[Front page] [Contents] [Previous] [Next] |
Toxicological Evaluation and Limit Values for 2-Ethylhexyl acrylate, Propylene carbonate, Quaternary ammonium compounds, Triglycidyl isocyanurate, and Tripropyleneglycol diacrylate
Evaluation of health hazards by exposure to
Triglycidyl isocyanurate
and estimation of a limit value in air.
Pia Berthelsen
The Institute of Food Safety and Toxicology
Danish Veterinary and Food Administration
1 General description
Commercial (technical) grade TGIC is a mixture of two optical stereoisomers, alpha and beta, which have different physicochemical properties. There are two main technical grades of TGIC. These are Araldite PT 810 (also known as TK 10622), and TEPIC. (NOHSC 1994).
| Molecular formula: | C12H15N3O6 |
| Structural formula: | 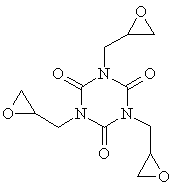 |
| Molecular weight: | 297.3 |
| CAS-no.: | 2451-62-9 |
| Synonyms: | Glycidylisocyanurate Isocyanuric acid triglycidyl ester N,N’,N’’- Triglycidylisocyanurate Teroxirone (a -TGIC) s-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris (2,3-epoxypropyl) TGT 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris (oxiranylmethyl) Tris (2,3-epoxypropyl) isocyanurate 1,3,5-Tris (2,3-epoxypropyl)-s-triazine- 2,4,6(1H,3H,5H)-trione 1,3,5-Triglycidylisocyanurate 1,3,5-Triglycidylisocyanuric acid 1,3,5-Tris (oxiranylmethyl)-1,3,5-triazine- 2,4,6(1H,3H,5H)-trione |
1.2 Physical / chemical properties
| Description: | White, granular solids with no discernible odour. | |
| Purity: | 90% (TEPIC) >97% (Araldite PT 810, TK 10622) | |
| Melting point: | 90-125° C (TEPIC) 95° C (Araldite PT 810, TK 10622) 105° C (a -TGIC) 156° C (b -TGIC) |
|
| Boiling point: | - | |
| Density: | 1.42 g/ml (TEPIC) 1.46 g/ml (Araldite PT 810, TK 10622) |
|
| Vapour pressure: | 0.05 x 10-6 mmHg (7.2 m Pa) at 20° C (Araldite PT 810, TK 10622) | |
| Concentration of saturated vapours: | - | |
| Vapour density: | - | |
| Conversion factor: | - | |
| Flash point: | >170° C (TEPIC) | |
| Flammable limits: | - | |
| Autoignition temp.: | >200° C (TEPIC) | |
| Solubility: | water: | 9 g/l (TEPIC at 25°C) 10 g/l (a -TGIC at 20°C) g/l (b -TGIC at 20°C) |
| epichlorohydrin: | <220 g/l (Araldite PT 810 at 25° C) | |
| DMSO: | >100 g/l at 20° C | |
| methanol: | 73 g/l (Araldite PT 810 at 25°C) < 1 g/l at 20°C | |
| toluene: | 30 g/l (Araldite PT 810 at 25°C) < 1 g/l at 20°C | |
| isopropanol: | 10 g/l (Araldite PT 810 at 25°C) | |
| 95 % ethanol: | < 1 g/l at 20°C | |
| acetone: | < 1 g/l at 20°C | |
| logPoctanol/water: | - 0.8 (TEPIC) | |
| Henry’s constant: | - | |
| pKa-value: | - | |
| Stability: | TGIC contains three epoxide groups which give alkylating and cross-linking properties to the chemical. TGIC, in its molten state, reacts easily with various functional groups like amines, carboxylic acids, carboxylic acid anhydrides, phenols, thiols and alcohols in the presence of catalysts or promoters. It can be polymerised by catalysts. Molten TGIC may undergo hazardous autopolymerisation after heating to >120°C for more than 12 hours. | |
| Incompatibilities: | - | |
| Odour threshold, air: | - | |
| Particle size distribution: | Technical grade granules (TEPIC): 0.003% <10 µm, 0.12% <150 µm, 99.6% > 400 µm. Powder coatings: |
|
| References: | Atassi et al. (1980), Chemfinder (1999), NOHSC (1994), NTP (1991), RTECS (1999), WHO (1998). |
|
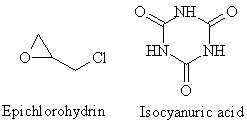
TGIC is produced from epichlorohydrin and isocyanuric acid using sodium hydrate and methanol (Nishioka et al. 1988). No production of TGIC occurs in Denmark.
 |
TGIC has been used as a curing agent for polyester resins in weather-resistant powder coatings in Europe for about 20 years. In Denmark TGIC has an extended use in powder coatings. A powder coating contains up to 10 % TGIC. It is sprayed directly onto metal objects by an electrostatic process. Powder coated objects include office and garden steel furniture, car parts, metal fencing, window and door frames, shelving, electrical equipment, and domestic appliances such as refrigerators, washing machines and ovens. (NOHSC 1994). TGIC is also used in electrical insulation materials, resin moulding systems, laminated sheetings, printed circuits, tools, inks, adhesives, lining materials, stabilisers for plastics and amine captures (Nishioka et al. 1988).
The alpha isomer of TGIC was used as an experimental anti-tumour agent under the names of Teroxirone, alpha-TGT, and Henkels compound. Clinical use of the alpha isomer was not pursued. (NOHSC 1994).
Environmental exposure to TGIC resulting form normal use in spray painting workplaces is expected to be low. Plants that formulate TGIC powder coatings do release some TGIC to the atmosphere and sewer. One Australian plant has estimated that it annually releases 26 kg to the atmosphere, 15 kg to sewer, and 7.5 tonnes to landfill. (NOHSC 1994).
As TGIC is an epoxide, any residues which enter the environment are expected to be rapidly degraded, either through microbial action or abiotic hydrolysis. (NOHSC 1994). However, when tested in a modified Sturm test, TGIC is not ready biodegradable (Ciba-Geigy Ltd. 1988 - quoted in NOHSC 1994). The result is likely to reflect complete primary degradation, with slow opening of the triazine ring restricting the rate of complete mineralisation. (NOHSC 1994).
Bioaccumulation
The reactivity of TGIC precludes any possibility of bioaccumulation (NOHSC 1994).
No data were found.
2 Toxicokinetics
Inhalation
Only 0.003 per cent of technical grade TGIC is < 10 µm and only 2.3 per cent of TGIC powder coatings is < 7 µm. The particles in this same powder coating were all < 130 µm and therefore have the potential to be inhaled. (NOHSC 1994). No toxicokinetic inhalation studies have been performed.
Oral intake
When 14C-labelled a -TGIC was administrated to rabbits by stomach tube, no parent drug was detected in plasma. Plasma concentration of metabolites were lower compared to those observed following i.v. administration. (Ames et al. 1984).
In a study in mice at least 17% of the administered dose was absorbed within 24 hours. TGIC was distributed to the liver, stomach and testes (the only tissues studied). Eight hours after treatment no free TGIC was detected. (Ciba-Geigy 1990 - quoted in WHO 1998).
Dermal contact
Absorption is probably low following dermal contact indicated by a high LD50 value (NOHSC 1994).
Metabolism
TGIC metabolism seem to involve hydrolysis catalysed by microsomal epoxide hydrolase. In a recently conducted study, induction of epoxide hydrolase activity in rat livers was associated with increased hydrolysis of TGIC. However, this study only examined oral and intraperitoneal administration and did not consider dermal and inhalational exposure. (Ciba-Geigy ltd 1993 - quoted in NOHSC 1994).
a -TGIC was metabolised in vitro by rat liver, but not lung, microsomal preparations by an NADPH-independent pathway. Epoxide hydrolysis metabolites were detected in the microsomal incubations, and cyclohexene oxide, a known inhibitor of microsomal epoxide hydrolases, inhibited a -TGIC metabolism. (Ames et al. 1984).
Epoxide hydrolase activity in some human tissues may be higher than in rodent tissues (Seidegard et al. 1986, Schmidt & Loeser 1985, Glat & Oesch 1987, Pacifici et al. 1988 - all quoted in NOHSC 1994). There is considerable variation in epoxide hydrolase activity between tissues and also significant (approximately 100-fold) interindividual variation of epoxide hydrolase activity in humans (Mertes et al. 1985 - quoted in NOHSC 1994).
Excretion
When 14C-labelled a -TGIC was administrated to rabbits by stomach tube, no parent drug was detected in plasma. Twenty-four-hour urinary recovery of radioactivity was about 30%. (Ames et al. 1984).
When humans were given i.v. administration of a -TGIC in doses up to 500 mg/m2 (= 15 mg/kg bw, calculated by using a standard weight of 60 kg and a standard surface area of 1.85 m2), less than 1 % a -TGIC is recovered in 24-hour urine. (Ames et al. 1984). In a phase I anticancer clinical trial with a -TGIC, i.v. infusion of 2000 mg/m2 (= 62 mg/kg bw, calculated) administered over 2-3 hours was measured during the study to correspond to a plasma concentration of about 1mg/ml (Piccart et al. 1981).
When 14C-labelled a -TGIC is administrated to rabbits by i.v. infusion, twenty-four-hour urinary recovery of parent drug is < 1%, while urinary recovery of 14C total radioactivity is 60 to 70%. (Ames et al. 1984).
Half-life
Rapid plasma elimination (t½< 5min.) and total body clearance (about 5 litres/min.) are observed following i.v. administration of a -TGIC to humans in doses up to 500 mg/m2 (= 15 mg/kg, calculated). When 14C-labelled a -TGIC is administrated to rabbits by i.v. infusion, plasma disappearance of parent drug is very rapid (t½< 5min.), while metabolites in the plasma are eliminated at a much slower rate (t½> 60 min.). (Ames et al. 1984).
The cytotoxicity of TGIC is probably related to the alkylating capacity of the epoxide moieties (Ames et al. 1984). a -TGIC was shown to alkylate a model compound 4-(p-nitrobenzyl)pyridine. E. coli strains defective in UV repair function were much more sensitive to a -TGIC than were the nondefective strains, suggesting that DNA may be the target of drug action. However, no in vitro interaction between a -TGIC and DNA or its components could be detected under physiological conditions by using a variety of biochemical and physicochemical techniques. (Wu & Le Pecq 1983). Two in vivo studies have demonstrated that TGIC is capable of covalently binding to DNA in mouse liver, stomach and testis tissues following oral administration and in rat liver tissue following intraperitoneal or oral administration. (Both quoted in NOHSC 1994).
3 Human toxicity
Inhalation
No data were found.
Oral intake
No data were found.
Dermal contact
No data were found.
Intravenous application
In human clinical trials a -TGIC was administered intravenously to cancer patients at doses up to 2700 mg/m2 (= 83 mg/kg b.w., calculated) using a variety of dosing regimens. The dose-limiting factor was local thrombophlebitis which was noted down to about 200 mg/m2 (= 6 mg/kg b.w., calculated). Its severity seemed to increase with higher doses and was most pronounced in patients previously treated with a -TGIC. At high doses a -TGIC induced leukopenia which resulted in life-threatening infections at doses of 2400 and 2700 mg/m2 (= 74 and 83 mg/kg b.w., calculated). Myelosuppression in patients given more than 1500 mg/m2 (= 46 mg/kg b.w., calculated) was observed in one of the trials where a -TGIC was more concentrated. Other toxic effects were mild to moderate and consisted of nausea, vomiting and hair loss. Nausea and vomiting was seen in one patient already at a dose of 33 mg/m2 (= 1 mg/kg b.w., calculated). (Dombernowsky et al. 1983, Neidhart et al. 1984, Piccart et al. 1981, Rubin et al. 1987).
3.2 Long term toxicity
Inhalation
TGIC has caused occupational asthma in a healthy 36-year old non-smoking man who worked mainly as a spray painter, using a powder paint containing 4% TGIC. Before the examinations he had been painting 5-8 hours daily for about 7 years. He was diagnosed with contact dermatitis as well as occupational asthma. (Piirilä et al. 1997).
Oral intake
No data were found.
Dermal contact
Allergic contact dermatitis due to exposure to TGIC is the main reported human health effect. Sixteen case reports exist in the literature. Patch tests confirmed that TGIC was the causative agent of the skin problems. (Craven et al. 1999, Dooms-Goossens et al. 1989, Foulds & Koh 1992, Jolanki et al. 1994, Mathias 1988, McFadden & Rygroft 1993, Munro & Lawrence 1992, Nishioka et al. 1988, Wigger-Alberti et al. 1997). Workers may become sensitised to TGIC from short-term exposure (less than 12 months) to the chemical in the production (Nishioka et al. 1988; 1 person) or the manufacture (Foulds & Koh 1992, Munro & Lawrence 1992, Wigger-Alberti et al. 1997; 9 persons) of it and during use of TGIC-containing powder paints (Dooms-Goossens et al. 1989, Mathias 1988, McFadden and Rygroft 1993, Piirilä et al. 1997; 4 persons). A chemist (Craven et al. 1999) and a silk-screen painter (Jolanki et al. 1994) working in the manufacture of circuit boards developed allergic dermal reactions to TGIC as well as to epoxy resins and acrylates after 12-15 years of exposure.
In the early nineties, the level of TGIC dust was monitored at several workplaces where TGIC was used throughout Australia. Different work practices significantly affected the level of exposure that was found to be from <0.001 to 6.5 mg/m3. Health effects in workers exposed to TGIC included allergic dermatitis which was confirmed with patch tests, aggravated asthma, nasal, eye and throat irritation, skin rash and nose bleed. Of 232 spray painters in Sydney, 11 had suffered health problems, mostly skin rashes, as a result of using TGIC powder coatings. (NOHSC 1994).
Epidemiological studies
No data were found.
3.3 Reproductive and developmental effects
No data were found.
3.4 Mutagenic and genotoxic effects
No data were found.
3.5 Carcinogenic effects
No data were found.
4 Toxicity, animal data
4.1 Short term toxicity
Inhalation
Two single, four-hour nose-only inhalation exposure studies in 10 rats of each sex per dose using technical grade TGIC revealed a LC50 of 300->650 mg/m3. A whole body exposure study in 5 male mice resulted in a LC50 of 2000mg/m3. More than 60% of the TGIC particles were within the respirable range (<7mm). No animals died when 2 rats of each sex were exposed nose-only for 30 minutes to technical grade TGIC at an atmospheric level of 3200 mg/m3. (All quoted in NOHSC 1994).
Groups of 12 male CD-1 mice were exposed nose-only to atmospheres containing 0, 10, 40 or 140 mg/m3 of technical grade TGIC for six hours/day for five days. More than 79% of the TGIC particles were less than 4 m m and therefore respirable. Two animals from each group were killed six hours after the final exposure to assess cytotoxicity. Approximately 400 metaphase germ cells were scored per animal. Cytotoxicity in germ cells was not increased in any of the treated groups indicating a lack of effect of TGIC on germ cell survival even at the two highest doses where high mortality of the rest of the mice were observed. The surviving animals were observed over a 17 day recovery period. Clinical signs of toxicity, increased body weight losses and high mortality were observed in the intermediate and high dose groups. The clinical signs included hunched posture, piloerection, lethargy and dyspnoea. Dead animals had lung damage. Besides pathology for animals dosed with 140 mg/m3 included pale livers, pale kidneys, and congestion of the small intestine. In the low dose group only one animal died and the death was unrelated to treatment. One animal had slightly reddened lungs in the low dose group. (Safepharm Laboratories Ltd. 1991 - quoted in NOHSC 1994).
A nose-only five day inhalational chromosomal aberration study was conducted in which groups of 10 male CD-1 mice were exposed to technical grade TGIC (dose of 7.8 mg/m3) or 10% TGIC powder coating (dose of 95.3 or 255.3 mg/m3) for 6 hours/day. The inhaled dust particles were for the main part respirable. There were no deaths during the study. The only adverse clinical signs noted were confined to one animal exposed to 255.3 mg/m3 of 10% TGIC powder coating on day four. This animal displayed hunched posture and piloerection. Bodyweight gain was unaffected in all groups. For the groups inhaling TGIC, cytotoxicity in spermatogonial cells was not significantly increased at the doses tested. (Safepharm Laboratories Ltd. 1992 - quoted in NOHSC 1994).
In an inhalational chromosomal aberration study, groups of 10 male CD-1 mice were whole body exposed to technical grade TGIC at concentrations of 0, 2.5, 10 and 50 mg/m3 for 6 hours/day for five days. The particle size range of TGIC was 2.5 to 3.5 µm. No deaths occurred and no adverse clinical signs were observed in the TGIC-treated animals. Body weight losses occurred in all groups. The study suggests that TGIC was cytotoxic to spermatogonial cells at doses of 10 and 50 mg/m3 because of a low number of animals in these groups with a sufficient number of scorable cells for chromosome aberrations. However, the cytotoxic ratios were not calculated. (Bushy Run Research Center 1992 - quoted in NOHSC 1994).
Oral administration
Five animals of each sex per dose were given technical grade TGIC and observed for 14 days to establish acute oral LD50. For rats LD50 was <100-950 mg/kg (4 studies). For hamsters LD50 was 1670 mg/kg (1 study). (All quoted in NOHSC 1994).
For seven consecutive days 10 CFE rats per sex per dose were fed 0, 54 or 216 mg/kg b.w./day (males) or 0, 43, or 172 mg/kg b.w./day (females) of technical grade TGIC dissolved in dimethylsulfoxide (DMSO) by gavage. Gross pathology was recorded for the lungs, kidney, liver, stomach, and intestines. Renal tubular, gastric and duodenal damage was observed in both sexes in the high dose groups. In the low dose groups renal tubular damage also occurred. (Shell Research Ltd. 1971 - quoted in NOHSC 1994 and WHO 1998).
A chromosomal aberration study was conducted in which a group of 10 male CD-1 mice were orally administered 115.0 mg/kg of technical grade TGIC for 5 days. There were no deaths during the study. Adverse clinical signs were noted in all animals treated with TGIC on day five. These animals displayed hunched posture and piloerection. Bodyweight gain was unaffected. TGIC significantly increased cytotoxicity in spermatogonial cells. (Safepharm Laboratories Ltd. 1992 - quoted in NOHSC 1994).
Dermal contact
In three acute dermal toxicity studies in 3-5 rats per sex per dose technical grade TGIC (in carboxymethylcellulose or arachid oil) was applied to intact shaven skin prior to the application of a semi-occlusive dressing. After 24 hours the skin was washed clean and the animals were observed for 14 days. LD50 was >2000 mg/kg. (All quoted in NOHSC 1994).
Very slight erythema and oedema were observed in five studies of acute skin irritation in rabbits. In each study, three rabbits of each sex were exposed for 24 hours to technical grade TGIC in the form of 0.5 ml as a 50% solution in polypropylene glycol or 0.5 g powder moistened with distilled water. The rabbits were observed for at least 72 hours. (All quoted in NOHSC 1994).
In two studies, skin sensitisation was observed in 4 out of 20 (half of each sex) or 12 out of 20 guinea pigs induced with technical grade TGIC and challenged two weeks later. For the induction in the first study, adjuvant was injected intradermally in the neck area and 40 mg TGIC was applied topically over the injection site and occluded for 24 hours. One week later, 120 mg of TGIC (in vaseline) was applied occlusively to the injection site for 48 hours. In the second study, the induction was carried out by injecting 0.5 mg of TGIC in arachid oil and 0.5 mg of TGIC in adjuvant intradermally to the shoulder area of each animal. One week later 100-150 mg of TGIC in arachid oil was applied topically to the injection site and held under an occlusive wrap for 48 hours. For the challenge, 20 mg (first study) or 50-100 mg (second study) of TGIC (in arachid oil for the second study) was applied occlusively for 24 hours to the flank. A positive response was seen as slight to moderate erythema and/ or oedema. (Both quoted in NOHSC 1994).
Eye contact
Severe eye reactions (moderate to severe corneal opacity, redness, chemosis and discharge) were noted in all treated, unwashed eyes in two out of three studies in which 3 rabbits of each sex in each study had 0.1 g of technical grade TGIC placed in the conjunctival sac of the left eye. The untreated right eye served as control. In three of the six rabbits in each study, the treated eye was flushed with saline. Eyes were assessed for irritation at 24, 48, 72 hours and four and seven days post-treatment. (All quoted in NOHSC 1994).
4.2 Long term toxicity
No data were found.
4.3 Reproductive and developmental effects
In a dominant lethal test, technical grade TGIC (in arachid oil) was administered by single gavage at doses of 0, 160 and 480 mg/kg b.w. to groups of 20 male Tif MAGf(SPF) mice. These mice were mated over three periods of 6 days to 40 female mice per dose group. The female mice were replaced at the end of each period. Females were killed on day 14 of gestation and the numbers of live and dead foetuses and foetal resorptions were noted. Females mated to males given 480 mg/kg of TGIC during the first period showed a significant increase in the number of embryonic deaths, compared with the negative control. No increase was seen in the females mated in the second and third periods at the same dose, nor in the females in the other treated groups. (Ciba-Geiga Ltd. 1986 - quoted in NOHSC 1994).
In a second dominant lethal test, technical grade TGIC (in peanut oil) was administered by single gavage at doses of 0, 138, 275 and 550 mg/kg b.w. to groups of 20 male ICR mice. These mice were mated over three periods of 5 days to 40 female mice per dose group. The female mice were replaced at the end of each period. No significant difference was observed in the number of embryonic deaths in test groups compared to the negative control. (Hazleton Laboratories America Inc. 1989 - quoted in NOHSC 1994).
In a third dominant lethal test, 10% TGIC in powder coating (doses of 0, 100, 1000, or 1700 mg/m3) was administered by whole body inhalational exposure to dust for six hours per day for five consecutive days to 30 male CD-1 mice per group. Following treatment each male was mated to two virgin females for eight weekly periods with the females being replaced at the end of each period. No increase in embryonic deaths was observed except in the positive control group and therefore TGIC did not induce heritable dominant lethal mutations under the conditions of the experiments. (Bushy Run Research Center 1991 - quoted in NOHSC 1994).
In a fourth dominant lethal test, technical grade TGIC (doses of 0, 2.5, 10, or 50 mg/m3) was administered by whole body inhalational exposure to dust for six hours per day for five consecutive days to 30 male CD-1 mice per group. Following treatment each male was mated to two virgin females for eight weekly periods with the females being replaced at the end of each period. TGIC did not induce heritable dominant lethal mutations. There was a slight increase in the number of non-viable implants and early resorptions in the third mating week, but this was not statistically significant. The study showed reduced fertility in males at 10 and 50 mg/m3 as seen by reduced number of males impregnating females in some of the mating weeks and a non-significant ten percent reduction in testes weight in the 50 mg/m3 group. The reductions in fertility were consistent with an effect on mature sperm, maturing spermatids and Type B spermatogonia at the 50 mg/m3 level and with Type B spermatogonia at the 10 mg/m3 level. (Bushy Run Research Center 1992 - quoted in NOHSC 1994).
In a 13-week toxicity/fertility study, groups of 10 male rats were given diets containing 0, 10, 30, or 100 ppm (0, 0.5, 1.5 or 5 mg/kg b.w., calculated) TGIC. This study followed a preliminary 19-day range-finding investigation in which signs of toxicity were observed in animals administered diets containing 160 or 640 ppm TGIC. In the full study after 64 days of treatment, each male was placed with two females until mating occurred. The females were then allocated to two subgroups (caesarean or normal delivery) on day 19 of pregnancy. Females from the caesarean group were killed on day 20 of pregnancy and the ovaries and uterus examined . The other group was allowed to deliver normally and the pups were examined for clinical signs and development. Between 22 and 25 days postpartum, the females in the normal delivery group were sacrificed and examined. In males at autopsy, all organs were examined in the high-dose and control groups. No exposure-related clinical effects or death were observed. Body weight gain was slightly lower over the first 6 weeks in animals from the 100 ppm test group. A dose-related reduction in the number of spermatozoa was noted but the spermatozoa viability was unchanged. No exposure-related infertility was noted in males, and no effects on embryonic and pup development were observed in the offspring. The highest concentration used in this study was not a maximum tolerated dose. (CIT 1995 - quoted in WHO 1998).
4.4 Mutagenic and genotoxic effects
In vitro tests
Technical grade TGIC (dissolved in DMSO) was positive in two Ames tests using five strains (TA1535, TA1538, TA1537, TA98 and TA100) of Salmonella typhimurium when tested both with and without metabolic activation systems. (Hazleton 1987, Ciba-Geiga Ltd. 1982 - both quoted in NOHSC 1994).
When tested in mammalian cells, technical grade TGIC (dissolved in DMSO) was positive in the mouse lymphoma assay with and without metabolic activation systems, in chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells with and without metabolic activation systems, and for unscheduled DNA synthesis in rat hepatocytes. Technical grade TGIC dissolved in DMSO also tested positive in a chromosome aberration assay in Chinese hamster lung cells without metabolic activation systems but was negative with metabolic activation. A reduction in the response to treatment was noted in the mouse lymphoma assay and in the two assays in Chinese hamster ovary cells when metabolic activation was present. TGIC was negative in two cell transformation assays in mouse embryo fibroblasts. (Loveday et al. 1990, Sofuni et al. 1990, NOHSC 1994).
In human cells, technical grade TGIC tested negative in a chromosomal aberrations assay in human lymphocyte cultures (TGIC doses between 0.063 and 10 mg/ml) and for unscheduled DNA synthesis in human fibroblast cultures (TGIC doses between 2.7 and 400 mg/ml) without metabolic activation systems. (Ciba-Geiga Ltd. 1985, Ciba-Geiga Ltd. 1988 - both quoted in NOHSC 1994).
In vivo tests
Technical grade TGIC was shown to be clastogenic in a nucleus anomaly test in Chinese hamsters. TGIC (in arachid oil) was administered by gavage to groups of three animals of each sex at dose levels of 0, 140, 280 or 560 mg/kg b.w./day for two days. The animals were sacrificed 24 hours after the second dose and femoral bone marrow samples were taken. One thousand bone marrow cells were scored per animal. Nuclear anomalies for the intermediate and high dose groups were significantly different from the negative control. (Ciba-Geigy Ltd. 1983 - quoted in NOHSC 1994).
Two studies were conducted to determine the ability of technical grade TGIC to induce sister chromatid exchanges (SCEs) in the bone marrow cells of Chinese hamsters. In each study, TGIC was suspended in arachid oil and administered by gavage. Twenty-five cells per animal were scored for SCEs. In one study, four animals of each sex per dose were treated with TGIC at dose levels of 0, 35, 70 and 140 mg/kg b.w.. In this study, no increases in the number of SCEs were observed. In the second study, groups of two animals per sex were treated with TGIC at doses of 0, 140, 280 and 560 mg/kg b.w. and a dose-related positive effect was observed. (Ciba-Geigy Ltd. 1984, Ciba-Geigy Ltd. 1983 - both quoted in NOHSC 1994).
Four oral and four inhalational studies have been conducted to determine chromosomal aberrations in mouse germ cells in animals exposed to technical grade TGIC in vivo. (Ciba-Geigy Ltd. 1986, Hazleton Laboratories America Inc. 1989, Hazleton Microtest 1991a, Hazleton Microtest 1991b, Bushy Run Research Center 1992, Bushy Run Research Center 1991, Safepharm Laboratories Ltd. 1992, Hazleton Microtest 1993 - all quoted in NOHSC 1994):
In tree chromosomal aberration studies, male mice were dosed orally with TGIC by gavage on five consecutive days in doses ranging between 0 and 350 mg/kg b.w. Chromosomal aberrations in the spermatogonia were observed in a dose-related manner starting at about 30 mg/kg b.w. Cytotoxicity was first observed at 57.5 mg/kg b.w. (Ciba-Geigy Ltd. 1986, Hazleton Laboratories America Inc. 1989, Hazleton Microtest 1991a - all quoted in NOHSC 1994).
In another chromosomal aberration study, groups of 15 male mice were given TGIC (in arachid oil) by gavage at dose levels of 0, 32 or 96 mg/kg b.w. on days 0, 2, 3, 5 and 9. Animals were killed 3 days after the final dose and tested for chromosomal aberrations in their spermatocytes. The results of this study were negative. (Hazleton Microtest 1991b - quoted in NOHSC 1994).
In an inhalational chromosomal aberration study, groups of 10 male CD-1 mice were whole body exposed to technical grade TGIC at concentrations of 0, 2.5, 10 and 50 mg/m3 for 6 hours/day for five days. The particle size range of TGIC was 2.5 to 3.5 µm. Animals were killed six hours after the end of the last exposure. The results of this study were inconclusive mainly because statistical analysis could not include the 10 and 50 mg/m3 groups due to a small number of animals in these groups with a sufficient number of scorable cells (>50 per animal). No statistically significant aberrations were found in the 2.5 mg/m3 group. The study suggests TGIC was cytotoxic to spermatogonial cells at doses of 10 and 50 mg/m3. However, the cytotoxic ratios were not calculated. (Bushy Run Research Center 1992 - quoted in NOHSC 1994).
In a second inhalational chromosomal aberration study, groups of 10 CD-1 male mice were whole body exposed to atmospheres containing 0, 100, 1000 or 1700 mg/m3 powder coating containing 10 % TGIC for 6 hours/ day for five days. The animals were killed six hours after the last exposure period. The test material significantly increased the number of chromosomal aberrations in spermatogonial cells of the animals exposed to 1700 mg/m3 powder coating but as for the above mentioned study, the number of animals with enough scorable cells was very low. (Bushy Run Research Center 1991- quoted in NOHSC 1994).
A nose-only five day inhalational chromosomal aberration study was conducted in which groups of 10 male CD-1 mice were exposed to technical grade TGIC (dose of 7.8 mg/m3) or 10% TGIC powder coating (dose of 95.3 or 255.3 mg/m3) for 6 hours/day for 5 days. Oral administration (dose of 115.0 mg/kg b.w.) of technical grade TGIC was included in this study for comparison. The animals were killed six hours after the final exposure to the test material. The inhaled dust particles were for the main part respirable. For the groups inhaling TGIC, cytotoxicity and chromosomal damage in spermatogonial cells was not significantly increased at the doses tested. Oral administration of TGIC, 115 mg/kg b.w., significantly increased both cytotoxicity and total chromosomal aberrations in spermatogonial cells. (Safepharm Laboratories Ltd 1992 - quoted in NOHSC 1994).
In a similar chromosomal aberration study, groups of six B6D2F1 male mice were exposed for 6 hours/day to powder coating containing 4.6% TGIC. The mice were exposed to atmospheres containing from 0 to 2000 mg/m3 of powder coating. A statistically significant increase in the number of spermatogonial cells with chromosomal aberrations was observed in animals exposed to the highest concentration - 2000 mg/m3. However, the increase was mainly due to chromosome damage in a single animal. (Hazleton Microtest 1993 - quoted in NOHSC 1994).
An in vivo study on TGIC’s ability to alkylate mouse liver, stomach and testis DNA demonstrated that TGIC is capable of covalently binding to DNA in these tissues following oral administration. Radiolabelled TGIC, in either aqueous solution or in oil, was administered by gavage to at least two male Tif:MAGf(SPF) mice per group at doses of 5, 17 and 200 mg/kg b.w. DNA was isolated at three, eight or 24 hours from the liver, stomach and testes and measured for radioactivity levels. Dose-dependent increases in TGIC-DNA adduct formation were observed. (Ciba-Geigy Ltd. 1990 - quoted in NOHSC 1994).
Another in vivo alkylating study was conducted in rats to assess the binding of technical grade TGIC to liver DNA. Two to four male Tif:RAIf(SPF) rats were pre-treated with trans-stilbene oxide (TSO) to induce epoxide hydrolase (EH) and glutathione S-transferase activities. Six days later these animals as well as untreated animals were intraperitoneally or orally administered 20 mg/kg b.w. radiolabelled TGIC. Twenty-four hours later the animals were killed and the livers excised. The results of this study indicated that increased microsomal EH activity was associated with increased hydrolysis of TGIC and a corresponding decrease in TGIC-DNA adduct formation in the rat liver. The study demonstrated that TGIC does bind to DNA in vivo in rats. However, the binding values were relatively low suggesting that only a small proportion of administered TGIC binds to DNA in the rat liver. (Ciba-Geigy Ltd. 1993 - quoted in NOHSC 1994).
TGIC was negative in the mouse spot test. This test system permits the detection of mutational events in the melanoblasts of embryos exposed in utero to a chemical. The mutational events resulting from the expression of recessive genes involved in coat colour determination are observed as spots in the fur of young mice. Technical grade TGIC was administered at doses of 13.5, 27.0 and 54.0 mg/kg b.w. in a single intraperitoneal injection to 96 female pregnant mice (C57 Bl/6) per group on the 10th day after conception. (Ciba-Geigy Ltd. 1986 - quoted in NOHSC 1994).
4.5 Carcinogenic effects
2.5% TGIC did not promote skin tumour formation in CF-1 mice painted twice weekly for 26 weeks. Four groups of 24 mice of each sex had a tumour initiating agent applied dermally to their shaved backs. Three weeks later (and twice weekly for the next 26 weeks), these mice were painted with either 2.5% TGIC, 2.5% of a tumour promoting agent, solvent or received no secondary treatment. After 27 weeks, the mice were killed and the skin from the treated areas was examined microscopically. Only mice who received the tumour promoting agent developed skin tumours. Of those mice exposed to TGIC, one female showed severe acanthosis and two males showed ulceration. (Shell research Ltd. 1971 - quoted in NOHSC 1994).
The final results of a chronic toxicity/carcinogenicity bioassay in rats conducted by the Centre Internationale de Toxicologie (CIT) in France were not available in 1998 (WHO 1998).
5 Regulations, limit values
Ambient air
Oklahoma, USA, has a maximum acceptable ambient concentration (MAAC) for TGIC of 0.5 m g/m3 (OAPCR 1999).
Drinking water
-
Soil
-
OELs
American Conference of Governmental Industrial Hygienists has set a threshold limit value of 0.05 mg/m3 (TWA) (ACGIH 1997 - quoted in Toxline pre 1981-1999).
Australia has a provisional occupational exposure limit of 0.08 mg/m3 (TWA) (NOHSC 1994).
Classification
TGIC is classified for acute toxic effects (T;R23/25 - toxic by inhalation and if swallowed), for local effects (Xi;R41 - risk of serious damage to eyes), for sensitising properties (Xi;R43 - may cause sensitisation by skin contact), for effects following repeated exposure (Xn;R48/22 - harmful: danger of serious damage to health by prolonged exposure if swallowed), for mutagenic properties (Mut2;R46 - may cause heritable genetic damage), and for environmental effects (N;R52/53 - harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment). (MM 1997).
EU
-
IARC/WHO
-
US-EPA
-
RD50
-
6 Summary
Description
Commercial (technical) grade TGIC is a mixture of two optical stereoisomers, alpha and beta, which have different physicochemical properties. It is a white powder or granule with no discernible odour. TGIC’s solubility in water is relatively low (9 g/l).
The main use of TGIC is as a three-dimensional cross-linking or curing agent in polyester powder coatings (paints) where it constitute up to 10%. The alpha isomer of TGIC has been used as an experimental anti-tumour agent.
Environment
Environmental exposure to TGIC resulting form normal use in spray painting workplaces is expected to be low. Plants that formulate TGIC powder coatings do release some TGIC to the atmosphere and sewer.
As TGIC is an epoxide, any residues which enter the environment are expected to be rapidly degraded, either through microbial action or abiotic hydrolysis. Hydrolysis proceeds more rapidly in the marine environment because of more rapid ring opening by chloride ions. TGIC is not expected to accumulate in soil or sediment because of high mobility. The reactivity of TGIC precludes any possibility of bioaccumulation.
Human exposure
No data were found.
Toxicokinetics
No toxicokinetic inhalation studies have been performed. If inhaled, most commercial TGIC particles are too big for reaching the lower bronchioles and alveolar regions of the lungs and will be deposited in the upper respiratory tract. TGIC is partially absorbed after oral administration to mice and rabbits. Absorption is probably low following dermal contact indicated by a high LD50-value. In mice in a oral study, TGIC was distributed to the liver, stomach and testes (the only tissues studied).
Clinical trials with a -TGIC in humans and studies in rabbits (intravenous administration) indicated that TGIC is rapidly eliminated from plasma with a half-life less than 5 minutes and a total body clearance at about 5 litres/minute. Less than 1% a -TGIC was recovered in 24-hour urine. Metabolites in the plasma were eliminated at a much slower rate (t½> 60 minutes). Twenty-four-hour urinary recovery of 14C total radioactivity was 60 to 70%.
TGIC metabolism seems to involve hydrolysis catalysed by microsomal epoxide hydrolase in the liver as indicated by both an in vitro and an in vivo study in rats. In the in vitro study, epoxide hydrolysis metabolites were detected in the microsomal incubations.
Human toxicity
Allergic contact dermatitis due to exposure to TGIC is the main reported human health effect. Sixteen case reports, in which patch tests confirmed that TGIC was the causative agent of the skin problems, exist in the literature. Workers may become sensitised to TGIC from short-term exposure (less than 12 months) to the chemical. Other health effects in workers exposed to TGIC include aggravated asthma, nasal, eye and throat irritation, skin rash and nose bleed. TGIC has caused occupational asthma in a healthy 36-year old non-smoking man who worked mainly as a spray painter, using a powder paint containing 4% TGIC. Before the examinations, he had been painting 5-8 hours daily for about 7 years.
In human clinical trials, a -TGIC was administered intravenously to cancer patients at very high doses. The dose-limiting factor was local thrombophlebitis. Leukopenia and myelosuppression was seen at the highest doses. Other toxic effects were mild to moderate and consisted of nausea, vomiting and hair loss.
Animal toxicity
Acute toxicity studies in animals have shown that TGIC is toxic by the oral (LD50 of <100-950 mg/kg for rats and LD50 of 1670 mg/kg for hamsters) and inhalational (LC50 of 300->650 mg/m3 for rats and LC50 of 2000 mg/m3 for mice) routes but has low acute dermal (LD50 of >2000 mg/kg for rats) toxicity.
TGIC causes serious eye effects in rabbits following instillation. Skin sensitisation was observed in guinea pigs. Very slight erythema and oedema were observed in studies of acute skin irritation in rabbits.
In a short term (6 hours/day for 5 days) nose-only inhalational study, mice were exposed to atmospheres containing 0, 10, 40 or 140 mg/m3 TGIC. Clinical signs of toxicity, increased body weight losses and high mortality were observed in the intermediate and high dose groups. The clinical signs included hunched posture, piloerection, lethargy and dyspnoea. Dead animals had lung damage. Besides pathology for animals dosed with 140 mg/m3 included pale livers, pale kidneys, and congestion of the small intestine. One animal had slightly reddened lungs in the low dose group. In another similar study, body weight gain was unaffected, no deaths occurred, and no adverse clinical signs were observed at a concentration of 7.8 mg/m3.
When rats were fed 0, 54 or 216 mg/kg b.w./day (males) or 0, 43, or 172 mg/kg b.w./day (females) of TGIC by gavage for 7 consecutive days, gross pathology was recorded for the lungs, kidney, liver, stomach, and intestines in both sexes in dosed animals. Renal, gastric and duodenal damage was observed in both sexes in the high dose groups.
In a 13-week toxicity/fertility study, male rats were given diets containing 0, 10, 30, or 100 mg/kg TGIC. This study followed a preliminary 19-day range-finding investigation in which signs of toxicity were observed in animals administered diets containing 160 or 640 mg/kg TGIC. In the 13-week study, no exposure-related clinical effects or death were observed. Body weight gain was slightly lower over the first 6 weeks in animals from the 100 mg/kg test group.
Reproductive and developmental effects
In the 13-week toxicity/fertility study, a dose-related reduction in the number of spermatozoa was noted in the male rats but the spermatozoa viability was unchanged. No exposure-related infertility was noted in males, and no effects on embryonic and pup development were observed in the offspring.
A dominant lethal test showed reduced fertility in male mice inhaling 10 and 50 mg/m3 of TGIC. No increase in embryonic deaths was observed in two dominant lethal studies where male mice inhaled up to 170 mg/m3 of TGIC for 6 hours/day for 5 consecutive days before mating the females. In a dominant lethal study with oral administration of TGIC, a dose of 480 mg/kg b.w. caused a significant increase in the number of embryonic deaths in females mated to the intoxicated males within the first week. This observation was not confirmed in a similar study where TGIC was given in doses up to 550 mg/kg b.w.
Mutagenic and genotoxic effects
TGIC is classified for mutagenic properties. The mutagenic and genotoxic effect of TGIC has been investigated in a wide range of in vitro and in vivo assays.
In vitro TGIC was positive both with and without metabolic activation systems in Ames test, in the mouse lymphoma assay, and in chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells. A reduction in the response to treatment was noted in the mouse lymphoma assay and in the two assays in Chinese hamster ovary cells when metabolic activation was present. TGIC also tested positive in a chromosome aberration assay in Chinese hamster lung cells without metabolic activation systems but was negative with metabolic activation. TGIC was positive for unscheduled DNA synthesis in rat hepatocytes. TGIC was negative in two cell transformation assays in mouse embryo fibroblasts.
In human cells in vitro, TGIC tested negative in a chromosomal aberrations assay in lymphocytes and for unscheduled DNA synthesis in fibroblasts.
In vivo, TGIC was shown to be clastogenic in a nucleus anomaly test in Chinese hamsters when they were administered oral doses above 280 mg/kg b.w./day for two days. TGIC also induced dose-related sister chromatid exchanges (SCEs) in the bone marrow cells of Chinese hamsters exposed orally to doses above 140 mg/kg b.w. Chromosomal aberrations in the spermatogonia were observed in a dose-related manner starting at about 30 mg/kg b.w. when male mice were exposed orally to TGIC for 5 days.
TGIC in a dose of 7.8 mg/m3 did not induce chromosomal damage in spermatogonial cells in mice exposed by nose-only inhalation for 6 hours/day for 5 days. Chromosomal aberrations were observed in male mice exposed nose-only to 2000 mg/m3 of powder coating containing 4.6% TGIC (that is 92 mg/m3 of TGIC). An in vivo inhalational study in mice exposed to TGIC in concentrations between 0 and 50 mg/m3 for 6 hours/day for 5 days was inconclusive regarding chromosomal aberrations in spermatogonia possibly because of cell cytotoxicity above 10 mg/m3. Chromosomal aberrations were observed at a concentration of 170 mg/m3 (10% TGIC in powder coating at 1700 mg/m3) but the number of scorable cells were very low.
Chromosomal aberrations in the spermatocytes were not observed in male mice given TGIC by gavage at doses up to 96 mg/kg b.w. for 5 days.
TGIC was negative in the mouse spot test following single intraperitoneal doses up to 54 mg/kg b.w.
The cytotoxicity of TGIC is probably related to the alkylating capacity of the epoxide moieties. Two in vivo studies have demonstrated that TGIC is capable of covalently binding to DNA in mouse liver, stomach and testis tissues following oral administration of doses between 5 and 200 mg/kg b.w. and in rat liver tissue following intraperitoneal or oral administration of 20 mg/kg b.w.
Carcinogenicity
2.5% TGIC did not promote skin tumour formation in CF-1 mice painted twice weekly for 26 weeks.
The final results of a chronic toxicity/carcinogenicity bioassay in rats conducted by the Centre Internationale de Toxicologie (CIT) in France were not available in 1998.
7 Evaluation
The critical effects in humans following exposure to TGIC is considered to be the possibly mutagenic effect and the skin sensitisation and asthma that it causes. This is based on the following:
In humans, the available data on health effects after exposure to TGIC in the air are limited to 16 case reports of allergic contact dermatitis, one report of occupational asthma and Australian surveys of health effects in workers exposed to TGIC. Workers may be exposed to TGIC in concentrations of up to 6.5 mg/m3. Health effects include aggravated asthma, nasal, eye and throat irritation, skin rash and nose bleed.
In humans, a -TGIC in the systemic circulation (given intravenously) in high doses induced leukopenia which resulted in life-threatening infections at plasma concentrations above 1.2 m g/ml. Other toxic effects were mild to moderate and consisted of nausea, vomiting and hair loss. In rats exposed orally to TGIC, gross pathology was recorded for the lungs, kidney, liver, stomach, and intestines. These studies show that systemic effects of TGIC are seen above certain dose levels but it is a question to what extent TGIC is absorbed after inhalation.
No toxicokinetic inhalation studies have been performed. If inhaled, most commercial TGIC particles are too big for reaching the lower bronchioles and alveolar regions of the lungs and will be deposited in the upper respiratory tract. Short term inhalation studies in rats and mice with respirable TGIC resulted mainly in lung damage indicating that the absorption of TGIC following inhalation probably is low. Pale livers, pale kidneys, and congestion of the small intestine seen in mice inhaling 140 mg/m3 short-term could be a side-effect of the heavily damaged lungs and not a direct toxic effect of TGIC since no cytotoxicity was observed in tested germ cells. Speaking against the low absorption is some in vivo inhalational genotoxicity studies in mice. A dose of 7.8 mg/m3 of TGIC did not induce chromosomal damage in spermatogonial cells but aberrations were observed in mice inhaling powder coating containing TGIC equivalent to 92 mg/m3. The question is whether TGIC was the toxic substance in the powder coating but some genotoxic substance in the powder coating must have been absorbed.
It is a cause of concern that several in vitro and in vivo genotoxicity studies are positive and TGIC seems to be a direct-acting mutagen. In vitro, TGIC tested positive in both gene mutation and clastogenic assays. In vivo, TGIC is not causing gene mutations as shown in the mouse spot test. Regarding a clastogenic effect, both positive and negative results exist. TGIC alkylates DNA but apparently it is not very potent. A 26 week study where mice were painted twice weekly with TGIC did not result in tumour formation. So far no results from long term toxicity/carcinogenicity studies have been published.
A long term carcinogenicity study would be valuable in assessing whether the genotoxic effect of TGIC can lead to cancer. But just as important is it to get established to what extent technical grade TGIC is absorbed when inhaled.
We know very little of which doses that are causing sensitisation. At the moment, the best study to base a NOAEL on is therefore a short term (6 hours/day for 5 days) nose-only inhalational study. In one such study, one mouse exposed to 10 mg/m3 of TGIC had slightly reddened lungs but in another similar study with 10 mice, body weight gain was unaffected, no deaths occurred and no adverse clinical signs were observed at 7.8 mg/m3. No cytotoxicity and chromosomal aberrations in spermatogonial cells were noted at this dose either. For the purpose of estimating a limit value in air, a level of 8 mg/m3 is therefore considered as a NOAEL for irritative effects and chromosomal aberrations in spermatogonial cells in mice inhaling TGIC short term.
8 Limit value in air
The limit value is calculated based on a NOAEL of 8 mg/m3 for irritative effects and chromosomal aberrations in spermatogonial cells in mice exposed to TGIC 6 hours/day for 5 days.
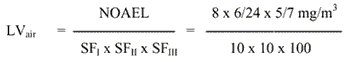 |
= 0.0001 mg/m3
The safety factor SFI is set to 10 assuming that humans are more sensitive than animals. The SFII is set to 10 to protect the most sensitive individuals in the population. The SFIII is set to 100 taking into account the quality of the study (few animals, only one dose level, poorly reported, short-term study) and because TGIC is a known genotoxic substance in in vitro and in vivo studies and it is still an open question whether chronic exposure will cause cancer. Finally, a NOAEL for asthma is not established and therefore cannot be considered for use in calculating the limit value.
9 C-value
A limit value of 0.0001 mg/m3 has been calculated. For substances having acute or subchronic effects, but for which activity over a certain period of time is necessary before the harmful effect occurs, the C-value is set at the limit value (MST 1990). A C-value of 0.0001 mg/m3 and placing in Main Group 1 is proposed. Placing in Main Group 1 is proposed because TGIC is a mutagenic substance which probably may cause cancer following chronic exposure.
C-value
0.0001 mg/m3, Main Group 1.
10 References
Ames MM, Kovach JS and Rubin J (1984). Pharmacological characterization of Teroxine, a triepoxide antitumor agent, in rats, rabbits, and humans. Cancer Res 44, 4151-4156.
Atassi G, Spreafico F, Dumont P, Nayer P and Klastersky J (1980). Antitumoral effect in mice of a new triepoxide derivative: 1,3,5-triglycidyl-s-triazinetrione (NSC 296934). Eur J Cancer 16, 1561-1567.
Chemfinder (1999). 1,3,5-Triglycidyl Isocyanurate. In: Chemfinder data base. Http.//www.chemfinder.com
Craven NM, Bhushan M and Beck MH (1999). Sensitization to triglycidyl isocyanurate, epoxy resins and acrylates in a developmental chemist. Cont Derm 40, 54-55.
Dombernowsky P, Lund B and Hansen HH (1983). Phase I study of a -1,3,5-triglycidyl-s-triazinetrione (NSC 296934). Cancer Chemother Pharmacol 11, 59-61.
Dooms-Goossens A, Bedert R, Vandaele M and Degreef H (1989). Airborne contact dermatitis due to triglycidylisocyanurate. Cont Derm 21, 202-203.
Foulds IS and Koh D (1992). Allergic contact dermatitis from resin hardeners during the manufacture of thermosetting coating paints. Cont Derm 26, 87-90.
Jolanki R, Kanerva L, Estlander T and Tarvainen K (1994). Concomitant sensitization to triglycidyl isocyanurate, diaminodiphenylmethane and 2-hydroxyethyl methacrylate from silk-screen printing coatings in the manufacture of circuit boards. Cont Derm 30, 12-15.
Loveday KS, Anderson BE, Resnick MA and Zeiger E (1990). Chromosome aberration and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. V: Results with 46 chemicals. Environ Mol Mutagen 16, 272-303.
Mathias CGT (1988). Allergic contact dermatitis from triglycidyl isocyanurate in polyester paint pigments. Cont Derm 19, 67-68.
McFadden JP and Rygroft JG (1993). Occupational contact dermatitis from triglycidyl isocyanurate in a powder paint sprayer. Cont Derm 28, 251.
MM (1997). The Statuary Order from the Ministry of the Environment no. 829 of November 6, 1997, on the List of Chemical Substances.
Munro CS and Lawrence CM (1992). Occupational contact dermatitis from triglycidyl isocyanurate in a powder paint factory. Cont Derm 26, 59.
Neidhart JA, Derocher D, Grever MR, Kraut EH and Malspeis L (1984). Phase I trial of teroxirone. Cancer Treat Rep 68, 1115-1119.
NOHSC (1994). Triglycidylisocyanurate (TGIC). National Industrial Chemicals notification and Assessment Scheme (NICNAS). National Occupational Health and Safety Commission (Worksafe Australia). Http://www.worksafe.gov.au/worksafe/fulltext/toc/h3-38.htm
NOHSC (1998). Triglycidylisocyanurate (TGIC). List of designated hazardous substances. National Occupational Health and Safety Commission (Worksafe Australia). Http://www.worksafe.gov.au/cgi-bin/wsresult.exe?txtID=2537
Nishioka K, Ogasawara M and Asagami C (1988). Occupational contact allergy to triglycidyl isocyanurate (TGIC, TepicŅ ). Cont Derm 19, 379-380.
NTP(1991). Tris (2,3-epoxypropyl) isocyanurate. In: NTP chemical Health and Safety Data. Http://ntp-db.niehs.nih.gov/ NTP_Reports/NTP_Chem_H&S/NTP_Chem2/ Radian2451-62-9.txt
OAPCR (1999). 1,3,5-Triglycidyl isocyanurate. In: Oklahoma Air Pollution Control Rules. Http://www.deq.state.ok.us/air1/toxdis.html
Piccart M, Rozencweig M, Dodion P, Cumps E, Crespeigne N, Makaroff O, Atassi G, Kisner D and Kenis Y (1981). Phase I clinical trial with alpha 1,3,5-triglycidyl-s-triazinetrione (NSC 296934). Eur J Cancer Clin Oncol 17, 1263-1266.
Piirilä P, Estlander T, Keskinen H, Jolanki R, Laakkonen A, Pfäffli P, Tupasela O, Tuppurainen M and Nordman H (1997). Occupational asthma caused by triglycidyl isocyanurate (TGIC). Clin Exp Allergy 27, 510-514.
RTECS (1999). S-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris(2,3-epoxypropyl)-. In: Registry of Toxic Effects of Chemical Substances.
Rubin J, Kovach JS, Ames MM, Moertel CG, Creagan ET and O“Connell MJ (1987). Phase I study of two schedules of teroxirone. Cancer Treat Rep 71, 489-492.
Sofuni T, Matsuoka A, Sawada M, Ishadate M, Zeiger E and Shelby MD (1990). A comparison of chromosome aberration induction by 25 compounds tested by two Chinese hamster cell (CHL and CHO) systems in culture. Mutat Res 241, 175-213.
Toxline (pre 1981-1999). Triglycidylisocyanurate. In: Toxline database.
Wigger-Alberti W, Hofmann M and Elsner P (1997). Contact dermatitis caused by triglycidyl isocyanurate. Am J Cont Derm 8, 106-107.
WHO (1998). Triglycidyl Isocyanurate. Concise International Chemical Assessment Document, no. 8. World Health Organisation, International Programme on Chemical Safety, Geneva.
Wu FYH and Le Pecq JB (1983). Mechanistic studies of a novel antitumor drug, a -1,3,5-triglycidyl-s-triazinetrione. Antitumor and cytotoxic effects. Mol Pharmacol 23, 182-189.
[Front page] [Contents] [Previous] [Next] [Top] |