[Front page] [Contents] [Previous] [Next] |
Toxicological Evaluation and Limit Values for 2-Ethylhexyl acrylate, Propylene carbonate, Quaternary ammonium compounds, Triglycidyl isocyanurate, and Tripropyleneglycol diacrylate
Tripropyleneglycol diacrylate
1 General description
1.1 Identity
| Molecular formula: | C15H24O6 |
| Structural formula: | 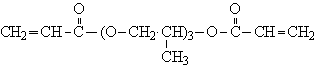 |
| Molecular weight: | 300.39 |
| CAS-no.: | 42978-66-5 |
| Synonyms: | Acrylic acid, propylenebis(oxypropylene) ester; 2-Propenoic acid, (1-methyl-1,2-diethanediyl)- bis(oxy(methyl-2,1-ethanediyl)) ester; Tripropyleneglycol diacrylate; TPGDA; TRPGDA. |
1.2 Physical / chemical properties
| Description: | Pale, yellow tinted liquid with a mild odour. |
| Purity: | Technical product: 80% pure monomer with > 18% oligomer (Nylander-French & French 1998). |
| Melting point | - |
| Boiling point: | - |
| Density: | 1.030 g/ml (at 20°C) |
| Vapour pressure: | < 0.01 mmHg (< 1.33 Pa) at 20°C 0.0106 mmHg (1.41 Pa) at 25°C |
| Concentration saturated vapours: | of 14 ppm (175 mg/m3 (calculated) at 20°C and 760 mmHg |
| Vapour density: | - |
| Conversion factor: | 1 ppm = 12.5 mg/m3 20°C 1 mg/m3 = 0.080 ppm 1 atm |
| Flash point: | > 110° C, closed cup |
| Flammable limits: | - |
| Autoignition temp.: | - |
| Solubility: | Insoluble in water. Soluble in many organic solvents. |
| logPoctanol/water: | - |
| Henry’s constant: | - |
| pKa-value: | - |
| Stability: | - |
| Incompatibilities: | - |
| Odour threshold, air: | - |
| References: | RTECS (1999), Nylander-French & French (1998), Mortensen (1991). |
1.3 Production and use
No data were found on production.
Tripropyleneglycol diacrylate is used in a variety of UV curable inks, lacquers and varnishes (surface coatings). A lacquer contains 56.4% TPGDA monomer (Tice et al. 1997). Surface coating materials in Sweden normally contain 10 - 30% TPGDA (Nylander-French et al. 1994).
1.4 Environmental occurrence
No data were found
1.5 Environmental fate
No data were found
1.6 Human exposure
No data on human exposure have been found. However, the general population may be exposed to tripropyleneglycol diacrylate by inhalation of contaminated air and through contact with TPGDA containing products.
2 Toxicokinetics
2.1 Absorption, distribution
Inhalation
No data were found.
Oral intake
No data were found.
Dermal contact
No data were found, but systemic effects have been observed following dermal application indicating that absorption through the skin takes place (Celanese Corporation - quoted from Mortensen 1991)
2.2 Elimination
Metabolism
The major route of detoxification of acrylates is their conjugation with glutathione via the Michaėlis addition reaction or glutathione-S-transferase. Conjugation of acrylates by glutathione is expected to be proportional to the number of functional acrylate groups. The available data suggest that the acrylates most likely act on the site of contact, conjugate available glutathione, and are hydrolysed by carboxylesterases. (Tice et al. 1997).
Excretion
No data have been found.
Half-life
No data have been found.
2.3 Toxicological mechanisms
Based on the chemical structure and molecular reactivity, acrylates have the potential to interact with biomolecules, including nucleic acids and nucleoproteins and thus to induce DNA damage, being limited in the biological activity only by the physico-chemical properties and access to biological systems (Tice et al. 1997).
3 Human toxicity
3.1 Short term toxicity
Inhalation
No data were found
Oral intake
No data were found
Dermal contact
Acrylates are generally potent contact allergens. Polyfunctional acrylates and epoxyacrylates are the most potent ones, whereas the polyfunctional methacrylates and cyanoacrylates are much weaker. Many of the acrylates cross-react.
The list of allergens contain some 60 acrylates which all cause contact sensitisation (National Institute of Occupational Health 1990). In the Nordic countries, 23 acrylates are considered contact allergens and a classification with R43 (may cause sensitisation by skin contact) has been agreed (Nordic Council of Ministers 1991).
One American woman working with silk screening of computer discs with UV curable inks developed acute allergic contact dermatitis on hands and forearms. Patch testing revealed a number of strong reactions to epoxy resins and many multifunctional epoxy resins. The only material listed on the safety data sheet to which she reacted was tripropyleneglycol diacrylate. The other positive reactions likely represent cross-reactions. (Skotnicki & Pratt 1998 - quoted from TOXLINE PLUS 1998).
Occupational allergic contact dermatitis due to tripropyleneglycol diacrylate exposure was observed in a male silk screen maker in Belgium (Goossens et al. 1998).
Fingertip paraesthesia and occupational allergic contact dermatitis caused by acrylics (one of which was tripropyleneglycol diacrylate) occurred in a dental nurse in Finland. (Kanerva et al. 1998 – quoted from TOXLINE PLUS 1998).
Three out of 59 workers exposed to polyfunctional acrylic monomers (including tripropyleneglycol diacrylate) in 5 Swedish furniture companies developed severe acute toxic skin reactions due to insufficient product knowledge and lack of introduction routines, handling directions and protective equipment. A follow-up period of three years upon implementation of new routines revealed no further cases of acute toxic eczema in the five companies. (Voog & Jansson 1992).
Based on 10 years of patch testing with the (meth)acrylate series, Kanerva et al. (1997) put a low score (ranked 22 of 24) on tripropyleneglycol diacrylate with respect to its sensitising capacity.
3.2 Long term toxicity
No data were found.
3.3 Reproductive and developmental effects
No data were found.
3.4 Mutagenic and genotoxic effects
No data were found.
3.5 Carcinogenic effects
No data were found.
4 Toxicity, animal data
4.1 Short term toxicity
Inhalation
No data were found.
Oral administration
An LD50-value after oral administration of a single dose of tripropyleneglycol diacrylate (TPGDA) to rats of 6200 mg/kg has been reported (NTIS - quoted from RTECS 1999).
Dermal contact
An LD50-value after single dermal application of TPGDA to rabbit skin of > 2000 mg/kg has been reported (Celanese Corporation - quoted from RTECS 1999).
A study on repeated dermal exposure was performed. Rabbits were painted with 500 mg/kg b.w. TPGDA 5 days a week for 2 weeks. The observation period was 4 week after the last application. This repeated exposure led to systemic toxicity, including convulsions, tremors and ataxia. The test methods and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
Irritation skin
TPGDA was tested for skin irritation after a single application to rabbits. The rabbits were examined 24 hours, 72 hours and 7 days after the application. The irritation was scored as moderately irritating both after 24 and 72 hours. At day 7, a delayed skin effect was observed. For most of the animals, skin necrosis and escar formation were noted. The delayed effects were assigned to category I (refers to corrosion). The dose was not stated. The test method is not described further and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
eye
When TPGDA was tested for eye irritation in rabbits, it was scored as category IV (slight irritation) after 72 hours. The test method is not described further and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
Sensitisation
TPGDA (purity not specified) was tested for sensitising capacity in guinea pigs using the Guinea Pig Maximization Test (GPMT). Groups of exposed and control animals consisted of 15 albino Dunkin-Hartley strain guinea pigs. For intradermal induction was used 1% TPGDA in olive oil:acetone (9:1), and for the topical induction was used a solution of 25% TPGDA in petrolatum. The challenge concentration was 0.2% TPGDA in petrolatum. The optimal test concentration was determined in a preliminary test. Of the guinea pigs treated with TPGDA, 11 of 15 (73%) became sensitised, which indicates that TPGDA is a strong sensitiser. (Björkner 1984).
The same study shows that other multifunctional acrylates such as 1,4-butanediol diacrylate, diethyleneglycol diacrylate, tetraethyleneglycol diacrylate and neopentylglycol diacrylate cross-react with TPGDA. (Björkner 1984).
The same test performed with acetone as vehicle show that the vehicle influences the allergic response to a very large extent. Of 15 animals tested, none was positive if acetone was used (11 of 15 were positive when petrolatum was used). The discrepancy between the results is due to a polymerisation process of the acrylates in acetone. Petrolatum prevents polymerisation of acrylic monomers. (Björkner & Niklasson 1984).
TPGDA stabilised with 500 ppm hydroquinone methylether was tested for sensitising properties in the Guinea Pigs Maximization Test (GPMT). The group of treated animals consisted of 10 male and 10 female and the control group of 5 male and 5 female Dunkin Hartley guinea pigs. On day 1, 0.1 ml of the test substance, in the presence of Freund’s adjuvant, was administered by intradermal route at a concentration of 0.5% in paraffin oil. On day 9, 0.5 ml of the test substance was applied by cutaneous route and kept on the application site for 48 hours. After a period of 15 days without treatment, a 0.5 ml challenge cutaneous application of the vehicle (left flank) and 0.5 ml of the test substance (right flank) at a concentration of 25% in paraffin oil was performed in all animals. The substances were held in place by means of an occlusive dressing for 24 hours. The cutaneous reactions were evaluated at the challenge application site, 24 and 48 hours after removal of the dressing. After the last scoring, the animals were sacrificed. No behavioural abnormalities, or cutaneous reactions were observed in any animal from the control or treated groups.
It was concluded that under the experimental conditions and according to the maximization method of Magnusson and Kligman, no cutaneous reactions related to a sensitisation potential of TPGDA were observed in the guinea pig.
(C.I.T. unpublished study 1989).
An identical study was performed by the same laboratory using exactly the same substance (TPGDA with 500 ppm hydroquinone methylether as stabiliser), same number of animals and same procedure, only was the concentration used for intradermal induction by day 1, 1% TPGDA in paraffin oil and the concentration used for the cutaneous induction on day 9, was 10% in paraffin oil. In this study, no behavioural abnormalities were observed in the animals throughout the study. Minimal to barely perceptible cutaneous reactions (very slight erythema) were noted 24 hours after removal of the dressing from the cutaneous challenge application, on the right flank (test substance) in 4 out of 10 males and in 2 out of 10 females in the treated group.
It was again concluded that under the experimental conditions and according to the maximization method of Magnusson and Kligman, no cutaneous reactions related to a sensitisation potential of TPGDA were observed in the guinea pig.
(C.I.T. unpublished study 1989).
Photomer 4061 (TPGDA with unspecified purity) was tested in the GMPT for sensitising properties using 10 and 5 female Dunkin Hartley guinea pigs as treated and control group, respectively. Six additional animals were used for the preliminary (dose finding) investigations. Alembicol D - a product of coconut oil was used as solvent. Based on the preliminary investigations, the following concentrations of Photomer 4061 were chosen: for the induction intradermal injection, 0.5% v/v in Alembicol D; for the induction topical application, - 25% v/v in Alembicol D; and for the topical challenge, 10% (anterior) or 5% (posterior) v/v in Alembicol D. Intradermal induction was performed on day 1 with 0.1 ml of each of 50% Freund’s complete adjuvant, 0.5% v/v Photomer 4061 in Alembic D and 0.5% v/v Photomer 4061 in 50:50 Freund’s complete adjuvant and Alembic D (control animals received the same but without Photomer 4061). One week later, the treated animals were inducted topically with 0.4 ml 25% v/v Photomer 4061 in Alembic D (controls with Alembic D alone) held on place for 48 hours with an occlusive dressing. Two weeks after this, all animals were challenged with 0.2 ml 10% and 5% v/v Photomer 4061 in Alembic D for 24 hours and the challenge sites were evaluated 24, 48 and 72 hours after removal of the patches. In this study, Photomer 4061 produced evidence of skin sensitisation (delayed contact hypersensitivity) in 8 of the 10 tested animals (2 were inconclusive). (Huntingdon Research Centre unpublished study 1993).
TPGDA was shown to induce contact sensitivity in Guinea pigs using the Polak method. In the test, 6 outbred Hartley guinea pigs were used. For intradermal induction, the guinea pigs received 1 mg TPGDA dissolved in ethanol:saline (1:4) and Freund’s complete adjuvant (FCA). Seven days later, the skin tests were performed with 0.2 ml of a solution (0.5% or 1% TPGDA in acetone:olive oil (4:1)) being applied to the skin. The test concentrations were 5% of the maximum concentration, which gave no irritation. The skin tests were repeated weekly up to 12 weeks. The time for the first positive skin reaction was at day 28. The number of sensitised animals was not mentioned. (Parker & Turk 1983).
4.2 Long term toxicity
Inhalation
No data were found.
Oral administration
No data were found.
Dermal contact
Rats were tested in a 90-day dermal test with TPGDA at dose levels of 20, 67, or 200 mg/kg b.w. for 5 days a week. Doses up to and including 67 mg/kg b.w. did not give rise to any systemic effects and is thus a NOAEL for systemic effects. All 3 doses led to skin irritation early in the study. The skin appeared to acclimatise and after 3 weeks, the severity of the irritation declined. There was no sign of irritation during the last few weeks of the study in rats treated with 20 mg/kg. The test methods and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
4.3 Reproductive and developmental effects
TPGDA was screened for teratogenic potential by repeated dermal exposure. Twenty pregnant rats were given a daily dermal dose of 250 mg/kg b.w. during day 6 to 15 of pregnancy. The dose level was based on results from a preliminary dermal maternal toxicity screening. TPGDA was not found to be teratogenic or foetotoxic based on maternal observations, including number of implantations, number of live and dead foetuses, number of early and late resorptions, and number of corpora lutea, as well as external, skeletal and visceral evaluation of foetuses for malformations. No further details of the test methods are described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
4.4 Mutagenic and genotoxic effects
TPGDA has been tested for mutagenicity in bacteria (Ames test) with and without metabolic activation; no mutagenic potential was found. The test method and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
In another test with mammalian cells in vitro, the mouse lymphoma cell mutagenicity assay, a positive mutagenic response was found both with and without metabolic activation. The test method and results are not further described and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
TPGDA or Lacquer A (an ultraviolet radiation curable lacquer containing 56.4% TPGDA as the active ingredient) were applied dermally to Tg.AC mice - 3 times a week for 20 weeks. Peripheral blood leukocytes were evaluated for DNA damage (single-strand breaks, alkali labile sites, and DNA crosslinking) at week 4, 8, 12, 16, and 20 by using the alkaline (pH:13) single cell gel (SCG) assay. Peripheral blood polychromatic erythrocytes (PCE) and normochromatic erythrocytes (NCE) were evaluated for the presence of micronuclei at week 20. The extent of DNA migration in leukocytes and the frequency of micronucleated erythrocytes were not significantly altered by treatment with TPGDA when administered alone or in Lacquer A, at doses that induced cell proliferation in keratinocytes. The absence of genotoxicity in these two cell populations suggests that these acrylates are not genotoxic or that they are not absorbed when applied dermally. However, a significant dose-dependent increase in the percentage of PCE relative to the vehicle control was present in mice treated with TPGDA, while a dose-dependent, but non-significant, increase in the percentage of PCE was observed in mice treated with Lacquer A. This observed rate of erythropoiesis may reflect bone marrow/blood toxicity. (Tice et al. 1997).
4.5 Carcinogenic effects
The carcinogenic potential of TPGDA was investigated in mice by dermal administration. The test group consisted of 50 male C3H/HeJ mice that were treated dermally with approximately 100 mg/kg TPGDA (2.5 mg/mouse) twice a week for 80 weeks or until tumours were diagnosed or animals died. The dose used in the study was selected in a preliminary pilot study, where male C3H/HeJ mice were treated with different dilutions of TPGDA. The concentration that only produced minimal skin irritation was selected for the study. Two negative control groups (one untreated and one treated with mineral oil) and one positive control group (treated with benzo(a)pyrene) were included. No obvious skin irritation was seen although fibrosis and changes in pigmentation occurred. At necropsy, skin and body cavities were examined and tissues were taken for histopathological analysis, which did not reveal any increased incidence of skin or visceral tumours. Ten of 50 mice died in the study. In the positive control group, 42 of 50 mice developed carcinomas and in the negative control groups, 1 of 50 mice developed skin papilloma among non-treated and mineral oil treated mice. The test method is not further specified and the data are unpublished. (Celanese Corporation - quoted from Andrews & Clary 1986).
According to Mortensen (1991), the study is not in accordance with OECD guidelines and is inadequate for an evaluation of the carcinogenic potential of TPGDA.
Insertion of the zeta-globin promoted v-Ha-ras transgene into the FVB mouse genome (Tg.AC) produce a defined lesion, which is critical but insufficient by itself to induce benign or malignant tumours in the skin unless activated. Groups of 10 female Tg.AC (v-Ha-ras) mice (12 weeks old) were administered dermal doses of 1, 5 or 10 µmoles/mouse of TPGDA either alone or mixed with acetone (totally 200 µl per application) or as an equimolar amount of a lacquer (lacquer A), three times per week for 20 weeks to the shaved dorsal skin (8 cm2). Negative controls were vehicle treated animals and positive controls were 12-O-tetradecanonylphorbol-13-acetate (TPA) treated mice. The treatment with 5 and 10 µmoles of TPGDA either as the substance itself or as lacquer A induced a dose related increase in papillomas between 6 and 12 weeks of treatment that reached a maximum number of papillomas per mouse between 19 and 20 weeks of treatment. These result indicate that TPGDA may be predicted to be carcinogenic at the site of contact in a long-term cancer bioassay. More studies are advised to clarify the significance of the role of the TPGDA-induced cellular proliferation in the induction of papillomas. (Nylander-French & French 1998).
5 Regulations, limit values
Ambient air
Denmark (C-value): -
Drinking water
Denmark: -
Soil
-
OELs
Denmark: -
Classification
TPGDA is classified for irritative effects (Xi;R36/37/38 - irritating to eyes, respiratory system and skin), for sensitising effects (R43 - may cause sensitisation by skin contact), and for environmental effects (N;R51-53 - toxic to aquatic organism, may cause long-term adverse effects in the aquatic environment) (EU 2000).
EU
-
IARC/WHO
-
US-EPA
-
RD50
-
6 Summary
Description
Tripropyleneglycol diacrylate (TPGDA) is a pale, yellow tinted liquid with a mild odour. It is insoluble in water but soluble in many organic solvents. It has a very low vapour pressure (0.0106 mmHg at 25° C).
Environment
No data were found.
Human exposure
No data on human exposure have been found. However, the general population may be exposed to tripropyleneglycol diacrylate by inhalation of contaminated air and through contact with TPGDA containing products.
Toxicokinetics
No data have been found with respect to absorption and distribution after inhalation or oral intake. Absorption after dermal contact has not been examined but systemic effects have been observed in rabbits after repeated topical application indicating that absorption through the skin takes place.
Detoxification of TPGDA would most likely be by conjugation with glutathione. Acrylates most likely react on the site of contact.
Human toxicity
A number of individuals using TPGDA occupationally have developed allergic contact dermatitis.
Animal toxicity
single dose toxicity
An LD50-value of 6200 mg/kg b.w. has been reported for oral administration to rats and of > 2000 mg/kg b.w. for dermal application to rabbits.
irritation
TPGDA was at first only moderately irritating to rabbit skin but had a delayed effect corresponding to a corrosiveness. It was only slightly irritating to the rabbit eye.
sensitisation
TPGDA has shown equivocal results in the Guinea Pig Maximisation Tests (GPMT) with both positive and negative results being observed. However, the vehicle strongly influences the sensitising response. Cross reactions with other multifunctional acrylates have been observed.
repeated dose toxicity
Systemic toxicity, including convulsions, tremors and ataxia was observed in rabbits following application to the skin of 500 mg TPGDA for 5 times a week for 2 weeks. Systemic toxicity was observed in rats receiving topically doses of 200 mg/kg b.w. for 5 days a week for 90 days; the NOAEL in this study was 67 mg/kg b.w.
Reproductive and developmental effects
TPGDA was not foetotoxic or teratogenic in female rats when 250 mg/kg b.w./day of TPGDA was applied to the skin during day 6 to 15 of gestation.
Mutagenic and genotoxic effects
Tripropyleneglycol diacrylate was not mutagenic in Ames’ test with or without metabolic activation, but gave a positive response in the mouse lymphoma cell mutagenicity assay both with and without metabolic activation.
Following dermal application (3 times a week for 20 weeks) to transgenic mice (Tg.AC (v-Ha-ras)), no DNA damage (single-strand breaks, alkali labile sites, DNA crosslinking) was observed in peripheral blood leukocytes using the alkaline single cell gel assay and the frequency of micronucleated erythrocytes (polychromatic and normochromatic) was not altered.
Carcinogenicity
No signs of carcinogenicity were found in male CH3/HeJ mice treated topically twice every week for 80 weeks with 2.5 mg TPGDA (100 mg/kg b.w.).
An increased number of skin tumours were observed in TPGDA-treated female Tg.AC (v-Ha-ras) mice in a twenty week short-term tumourigenesis study. TPGDA was applied topically 3 times a week for 20 weeks in doses of 1, 5 or 10 µmoles/mouse either in form of technical quality TPGDA or as a lacquer intended for UV cured coatings. Negative controls were vehicle treated animals and positive controls were 12-O-tetradecanoylphorbol-13-acetate treated mice (10 animals per dose group). Number of papillomas was increased in mice treated with 5 and 10 µmoles and all doses of the lacquer and likewise the latency periods (time to occurrence) were shorter than for the negative controls.
7 Evaluation
The available data on human health effects are limited to some cases of allergic contact dermatitis. No data on respiratory sensitisation have been found.
The critical effects in humans following exposure to tripropyleneglycol diacrylate (TPGDA) is considered to be the irritative effects on the respiratory system as also observed following exposure to other acrylates, the skin damaging effects observed in animal studies upon repeated dermal exposure, and the sensitising potential (delayed contact hypersensitivity) observed among workers as well as in animal studies.
Systemic effects (not further specified in the publication) have been observed in one study of rats following dermal application. Based on the available data, it cannot be excluded that systemic effects may occur in humans following inhalation of TPGDA.
The available data on TPGDA are considered to be inadequate for the purpose of estimating a health based limit value in air. As TPGDA, in analogy to other acrylates as e.g., 2-ethylhexyl acrylate, is irritative to the respiratory system and a skin sensitiser, the C-value of 0.01 mg/m3 proposed for 2-ethylhexyl acrylate is proposed for TPGDA as well.
8 C-value
The available data on TPGDA are considered to be inadequate for the purpose of estimating a health based limit value in air. As TPGDA, in analogy to other acrylates, is irritative to the respiratory system and a skin sensitiser, the C-value of 0.01 mg/m3 proposed for 2-ethylhexyl acrylate and placing in Main Group 2 is proposed for TPGDA as well.
C-value
0.01 mg/m3, Main Group 2.
9 References
Andrews LS and Clary JJ (1986). Review of the toxicity of multifunctional acrylates. J Toxicol Environ Health 19, 149-164.
Anonymous (1999). Vurdering af UV-hærdende trykfarver og lakker i et samlet miljøperspektiv. Miljø og Energiministeriet, Miljøstyrelsen.
Björkner B (1984). The sensitising capacity of multifunctional acrylates in the Guinea pig. Cont Derm 11, 236-346.
Björkner B and Niklasson B (1984). Influence of the vehicle on contact allergic reactions to acrylic compounds in the Guinea pig. Cont Derm 11, 268-278.
Clouzeau J (1989). TPGDA sensitisation test in the guinea-pig (study No. 4680 TSG). Centre International de Toxicologie (C.I.T.), France. Unpublished report.
Clouzeau J (1989). TPGDA sensitisation test in the guinea-pig (study No. 4683 TSG). Centre International de Toxicologie (C.I.T.), France. Unpublished report.
Denton SM (1993). Photomer 4061 Bx.92315 skin sensitisation in the guinea-pig. HCR Report. Huntingdon Research Centre. Unpublished report.
EU (2000). Commission directive 2000/32/EC of 19 May 2000 adapting to technical progress for the 26th time Council Directive 67/548/EEC on the approximation of the laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Official Journal of the European Communities L 136.
Golden R (1997). Safety and handling of UV/EB curing materials. J Coat Technol 69, 83-89.
Goossens A, Connix D, Rommens K and Verhamme B (1998). Occupational dermatitis in a silk-screen maker. Cont Derm 39, 40-42.
Kanerva L, Jolanki R and Estlander T (1997). 10 years of patch testing with the (meth)acrylate series. Cont Derm 37, 255-258.
Kanerva L, Mikola H, Henriks-Eckerman ML, Jolanki R and Estlander T (1998). Fingertip paresthesia and occupational allergic contact dermatitis caused by acrylics in a dental nurse. Cont Derm 38, 114-116.
Mortensen B (1991). Tripropylene glycol diacrylate. In: Health effects of selected chemicals. Nordic Chemicals Group Vol. 1, 123-132.
Nylander-French LA, Fischer T, Hultengren M, Lewné M and Rosén G (1994). Exponering vid ytbehandling med ultravioletthärdande akrylatlacker i träindustrin. Arbete och Hälsa 13, 1-33.
Nylander-French LA and French JE (1998): Tripropyleneglycol diacrylate but not ethyl acrylate induces skin tumours in a twenty-week short-term tumourigenesis study in Tg.AC (v-Ha-ras) mice. Toxicol Pathol 26, 476-483.
Nylander-French LA, Priha E, Berglund GB and Rosén G (1994). A method for monitoring worker exposure to airborne multifunctional acrylates. Appl Occup Environ Hyg 9, 977-983.
Parker D and Turk JL (1983). Contact sensitivity to acrylate compounds in guinea pigs. Cont Derm 9, 55-60.
RTECS (through April 1999).
Roberts DW (1987). Structure-activity relationships for skin sensitisation potential of diacrylates and dimethacrylates. Cont Derm 17, 281-289.
Tice RR, Nylander-French LA and French JE (1997). Absence of systemic in vivo genotoxicity after dermal exposure to ethyl acrylate and tripropylene glycol diacrylate in Tg.AC (v-Ha-ras) mice. Environ Mol Mutagen 29, 240-249.
Voog L and Jansson B (1992). Identification and control of contact dermatitis from polyfunctional acrylic monomers in five Swedish furniture companies. J Environ Sci Health 27, 1925-1938.
[Front page] [Contents] [Previous] [Next] [Top] |