Survey and Health Assessment of Possible Health Hazardous Compounds in Proofing Sprays
8 Health Assessment
- 8.1 Butyl acetate
- 8.2 Butanone
- 8.3 1-Butanol
- 8.4 Cyclohexane
- 8.5 Perfluoroctane-1-ol
- 8.6 Dodecamethylpentasiloxane
- 8.7 Recapitulation on health assessment and information collection
In consultation with the Danish Environmental Protection Agency the following substances were selected for health assessment: cyclohexane, butan-2-on, 1-butanol, butyl acetate, perfluoroctane-1-ol and dodecamethylpentasiloxane. In this chapter, the toxicological profiles of the 6 chemical substances have been set up. The four first mentioned substances are assumed to be used in spray products in their capacity of propellants and solvents and therefore they are subject to special control in Regulation 571 dated 29/11/1984 (the Danish Environmental Protection Agency). All four substances are included on the list of permitted propellants and solvents in enclosure 1 of the Regulation, but all four substances are forbidden (in concentrations exceeding 1 %) in products intended for indoor use (all tested spray products) with propellant. The two last-mentioned are the two actual proofing substances where the occurrence has been best documented in the spray products selected for analysis.
All the substances were found in products that are sprayed from aerosol cans with propellant. None of the below assessments deal with the fact that the substances also appear as very fine aerosol mists. That is because it has not been possible to find experimental toxicological data for these substances in the form of aerosols. The end of this chapter examines the importance of the very fine aerosol mists that have been measured for all aerosol products with propellants in this investigation.
8.1 Butyl acetate
8.1.1 Application
Butyl acetate is mainly used as solvent in varnish, artificial leather, photographic film and plastics. To a minor degree, butyl acetate is used in the perfume industry and for the production of artificial aromatic compounds (HSDB, 2007).
8.1.2 Identification
At room temperature, butyl acetate is a clear, colourless liquid with a pleasant smell that often is described as banana-like. It is not easily soluble in water, but it is miscible with most hydrocarbons and very easily soluble in ethanol and ether and soluble in acetone (HSDB, 2007). The odour limit in water is 0.066 mg/m³ (HSDB, 2007). Butyl acetate is included on the list of organic solvents of the Danish Working Environment Authority.
| Identification: | |
| Substance name: | Butyl acetate |
| Synonyms: | 1-Butyl acetate; n-Butyl acetate; 1-Butyl acetate; Acetic acid, butyl ester (ECB, 2007) Butyl ethanoate (HSDB, 2007) |
| CAS no.: | 123-86-4 |
| EINECS No.: | 204-658-4 |
| Molecule formula | C6H16O2 |
| Molecule structure | 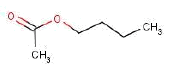 |
| Legislation: Classification according to the list of hazardous substances (Danish Environment Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value of the Danish Working Environment Authority (ppm, mg/m³) |
R10; R66; R67 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 150 ppm; (710 mg/m³) for all butyl acetates |
8.1.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Colourless liquid (HSDB, 2007) |
| Molar weight | 116.16 (HSDB, 2007) |
| Density | 0.8826 g/cm³ at 25°C (HSDB, 2007) |
| Melting point | -78°C (HSDB, 2007) |
| Boiling point | 126.1°C (HSDB, 2007) |
| Vapour pressure at 25 ?C | 11.5 mm Hg (HADB, 2007) |
| Octanol water (logPow) | 1.78 (HSDB, 2007) |
| Solubility in water | 14 g/L at 20°C; 5 g/L at 25°C (HSDB, 2007) |
| Odour limit in water | 0.066 mg/m³ (HSDB, 2007) |
8.1.4 Toxicological data
8.1.4.1 Absorption
Butyl acetate is quickly absorbed in the blood by inhalation. No measurements exist of gastrointestinal or dermal absorption, but effectuated oral and dermal LD50 studies indicate that the substance also is absorbed through these routes.
8.1.4.2 Acute effects, humans
Butyl acetate has a low systemic effect (HSDB, 2007). The lowest toxic concentration on inhalation was found to be 200 ppm (920 mg/m³), and changes were found on the sensory organs and especially the olfactory sense, on eyes (irritation) and on lungs, on chest and respiration (other changes) (ChemIDPlus, 2007).
Possible toxic symptoms are central nervous system (CNS) effects: headache, muscular weakness, dizziness, stiffness, confusion, delirium and coma. Gastrointestinal tract effects are: nausea, vomiting, and diarrhea (with smell of the alcohol from the faeces); irritation in eyes and neck from vapour as well as liquid, coughing and dyspnoea; ictus disturbance; death due to respiratory failure. (HSDB, 2007).
Butyl acetate is described as a mildly irritating substance, but more irritating than ethyl acetate, and as a CNS depressor. These effects are considered to originate from the physical properties of the substance (HSDB, 2007)).
Skin exposure: prolonged or frequently repeated exposure can lead to drying of the skin.
Butyl acetate vapours lead to eye irritation and inhalation irritates the respiratory passages.
Occupational inhalation has led to effects on the liver (HSDB, 2007).
8.1.4.3 Acute effects, animals
In connection with oral administration, the LD50 values are between 3200 mg/kg bw (rabbit) and >10.000 mg/kg bw (rat). Dermal LD50 17.600 mg/kg bw (rabbit); LD50 values by direct administration in the abdominal cavity was 1230 and 1500 mg/kg bw in guinea pigs and mice, respectively. LC50 was 6000 mg/m³ after 2 hours of inhalation in mice and 390 ppm corresponding to 1850 mg/m³ after 4 hours of inhalation in rats (ChemIDPlus, 2007).
8.1.4.4 Subchronic effects
No studies have been found with repeated dosage in animals apart from one single study in cats where no local changes were found in the cornea or the conjunctival sac of cats dosed either with 500 ppm for 20 days or with 1000 ppm for 4 days. However, according to ACGIH, animals (species of animal not informed) exposed 6 hours a day for 6 days to 3100 ppm showed blood changes (HSDB, 2007).
8.1.4.5 Mutagenicity
Butyl acetate showed no mutagenic properties in Ames' test (Salmonella typhimurium strands TA98, TA100, TA1535, TA1537, TA1538 and Escherichia coli (WP2uvrA strand)) during testing with and without activation with rat microsomal fraction.
8.1.4.6 Chronic effects
No long-term tests with butyl acetate have been carried out in any species of animal. IARC has not considered the carcinogenic properties of butyl acetates. On the other hand, ACGIH in the USA has decided that within a two-year period the substance shall be transferred to an approval list: Cannot be classified as a human carcinogen (HSDB, 2007).
8.1.4.7 Summary
Butyl acetate is not acute toxic on intake or inhalation or during exposure of the skin. Due to the physical/chemical properties – solvent with large vapour pressure – the substance has irritating effects on skin and mucous membrane (eyes and upper respiratory passages) and a number of effects on the CNS after inhalation. No information has been found stating that butyl acetate should be sensitizing.
It is assessed that people working in the chemical industry who have skin diseases, nephropathy, chronic respiratory diseases or hepatic diseases can have increased risk in connection with exposure to butyl acetate.
Toxicological data (animals) |
|
| LD50, mg/kg, oral, rat | 10768 (ChemIDPlus, 2007) |
| LD50, mg/kg, oral, guinea pig | 4700 (ChemIDPlus, 2007) |
| LD50, mg/kg, oral, mouse | 6000 (ChemIDPlus, 2007) |
| LD50, mg/kg, oral, rabbit | 3200 (ChemIDPlus, 2007) |
| LD50, mg/kg, dermal, rabbit | >17600 (ChemIDPlus, 2007) |
| LC50, mg/m³, inhalation, 2 hours, mouse | 6000 (ChemIDPlus, 2007) |
| LC50, mg/m³, inhalation, 4 timer, rat | 1846 (corresponding to 390 ppm) (ChemIDPlus, 2007) |
| Toxicological data (humans) | |
| LCLo mg/m³, inhalation (time not informed) | 947 (corresponding to 200 ppm) (ChemIDPlus, 2007) |
8.1.5 Health assessment of butyl acetate
Occurrence in investigated spray products:
| Butyl acetate measured in analysed products | Product no. | ||||||
| 1 | 3 | 9 | 14 | 15 | 16 | 25 | |
| Semi-quantitative g/kg (%) | 23 (2.3) |
39 (3.9) |
|||||
| Quantitative g/kg (%) | 98 (9.8) |
20 (2.0) | 80 (8.0) |
0.058 (0.58) |
0.065 (0.65) | ||
| Butyl acetate declared (other remarks) | No (discontinued product) |
Yes | No | Yes | Yes | No | No |
The absolute worst case scenario is that 1 spray can is emptied into a 20 m³ room and that the person stays in the same room for 8 hours without airing.
The aerosol product with the highest concentration of butyl acetate is product no. 1 that true enough has been discontinued, but product no. 14 contains almost as much. The calculation was most logically carried out in product no. 14 which still is marketed.
Product no. 14 is sold in Denmark in 200 ml spray cans but in other countries it is marketed in 400 ml cans.
The density of the spray liquid is not known but a conservative estimate is a density of 1 g/cm3 which means that 200 ml weighs 200 g.
Therefore, a spray can will contain 16 g of butyl acetate and distributed in a 20 m³ large room that will give a concentration of 800 mg/m³.
That is 12 % above the limit value of the Danish Working Environment Authority which is 710 mg/m³ (150 ppm).
The limit value is a “time weighted average” determined according to extensive toxicological estimates as the value to which a worker may be exposed 8 hours daily in an entire working life.
As the product is a consumer product where exposure only will take place now and then, the calculated value can instead be compared with the ceiling value of the Danish Working Environment Authority, at the double of the ordinary limit value.
Therefore, it must be assessed that the use of spray no. 14 is not injurious to health even during the absolute worst case scenario. Presumably, passing and acute sickness can arise (irritation of the eyes and respiratory passages).
8.1.6 Conclusion on butyl acetate (n-butyl acetate)
The content of butyl acetate in the examined spray products for textile proofing is not in itself a health hazard to the consumers.
8.2 Butanone
8.2.1 Application
Is mainly used as solvent in surface coating industries, paint and varnish industries, for polymer and glue production and as an intermediate for chemical syntheses in the chemical and pharmaceutical industry. In addition, it is used to some extent in the aromatics industry.
The Food and Drug Administration (FDA) in the USA has set an acceptable daily intake value (ADI) of 3.2 mg/day (oral intake) (HSDB, 2007).
8.2.2 Identification
Butanone is a clear liquid with a sweet, pleasant, lightly pricking, acetone-like odour. Butanone is easily soluble in water at low temperatures, but the solubility declines with increasing temperatures. The substance is soluble in alcohol, ether, acetone and benzene. (HSDB, 2007).
The substance is included on the list of organic substances of the Danish Working Environment Authority.
| Identification: | |
| Substance name: | Butanone |
| Synonyms: | Methyl ethyl ketone; butan-2-on; 2- butanone; methyl ethyl ketone |
| CAS no.: | 78-93-3 |
| EINECS No.: | 210-159-0 |
| Molecule formula | C4H8O |
| Molecule structure | 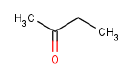 |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value (ppm, mg/m³) (The Danish Working Environment Authority, 2007) |
F;R11 XI;R36 R66 R67 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 50 ppm; 145 mg/m³ (H, can be absorbed through the skin) |
8.2.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Colourless liquid (HSDB, 2007) |
| Molar weight | 77.11 (HSDB, 2007) |
| Density | 0.805 g/cm³ at 20°C (HSDB, 2007) |
| Melting point | -86°C (HSDB, 2007) |
| Boiling point | 79.6°C (HSDB, 2007) |
| Vapour pressure at 25°C | 90 mm Hg (HSDB, 2007) |
| Octanol water (logPow) | 0.29 (HSDB, 2007) |
| Solubility in water | 353 g/L at 10°C (HSDB, 2007); 27.1 g/L at 20°C (IUCLID)(IPCS, 1992) |
| Odour limit | low: 0.7375 mg/m³; high = 147.5 mg/m³ (HSDB, 2007) |
8.2.4 Toxicological data
8.2.4.1 Absorption
Butanone is absorbed quickly in the body no matter if oral or dermal exposure is in question or if absorption takes place on inhalation. Butanone seems to be distributed on all tissue. Butanone and its metabolites are eliminated completely in the course of 24 hours. Elimination especially takes place with the expiratory air even though small amounts are eliminated in transformed form via the kidneys (IPCS, 1992).
8.2.4.2 Acute effects, humans
Exposure to 590 mg/m³ (200 ppm) did not cause changes in different behaviour or psychological tests. Nor did experimental exposure to 794 mg/m³ (270 ppm) 4 hours/day have greater effect on behaviour and 5 min. contact with liquid butanone only caused passing bleaching of the skin (IPCS, 1992).
8.2.4.3 Acute effects, animals
Very low acute toxicity was present in the tested species of animals for all routes of administration. The LD50 values for oral studies are 2700 and 5520 mg/kg bw in rats and 34140 mg/kg bw in mice. The inhalation studies carried out on mice and rats are all very old and were not carried out in accordance with the current guidelines but the lethal concentration for 50 % of the animals (LC50) in mice after 45 min. of exposure can be calculated to 205025 mg/m³ (69500 ppm) and in rats after 4 hours of exposure to 23600 mg/m³ (8000 ppm). A dermal LD50 value was found in rabbits at 8000 mg/kg bw with 24 hours of exposure (IPCS, 1992).
Minor to moderate irritation of the skin and moderate to serious irritation of rabbit eyes were observed. Other skin studies did not show irritation (IPCS, 1992).
8.2.4.4 Subchronic effects
Most studies with repeated dosage were carried out on rats with exposure on inhalation. Only doses of 5000 ppm (14750 mg/m³) in the one and 5041 ppm (14870 mg/m³) in the other given 6 hours/day 5 days a week for 90 days had effects. Reduced body weight, brain and spleen weight and increased liver weight and changed blood parameters were found and females were more sensitive than males. No histopathological changes or influence on the reproductive organs or morphological changes in CNS or peripheral nervous systems (PNS) were found (IPCS, 1992).
In a test, mice were exposed to increasing concentrations of butanone from 300 to 10000 ppm (total of 5 levels). The dosage time of each concentration was 30 min. and the number of mice that did not react to visual stimuli was counted. The dose at which 50 % of the animals no longer reacted could be calculated to 8528 mg/m³ corresponding to 2891 ppm (IPCS, 1992).
In a teratogenic test in mice, a no observed adverse effect concentration (NOAEC) of 2980 mg/m³ (1010 ppm) given on day 6-15 of the gestation period, 7 hours/day could be determined. No significant toxicity signs were found in the dam, but there was a minor increase in the relative liver weight in the highest dosed group. In the same group, lower foetal body weight was observed and it was significant for the males. Lowest observed adverse effect concentration (LOAEC) was set to 3000 ppm (IPCS, 1992) due to the developmental effects.
8.2.4.5 Mutagenicity
Butanone was not found mutagenic in a number of Ames' tests that were carried out and in vivo micronucleus studies in mice or Chinese guinea pigs showed no positive effects. However, some studies showed that butanone and a number of similar substances induce aneuplodi in yeast cells; an effect that was significantly strengthened by simultaneous exposure to ethyl acetate (IPCS, 1992).
In the light of this, butanone cannot be assessed to be genotoxic in short-term tests in vitro and in vivo.
8.2.4.6 Chronic effects
The only longer study that has been carried out is a one-year dermal study in male mice with application twice weekly of 8 mg (50 mg of a 17 % solution). No papilloma were found after 1 year (7).
Butanone cannot be classified with regard to carcinogenic effect in humans as no information exists about the substance concerning cancer in humans and sufficient data from experiments on animals does not exist.
8.2.4.7 Summary
Butanone is easily absorbed in the body after exposure via the gastrointestinal tract, the skin or the lungs. Absorbed butanone is eliminated in the course of 24 hours. Butanone has a very low acute toxicity in humans as well as in animals.
The results from testing butanone for irritation of skin and mucous membrane conflict a bit, but some irritation was found in most studies. Exposure of human skin to undiluted butanone results in passing bleaching of the skin. Butanone is classified with regard to eye irritation (R36) but not with regard to irritation of skin although repeated exposure can give dry or cracked skin (R66), In addition, a product should be marked, stating that vapours can cause lethargy and dizziness (R67) if it contains 15% or more butanone plus possibly other chemical substances with the same effect.
The critical effect is found to be lower foetal body weight in a teratogenic test with mice with a NOAEC of approx. 3000 mg/m³ corresponding to a bit more than 1000 ppm, treatment time was 7 hours/day on gestation day 6-15.
| Toxicological data (animals) | |
| LD50, mg/kg bw, oral, rat | 2737 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, oral, mouse | 4050 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, dermal, rabbit | 6480 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, dermal, rabbit | 8000 (IPCS, 1992) |
| LC50, mg/m³, inhalation, 8 hours, rat | 23500 (ChemIDPlus, 2007) |
| LC50, mg/m³, inhalation, 4 hours, mouse | 32000 (ChemIDPlus, 2007) |
| NOAEC, mg/m³, inhalation, day 6-15 of gestation period, 7 hours/day, mouse | 2980 |
| Toxicological data (humans) | |
| NOAEC, mg/m³, inhalation, (time not stated) | 590 (IPCS, 1992) |
| NOAEC, mg/m³, inhalation, 4 hours | 794(IPCS, 1992) |
8.2.5 Health assessment of butanone
The semi-quantitative screening of all products for textile proofing showed no results stating the content of butanone.
The more sensitive SPME-GC/MS screening of all products registered the occurrence of butanone in product no. 8 and 21, but without measured concentrations.
In connection with the quantitative analyses, butanone was not found in amounts exceeding the detection limit in any of the products – and not in product no. 8 and 21.
8.2.6 Conclusion on butanone in textile proofing sprays
Butanone has been identified in product no. 8 and 21. However, in the quantitative analyses of spray products, butanone was not found in amounts exceeding the detection limit of 0.02 mg/g.
Therefore, butanone is not in itself hazardous to health for consumers in the investigated spray products for textile proofing.
8.3 1-Butanol
8.3.1 Application
1-Butanol is used as solvent in the dyestuff and the varnish industry when making natural and synthetic resins, vegetable oils, dyes and alkaloids. It is used as an intermediate when making medicine and chemicals and is used in industries that make artificial leather, textiles, rubber adhesives, photographic film and perfume (HSDB, 2007). 1-Butanol is included on the list of organic solvents of the Danish Working Environment Authority.
8.3.2 Identification
1-Butanol is a clear colourless liquid with a very characteristic (rancid and sweet) faint smell of alcohol. The substance is rather soluble is water, miscible with ethanol and ether and very easily soluble in acetone. The solubility in benzene exceeds 10 % (HSDB, 2007).
| Identification: | |
| Substance name: | 1-Butanol |
| Synonyms: | 1-Butanol; n-butanol |
| CAS no.: | 71-36-3 |
| EINECS No.: | 200-751-6 |
| Molecule formula | C4H10O |
| Molecule structure | |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value (ppm/mg/m³) (Danish Working Environment Authority, 2007) |
R10 XN;R22 XI;R37/38-41 R67 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 50 ppm; 150 mg/m³ for all butanol-isomers (L, ceiling value; H, absorbed through the skin) |
8.3.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Colourless liquid |
| Molar weight | 74.1(HSDB, 2007) |
| Density | 0.8098 at 20°C (HSDB, 2007) |
| Melting point | -89°C(HSDB, 2007) |
| Boiling point | 117.7°C (HSDB, 2007) |
| Vapour pressure at 25 ?C | 7.0 mmHg (HSDB, 2007) |
| Octanol water (logPow) | 0.88 (HSDB, 2007) |
| Solubility in water | 63.2 g/L (HSDB, 2007); 74 g/L (IUCLID (ECB, 2007)) both at 25°C |
| Odour limit | In water 7.1 mg/L; in air 0.83 ppm (HSDB, 2007) |
8.3.4 Toxicological data
8.3.4.1 Absorption
1-Butanol is absorbed in the body via the lungs, the gastrointestinal tract and the skin. Absorbed substance is quickly distributed to the tissue where the substance is transformed considerably. The main part of absorbed substance is eliminated as CO2 via the lungs; but only a minor part is eliminated via the kidneys (HSDB, 2007).
8.3.4.2 Acute effects, humans
High concentrations in the air cause inhibition of CNS (tiredness, headache, muscular weakness, dizziness, stiffness, confusion, delirium, coma) (HSDB, 2007; IPCS, 1992). In addition, there might be gastrointestinal effects such as nausea, vomiting and diarrhea. Possible lethal toxification would be due to respiratory failure (HSDB, 2007).
1-Butanol is very irritating on the mucous membrane. Irritation of skin, eyes and neck has been observed during exposure to the liquid and vapours. In addition, coughing and difficulty in breathing have been observed.
8.3.4.3 Acute effects, animals
The oral LD50 values of 1-Butanol in rats vary between 700 mg and 2100 mg/kg bw.
The main effects from exposure to the vapour for a shorter time consist of different degrees of irritation of the mucous membrane and inhibition of CNS. Several sources state that it is believed to be approx. 6 times as toxic as ethanol (IPCS, 1987).
The substance seems distinctly irritating during testing with liquid in the eyes and moderately irritating on the skin (HSDB, 2007).
The skin sensitizing potential of 1-Butanol (IUCLID (ECB, 2007)) has not been tested.
8.3.4.4 Subchronic effects, animals
The effect of repeated inhalation comprises pathological changes in lung tissue and degenerative injuries in liver and kidneys (IPCS, 1987).
That was found in a number of inhalation studies carried out on rodents with different dosages (from 0.03 to approx. 40 ppm) and set ups varied from dosage in measured hours/day in a certain number of days to continuous exposure for 30 days, 4 months or 92 days (IUCLID).
The available animal studies are not suited for determining a no observed adverse effect level (NOAEL) to be used in risk assessments.
An inhalation study with exposure of pregnant female rats from day 1 to day 19 during gestation periods 7 hours a day with 3500, 6000 or 8000 ppm, revealed a NOAEC in the dam of 3500, but there was a minor increase in the number of rudimentary cervical vertebra in the offspring in the highest dosed group, and therefore NOAEC for development/teratogenecity was 6000 ppm (corresponding to 18000 mg/m³) (IUCLID from (ECB, 2007)).
No other reproduction toxicity studies are suited for determination of NOAEL.
8.3.4.5 Chronic effects
A wide range of short-term studies especially in vitro, showed no signs of mutagenic or genotoxic properties in 1-Butanol.
Environmental Health Criteria no. 65: Butanols - four isomers, 1987, as well as IUCLID from (ECB, 2007) refer to the fact that 2 long-term studies of very poor quality are supposed to exist, but it has not been possible to find further reference to these studies.
No studies exist with a route of administration that makes it possible to evaluate the chronic effects – not to mention the carcinogenic potential of 1-Butanol in humans.
IARC has not assessed 1-Butanol with regard to carcinogenicity in animals or humans.
8.3.4.6 Summary
1-Butanol is an ignitable colourless liquid that is used as organic solvent in many industrial connections. It has a low acute toxicity regardless of the exposure method. The substance is easily absorbed with the inhaled air, after intake or via the skin and it is distributed very quickly and evenly to all tissue.
High concentrations with the inhaled air induce signs of inhibition of CNS such as drowsiness, headache (in humans) and dizziness in animals as well as in humans.
Pathological changes in the lung tissue and degenerative changes in liver and kidneys appear in animals after repeated dosage via inhalation and anaesthesia is constantly developed.
Another predominating effect of 1-Butanol is skin and especially mucous membrane irritation, so irritation of eyes, nose and throat are effects that are registered at low exposures.
Sensitizing potential tests have not been carried out.
No trustworthy long-term studies have been found in any species of animal but the substance has proved to be non-mutagenic after substantial testing in vitro.
A minor occurrence of developmental disturbance was found at doses where toxic effect on the dam also was observed in a development/teratogenic test.
One of the very sensitive effects is eye irritation on exposure to vapour from 1-Butanol. In that connection, the effect level is 153.9 mg/m³ corresponding to 50 ppm in humans.
| Toxicological data (animals) | |
| LC50, ppm, inhalation, rat, 4 hours | 8000 (ChemIDPlus, 2007) |
| LD50, mg/kg bw, oral, rat | 700 (IPCS, 1987) |
| LD50, mg/kg bw, oral, rat | 800-2000 (IPCS, 1987) |
| LD50, mg/kg bw, oral, rat | 2100 (IPCS, 1987) |
| LD50, mg/kg bw, oral, mouse | 2680 (IPCS, 1987) |
| LD50, mg/kg bw, oral, rabbit | 3500 (IPCS, 1987) |
| LD50, mg/kg bw, dermal, rabbit | 4200 (IPCS, 1987) |
| LD50, mg/kg bw, dermal, rabbit | 5300 (IPCS, 1987) |
| NOAEC1,ppm, 7 hours/day, gd 1-19, female rat | 3500 (IPCS, 1987) |
| NOAEC²,ppm, 7 hours/day, gd 1-19, female rat | 8000 (IPCS, 1987) |
| Toxicological data (humans) | |
| NOAEC³, ppm inhalation – time not stated | 50 (IPCS, 1987) |
gd = gestation day
1General toxic effects
²Developmental toxic effects
³Eye irritation
8.3.5 Health assessment of 1-Butanol
No results were found for content of 1-Butanol during the semi-quantitative screening of all products for textile proofing.
The more sensitive SPME-GC/MS screening of all products registered the occurrence of 1-Butanol in product no. 18, 20, 25 and 26.
The quantitative analyses did not show 1-Butanol in amounts exceeding the detection limit in any product – and not in product no.18, 25 and 26 that were analysed quantitatively.
8.3.6 Conclusion on 1-Butanol in textile proofing spray
1-Butanol was not found in amounts exceeding the detection limit (0.2 mg/g) in the quantitative analyses in any spray.
1-Butanol in the investigated spray products for textile proofing is therefore in itself not a health hazard to consumers.
8.4 Cyclohexane
8.4.1 Application
The main application is as solvent for varnishes and resins, as paint and varnish remover, for extraction of "essential oils" in the analytic chemistry for determination of molar weight, for making adipic acid, benzene, cyclohexanon, cyclohexanol, cyclohexyl chloride, nitrocyclohexane, solid fuel, for industrial re-crystallization of steroids and in fungicides (HDSB, 2007).
8.4.2 Identification
Cyclohexane is a colourless, easily flowing liquid with a mild, sweet petroleum or chloroform-like odour. It is very flammable. Cyclohexane is practically insoluble in water but is soluble in ethanol, ether and acetone and is miscible with olive oil (HDSB, 2007). The odour limit is approx. 25 ppm in air. Cyclohexane is included on the list of organic solvents of the Danish Working Environment Authority.
| Identification: | |
| Substance name: | Cyclohexane |
| Synonyms: | Cyclohexane (IUPAC) from (8) Hexahydrobenzene, hexamethylene, |
| CAS no.: | 110-82-7 |
| EINECS No.: | 203-806-2 |
| Molecule formula | C6H6 |
| Molecule structure | 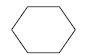 |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans. Limit value of the Danish Working Environment Authority (ppm, mg/m³) (Danish Working Environment Authority, 2007) |
F;R11 Xi;R38 Xn;R65 R67 N;R50/53 The substance is stated in enclosure 1 of the Regulation. Must not be used in aerosols intended for indoor use. 50 ppm; 172 mg/m³ |
8.4.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Clear liquid |
| Molar weight | 84.16 (ECB, 2004) |
| Density | 0.778 g/cm³ at 20°C (HSDB, 2007) |
| Melting point | 6.47°C (HSDB, 2007) |
| Boiling point | 80.7°C (ECB, 2004) |
| Vapour pressure at 25 ?C | 96.9 mm Hg (HSDB, 2007) (103 hPa at 20°C (ECB, 2004) |
| Octanol water (logPow) | 3.44 |
| Solubility in water | 58 mg/L at 25°C (ECB, 2004) |
| Odour limit | Approx. 25 ppm (HSDB, 2007) |
8.4.4 Toxicological data
8.4.4.1 Absorption
Cyclohexane is almost completely absorbed via the gastrointestinal tract and after inhalation. Approx. 50% absorption via the skin has been measured of small doses in the form of vapour, but substantially lower absorption has to be expected from liquid cyclohexane placed directly on undamaged skin (ECB, 2004).
Cyclohexane is distributed in the body with highest concentrations in fatty tissue. Elimination mainly takes place via the lungs either unchanged or as CO2 (ECB, 2004).
8.4.4.2 Acute toxic effects, humans
In a recent study, human volunteers were exposed to 25 or 250 ppm cyclohexane for a 4-hour period. No neuro behaviour effects were found in connection with any of the doses. The 250 ppm (corresponding to 860 mg/m³) is therefore assessed to be a no observed adverse effect concentration (NOAEC) for neuro behaviour toxicity (ECB, 2004).
Skin irritation appears after repeated dermal exposure. That is because cyclohexane has degreasing properties.
Skin sensitizing properties are not expected (ECB, 2004).
8.4.4.3 Acute toxic effects, animals
Oral LD50 values of more than 5000 mg/kg, 29800 mg/kg and 8000-39000 mg/kg were found for cyclohexane in rats. The lowest lethal oral dose in rabbits is 6000 mg/kg; the study showed that toxicity involved the CNS (narcotic effect and cramps).
The dermal LD50 in rabbits is larger than 2000 mg/kg which is the highest dose that has been tested (ECB, 2004).
Exposure of rabbits to cyclohexane vapour for 1 hour gave CNS effects (cramps, shaking, quick respiration, cyanosis and diarrhea). All animals exposed to 26000 ppm (89600 mg/m³) died. LC50 for exposure of rats for 4 hours exceeded 9500 ppm (32800 mg/m³) as no death occurred (ECB, 2004).
NOAEC was 2000 ppm (6880 mg/m³) for neuro toxicity in rats after 6 hours of wholebody exposure (ECB, 2004). A NOAEC of 400 ppm (1400 mg/m³) was found for neuro toxic effects in a sub-acute rat study with 8 hours of exposure daily for 6 days (ECB, 2004).
8.4.4.4 Subchronic effects
After repeated dosage on inhalation, the systematic effects in both mice and rats in the course of the 28 and 90 day studies were limited to effects on the liver: increase in absolute and relative liver weight, increase in mitotic index figures and centrolobular hypertrophy. The study lead to a no observed adverse effect concentration (NOAEC) of 2000 ppm (6880 mg/m³) (ECB, 2004).
It is true that an older study showed a NOAEC of 425 ppm, but the study is very insufficient and therefore this value cannot be used for health assessments (ECB, 2004).
No studies of subchronic effects from oral exposure exist.
An old study exists for rabbits of subcronic effects from dermal exposure but it was not possible to derive a NOAEL value (ECB, 2004).
In a 2-generation rat study (inhalation) no effects were found on fertility and only small weight reductions were found in the newly born offspring at 7000 ppm and toxicity in the dam also appeared. In the study, there was NOAEC of 500 ppm (1720 mg/m³) for systematic toxicity (sedation) and of 2000 ppm (6880 mg/m³) for reproduction.
2 inhalation studies were carried out for toxicity on the development (teratogenecity studies) – one in rats and one in rabbits. Concentrations of up to 7000 ppm, 6 hours a day on gestation day 7-16 (in rats) or on gestation day 7-19 (in rabbits) were used. In rats, there was systematic toxicity in the form of reduced number of implantations and the dam showed reduced body weight and feed consumption at 2000 and 7000 ppm, but no effects were seen in the development of the foetuses. In rabbits, no toxicity was seen in the dam or in the foetuses. Therefore, there is a NOAEC of 500 ppm (1.720 mg/m³) for systematic effects in rats, but with regard to the development of the foetuses there is a NOAEC of 7000 ppm (24.080 mg/m³). In the rabbit study, both NOAEC values are 7000 ppm (24.080 mg/m³) (ECB, 2004).
8.4.4.5 Mutagenicity
Cyclohexane neither appeared genotoxic in short-term in vitro nor in vivo studies (ECB, 2004).
8.4.4.6 Chronic effects
In a doubtful study it appeared that cyclohexane might have a weak cancer promoter potential (ECB, 2004). However, no conventional 2-year carcinogenic study exists, but the EU believes it is unlikely that the substance should be carcinogenic.
IARC has not assessed cyclohexane with regard to carcinogenic potential.
8.4.4.7 Summary
Cyclohexane is absorbed easily via the gastrointestinal tract and on inhalation and it to some degree it is also absorbed via the skin.
There is low acute toxicity from all routes of exposure. The acute effects as well as the effects after repeated dosage are mainly effects from the CNS. In addition, liver effects appear as increased weight and growth of central cells in the liver in subchronic studies in rodents. Cyclohexane has no toxic effects on reproduction.
The critical study is an acute human study with 4-hour exposure to 250 ppm corresponding to 860 mg/m³ for neuro behaviour effect. No effects were seen with this concentration. The critical effect is general toxicity in the dam in the rat teratogenic test. Effects are seen at 500 ppm.
Cyclohexane is not mutagenic and even though no regular carcinogenic study exists it is assessed to be unlikely that cyclohexane should have carcinogenic potential. The substance has not been assessed by IARC.
| Toxicological data (animals) | |
| LC50, ppm, inhalation, rat, 4 hours | >9500 (ECB, 2004) |
| LD50, mg/kg bw, oral, rat | 29820 (HSDB, 2007) |
| LD50, mg/kg bw, oral, rat | 8000 (HSDB, 2007) |
| LD50, mg/kg bw, oral, rat | 12705 (ChemlDPlus, 2007) |
| LD50, mg/kg bw, oral, mouse | 1300 (HSDB, 2007) |
| LD50, mg/kg bw, oral, mouse | 813 (ChemlDPlus, 2007) |
| LD50, mg/kg bw, oral, rabbit | 6000 (ECB, 2004) |
| LD50, mg/kg bw, dermal, rabbit | 18000 (ChemlDPlus, 2007) |
| LD50, mg/kg bw, dermal, rabbit | >2000 (ECB, 2004 |
| NOAEC1,ppm, 6 hours/day, gd 7-16, rat | 500 |
| NOAEC²,ppm, 6 hours/day, gd 7-16, rat | 7000 |
| NOAEC³,ppm, 6 hours/day, gd 7-19, rabbit | 7000 |
| Toxicological data (humans) | |
| NOAEC, ppm, inhalation, 4 hours | 250 |
gd = gestation day
1General systematic toxic effects
²Developmental toxic effects
³Both general and developmental toxic effects
8.4.5 Health assessment of cyclohexane
8.4.5.1 Exposure and health assessment
Occurrence in investigated sprays:
| Cyclohexane measured in analysed products | Product no. | ||||
| 1 | 3 | 6 | 8 | 9 | |
| Identified in SPME-GC/MS screening | X | X | X | X | X |
| Quantitative g/kg (%) | 6.5 (0.65) |
0.29 0.029 |
Not analysed | 6.0 (0.60) |
Not analysed |
The absolute worst case scenario is that 1 spray can is emptied into a 20 m³ room and that the person stays in the same room for 8 hours without airing.
The aerosol product with the highest concentration is product no. 1 that true enough has been discontinued but product no. 8 contains almost as much. The calculation was most logically carried out in product no. 8 that still is marketed.
An aerosol can filled with product no. 8 can hold 500 ml. If the density of the product is fixed at 1 g/cm³, then the spray container can liberate 3.0 g cyclohexane at the most, which distributed in the 20 m³ gives a maximum concentration of 150mg/m³.
Cyclohexane has a limit value determined by the Danish Working Environment Authority of 172 mg/ m³. The obtained concentration in the absolute worst case scenario amounts to approx. 87% of the limit value of the Danish Working Environment Authority.
The limit value is a ”time weighted average” that has been determined according to extensive toxicological estimates as the value to which a worker may be exposed 8 hours daily in an entire working life.
As the product is a consumer product where exposure only will take place now and then, the calculated value can instead be compared with the ceiling value of the Danish Working Environment Authority, at the double of the “ordinary” limit value.
Therefore, it must be assessed that the use of spray no. 8 is not injurious to health compared to exposure to cyclohexane. Even the absolute worst case scenario where 500 ml aerosol liquid is sprayed into a room of only 20 m³ will not lead to passing, acute sickness.
8.4.6 Conclusion on cyclohexane in aerosol products for textile proofing
The content of cyclohexane in the investigated spray products for textile proofing on the Danish market is in itself not a health hazard to the consumers.
8.5 Perfluoroctane-1-ol
8.5.1 Application
Perfluoroctane-1-ol forms part of several goods marked with "Fluortelomer Intermediate”, of which perfluoroctane-1-ol amounts to 27 - 34 %. The rest is formed by homologous substances of which approx. 1 % has fewer -CF2 – and the rest has more -CF2 links (always an equal number C atoms in the substances). These so-called fluortelomer alcohols are used in the production of products that require protective surface properties within the surface coating, pressure, textile and chemical industry.
8.5.2 Identification
telomer alcohols consist of an equal number of fluoridised carbon atoms connected to an ethanol part. Perfluoroctane-1-ol is a wax-like solid substance with a light to yellowish brown colour. The substance has a wax-like smell. It is almost insoluble in water, but soluble in acetone, butanone and isobutanol. The melting point is between 55 and 65°C.
| Identification: | |
| Substance name: | Perfluoroctane-1-ol |
| Synonyms: | 1,1,2,2-Tetrahydroperfluor-1-octanol; 1H,1H,2H,2H-perfluoroctanol; 1-Octanol, 3,3,4,4,5,5,6,6,7,7,8,8,8-tridecafluor- (systematic name) (ChemlDPlus): 6:2 FTOH or fluortelomer alcohol 6-2 |
| CAS no.: | 647-42-1 |
| EINECS No.: | 211-477-1 |
| Molecule formula | C8H4F13O |
| Molecule structure | 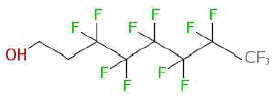 |
| Legislation: Classification according to the list of hazardous substances (Danish Environmental Protection Agency, 2005) Limit value of the Danish Working Environment Authority (ppm, mg/m³) (Danish Working Environment Authority) |
Not on the list Not on the list |
8.5.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Solid wax-like yellowish brown substance |
| Molar weight | Approx. 370 |
| Density | Approx. 1.7 g/cm² |
| Melting point | 55-65°C |
| Boiling point | 145 - 245°C |
| Vapour pressure at 25 ?C | - |
| Octanol water (logPow) | - |
| Solubility in water | Insignificant |
| Odour limit | No accessible information |
8.5.4 Toxicological data
As (hardly) any information was found for the substance perfluoroctane-1-ol itself, most data originates from investigations on the immediately higher homolog – the substance with 8 perfluoridised carbon atoms in addition to the 2 surrounded by hydrogen atoms. The terminology in English is often fluortelomer alcohol 6-2 (octanol compound), while the compound on which much data was found is called fluortelomer alcohol 8-2 (possibly 8:2) (decanol compound). These substances are generally written as 6:2 FTOH or 8:2 FTOH, respectively, in scientific literature.
It has been chosen to generalise in the light of the specific substance 8:2 FTOH and the term fluortelomer alcohols will also be used.
8.5.4.1 Absorption
Fluortelomer alcohols (8:2 FTOH) are absorbed quickly after oral intake, but the systematic concentration after 6 hours of skin exposure is insignificant. After oral intake, the plasma concentration is maximal when 1 hour has passed. The half-life period in the blood is 5 hours. The largest part of 8-2 FTOH is eliminated with faeces; the main part in unchanged form. Less than 4 % of an administered dose is eliminated with the urine. Of this, a small amount is oxidized to perfluoroctanoate (PFOA). Absorption is the same in male and female rats (Fasano et al, 2006).
8.5.4.2 Acute toxic effects, humans
No information has been found with regard to the acute effects of fluortelomer alcohols on humans.
An estrogenic effect of fluortelomer alcohols appeared on some human estrogenic receptor isoforms (in a test carried out on yeast cells) (Ishibashi et al, 2007) but neither perfluoroctanoate (PFOA) or perfluoroctane sulphonate (PFOS) had that effect. It is uncertain, what the specific biological importance of this is.
8.5.4.3 Acute toxic effects, animals
No information was found with regard to the acute effects of fluortelomer alcohols on animals (Herzke et al., 2007).
8.5.4.4 Subchronic effects
In a 90-day oral rat study, with 8-2 FTOH with daily doses of 1, 5, 25 and 125 mg/kg bw a no observed adverse effect level (NOAEL) was found on 5 mg/kg bw for male rates and 25 mg/kg bw for female rats. The effects at higher doses were liver necrosis and kidney injuries. There were signs of peroxisom proliferation in females at 25 mg/kg bw/day and in both sexes at 125 mg/kg bw/day (Fasano et al., 2006).
In a test concerning the toxic effects on development/teratogenecity it appeared that 8-2 FTOH does not effect the foetus development selectively (Fasano et al., 2006).
8.5.4.5 Mutagenicity
No information was found that could shed light on the mutagenic potential of fluortelomer alcohols.
8.5.4.6 Chronic effects
No studies of longer duration that could illustrate the chronic effects or carcinogenic potential of fluortelomer alcohols were found.
8.5.4.7 Summary
Nearly all accessible data on fluortelomer alcohols with 8 or with 10 carbon atoms was found as short background information in a larger investigation about absorption, distribution, metabolism and elimination (ADME study) of perfluordecan-1-ol. The background information originates from non-publicised studies.
Fluortelomer alcohols are absorbed in rats after oral administration but not after dermal exposure to the substance. ADME is the same in male and female rats.
No information was found about acute human effects.
After 90 days of oral administration effects were found in rodents on liver and kidneys. No observed adverse effect level (NOAEL) was found at 5 mg/kg bw in male rats and 25 mg/kg bw in female rats. That is in accordance with the demonstration that the substances give peroxisom proliferation in rodents.
| Toxicological data (animals) | |
| NOEL, mg/kg bw/day, oral, 90 days, female rat | 25 |
| NOEL, mg/kg bw/day, oral, 90 days, male rat | 5 |
8.5.5 Health assessment of perfluoroctane-1-ol
In the semi-quantitative analyses a substance that is expected to be perfluoroctane-1-ol (called 1H,1H,2H,2H–perfluoroctane-1-ol) (6:2 FTOH) was found in 3 products. On analysis of the procured standard it appeared that another substance was in question that is closely related to perfluoroctane-1-ol. In 2 additional products a sum of fluorine compounds was measured.
The quantitative analyses showed no 6:2 FTOH in the analysed products, but substances similar to this were measured in 3 products.
| Different fluorine compounds measured in analysed products | Product no. | ||||
| 6 | 8 | 14 | 21 | 25 | |
| Semi-quantitative screening results | |||||
| 6:2 FTOH g/kg | 0.17 | 0.29 | 0.03 | ||
| Sum of fluorine compounds (g/kg) | 0.03 | 0.17 | |||
| Quantitative analysis results | |||||
| Other fluorine compounds (g/kg) | Not analysed | 0.61 | 0.68 | 0.33 | Not found |
As the other quantitatively determined fluorine containing substances are very similar to 6:2 FTOH it was chosen to assess the content in these products as if fluortelomer alcohols were in question.
The absolute worst case scenario is that 1 spray can is emptied into a 20 m³ room and that the person stays in the same room for 8 hours without airing.
The highest concentration is found in product no. 14 where 1 kg spray liquid contains 680 mg. Product no. 14 is sold in Denmark in spray cans with a content of 200 ml, but in other European countries it is sold in 400 ml spray cans.
If a spray can of 200 ml (200 g) is emptied completely into the 20 m³, an average concentration of fluortelomer alcohol of (680 x 0.2/20 mg/m³) = 6.8 mg/m3 per m³ air is obtained.
In Technical Guidance Document on Risk Assessment (TGD, part 1), European Chemicals Bureau (European Commission, 2003), the inhalation rate for adults has been determined to an average of 0.83 m³/hour. And if we anticipate that the person remains in the small room non-stop (and without ventilation) for 8 hours, then the inhaled amount is 6.8 x 0.83 x 8 mg = 45 mg.
We have no data of how much of the substance will be absorbed in the body from the inhalation air. Therefore, absorption must be set to 100 %.
In TGD, part 1, the standard average weight is 60 kg for females and 70 kg for males.
Exposure can be calculated to 0.75 mg/kg bw for a female and 0.64 mg/kg bw for a male.
In connection with an aerosol for household use it can be anticipated that spray treatment corresponding to worst case scenario only happens at long intervals between treatments and therefore it would be relevant to compare the actual exposure with a no effect level from an acute study. However, that is not possible as only few data exist for flurotelomer alcohols.
In the toxicological data there is no observable effect level (NOEL) for male rats of 5 mg/kg bw in a 90-day test.
If that value is compared with the calculated exposure for a female, then there is a margin of safety (MOS) of 5/0.75 = 6.7.
MOS becomes a bit higher for a male: 5/0.64 = 7.8.
For chemical substances in consumer products a MOS of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account.
8.5.5.1 Discussion
The calculated margin of safety (MOS) that is less than 10 does not give sufficient safety in connection with use of spray product no. 14 in accordance with the scenario set up for spray proofing.
The analysis results of the fluorine compounds in product no. 8 are only approx. 10 % lower than for product no. 14. For this product, the margin of safety is also below 10.
It should also be considered that neither of the two products state a content of fluorine compounds on the label (product no. 8) or in the safety data sheet (product no. 14), respectively. The low content and the fact that these fluorine compounds are not included on the list of hazardous substances (the Danish Environmental Protection Agency, 2005) of the Danish Environmental Protection Agency result in no absolute demand for declaration, but the impression easily arises from the given declarations that they are exhaustive.
The consumer might get the impression that the proofing agent itself in both cases is low-boiling, hydrogenated naphta-fractions.
In connection with the screening investigations a high content of fluorine was found in more products than in which fluortelomer alcohols were analysed. Therefore, only a small part of this fluorine has been accounted for. A polymerisation might have taken place in connection with the analysis. However, it is possible that the consumer could be exposed to non-polymerised fluorine compounds in rather high concentrations. The problem is especially that we do not know the identity of the substances but if it is assumed that they can be compared to FTOH 6:2 then they might constitute a substantial problem that we cannot include in our conclusion because it only considers the substances found through analyses.
8.5.6 Conclusion on fluortelomer alcohol-like substances in proofing spray
Based on the very small amount of data material for the industrially very widespread fluortelomer alcohols a no observed effect level (NOEL) can be determined on male rats of 5 mg/kg bw/day from a 90-day study.
A quantitative analysis showed a content of similar substances of 0.61 g/kg (product no. 8), 0.68 g/kg (product no. 14) and 0.33 g/kg (product no. 21), respectively.
By calculating the margin of safety (MOS) of the two products with the highest concentrations, values arise that are less than 10. For chemical substances in consumer products a MOS of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account.
Data has not been found that would render an assessment of a possible mechanical effect of fluortelomer alcohols on the lungs possible. In aerosols consisting of fluortelomer alcohols (with extremely low steam pressure) and solvents with rather high steam pressure the solvent would quickly evaporate – the smaller the aerosols, the quicker the evaporation. In practice that means that aerosols that are inhaled mainly will consist of the heavy volatile proofing agent (fluortelomer alcohols). In concentrated form that could influence the ratio of the surface tension in the lungs and in that way result in a change in the lung function.
8.6 Dodecamethylpentasiloxane
8.6.1 Application
Dodecamethylpentasiloxane is one of several linear polydimethylsiloxanes that when mixed often creates a group of artificial polymers that are among the most produced silicone substances. They are very widespread because of their physical-chemical properties and are used in many connections for production of cosmetics and foodstuffs, for surface treatment and many other things including the production of breast implants. In addition, they are often used in the textile industry and for the production of proofing liquids.
8.6.2 Identification
It has not been possible to find very many physical-chemical data for precisely dodecamethylpentasiloxane, but the substance is one of many linear polydimethylsiloxanes that are very similar to each other. Dodecamethylpentasiloxane is a viscous liquid with a low vapour pressure.
As other polydimethylsiloxanes, the substance is almost insoluble in water but is soluble in methylene chloride, ether, xylene and methyl ethyl ketone (butanone).
No data was found with regard to specific appearance or odour.
| Identification: | |
| Substance name: | Dodecamethylpentasiloxane |
| Synonyms: | |
| CAS no.: | 141-63-9 |
| EINECS No.: | 205-492-2 |
| Molecule formula | C12H36O4Si5 |
| Molecule structure | 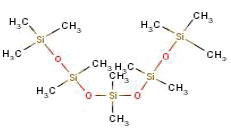 |
| Legislation: Classification according to the list of hazardous substances Limit value of the Danish Working Environment Authority (ppm, mg/m³) |
Not on the list Not on the list |
8.6.3 Physical-chemical data
| Physical-chemical properties | |
| State of matter | Liquid |
| Molar weight | |
| Density | 0.940 g/cm³ at 25°C |
| Melting point | |
| Boiling point | 232°C (ChemlDPlus, 2007) |
| Vapour pressure at 25 ?C | |
| Octanol water (logPow) | 6 |
| Solubility in water | Almost insoluble |
| Odour limit | Not found |
8.6.4 Toxicological data
8.6.4.1 Absorption
The absorption, distribution and elimination of dodecamethylpentasiloxane after one single oral dose were measured in rats. It was calculated that approx. 25 % of an oral dose is absorbed from the gastrointestinal tract. In the course of the first day, approx. 65 % of the administered dose is eliminated; most of it through faeces. In the course of the next 24 hours, an additional 34 % is eliminated. Around 23 % is eliminated with the expiratory air and approx. 2 % with the urine (TOXNET, 1984).
8.6.4.2 Acute toxic effects, humans
The descriptions of effects in humans is to a high degree limited to the use of polydimethylsiloxanes in implants of different kinds or the use of the substances for direct injection in the vitreuos body of the eye in connection with treatment of glaucoma (HSDB, 2007). These are not relevant in this connection.
No reports were found on allergy in connection with polydimethylsiloxanes in cosmetic products (Fischer, 1986).
8.6.4.3 Acute toxic effects, animals
One single oral dose of 600 mg/kg bw has not provoked systematic effects in rats (TOXNET, 1984).
Polydimethylsiloxanes cause irritation in rabbit eyes but do not damage cornea (HSDB, 2007).
8.6.4.4 Subchronic effects
Injected doses of up to 20 mg/kg bw did not give developmental toxicity in rats (HSDB, 2007).
8.6.4.5 Mutagenicity
No genotoxic or mutagenic properties were found of linear polydimethylsiloxanes (HSDB, 2007).
8.6.4.6 Chronic effects
In a two-year investigation on rats with polydimethylsiloxane concentrations in the feed of up to 0.28 % there were no signs of unwanted effects (HSDB, 2007). There is a no observed adverse effect level (NOAEL) of 0.28 % in the feed, corresponding to 140 mg/kg bw/day, as a rat according to OECD eats 20 g feed a day and in average weighs 0.4 kg.
In another test, mice were dosed with polydimethylsiloxane in a concentration of 2.35 % in the feed for 80 weeks. That did not give rise to any significant increase in deaths or significant increase in the number of benign or malignant tumours (HSDB, 2007). As mice according to OECD eat 3 g feed a day and weigh 0.020 kg, 2.35 % in the feed corresponds to a NOAEL of 3525 mg/kg bw/day.
8.6.4.7 Summary
Polydimethylsiloxanes are often referred to as practically inert (biologically and chemically inactive) substances.
Despite the widespread use of linear polydimethylsiloxanes, including dodecamethylpentasiloxane in many industrial connections and consumer products, these substances seem to be very poorly investigated in experiments on animals.
In the light of 2 long-term feed tests, NOAEL values of 140 mg/kg bw in rats and 3525 mg/kg bw in mice, respectively, were found calculated on the basis of the highest tested concentrations in feed.
| Toxicological data (animals) | |
| NOEL, mg/kg bw, oral, rat, acute | >600 |
| NOAEL, mg/kg bw/day, oral, rat, 2 years | >140 |
| NOAEL, mg/kg bw/day, oral, mouse, 18 months | >3525 |
| Toxicological data (humans) | |
| No relevant data found |
8.6.5 Health assessment of dodecamethylpentasiloxane
The content of dodecamethylpentasiloxane could only be determined quantitatively for product no. 18. The product contains 0.66 g/kg.
The absolute worst case scenario is that 1 spray can is emptied in a 20 m³ room and that the person stays in the same room for 8 hours without airing.
During spraying of up to 1 kg of proofing liquid, corresponding to the content in the largest aerosol can that is allowed for non-industrial use, an average concentration of 33 mg/m³ is obtained.
If a human remains in the room for 8 hours 33 mg/m³ x 0.83 m³/hour x 8 hours = 219 mg (European Commission, 2003) is inhaled.
No data states to which high degree polydimethylsiloxanes are absorbed on inhalation, so here it is anticipated that 100 % is absorbed.
A male will therefore be exposed to 219/70 mg/kg bw = 3.13 mg/kg bw and a female correspondingly to 219/60 mg/kg bw = 3.65 mg/kg bw.
The margin of safety (MOS) is calculated in the light of it having been informed that no systematic effects were seen of the individual dose of 600 mg/kg bw in connection with the investigation of absorption from the gastrointestinal tract.
Therefore, MOS amounts to: 192 for males and 164 for females which are acceptable rates. For chemical substances in consumer products a MOS of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account.
8.6.6 Conclusion on the appearance of dodecamethylpentasiloxane in proofing spray
A study with oral absorption, distribution, metabolism and elimination (ADME) of polydimethylsiloxanes in rats has been reported and therefore an acute no observed effect level of 600 mg/kg bw could be determined.
Compared with the worst case scenario, margins of safety (MOS) could be calculated of at least 192 for males and 164 for females for the only spray liquid in which dodecamethylpentasiloxane was measured. These safety margins are acceptable.
Data has not been found that would render an assessment of a possible mechanical effect of fluortelomer alcohols on the lungs possible. In aerosols consisting of fluortelomer alcohols (with extremely low steam pressure) and solvents with rather high steam pressure the solvent would quickly evaporate – the smaller the aerosols, the quicker the evaporation. In practice that means that aerosols that are inhaled mainly will consist of the heavy volatile proofing agent (polydimethylsiloxanes). In concentrated form that could influence the ratio of the surface tension in the lungs and in that way result in a change in the lung function.
8.7 Recapitulation on health assessment and information collection
8.7.1 Chemical substances
In this chapter, health assessments were carried out on 6 substances found either through semi-quantitative screenings or through quantitative analyses of chemical substances in spray products intended for textile proofing. Assessments of the health related conditions were carried out in the light of the worst case scenarios that had been set up.
The assessments demonstrated that the content of organic solvent in these spray products in itself does not compose a health hazardous problem.
The content of polydimethylsiloxane found in one single spray product based on the calculations that were carried out cannot constitute a health hazardous risk.
Based on measurements of substance concentrations that look like a certain fluortelomer alcohol and compared with the small amount of toxicological data that is available for this and similar substances only a very low safety margin was found compared to the worst case scenario that was set up. On the basis of the analysis data, the products should not during use in the present form in themselves compose a health hazardous risk, but for chemical substances in consumer products a margin of safety (MOS) of at least 100 is required and a factor 10 is used to extrapolate from animal studies to exposure of humans and another factor 10 is used to take particularly sensitive groups or individuals into account. It is believed that several of the products do not fulfil that requirement.
In connection with these substances there are additional reasons to recommend cautiousness and to use a large safety margin. The literature study that was carried out by using available information about cases of poisoning caused by textile proofing agents demonstrated that the main part of all registered cases of poisoning precisely have occurred when using spray liquids containing organic perfluorinated polymers.
In addition, the same obersvation was reported by Lyngenbo et al. (2007). That investigation specifies the cases of poisoning that were reported to the Danish Poison Information Centre from 1991 to 2007 and that have involved sprays for surface treatment of many different materials. In 84 of the cases, the majority of the sprays reported for cases of poisoning contained a fluorine compound. However, it is concluded: the cause and mechanism of the lung diseases is not known and prevention of the problem is not straightforward.
Finally, the problem might be greater and more confusing than the analysis results in this project disclose. In connection with the screeing investigations a high content of fluorine was found in more products than in which substances similar to fluortelomer alcohol were analysed. Therefore, an account has only been given for a small part of that fluorine.
However, it is possible that the consumer can be exposed to non-polymerized fluorine compounds in rather high concentrations. The exact identities of the substances are not known but if it is assumed that they can be compared to FTOH 6:2, then they can form a substantial problem which it has not been possible to include in the health assessment that was carried out.
Data has not been found that would render an assessment of a possible mechanical effect of fluortelomer alcohols on the lungs possible. In aerosols consisting of fluortelomer alcohols (with extremely low steam pressure) and solvents with rather high steam pressure the solvent would quickly evaporate – the smaller the aerosols, the quicker the evaporation. In practice that means that aerosols that are inhaled mainly will consist of the heavy volatile proofing agent that in concentrated form that could influence the ratio of the surface tension in the lungs and in that way result in a change in the lung function.
8.7.2 Products
Spray cans are only allowed to contain the propellants and solvents stated in the enclosure to Regulation no. 571 dated 29/11/1984 on the use of propellants and solvents in aerosol cans from the Danish Environmental Protection Agency. In addition, it appears from that enclosure that a number of allowed propellants or solvents must not be used in cosmetics or in products for indoor household use. That means that they must not appear in concentrations of more than 1 % unless the Danish Environmental Protection Agency has given their permission (§8 in the Regulation).
Most of the surveyed spray products are marketed principally for indoor use as none of the products are marked and it has not been stated in any other way that the product must only be used outdoors, e.g ”only for outdoor use”. On other products it is stated that they have to be used in the open or only in places with good ventilation. Directions for use often recommend ventilation at the place of treatment.
8.7.2.1 Butyl acetate in the investigated products
In the enclosure of the previously mentioned Regulation the amount of butyl acetate is stated comprising 1-butyl acetate (n-butyl acetate), 2-butyl acetate and tert-butyl acetate. The 2 last mentioned were not found in any product by semi-quantitative screening. Therefore, butyl acetates must not be used as solvents in spray cans for indoor household use unless the Danish Environmental Protection Agency has given dispensation.
In connection with product no. 3, 14 and 15 the content of butyl acetate has been declared on the safety data sheet. They contain 2, 8 and 3.9 %, respectively. On the safety data sheet of product no. 14 the content of n-butyl acetate has been declared to 1-5 %.
In connection with product no. 1 and 9 the content of butylacetate has not been declared, but they contain 9.8 and 2.3 %, respectively.
In connection with product no. 16 and 25 the content of butylacetate has not been declared. Analyses have shown 0.0058 and 0.0065 %, respectively. The content is very low and therefore it does not have to be declared.
Compared to the rules in Regulation no. 571 dated 29/11/1984 concerning the use of propellants and solvents in aerosol cans product no. 1, 3, 9, 14 and 15 exceed the allowed concentration of butyl acetate in aerosols intended for indoor household use.
8.7.2.2 Butanone in the investigated products
In the enclosure of the previously mentioned Regulation butanone is stated under the description methyl ethyl ketone.
Butanone was identified in product no. 8 and 21 by SPME-GC/MS analysis. However, in the quantitative analyses of spray products butanone was not found in amounts exceeding the detection limit of 0.02 mg/g.
8.7.2.3 1-Butanol in the investigated products
In the enclosure of the previously mentioned Regulation, the amount of butanol is stated comprising 1-Butanol (n-Butanol), 2-Butanol and tert-butanol. The 2 latter were not found in any product by semi-quantitative screening.
1-Butanol was identified in product no. 18, 20, 25 and 26 by SPME-GC/MS screening of all products. In connection with the quantitative analyses 1-Butanol was not found in amounts exceeding the detection limit in analysed products (no. 18, 25 and 26).
8.7.2.4 Cyclohexane in the investigated products
Cyclohexane is stated in the enclosure of the previously mentioned Regulation.
The three analysed products no. 1, 3 and 8 contain cyclohexane in concentrations of 0.65, 0.029 and 0.60 %, respectively. The content is very low and therefore it does not have to be declared.
8.7.2.5 Perfluoroctane-1-ol in the investigated products
Perfluoroctane-1-ol is not stated in the enclosure of the previously mentioned Regulation as it solely deals with propellants and solvents.
Perfluorctane-1-ol was not found in the products. However, screening identified fluortelomer alcohols that are closely related to perfluoroctane-1-ol in product no. 6, 8, 14, 21 and 25 and quantitatively determined in product no. 8, 14 and 21 at 0.61, 0.68 and 0.33 mg/kg, respectively. Based on a worst case scenario, MOS was calculated for product no. 14 to 7.8 for males and 6.7 for females which is less than 1/10 of the MOS of 100 that is required for consumer products. The same goes for product no. 8 and 21.
None of the analysed products declare the content of fluorine compounds as there is no requirement. The consumer could get the impression that the proofing agent itself is low boiling, hydrogenated naftafractions.
8.7.2.6 Dodecamethylpentasiloxane in the investigated products
Dodecamethylpentasiloxane has not been stated in the enclosure of the previously mentioned Regulation.
Dodecamethylpentasiloxane is only identified in product no. 18 and determined quantitavely to 0.66 g/kg. Based on a worst case scenario, MOS is calcualted to 192 for males and 164 for females which is acceptable as a MOS of at least 100 is required for chemical substances in consumer products.
8.7.3 Effects of propellants in spray cans
Cases of toxification when using marketed proofing sprays in Germany, the Netherlands and Switzerland have not led to serious health problems such as respiratory diseases or pulmonary edema if the aerosol mists cannot reach the alveolar tissue in the lungs. In order to reach those parts of the lungs (respirable) the drop sizes have to be less than approx. 4 µm. That drop size is easily obtained when the product is applied when using a propellant and a correspondingly small nozzle in the spray head - as demonstrated in this investigation. When the same liquids are used when using a pump mechanism the drops do not become smaller than approx. 100 µm and therefore they cannot reach the alveolars. A new investigation shows that the registered cases of toxification in Denmark apparently all have comprised products with a propellant (see enclosure 1).
This project has demonstrated that the consumer can be exposed to high local concentrations of aerosol mists with respirable aerosols. In connection with using textile proofing agents considerable concentrations of fine (<1 µm) and ultra fine aerosols (nanoaerosols) (<100 nm) can be created and they must be regarded as being 100 % respirable.
The toxicological effect from inhaling nanoaerosols is not yet known. Existing knowledge in the field cannot document that small aerosols in themselves are harmful. Aerosols can be carriers of (re)active chemical substances, e.g. fluorcarbon monomers, but the effect is not know either, as the chemical structure of the (re)active substances is not known and it has not been possible to determine it on the basis of the chemical analyses that were carried out.
Cases of toxification in Germany with claimed nanoaerosol containing spray liquids have been discussed by a number of German experts (BfR, 2006 a). They could not agree on a final toxicological assessment of the effect on the lungs. The experts pointed out that the classic toxicological assessments of the individual substances in a product are not sufficient when the product is sprayed by means of a propellant. Physical properties, e.g. aerosol size are determining factors for if and which toxicological effect could arise in the respiratory passages. Therefore, it was not possible to disregard the possibility that the observed toxic effects could have arisen solely as a result of the aerosol use, meaning not an effect from inhalation of nanoaerosols.
The experts agreed that the health effects of spray products with propellant only can be determined by means of a test streategy that copies the actual conditions of use indoors. Toxic effects are only seen when the product itself, meaning the complete mixture of substances in the consumer product, is inhaled as a fine aerosol with the corresponding small drop size. That goes for products with as well as without nanoaerosols.
As mentioned, the toxicological effect from inhaling nanoaerosols in not yet known. Several international research activities are taking place concerning the toxicity of nanoaerosols and in a couple of years they will hopefully shed more light on the problem.
8.7.4 Proposals for further investigations
In order to carry out a more complete health assessment and clarify the reasons for the cases of illness that have been observed in Denmark and abroad it is necessary to have:
- an improved experimental basis to describe the toxicity of fluorcarbon compounds.
- an understanding of whether or not the toxicity of substances in aerosol form, including fluorcarbon compounds increases additionally when the aerosol size in the aerosol mists declines to nanosizes (< 0.1 µm).
- develop completely new analysis methods that take the reactivity of the components to be analysed into account.
8.7.5 Good advice to consumers when using textile proofing spray
- As far as possible use textile proofing sprays outdoors. Avoid standing in the wind direction.
- If the product has to be used indoors it is important to provide good ventilation in the room during and after use.
- Only use small amounts indoors.
- Spray for a short period of time and avoid inhaling aerosol mists.
- Keep the spray can as far away from your face as possible.
- Read possible user instructions on the product and follow them carefully.
- Max. use the amount recommended on the product.
- Use pump spray rather than spray with propellants.
- Do not use spray products when children are around.
- Do not let children use spray products.
- If possible, use a dust filter mask and rubber gloves to reduce inhalation and skin contact.
Version 1.0 October 2008, © Danish Environmental Protection Agency