Survey and risk assessment of chemical substances in deodorants
5 Risk assessment
- 5.1 Deodorants and contact allergy
- 5.2 Risk assessment – in general
- 5.3 The selected fragrance substances
- 5.4 Farnesol
- 5.5 Comments concerning other fragrance substances
- 5.6 Allergen load of fragrance substances
- 5.7 Triclosan
5.1 Deodorants and contact allergy
The use of perfumed deodorants is associated with an increased risk of perfume allergy. In a study of 925 eczema patients and a control group of 806 persons, randomly selected from the population, a statistically significant correlation was found between skin rash to a perfumed deodorant as first time symptom (odds ratio: 2,3-2,9) and later diagnosis of perfume allergy (14). In a German study, eczema patients were patch tested with their own deodorants (15). All-in-all 1069 deodorants were tested, of these 6.7% produced allergic reactions. There was a statistically significant correlation between an allergic reaction to ones own deodorant and perfume allergy, among these allergy to HICC and cinnamal (15).
The environment in the armpit is moist and occluded, which like the presence of hair follicles can increase penetration of certain allergens (16-18). Shaving also increases penetration and thus the risk for skin allergy (19). In a case study, 14 perfume allergic patients were asked to use one of their own deodorants in the armpit and as well as on the upper arm for one week. Twenty deodorants were tested, and 12 of these (60%) produced eczema in the armpit, while only 4 (20%) produced eczema on the upper arm (20). The deodorants, which gave a positive test, contained 1.3-8.6 times higher concentration of allergenic fragrance substances than those products which were negative in the study (20)
Deodorants are available in different formulations, such as aerosol sprays, roll-ons, and sticks. There may be differences in bioavailability of allergenic fragrance substances in different formulations of deodorants. In a small study, a deodorant spray and a deostick with the same concentrations of allergenic fragrance substances were tested in the cubital fossa of 7 perfume allergic patients. Five of these persons reacted to deospray, while only one reacted to deostick. (21). The influence of deodorant matrix on allergy has not been investigated systematically. Other products are also important for perfume allergy, especially perfumes, colognes, aftershave lotion and creams/lotions (14-15).
5.2 Risk assessment – in general
Exposure in the form of dose/cm² of an allergen is a decisive determinant for the development of allergy (22, 23). Thus, the concentration of allergen in a given product is important. The EU Commission has published guidelines for risk assessment. The standard dose of deodorant is 0.5 g/day on a total surface area of 100 cm², i.e. 5 mg/ cm² (24). The Research Institute for Fragrance Materials (RIFM), which is financed by the perfume producers association called International Fragrance Association (IFRA) use, in a new model for risk assessment, 9.1mg/cm²/day. In several risk assessments, different doses and surface areas have been used (18, 24, 25), for example the total skin area exposed to deodorants has been estimated to be 100 cm² -240 cm².
In a recent study, the use of 6 different cosmetic products by American women of 19-65 year of age was measured during a 14 days period (26), including use of
deostick. The mean dose per application of deostick was 0.61 g (median 0.45 g), which corresponds to standard dose, and the average application frequency was 1.3 per day (0-4). The 10% of the participants, who were the most frequent users, used deodorant as a minimum twice a day (26). Other deodorant formulations were not investigated in this study. Other factors, than allergen dose, are of importance for development of allergy, e.g. the skin area of the body, vehicle, simultaneous ocurrence of skin irritants in the formulation and preexisting eczema (27).
Since 1973, the perfume industry’s’ association IFRA has published recommendations for the use of allergenic fragrance substances. (28), which are based on tests conducted in healthy voluenteers. In their earlier used model, which is still in force for the majority of the substances, the scientific rationale for the recommendations was seldom given. The problems of fragrance allergy are a consequence of these recommendations being insufficient (29, 30).
Toxicologists from cosmetic industry have worked several years on the development of a model for risk assessment. This is based on the results of a predictive test in mice, the so called Local Lymph Node Assay (LLNA), in some cases supplemented with tests performed in healthy voluenteers (18, 23, 31). The potential of a substance to induce skin allergy is predicted from the data obtained through these tests (31, 33). The basis for the calculation includes exposure estimate and uncertainty factors (18, 32).
The risk assessment model developed by the scientists in the cosmetic industry includes scientific elements and it establishes safe limits, but it is not validated with respect to whether these limits will be able to prevent allergy. Besides, the model aims exclusively at prevention of new cases of allergy and it does not take into account the existing allergy problem in the population. It has been demonstrated that limit values, which are based on data from tests performed in persons with allergy, are very effective in preventing new cases of allergy as well as in minimizing the consequences of the disease in persons, who have already developed allergy (27). The results of such dose-response investigations employing allergic persons have been shown to be highly reproducible, even when these are performed in different clinics and in different European countries (34).
The risk assessment presented here, is based on the clinical data derived from tests employing patients with allergy. For comparison, an example of risk assessment based on animal experiments and an overview of actual limit values, which are in force or are proposed as official limit values as well as those recommended by perfume industry’s’ organisations (RIFM/IFRA), are presented.
5.3 The selected fragrance substances
5.3.1 Hydoxyisohexyl 3-cyclohexene carboxaldehyde (HICC)
Hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC) with the trade name Lyral®, is one of the most frequent causes of perfume allergy. In studies performed in European Dermatology clinics, 1.6%-2.7% of eczema patients have been found to be allergic to HICC (35, 36). In a multicenter study in Germany, 1.9% of eczema patients were found to be allergic to HICC (37) without any significant sex difference. Similar results have also been found in the Danish monitoring network of dermatologists. Here, 2.3% of men and 2.6% of female eczema patients were found to be allergic to HICC in 2005; and in 2006, 1.3% of men and 2.7% of female eczema patients were allergic to HICC (38). The frequency of HICC allergy among the tested eczema patients in a dermatology department in Copenhagen is shown in Figure 1. There is a tendency that patients with HICC allergy have eczema in the armpits more frequently than other eczema patients, p = 0.065 (37).
Figure 1. Frequency of eczema patients with allergic reaction to selected fragrance substances among all eczema patients (n = 3179) allergy tested at the Dermatology Department of Gentofte Hospital in the period 2002-2005 (unpublished results)
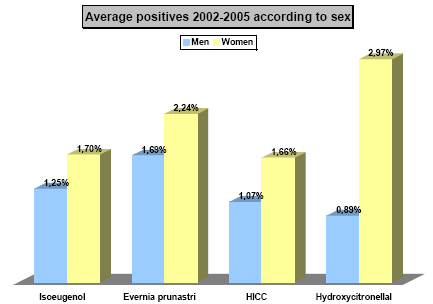
HICC: Hydroxyisohexyl 3-cyclohexene carboxaldehyde (HICC). Isoeugenol and Evernia Prunastri extract (oak moss abs.) are tested at 1 %, while hydroxycitronellal and HICC are testet at 5 % in petrolatum.
HICC is included in the new perfume mixture, fragrance mix II, which is routinely used for the diagnosis of perfume allergy. Furthermore, it is recommended to test separately with HICC, because it is a very frequent allergen (36).
In a European experimental study, 18 HICC allergic persons were patch-tested with a serial dilution of HICC (6%-0.0006%), i.e. tested under occlusion for 48 hours (39). It was possible from the dose response curve to calculate the dose, which will cause an allergic reaction in 10% of allergic patients. This dose was found to be 29 ppm HICC (CI: 7-69) (39). In a later investigation employing a group of Danish eczema patients, an identical dose-response curve was obtained (7), i.e. 10% reacted to 25 ppm HICC (CI: 0.8-120).
HICC allergy is less frequent in North America, where this allergy has been demonstrated in approximately 0.4 % of eczema patients. The possible explanation is that HICC is less frequenly used in deodorants in North America, and when used, it is in lower concentrations (40), while HICC is shown to be very common on the European market. In a study in 1996, HICC was found in 53% of deodorants (6), and in the present study HICC was present in 33% of the deodorants (Table 2). On the basis of the high frequency of allergy to HICC, EU’s Scientific Committee on Consumer Products (SCCP) has recommended 200 ppm as maximum amount of HICC in cosmetic products (41) (Table 8).
Table 8. Recommendations for limit values of selected fragrance substances by the EU Scientific Committee (SCCP) and perfume industry’s organisation IFRA.
| Fragrance substance | SCCP* | Year | IFRA** | Year |
| HICC | 200 ppm*** | 2003 | 15.000 ppm | 2004 |
| Hydroxycitronellal | 10.000 ppm | 2001 | 10.000 ppm | 1992 |
| Isoeugenol | 200 ppm | 2001 | 2000 ppm 200 ppm |
before 1998 1998 |
| Cinnamal | 1000 ppm | 2001 | None 500 ppm |
before 2007 2007 |
| Cinnamyl alcohol | 8000 ppm | 2001 | 8000 ppm 4000 ppm |
before 2004 2004 |
* EU Scientific Committee on Consumer Products.
** International Fragrance Association publishes guidelines and establishes limit values for the use of fragrance substances with regard to health effects. Limit values in Table 8 are established on the basis of allergenic effects (28).
*** Recommended through SCCP Opinion on HICC (41), but not yet implemented.
The limits of other substances are taken from the public hearing, prior to implementation in Cosmetic Directive (46).
In the present investigation, 6 deodorants were found to contain more than 200 ppm HICC. This means that approximately 1/3 of the analysed deodorants and at least 6.8% of the 88 perfumed deodorants, within this study, contained more HICC than recommended due to the risk of allergy (41). There was one deodorant, which contained 4431 ppm HICC, i.e. more than 20 times the limit recommended by the SCCP (41), and more than twice the maximum concentration (1874 ppm) which was found in a similar study 10 years back (6). Use of deodorants implies a special kind of exposure as described above, and the mentioned 200 ppm limit value of HICC is based on a model calculation. This limit value has been tested later in a study employing 14 HICC allergic persons and 10 controls (7). They were supplied with a deodorant containing 200 ppm HICC for the use two times daily for 14 days in one armpit, and a similar deodorant without HICC to be used in the contralateral armpit. Within 14 days, 9/14 (69%) of the HICC allergic persons developed an allergic eczema soley in the armpit where HICC containing deodorants had been applied. A control group of healthy voluenteers used deodorants in the same way, but none developed eczema (Table 9). Thus, there was a statistically significant correlation between the exposure to 200 ppm HICC in a deodorant and development of eczema (p=0.02). When the exposure dose was increased from 200 ppm HICC to 600 ppm and 800 ppm, all persons in the test group developed eczema, but none in the control group (Table 9). The amount of the deodorant used per day was estimated as realistic (7). It was concluded that 200 ppm is not a safe limit value for HICC in deodorants with regard to elicitation of the allergy, and it should be considered whether HICC should be used at all in this type of product (7). HICC was present in 33% of the perfumed deodorants in the present study.
Table 9. Overview of results of deodorant provocation investigations with different allergens. Frequency in % of test groups, which reacted at different doses of allergen applied in a roll-on antiperspirant, is given.
| Isoeugenol | Cinnamal(1) | Cinnamal(2) | Hcitron | HICC | |
| Dose in ppm | |||||
| 0 | 0 | 0 | 0 | 0 | 0 |
| 63 | 23 | ||||
| 100 | 11 | ||||
| 200 | 69 | 64 | |||
| 320 | 75 | 55 | 57 | ||
| 600 | 85 | ||||
| 630 | 76 | 100 | |||
| 1000 | 99 | 71 | |||
| 1800 | 100 | ||||
| 3200 | 100 | ||||
| No. test persons | 13 | 8 | 9 | 7 | 14 |
| No. of control persons | 10 | 20 | 7 | 10 | |
| % control persons, who reacted | 0 | 0 | 0 | 0 | |
| Exposure should be: | < 63 ppm | <100 ppm | <320 ppm | < 200 ppm | |
| Reference | (10) | (9) | (8) | (7) | |
Hcitron: Hydroxycitronellal; HICC: hydroxyisohexyl cyclohexenecarboxaldehyde (Lyral®).
As a major part of the test persons reacted to deodorants with the lowest concentration of HICC, it was attempted to statistically evaluate the data to determine a concentration level, which can elicit allergic eczema in 10% of the allergic persons, the so called ED 10% (Table 10). Although the data permits such a calculation, i.e. the logistic dose-response model is statistically acceptable; the relative low number of test persons influences the strength and give a low precision of the estimates as well as large confidence intervals. For HICC, it was estimated that 10% of HICC allergic patients will react to the use of a deodorant with 29 ppm HICC in the product (Table 10). Only four of the analysed deodorants in the present study contained less than 29 ppm HICC. The estimates for the other selected fragrance substances have also a low presicion and therefore, these data are not used further in the risk assessment.
Table 10. The allergen concentration in deodorant (roll-on), which provokes allergic eczema in persons with allergy to the substance. Estimates on the basis of fitted dose-response curves with corresponding confidence intervals (58).
| Substance | ED10 % (95 %CI) | ED25 % (95 % CI) | ED50 % (95 % CI) | Ref. |
| HICC | 29 ppm (<5ppb-89) | 62 ppm (0,05-148) | 136 ppm (4,3-296) | 7 |
| Hydroxycitronellal | 38 ppm (*) | 103 ppm (*) | 276 ppm (*) | 8 |
| Isoeugenol | 20 ppm (0,4-56) | 53 ppm (5,8-130) | 146 ppm (47-478) | 10 |
| Cinnamal (1+2) | 109 ppm (39-169) | 169 ppm (86-245) | 263 ppm (170-397) | 9 |
ED: Elicitation dose (concentration) for a defined part of the test population, for example 10 %.
CI: Confidence interval.
*: can not be calculated. Group size in some studies is small (see Table 9), thus there is a considerable uncertainty of the estimates.
For comparison, the levels of allergenic fragrance substances, which will be acceptable according to the risk assessment model used by the cosmetic/perfume industry, are presented in Table 11. Characteristic for this model is that as a starting point it uses the amount of the allergenic substance which can induce allergy in experimental animals (mice) or in healthy voluenteers. The model, which can be used in different ways, is based on several assumptions. Furthermore, the model is not validated with regard to the levels of the allergens which in fact are effective in prevention of development of new cases of allergy, and it does not consider the existing allergy problems in the population. Generally, the levels which will be considered acceptable according to this model will be higher than those based on clinical data. This is also seen for HICC, where the safe limit value is calculated to 2000 ppm. (Table 11).
Table 11. Example of risk estimates for induction. Dose per application (concentration in deodorant), which is theoretically acceptable and calculated on the basis of induction experiments using animals/healthy voluenteers (59).
| Fragrance substance | Potency (31,59) | Reference dose* | Acceptable conc. |
| HICC | Weak | 1 µg/cm² | < 2000 ppm |
| Hydroxycitronellal | Weak | 1 µg/cm² | < 2000 ppm |
| Isoeugenol | Moderate | 0,1 µg/cm² | < 200 ppm |
| Cinnamal | Moderate | 0,1 µg/cm² | < 200 ppm |
*The reference dose for sensitisation is based on the potency of the substances (derived from experiments) as default values and uncertainty factors. In the calculation, the maximum possible uncertainty factors have been used: a factor 10 for inter-individual variation, a factor 10 for difference in matrix between the experiment and the real product, a factor 10 for experimental conditions (armpit), all together 1000. The acceptable concentration is calculated from exposure (0.5 g deodorant/day on a total of 100 cm² skin and the reference dose, so that the ratio of the reference dose and exposure in µg/cm² is below 1.
The perfume industry (IFRA) has since April 2004 recommended that maximum 1.5 % (15.000 ppm) HICC should be used in cosmetics (28), a limit, which is associated with risk of allergy, independently of the model used for the risk assessment (Tabel 9-11).
5.3.2 Hydroxycitronellal
Hydroxycitronellal is included in fragrance mix I, a perfume mixture containing eight fragrance substances, which is used for the diagnosis of perfume allergy. It is the 3rd most frequent cause of allergy among the 8 substances in the mixture (42). It is responsible for allergic reactions on an average in 13% of the test persons, who show a positive reaction to fragrance mix I or who are suspected of fragrance allergy. In a European study from the beginning of 1990’s, 0.75% of 1072 eczema patients reacted to hydroxycitronellal (43). Danish data reveals that among eczema patients tested at a dermatology department in Copenhagen, 3% among women and 0.9% of men show an allergic reaction to hydroxycitronellal (Figure 1).
The perfume industry’s association IFRA has for several years recommended the use of maximum 1% hydroxycitronellal in the products, because of its allergenic properties (28). This limit is based on experiments employing healthy voluenteers (44). In the present investigation, hydroxycitronellal was found in 27% of the products with up to 0.17%; chemical analysis of the 23 selected products revealed a median concentration of 203 ppm hydroxycitronellal.
In a case study, it was found that persons with the diagnosis of hydroxycitronellal allergy had used products containing an average of 0.18% hydroxycitronellal compared to products containing 0.032 % used by the persons, who did not have hydroxycitronellal allergy (45). In a provocation study with 14 eczema patients, among these seven with allergy to hydroxycitronellal, the patients were supplied with two deodorants in a blind and randomized way, one with hydroxycitronellal and the other without hydroxycitronellal (8). Start concentration of hydroxycitronellal was 320 ppm (0.032%), which was applied, in an armpit, two times daily up to 14 days; and the unperfumed deodorant was applied in the contralateral armpit as control. None in the control group reacted, while all who were allergic to hydroxycitronellal reacted to deodorants containing hydroxycitronellal: four persons to the deodorant with 320 ppm (0.032%) hydroxycitronellal, one to 1000 ppm (0.1%) hydroxycitronellal and yet two to the deodorant containing 3200 ppm (0.32%) hydroxycitronellal (Table 9). The amount of deodorants used was estimated as realistic (8). This means that approximately half of the patients will react to concentrations, which are found in 10% of the deodorants (8/88) on the market. This is a pattern similar to HICC, which is structurally related. The study employing allergic patients indicated that deodorants should not contain more than 320 ppm hydroxycitronellal (8). Concentrations at this level or higher were found in six products, corresponding to approximately 1/3 of the products declared to contain hydroxycitronellal and 6.8% of the of the 88 perfumed products included in the study.
5.3.3 Isoeugenol
Isoeugenol is a moderate/strong allergen. It is included in fragrance mix I, a perfume mixture containing eight fragrance substances, which is used for the diagnosis of perfume allergy. This is the second most frequent allergen among the eight substances in the mixture (42). It is responsible for allergic reactions on an average in 18.9% of the tested persons, who show a positive reaction to fragrance mix I or who are suspected of having fragrance allergy (42). Danish data show that among eczema patients tested at a dermatology department in Copenhagen, 1.5% show an allergic reaction to isoeugenol (Figure 1). In an earlier investigation, 20/73 (29%) of the deodorants on the European market were found to contain 1-458 ppm isoeugenol (6). In the present investigation eight products (9%) contained a median concentration of isoeugenol at 32 ppm and a maximum concentration of 138.4 ppm. The perfume industry has since 1992 recommended a maximum isoeugenol concentration in cosmetic products at 0.2%, and from May 1998, a maximum concentration of 0.02%. This limit is in public consultation within EU with regard to implementation in the Cosmetic Directive (46). Deodorants in the present investigation conform to this limit.
In a provocation study with 23 eczema patients, among these 13 with allergy to isoeugenol, these were supplied with two deodorants, one with and another without isoeugenol (10). The start concentration was 63 ppm (0.0063%) isoeugenol in the deodorant, which was applied in an armpit two times daily up to 14 days, and the unperfumed deodorant was applied in the other armpit as control. If no reaction was observed, a deodorant with higher concentration of isoeugenol, 200 ppm (0.02%), was applied for additional 14 days, and if still no reaction was observed then a deodorant with higher isoeugenol concentration, 630 ppm (0.063%), was applied for addition 14 days (Table 9). None in the control group reacted, while 10 of the 13 patients with isoeugenol allergy reacted to deodorants with isoeugenol. Of these, three patients reacted to a deodorant with 63 ppm isoeugenol, 4 patients to a deodorant with 200 ppm isoeugenol and 3 more reacted to the use of a deodorant with 630 ppm isoeugenol. None of the control persons reacted to the deodorants. This means that seven of the 13 (53%) allergic person got allergic reactions from the use of deodorants containing isoeugenol at 200 ppm concentration level, the maximum concentration recommended by the industry. On the basis of this study the isoeugenol level in products should be kept below 63 ppm. This was the case in 5/8 isoeugenol containing deodorants in the present study. This means that isoeugenol concentration in at least 3.4% of the 88 perfumed deodorants in the present study was higher than recommended.
Although the perfume industry’s organisation IFRA in 1998 reduced the recommended concentration of isoeugenol from 2000 ppm to 200 ppm, the incidence of isoeugenol allergy has not reduced (47). This is possibly due to the use of structurally related substances (for example isoeugenol acetate) instead of isoeugenol (48). It will be important to make a survey of the use of substances structurally related to isoeugenol.
5.3.4 Cinnamal/cinnamyl alcohol
Cinnamal is a moderate/strong allergen, which has been in focus for several years due to its allergenic properties (1, 49). In the earlier mentioned investigation cinnamal was found in 17% of the deodorants (6). In the present investigation, cinnamal was present in only one deodorant (1.1%) at a concentration level of 5 ppm. A limit of 0.1% cinnamal is in public consulation for the implementation in the Cosmetic Directive (46). The risk estimate, performed by the cosmetic industry, indicates that there is a significant risk of sensitisation by exposure to 0.1% cinnamal (31). Perfume industry has recently recommended use of maximum 0.05% (500 ppm) in cosmetic products, fully implemented in 2007 (28). Earlier, perfume industry did not give any recommendation to a maximum limit for cinnamal. Both in a Danish and a British investigation, the frequency of allergy to cinnamal and cinnamyl alcohol was found to be reduced over a long period (50, 51), possibly related to reduction in use of these substances. In a study of eczema patients allergic to cinnamal, allergic eczema was developed in one of nine patients (11%) by the use of a 100 ppm cinnamal containing deodorant, and in 99% of the patients by the use of deodorant containing 1000 ppm cinnamal (9). An unperfumed control deodorant did not give any reaction. In the paper (9), it is recommended to keep the cinnamal concentration in the products below 100 ppm. The products in the present investigation conform to this recommendation.
In the analysis of deodorants cinnamyl alcohol, which can transform to cinnamal in the skin (52-55) was included. Animals which were sensitised to one of these substances also reacted to the other substance (52). Simultaneous reaction of the two substances is also often seen in humans (56). Cinnamal is a more potent allergen and induces allergy in animals at 15 times lower concentration than cinnamyl alcohol (52). In humans, 80% of the persons allergic to cinnamal will also react to cinnamyl alcohol in identical concentrations at patch testning (56). In the present investigation, cinnamyl alcohol was found in 11 deodorants (12 %) in concentrations from 1.7 ppm to 503 ppm, median 40 ppm. A limit of 0.8 % cinnamyl alcohol is in public consultation for implementation in the Cosmetic Directive (46). The Perfume industry’s organisation IFRA has, since 2002 with final implementation in 2004, recommended its members not to use cinnamyl alcohol in concentrations above 0.4% (28). This is based on a number of old studies, where the concentration which did not produced allergy in healthy persons was 4 % cinnamyl alcohol (no-effect level) (55, 57). In perfume industry’s earlier risk assessments, this level was divided by an uncertainty factor of 10, to define the safe limits (58). Although the data has been available for many years, the limit has not previously been in accordance to this principle.
Cinnamal was found at 5 ppm in one product in this investigation, which does not pose any risk. The level of cinnamyl alcohol is difficult to assess as there is no quantitative clinical data. However, the data illustrates that persons allergic to cinnamal can be exposed to substances in the products, which may produce ezema, even though cinnamal is not present in the products. For this reason, cinnamyl alcohol should be used under the same conditions as cinnamal, i.e. below 100 ppm (Table 9), which was the case for about half of the products containing cinnamyl alcohol in the present investigation.
5.4 Farnesol
Farnesol can be used in deodorants both as an antimicrobial substance and as a fragrance substance. It is considered to be an important allergen (6). Farnesol is included in the new perfume mixture, fragrance mix II, which is used routinely for the diagnosis of perfume allergy. Farnesol allergy was detected in 0.4%-0.5% of eczema patients tested in two different European multicenter studies (35, 36).
In a German multicenter study, 2021 eczema patients were tested and 1.1% was found to be allergic to farnesol. On this basis, it was estimated that 10.000 persons in Germany were allergic to farnesol (61). In the present investigation 13 products (14.8%) were found to contain farnesol, and in the subgroup of 23 deodorants, subjected to chemical analysis, 9 of the products contained farnesol. The median concentration of farnesol in these products was 661 ppm (range 9-1771 ppm, Table 3 and Table 7).
Perfume industry’s organisation IFRA has adopted a new model for quantitative risk assessment, which is based on same principles as in Table 11, except that the concrete reference value is calculated specifically on the basis of experimental results employing humans and not on the basis of normal values as in Table 12 (28). Until now, no limit values for farnesol have been adopted, but for new formulations limit values are introduced from June 2007 and for old formulation from June 2008. The limit values are laid down because of allergenic properties and introduced for 11 different product categories. The limit value for deodorants is 0.11% (1100 ppm). In the present investigation, only two deodorants were found to contain more than 1100 ppm farnesol. Thus in the present situation, this limit will probably not affect the Danish market or incidence of allergy. A safe limit is, however, required to prevent the use of higher concentrations in future. The proposed limit value can be validated by dose-response study employing persons with farnesol allergy.
5.5 Comments concerning other fragrance substances
The 26 fragrance substances, which should be declared when used in cosmetics, are very different with regard to both allergenic potency as well as incidence of allergy. The most allergenic and frequently occurring allergens are the 14 fragrance substances, which are routinely used for the screening of perfume allergy, i.e. the ingredients of fragrance mix I and fragrance mix II (indicated in Table 5). However, there are differences in allergenic properties of the individual substances even within this group (62). Besides, methyl 2-octynoate is a very potent allergen (28), and oxidation products of d-limonene and linalool are frequent allergens (63, 64).
Evernia Prunastri extract (oak moss) is a natural extract, which is included in fragrance mix I and this is also an ingredient that frequently gives positive reaction (42). It contains the most potent known allergens, atranol and chloroatranol (65, 66). On the basis of allergy risk, the SCCP has recommended that these two substances should not be present in cosmetic products (67). Four of the products in the present investigation contained evernia prunastri extract. Even though the content is not quantified, this is inappropriate.
Methyl 2-octynoate has been shown to be an extreme allergen in experiments using healthy voluenteers (28). It was declared on one deodorant (Table 4). On the basis its of sensitisation properties the recommended limits of use by IFRA is 0.01% and this limit is proposed for adoption in the Cosmetic Directive (46). Methyl 2-octynoate allergy is not frequent, because it is seldom used and has had a low limit value compared to other fragrance substances.
5.6 Allergen load of fragrance substances
The present investigation included 88 perfumed deodorants from the Danish market. According to ingredients listing on the products, up to 65.9 % of these contained one or more of the fragrance substances which were selected for the study. Among these cinnamal was present in 1% of the deodorants, isoeugenol in 9%, hydroxycitronellal in 27.3% and HICC in 33 % of the deodorants. Approximately one fourth of the deodorants (n=23), which was selected for the quantitative analysis of fragrance substances, contained 5-17 of the 26 target fragrance substances in the products, median eight fragrance substances per product. Thus, there is a considerable allergen load in a considerable part of deodorants on the Danish market, further the same allergenic substances are commonly used. There are approximately 2500 fragrance substances used in the formulation of perfumes. Of these, about 10% have been described as allergenic in humans (68).The products in the present investigation concerned 1% of available fragrance ingredients, even so it was the same substances which were used in many of the deodorants.
Although, there is a great difference in allergenic potency and use concentration of individual substances, as described above, the more potent allergens were found in a number of products and in combination with other allergens. This may be of concern, as several substances are structurally alike and thus they will contribute to additional allergy risk when they are used together. How the simultaneous allergen exposure affects the risk of allergy is not known, but simultaneous exposure to several allergens in allergic patients can produce synergistic reactions (69)
5.7 Triclosan
Triclosan is an antibacterial substance, which is used in deodorants and other cosmetics (70) and also in other types of products, such as cutting oils (71). The Swiss Contact Dermatitis Group tested 2295 eczema patients with tricolsan in the years 1989/90 and found a positive reaction in 0.8% of these. In the same study, 5.7% patients gave a positive reaction to the preservative formaldehyde and 5.5% reacted positive to methylchloroisothiazolinone/methylisothiazolinone (72). In a study from 1970’s, no positive reaction was recorded when 902 eczema patients were tested with 0.5% and 1% triclosan in a 16 months period, but two cases of positive reaction were recorded when tests were performed with 2% triclosan in another period of 17 month (73). Both of these patients were sensitised by tricosan containing deodorants, and one of these also had used a soap containing triclosan. In another study from the same period, 292 patients tested negative to triclosan while positive reaction was observed in two cases. One was sensitized by the use of triclosan containing foot powder deodorant and the other by the use of a deostick containing 0.12% triclosan for a couple of years. This last patient later performed a use-test with a soap containing 0.5% triclosan and developed eczema on one arm, where the soap was used, while the other arm, where a trriclosan free soap was applied, was eczema free. Also in the same time period a case from England was reported, where a woman developed allergic eczema after using a deodorant spray containing triclosan for 3 months (74).
In a Norwegian study, three persons were found to be sensitised to triclosan among 103 tested patients, in two of the persons from the use of a room disinfection product containing 3% triclosan, and in the third case for unknown reason (75). Three cases of allergy to triclosan as result of the use of the same room disinfection product were also reported from Italy (76). A nurse trainee with allergy to triclosan as a result of the use of a hand disinfection product is reported from England (77) and a patient with allergy to triclosan and other preservatives from a soap (78). In a recent report from Finland, 0.2% of the 5376 tested patients with suspected cosmetic allergy were shown to be allergic to triclosan in the period 1995-1996 and 0.1% of the 6598 patients tested in the period 2000-2002 (79).
Allergy to triclosan is not tested routinely, and thus, the cases of allergy to triclosan are possibly overseen. In the present investigation, triclosan was found in 15 products (17%) in concentrations from 0.05%-0.24%, which is within the maximum permitted limit in cosmetic products. In USA, triclosan is typically used in the concentration range 0.15-0.30% (80), which also corresponds to that found in the present investigation. Triclosan is an allergen, the frequency of allergy to this substance has not been mapped, but it is possibly less frequent compared to that due to other preservatives, for example methylchloroisothiazolinone/ methylisothiazolinone. A dose-response study of triclosan’s allergenic effects has not been performed, and therefore, it is not possible to perform a risk assessment.
Version 1.0 October 2007, © Danish Environmental Protection Agency