Survey and health assessment of chemical substances in essential oils and fragrance oils
5 Health assessment
- 5.1 Search of data
- 5.2 Toxicity in selected substances by inhalation
- 5.3 Exposure to selected substances by inhalation
- 5.4 Risk assessment of health aspects
The aim of aromatherapy is to achieve a certain psychical and physical state of the treated person. The result is achieved by the scent from the oil influencing the smell receptors in the mucous membranes in the nose, which then sends impulses via the olfactory nerve to different brain centres.
This report is about the possible health damaging effects caused by inhalation of certain chemical substances being part of aromatherapy oils. The survey concentrates on effects that can be achieved in the respiratory tract (nose, trachea, bronchi) or in the lower part of the lungs (alveolus) or in the remaining part of the body after the substances have gone through the bloodstream via the lungs.
The effect of the fragrances on the olfactory nerve is not included in the assessment. Effects by exposure to the skin or by swallowing are also not included in this assessment.
Actual health damaging inhalation effects may occur as irritation of the respiratory tract or by toxic impact on the respiratory tract and the lower pulmonary segments. When a substance has reached the bloodstream via the lungs it is transported round in the body to all organs and by this way the substance may have a hazardous effect (systemic effect). Apart from this, certain substances may have negative health effects such as being carcinogenic, genotoxic or influencing the immune system.
An additional health damaging effect of the examined substances is the possibility for sensitizing the respiratory tract i.e. an effect that may cause asthma. This is reasonable as several of the substances in focus have a sensitizing effect on the skin (so-called delayed type 4 allergy) and is called for allergens. Sensitizing in the nose or the respiratory tract follows another immunological mechanism than the skin. It is a so-called type-1 immediate allergy causing hay fever (rhinitis allergica) and asthma (asthma bronchiale). There is however no direct relation between the two allergy mechanisms. A substance that is skin allergen does not necessarily also cause allergy in the respiratory tract.
By reviewing the literature for the 6 selected substances the above considerations have been used for guidance. Comments on the substances' health damaging effects by inhalation are limited to the described area and there has not been searched for information on the substances' allergenic effect by exposure via the skin and the substances' effect on the skin have not been assessed (most of them have been assessed earlier) whereas certain systemic effects to a certain extent will be described.
5.1 Search of data
Background data for toxic effects by inhalation have been found in:
- DTV-online search in HSDB and RTECS;
- The European Chemicals Bureau (ECB);
- ChemID light with relevant underlying databases;
- IARC Website;
- NIOSH Website.
Apart from the above, original literature has been searched for using the search parameters: substance name and "inhalation" and substance name and "exposure" in the following databases:
- PubMed;
- Medline;
- Scopus.
Furthermore there has been searched for original literature by a DTV-online search for CAS no. and "inhalation" in Chemical Abstracts.
Information has also been collected from Danish experts from the National Research Centre for the Working Environment, The Danish Research Centre for Chemical Sensitivities, and Department for Environmental and Occupational Medicine, Institute of Public Health, University of Aarhus and from international experts from Research Institute for Fragrance Materials in USA and from the Tisserand Institute in Great Britain.
A review of several survey reports from the Danish Environmental Protection Agency has given useful information about some of the substances and the procedures, for example survey report no. 36 on chemical substances in printed matter (Hansen & Eggert 2003), no. 49 on emission of chemical substances from exotic wood (Witterseh 2004) and no. 82 on selected respiratory tract sensitizing substances in consumer products (Boyd & Mogensen 2007), as they threat several of the substances investigated in this report (e.g. d-limonene, alpha-pinene, citral).
5.2 Toxicity in selected substances by inhalation
5.2.1 D-limonene
| Chemical name | (R)-p-mentha-1,8-diene |
| Synonym | +Dipentene (R)-p-mentha-1,8-diene (S)-p-mentha-1,8-diene trans-1-methyl-4-(1-methylvinyl)cyclohexene (±)-1-methyl-4-(1-methylvinyl)cyclohexene Limonene D-limonene L-limonene |
| CAS-No. | 5989-27-5 |
| EINECS No. | 227-813-5 |
| Molecular formula | C10H16 |
| Molecular structure | 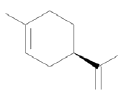 |
| Legislation: | |
| Classification as per list of dangerous substances | R10 XI;R38 R43 N;R50/53 |
| List of undesirable substances. | On the list as the substance has been assessed as allergenic by skin contact and is one the 26 allergenic fragrances assessed by SCCNFP. |
| IUCLID-dataset | Yes |
| Cosmetics | The fragrance is declared in cosmetics if used in quantities above o.01% in products that are cleaned and o.001% in products that are not cleaned. |
| Physical chemical substances: | |
| Melting point, °C | -89 - -96.9 |
| Boiling point, °C | 170-180 |
| Vapour pressure (Pa) | 2.66644 hPa at 25° C |
| Physical state | Liquid |
| Octanol-water partition coefficient, (log Pow) | 4,.57 |
| Water solubility (mg/L) | very low (<0.1 g/100 mL at 19.5 C) |
Available information on toxicity related to inhalation
D-limonene has very low acute toxicity in humans and test animals. There is no genotoxic, teratogenic or embryo-toxic effect described in the found literature. Its effect on the respiratory tract is controversial. By inhalation the substance is readily absorbed into the blood wherefrom it is absorbed by fatty tissues and eliminated through the kidneys. D-limonene is a strong immunological active substance at a relatively low concentration (Josefson 1993).
Human exposure by inhalation of 450 mg/m³ d-limonene gives significant reduction of lung capacity, but not of the other respiratory functions. No irritating effect on eyes, nose, throat or the upper and lower respiratory tract and no influence of the central nervous system has been reported. Tests have shown a ready uptake of 70% of the dose during two hours exposure (Falk-Filipson et al. 1993, Beije and Lundberg 1993).
Inhalation tests with mice show a decrease in respiratory rate at 1076 ppm as a result of sensory irritation. This reaction seems to resemble the human response, as NOEL for sensory irritation is 80 ppm in humans whereas it is 100 ppm in mice. A mild bronchoconstrictive effect at mice is seen at 1000 ppm (Larsen et al. 2000).
Inhalation of d-limonene prevents bronchial obstruction in sensitized rats by reaction with ozone. Histologically there is an inflammatory inhibitory effect. (Keinan et al. 2005).
Airways irritants in the form of ultrafine particles can be formed by reaction between ozone and unsaturated volatile organic compounds - especially limonene and alpha-pinene. (Wolkoff et al. 2000; Rohr et al. 2003). Nøjgaard et al. (2005) report that oxidation products of terpenes (e.g. limonene) contain unidentified irritants that may be responsible for a part of the reported eye and airway complaints in indoor environments.
Exposure of rats to 6 ppm d-limonene and 0.8 ppm ozone for three hours causes inflammatory changes in the lungs (Sunil et al. 2007).
D-limonene is not in itself an allergen, but allergens are created by auto-oxidation (Karlberg et al. 1992).
According to IARC (1999) it is estimated that the substance d-limonene cannot be classified in relation to its carcinogenic effect on humans (Group 3).
When humans inhale d-limonene a stimulation of the autonomic nervous system is observed with increased b blood pressure, subjective alertness and restlessness as well as subjective mental and emotional reactions (Heuberger et al. 2001).
Limit values for d-limonene:
AT limit value (AT 2007): 75 ppm (tentative)
NIK (AgBB 2005): 1400 µg/m³
LCI (Jensen et al. 2001): 300 µg/m3
5.2.2 Alpha-Pinene
The substance is linked to several CAS numbers. In the following data have been collected for three:
1) 80-56-8: alpha-Pinene (non-specified mixture of below substances)
2) 7785-26-4: (-)-alpha-Pinene
3) 7785-70-8: (+)-alpha-Pinene
Both enantiomers, (-)-alpha-pinene and (+)-alpha-pinene, are present in natural oils.
| Chemical name | alpha-Pinene |
| Synonym 1) | 2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene 2-Pinene Acitene A alpha-Pinene Cyclic dexadiene pin-2(3)-ene |
| CAS-No. | 80-56-8 |
| EINECS No. | 201-291-9 |
| Molecular formula | C10H16 |
| Molecular structure | 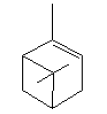 |
| Legislation: | |
| Classification according to list of dangerous substances | Not on the list |
| Lists of undesirable substances…….. | No |
| IUCLID-dataset | Yes |
| Cosmetics | |
| Physical chemical properties: | |
| Melting point, °C | -64 |
| Boiling point, °C | 155 |
| Vapour pressure (Pa) | Not identified |
| Physical state | Liquid |
| Octanol-water partition coefficient, (log Pow) | 4.83 |
| Water solubility (mg/L) | Not identified |
| Chemical name [1]9 | (1S)-(-)-alpha-Pinene |
| Synonym 1) | (1S)-(1)-alpha-Pinene (1S)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene (1S)-(-)-alpha-Pinene (-)-alpha-Pinene Dipentene Pinen |
| CAS-No. | 7785-26-4 |
| EINECS No. | 232-077-3 |
| Molecular formula | C10H16 |
| Molecular structure | 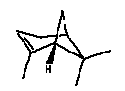 |
| Legislation: | |
| Classification as per list of dangerous substances | Not on the list |
| List of undesirable substances. | No |
| IUCLID-dataset | No |
| Cosmetics | |
| Physical chemical properties | |
| Melting point, °C | -64 |
| Boiling point, °C | 155 - 156 |
| Vapour pressure (Pa) | NA |
| State | Liquid |
| Octanol-water partition coefficient, (log Pow) | 4.4 8) |
| Water solubility (mg/L) | NA |
| Chemical name [2]9 | (1R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene |
| Synonym 1) | (+)-alpha-Pinene (1R)-(+)-alpha-Pinene (1R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene |
| CAS-No. | 7785-70-8 |
| EINECS No. | 232-087-8 |
| Molecular formula | C10H16 |
| Molecular structure | ![Molecular structure: (1R)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene](images/s82.gif) |
| Legislation: | |
| Classification as per list of dangerous substances | Not on the list |
| List of undesirable substances | No |
| IUCLID-dataset | No |
| Cosmetics | |
| Physical/chemical properties | |
| Melting point, °C | -62 |
| Boiling point, °C | 155 |
| Vapour pressure (Pa) | Not identified |
| Physical state | Not identified |
| Octanol-water partition coefficient, (log Pow) | Not identified |
| Water solubility (mg/L) | Not identified |
Available information on toxicity related to inhalation:
The substance may cause the same effects as turpentine. The substance may, if inhaled in high concentrations cause heart beat, dizziness, disturbance of the nervous system, chest pain, bronchitis and inflammation of the kidneys (Gosselin et al. 1984).
Alpha-Pinene is toxic to rats and mice when inhaled (Lewis 1999).
For humans there are no subjective inconveniences or influence of the lung function by inhalation of alpha-pinene at concentrations of 450 mg/m³. The main relative blood uptake of the substance was 62% of the amount supplied (Edman et al. 2003; Filipson 1996).
The substance may cause irritation in the lungs (Rohr et al. 2002).
An inhalation study with mice shows that the substance causes irritation in the upper respiratory tract (reduced respiration rate) at doses between 100 and 3691 ppm (Nielsen et al. 2005). Limit value for effect from (+)-alpha-pinene is 70 ppm, equivalent to GV dose of 40 ppm for humans. At concentrations above 200 ppm, contraction of the respiratory tract is seen. NOEL for sensory irritation is 72 ppm.
At concentrations of (+/-)-alpha-pinene below 81 ppm there was no contraction of the respiratory tract in humans. Neither (+) or (–)-alpha-pinene below 82 ppm has shown any effect on the central nervous system in humans (Falk et al. 1990).
Animal inhalation studies show that at 6-12 g/m³ there is irritating effect of the respiratory tract for (+)- alpha-pinene, but not for (–)-alpha-pinene. No risk for harmful health effects on humans (Mersch-Sundermann 2007).
Inhalation of alpha-pinene has a moderate effect on the autonomic nervous system resulted in increased blood pressure and increased concentration of stress hormones in the blood (catecholamine) (Haze et al. 2002).
The following animal toxicological data have been identified in IUCLID:
- LCLO (inhalation, rat) = 625 µg/m³
- LCLO (inhalation, porpoise) = 572 µg/m³
- LCLO (inhalation, mouse) = 364 µg/m³.
- (LCLO = lowest concentration at which death occurred)
Limit values for alpha-pinene
AT limit value (AT 2007): None
NIK (AgBB 2005): 1400 µg/m³
LCI (Jensen et al. 2001): 250 µg/m³ (CAS No.: 80-56-8)
NOEL for lung symptoms: 25 mg/m³ (Larsen et al. 1999).
5.2.3 Benzyl alcohol
| Chemical name | Benzyl alcohol |
| Synonym | alpha-Hydroxytoluene alpha-toluenol Benzyl alcohol Benzenecarbinol Benzenemethanol Benzoyl alcohol (hydroxymethyl)benzene Hydroxytoluene Phenylcarbinol Phenylmethanol Phenylmethyl alcohol |
| CAS-No. | 100-51-6 |
| EINECS No. | 202-859-9 |
| Molecular formula | C7H8O |
| Molecular structure | 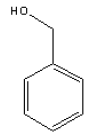 |
| Legislation: | |
| Classification as per list of dangerous substances | Xn;R20/22 (at conc.>=25%) |
| List of undesirable substances. | No |
| IUCLID-dataset | Yes |
| Cosmetics | The fragrance is declared in cosmetics if used in quantities above 0.01% in products that are cleaned and 0.001% in products that are not cleaned. |
| Physical/chemical properties: | |
| Melting point, °C | -15.3 |
| Boiling point, °C | 205 |
| Vapour pressure (Pa) | not identified |
| Physical state | oily liquid |
| Octanol-water partition coefficient, (log Pow) | 1.10 |
| Water solubility (mg/L) | 4.29 g/100 mL |
Available information on toxicity related to inhalation
Vapours may cause irritation of eyes, nose and throat (US Coast Guard referred in HSDB and RTECS). Vapours cause irritation in eyes, nose and throat with cough and bad throat, but there is no quantitative data and benzyl alcohol is not classified as irritant. (Koniezko and Czerczak 2003).
According to Cosmetic Ingredient Review the substance is not carcinogenic or genotoxic (CIR 2001).
There is uncertainty as regards the human toxicological data. The following has been identified in RTECS (2007 data without reference) regarding inhalation:
- LC50 (mouse, inhalation): > 500 mg/m³.
- LC100 (rat, inhalation, 8 hours): 200 - 300 ppm - a value that must be seen in relation to the following value which is also referred to in (RTECS, data without reference. 2007):
- LC50 (rat, inhalation, 8 hours): 1000 ppm, also referred to as LCLo(RTECS, data without reference. 2007).
According to IUCLID there is the following data for inhalation:
- LC50 (rat, inhalation, 4 hours): results range from >4,178 to >9 mg/l.
Vapour from the substance is assessed to be able to penetrate intact skin (Opdyke 1979).
Inhalation of the substance may cause cough, dizziness and headache (IPCS, 2000).
The substance has only caused negative results in Ames Tests (CCRIS database, 2007).
Limit values for benzyl alcohol
AT Limit values (AT 2007): None
NIK (AgBB 2005): 440 µg/m³
LCI (Jensen et al. 2001): 100 µg/m3.
5.2.4 p-Cymene
| Chemical name | p-Cymene |
| Synonym | 1-Methyl-4-isopropylbenzene 1-Methyl-4-isopropylbenzene para-Cymene 4-isopropyltoluene p-methyl cumene 4-methyl isopropylbenzene Cymol Dolcymene Methyl-4-(1-methylethyl)benzene |
| CAS-No. | 99-87-6 |
| EINECS No. | 202-796-7 [3]) |
| Molecyle formula | C10H14 1) |
| Molecyle structure | 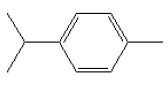 1) 1) |
| Legislation: | |
| Classification as per list of dangerous substances | Not on the list |
| List of undesirable substances. | No |
| IUCLID-dataset | Yes |
| Cosmetics | |
| Physical/chemical properties: | |
| Melting point, °C | -67 |
| Boiling point, °C | 176 - 178 |
| Vapour pressure (Pa) | Not identified |
| Physical state | Flammable liquid |
| Octanol-water partition coefficient, (log Pow) | 4.10 |
| Water solubility (mg/L) | Unsolvable |
Available information on toxicity related to inhalation
Vapours have been assessed not to cause irritation in the throat (IPCS 2000).
Inhalation is mentioned to cause dizziness, drowsiness and vomiting, but there is no information about concentration (NIOSH 1997).
Inhalation tests with humans show a significant increase in amylase content in sputum, which seems to be provoked by stimulation of the olfactory nerve rather than nerves in the respiratory tract (Hanawa 2007).
Rat inhalation tests with 0.50 and 250 ppm for 4 weeks show changes in the brain that resemble the toxicity of solvents (Lam 1996).
Rats and porpoise inhalation, 100 mg/kg. During 48 hours 60-80% of the dose had been eliminated through urine in form of 18 metabolites (Walde 1983).
The following human toxicological data for inhalation has been identified for the substance:
LC50 (mouse, inhalation) = 19,500 mg/m3 (RTECS, data without reference. 2007).
Limit values
AT Limit values (AT 2007): 25 ppm, 135 mg/m³
NIK (AgBB 2005): None
LCI: None
5.2.5 Citral
| Chemical name | Citral |
| Synonym | 3,7-Dimethyl-2,6-octadienal cis-3,7-Dimethyl-2,6-octadienal cis-Citral' cis/trans-3,7-Dimethyl-2,6-octadienal Citral A Citral B Citral, mixture of cis and trans CITRAL NATURAL CITRAL SINTETICO Geranal Geranial Geranialdehyde Lemarome n Neral trans-3,7-Dimethyl-2,6-octadienal |
| CAS-No. | 5392-40-5 |
| EINECS No. | 226-394-6 [4]) |
| Molecular formula | C10H16O |
| Molecular structure | 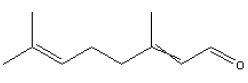 |
| Legislation: | |
| Classification as per list of dangerous substances | Xi; R38 - R43 |
| List of undesirable substances. | Yes |
| IUCLID-dataset | Yes |
| Cosmetics | The fragrance is declared in cosmetics if used in quantities above 0.01% in products that are cleansed off and 0.001% in products that are not cleansed off. |
| Physical/chemical properties: | |
| Melting point, °C | < 20 [5]) |
| Boiling point, °C | 225 5) |
| Vapour pressure (Pa) | < 100 at 50 degrees C |
| Physical state | Liquid |
| Octanol-water partition coefficient, (log Pow) | Not identified |
| Water solubility (mg/L) | 0.01 - 0.1 g/100 ml at 18 degrees |
Available information on toxicity in relation to inhalation
Inhalation tests with pregnant rats during 6-15 days with 10, 35 and 68 ppm. The pregnant animals show toxic effects at 68 ppm, but no effect on foetus has been observed at this concentration. The substance is not teratogenic (Gaworksi et al. 1992).
Rat/mouse inhalation tests give LC50 of 12,500 ppm. The substance is moderately toxic (Luo et al. 2005).
The substance is in the Chemical Carcinogenesis Research Information System (CCRIS) referred with negative Ames Tests and has not been assessed by IARC.
According to York et al. (1989) the substance is not teratogenic.
Limit values
Limit value (AT 2007): None
NIK (AgBB 2005): None
LCI: None.
5.2.6 Camphor
| Chemical name | Camphor |
| Synonym 1) | (±)-Camphor 1,7,7-Trimethylbicyclo[2.2.1]-2-heptanone 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one 1,7,7-Trimethylnorcamphor 2-Camphanone 2-camphonone Camphore Caladryl Camphor Oil Gum camphor Radian B |
| CAS-No. | 76-22-2 |
| EINECS No. | 200-945-0 |
| Molecular formula | C10H16O |
| Molecular structure | 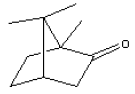 |
| Legislation: | |
| Classification as per list of dangerous substances | Not on the list |
| List of undesirable substances. | No |
| IUCLID-dataset | No |
| Cosmetics | |
| Physical chemical properties: | |
| Melting point, °C | 177 |
| Boiling point, °C | 207 |
| Vapour pressure (Pa) | |
| Physical state | Liquid |
| Octanol-water partition coefficient, (log Pow) | 2.38 |
| Water solubility (mg/L) | 0.12 g/100 mL |
Short summary on available information on toxicity in relation to inhalation
Nose in and expiratory resistance was not changed by inhalation of the substance by humans (Eccles et al. 1987).
Five minutes inhalation provokes a subjective sense of chill and improved air flow through the nose. The substance stimulates chill receptors in the mucous membrane in the nose (Burrow et al. 1983).
Inhalation of concentration above 2 ppm may cause irritation in nose and throat (IPCS 1989).
At concentrations above 6 mg/m³ the substance may cause serious health effects on animals (OHSA, 1989).
Porpoise inhalation tests at 500 µg/l reduce chemically provoked cough reflex. At lower concentrations no effect was observed (Laude et al. 1994).
According to HSDB web the substance is not carcinogenic and IARC has not evaluated the substance.
The following human toxicological data have been identified:
- LC50 (rat, inhalation) = 500 mg/m³ (RTECS data without reference, 2007)
- LC50 (mouse, inhalation) = 450 mg/m³ (RTECS data without reference, 2007).
Limit values
Limit value (AT 2007): 2 ppm, 12 mg/m³
NIK (AgBB 2005): None
LCI (Jensen et al. 2001): 250 µg/m³
5.3 Exposure to selected substances by inhalation
Concentrations of the selected substances in the model room calculated based on tests in the climate chamber can be seen in table 5.1. What is indicated is an average concentration for four hours after start. For calculation of the average concentration during the four hours the exposure model, presented in chapter 3, has been used. It is assumed that the substances are emitted with a constant rate for 2 hours and that the concentration in the room, subsequently, will gradually decrease due to air circulation.
As background for the calculations, data for those of the tested oils - in each of the experimental setups - where the substances have the highest concentration has been used. have been used in each arrangement
In order to take the uncertainty on the interpretation of the measuring results into account, the calculated values are both based on actual emission tests and worst case scenarios.
Concentrations in model rooms based on actual tests
The emission rate during the first two hours is calculated differently for the two setups.
For the tests with the candle diffuser it has been assumed that the emission during the 2 hours is at the same level as measured in the period 15-25 minutes. From the measurements it is obvious that this is not the case - after 2 hours the emission is considerably lower. By the calculation it is considered that there will be an inherent tendency that the measured rates are lower than the actual rates as a steady state has not yet been established in the climate chamber.
For the tests with Aroma Stream a regression line has been used as described in chapter 4.5.1.
"Worst case" scenario based on examined oils
It seems to be quite certain that the emission of substances is considerably larger than the quantities collected and measured but it is not obvious why. The differences may be due to a combination of many factors: the substances are broken down or creating reaction products before the measurement; the collection is not efficient because some of the substances are absorbed by water particles; the measuring uncertainty is biased, or a steady state in the test chamber has not yet been established before the first measurement and the emission is consequently underestimated. For Aroma Stream an alternative explanation could be that the substances are absorbed to the filter, but this has not been confirmed by a simple weight loss test. It can thus not be rejected that the actual emissions would be higher, and as the oxidation products for several of the substances have been demonstrated to also have sensitizing effects on the respiratory tract, there is, to be on the safe side in the assessments, used a worst case where 50% is emitted during the first hours.
As a result of the measuring results for tea tree oil in Aroma Stream shown in figure 4.2, there will be a difference depending on how large a part is actually emitted, but the data material is too vague to make precise calculations for each substance.
In the table is also given AT limit values and LCI and NIK values used for assessment of exposure level in the indoor climate.
Table 5.1 Concentration of selected substances calculated based on measurement in climate chambers.
| Substance | Product | Concentration in product % |
Concentration in model room based on measurements (average first 4 hours), µg/m3 *1 |
Worst case concentration in model room (average first 4 hours), µg/m3 *2 |
AT limit value µg/m³ |
LCI µg/m³ |
NIK µg/m³ |
| Experiments with candle diffuser: | |||||||
| d-Limonene | No. 34 |
20.5 | > 190 | 665 | 75 ppm (tentative) |
300 | 1,400 *3 |
| alpha-Pinene | Rosemary oil |
11.5 | 175 | 580 | 250 | ||
| Camphor | Rosemary oil |
11 | 25 | 560 | 2 ppm 12,000 (synthetic) |
250 | |
| Citral | No. 34 | 1.9 | 0.2 | 60 | |||
| p-Cymene | Rosemary oil | 2.2 | 12.7 | 110 | 25 ppm 135,000 |
||
| Benzyl alcohol | Rosemary oil |
0.1 | <0.1 *4 | 10 | 100 | 440 | |
| Experiments with Aroma Stream: *2 | |||||||
| d-Limonene | Tea tree oil | 1.1 | 20 | 60 | 75 ppm (tentative) |
300 | 1,400 *3 |
| alpha-Pinene | Tea tree oil | 2.3 | 100 | 120 | 250 | ||
| Camphor | - | - | - | - | |||
| Citral | Tea tree oil | 0.5 | 0 | 30 | |||
| p-Cymene | Tea tree oil |
2.5 | 35 | 130 | 25 ppm 135,000 |
||
| Benzyl alcohol | No. 38 |
0.6 | 0.2 *4 | 25 | 100 | 440 | |
*1 The concentrations for fragrance oils have been calculated based on the assumption that the emission continues for two hours with the same rate as measures in the period 15-25 minutes. The emission for Aroma Stream has been calculated based on the regression line between the two measuring points in 20 min and 130 minutes, respectively.
*2 Worst case concentration is for both scenarios calculated by roughly estimating that 50% of the substances in the added oil has been emitted and are in the room either in form of pure substances or reaction products.
*3 NIK values for limonene (CAS No. 138-86-3).
*4 There is no explanation to the measured low values for benzyl alcohol, but the result should be interpreted with caution.
"Worst case" scenario based on oils with the highest concentration
The oils that have been included in the climate chamber tests are not necessarily the oils with the highest concentration. For example is the highest concentration of d-limonene in the examined oils 20.5% in no. 34, but the concentration of the d-limonene in citrus oil was measured to 72.5%. It must thus be expected that there could be a considerably higher concentration in the model room if citrus oil was used in the setups.
In view of describing a "worst case scenario", a calculation has been made using the exposure model using the highest registered concentration of the substance in any product. For products where the concentration in safety data sheets has been indicated with an range the highest value in the range has been used. It has been assumed that for the setups 0.4 g product is added equivalent to 10 drops (based on the measured average of the 10 climate chamber tests).
It has furthermore been assumed that 50% of the quantity of each substance added evaporates to the room during a 2 hour period. There are several circumstances indicating that the actual emission rates could be of that size even though it would vary from substance to substance depending on the substances' physical/chemical properties. Alternatively you could argue for a worst case scenario where 100% is emitted, but this does not seem to be the case in the actual use situations.
The average concentration of a number of terpenes (d-limonene, alpha-pinene, camphene, p-mentha-1.4-diene, p-mentha-1.3-diene, beta-pinene and 3-carene) during the first four hours is considerably above the LCI values. The highest concentration is for d-limonene in citrus oil, where the concentration in the room is more than 10 times higher than the LCI value.
For diethyl phthalate the average concentration is 2,400 µg/m³ close to the Danish Working Environment Agency's limit value of 3,000 µg/m³.
Table 5.2 Worst case scenario where it is assumed that half of the added quantity of substance is emitted during the first two hours.
| Substance | Example of product *1 | Maximum concentration in product % *2 |
Concentration in model room (average first 4 hours) µg/m3 *3 |
AT limit value µg/m³ or ppm |
LCI µg/m3 *4 |
NIK µg/m³ *4 |
| d-Limonene | lemon oil | 72.5 | 3,490 | 75 ppm (tentative) | 300 | 1,400*1 |
| alpha-Pinene | Pine needle oil | 50 | 2,400 | 250 | 1400 | |
| p-Cymene | Tea tree oil | 2.5 | 120 | 25 ppm 135,000 |
||
| Benzyl alcohol | No. 15 | 10 | 480 | 100 | 440 | |
| Camphor | Rosemary oil | 11 | 530 | 2 ppm 12,000 synthetic | 250 | |
| Citral | Lemongrass oil | 75.5 | 3,630 | |||
| Dietylphthalat | No. 39 | 50 | 2,400 | 3,000 | ||
| Myrcene | Orange oil | 2.5 | 120 | 1000 *4 | ||
| n-Decalaldehyd | No. 34 | 5 | 240 | 400 | 1400 | |
| Camphene | Rosemary oil | 10 | 480 | 250 | ||
| beta-Caryophyllene | Nutmeg oil | 5 | 240 | 1000 *4 | ||
| p-Mentha-1,4-diene | Camphor oil | 20 | 960 | 250 | ||
| p-Mentha-1,3-diene | Camphor oil | 10 | 480 | 250 | ||
| p-Mentha-1,4(8)-diene | Tea tree oil | 5 | 240 | 1000 *4 | ||
| beta-Pinene | Lemon oil | 20 | 960 | 250 | 1400 | |
| 3-Carene | Pine needle oil | 20 | 960 | 250 | 1400 |
*1 Examples of products where the substance has the highest concentration; the substance may be present in similar concentrations in other products.
*2 Represents highest reported concentration in safety data sheets or actual measurements. In cases where the safety data sheet indicated ranges the highest value of the range is listed.
*3 It has been estimated that 0.4 g product is used, equivalent of 10 drops (average of measurements at climate chamber tests). It is assumed that 50% of the added quantity of the substance is emitted to the air during 2 hours. The concentration shows a total concentration of the substance and possible reaction products.
*4 Based on Jensen et al. 2001. For substances marked with *4 there is no values in Jensen et al. 2001, and instead the LCI values from ECA-IAQ (1999) have been listed.
5.4 Risk assessment of health aspects
The risk assessment of health aspects for the six examined substances is very difficult to carry out. Partly there is not sufficient data from human inhalation tests; partly the measured concentrations and the calculated worst case scenario values are very uncertain. The risk assessment has consequently been made on an uncertain basis and must be taken with many different reservations for the results and conclusions of the assessment.
Lacking and defective data in literature
By going through the literature and by personal inquiries to Danish and foreign experts, not much useful and valid information about the six examined substances have come forward on the health effects by inhalation.
For two substances, d-limonene and alpha-pinene there are systematic reviews of inhalation tests in humans. For the other four substances, the information is very scarce and defective. One substance, benzyl alcohol, has been classified as hazardous by inhalation in concentrations above 25%. For several of the substances there are experimental tests of inhalation by rats and mice. Based on these animal tests, the LCI values have been calculated by using correction factors 100 and 1000. It is problematic to use these surveys as a scientific basis for a risk assessment of health impacts.
The Danish experts with expertise in climate chambers and indoor climate problems have no supplementary information about health effects caused by inhalation of the six selected substances. Dr. Elberling from the Danish Research Centre for Chemical Sensitivities says that according to a Danish survey there are people who get nuisance in the upper and/or lower respiratory tract by inhalation of fragrance and these persons have a so-called "bronchial hyper-reaction" meaning that they - as a contracts to none-hypersensitive persons - react with contractions in the respiratory tract when tested with specific substances. This hyper activity is not related to an allergic reaction such as asthma. It is uncertain whether the mentioned fragrances contain any of the six tested substances.
Missing data for standards
There is insufficient information for the current standards for the six substances. There are AT limit values for three of the substances valid for the working environment i.e. 8 hours exposure per day. Otherwise there are LCI/NIK values for respectively five and three of the substances used for indoor climate assessments. These values relate to exposure in the indoor climate 24 hours a day, 7 days a week.
There is a considerable difference between the Danish LCI-values and the German NIK-values with a factor 5 difference for d-limonene and alpha-pinene, and a factor 4 for benzyl alcohol. It is not obvious what the differences are due to, but it illustrates that caution should be taken when health assessments are based on these values.
There is a considerable quantitative difference of up to 300 times between the AT limit values and the LCI values which is linked to the different exposure situations the values are used for.
Uncertainty in own test results
The quantitative climate chamber tests of concentration of the six substances during evaporation from Aroma Stream and candle diffusers have an uncertainty that makes it difficult to determine a concentration as basis for the risk assessment of health impacts as it is not obvious in which form the substances are.
Selection of LCI-value as a basis for health related risk assessment
The risk assessment is based on LCI values for the five substances whereas there is no immediately accessible values for citral. It is estimated that concentrations listed as LCI values are so low that they should be considered as safe for a health assessment at a maximum 4 hours exposure by inhalation.
The definition of LCI is described in chapter 2.4.1. For several of the substances the LCI value is determined based on the very poor knowledge on effects. There are considerable safety margins used by the determination of the LCI. Irritation was the most decisive health effect of the substances for the determination of the LCI value. More serious health effects were found at a much higher concentrations.
We have found one single (human) NOEL value for lung symptoms for alpha-pinene at 25,000 µg/m³. This value equals the set LCI value for the substance with a safety factor of 100. This example again shows that the LCI-values are considered to be very certain for this risk assessment.
Health related risk assessment has been made for:
- (A) calculated concentrations in model rooms based on actual measurements in climate chamber tests for the six examined products;
- (B) calculated "worst case" concentrations in model room based on climate chamber test for the six examined products;
- (C) calculated "worst case" concentrations in model rooms for products with highest content of the six substances based on information of concentrations on safety data sheets.
A. The calculated concentrations above 4 hours in model rooms (table 5.1) are all below the listed LCI values, for d-limonene and alpha-pinene, however the concentrations are of the same size. For p-cymene, benzyl alcohol and camphor one to two times lower. There is consequently not expected to be any health risk related to the intended use.
B. The calculated "worst case" concentrations in model rooms (table 5.1) are approximately twice as high as the LCI values for d-limonene, alpha-pinene and camphor, whereas they are about the LCI value for p-cymene and benzyl alcohol. Under these circumstances a health risk for the first mentioned three substances cannot be ignored completely - it is however very limited - but it is assessed that there will be no risk for the remaining three.
C. Here the listed concentrations for d-limonene and alpha-pinene exceed the LCA value with at factor 10, for benzyl alcohol with a factor 5 and camphor a factor 2 (table 5.2). Similar exceeding is seen for at number of other substances that are not further assessed here. The exceeding indicates a possible; however low health risk at constant exposure in the indoor climate. Bearing in mind that the NOEL value for lung effects in humans is approx. 25,000 µg/m³ there is a good safety margin, at least for alpha-pinene/terpenes.
Assessment of toxicity
For none of the substances a significant toxicity has been observed, neither in human inhalation tests nor in animal inhalation tests.
Assessment of irritating effects on the respiratory tract
For several of the substances there have been described irritating effects on humans and animals but only at high concentrations.
D-limonene in a (high) concentration of 450,000 µg/m³ causes a reduction of the lungs vital capacity for humans. At high concentration it causes a fall in the respiration rate in rats. These effects have been observed at extremely high concentrations.
D-limonene and alpha-pinene are known as potential irritant for the respiratory tract after oxidation, e.g. with ozone. In this process ultrafine particles are created.
By inhalation of camphor, test persons experience a sensation of chill in the nose with a better air passage. Objective measurements have not been able to confirm an improvement of the nose's air flow. The sensation of chill is due to influence of the nervous receptors in the mucous membranes in the nose
Assessment of sensitizing
None of the examined substances has a respiratory sensitizing effect. In 2008 on Institute for Research on Fragrance Materials (IRFM) a study is planned of benzyl alcohol's possible sensitizing/allergic effect on the respiratory tract.
Assessment of systemic and other biological effects
When inhaled, all substances are quickly absorbed in the blood. Adult test persons who inhaled d-limonene while they were doing a light manual labour of 50 W (expression of working intensity), 70% of the given dose was found in the blood after 2 hours. A large part of the substances are subsequently absorbed into the fatty tissue. They are transformed to other chemical substances (metabolites) and the major part is excreted through the kidneys. A small part of the inhaled dose is found in the expired air.
Inhalation of camphor by humans showed sign of stimulation of the autonomic nervous system in form of increased blood pressure immediately after inhalation whereas other fragrances have the opposite effect. Inhalation of d-limonene and alpha-pinene causes increased blood pressure and a stress-like condition in rats.
There have not been described any biological effects on other organs.
For none of the examined substances any carcinogen, genotoxic or foetus damaging effects have been observed.
Discussion
This risk assessment focuses primarily on health effects as a consequence of a short (2-4 hours) inhalation of one single substance. It is not likely that health effects arise long time after a short-period inhalation. But the possibility for a long-term health effect, such as chronic bronchitis or pneumonia, emerging after repeated short-period inhalations over a longer time period cannot with certainty be precluded. According to literature and contacted experts there is however no basis for this assumption.
Most aromatherapy oils contain several active substances that are inhaled during the treatment. The risk assessment only concentrates on one of the active substances. This makes a health assessment further complicated and uncertain because several substances in one and same aroma oil product may have different, partly additive or reverse health effects by inhalation.
This risk assessment, comparing the measured concentrations in a climate chamber with a fixed LCI value, does not consider a group of people reacting with respiratory symptoms by exposure to fragrances in a very low concentration as e.g. persons with multiple chemical sensitivity.
Conclusions
The results of the survey do not indicate that, apart from people being sensitive to fragrances, there are considerable health problems by inhalation of aromatherapy oils occasionally with the reservation that only a limited number of constituents have been examined. On the contrary, based on the results of the examination it cannot be rejected that daily use of 5-10 drops of oil in a small room for a prolonged period in the long term may result in airway irritation.
Recommendations
It is thus recommended, until more certain results are available, that small quantities are used, to ventilate the room carefully air when the fragrance effect is no longer wanted and that use of fragrance oil is not a daily occurrence.
It is recommended that the consumers only use oil specifically recommended by the producers for this purpose and follow the recommendations given on the packaging. It is also recommended that before using a diffuser candle or diffuses the fragrances in another way to read the safety recommendations given by the producers and suppliers at their web-sites or given in books on the subject.
It is recommended that more and better climate chamber and inhalation tests on humans are made if there is a suspicion of health effects on humans. Targeted provocation tests can also be made as the expertise and interest for the problem is present among Danish experts. It is recommended that further research is made on the possible effects of long term impact of inhalation of aroma substances in concentrations relevant in the indoor climate and on the effects of simultaneously exposure to a long series of chemical substances.
[3] http://ecb.jrc.it/esis/index.php?PGM=ein
[4] http://ecb.jrc.it/esis/index.php?PGM=ein
[5] IUCLID dataset on http://ecb.jrc.it
Version 1.0 April 2008, © Danish Environmental Protection Agency