Survey and health assessment of selected respiratory sensitizers in consumer products
3 Results of emission analysis from selected consumer products
- 3.1 Analysis of MDI emission from various consumer products
- 3.1.1 Sample treatment and collection
- Polyurethane one component sealant and adhesive
- Floor adhesive
- Hair conditioner
- Hair spray with polyurethane as declared ingredient
- Polyurethane rain coat
- Foam mattress and spring mattress
- 3.1.2 Method of analysis
- Extraction
- Chromatography
- 3.1.3 Calculation of detection limit
- 3.1.4 Results
- 3.2 Analysis of phthalic anhydride derivatives in consumer products
- 3.3 Analysis of glutaraldehyde in consumer products
The products that were purchased and submitted for analysis are listed in annex 2.
3.1 Analysis of MDI emission from various consumer products
Collection and analysis of air samples were carried out according to OSHA (Occupational Safety and Health Administration, U.S. Department of Labour), method 47 for analysis of MDI in the occupational environment.
Air samples are collected by sucking a known amount of air through a glass fibre filter, which is coated with 1-(2-pyridyl)piperazin (1-2PP). Together with MDI 1-2PP forms a complex, which absorbs UV-light. The filter is extracted with acetonitril:dimethylsuphoxide (90:10) and is analysed by HPLC with UV detector (254 nm).
3.1.1 Sample treatment and collection
The purpose of the collection was to treat the sample in a way as close to the user situation as possible.
Car window adhesive
A car window was placed on a table (see figure 1). The sample was opened, and the adhesive was squeezed out of the container with a joint gun while distributing it along the edge of the window pane. After this, the adhesive was covered with the moulding that protects the window pane during transport, mimicking the placement of the window in the car frame (realistic work scenario).
During the out-squeezing and distribution of the adhesive, air was sampled from a height equal to the position of the nose of the user. Application of the adhesive lasted 12 minutes (realistic work scenario).
After this, the filter was exchanged with an un-exposed filter, and air was sampled right over the adhesive in nose height for two hours.
The remainder of the adhesive was squeezed out of the container, and air was sampled over the adhesive for six minutes (maximum exposure).

Figure 1. Application of adhesive to car window
Polyurethane one component sealant and adhesive
Sealant (160-180 g) was squeezed out in stripes on a non-absorbing surface placed in a plastic tray (see figure 2 below). Collection of sample was carried out 25 cm above the sealant. The collection of air and expression of sealant was started simultaneously and continued for 15 minutes.
Floor adhesive
Approximately 200 g of the thin fluid adhesive was spread over an area of approximately 25x40 cm². (see figure 2 below). Suction took place 25 cm above the surface for 15 minutes counting from the opening of the bottle.
Hair conditioner
A large handful of conditioner (app. 25 g) was spread over an area of app. 25x30 cm² (see figure 2 below). Air was collected 25 cm above the area for 15 minutes counting from the opening of the container.

Figure 2. Hair conditioner. The plastic tray with non-absorbing material was used for sealant, adhesive, hair conditioner, and hair spray.
Hair spray with polyurethane as declared ingredient
Hair spray was sprayed out over a non-absorbing surface of 25x30 cm² placed in a plastic tray (see figure 2 above). Spray for 10 seconds, pause for 10 seconds, and spray for 10 seconds. Air was collected 25 cm above the area for 15 minutes.
Polyurethane rain coat
Collection of air was started as soon as the plastic bag around the rain coat was opened. The rain coat was spread out and turned over and around several times during the air collection. Air was collected 25 cm above the rain coat for 15 minutes.
Foam mattress and spring mattress
Collection of air was started when the plastic cover was removed. The mattress was placed on the floor, and air was collected 25 cm above the surface for 7 hours (see figure 3 below). During this time the mattress was sat on and walked on every half hour.
The sampling air flow velocity was 1 L/min in all samplings.

Figure 3. Collection of air sample over mattress.
3.1.2 Method of analysis
Extraction
The filters were extracted with 4 mL acetonitril:dimethyl sulfoxide (90:10).
Chromatography
The extract was analyzed on HPLC (Agilent 1100)
Method 1:
Column: Zorbax XDB 5μ C8 from Agilent, length 150 mm, diameter 4,6 mm. Mobile phase: 50% acetonitril and 50% 0,05M ammoniumacetate in MilliQ water (pH 6.07), isocratic. Flow velocity 1 mL/min. Temperature 25° C. Injection volume 25 μl.
Retention time 4.333 min.
Method 2:
Column: Prodigy, 5μ from Phenomenex, length 250 mm, diameter 4,6 mm. Mobile phase 50% acetonitril, 50% ammoniumacetate in MilliQ water (pH 6.07), isocratic. Flow velocity 1 mL/min. Temperature 25° C. Injection volume 25 μl.
Retention time 10.98 min.
Detection
Detection was carried out at 254 nm on a G1314A VWD variable wavelength detector from Agilent.
The extracted samples were analyzed against a row of standard solutions of MDI derivatized with 1-2PP in the concentrations 10, 25, 50, 100, 250, and 500 ng/ml. The standard curve was linear within the entire concentration span.
The lowest concentration of standard solution which gave a signal significantly different from the base line was 25 ng/ml.
3.1.3 Calculation of detection limit
The detection limit depends on the collected amount of air. Collection in 12 minutes corresponds to 12 litres of collected air.
The filter was extracted with 4 ml of solvent. 25 ng/ml in 4 ml corresponds to 100 ng in 12 l of air, corresponding to 8 ng/ l air or 8 μg/m³ of air.
The detection limits for the different analyses appear from table 1 below.
3.1.4 Results
In some of the chromatogrammes, which were obtained by the HPLC method 1, one peak was situated very close to the MDI peak. All samples were therefore reanalyzed with HPLC method 2, which showed that there was no MDI in any of the samples. The analytical result for the collected air samples are shown in table 1 below.
Table 1 Concentration of MDI in the collected air samples
| Sample | DMU no. | Collected amount of air, L | Concentration of MDI, μg/m³ | Limit of detection μg/m³ |
| 1 Car window adhesive Realistic work scenario |
ATMI 2006-460 |
12 | n.d. | 8 |
| 1 2 hours following work process |
- | 120 | n.d. | 0.8 |
| 1 Maximum exposure |
- | 6 | n.d. | 17 |
| 11 Sealant |
ATMI 2006-918 |
15 | n.d. | 7 |
| 17 Adhesive /sealant |
ATMI 2006-924 |
15 | n.d. | 7 |
| 6 Floor adhesive |
ATMI 2006-913 |
15 | n.d. | 7 |
| 5 Hair conditioner |
ATMI 2006-912 |
15 | n.d. | 7 |
| 4 Hair spray |
ATMI 2006-911 |
15 | n.d. | 7 |
| 9 Rain coat |
ATMI 2006-916 |
15 | n.d. | 7 |
| 19 Foam mattress |
ATMI 2006-1037 |
420 | n.d. | 0.2 |
| 18 Spring mattress |
ATMI 2006-1038 |
420 | n.d. | 0.2 |
3.2 Analysis of phthalic anhydride derivatives in consumer products
The following phthalic anhydride derivatives are comprised by the analytical method:
Cyclohexane-1,2-dicarboxylic anhydride (unspec.) (HHPA), CAS 85-42-7
Hexahydro-4-methylphtalic anhydride (HHMPA), CAS 19438-60-9
Methyltetrahydrophtalic anhydride (unspec.) (MTHPA), CAS 11070-44-3
Collection and analysis of phthalic anhydride derivatives was carried out after a method described by Welinder and Gustavsson in 1992 (12). The method was described for MTHPA, but it is also applicable to other phthalic anhydride derivatives.
Air samples were collected by sucking a known amount of air through a little glass column packed with glass wool and two layers of XAD-2, which adsorbs the anhydrides. XAD-2 is extracted with toluene which is analyzed for phthalic anhydride derivatives by gas chromatography with mass spectrometry detection (GC-MS).
3.2.1 Sample treatment and collection
The purpose of the sample collection was to treat the samples in a way, which as close as possible to the normal way of application.
Nail Lacquer
A glass plate was placed on top of a drawing of ten rectangles the size of fingernails (1.2 cm x 2 cm). Nail Lacquer was applied in an even, thick layer with the supplied brush, which was dipped in the bottle. Air was collected 25-32 cm above the glass plate for 10 min., and the pump was started when the bottle was opened and the application started (see figure 4 below).
In addition, 10 drops of nail lacquer was put on a watch glass, and air was sucked from right above the watch glass for 10 min. to simulate maximum exposure.
Figure 4: Application of nail lacquer on glass plate. Air is sucked through XAD-2 column, which is placed in nose height over the nail lacquer.
Epoxy adhesive
Epoxy adhesive is a two-component adhesive. Half of each tube was squeezed out on a glass plate, and the two parts were mixed with the supplied spatula or a cotton swab. As long as the adhesive was liquid, it was used for gluing various surfaces (app. 8 min.). The air sample was taken in nose height above the work surface for 10 minutes with start of the pump when the adhesive was first squeezed out (see figure 5 below)

Figure 5: Application of epoxy adhesive. Air is sucked through an XAD-2 column, which is placed in nose height above the work surface.
The sampling air flow velocity was 1 l/min during all collections.
Control of method
A standard solution of the three phthalic anhydride derivatives in toluene was aerated with nitrogen at 37° C. Air was collected just above the surface for 10 min. During this a part of the anhydrides were liberated, and it was made possible to perform a positive qualitative control of the method of collection and extraction.
Analytical method
Extraction: The tips of the XAD-2 column were removed with a pair of pliers. 500 μL toluene was added and the content of the column (XAD and glass wool) was pushed out into a 4 mL capped vial and the column was rinsed with an additional 500 μL of toluene. The extraction was performed for 20 minutes in ultrasound bath. The toluene extract was aspirated with a 1 mL plastic syringe and filtered through a 0.2 μm filter.
Chromatography: The extracts were analyzed by GC-MS (Turbomass from Perkin Elmer)
Column: Rtx 200 MS from Retstek, length 30 m, diameter 0.25 mm, film thickness 0.25μm. Carrier gas: helium with a flow velocity of 2 mL/min. Injection volume 1μL. Injection temperature 300° C. Temperature program of the oven: 100° C for 5 minutes increasing to 230° C with 5° C a minute, hold for 10 minutes, then increasing to 280° C with 25° C a minute, hold for 10 minutes. The column was changed to a new one of same type after analysis of the two first samples.
Detection: The substances were detected by mass spectrometry after electro impact (EI+) ionisation. Collection of masses appears from table 2 below.
Table 2: Analysis parameters for phthalic anhydrides on GC-MS
| Substance | CAS nr | Collection time [Minutes] |
Retention time [Minutes] |
Mass m/z |
| Cyclohexane-1,2-dicarboxylic anhydride (unspec.) (HHPA) | 85-42-7 | 7-12 | 11.43 | 54 67 82 |
| Hexahydro-4-methylphthalic anhydride (HHMPA) | 19438-60-9 | 12-18 | 12.72 12.79 |
54 81 96 |
| Methyltetrahydrophthalic anhydride (unspec.) (MTHPA), | 11070-44-3 | 12-18 | 12.94 13.01 13.36 |
79 93 94 |
The samples were analyzed against a series of standard solutions of 1, 5, 10, 50, and 100 ng/mL. The calibration curve was linear in the entire area of measurement. The lowest concentration of standard solution, which gave a signal significantly different from the noise on the baseline was 5 ng/mL.
Calculation of detection limit
The detection limit depends on the collected amount of air. In the realistic work scenarios the exposure time was 8-10 minutes corresponding to 8-10 litres of air.
The column was extracted with 1 mL solvent. 5 ng/mL in 1 mL corresponds to 5 ng in 8-10 L of air corresponding to 0.5-0.6 μg/m³ of air.
3.2.2 Results of phthalic anhydride measurements
The chromatogrammes for a standard solution and the method control run before change of GC column is seen in figure 6 and 7 below.
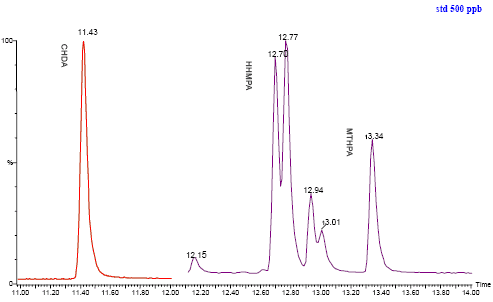
Figure 6: Chromatogram of standard solution of the three phthalic anhydride derivatives.
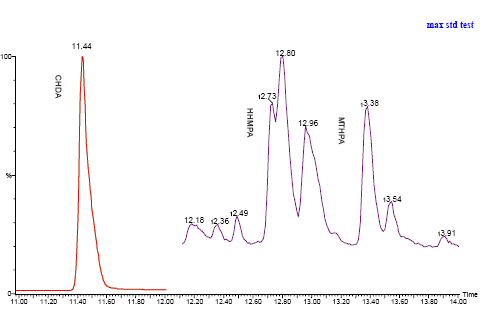
Figure 7: Chromatogram for control of method for collection and extraction of three phthalic anhydride derivatives.
None of the phthalic anhydride derivatives were detected in any of the air samples (see table 3 below)
Table 3: Concentration of phthalic anhydride in selected products
| DMU no. | Collected amount of air [L] | Concen-tration | Detection limit [μg/m³] |
|
| 2 Nail lacquer |
ATMI 2006-461 |
10 | n.d. | 0.5 |
| 3 Two-component adhesive |
ATMI 2006-462 |
10 | n.d. | 0.5 |
| 10 Two-component adhesive |
ATMI 2006-917 |
10 | n.d. | 0.5 |
| 12 Nail lacquer |
ATMI 2006-919 |
10 | n.d. | 0.5 |
| 13 Nail lacquer |
ATMI 2006-920 |
10 | n.d. | 0.5 |
| 14 Nail lacquer |
ATMI 2006-921 |
10 | n.d. | 0.5 |
| 15 Nail lacquer |
ATMI 2006-922 |
10 | n.d. | 0.5 |
| 16 Nail lacquer |
ATMI 2006-923 |
10 | n.d. | 0.5 |
3.3 Analysis of glutaraldehyde in consumer products
Collection and analysis of air samples was carried out according to OSHA method 64 (13) for analysis of glutaraldehyde in the occupational environment.
Air samples were collected by sucking a known amount of air through a silica column (Sep-Pak) coated with 2,4-dinitrophenylhydrazine (DNPH). Together with DNPH glutaraldehyde forms a derivative, which can be analyzed by HPLC and UV-detection.
Sample treatment and collection
The intention with the air collection was to treat the sample in a way that closely resembles the use scenario.
Kitchen roll
Collection of air was begun when the package of 4 kitchen rolls was opened. One roll was rolled out and crumpled up as if it was going to be used for wiping, while the three other rolls were left standing on the table. The collection time was 10 minutes.
Toilet paper
Collection of was begun when the package of 8 toilet rolls was opened. All 8 rolls were put out on the table. One roll was unrolled partly (approx. 2 m). The other rolls were squeezed and turned somewhat during the first 5 minutes. The collection time was 10 minutes.
The flow velocity of the air sampling was 1 L/min. at all collection sessions. The collection was performed with Sep-Pak DNHP-Silica cartridge from Waters (14).
Analytical method
Elution: the Sep-Pak column was eluted with 5 ml of acetonitril.
Chromatography: The eluate was analyzed on HPLC (Agilent 1100)
Column: Nova-pak, 4μ C18 from Waters, length 150 mm, diameter 3,9 mm.
Pre column C18 (Waters).
Method 1
Mobile phase A: 100% acetonitril, B: 0,1% phosphoric acid in Milli-Q water. Flow velocity 1,0 ml/min. Gradient: 55% A increasing to 100% A at 8 minutes. Falling to 55% A at 9 minutes, hold until 20 min.
Method 2
Mobile phase A: 100 % acetonitril, B: 50% metanol +50% MilliQ water. Flow velocity 1 mL/min. Gradient: 40% A increasing to 100% A at 40 min. Falling to 40% A at 41 min, hold until 55 min.
Temperature 25° C. Injection volume: 20 μl.
Detection: The samples were detected at 360 nm on a G1314A VWD variable wavelength detector from Agilent. Retention time: 5.66 minutes.
The samples were analyzed with elution method 1 against standard solutions of 10, 25, 50, 100, 250, and 500 ng/mL. The calibration curve was linear in the entire measuring range. The lowest concentration of standard solution, which gave a signal significantly different from the noise on the base line, was 10 ng/mL.
With elution method 1, the samples and the blank gave a signal in the chromatograms close to glutaraldehyde. The samples were therefore analyzed again with elution method 2, and all samples were analyzed with and without spiking with 500 ng/ml glutaraldehyde. Elution method 2 could separate glutaraldehyde from the peak in the samples. Hence, this peak does not come from glutaraldehyde. The applied method of analysis detects a host of aldehydes and ketones, and one of these may have been present in the ambient air.
Calculation of detection limit
The detection limit depends on the collected amount of air. At the realistic use scenarios the exposure time was 10 minutes corresponding to 10 L of air.
The column was extracted with 5 mL of solvent. 10 ng/mL in 5 mL corresponds to 50 ng in 10 L air corresponding to 5 μg/m³ air.
3.3.1 Results of glutaraldehyde measurements
Table 4. Concentration of glutaraldehyde in selected consumer products
| Product | DMU no. | Collected volume of air [L] | Concen-tration | Limit of detection [μg/m³] |
| 7 Kitchen roll made from recycled paper |
ATMI 2006-914 |
10 | n.d. | 5 |
| 8 Toilet paper made from recycled paper |
ATMI 2006-915 |
10 | n.d. | 5 |
Version 1.0 July 2007, © Danish Environmental Protection Agency