[Front page] [Contents] [Previous] [Next] |
Vibrio vulnificus in Denmark
4. Occurence and virulence of Vibrio vulnificus
4.1 Ecology
V. vulnificus in aquatic ecosystems
V. vulnificus can be isolated from a wide variety of aquatic ecosys-tems, but the occurrence of the organism is favored by high tempera-tures (>20°C) and intermediate salinities (15-25l) (Motes et al., 1998). V. vulnificus has been reported on the Atlantic, Pacific, and Gulf coasts of the US (Kelly, 1982; Oliver et al., 1982; Tamplin et al., 1982; Kaysner et al., 1987; O'Neill et al., 1992). In temperate areas, V. vulnificus is less abundant than in subtropical waters, but V. vulnificus has been isolated from coastal waters or implicated in human infec-tions during the summer months in Denmark, Sweden, Germany, Holland, and Belgium (Mertens et al., 1979; Veenstra et al., 1994; Hoyer et al., 1995; Melhus et al., 1995; Dalsgaard et al., 1996b). V. vulnificus was recently isolated from the Mediterranean for the first time despite the high salinity (35l) that do not favor growth of V. vulnificus (Arias et al., 1998a). However, no clinical cases has been reported from this area in recent time even though millions of tourists swim in the Mediterranean each year. V. vulnificus occurs most likely only in very low concentrations in these waters because of the high salinity, and the concentration is apparently too low to cause human infections. V. vulnificus has also caused disease in eel farms in Japan, Spain, Norway, Sweden, and Denmark (Muroga et al., 1976, Biosca et al., 1991; Høi et al., 1998b; Dalsgaard et al., in press).
Reservoirs and vehicles of transmission
Oysters, clams, mussels, fish, plankton, as well as water and sediment have all been described as reservoirs and vehicles for V. vulnificus (Oliver et al., 1983; Kaysner et al., 1987; Oliver, 1989; DePaola et al., 1994; Dalsgaard et al., 1996a; Wright et al., 1996; Biosca et al., 1997b). V. vulnificus has been isolated from waters with temperatures from 7°C to 31°C and salinities between 1 to 35l (Kaysner et al.,1987; Wright et al.,1996; Arias et al.,1998a; Høi et al.,1998c). V. vulnificus tolerates wide ranges of salinities and temperatures and is abundant in water with temperatures above 20°C and salinities be-tween 15 to 25l (Kelly, 1982; O'Neill et al., 1992; Kaspar & Tamplin, 1993; Motes et al., 1998). Additional factors (e.g., sunlight, pH, nutrient factors, presence of competing bacterial populations, and grazing) may also affect the distribution of V. vulnificus in the envi-ronment.
Grazing
Grazing by protozoa is one of the main biological processes that control bacterial density in marine environments but at the present time it is not known to what extent grazing effects the ecology of V. vulnificus (Barciana et al., 1997). Bacteriophages lytic to V. vulnificus have recently been reported in estuarine waters, sediments, plankton, shellfish, and the intestines of finfish from the Gulf of Mexico (DePaola et al., 1997a; DePaola et al.,1998). The number of plaque forming units (PFU) did not correlate with densities of V. vulnificus - the lowest number of PFU was found in intestinal contents from fish where the number of V. vulnificus was highest (DePaola et al., 1997a). The greatest variety and abundance of phages lytic to V. vulnificus were isolated from Gulf of Mexico oysters. The abundance of phages ranged from 101 to 105 PFU per gram of oyster tissue (DePaola et al., 1997a). The V. vulnificus-specific-phages from the Gulf of Mexico were able to lyse V. vulnificus isolated from diseased eels in Denmark which suggests that the unidentified attachment sites for these phages are present on V. vulnificus strains worldwide (Høi et al., 1998b). Future research will probably reveal that phages play a significant role in the ecology and perhaps even in virulence of V. vulnificus as has been described for other bacteria (Hennes & Simon, 1995; Waldor & Mekalanos, 1996; Cochran & Paul, 1998).
No correlation with fecal indicators
Oliver et al. (1982, 1983) investigated the distribution and ecology of V. vulnificus along the East coast of the United States and found no correlation between the prevalence of V. vulnificus and faecal coliforms. Tamplin et al. (1982) described a negative correlation between V. vulnificus and faecal coliform; V. vulnificus was most frequently isolated from samples with less than three faecal coliform per 100 ml. An inverse correlation between faecal coliforms and counts of V. cholerae has also been described by Dalsgaard (1994).
Seasonal occurrence
The occurrence and prevalence of V. vulnificus is seasonal (Kelly, 1982; O'Neill et al., 1992). The mechanism of the seasonal variation was speculated to be similar to that of V. parahaemolyticus which has been described to overwinter in bottom sediments and enter the water column again when warm temperatures return (Kelly, 1982). How-ever, several studies have speculated that the disappearance of V. vulnificus in cold periods is not due to die-off but to entry into the Aviable but non-culturable@ (VBNC) state (Oliver, 1995). In this state, the cells have been shown to be viable with several direct viability assays but can no longer be cultured on routine media. VBNC cells can resuscitate and are virulent in an iron-overload adult mouse model according to Oliver & Bockian (1995). The majority of human infec-tions occur in the warm summer months when densities of culturable V. vulnificus cells are high and the importance of VBNC cells in human infections remains to be definitively determined (Klontz et al., 1988). The ability of VBNC cells to resuscitate when the stress (cold temperature) is eliminated or if reappearance is due to regrowth by a few non-detectable culturable cells is debatable (Nilsson et al., 1991; Firth, et al., 1994; Oliver, 1995; Oliver et al., 1996; Weichart & Kjelleberg, 1996). Recently Bloomfield et al. (1998) suggested a new plausible model to account at least partially for the VBNC phenome-non. They hypothesized that VBNC cells are not unculturable but that "we are simply failing to provide appropriate conditions to support culture" (Bloomfield et al., 1998). Further, they provided evidence that the transfer of "so-called VBNC cells" to a nutrient-rich agar cause cell death. Bloomfield et al. (1998) proposed Athat sudden transfer of cells to nutrient-rich agar at temperatures optimal for enzyme activity initiates an imbalance in metabolism, producing a near-instantaneous production of superoxide and free radicals. In the absence of phenotypic adaptation, the cells are not equipped to detoxify superoxide. As a result, a proportion or all of these cells die".
4.2 Clinical manifestations, epidemiology and treatment of V. vulnificus infections in humans
V. vulnificus causes primary septicemias and wound infections (Blake et al., 1979). Most primary septicemias are associated with raw sea-food consumption, especially raw oysters and in almost every case the patient has a chronic underlying disease. V. vulnificus differs from other food borne pathogens as it is seldom reported to cause diarrhea and vomiting (Hollis et al., 1976; Blake et al., 1979; Hlady & Klontz, 1996). V. vulnificus causes only sporadic disease and outbreaks (i.e., two or more culture- confirmed cases linked to a common meal or lot of oysters) have never been reported (Whitman, 1995). The fatality rate is high with almost 60% of the patients with primary septicemia dying within a few days (Oliver, 1989). A high prevalence of liver disease and alcoholism among patients with septicaemia may reflect a requirement of V. vulnificus for free iron via saturated transferrin or excess of iron (Hlady & Klontz, 1996). Other risk factors include the use of immunosuppressive agents, gastric diseases, and blood disor-ders (Oliver, 1989).
Wound infections
V. vulnificus causes wound infections by entering a pre-existing skin lesion during exposure to saline waters. Patients are often employed as fishermen or in other jobs with close contact to the marine environ-ment (Dalsgaard et al., 1996b; Hlady & Klontz, 1996). The fatality rate of reported cases is approximately 20% but amputation or surgical debridement is often necessary (Oliver, 1989).
In Denmark, four of 11 patients in 1994 developed septicemia, of which one subsequently died. Nine patients exhibited skin manifesta tions and six underwent surgical debridement. Four patients contracted their disease during fishing and at least one patient had been handling eels (Dalsgaard et al., 1996b).
Treatment
V. vulnificus is sensitive to most antibiotics and infections have been treated with antibiotics, e.g. ampicillin, tetracycline, chloramphenicol or third-generation cephalosporins (Klontz et al., 1988; Chuang et al., 1992; Fang, 1992; Dalsgaard et al., 1996b). Antibiotic treatment is often ineffective unless initiated as soon as the first clinical symptoms appear (Oliver, 1989). However, in cases of serious wound infections, the primary treatment is a proper surgical debridement with antibiotics playing a secondary role (Dalsgaard et al., 1996b). A vaccine against V. vulnificus has been developed but has not been tested beyond the pre-clinical trials in mice (Devi et al., 1995; Devi et al., 1996). The vaccine antiserum is raised against the capsule of V. vulnificus and the achieved protection is capsule-type-specific. Clinical strains of V. vulnificus exhibit various capsule types and far from all types have yet been identified (Hayat et al., 1993; Simonson & Siebeling, 1993). Because risk factors for V. vulnificus infection are unclear and illness are rare, vaccination may not be an appropiate effective control strat-egy (Blake et al., 1979; Mertens et al., 1979; Kelly & Avery, 1980; Oliver, 1989). However, a serosurvey in the Chesapeake Bay Region revealed that asymptomatic infection with V. vulnificus may be rela-tively common among persons like shellfish industry workers with high levels of exposure to shellfish (Lefkowitz et al., 1992). Danish eel farmers are exposed to high concentrations of V. vulnificus during outbreaks but at the present time it is not known if asymptomatic infections of V. vulnificus occur in Denmark.
Warning labels
In California, Florida and Louisiana, where most cases of V. vulnificus occur, restaurants that serve raw oysters are required to post warnings. In the state of Louisiana all shucked shellfish products and shellstock must be labeled with the warning tag: Raw oysters, raw clams, and raw mussels can cause serious illness in persons with liver, stomach, blood, or immune system disorders (Dayal et al., 1993).
Infectious dose unknown
The infectious dose of V. vulnificus is unknown and is due in part to the inability to match clinical isolates with isolates from implicated lots of oysters, the lack of opportunities to trace a human infection (especially wound infections) back to the implicated source and finally because human volunteer studies are too dangerous.
4.3 V. vulnificus infections in eels
V. vulnificus biotype 2 causes serious economic losses in aquaculture that keep eels in brackish water around 24°C (Amaro et al., 1995). Under laboratory conditions, immersion challenge with 105 CFU/ml or between 102 and 105 CFU/fish by intraperitonal injection leads to the development of vibriosis in less than 24 h (Amaro et al., 1995). The disease is a septicemic infection and bacteria are easily isolated from blood samples from moribund eels (Amaro et al., 1997).
Pathological changes
The first isolates of V.vulnificus biotype 2 were recovered from Japa-nese eels (Anguilla japonica) between 1975 and 1977 (Muroga et al., 1976; Nishibuchi et al., 1980). The disease was characterized by reddening of the body, especially tails and fins, and hemorrhages of the dorsal area (Miyazaki et al., 1977). In progressed cases, pathologi-cal changes could be observed in the gastrointestinal tract, gills, heart, liver and kidney (Miyazaki et al., 1977).
V. vulnificus biotype 2 was first described in Europe in 1989 where recurrent outbreaks occurred in cultured European eel (A. anguilla) in Spain (Fig. 4) (Biosca et al., 1991). Early clinical features were that the eels became lethargic, external lesions appeared first as petechiae on the abdomen, hemorrhages of the anal fin and a reddening in the opercular region. Large wounds (2-4 cm diameter) with central ne-crotic tissue occurred in some eels. Pathological changes and inflam-mation of internal tissues, liver, kidney, and in the abdominal cavity were observed (Biosca et al., 1991). The eel farm in Spain experienc-ing these disease outbreaks used brackish well-water (17l NaCl and 22°C) in a recirculating system (Biosca et al. ,1991). The diseased eels had different origins and therefore it was impossible to ascertain the source of V. vulnificus (Biosca et al., 1991). Earlier environmental studies in the area of the eel farm did not reveal the presence of V. vulnificus (Pujalte et al., 1983; Ortigosa et al., 1989; Garay et al., 1985).
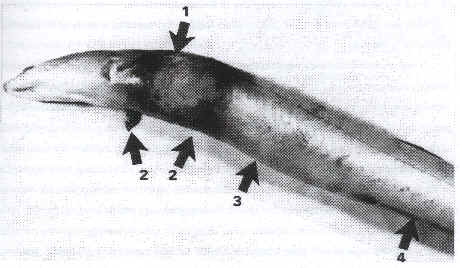
Figure 4: Gross appearance of eel suffering from V. vulnificus biotype 2 infection: (1) Wound with central perforation (2) Reedening in the opercular region (3) distended abdomen (4) hemorrhegic anal fin (Biosca et. al., 1991).
For information on V. vulnificus in Danish eel farms see section 4.5.
4.4 Virulence factors
4.4.1 Virulence factors of V. vulnificus in human infections
Multifaceted bacterial and host factors affect the development and severity of V. vulnificus infections in humans.
Capsule production
V. vulnificus displays two distinct colony morphologies, opaque and translucent. The opaque variant is encapsulated, virulent to mice and capable of survival in human serum. Nonencapsulated, translucent strains arise spontaneous during routine bacteriological mani pulation of opaque strains (Fig. 5 and 6) (Simonson & Siebeling, 1993; Zuppardo & Siebeling, 1998). Opaque variants possess a ruthenium red-staining layer identified as an acidic polysaccharide (Kreger et al., 1981). Reversion from both colony variants has been reported to occur under laboratory conditions at very low frequencies (~10-4), which sug-gests that capsular production is con-trolled by a revers-ible genetic rearrangement (Yoshida et al., 1985; Wright et al., 1990). Genetic factors that regulate these changes in colony morphology are not fully understood. An epimerase3 gene has been shown to be essential for capsule synthesis (Zuppardo& Siebeling, 1998). The loss of a functional epimerase, e.g. by transposition, can cause the disrup-tion of capsule production by a depletion of precursors in the early stage of capsule production (Zuppardo & Siebeling, 1998).
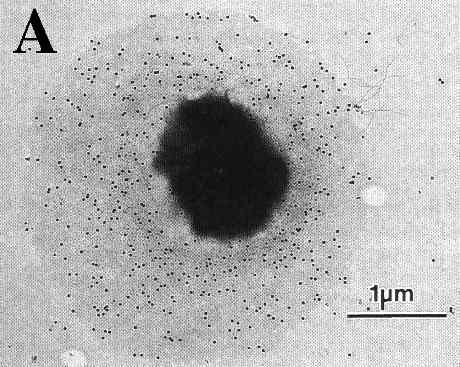
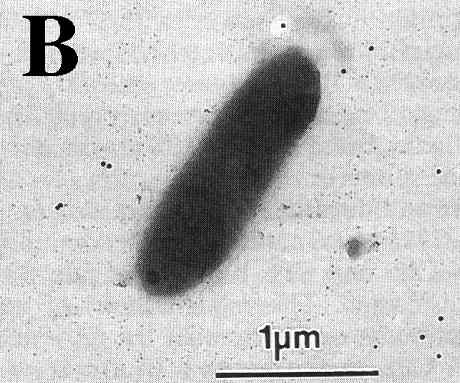
Figure 5: Transmission electron micrographs of a opague (A) and translucent (B) variant of the same V. vulnificus strain reacted with anti-capsular rabbit sera and then stained with protein A-colloidal gold conjugates (Simmonson & Sibeling, 1993).
The importance of capsule in V. vulnificus virulence has been demon-strated in a number of studies (Oliver, 1989; Simpson et al.,1987). The capsule permits V. vulnificus to evade nonspecific host de-fense mechanisms such as activation of the alternative pathway of the complement system and complement-mediated opsonophagocytosis (Tamplin et al., 1985; Yoshida et al., 1985; Shinoda et al., 1987). Loss of capsule in transposon mutants is accompanied by a several log higher LD50 in mice and decreased serum resistance (Wright et al., 1990; Zuppardo & Siebeling, 1998). Antibodies generated against capsule antigens protected vaccinated mice when challenged intraperitoneally with live V. vulnificus cells (Kreger et al., 1984). However, the importance of capsule production for the virulence of V. vulnificus in humans is not fully elucidated as most environmental strains are encapsulated and yet vary in virulence to mice (Stelma et al., 1992; Kaysner et al., 1987). The presence of capsule may there-fore be regarded as one among several virulence factors.
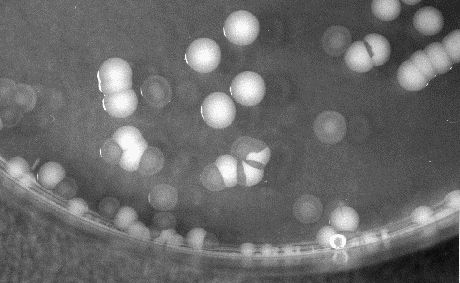
Figure 6: Opaque and translucent colonies of V. vulnificus strain on an agar plate.
Serum iron
Serum iron availability plays an important role in host susceptibility to V. vulnificus. Serum iron levels is strongly correlated to the size of V. vulnificus inoculum required to cause mortality in mice. The LD50 value dropped from 106-108 to less than 102 cells in mice injected with iron before bacterial challenge (Wright et al., 1981). Virulent strains of V. vulnificus produce both phenolate and hydroxymate siderophores, which enable them to acquire iron from fully saturated transferrin, lactoferrin, ferritin, haemoglobin and haptoglobin com-plexes (Simpson et al., 1987; Zakaria-Meehan et al., 1988; Oliver, 1989; Stelma et al., 1992). An exocellular protease has been described to cleave transferrin and lactoferrin thereby making bound iron more accessible to the siderophores (Okujo et al., 1996).
Non-encapsulated transposon mutants are not defective in iron acqui-sition but are less virulent in mice than encapsulated suggesting iron acquisition is less important than capsule production (Wright et al., 1990). Biosca et al. (1996b) demonstrated that iron-uptake by V. vulnificus from human transferrin is independent of capsule produc-tion in both biotypes.
Other virulence factors
Published data on the role of other putative virulence factors include the production
of a cytolysin (Gray & Kreger, 1985), a protease (Miyoshi et al., 1987), a
phospholipase (Testa et al., 1984), piluslike structures (Gander & LaRocco, 1989) and
LPS endotoxin (McPherson et al., 1991). The cytolysin found in all V. vulnificus strains
lyses mammalian erythrocytes and Chinese hamster ovary cells, produces a vascular
permeability factor activity in guinea pig skin and is lethal to mice (Gray & Kreger,
1985). One study reported that mice and hu-mans surviving V. vulnificus infections
produced antibodies against the cytolysin, indicating that the cytolysin was produced in
vivo (Gray & Kreger, 1986). However, Wright & Morris (1991) inactivated the
structural gene for the cytolysin of a virulent clinical strain and found that it did not
affect virulence in mouse models. Other authors have also been unable to detect any
correlation between production of cytolysin and virulence (Oliver et al., 1986; Morris et
al., 1987; Massad et al.,1988). The protease exhibits both caseinolytic, elastinolytic,
and collagenolytic activities (Kothary & Kreger, 1987; Miyoshi et al., 1987), but is
produced by both virulent and avirulent strains and differences in titre do not correlate
with virulence in animal models (Morris et al., 1987b). LPS from V. vulnificus is
pyrogenic and can cause cardiovascular injuries leading to death in rats (McPherson et
al., 1991). V. vulnificus LPS, once in the blood compartment in humans, may trigger the
sepsis cascade leading to septic shock and possible death. The septic shock may be
mediated by several factors including (i) degranulation of neutrophils onto the
endothelium surface which causes vessel wall damage, (ii) intravascular activation of the
complement cascade by LPS through the alternative pathway, (iii) activated macrophages
overproduction of
the cytokines IL-1 and TNF which cause endothelium injuries, and (iv) activate additional
secondary inflammatory mediators (e.g. platelet activation factor and arachidonic acid)
(Siebeling, 1997).
Plasmids
Plasmids play an important role in the virulence of Enterobactericeae. but their role in virulence of V. vulnificus biotype 1 has received little attention (see section 5.1).
Numerous studies have reported no differences in virulence character-istics of clinical and environmental isolates of V. vulnificus (see Chapter 5) (Oliver et al., 1986; Tison & Kelly, 1986; Kaysner et al., 1987; Stelma et al., 1992). However, this is inconsistent with the low attack rate in susceptible populations consuming seafood contami-nated with V. vulnificus. Less than one illness occur per 10,000 meals of raw Gulf oysters served to the highest risk population, people with liver diseases, suggesting that environmental strains are not equally virulent or not all people with liver disease are equally susceptible (Hlady, 1997).
The high virulence of V. vulnificus can not be assigned to a single factor but is influenced by capsule production, ability to acquire iron in human serum, LPS type, production of exoenzymes and exotoxins, and a susceptible host.
4.4.2 Virulence factors of V. vulnificus in eel infections
The two biotypes share many of the same virulence factors, including (i) the capsule, a
protective surface antigen that allows cells to resist phagocytosis and lysis by human
serum but not by eel complement (Biosca et al., 1993b; Amaro et al.,1994); (ii) various
iron uptake systems, including siderophore production and the ability to use hemoglobin
and hemin as iron sources (Amaro et al., 1994; Biosca et al., 1996b); and (iii) a
cytolysin, with hemolytic activity together with potent proteases, which are active
involved in the lesions produced in different organs (Amaro et al., 1992). Exotoxins
produced by both
biotypes are equally lethal for eels; they produce the main symptoms of vibriosis when
injected intraperitoneally as crude extracts of extracellular products (Biosca &
Amaro, 1996). The capsule of biotype 2 strains has been suggested to favor adherence to
eel mucus and to be essential for virulence under natural conditions (Amaro et al., 1995).
The existence of different capsule types has been studied with 10 polyclonal capsular
antisera in V. vulnificus strains from diseased eels in Denmark (Høi et al., 1998b). The
isolates were either non-typeable or possessed capsule type 9 which indicates that this
type of capsule may enhance the capability of V. vulnificus to infect eels (Høi et al.,
1998b).
A relationship between high molecular weight plasmids and eel virulence was first suggested by Biosca et al. (1997a), who found that a plasmid-free biotype 2 strain had a significantly higher LD50 in eels than biotype 2 strains harboring high molecular weight plasmids. These findings are corroborated by Høi et al. (1998b) who found that 93 of 97 biotype 2 strains isolated from diseased eels contained one to three high molecular weight plasmids of varying sizes. Restriction digests of plasmids from a number of biotype 2 strains from Denmark revealed a high degree of homology (Lewin, 1998). The role of plasmids in virulence requires further studies including DNA sequencing.
LPS
Biotype 2 strains constitute a relatively homogenous LPS based O serovar according to Biosca et al. (1997a). The O side chain of this LPS type may determine the selective virulence of biotype 2 for eels (Amaro et al., 1997). The crude extract of this LPS type, termed serovar E, is not toxic to eels, which suggests that this surface antigen acts as a protective factor against nonspecific immune mechanisms such as nonopsonic phagocytosis and/or bactericidal action of serum complement (Amaro et al., 1997). Biotype 1 strains are lysed by eel serum through activation of the alternative pathway of the comple-ment system (Amaro et al., 1997). Biotype 2 strains are resistant to non-immune eel serum whereas rough mutants of biotype 2 lacking the O polysaccharide chain are sensitive to non-immune eel serum and avirulent for eels. The authors suggest that only strains possessing this particular type of LPS are able to colonize eels (Amaro et al., 1997). To cause septicemic infections the strains must also be able to express other virulence factors (e.g. exotoxins and iron uptake systems) which may be plasmid encoded.
4.5 V. vulnificus in Denmark
4.5.1 Occurrence of V. vulnificus in coastal water, sediment, wild fish, and shellfish in Denmark
A comprehensive environmental survey of V. vulnificus in Danish marine environments was done during 1996 (Høi et al., 1998c). The aims of this survey were to investigate the occurrence of V. vulnificus in Danish coastal waters, shellfish, and wild fish and especially to investigate the distribution of V. vulnificus biotype 2 strains (Høi et al., 1998c).
From May to October 1996, water was sampled weekly at sites 1 to 7 (Fig. 8) and sediment samples were collected weekly from sites no. 1 and 2. Blue mussels (Mytilus edulis) and oysters (Oestra edulis and Crassostrea gigas) were sampled from July until December 1996 from a total of 13 sites (Fig. 7). From July until October 1996, a total of 136 wild fish were analyzed, including 29 flounders (Platichthys flesus), 14 eel pouts (Zoarches viviparus), and 93 eels (A. anguilla) that were caught at various locations in Køge Bay and in the waters close to location no. 2 (Fig. 7) (Høi et al., 1998c).
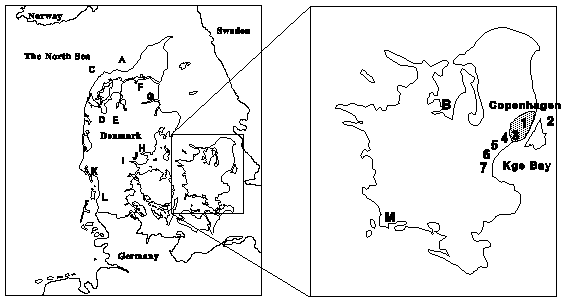
Figure 7: Geographical location of sampling sites. Coastal water sam-ples were collected at locations 1 to 7. Sediment samples were obtained from locations 1 and 2. Oysters were harvested in areas A and B and blue mussels in the areas C to M. Wild fish were caught in Køge Bay and at location 2.
Biotype 2 in coastal water
Until recently, V. vulnificus biotype 2 had never been isolated from coastal water suggesting that disease was transmitted from one eel to another by direct contact, and that biotype 2 did not survive well in brackish water (Høi et al., 1997).
In Denmark, biotype 2 strains were isolated from sediment and coastal water samples although the frequency of isolating such strains was very low (3 of 706 strains were indole negative and designated biotype 2). The low incidence of V. vulnificus biotype 2 strains in environmental samples may explain why its occurrence in coastal water has not been reported earlier. The isolation procedure may also influence which biotype is detected. A few biotype 2 colonies on a CC agar plate may not be picked for identification and characterization if numerous biotype 1 colonies are present on the plate. While virulence determinants, phenotypic properties, and physiology of the two bio types are similar (Amaro et al., 1992; Biosca & Amaro, 1996; Biosca et al., 1996a; Biosca et al., 1996b; Biosca et al., 1997a), our study suggests differences in their ecology: biotype 1 was isolated from various environmental sources whereas biotype 2 was rarely isolated from coastal water and sediment. Danish marine environments are potential reservoirs of V. vulnificus biotype 2. The distribution of eel-pathogenic V. vulnificus strains in Danish coastal waters may have been underestimated because not all eel-pathogenic V. vulnificus strains are indole negative (see Chapter 5). This knowledge is particu-lar important to fish farmers since the use of brackish water for cultur-ing eels may introduce serious V. vulnificus infections in the farms.
Occurrence correlated with to water temperature
The occurrence of V. vulnificus at the seven coastal sites was strongly correlated to water temperatures, as reported by other researchers (Fig. 8) (Wright et al., 1996; O'Neill et al., 1992; Kelly, 1982). V. vulnificus was rarely isolated when water temperatures were below 15°C. However, V. vulnificus was detected in coastal waters at a mussel farm at 7°C which is lower than previously reported (Wright et al., 1996).
Figure 8: Mean densities of V. vulnificus in samples of coastal
water and water temperatures from the seven sites studied.
Detection at low temperature
Detection of V. vulnificus at low temperatures as seen in the present study may be a function of the sensitivity of the isolation method and/or of adaptation of V. vulnificus to colder temperatures. Our inability to isolate V. vulnificus from water samples with temperatures below 7°C may be caused by inappropriate culture conditions for these cold-stressed cells or the absence of culturable cells (Oliver, 1995; Bloomfield et al., 1998).
No correlation with fecal indicators
The control of Danish coastal bathing water is based on presumptive Escherichia coli and coliforms as indicators of water quality. As reported elsewhere, analysis of the data collected in Denmark in 1996 did not reveal any correlation between presumptive E. coli and V. vulnificus (Høi et al., 1998c).
Low levels of V. vulnificus in Danish shellfish
Danish oysters and mussels and their surrounding waters were ana-lyzed for V. vulnificus. V. vulnificus was isolated from both water and blue mussels from one cultivation area of a total of 13 areas tested (area G, Fig. 7). Temperature and salinity in area G was similar to others in the study. Concentrations of V. vulnificus in blue mussels were very low (# 10 CFU per gram of mussel tissue) and the low probability of detecting a viable V. vulnificus cell may explain why it was not isolated from other areas. However, cultivation of mussels in area G differed in several ways from the other areas investigated: (i) mussels are cultivated on suspended ropes. In this cultivation method mussels are raised above the sea-bed to give a more efficient exploita-tion of food at all depths as compared to cultivation on the sea-bed (ii) cormorants (Phalacrocorax carbo) are present in high numbers at the mussel farm (iii) the area is among the most nutrient rich waters in Denmark. Location of mussels in surface waters provides more favor-able temperatures for growth of V. vulnificus during summer time, and the high level of nutrients may provide conditions favorable for growth of V. vulnificus, even at low temperatures.
Transmission through birds
The presence of high numbers of defecating cormorants above the mussel farm may be of importance for the prevalence of V. vulnificus in this particular mussel farm. One could hypothesize that when cormorants eat mussels containing V. vulnificus, the organisms may survive or even replicate in their intestines and be released back into the environment with feces. The body temperature of birds is 41-42°C which allows growth of V. vulnificus (Freeman, 1983). Further, V. vulnificus and V. cholerae have been isolated from cloacal swaps and bird droppings from a variety of seabirds from the Alabama coast of The Gulf of Mexico (DePaola, 1998). V. vulnificus was isolated from eight of 22 samples from seabirds, and concentrations as high as 106 CFU per gram of bird droppings were registered (DePaola, 1998). Birds have also been reported to be a potential vector for the fish pathogenic bacterium Yersinia ruckeri which has been isolated from the intestines of several fish-eating birds (Willumsen, 1989; Furones et al.,1993). The role of seabirds in the ecology of V. vulnificus in Denmark was investigated in the summer of 1997 (Hein, 1998). Thirty-seven cloacal swaps from seagulls and ducks (Larus fuscus, L. Canus, L. Argentatus, Anas clypeata, A. Crecca, A. Platyrhynchos) from two coastal locations on Zealand were investigated but all sam-ples were negative for V. vulnificus (Hein, 1998). V. vulnificus was isolated from water samples relatively near one of the sampling areas in the same period as the birds were investigated (Hein, 1998). The negative result may be explained by one or more of the following factors: (i) the birds did not consume any V. vulnificus, (ii) some V. vulnificus cells may get killed during passage through the intestinal tract, and therefore intake of only a few V. vulnificus cells may not result in a positive cloacal sample, (iii) V. vulnificus may not survive passage through the bird species examined, or (iv) V. vulnificus occur with such low prevalence that more than 37 samples would have been necessary to isolate this bacterium. Further studies are necessary to determine if seabirds can be a reservoir or vehicle for V. vulnificus in Denmark.
US shellfish
Gulf of Mexico shellfish are implicated in nearly all V. vulnificus primary septicemia infections in the United States (Klontz et al., 1988; Klontz et al., 1994; Hlady & Klontz, 1996). High concentrations of V. vulnificus (105 -106 CFU per gram) are reported in raw oysters from April to October where more than 90% of primary septicemia cases occur (Klontz et al., 1988; Tamplin, 1995; Hlady & Klontz, 1996). Wound infections due to occupational activities with contact to saline water have been reported to show a similar seasonal pattern (Hlady & Klontz, 1996). In Denmark, V. vulnificus primary septicemia has not been reported and all wound infections occurred in August during extremely warm summers (Dalsgaard et al.,1996b). V. vulnificus infections have not been associated with consumption of raw shellfish in Denmark or elsewhere in Europe. These findings suggest minimal risk associated with consumption of raw shellfish containing V. vulnificus in low numbers.
Role of fish in ecology
Bottom-feeding fish have been reported to play a role in the ecology of V. vulnificus in Alabama and Gulf of Mexico waters (DePaola et al., 1994). High densities (108 CFU/g) of V. vulnificus were found in the intestinal contents of fish that consumed mollusks and crustaceans and it was suggested that fish may play an ecological role in the growth and transport of V. vulnificus (DePaola et al., 1994). V. vulnificus was also isolated from the intestines from brackish-water fish caught on the west coast of India (Thampuran & Surendran, 1998). MPN counts in the Indian study ranged from 15 to 910 CFU per gram of intestinal content, but concentrations were probably underestimated since the selective agar TCBS, which offer poor recovery of V. vulnificus, was used (Thampuran & Surendran, 1998). In Denmark, V. vulnificus was isolated with higher a prevalence from gills (21%) (P<0,001, ?2 test, SigmaStat7 version 3.2) compared to intestines (5%) and mucus (10%) from the three fish species eel, eel pout, and flounder (Table 3).
| Fish species | No. of positive samples / total no. of samples | ||
| gills | mucus | intestinal content | |
| Eels | 19/73 | 3/20 | 4/73 |
| Eel pouts | 2/27 | 0/6 | 0/27 |
| Flounders | 1/5 | 0/5 | 1/5 |
| Total | 22/105 | 3/31 | 5/105 |
Table 3. Occurrence of V. vulnificus in samples of gills, mucus, and intestinal contents from different fish species collected in Denmark in 1996 (Høi et al., 1998c).
Possible bactericidal ef-fect of fish mucus
The low prevalence of V. vulnificus in fish mucus may be explained by a possible bactericidal effect since fish mucus has been reported to contain proteins (complement, antibodies) and glycoproteins that react with environmental antigens and serve as a defense barrier to bacterial colonization (Hjelmeland et al., 1983; Alexander & Ingram, 1992). V. vulnificus biotype 2 strains were reported to be resistant to the antimicrobial activity of eel mucus in in vitro experiments (Amaro et al., 1995). The mucus solution used in these experiments were filter-sterilized, stored at -20°C, and dried onto disks which may decrease any existing antimicrobial effect. Further, no appropriate controls were included to test the mucus-assay. The low prevalence in the intestinal contents may be caused by competing bacterial populations and low pH.
The prevalence of V. vulnificus in samples from the 3 fish species were not statistically different (?2 test, SigmaStat7 version 3.2). Detection of V. vulnificus in estuarine fish in Denmark supports their involvement in the ecology of V. vulnificus as suggested by DePaola et al. (1994). Migrating fish containing V. vulnificus may facilitate the spread to new areas where the bacterium can survive. Serotyping of the fish isolates revealed that healthy wild eels are asymptomatic carriers of eel-pathogenic V. vulnificus (biotype 2) strains in their gills (unpublished results).
Risk of infection corre-lated with water temperature
Findings of V. vulnificus in Danish wild fish during the summer suggest that fishermen, especially those with abrasions on their hands may be at risk for V. vulnificus wound infections. In 1994 and 1995, seven people contracted V. vulnificus wound infections while fishing or handling eels (Dalsgaard et al.,1996b; Bruun, 1997). V. vulnificus cases were not reported during the summer of 1996 when low concen-trations (<2 CFU/100 ml) were observed in coastal waters. V. vulnificus levels were either too low to cause infection, even in sus-ceptible individuals, and colder temperatures discouraged bathers from contact with coastal waters. Epidemiological data from 1994 and 1995 suggest that the risk of contracting a V. vulnificus infection following exposure to coastal water was correlated with water temperature. Thus, surveillance and monitoring efforts should be increased when water temperatures exceed 20°C.
4.5.2 Occurrence of V. vulnificus in frozen seafood imported into Denmark
Low and safe levels in tropical shrimp
V. vulnificus is a naturally occurring bacterium in warm estuarine environments and is therefore expected in shrimp produced in brackish-water aquaculture in South East Asia. The European Union imports approximately 75 metric tonnes5 of these warm-water shrimp through Denmark each year (Dalsgaard & Høi, 1997). The prevalence of V. vulnificus in a total of 107 samples representing 37 consignments of frozen shrimp imported from South East Asia was determined. V. vulnificus was detected in three of 46 (7 %) frozen raw shrimp sam-ples but was not recovered from any of the 61 frozen cooked products. Absence of V. vulnificus in frozen cooked shrimp products indicated proper processing such as adequate heat treatment and sanitation (Dalsgaard & Høi, 1997). The low prevalence of V. vulnificus in frozen raw shrimp products was likely due to its poor resistance to cold (Oliver, 1981; Boutin et al., 1985; Parker et al., 1994). Frozen shrimp products are usually kept at temperatures at -20EC before and after shipping, often for substantial periods, and a significant decrease in any number of V. vulnificus would be anticipated. The absence of V. vulnificus in frozen cooked shrimp products and the low prevalence of V. vulnificus in frozen raw shrimp suggested that V. vulnificus does not constitute a hazard to public health in Denmark if the shrimp products are correctly handled and cooked (Dalsgaard & Høi, 1997).
4.5.3 Occurrence of V. vulnificus in Danish eel farms
The Danish production of eels in aquaculture is expanding with a production in 1999 expected to double over 1998 production (3,000 metric tonnes). Danish eel production is dependent on import of elvers (A. anguilla) from France and the United Kingdom. The majority of eel farms in Denmark use fresh water to culture eels. Disease out-breaks caused by V. vulnificus and other pathogenic Vibrio spp. have not been reported in Denmark with recirculating systems that use freshwater continuously (Mellergaard & Dalsgaard, 1987). However, it is preferable to culture eels in brackish water instead of fresh water since it leads to higher growth rates, better feed conversion and taste. However, brackish water can be a reservoir or vehicle of V. vulnificus biotype 2 and might facilitate the spread to cultured eels (Høi et al., 1998c). Further, water temperatures (approximately 24EC) in eel farms favor V. vulnificus growth (Høi et al.,1998c). V. vulnificus biotype 2 was isolated from wound infections in humans and water in Denmark in 1994 but was not isolated from diseased eels in Danish farms using brackish water until 1995 (Dalsgaard et al., 1996b; Høi et al., 1997; Høi et al., 1998b; Høi et al., 1998c; Dalsgaard et al, in press). Since 1995 recurrent outbreaks of V. vulnificus have occurred in two Danish eel farms both using brackish water causing serious eco-nomic losses (Høi et al.,1998b; Dalsgaard et al.,in press). Recent findings have shown that V. vulnificus also can cause disease in freshwater eel-farms if the farms have used intake of brackish water in the past (unpub-lished results).
Clinical findings in diseased eels
In the first outbreak in one of the farms, the diseased eels were lethargic and exhibited clinical signs typical of bac-terial septicemia and also external hemorrhages in the ocular area and in some cases bilateral exophthalmia (Fig.10A). Erosive lesions developed on the operculum area and in the jaw region (Fig. 10B). The severe tissue necrosis seen in the jaw region of the eels has not been described in the literature and remains unexplained. When these V. vulnificus strains were injected intraperitoneally in challenge experi-ments, the eels died from septicemia within 1-2 days which was not enough time for a possible jaw necrosis to develop (Dalsgaard et al., in press).
Antibiotic treatment
Antibiotic treatment of V. vulnificus infections in eels has limited effect and outbreaks are recurrent. No antibiotic resistance has been demonstrated so far (Dalsgaard et al., in press). Changes to production in freshwater usually reduce the eel mortality, but in one Danish eel farm the infection with V. vulnificus is persisting at the present time. Research investigating survival and spread of V. vulnificus in eel farms and the efficiency of vaccination is needed to make culturing eels in brackish water profitable. Future research should use routine culture methods as well as direct detection techniques to determine the role of non-culturable cells in the ecology of V. vulnificus in eel farms. V. vulnificus has recently been isolated from the gills and intestinal contents of eels cultured in freshwater but these eels had been kept in brackish water in the past (unpublished results). These findings sug-gest that once V.vulnificus enter the eel farm and colonize the eels, then the Na+ ions present in the eel may be sufficient for growth and persistence of this halophilic bacterium. The concentration of Na+ in blood and extracellular fluids in eels is approximately 150 mmol (8,8 g/L) which theoretically is sufficient for growth of V. vulnificus (Scholz & Zerbst-Boroffka, 1994).
4.6 Conclusions from Chapter 4
Human infection with V. vulnificus following exposure to coastal water in Denmark occur when water temperatures exceed 20°C and fishermen appear to be at the greatest risk. Consumption of raw shellfish has not been associated with V. vulnificus infections in Denmark, although V. vulnificus occasionally was isolated in low numbers. V. vulnificus was detected in a few imported frozen shrimp products and does not at present constitute a potential hazard to public health. V. vulnificus presents a serious economic problem to the Danish eel farmers wanting to use brackish water and warrant for both therapeutic and prophylactic measures. Research is needed to understand the ecology of V. vulnificus in eel farms and to develop a vaccine against eel-pathogenic V. vulnificus strains.
[Front page] [Contents] [Previous] [Next] [Top] |