particulate matter is itself a complex mixture and can be fractionated as inorganic ash material, soot, and condensed organic material.
In addition to temporal and seasonal variations, the physico-chemical characteristics of particulate matter as well as the associated adverse health effects exhibit large regional differences, even when the particles are from the same type of source. Such variations will complicate an evaluation of the association between exposure to particulate matter and adverse health effects. The most important determinants of the toxicity of particles are the particle size and the chemical composition. These aspects will be addressed in the following sections.
2.1 Particle size distribution
The particle size is believed to be an important parameter in relation to health effects. The particle size can also reveal the origin / sources and the history of the particles. Furthermore, the particle size determines the atmospheric lifetime. (Palmgren et al. 2003).
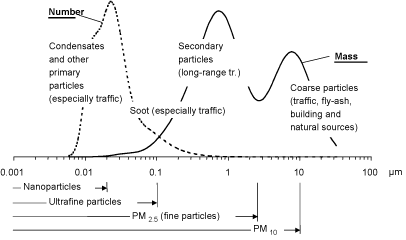
The particles size distribution is traditionally described as appearing in three modes according to the aerodynamic diameter of the particles: Coarse particles (2.5-10 µm), fine particles (0.1-2.5 µm), and ultrafine particles (<0.1 µm). The particle fractions most often used in epidemiological studies are PM10 and PM2.5, referring to the mass of particles with an aerodynamic diameter ≤ 10 and 2.5 µm, respectively. (Trafikministeriet 2003, Palmgren et al. 2003). Figure 1 shows the particle size distribution in urban air.
In urban areas, the coarse mode particles are typically formed mechanically by abrasion of road material, tyres and brake linings, dust raised by wind, and traffic turbulence etc. Natural sources are soil dust and sea spray (sea salt). Their atmospheric lifetime is short (minutes to hours). Coarse particles contain inorganic ions (for example calcium, aluminium, silicon, magnesium and iron) and components with biological activity, e.g. allergens in pollen and toxins in mould spores. (Palmgren et al. 2003, Trafikministeriet 2003).
Fine mode particles are typically formed by atmospheric chemical/physical processes during long-range transport (e.g., sulphur dioxide and nitrogen oxides are transformed into sulphate and nitrate – secondary particles), or by other relatively slow processes in the atmosphere. (Palmgren et al. 2003). The fine particles are therefore commonly aged particles and transported over long distances. The main sources of the primary emissions of PM2.5 are vehicle exhausts, fossil fuel combustion (especially coal and wood heating), industrial processes, other biomass burning, and fugitive dust. The main emissions of precursors for secondary PM2.5 formation are nitrogen oxides, sulphur dioxide, ammonia, and volatile organic hydrocarbons from different sources (Schlesinger & Cassee 2003). Fine particles contain especially elemental carbon and different metals, as well as organic compounds. In the PM2.5 fraction, fine particles (0.1-2.5 µm) account for the largest mass, but the largest numbers are ultrafine particles (over 90%), and the largest surface area per mass is in the accumulation mode fraction (0.1-1 µm).
Ultrafine particles are primarily formed from gases by nucleation in air, and their atmospheric lifetime is short. Ultrafine particles are fuel or oil in the form of aerosols or solid elemental and organic carbon (soot) as well as heavy metals. The predominant source of ultrafine particles is traffic, particularly diesel engines, and these particles are therefore dominating in heavily trafficked areas. In general, there is limited information on the concentrations of ultrafine particles in ambient air because they have not been monitored comprehensively (Trafikministeriet 2003, Palmgren et al. 2003).
Figure 1. The particle size distribution in urban air. From Trafikministeriet (2003).
The particle size varies strongly during the different phases of the wood combustion process. The emissions are largest during the start-up phase when the particle size is in the range 60-70 nm. During the intermediate and smouldering phases, the emissions decrease and the particle size distribution has two modes, one in the 20-30 nm range and another in the 100-200 nm range. Measurements in a neighbourhood dominated by wood combustion emissions showed a particle size distribution composed of all three combustion phases, with particles from the start-up phase as dominating. It is likely that particles from different phases and size modes have a somewhat different chemical composition. (Forsberg et al. 2005).
Boman et al. (2004) have studied the emission of fuel pellets, a new biomass fuel. The emitted particles were mainly found in the fine (<1 µm) mode with mass median aerodynamic diameters of 0.20-0.39 µm and an average PM1 of 89.5% ± 7.4% of total PM.
Kocbach et al. (2006) found a small difference in the particle diameter between primary carbon particles from vehicle exhaust and residential wood smoke. A mean diameter of 31 ± 7 nm was found for primary carbon particles of wood smoke sampled from a single-stage combustion stove compared to a mean diameter of 24 ± 6 nm for carbon particles sampled from a road tunnel.
2.2 Chemical characterisation of wood smoke
Wood consists of cellulose (50-70%), lignin (about 30%), and small amounts of resinous materials and inorganic salts. In wood, cellulose compounds form a supporting mesh that is reinforced by lignin polymers. Together these compounds form the rigid wood structure. Upon heating, these structures break apart producing a large variety of smaller molecules including methoxy phenols, methoxy benzenes, phenols, catechols, benzene, and alkyl benzenes. Non-wood biomass does not contain lignin and therefore the methoxy phenols and methoxy benzenes are unique tracers of wood smoke combustion. Conifers (softwoods) produce large amounts of resin acids while deciduous (hardwoods) trees do not. Combustion of hardwoods produces more ash and therefore more trace elements than softwoods. Approximately 5-20% of wood smoke particulate mass is elemental carbon, while the composition of the organic carbon fraction varies with the specific biomass fuel being burned and with the combustion conditions. Potassium is the trace element found at highest concentrations in wood smoke and has often been used as a wood smoke tracer. Table 1 summarises the chemical composition of wood smoke. (Larson & Koenig 1994).
In a detailed analysis of organic wood smoke aerosol, nearly 200 distinct organic compounds were measured in wood smoke, many of them derivatives of wood polymers and resins (Rogge et al. 1998).
Table 1. Chemical composition of wood smoke. From Larson and Koenig (1994).
| Species | g/kg wood |
| Carbon mono×ide | 80-370 |
| Methane | 14-25 |
| VOCs (C2-C7) | 7-27 |
| Aldehydes (formaldehyde, acrolein, propionaldehyde, butyraldehyde, acetaldehyde, furfural | 0.6-5.4 |
| Substituted furans | 0.2-1.6 |
| Benzene | 0.6-4.0 |
| Alkylbenzenes | 1-6 |
| Acetic acid | 1.8-2.4 |
| Nitrogen o×ides | 0.2-0.9 |
| Sulfur dio×ide | 0.16-0.24 |
| Naphthalene | 0.24-1.6 |
| Substituted naphthalenes | 0.3-2.1 |
| O×ygenated monoaromatics | 1-7 |
| Total particle mass | 7-30 |
| Particulate organic carbon | 2-20 |
| Particulate elemental carbon | 0.3-5 |
| O×ygenated PAHs | 0.15-1 |
| PAHs | 10-5-10-2 a) |
| Trace elements | 10-4-10-2 b) |
| Normal alkanes (C24-C30) | 1×10-3-6×10-3 |
| Cyclic di- and triperpenoids | 10-6-0.1 |
| Chlorinated dio×ins | 1×10-5-4×10-5 |
| Particulate acidity | 7×10-3-7×10-2 |
a) 18 different PAHs listed with concentrations ranging from 10-4-10-2 g/kg wood.
b) 18 different trace elements listed with concentrations ranging from 10-4-10-2 g/kg wood.
Organic compound emission rates for volatile organic compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds have been measured from residential fireplace combustion of wood. Firewood from a conifer tree (pine) and from two deciduous trees (oak and eucalyptus) was analysed to determine organic compounds emissions profiles for each wood type. The results are summarised in Table 2. (Schauer et al. 2001).
Table 2. Average fine particle emissions rate and fine particle chemical composition of emissions from fireplace combustion of wood. Reproduced from Schauer et al. (2001).
| Pine | Oak | Eucalyptus | |
| Fine particle emissions rate (g/kg wood burned) | 9.5 ± 1.0 | 5.1 ± 0.5 | 8.5 ± 0.8 |
| Organic carbon (wt% FPMa) | 56.0 ± 2.8 | 59.1 ± 3.0 | 43.7 ± 2.2 |
| Elemental carbon (wt% FPMa) | 1.4 ± 0.1 | 3.2 ± 0.2 | 2.6 ± 0.2 |
| Chloride (wt% FPMa) | 0.29 ± 0.04 | 0.20 ± 0.01 | 1.70 ± 0.05 |
| Nitrate (wt% FPMa) | 0.19 ± 0.01 | 0.44 ± 0.01 | 0.45 ± 0.01 |
| Sulphate (wt% FPMa) | 0.12 ± 0.01 | 0.41 ± 0.01 | 0.24 ± 0.01 |
| Ammonium (wt% FPMa) | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.45 ± 0.01 |
| Sodium (wt% FPMa) | 0.09 ± 0.01 | 0.10 ± 0.01 | 0.18 ± 0.01 |
| Sulphur (wt% FPMa) | 0.059 ± 0.002 | 0.148 ± 0.004 | 0.056 ± 0.003 |
| Chlorine (wt% FPMa) | 0.181± 0.003 | 0.127 ± 0.006 | 01.290 ± 0.008 |
| Potassium (wt% FPMa) | 0.277 ± 0.003 | 0.647 ± 0.007 | 0.809 ± 0.005 |
a) FPM: Fine Particle Mass
Kocbach et al. (2006) found that residential wood smoke particles and diesel particles had a very comparable total carbon content (83 versus 80%). However, the organic content was 35% for wood smoke particles compared to 16% for diesel particles. The PAH content in wood smoke was found to be 11800 ng/mg, which was significantly higher than the PAH content in diesel particles of 84 ng/mg.
The emissions of particles, PAHs and dioxins were measured in smoke samples from private wood stove chimneys in Denmark (Glasius et al. 2005). The results are summarised in Table 3 (I-TEQ are International Toxicity Equivalents). The emissions of PAHs were 43.4±46.2 mg/kg wood (with values in the interval 4.4-81.2 mg/kg wood) and the particle emission 21.1±28.9 g/kg wood (3.2-82.9 g/kg wood). Insufficient combustion conditions will lead to increased emission of both particle mass and PAHs, and a positive correlation between the emissions of PAHs and particles was seen. The dioxin emission was 6.1±6.1 ng I-TEQ/kg wood (0.3-17.7 ng I-TEQ/kg wood), and no correlation between the emission of dioxins and particles were found. Dioxins and particles are formed via two different processes and therefore no correlation was expected. Dioxins are formed by a chemical reaction between chlorine and organic substances while particles are formed via condensation of the flue gas.
In a recent follow-up study on 13 appliances of which six were also included in the study reported in Glasius et al. (2005), the average emissions were PAH 57±83 ng/kg wood, dioxins 19±32 ng I-TEQ/kg wood and particles 6.2±5.4 mg/kg wood (Glasius et al. 2007). Generally the results were in agreement with the first study, except for particle emissions that were lower in the second study. The large relative standard deviations in the measurements are caused by the large variation in emissions between appliances.
A recent inventory shows that the total Danish dioxin emission to air was about 22.0 g I-TEQ in 2004. The main sources were residential wood combustion (40%) and fires (28%) (Henriksen et al. 2006).
Table 3. Wood smoke emissions from private wood burning stoves. Reproduced from Glasius et al. (2005).
| New stoves < 3 years | Old stoves > 5 years | Old boiler | |
| Number of determinations | 3 | 6 | 2 |
| Dioxin (ng I-TEQ/kg wood) | 0.3-3.0 | 5.1-17.7 | 0.3-0.6 |
| Dioxin (ng I-TEQ/m³ smoke) | 0.05-0.46 | 0.79-2.7 | 0.045-0.094 |
| PAH (mg/kg wood) | 4.4-7.8 | 5.5-81.2 | 15.4-23.7 |
| PAH (mg/m³) | 0.7-1.2 | 0.8-12.4 | 2.3-3.6 |
| Particles (g/kg wood) | 4.3-11.4 | 3.2-82.9* | 20.3-24.2 |
| Particles (mg/m³) | 0.7-1.7 | 0.5-12.7* | 3.1-3.7 |
* 4 determinations.
The type of wood burned was mostly dried birch and beech. Not included in this Table was a smoke sample taken from a new stove during combustion of pallets and painted wood. In this sample, the emission per kg wood of PAH, dioxin, and particles compared to clean wood was increased 5-, 4-, and 8-fold, respectively.
Version 1.0 May 2008, © Danish Environmental Protection Agency