Survey and health assessment of selected respiratory sensitizers in consumer products
4 Exposure and risk assessment
- 4.1 Cyclohexan-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
- 4.2 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
- 4.3 Phthalic anhydride, Methyltetrahydro- (unspec.), CAS 11070-44-3
- 4.4 Methylenediphenyldiisocyanate, CAS 26447-40-5, 5873-54-1 and 101-68-8
- 4.5 Glutaraldehyde, CAS 111-30-8
4.1 Cyclohexan-1,2-dicarboxylic anhydride (unspec.), CAS 85-42-7
Synonyms: hexahydrophthalic anhydride, HHPA
hexahydro-1,3-isobenzofurandione
Molecular formula: C8H10O3
Molecular weight: 154.17
Structural formula:
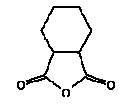
Cyclohexan-1,2-dicarboxylic anhydride (HHPA) is a solid substance at room temperature. (15).
Melting point: 34° C (15)
Vapour pressure: 0.01 hPa at 20° C (15)
Boiling point: 296° C at 1013 hPa (15)
Vapour density: not found
Water solubility: very low solubility in water, and is slowly reacting with water (15).
Odour threshold, air: not found
Conversion factor at 20° C, 1 atm.:1 ppm = 6.293 mg/m³
1 mg/m³ = 0.159 ppm (16)
4.1.1 Hazards
Cyclohexan-1,2-dicarboxylic anhydride (HHPA) has the following classification:
Xi: Irritant
Xn: Sensitising
R41: Risk of serious damage to eyes
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of HHPA in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
As other cyclic acid anhydrides, HHPA is an irritant because of formation of corresponding acids in wet surroundings.
HHPA rarely induces contact allergy of the skin (delayed type hypersensitivity), but more easily induce IgE-mediated contact urticaria. This only comes about after initial respiratory sensitization, and subsequent skin contact.
The mechanism of respiratory sensitization is mainly IgE mediated allergy both in animal studies and when exposed workers have been investigated. In the respiratory challenge tests bronchial obstruction has been verified, as well as development of inflammation (16).
HHPA has caused both sensitization and work-related symptoms at exposure levels as low as 10-50 μg/m³. The level of exposure needed to cause specific IgE antibody production and work-related symptoms in mucous membranes and respiratory organs may be less than 10 μg/m³ (16). No information on the duration needed to induce respiratory sensitization was found.
There is cross sensitivity to MHHPA (see below).
The critical effect is sensitization.
4.1.2 Limit values
No health based limit values for HHPA have been found, but a limit value should be below 10 μg/m³ to ensure absence of sensitizing effect.
4.2 Hexahydro-4-methylphthalic anhydride, CAS 19438-60-9
Synonyms: MHHPA, 5-methyl- hexahydro-1,3-isobenzofurandione
Molecular formula: C9H12O3
Molecular weight: 168.19
Structural formula:
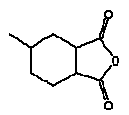
MHHPA is an oily liquid at room temperature.
Melting point: - 30° C- -29° C (16,17)
Boiling point: 120° C at 130 Pa (16)
Vapour density: not found
Vapour pressure: not found
Water solubility: 36 g/l at 20° C (17)
Odour threshold, air: not found
Conversion factor at 20° C, 1 atm.: 1 ppm = 6.865 mg/m³
1mg/m³ = 0.146 ppm
4.2.1 Hazards
MHHPA has the following classification:
Xi: Irritant
Xn: Sensitising
R41: Risk of serious damage to eyes
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of MHHPA in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
As other cyclic acid anhydrides, MHHPA is an irritant because of formation of corresponding acids in wet surroundings. It rarely induces contact allergy of the skin but more easily induces IgE-mediated contact urticaria. The mechanism of respiratory sensitization is mainly IgE mediated allergy both in animal studies and when exposed workers have been investigated. In the respiratory challenge tests bronchial obstruction has been verified, as well as development of inflammation (16).
The critical effect is sensitization.
There is cross-sensitivity to HHPA.
MHHPA has caused both sensitization and work-related symptoms at exposure levels as low as 10-50 μg/m³. The level of exposure needed to cause specific IgE antibody production and work-related symptoms in mucous membranes and respiratory organs may be less than 10 μg/m³ (16).
4.2.2 Limit values
No health based limit values for MHHPA have been found, but a limit value should be below 10 μg/m³ to ensure absence of sensitizing effect.
4.3 Phthalic anhydride, Methyltetrahydro- (unspec.), CAS 11070-44-3
Synonyms: Tetrahydromethylphthalic anhydride, 1,2,3,6-tetrahydro-4-methylphthalic anhydride, MTHPA.
Molecular formula:C9H10O3
Molecular weight: 166.19
Structural formula (1,2,3,6-tetrahydro-4-methylphthalic anhydride, CAS 26590-20-5, shown):
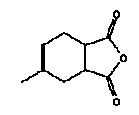
Melting point: -38° C (18)
Boiling point: 150° C at 13.5 hPa, 210° C at 136 hPa (18)
Vapour density: none found
Vapour pressure: not found
Water solubility: 176.4 g/l at 20° C (18)
Odour threshold, air: none found
Conversion factor at 20° C, 1 atm.: 1 ppm = 6.783 mg/m3
1 mg/m³ = 0.147 ppm (16)
4.3.1 Hazards
MTHPA has the following classification:
Xi: Irritant
Xn: Sensitising
R41: Risk of serious damage to eyes
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of MTHPA in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
As other cyclic acid anhydrides, MTHPA is an irritant because of formation of corresponding acids in wet surroundings. It rarely induces contact allergy of the skin but more easily induces IgE-mediated contact urticaria. The mechanism of respiratory sensitization is mainly IgE mediated allergy both in animal studies and when exposed workers have been investigated. In the respiratory challenge tests bronchial obstruction has been verified, as well as development of inflammation (16).
Among MTHPA-exposed workers, even at low levels of exposure (5-20 μg/m³) 56% had allergy symptoms of the eyes and upper airways, 9% had asthma, and 16% had MTHPA specific IgE antibodies. The corresponding numbers were 65%, 11%, and 22% in the more heavily (20-150 μg/m³) exposed groups (16).
The critical effects for MTHPA are irritation of mucous membranes of the eyes and airways and sensitization-induced work-related diseases. Sensitization, work-related rhinoconjunctivitis, and asthma have been verified for workers exposed to MTHPA levels of 5-20 μg/m³ (16).
4.3.2 Limit values
No health based limit values for MTHPA have been found, but a limit value should be below 5 μg/m³ to ensure absence of sensitizing effect.
4.3.3 Exposure and risk assessment for consumers for all the above phthalic anhydride derivatives
The emission of the three phthalic anhydride derivatives was measured during realistic use scenarios for 6 nail lacquers and 2 two-component epoxy adhesives.
The results of these emission measurements were all below the detection limit of 0.5 μg/m³. This is at least 10 times below the lowest level of 5 μg/m³, which has been found to induce respiratory sensitization.
Nail lacquers were chosen for emission measurements, because allergic contact dermatitis cases have been found in the literature. These cases were caused by phthalic anhydride used in the copolymer base of the nail lacquers (19). The nail lacquers chosen were selected among those with phthalic anhydride copolymers listed among the very first on the ingredients list, meaning that the highest concentrations of phthalic anhydride derivatives would be expected in these particular nail lacquers.
Epoxy adhesives were chosen because phthalic anhydrides are known to be part of these products, and occupational exposure via epoxy resins has been reported to cause allergic rhinitis and conjunctivitis (20).
Since no measurable emission from the selected nail lacquers and epoxy adhesives could be found, the risk of respiratory sensitization must be considered to be low.
There is a slight possibility that people who have acquired respiratory allergy from other sources, e.g. occupationally, may react to very minute amounts in consumer products. However, no such reactions toward consumer products have been found in the literature.
4.4 Methylenediphenyldiisocyanate, CAS 26447-40-5, 5873-54-1 and 101-68-8
Synonyms: MDI
The possible isomeric forms of MDI are:
4,4’-methylenediphenyl diisocyanate, CAS 101-68-8
2,4’-methylenediphenyl diisocyanate, CAS 5873-54-1
2,2’-methylenediphenyl diisocyanate, CAS 2536-05-2
Molecular formula:C15H10N2O2
Molecular weight: 250.26
Structural formula (4,4’-MDI, CAS no. 101-68-8 shown):
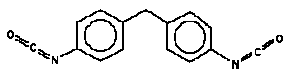
Polymeric MDI is a dark amber viscous liquid while the pure 4,4’ MDI is a white waxy solid. The odour of MDI is slightly musty (10).
Melting point: 34 - 43° C (10)
Boiling point: 314- 364° C (10)
Vapour density: not found
Vapour pressure: <0.014 Pa (2,4’-MDI)
<0.002 Pa (4,4’-MDI)
<0.005 Pa (polymeric MDI), all at 20° C (10)
Water solubility: Determination of the MDI solubility in water is difficult because of the high reactivity of the NCO groups towards OH groups, e.g. in water giving rise to aromatic amines. Consequently it is not possible to measure the solubility of MDI in water using the EC standard methods.
Odour threshold, air: none found
Conversion factor at 20° C, 1 atm.: 1 ppm = 10.22 mg/m3
1 mg/m³ = 0.098 ppm (21)
4.4.1 Hazards
MDI has the following classification:
Xn: Harmful
Xi: Irritant
Xn: Sensitising
R20: Harmful by inhalation
R36/37/38: Irritating to eyes, respiratory system, and skin
R42/43: May cause sensitization by inhalation and skin contact
The occurrence of MDI in chemical consumer products (preparations), other than cosmetics, will not appear on the label, when the concentration is below 0.1%. However, even below 0.1% the product should be labelled. “Contains isocyanates. See information provided by the manufacturer”. If the product is an article there is no such requirement for labelling.
In the EU risk assessment report (10) a different classification was proposed:
Xn: Carcinogenic, category 3
Xn: Harmful
Xi: Irritant
Xn: Sensitising
R20: Harmful by inhalation
R36/37/38: Irritating to eyes, respiratory system, and skin
R42/43: May cause sensitization by inhalation and skin contact
R 40: Limited evidence of a carcinogenic effect
R 48/20: Harmful: danger of serious damage to health by prolonged exposure through inhalation
Strictly speaking MDI should be classified as toxic by inhalation on the basis of a 4-hour LC50 of 490 mg/m³. However, a consensus was reached among European experts (Directive 67/548/EEC; 25th ATP, i.e. Dir. 98/8/EC, O.J.30.12.1998) to
consider this value as irrelevant in terms of real-life exposure, because such high values are said not to be achievable except under experimental testing conditions. This pragmatic reasoning is acceptable provided that such high concentrations are indeed never achieved, even through misuse or (further) technological changes in work processes. Consequently it is proposed to classify MDI as harmful by inhalation. Taken together, in terms of pure hazard characterisation MDI is toxic by inhalation. However, if one considers the exposure assessment, it is reasonable to consider MDI as harmful only and to apply the risk management phrase ‘harmful by inhalation’(10).
It should be noted that since the vapour pressure of MDI (4,4’) is only 0.002 Pa at room temperature it is only possible to reach a saturated air concentration of 0.0197 ppm or 0.2 mg/m³. In order to reach a concentration of as much as 490 mg/m³ it is necessary to heat the MDI, which in turn will condensate and form particles, not vapour, in the indicated concentration.
For irritant effects a NOAEL of 0.5 mg/m³ was found (10).
Animal data as well as studies in humans provide clear evidence of possible skin sensitization due to MDI. Animal studies indicate that MDI is a strong allergen. Human case reports describe the occurrence of allergic contact dermatitis due to MDI skin exposure (10).
MDI is a potential respiratory sensitizer in animals and humans. Animal studies have shown that respiratory sensitization can be induced by skin contact with MDI. The quantitative relationships between exposures (concentration, duration, rate of exposure, route of exposure) have not been established. At the present time it is not possible to define reliable exposure-response relationships with regard to the risk of sensitization for MDI. The current knowledge/state of the art in this field does not yet allow deciding a threshold level for sensitization. Because animal data support the hypothesis that respiratory hypersensitivity may be induced by skin contact and because such possibility has not been excluded in studies involving humans, it is reasonable to consider that it is not only important to reduce inhalation exposure but also to avoid skin contact (10).
The mechanism behind isocyanate-related hypersensitivity is still obscure. Several publications indicate that complex immunological reactions are involved in the sensitization process to MDI. Immediate allergic, late allergic and dual-phase responses can occur. Humoral as well as cellular immunity may be involved in the pathogenesis of hypersensitivity due to isocyanates. The specific humoral response can be IgE as well as IgG mediated. Cross-reactivity with other isocyanates has been described in several publications (10).
There is inadequate evidence of carcinogenicity in humans and limited evidence in experimental animals (10), which is the reason the EU risk assessment report proposed as classification as carcinogenic in category 3.
In conclusion, dermal and respiratory sensitization seems to be the most critical effects.
4.4.2 Limit values
The Danish occupational limit value is 0.005 ppm or 0.05 mg/m³ (22). This is a time weighted average (TWA) over 8 hours. In practise, the Danish ceiling limit (for a random 15 minute measuring period) is twice the TWA, i.e. 0.01 ppm or 0.1 mg/m³.
The American Conference of Governmental Industrial Hygienists (ACGIH) has recommended the same limit as a time weighted average (21). The ACGIH notes that the recommended limit may not necessarily protect susceptible workers from possible sensitization or an allergic reaction in previously sensitized individuals.
The Occupational Safety and Health Administration (OSHA) of the US department of Labor has set a permissible exposure limit of 0.02 ppm or 0.2 mg/m³ as a ceiling limit (23).
4.4.3 Exposure and risk characterisation for consumers
The EU risk assessment report finds that as respiratory hypersensitivity may be induced by skin contact, respiratory and skin sensitisation due to MDI cannot be excluded during spray painting, the use of one component foam, during gluing or using a putty/filler cartridge or during the use of a hot melt adhesive. However, there is already sufficient information available upon which to base a conclusion (iii) for this endpoint for all scenarios: there is a need for limiting the risks; risk reduction measures which are already being applied shall be taken into account. (Specific attention should be paid to the situation where a subject has an occupationally acquired sensitisation to MDI) (10).
The EU risk assessment report considers chronic toxicity from the use of consumer products containing MDI of less concern as consumer exposure of the identified products is expected to occur on occasional events of short duration. For chronic toxicity and carcinogenicity, conclusion (ii) is reached for all scenarios: there is at present no need for further information or testing or risk reduction measures beyond those which are being applied already (10).
In the present investigation we were not able to find hot melt adhesive containing MDI nor MDI containing spray paint for private consumers on the Danish market.
The products that were tested for emission of MDI were: car window adhesive, mattresses, one component adhesives and sealers, polyurethane rain-coat, floor adhesive, and a hair conditioner.
No emission of MDI could be detected under the testing conditions.
We have not examined exposure scenarios involving the grinding or thermal removal of MDI containing material. Such secondary exposure is known to be a hazard in the occupational setting (9), but only normal, predictable consumer exposure has been the scope of this project.
With limits of detection varying from 0.2-17 μg/m³ and no emission found in the different exposure scenarios, the exposure is well below the occupational exposure limit of 50 μg/m³. Hence, the risk of inducing hypersensitivity during the use of the available consumer products made with MDI seems very low.
It is important that consumers follow instructions to avoid skin contamination, since dermal sensitization can lead to general sensitization, thus causing risk of respiratory allergy with asthma-like symptoms upon later exposure by inhalation.
In the case of already acquired hypersensitivity towards MDI or other isocyanates, occupationally or accidentally, even small exposures by inhalation from consumer products like window sealing foams, may cause an outbreak of respiratory allergy with asthma-like symptoms (10).
4.5 Glutaraldehyde, CAS 111-30-8
Synonyms: Glutaral; 1,5-pentanedial
A colourless oily liquid. Commercial solutions often have an amber tint and an odour similar to spoiled fruit.
Molecular formula: C5H8O2
Molecular weight: 100.12
Structural formula:
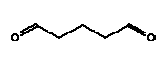
Boiling point: 187-189° C (with decomposition)
Vapour density: 3.4 (air=1)
Vapour pressure: 16.5 mmHg (2.2 kPa) at 20° C. There is some confusion
about the purity of the test substance used: IUCLID (1996) gives the same value for pure glutaraldehyde and for a 50% aqueous solution, ACGIH (1991) gives a vapour pressure of 0.0152 mmHg (2.0 Pa at 20° C) for a 50% solution. In
SUBFAC (a computer model), vapour pressures (20° C) has been calculated to 24.9 mmHg for a 100% solution, 19.4 mmHg for a 50% solution, and 2.7 mmHg for a 2% solution (24). In 2001, ACGIH gave a vapour pressure of 0.102 mmgHg for a 50% solution, and 0.003 mmHg for a 2% solution (21).
Odour threshold, air: 0.14 ppm (0.17 mg/m³)
Conversion factor at 20° C, 1 atm.: 1 ppm = 4.2 mg/m³
1 mg/m³ = 0.240 ppm
Data taken from (24).
4.5.1 Hazards
Glutaraldehyde has the following classification:
R23/25: Toxic by inhalation and if swallowed.
R34: Causes burns.
R42/43: May cause sensitization by inhalation and skin contact.
R50: Very toxic to aquatic organisms.
with the following classification limits in products:
| Concentration | Classification |
| C = 50 % | T, N; R23/25-34-42/43-50 |
| 25 % = C < 50 % | T; R22-23-34-42/43 |
| 10 % = C < 25 % | C; R20/22-34-42/43 |
| 2 % = C < 10 % | Xn; R20/22-37/38-41-42/43 |
| 1 % = C < 2 % | Xn; R36/37/38-42/43 |
| 0,5 % = C < 1 % | Xi; R36/37/38-43 |
The occurrence in chemical consumer products, other than cosmetics, will not appear on the label, when the concentration is below 0.1%.
Inhalation studies in mice and rats showed no carcinogenic activity when exposed to air concentrations of 62.5 – 250 ppb (0.0625 – 0.250 ppm) 6 hours a day, 5 days a week for 104 weeks (25).
Inhalation allergy in the occupational setting has occurred in several cases, particularly during cold sterilization of hospital equipment and use of glutaraldehyde containing chemicals during radiographic processing. Symptoms vary from watering of eyes, rhinitis, respiratory difficulty, nausea to headache. The vapours from glutaraldehyde may act as an irritant to bronchial and laryngeal mucous membranes, and prolonged exposure could produce localized edema and other symptoms of allergic response, including asthma. In these cases, the air concentrations in the breathing zone varied from 0.05 ppm to 0.12 ppm. In the cases where information on air concentration was not given, the workers had worked with preparations containing 2 – 3.6 % glutaraldehyde (25).
Numerous symptoms have been found in individuals with exposure to less than 0.05 ppm glutaraldehyde, which is the recommended peak exposure limit in many countries (26). One such case was described in a 61-year-old nurse, an ex-smoker, who began working in a renal dialysis unit in 1976 (22 years before).
Ten years later, she experienced sporadic and mild episodes of chest tightness and shortness of breath related to exposure to formalin, which was used to sterilize artificial kidney machines. In 1994, formalin was replaced with 2% glutaraldehyde, which she handled daily in an open environment. She was symptomless until February 1998, when she developed symptoms of irritation of the eyes and upper respiratory tract, dyspnea on exertion, dry cough, and episodic attacks of wheezing, which she associated which glutaraldehyde exposure.
Her symptoms were progressively severe, and she had an acute asthma attack requiring hospitalization. She then took sick leave, during which she slowly recovered. After 3 months with medical treatment, she was symptom free, and she was clinically evaluated.
She underwent a the specific bronchial challenge test with activated 2% glutaraldehyde aqueous solution painted on a cardboard in a 7 m³ challenge chamber for 10 min. No changes FEV1 (forced expiratory volume in one second) were observed in a 24 hour monitoring period. However, at the end of this period, the methacholine inhalation test became positive (PC20 0.74 mg/ml, PC20: precise concentration of methacoline where FEV1 falls by 20%). One week later, the challenge test with 2% glutaraldehyde was repeated, and it elicited an early asthmatic response. Although no late reaction was observed, a recurrent nocturnal asthmatic reaction occurred in the following days.
Similar cases of rhinitis and asthma have been diagnosed by specific bronchial challenge with glutaraldehyde concentrations in the range of 0.064-0.081 mg/m³, which is well below the ceiling limit of 0.8 mg/m³ (0.2 ppm) in Denmark or even the more stringent 0.05 ppm ceiling limit in many other countries. Cross reactivity between formaldehyde and glutaraldehyde has been suggested.
The type of mechanism responsible for glutaraldehyde induced asthma is not known because IgE specific antibodies have not been demonstrated in affected subjects, or can be detected in only a small percentage of workers with symptoms related to work with glutaraldehyde (27).
According to the American Conference of Industrial Hygienists (ACGIH) no clear dose-response relationships in humans have been established for airborne glutaraldehyde exposure. Reported industry experience indicates an absence of glutaraldehyde-induced skin or respiratory sensitizations for workers routinely exposed to airborne glutaraldehyde concentrations ranging from 0.01 to 0.34 ppm (21,26).
4.5.2 Limit values
In Denmark, the occupational exposure limit is 0.2 ppm (0.8 mg/m³), and this a ceiling limit (22).
The American Conference of Industrial Hygienists (ACGIH) recommends a threshold limit value – ceiling of 0.05 ppm (21).
In Denmark, a general limit (a so-called C-value) of 0.001 mg/m³ for ambient air has been calculated (24). The C-value is a limit value for how much an installation may contribute to air pollution.
4.5.3 Exposure and risk characterisation for consumers
In general, it was very difficult to obtain consumer products with a content of glutaraldehyde. No cosmetic products were found, even though glutaraldehyde is a permitted ingredient with restrictions.
Glutaraldehyde may sometimes be used as a disinfectant during the manufacture of paper towels, toilet paper and the like. The two samples taken did not emit any detectable amounts of glutaraldehyde, i.e. the emission was below 5 μg/m³. This is well below the Danish occupational exposure limit, and at the level of general limit for ambient air.
Hence no risk of respiratory sensitization to glutaraldehyde can be attributed to these paper samples.
No cases of sensitization to glutaraldehyde in consumer products have been found in the literature.
The risk of respiratory sensitization to glutaraldehyde via consumer products must be considered very low, both because of low availability, non-detectable emissions, and absence of cases in the literature.
It is possible that consumers with already acquired sensitization towards glutaraldehyde or formaldehyde from other sources may react to even small residual concentrations of glutaraldehyde in consumer products. However, since no such cases were found in the literature to confirm this, the risk must be considered very low.
Version 1.0 July 2007, © Danish Environmental Protection Agency