|
[Front page] [Contents] [Previous] [Next] |
Toxicological Evaluation and Limit Values for Nonylphenol, Nonylphenol Ethoxylates, Tricresyl, Phosphates and Benzoic Acid
1. General description
1. General description
1.1 Identity
1.2 Physical/chemical properties
1.3 Production and use
1.4 Environmental occurrence
1.5 Environmental fate
1.6 Human exposure
1.1 Identity
Nonylphenol (NP) is the commercially most important member of the group of alkyl
phenols. The term "nonylphenol" represents a large number of isomeric compounds,
varying in the point of attachment of the nonyl group to the phenol molecule, and in the
degree of branching in the nonyl moiety. Commercially produced nonylphenols are
predominantly 4-nonylphenol (para-nonylphenol) with a varied and undefined degree of
branching in the alkyl group, while very little straight chain nonylphenol is present
(EU-RAR 1998).
Similarly, nonylphenol ethoxylate (NPE) is the most important alkylphenol ethoxylate. NPE
accounts for approximately 85% of alkylphenol ethoxylate production (Anonymous 1997). An
NPE is composed of a nonyl chain, usually branched, attached to a phenol ring (hydrophobe
moiety) which is combined, via an ether linkage, with one or more ethylene oxide or
polyoxyethylene units (hydrophile moiety).
A particular NPE is identified by the length of the polyoxyethylene chain (average number
of ethylene oxide units, abbreviated EO). NPEs of a particular average ethylene oxide
chain length, produced by different manufacturers, may differ in ranges of ethylene oxide
chain lengths.
Molecular formula: NP: C15H24O
NPE: C15H24O(C2H4O)n
Structural formula:
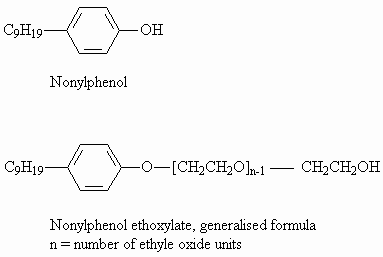
| Molecular weight: | NP: 220 NPE: 220 + n x 44 where n is the number of ethoxylene units (n can vary from 1-100). |
| CAS-no.: | Nonyl phenol: 84852-15-3 Nonylphenol ethoxylate: A number of CAS numbers exist for the various NPEs with EO=1: 104-35-8, 27986-36-3 with EO>1: 9016-45-9, 26027-38-3, 68412-54-4 with EO=2: 20427-84-3, 27176-93-8 with EO=4: 7311-27-5 with EO=8: 27177-05-5 with EO=9: 26571-11-9 with EO=10: 27177-08-8 |
| Synonyms: | Nonylphenol: NP; isononylphenol; phenol, nonyl-, branched;
para-nonylphenol; monoalkyl (C3-9) phenol. Nonylphenol ethoxylate: NPE; nonylphenol polyoxyethylene ether; nonylphenol polyethylene glycol; nonylphenol polyethylene glycol ether; polyoxyethylene nonylphenol ether. |
1.2 Physical / chemical properties
| Description: | NP is a clear to pale yellow viscous liquid with a slight phenolic odour
(Merck 1996). NPEs with between 1 and 13 ethylene oxide units are liquid. NPEs with 14 and 15 ethylene oxide units are paste-like liquids. Viscosity increases with increasing ethylene oxide chain length, and NPEs with 20 or more ethylene oxide units are waxy solids. Colour varies from colourless to light amber. NPEs with higher numbers of ethylene oxide units are opaque. (CIR 1983). |
| Purity: | NP: 90% w/w. Impurities: 2-nonylphenol (5% w/w); 2,4-dinonylphenol (5% w/w) (EU-RAR
1998). NPE: no data have been found. The same impurities as in the starting material (NP) may be expected. Possible content of traces of ethylene oxide or its degradation product, 1,4-dioxane (CIR 1983). |
| Melting point: | NP: Substances of this type (oily) do not have a clear melting point. Various values
have been reported, probably owing to differences in the alkyl chain structure. The values
are: -10° C, -8° C, 10 ° C , and <20 ° C. NPE: "Solidification points" for nonylphenols with varying average lengths of the ethylene oxide chain: -6° C (EO=7), -1.0-1.1 ° C (EO=8.5), -2.5-4.0 ° C (EO=9.5), 6 ° C (EO=13), 19-21 ° C (EO=15), -5.0- -3.0 ° C (EO=30), 67 ° C (EO=40). |
| Boiling point: | NP: 290-310° C. However, some thermal decomposition probably occurs before this
temperature is reached. NPE: - |
| Density: | NP: 0.95 g/ml (at 20° C) NPE: 0.98-1.08 (at 25° C) |
| Vapour pressure: | NP: 0.0023 mmHg (0.3 Pa) at 20° C NPE (9 EO): <0.075 mm Hg (<10 Pa) |
| Concentration of saturated vapours: | - |
| Vapour density: | - |
| Conversion factor: | 1 ppm = 9.15 mg/m3 20° C 1 mg/m3 = 0.109 ppm 1 atm |
| Flash point: NP: | 141-155° C NPE: >150 ° C |
| Flammable limits: | - |
| Autoignition temp.: | NP: 370° C NPE: (with 9 ethylene oxide): 425 ° C |
| Solubility: | NP: Water: Practically insoluble, 6 mg/100 ml (at 20° C). NPE: Water: >0.1 g/100 ml (at 20° C) (9 EO). For NPEs, water solubility is increased by alkyl branching and is directly proportional to the number of ethylene oxide units. NPEs are water soluble when the number of ethylene oxide units exceeds 6. The NPE most commonly used in cleaning products has 9-10 ethylene oxide units. |
| logPoctanol/water: | NP: 4.48 NPE: - |
| Henry’s constant: | NP: 1.02 Pa m3 /mole NPE (with 6 ethylene oxide units):4.1 x 10-12 atm x m3/mol (HSDB 1997). |
| pKa-value: | NP: Estimated value 10.25 NPE: - |
| Stability: | - |
| Incompatibilities: | - |
| Odour threshold, air: | - |
| References: | CIR (1983), EU-RAR (1998), HSDB (1997), IUCLID (1996), Superfos Kemi a/s (1997) Talmage (1994). |
1.3 Production and use
Nonylphenol
NP is manufactured by reacting mixed nonenes with phenol. The nonyl group, which may be branched or linear, may be linked to the ring either ortho, meta or para to the OH group. In the EU, 78,000 tonnes of nonylphenol were produced in 1994 (EU-RAR 1998).
NP is used as a starting material in the synthesis of NPEs, and as a monomer in polymer production.
Nonylphenol ethoxylate
NPEs are manufactured by reacting NP with ethylene oxide. A polyethylene oxide chain of
any desired length can be built up by continued introduction of ethylene oxide into the
reaction mixture. Such a reaction yields NPEs with a mixture of ethylene chain lengths,
and the number of ethylene units used to describe the product is the average number
(Swisher 1970). During base catalysed ethoxylation of nonylphenol, ethylene oxide
preferentially reacts with the free nonylphenol and only when this has all reacted do
longer ethylene oxide chains form. Nonylphenol ethoxylates have much narrower homologue
distributions than alcohol ethoxylates (ICI datasheet for Synperonic NP surfactants).
880 million pounds (4 mio metric tonnes) of alkyl phenol ethoxylates are used annually
throughout the world. 80-85% are sold as nonylphenol ethoxylate (NPE), over 15% as
octylphenol ethoxylate, and 1% each as dinonylphenol and dodecylphenol ethoxylates
(Anonymous 1997).
In the EU, 109,808 tonnes of nonylphenol ethoxylates were produced in 1994 (EU-RAR 1998).
NPEs are non-ionic surfactants. They are used industrially, as ingredients in institutional cleaners and detergents, and in household cleaning and personal-care products. Industrially, NPEs are used for emulsion polymerisation and polymer stabilisation, textile processing, in agricultural chemicals, pulp and paper processing, metal and mineral processing, latex paints, wetting agents and emulsifiers, foaming agents, inks, adhesives, and pharmaceuticals (Anonymous 1997).
1.4 Environmental occurrence
NP and NPE are not known to exist in nature.
NP is released to the environment from production and use as a chemical intermediate, in
the polymer industry, and as nonylphenol itself. The major part (95%) is released to
water. The proportion released to air is low (1%), while approximately 4% is released to
soil. Further, NP is released from NPE. NP has a low vapour pressure, a low water
solubility, and has a strong tendency to adsorb to soils and sediments.
NPE is primarily released to the environment as a result of use. As for NP, the major part
(85%) is released to water, while the release to soil amounts to 13%, and to air 2.5%
(EU-RAR 1998).
Air
NP: NP has not been measured in the atmosphere (EU-RAR 1998).
NPE: No data have been found.
Water
NP has been found in surface water, sea water, and ground water (data from Switzerland, United Kingdom, USA, and Croatia). A typical value was 1 mg/l, and the highest value measured was 180 mg/l (River Aire, UK) (EU-RAR 1998).
NPEs have been found in European surface water in concentrations ranging from below the limit of detection to 59mg/l (Talmage 1994).
Soil
In sludge from waste water treatment plants, concentrations of NP in the order of gram per kg dry weight have been found. In soil treated with sludge, 4.7 mg/kg has been found (EU-RAR 1998).
In river sediments, NPE concentrations of the order of mg per kg dry weight have been measured (Talmage 1994).
Foodstuffs
NP was detected in raw beef samples, concentration not stated (HSDB 1997).
1.5 Environmental fate
NP is not readily biodegradable. Several mechanisms of microbial aromatic ring degradation have been reported, the most common being formation of catechol from phenol, followed by ring scission between or adjacent to the two hydroxyl groups (Talmage 1994).
NPEs may degrade into nonylphenol. During degradation NPEs’ ethylene oxide units are cleaved off the ethylene oxide chain until only short-chain NPE remain, typically mono- and diethylene oxides. Oxidation of these oligomers creates the corresponding carboxylic acids. This leaves several degradation products: short-chain ethoxylates, their carboxylic acids, and nonylphenols (Anonymous 1997). The rate of biodegradation seems to decrease with increasing length of the ethylene oxide chain (Talmage 1994).
Air
NP released to the atmosphere will exist in the vapour phase and is thought to be degraded by reaction with photochemically produced hydroxyl radicals, with a calculated half-life of 0.3 days.
No data have been found for NPE.
Water
Abiotic degradation of NP is negligible. Biodegradation does not readily take place. The half-life in surface water may be around 30 days (EU-RAR 1998).
No data have been found for NPE.
Soil
NP in soil will have no mobility (HSDB 1997). Biodegradation of NP in soil is slow, and may occur in steps, each step characterised by a certain rate of degradation, as shown in field tests. The half-life in soil is probably around 30 days (EU-RAR 1998).
No data have been found for NPE.
Bioaccumulation
NP bioconcentrates to a significant extent in aquatic species. Excretion and metabolism is rapid (EU-RAR 1998).
No data have been found for NPE.
1.6 Human exposure
The possible routes of human exposure to NP and NPEs are dermal contact and inhalation by workers involved in the manufacture and use, dermal and inhalation exposure of consumers from household pesticide products, dermal contact to cleaning products and cosmetics, mucous membrane contact to spermicides; inhalatory exposure via the environment through air, and oral exposure via the environment through drinking water and food.
|
[Front page] [Contents] [Previous] [Next] [Top] |