[Front page] [Contents] [Previous] [Next] |
Vibrio vulnificus in Denmark
3. Isolation of Vibrio vulnificus from environmental samples
3.1 Use of pre-enrichment broths
The choice of including a pre-enrichment step in isolation of V. vulnificus depends on four factors: (i) the expected concentration of V. vulnificus in the samples, (ii) if a quantitative or qualitative result is needed, (iii) the conditions of the cells, and (iv) the level and composition of background flora. The pre-enrichment step should improve the ratio of target to background flora before a selective plating step.
Direct plating
The sensitivity of direct plating procedures are limited by the capacity of the agar surface of the plating medium to absorb inoculum. Therefore, the detection limit for direct plating procedures is higher than for procedures including a pre-enrichment step and samples with low concentrations (less than 10 CFU per gram) of V. vulnificus should be pre-enriched (DePaola et al., 1997b). The sensitivity of direct plating procedures are also limited by the presence of selective substances in the agar, e.g. antibiotics, which may inhibit part of the target flora.
MPN methods
Results obtained by most-probable-number (MPN) methods including a pre-enrichment step are not as precise as results obtained by direct plating procedures. In one study, the measurement variance of a MPN procedure was 0.118 compared to 0.004 when using a direct plating procedure; and even a 10 tube MPN method would still have more than 8 times the measurement variance than the direct plating procedure (DePaola et al., 1997b). Pre-enrichment procedures often give improved recovery of V. vulnificus compared to direct plating on selective agars but the choice of procedure should always depend on the sample type (Kaysner et al., 1989; Biosca et al., 1997b; DePaola et al., 1997b; Arias et al., 1998a).
Alkaline peptone water and TCBS agar
The isolation of pathogenic Vibrio spp. is usually accomplished by culture methods that start with pre-enrichment in alkaline peptone water (APW; 1% peptone, pH 8.6) with 1% NaCl to recover sublethal injured organisms, followed by plating onto thiosulfate-citrate-bile salts-sucrose (TCBS) agar (Colwell, 1984). Earlier studies of the environmental distribution of V. vulnificus, as well as clinical investigations, used this protocol, which was developed for other Vibrio spp. and not optimized for the isolation of V. vulnificus (Oliver et al., 1982). Various enrichment broths have been tested for their capability to support the isolation of V. vulnificus, including APW with various salt concentrations, marine broth, salt-polymyxin B broth, Horie=s broth, Monsur=s broth and glucose-salt-teepol broth (Sloan et al., 1992; Hagen et al., 1994; Biosca et al., 1997b; Arias et al., 1998a). Overnight pre-enrichment in APW with 1% NaCl at 35-37°C generally gives the best recovery of V. vulnificus and this procedure is recommended in the Bacteriological Analytical Manual of the USFDA (US Food and Drug Administration, 1995).The use of APW in combination with cellobiose-polymyxin B-colistin (CPC) agar and modified CPC (mCPC) agar has been reported to be effective in recovering V. vulnificus from oyster and water samples (Tamplin et al., 1991; Tamplin & Capers, 1992).
APW with polymyxin B
Overnight pre-enrichment in APW with polymyxin B (20 U/ml; APWP) gave higher recovery rate than pre-enrichment in regular APW in combination with mCPC agar when analyzing samples of coastal water and sediment in Denmark (Dalsgaard et al., 1996a). APWP and mCPC agar was subsequently used with success for isolation of V. vulnificus from fresh and frozen seafood (Dalsgaard & Høi, 1997; Høi et al., 1998c). However, when analyzing gills, mucus, and intestinal content from cultured diseased eels and wild fish from Danish coastal waters pre-enrichment in APW for 6-8 h proved more favorable than overnight pre-enrichment in APWP (Høi et al., 1998c; unpublished results). This finding may be explained by a lower background flora in samples from healthy wild fish compared to samples of coastal water, sediment, and seafood and therefore there may be less need for adding antibiotics. Samples from diseased eels may be dominated by V. vulnificus and therefore a short pre-enrichment period may be sufficient. Recent studies with heavily infected eels showed that direct plating of tissue samples homogenized in phosphate-buffered-saline (PBS) gave the same or sometimes even better recovery of V. vulnificus than by pre-enrichment (unpublished results). Other studies have also reported that different sample types requires different isolation strategies for V. vulnificus (Biosca et al., 1997b; Kaysner et al., 1989). Arias et al. (1998a) reported that 3 h pre-enrichment in APW with 3% NaCl followed by streaking onto CPC agar was optimal for recovering V. vulnificus from seawater and shellfish samples from the Western Mediterranean coast and that this culture technique gave more positive results than detection by direct PCR. The high salt concentration in the pre-enrichment may favor isolation of V. vulnificus cells adapted to the high salinity in the Mediterranean (around 35l).
Recently, the components of a possible enrichment broth were examined in laboratory testing using pure cultures. An enrichment broth containing 5% peptone, 1% NaCl, and 0.08 % cellobiose amended with 1 to 4 U colistin per ml (PNCC; pH 8.0) was suggested for future field studies (Hsu et al., 1998). Studies with 50 V. vulnificus strains from various sources and countries showed that no strains had a minimal inhibitory concentration (MIC) lower than 779 U colistin/ml (Høi et al., 1998a); thus recovery should not be reduced by adding as high a concentration as 20 U colistin/ml to the enrichment broth (Høi et al., 1998a).
3.2 Use of selective agars
The use of a selective and indicative medium for isolation of V. vulnificus serves two purposes: (i) to allow growth of V. vulnificus while inhibiting growth of more abundant marine species, (ii) to allow differentiation of V. vulnificus from other bacterial species present so suspect colonies can be further identified.
CPC and mCPC selective and indicative agars
New media have been developed and recommended for the isolation of V. vulnificus from the environment (Brayton et al., 1983; Bryant et al., 1987; Massad & Oliver, 1987; Miceli et al., 1993). Cellobiose-polymyxin B-colistin (CPC) agar was first described in 1987 for isolation and differentiation of V. vulnificus (Massad & Oliver, 1987). The medium takes advantage of the colistin and polymyxin B resistance of V. vulnificus and the fermentation of cellobiose for differentiation. Further, high temperature incubation (40°C) eliminates many marine bacteria. CPC agar was clearly superior to TCBS, sodium dodecyl sulfate-polymyxin B-sucrose (SPS) agar, and V. vulnificus enumeration (VVE) agar for the isolation of V. vulnificus from environmental samples (Sun & Oliver, 1995; Oliver et al.,1992). Tamplin et al. (1991,1992) described a less selective modification of the CPC agar termed mCPC with a reduced concentration of colistin. This medium has been reported to be effective in isolating V. vulnificus from environmental sources (Tamplin et al., 1991; Tamplin & Capers, 1992; Dalsgaard & Høi, 1997; Høi et al., 1998c). In Denmark, more than 95% of presumptive colonies on mCPC agar could be identified as V. vulnificus when taking into consideration the typical colony morphology of V. vulnificus on this medium (flat, yellow colonies of approximately 2 mm in diameter) (Høi et al., 1998a; Høi et al., 1998c). However, in Spain only 8% of the presumptive colonies from CPC agar were identified as V. vulnificus by PCR (Arias et al.,1998a). Possible explanations for this disagreement could be: (i) lack of experience in recognizing presumptive colonies, (ii) the background flora in samples from the Mediterranean differs from the background flora in Danish environmental samples, (iii) the description of presumptive colonies as Ayellow colonies surrounded by a yellow halo@ is not sufficient, or (iv) the agar may have been made with different brands of reagents than used in Denmark or in a slightly different way, and this may have affected the colony appearance on the agar (Arias et al., 1998a). In Denmark, we experienced that it was very important for the identification-success rate to include the criterion Aflat@ in the evaluation of suspect colonies (Høi et al., 1998a; Høi et al., 1998c).
Bactericidal activity of polymyxins
Arguments for using both polymyxin B and colistin in a V. vulnificus-selective agar have not been provided (Massad & Oliver, 1987; Tamplin et al., 1991). Colistin and polymyxin B are both fatty acyl decapeptide antibiotics with bactericidal activity against most Gram-negative bacteria and are known by the name Apolymyxins@(Søgaard, 1982). The chemical composition of colistin and polymyxin B differs only in a single amino acid, and their mode of action and microbiological activity are identical (Søgaard, 1982). The basic polymyxin antibiotics act specifically on Gram- negative bacteria by electrostatic and hydrophobic interactions with anionic phospholipids and the lipid A group of lipopolysaccharide (LPS). Ultimately, a lethal effect is exerted by disruption of the cytoplasmic membrane resulting in leakage of periplasmic contents and small cytoplasmic molecules (Søgaard, 1982). The following mechanisms can be proposed to explain why V. vulnificus is relatively resistant to polymyxins: (i) polymyxins may not bind to the cell wall because of the fatty acid composition of the phospholipids in the outer membrane, (ii) the cytoplasmic membrane of V. vulnificus may contain low amounts of phosphatidylethanolamine, a substance that increases susceptibility to polymyxins, (iii) the access of polymyxins to the susceptible cytoplasmic membrane may be blocked by the polysaccharide capsule, or (iv) initial binding of polymyxins occur where divalent cations are present in the outer membrane, and the net ionic charge of the V. vulnificus membrane may differ from that of susceptible bacteria.
CC agar
Høi et al. (1998a) examined a collection of V. vulnificus strains for their sensitivity to colistin and recommended a new medium termed cellobiose colistin (CC) agar. CC agar gave a better V. vulnificus recovery than TCBS, CPC and mCPC agar in laboratory studies with pure cultures and with Danish water and sediment samples. V. vulnificus was isolated from 179 of 446 (40%) APWP pre-enrichments using CC agar and from 154 (35%) of the same 446 APWP pre-enrichments using mCPC agar in the investigation of 26 water samples and 14 sediment samples (Høi et al., 1998a). The recovery rate on CC agar was significantly better than on mCPC agar (Høi et al., 1998a). TCBS agar gave a very low plating efficiency (1%) of both clinical and environmental V. vulnificus strains and should not be recommended for the isolation of V. vulnificus (Høi et al., 1998a). This is in agreement with other reports of low recovery of V. vulnificus on TCBS (Brayton et al., 1983; Beazley & Palmer, 1992). Fig. 3 shows an overview of the methods used for isolation of V. vulnificus in Denmark.
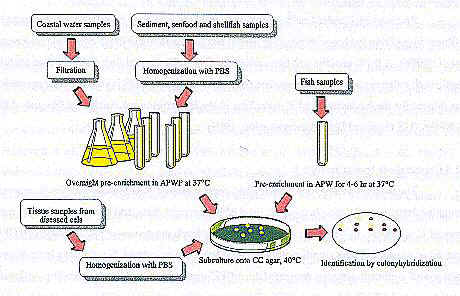
Figure 3: Flow diagram for isolation of V. vulnificus in Denmark
Although, V. vulnificus has been described to be resistant to colistin and polymyxin B (Massad & Oliver, 1987), plating efficiency2 experiments showed that CC agar, which has the lowest concentration of colistin compared to the other media tested, gave the best recovery of V. vulnificus. The results showed that V. vulnificus was inhibited by increasing concentrations of colistin and polymyxin B and that a proportion of the V. vulnificus strains present in Danish marine environments were inhibited by the concentration of colistin and polymyxin B in CPC and mCPC agar, and that the use of CC agar increased the isolation rate of V. vulnificus. Even though MIC testing suggested that mCPC and CC agar both were suitable for the use in isolation of V. vulnificus, further investigations proved that CC agar was superior to mCPC in isolation of V. vulnificus from samples of coastal water and sediment (Høi et al., 1998a). This underlines the importance of testing selective agars for Aruggedness@ using a variety of naturally contaminated samples.
The confirmation rate of presumptive isolates from CC agar was as high as previously reported for mCPC (approximately 95%) (Høi et al., 1998a).
Isolation strategy
Recovery of V. vulnificus from environmental samples may be influenced by the choice of diluent in addition to choice of selective media and pre-enrichment broth according to Azanza et al. (1996). A 0.1% peptone solution containing 3% NaCl gave higher recovery than PBS in both broth cultures and oyster homogenate (Azanza et al., 1996). PBS is currently recommended as diluent for the enumeration of V. vulnificus in the Bacteriological Analytical Manual of the USFDA (US Food and Drug Administration, 1995).
3.3 Conclusions from Chapter 3
The isolation strategy for recovery and enumeration of V. vulnificus from environmental samples depends on the sample type, the level of background flora, and the expected concentration of V. vulnificus. In Denmark, where V. vulnificus levels are generally low (less than 10 CFU per ml or gram), CC agar significantly increased the isolation rate of V. vulnificus from coastal water and sediment samples compared to mCPC agar when used in combination with pre-enrichment in APWP. More than 95% of the presumptive colonies on CC agar were identified as V. vulnificus with the VVAP probe. TCBS gave very low plating efficiencies and can not be recommended for the isolation of V. vulnificus.
[Front page] [Contents] [Previous] [Next] [Top] |