Survey and health assessment of chemical substances in essential oils and fragrance oils
4 Results of analysis program
- 4.1 Selection of products for analysis
- 4.2 Methods of analysis
- 4.3 Quantitative determination of constituents
- 4.4 Climate chamber test
- 4.5 Interpretation of analysis results
- 4.6 Selection of substances for health assessment
4.1 Selection of products for analysis
The preliminary contact to Danish knowledge centres indicated that there is not much knowledge about these products and the possible impact from their constituents via exposure to the respiratory tract. There is considerable material on the substances impact to the skin by exposure, but this knowledge is not immediate useful to assess exposure via the respiratory tract.
At least 17 of the substances have previously been assessed in connection with other consumer product projects. Only one of the reviews of health impacts of the substances contained information about possible effects at exposure via the respiratory tract.
Tisserand & Balacs (1995) give in the book "Essential oil safety. A guide for health care professionals" a thorough review of health aspects related to essential oils. There is a number of essential oils that may contain various dangerous substances, but these oils are not among the oils identified in this project and marketed for aromatherapy.
The book also concludes about exposure via the respiratory tract that: "Inhalation is an important route of exposure because the role of odour in aromatherapy, but from a safety standpoint it presents a very low level of risk to most people…..The only risk would be from prolonged exposures(perhaps 1 hour or more) to relatively high levels of essential oil vapour, such as could occur when directly sniffing from a bottle of undiluted oil. This could lead to headaches, vertigo, nausea and lethargy".
In the book a number of substances are pointed out having a possible health effect, however not particularly by exposure via the respiratory tract. The following has a critical effect:
- safrole (CAS No 94-59-79) - carcinogenic;
- estragole (CAS No 140-67-0) - carcinogenic;
- trans-anethole (CAS No 4180-23-8) - weak oestrogenic activity;
- thujone (CAS No 546-80-5) - acute poisonous;
- methyleugenol (CAS No 93-15-2) - genotoxic.
It should be noticed that none of the 5 substances is included in the list of dangerous substances. Out of the 5 substances, thujone is not listed as a constituent of the oils used for aromatherapy.
Four of the oils have previously been examined for presence of the 26 sensitizing substances. The results are in accordance with the results presented here based on information from producers considering the variations to be expected.
Some of the constituents of the oils are also part of wooden building materials and are in this connection assessed to be released to the indoor climate. LCI values have been found for 11 substances as listed in table 4.1. It has been found relevant to include substances with LCI values, as it will be possible to relate the LCI values to the calculated room concentrations when using candle diffusers.
According to one of the suppliers, the most popular essential oils for candle diffusers are lavender, eucalyptus, bergamot and orange, whereas another supplier lists eucalyptus, peppermint, citronella, lemongrass, citrus oils (lemon, orange, bergamot, grape, and other), rosemary, ylang ylang, patchouli and litsea.
An initial literature search showed that reviews have been made on impacts from exposure via the respiratory tract for d-limonene and alpha-pinene.
The table below shows the substances that at first were selected for quantitative analysis of constituents. The list was subsequently reduced to 15 substances by taking out substances that are only present in smaller quantities in few oils and omit substances that structurally are similar to other substances on the list and that have the same LCI value.
A number of products have then been selected based on 3 criteria: 1) all substances should be covered; 2) products should specifically be used for candle diffusers; 3) relatively many fragrance oils are selected because their constituents are less known. A number of the fragrance oils contain essential oils whose constituents are not shown, and it should thus be expected that many of the fragrance oils contain more of the selected substances than listed in table below. These fragrance oils will contain a large part of the selected substances and are thus appropriate for climate chamber tests.
The selected products are:
- Essential oils: Rosemary oil, citrus oil, tea tree oil, lemon grass oil;
- Fragrance oils: No. 38, No. 5, No. 2, No. 6, No. 27, No. 34.
The following oils were selected for climate chamber tests based on the criteria that they in total cover the main part of the 15 constituents: Rosemary oil, tea tree oil, No. 38, No. 5, and No. 34.
The following climate chamber tests are made:
- Candle diffuser with rosemary oil, No. 34 and No. 5;
- Aroma Stream with tea tree oil and No. 38.
Table 4.1 Background data for selection of substances and products.
4.2 Methods of analysis
4.2.1 Quantitative description of constituents
A partial test of the product is extracted with dichloromethane for one hour on a shaking table and is left over night. A partial test of the extract is taken out and analyzed directly by a combined gas chromatography and mass spectrometry (GC/MS). The content is calculated quantitatively. Analyses are carried out as duplicate determination, i.e. two determinations on the same product. Standards are made for all 15 specific components.
The uncertainty of the analyses is 10-15% RSD. The detection limit is 10-100 mg/kg.
4.2.2 Climate chamber test
The products are tested in two relevant user situations. 10 drops of the products (weighed) for each climate chamber test. The arrangements were placed in climate chambers with a known air circulation and humidity and temperature of the intake air.
Candle diffuser: 20 ml water was filled in the bowl. 10 drops of oil was added to the water. A tea light was lit under the bowl just before test start.
Aroma Stream: The device has a filter in the bottom that can be taken out and oils are added. 10 drops of oil were added to the filter and the device was assembled. The fan was started just before test start.
 |
 |
| The used candle diffuser | Aroma Stream. Source: BodyMind Company http://bodymindcompany.zafi. netimage.dk/index.php?pid=3&item_id=274 |
The emissions from the products is collected on ATD-tubes with the absorption material Tenax starting after 15 minutes, 2 hours and 4 hours respectively. With this absorption material, substances can be collected which are in a gas phase.
After 15 minutes, collection is made for 10 minutes and for the two subsequent tests for 20 minutes. The short test period after 15 minutes is due to the fact that otherwise the amount of emitted substance would exceed the capacity of the tubes.
The ATD-tubes are desorbed thermally and the content is analyzed for 15 specific components and TVOC (Total Volatile Organic Compounds) by combined gas chromatography and mass spectrometry (GC/MS). Reference method for ATD: ISO 16000-6.
The reporting limit is 1 µg/hour. Total uncertainty for test and analysis: 20-30% RSD.
The climate chamber tests were made using a method identical to the method used for emission test of VOC (Volatile Organic Compounds) from building materials after ISO 16000-9, 2006. These tests are reported as a steady state room concentrations in µg/m³ air in a standard room (accordingly) with a volume of 17.4 m³, air circulation of 0.5 times/hour, temperature of 23° C and relatively humidity of 50% RH. As this calculation method assume a constant emission at the measured rate of approx. 8 hours before, a steady state situation is achieved which is not fulfilled in these tests and instead it has been chosen to calculate room concentrations subsequently based on the exposure model with the same room size and air circulation, but with the emission over a shorter period.
Chamber conditions
Material: Plexiglas. Volume: 28 litre. Multistage air clean-up. A blind test was made in the empty room with or without light before each test. The added air had a temperature of 23° C and a relatively humidity of 50% RH. The relative humidity increased during the tests in the tests where the oil was added to 20 ml of water. The air circulation was 4.3 times per hour.
It will take some time before a steady state has been achieved where the quantity of the emitted substance sucked out of the climate chamber equals the quantity emitted from a source within the chamber. As it can be seen from the below figure, a steady state situation is not established in the climate chamber after approx. one hour if a source is placed in the room with a constant source strength. This means that after 15 minutes considerably lower concentrations are measured than the actual, but it has been chosen to make the test at this early point in time to ensure that not all the fragrances were emitted before the start of the collection.
At constant source strength, after 15 and 25 minutes, there will be measured 67% and 85% respectively of the actual emission rates and totally seen a measurement from 15 to 25 minutes will be 76% of the actual emission rate. As the results, at least for Aroma Stream, shows falling source strength, this underestimation will partly be outweighed by the fact that the source strength is falling. As there are no grounds for a correct correction of this uncertainty in measuring, no adjustments have been made, but this uncertainty is considered by the assessment of the results.
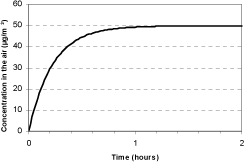
Figure 4.1 Course of concentration in a climate chamber with air circulation of 4.3 times per hour in which a source with constant source strength is placed.
4.3 Quantitative determination of constituents
Results of the quantitative determination of constituents of 15 oils are shown in table 4.2. Results of two parallel determinations are given on the same oil.
The measured concentrations in the four essential oils correspond to the information on the safety data sheet considering that a certain variation must be expected in the content of the natural oils. The very high concentrations of more than 70% d-limonene in citrus oil and above 70% citral in lemongrass oil have thus been confirmed by these analyses.
For the six fragrance oils it is for most of the substances not possible to compare the measured concentration with the concentrations stated in the safety datasheets as the safety data sheets typically only give information on a substance level for the synthetic substances, whereas for the essential oils being part of the fragrance oils, only information on the quantities is given. The measured content benzyl benzoate and diethyl phthalate is in accordance with the information on the safety data sheets. It can be seen that d-limonene is present in most of the oils in high concentrations, but by comparing to the safety data sheets it is seen that d-limonene primarily will be part of the fragrance oils as constituents of citrus oil and other essential oils.
Table 4.2 Results from the analysis of 15 specific substances in essential oils and fragrance oils. The unit is mg/kg. A and B show the result of two parallel analysis of the same oil.
| D.l. | Rosemary oil | Lemon oil | Tea tree oil | Lemongrass oil | |||||
| A | B | A | B | A | B | A | B | ||
| alpha-Pinene | 100 | 110,000 | 120,000 | 21,000 | 21,000 | 23,000 | 23,000 | 1,200 | 1,100 |
| Camphene | 100 | 52,000 | 53,000 | 860 | 870 | - | - | 5,300 | 5,000 |
| Myrcene | 100 | 11,000 | 11,000 | 23,000 | 22,000 | 11,000 | 10,000 | 15,000 | 13,000 |
| Benzylalcohol | 100 | <1,000* | <1,000* | - | - | - | - | - | - |
| para-Cymene | 100 | 22,000 | 22,000 | 8,800 | 9,600 | 31,000 | 18,000 | 560 | 490 |
| d-Limonene | 100 | 41,000 | 42,000 | 730,000 | 720,000 | 11,000 | 11,000 | 2,900 | 2,400 |
| Terpinolene | 100 | 480 | 490 | 2,900 | 2300 | 38,000 | 38,000 | 220 | 168 |
| Camphor | 100 | 110,000 | 110,000 | - | - | - | - | 2,500 | 2,300 |
| Estragole | 100 | - | - | - | - | - | - | - | - |
| n-Decylaldehyde | 100 | - | - | 820 | 880 | - | - | 3,800 | 3,500 |
| Citral ** | 100 | - | - | 22,000 | 22,000 | 470 | 470 | 780,000 | 730,000 |
| trans-Anethole | 100 | - | - | - | - | - | - | - | - |
| beta-Caryophyllene | 100 | 33,000 | 33,000 | 2,400 | 2,300 | 4,900 | 4,800 | 18,000 | 16,000 |
| Diethyl phthalate | 100 | - | - | - | - | - | - | - | - |
| Benzylbenzoate | 100 | - | - | - | - | - | - | - | - |
D.l.:
detection limit
-:
not detected above the detection limit
*:
Increased detection limit due to interference
**
Sum of cis and trans-citral.
Table 4.2 continued
| D.l. | No. 5 | No. 2 | No. 6 | No. 38 | |||||
| A | B | A | B | A | B | A | B | ||
| alpha-Pinene | 100 | 1,800 | 1,800 | 4,900 | 5,200 | 1,700 | 1,700 | - | - |
| Camphene | 100 | 290 | 300 | 380 | 410 | 220 | 210 | - | - |
| Myrcene | 100 | 50,000 | 49,000 | 50,000 | 52,000 | 45,000 | 45,000 | 660 | 620 |
| Benzyl alcohol | 100 | - | - | <1,000* | <1,000* | - | - | 5,800 | 5,800 |
| para-Cymene | 100 | 1,600 | 1,600 | 4,100 | 4,300 | 870 | 850 | - | - |
| d-Limonene | 100 | 83,000 | 82,000 | 250,000 | 240,000 | 74,000 | 72,000 | 1,100 | 1,000 |
| Terpinolene | 100 | 1,200 | 1,200 | 8,600 | 9,200 | 1,200 | 1,200 | - | - |
| Camphor | 100 | <1,000* | <1,000* | 5,700 | 6,000 | <2,000* | <2,000* | - | - |
| Estragole | 100 | - | - | - | - | - | - | - | - |
| n-Decylaldehyde | 100 | 6,100 | 6,200 | - | - | 6,500 | 6,500 | - | - |
| Citral ** | 100 | 13,000 | 13,000 | 9800 | 10,000 | 16,000 | 16,000 | 160 | 160 |
| trans-Anethole | 100 | - | - | - | - | - | - | - | - |
| beta-Caryophyllene | 100 | 4,300 | 4,700 | <5,000* | <5,000* | 4,600 | 4,400 | <10,000* | <10,000* |
| Diethyl phthalate | 100 | - | - | - | - | - | - | 96,000 | 95,000 |
| Benzyl benzoate | 100 | 110,000 | 140,000 | 100 | - | 140,000 | 120,000 | 5,500 | 5,400 |
Table 4.2 continued
| D.l. | No. 27 | No. 34 | |||
| A | B | A | B | ||
| alpha-Pinene | 100 | - | - | 960 | 930 |
| Camphene | 100 | - | - | - | - |
| Myrcene | 100 | - | - | 3,700 | 3,400 |
| Benzyl alcohol | 100 | <500* | <500* | - | - |
| para-Cymene | 100 | - | - | 240 | 230 |
| d-Limonene | 100 | 260 | 220 | 210,000 | 200,000 |
| Terpinolene | 100 | - | - | - | - |
| Camphor | 100 | - | - | - | |
| Estragole | 100 | - | - | - | - |
| n-Decylaldehyde | 100 | - | - | 58,000 | 55,000 |
| Citral ** | 100 | - | - | 19,000 | 18,000 |
| trans-Anethole | 100 | - | - | 2,300 | 2,200 |
| beta-Caryophyllene | 100 | - | - | - | 100 |
| Diethyl phthalate | 100 | - | - | - | - |
| Benzyl benzoate | 100 | 240,000 | 240,000 | - | - |
D.l.: detection limit
-: not detected above the detection limit
*: Increased detection limit due to interference
** Sum of cis and trans-citral.
4.4 Climate chamber test
4.4.1 Candle diffuser
The measurement results of the emission of 16 substances from rosemary oil and the two fragrance oils No. 5 and No. 34 from the candle diffuser can be seen in table 4.3.
Table 4.3 Emissions of 15 specific substances from rosemary oil and the two fragrance oils no. 5 and no. 34. The unit is µg/time (emission rate). A and B show the results of two parallel climate chamber tests on the same oil.
| Substance | R.g. | Rosemary oil | No. 5 | No. 34 | |||
| A | B | A | B | A | B | ||
| Gram sample (10 drops) | 0.45 | 0.47* | 0.39 | 0.41 | 0.29 | 0.30 | |
| 15-25 min. after start | |||||||
| alpha-Pinene | 1 | 6,000 | 2,400* | 390 | 410 | 140 | 110 |
| Camphene | 1 | 3,900 | 1,400* | 120 | 100 | - | - |
| Myrcene | 1 | 360 | 88* | 190 | 310 | 140 | 110 |
| Benzyl alcohol | 1 | 5.7 | 1.2* | - | - | - | 1.3 |
| para-Cymene | 1 | 420 | 260* | 480 | 270 | 84 | 95 |
| d-Limonene | 1 | 340 | 340* | 2,500 | 4,600 | >5,300 | >4,900 |
| Terpinolene | 1 | 18 | 5.8* | 29 | 28 | - | - |
| Camphor | 1 | 840 | 480* | 370 | 270 | - | - |
| Estragole | 1 | - | - | - | - | - | - |
| n-Decylaldehyde | 1 | - | - | - | - | 55 | 36 |
| Citral ** | 1 | - | - | 13 | 16 | 13 | 11 |
| trans-Anethole | 1 | - | - | - | - | - | - |
| beta-Caryophyllene | 1 | 12 | 2.8* | 2 | 8.0 | - | 1.2 |
| Diethyl phthalate | 1 | - | - | - | - | - | - |
| Benzyl benzoate | 1 | - | - | 20 | 42 | - | - |
| TVOC | 43,000 | 18,000* | 10,000 | 14,000 | >11,000 | >10,000 | |
| 120-140 min. after start | |||||||
| alpha-Pinene | 1 | 18 | 45 | - | 1 | - | - |
| Camphene | 1 | 14 | 29 | - | - | - | - |
| Myrcene | 1 | - | - | 8 | 22 | - | - |
| Benzylalcohol | 1 | - | - | 1 | 3 | - | - |
| para-Cymene | 1 | 13 | 28 | 3 | 5 | - | - |
| d-Limonene | 1 | 10 | 28 | 28 | 58 | 14 | 15 |
| Terpinolene | 1 | - | - | 1 | 2 | - | - |
| Camphor | 1 | 93 | 330 | 1 | - | - | - |
| Estragole | 1 | - | - | - | - | - | - |
| n-Decylaldehyde | 1 | - | - | - | - | - | - |
| Citral ** | 1 | - | - | 33 | 22 | - | - |
| trans-Anethole | 1 | - | - | - | - | - | - |
| beta-Caryophyllene | 1 | 28 | 73 | 3 | 2 | - | - |
| Diethyl phthalate | 1 | - | - | - | 3 | - | - |
| Benzylbenzoate | 1 | - | - | 1,300 | 350 | 1.8 | - |
| TVOC | 730 | 1,800 | 2,700 | 1,800 | 96 | 84 | |
| 240-260 min. after start | |||||||
| alpha-Pinene | 1 | 11 | 13 | - | - | - | - |
| Camphene | 1 | 9.5 | 9.8 | - | - | - | - |
| Myrcene | 1 | - | - | 2 | 11 | - | - |
| Benzyl alcohol | 1 | - | 1.7 | - | - | - | - |
| para-Cymene | 1 | 7.6 | 9.7 | 2 | 3 | - | - |
| d-Limonene | 1 | 6.4 | 11 | 12 | 26 | 11 | 13 |
| Terpinolene | 1 | - | - | - | 1 | - | - |
| Camphor | 1 | 19 | 24 | 1 | - | - | - |
| Estragole | 1 | - | - | - | - | - | - |
| n-Decylaldehyde | 1 | - | - | - | - | - | - |
| Citral ** | 1 | - | - | 7 | 24 | - | - |
| trans-Anethole | 1 | - | - | - | - | - | - |
| beta-Caryophyllene | 1 | 10 | 2.7 | - | 1 | - | - |
| Diethyl phthalate | 1 | - | - | - | - | - | - |
| Benzyl benzoate | 1 | - | - | 23 | 17 | - | - |
| TVOC | 270 | 720 | 180 | 840 | 6.0 | 6.0 | |
R.g: Reporting limit
- : Not detected above reporting limit
* At the test 15-25 min only 0,329 gram was added at chamber B.
** Sum of cis- and trans-citral.
TVOC The sum of Volatile Organic Components calculated as toluene.
4.4.2 Aroma Stream
Measurement results of emission of 15 substances from tea tree oil and fragrance oil No. 38 evaporated with Aroma Stream are shown in table 4.4.
Table 4.4 Emissions of 15 specific substances from tea tree oil and fragrance oil no. 38. The unit is µg/time (emission rate). The two results show double indications on the same oil.
| R.g. | Tea tree oil | No. 38 | |||
| A | B | A | B | ||
| Gram sample (10 drops) | 0.46 | 0.48 | 0.34 | 0.39 | |
| 15-25 min. after start | |||||
| alpha-Pinene | 1 | 4,300 | 4,000 | 84 | 47 |
| Camphene | 1 | - | - | 29 | 6 |
| Myrcene | 1 | 840 | 770 | 140 | 92 |
| Benzyl alcohol | 14 | 6 | 4 | - | |
| para-Cymene | 1 | 1,100 | 1,200 | 880 | 370 |
| d-Limonene | 1 | 920 | 550 | 360 | 140 |
| Terpinolene | 1 | 1,300 | 1,600 | - | - |
| Camphor | 1 | - | - | - | - |
| Estragole | 1 | - | - | - | - |
| n-Decylaldehyde | 1 | - | - | - | - |
| Citral ** | 1 | - | - | - | - |
| trans-Anethole | 1 | - | - | - | - |
| beta-Caryophyllene | 1 | 1.3 | 2.8 | 1.8 | 1.2 |
| Diethyl phthalate | 1 | - | - | - | - |
| Benzyl benzoate | 1 | - | - | - | - |
| TVOC, 2 timer | 36,000 | 36,000 | 8,500 | 5,200 | |
| 120-140 min. after start | |||||
| alpha-Pinene | 1 | 39 | 140 | - | - |
| Camphene | 1 | 0.4 | - | - | - |
| Myrcene | 1 | - | 120 | 3.6 | 18 |
| Benzyl alcohol | 1 | - | - | 4.6 | 16 |
| para-Cymene | 1 | 390 | 820 | 1.6 | 5.3 |
| d-Limonene | 1 | 140 | 240 | 11 | 17 |
| Terpinolene | 1 | 670 | 1,700 | - | 1.7 |
| Camphor | 1 | - | - | - | - |
| Estragole | 1 | - | - | - | - |
| n-Decylaldehyde | 1 | - | - | - | - |
| Citral ** | 1 | - | - | - | - |
| trans-Anethole | 1 | - | - | - | - |
| beta-Caryophyllene | 1 | 110 | 220 | 1.4 | 3.5 |
| Diethyl phthalate | 1 | - | - | - | - |
| Benzyl benzoate | 1 | - | - | - | - |
| TVOC, 2 timer | 22,000 | 45,000 | 960 | 2,200 | |
| 240-260 min. after start | |||||
| alpha-Pinene | 1 | 14 | 24 | - | - |
| Camphene | 1 | - | - | - | - |
| Myrcene | 1 | - | 6.0 | 3.6 | 4.0 |
| Benzyl alcohol | 1 | - | - | 4.0 | 7.6 |
| para-Cymene | 1 | 58 | 130 | 1.2 | - |
| d-Limonene | 1 | 18 | 40 | 4.6 | 4.3 |
| Terpinolene | 1 | 96 | 240 | - | - |
| Camphor | 1 | - | - | - | - |
| Estragole | 1 | - | - | - | - |
| n-Decylaldehyde | 1 | 2.3 | - | - | - |
| Citral ** | 1 | - | - | - | - |
| trans-Anethole | 1 | - | - | - | - |
| beta-Caryophyllene | 1 | 5.2 | 17 | 1.6 | 3.0 |
| Diethyl phthalate | 1 | - | - | - | - |
| Benzyl benzoate | 1 | - | - | - | - |
| TVOC, 4 timer | 2,300 | 5,700 | 580 | 980 | |
R.g: Reporting limit
- : Not detected above reporting limit
* At the test 15-25 min only 0,329 gram was added at chamber B.
** Sum of cis- and trans-citral.
TVOC The sum of Volatile Organic Components calculated as toluene.
4.5 Interpretation of analysis results
At all the climate chamber tests it can be seen that the emission rates are relatively low considering the quantities of each substance being part of the 10 drops of oil added to the candle diffuser or Aroma Stream. In the following the results will be elaborated further.
4.5.1 Aroma Stream
Tea tree oil
The measured emission rates with results for tea tree oil in Aroma Stream can be seen in figure 4.2. For each measuring period, a measuring point is given in the middle of the interval at 20, 130 and 250 minutes respectively.
There is a regular fall in the emissions rates for four of the substances whereas for alpha-pinene the rates are significantly higher in the beginning and at the same time a relatively low rate after 130 minutes.
The emission rate for beta-caryophyllene was unexpectedly low for the period 15-25 minutes. The same is the case for the emission measurement of beta-caryophyllene from rosemary oil, No. 38 and No. 5 from this testing period, but there is no explanation to this.
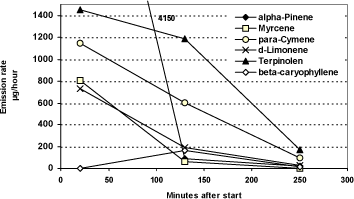
Figure 4.2 Measured emission rates from tea tree oil in Aroma Stream. Value of 4,150 for alpha-Pinene is out of scale. The unit is µg/time. The average of two tests.
In order to normalize the rates to the content of the substances in tea tree oil the same results is shown in figure 4.3, where emission factors indicating the emission in % of the quantity of the substances added to Aroma Stream.
It can be seen that substances with a relatively high emission factor after 20 minutes have an equivalently low emission factor after 130 minutes which indicates that the relatively low rate after 130 minutes is due to the fact that a large part of the substances have already disappeared. Alpha-pinene and myrcene with high rates in the beginning thus have low rates after 130 minutes where the rates are less than 1/10 of the rates in the beginning. It should be noted that the emission factor has been calculated in relation to the starting quantity of the substance and not the remaining quantity at different points in time. Held together with the very low rates after 250 minutes, the results indicate that the main part of the oils have disappeared after four hours.
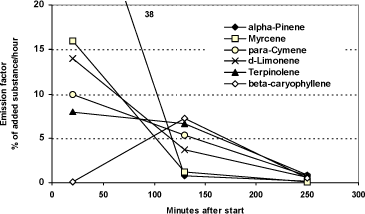
Figure 4.3 Measured emission factors from tea tree oil in Aroma Stream. Value at 38% for alpha-Pinene is out of scale. The emission factor in percentage shows how large a part of the added substance will be emitted during an hour with the measured emission rates. The average of two measurements.
A calculation based on the measured emission rates indicates however that for most of the substances less than 25% has been collected on the ATD-tubes, whereas for alpha-pinene there has been collected 51% of the added quantity (table 4.5). The total quantity emitted has been calculated for each time interval based on a line drawn through the two nearest measuring points. The emission in the period 0-2 hours has thus been calculated as 2 hours emission with the emission rate at 60 minutes (stated in µg/hour) based on the line through the measuring points for 20 and 130 minutes. For some of the substances an exponential fall probably better describes the actual development in the emission rates, but there are too few measuring points to make somewhat certain fits for an exponential tendency line. Control measurements show that the calculated emissions, if exponential functions are used, are close to the emissions stated in table 4.5 calculated on the basis of linear functions.
A simple test where the weight loss was measured after Aroma Stream had functioned for 2 hours showed for the fragrance oil No. 38 that approx 40% of the oil had disappeared after 2 hours. Similar tests were not made for the other oils. The tests indicate that the low rates are probably not due to the fact that the substances generally are absorbed strongly to the filter or diffuse into the plastic material.
Table 4.5 Tea tree oil in Aroma Stream. Total emission in respectively first 2 hours and first 4 hours in µg and in percentage of the content of the substances in the added oil. Average of two measurements.
| Content of 0.46 g oil added |
Estimated total
emission First 2 hours |
Estimated total
emission First 4 hours |
||||
| % | µg | µg | % of addef |
µg | % of added |
|
| alpha-Pinene | 2.30 | 10,810 | 5,347 | 49 | 5,462 | 51 |
| Myrcene | 1.05 | 4,935 | 1,068 | 22 | 1,137 | 23 |
| para-Cymene | 2.45 | 11,515 | 1,904 | 17 | 2,649 | 23 |
| d-Limonene | 1.10 | 5,170 | 1,074 | 21 | 1,307 | 25 |
| Terpinolene | 3.80 | 17,860 | 2,707 | 15 | 4,153 | 23 |
| beta-Caryophyllene * | 0.49 | 2,280 | 123 * | 5 | 313 | 14 |
* The measurements of beta-caryophyllene in the time period 15-25 minutes are unexpectedly low for all oils. There has been found no explanation of this.
Creation of decomposition and oxidation products
A possible explanation of the relatively low emission factors at the same time as the substances seems to disappear may be that the substances are either broken down or react with other substances after they have been transmitted to the air where substances are formed that are not part of the measuring program. The reactions will partly happen as gas phase reactions in the room, on surface particles or in the actual ATD-tubes before measuring.
The fact that e.g. terpenes oxidize in the air is a well known case, but the question is whether it happens to an extent so that 50-70% of the substances have reacted before measured. The substances are in relatively high concentrations in the chamber and it should be expected that the half life period depend on the relationship between the substances and the other reactants.
In an assessment on d-limonene from the International Programme for Chemical Safety (IPCS 1999) it is indicated that d-limonene emitted to the atmosphere is expected to quickly become part of a gas phase reaction with photo chemically made radicals, ozone and nitrate radicals. It has further been mentioned that it is important when analyzing limonene in the air also to analyze the oxidation products as limonene is quickly oxidized in the air. Based on the experimentally determined rate constants, a lifetime has been calculated for d-limonene at the reaction with photo chemically made hydroxyl radicals in 0.3-2 hours. The equivalent life times at reaction with ozone is 2-2.6 hours, whereas the life time at night hour reactions with nitrate radicals has been calculated to 0.9-9 minutes. Further circumstances and concentrations of reaction substances are not listed in the statements, but it is said that the atmospheric lifetime for d-limonene during daytime is estimated at 12-48 minutes. It is not perfectly clear whether life time actually means average lifetime.
Another reference shows a half-life time for d-limonene at 46 minutes at an ozone concentration of 50 ppb (Wainman et al 2000). Indoor ozone concentrations are in Denmark from approx. 10 up to approx. 70 ppb (Wolkorff 2004).
There is a long series of studies describing how the oxidation of d-limonene has an effect on the creation of fine particles in the air of the indoor climate (Vartiainena et al. 2006; Weinman et al. 2000).
It should be expected that the other substances in a similar way are part of the reactions even though the rates would be different. It is difficult to say whether the reactions could take place in such high rates in the climate chamber with high concentrations of a number of VOCs at the same time, but the stated reaction times for limonene indicated that such reactions may have a significant influence on the quantity of pure substances in the atmosphere in the climate chamber.
A completely different question is whether the reaction products will have any of the effects seen for the pure substances, so that by a health assessment it is necessary also to include the reaction products. This is further discussed in chapter 5 under health assessment.
Fragrance oil No. 38
Calculated emission factors for 5 substances from fragrance oil No. 38 can be seen in table 4.6. Emissions of alpha-pinene, camphene and para-Cymene were also measured above the reporting limit, but the measured concentrations in the oil were below the detection limit and it was thus not possible to calculate emission rates for these substances. The results show emission rates for Myrcene and d-limonene that are relatively high compared to the emission rates measured for tea tree oil (figure 4.3). The calculated emission factors for benzyl alcohol, Diethyl phthalate and benzyl benzoate in the period 15-25 minutes is 0.08%/hour for Benzyl alcohol and 0 for the two other substances. There has been found no explanation why no emission has been measured from the three substances.
A subsequent test, where the filter was measured at start and after 2 hours, it showed that 39% of the oil had evaporated after 2 hours. The emission of TVOC the first two hours may, based on a regression line between the measurements of the emission of TVOC in the periods 15-25 minutes and 220-240 minutes, be calculated to 9,990 µg equivalent to 2.7% of the weight of the added oil.
The composition of the oil is not known, but the figures indicate that together with data for rosemary oil, the measured emissions of the VOCs are considerably lower than the evaporated quantity.
Table 4.6 Fragrance oil no. 38 in Aroma Stream. Emission factors for constituents of the three measuring periods and content in added oil. Average of two measurements.
| Content of added oil (0.365 g) |
Emission factor (% of content of oil per hour) |
||||
| % | µg | 15- 25 min | 120-140 min | 220-240 min | |
| Myrcene | 0.06 | 234 | 50 | 4.6 | 1.6 |
| Benzyl alcohol | 0.58 | 2,117 | 0.08 | 0.5 | 0.3 |
| d-Limonene | 0.11 | 383 | 65 | 3.7 | 1.2 |
| Diethyl phthalate | 9.6 | 34,858 | 0 | 0.001 | 0 |
| Benzyl benzoate | 0.55 | 1,989 | 0 | 0.001 | 0 |
4.5.2 Candle diffuser
The candle diffuser generally shows a more pronounced decrease in the emission between the first and the second measurement than in the tests with Aroma Stream. The measured emission rates for rosemary oil shown in figure 4.4 indicate that the main part of the substances evaporates within the first two hours.
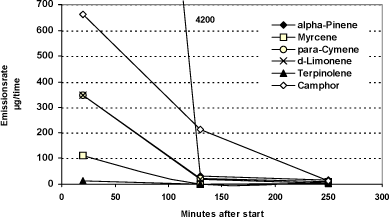
Figure 4.4 Measured emission rates from rosemary oil in candle diffuser. Value of 4,200 for alpha-pinene is out of scale.
But as for the measurements of Aroma Stream, the measured rates are considerably below the quantities that seem to disappear.
In table 4.7 is shown the calculated rates in percentage of the substance quantities added to the lamp in form of rosemary oil. The total emission has been calculated by assuming that the measured rate at 15-25 minutes represents an average rate for the first 2 hours. On the contrary to what is the case with Aroma Stream, it cannot - in this set up - be assumed that the emission gradually is reduced as it is a fact that the oil is dissolved in water and that the temperature increases. However, the uncertainty of this calculation can hardly explain the low rates that more likely are due to some of the same mechanisms resulting in low rates in the tests with Aroma Stream.
Apart from the creation of reaction products it may in this test also have an effect that part of the substances adsorb to or are dissolved in water drops created in the chamber due to the high humidity. With the used ATD-tube, substances adsorbed to water particles will not be collected and measured.
In order to illustrate the results of the climate chamber test, a simple test has been made with the evaporation of rosemary oil from the candle diffuser (same model as used in the chamber). After 15 minutes the water was well heated and there was still a visible layer of oil on the surface. All the water had evaporated after 1 hour and 40 minutes. No oil, after the last bit of water had evaporated, could be observed in the bowl and in the following 15 minutes there were no significant odour from the lamp (subjectively determined by the author) which is accordance with the fact that the test after 130 minutes showed very limited emission of fragrances.
There was not observed any change in the odour either after the water had evaporated. Addition of one drop of oil to the hot diffuser immediately gave a very strong odour and the oil disappeared within a few minutes. It seems as if the substances in the oil evaporates with the water, but it is not possible to say whether the rates measured in the period 15-25 minutes is representative for the rates through the whole period. It seems to be pretty certain that approx. 99% of the substances are emitted to the air shortly after the water has evaporated.
It should be noticed that some aroma therapists recommend to turn off the diffuser before all the water has evaporated, but it is not indicated on any recommendations for the used diffuser. If the diffuser is blown out after an hour where half of the water has evaporated and then left in the room there will of course evaporate a smaller quantity than if you let all the water evaporate, but it is reasonable to assume that at least half of the substances in the added oil will still evaporate.
Table 4.7 Rosemary oil in candle diffuser. Calculated emission in the first 2 hours in µg and in percentage of the substances in the added oil (emission factor).
| Content of added oil (0.46 g) | Estimated total emission First 2 hours |
|||
| % | µg | µg | Emission factor % of added |
|
| alpha-Pinene | 11.50 | 54,050 | 9,429 | 17 |
| Camphene | 5.25 | 24,675 | 5,900 | 24 |
| Myrcene | 1.10 | 5,170 | 486 | 9 |
| para-Cymene | 2.20 | 10,340 | 791 | 8 |
| d-Limonene | 4.15 | 19,505 | 826 | 4 |
| Terpinolene | 0.05 | 228 | 26 | 12 |
| Camphor | 11.0 | 51,700 | 1,526 | 3 |
| beta-Caryophyllene | 3.3 | 15,51o | 16 | 0.1 |
4.6 Selection of substances for health assessment
Out of the 15 substances examined, six substances have been selected for a closer health assessment.
The substances have been selected based on the following criteria:
- The substances are part of a number of the oils in significant quantities;
- It has been shown that the substances are emitted in such large quantities from the oils examined in the climate chamber that it is possible to calculate a concentration in the air;
- There are AT limit values and/or LCI/NIK values (can both be used directly for comparison, but also indicates that data is available);
- The substances are on the list of dangerous substances;
- The substances are mentioned in literature on aroma therapy as having a possible health effect;
- It is realistic to find information on the substances' possible effect by exposure via the respiratory tract;
- The selected substances should represent several substance groups (e.g. not all should be terpenes).
- Four of the substances were not chosen because the emissions were below the detection limit so that concentration could not be calculated in the test room: estragole, n-decylaldehyde, trans-anethole and Diethyl phthalate.
Based on these criteria the following substances have been selected for health assessment:
| d-Limonene CAS No. 5989-27-5 |
Part of all tested oils (up to 73%) and in most other oils. AT limit value and LCI/NIK values On the list of dangerous substances. Knowledge of literature on exposure via the respiratory tract |
| alpha-Pinene CAS No. 7785-26-4 and 7785-70-8 |
Part of 9 of the examined oils (op to 11%) and concentrations of up to 50% other oils NIK value Knowledge of literature on exposure via the respiratory tract |
| p-Cymene CAS No. 99-87-6 |
Part of 9 of the examined oils (up to 2%) and in concentrations of up to 5% in other oils AT limit value |
| Benzyl alcohol CAS No.100-51-6 |
Part of 4 of the examined oils (up to 0.5%) and in concentrations up to 10% in other oils. On the list of dangerous substances NIK value |
| Camphor | Part of the 5 examined oils (up to 11%) and in concentrations of up to 10% in other oils |
| Citral | Part of 8 of the examined oils (up to 75%) and in a number of other oils On the list of dangerous substances |
Version 1.0 April 2008, © Danish Environmental Protection Agency